Abstract
Background
The neutrophil-to-lymphocyte (N/L) ratio has been associated with poor prognosis in patients with heart failure, but it has not been compared with N-terminal pro-brain natriuretic peptide (NT-proBNP) in elderly patients with chronic heart failure (CHF). We sought to make this comparison.
Methods
A total of 1355 elderly patients with CHF were analyzed. A multivariate logistic regression model was used to analyze the variables associated with atrial fibrillation (AF). Cox regression analysis was used to assess the multivariable relationship between the N/L ratio, NT-proBNP level, and subsequent major cardiovascular events (MCE).
Results
In the multiple logistic regression analysis, the N/L ratio was demonstrated as a risk factor for AF in elderly patients with CHF [odds ratio (OR): 1.079, 95% confidence interval (CI): 1.027–1.134, P = 0.003]. The median follow-up period was 18 months. In a multivariable model using tertiles of both variables, the highest tertile of the N/L ratio was significantly associated with MCE [hazard ratio (HR): 1.407, 95% CI: 1.098–1.802, P = 0.007] compared with the lowest tertile. Similarly, the highest NT-proBNP tertile was also significantly associated with MCE (HR: 1.461, 95% CI: 1.104–1.934, P = 0.008).
Conclusions
In elderly patients with CHF, the N/L ratio is one of the important risk factors for AF and it is an inexpensive and readily available marker with similar independent prognostic power to NT-proBNP. The risk of MCE increases 1.407-fold when the N/L ratio is elevated to the highest tertile.
Keywords: Atrial fibrillation, Chronic heart failure, Elderly patients, Neutrophil-to-lymphocyte ratio, N-terminal pro-brain natriuretic peptide
1. Introduction
Chronic heart failure (CHF), commonly the end-stage of cardiovascular diseases, is highly prevalent in aging populations worldwide and is becoming a significant health care burden in developed countries.[1] The prevalence of other diseases such as atrial fibrillation (AF) is common in elderly patients with heart failure. To target rational diagnosis, therapy, and prognosis monitoring to the most appropriate patients, simple and readily available biomarkers are needed for these patients. Such biomarkers should independently predict outcome, and the more widely available these variables are, the more likely the biomarker will be adopted into routine clinical practice.
Brain natriuretic peptide (BNP) is mainly secreted by the heart and released into the circulation in response to increased wall stretch due to increased volume and pressure overload of the heart.[2] Currently, the measurement of BNP and its biologically inactive N-terminal fragment (NT-proBNP) are viewed as the most powerful prognostic biomarkers available in heart failure.[3]–[5] Therefore, every novel potential biomarker should be compared against these peptides.
The neutrophil-to-lymphocyte (N/L) ratio is a composite inflammatory biomarker that combines two different immune pathways: neutrophils are involved in active nonspecific inflammation, and lymphopenia is associated with physiological stress.[6] The N/L ratio has been associated with poor outcomes in patients with heart failure in several studies,[7],[8] but has not yet been compared against either BNP or NT-proBNP. In this study, we intended to demonstrate that the N/L ratio is independent of NT-proBNP as a prognostic biomarker in elderly patients with CHF.
2. Methods
2.1. Patients
We enrolled 1355 elderly patients (mean age, 72.6 ± 8.0 years) referred to the Department of Cardiology, Chinese PLA General Hospital (Beijing, China) with CHF between January 2011 and January 2014. Patients were included in the analysis if they had a diagnosis of CHF and total white blood cell (WBC) and differential counts and NT-proBNP measurements were performed at the time of admission. The diagnosis of CHF was made according to the criteria of the European Society of Cardiology[9] by symptoms or signs, electrocardiogram, chest radiograph, and echocardiography. Patients were categorized on the basis of left ventricular ejection fraction (LVEF) into heart failure with reduced ejection fraction (HFrEF, LVEF < 40%), heart failure with mid-range ejection fraction (HFmrEF, 40% ≤ LVEF ≤ 49%), and heart failure with preserved ejection fraction (HFpEF, LVEF ≥ 50%) groups. Of the 1355 patients, 339 (25.0%), 375 (27.7%), and 641 (47.3%) patients were in the HFrEF, HFmrEF, and HFpEF group, respectively. The N/L ratio was calculated as the ratio of the neutrophils to lymphocytes from the differential counts. All referred patients had complete medical records, including demographic variables, laboratory values, echocardiographic parameters and information on past medical history and medications.
Patients with known evidence of acute myocardial ischemia, hematological diseases, a history of cancer and/or chemotherapy treatment, infection, chronic inflammatory conditions, glucocorticoid therapy and/or a history of glucocorticoid use three months before admission, which could affect the total white blood cell (WBC) and differential counts, were excluded. After a median follow-up period of 18 months (interquartile range 12 to 29), major cardiovascular events (MCE), including cardiac death and rehospitalization for heart failure, were documented through telephone follow-up and the hospital's medical records. All subjects provided informed consent. This study was approved by the Ethical Committee for Medical Research of Chinese PLA General Hospital and was conducted in accordance with the Helsinki Declaration.
2.2. Statistical analysis
Data analysis was performed using the Statistical Package for Social Sciences (SPSS 19.0). All continuous variables are given as the means ± SD, whereas discrete variables are presented as frequency counts and percentages. The continuous variables were compared between the MCE and non-MCE group using Student's t-tests and Mann-Whitney U tests for variables with normal and skewed distributions, respectively. The discrete variables were compared between the MCE and non-MCE group using the Chi-square test. A Spearman correlation test was employed to study the variables related to NT-proBNP levels and the N/L ratio. A multivariate logistic regression model was used to analyze the variables associated with AF. Odds ratio (OR) with 95% confidence intervals (CIs) were calculated. We used receiver operating characteristic (ROC) curves based on a univariate model to examine the power of NT-proBNP levels and the N/L ratio to predict MCE. The Kaplan-Meier method was used to assess the cumulative survival for MCE, and the log-rank test was used to compare differences among tertiles of NT-proBNP levels and the N/L ratio. Univariate and multivariate Cox regression models using tertiles of both variables were constructed to explore the relationship between the variables and outcome. The hazard ratios (HRs) with their 95% CIs were recorded. The variables for which a P value < 0.1 was obtained in the univariate analysis were considered for use in the multivariate model. A P value of less than 0.05 was considered significant.
3. Results
3.1. Patient demographics
The clinical characteristics of the total 1355 patients in the study cohort and a comparison of the baseline characteristics of patients who experienced MCE with those who did not are shown in Table 1. More male (60.2%) elderly patients with CHF (mean age, 72.6 ± 8.0 years) were included in this study. The basic diseases of CHF were mostly hypertension (74.6%) and coronary artery disease (CAD) (77.7%). Beta-blockers (74.0%), nitrates (63.8%), aspirin (70.7%), and statins (78.4%) were the main drugs used by the patients.
Table 1. Characteristics for the total study cohort and comparisons between patients that had MCE and those did not have them during the follow-up period.
| Total cohort (n = 1355) | MCE (n = 422) | Non-MCE (n = 933) | P value | |
| Age, yrs | 72.6 ± 8.0 | 73.9 ± 8.2 | 71.9 ± 7.8 | < 0.001 |
| Male | 60.2% | 66.4% | 57.4% | 0.002 |
| Body mass index, kg/m2 | 24.8 ± 3.8 | 24.7 ± 3.9 | 24.8 ± 3.8 | 0.544 |
| Systolic blood pressure, mmHg | 135.2 ± 20.4 | 134.7 ± 20.3 | 135.4 ± 20.5 | 0.547 |
| Diastolic blood pressure, mmHg | 75.3 ± 12.3 | 74.1 ± 12.7 | 75.8 ± 12.1 | 0.020 |
| Heart rate, bpm | 77.4 ± 15.6 | 77.3 ± 14.7 | 77.5 ± 16.0 | 0.762 |
| Hypertension | 74.6% | 75.1% | 74.4% | 0.774 |
| Coronary artery disease | 77.7% | 82.0% | 75.8% | 0.011 |
| Diabetes mellitus | 36.1% | 38.2% | 35.2% | 0.288 |
| Atrial fibrillation | 51.1% | 44.8% | 54.0% | 0.002 |
| Renal failure | 34.4% | 48.8% | 27.9% | < 0.001 |
| Total protein, g/L | 66.8 ± 6.2 | 66.9 ± 6.4 | 66.8 ± 6.1 | 0.664 |
| Albumin, g/L | 39.2 ± 4.1 | 38.8 ± 4.1 | 39.4 ± 4.1 | 0.022 |
| Blood urea nitrogen, mmol/L | 7.4 ± 3.9 | 8.5 ± 4.8 | 6.9 ± 3.3 | < 0.001 |
| Creatinine, umol/L | 97.2 ± 50.9 | 110.1 ± 59.7 | 91.4 ± 45.3 | < 0.001 |
| eGFR, mL·min−1 per 1.73 m−2 | 68.2 ± 21.3 | 61.7 ± 22.4 | 71.1 ± 20.1 | < 0.001 |
| Sodium, mmol/L | 140.6 ± 3.9 | 140.1 ± 3.9 | 140.7 ± 3.9 | 0.009 |
| Cholesterol, mmol/L | 3.9 ± 1.0 | 3.8 ± 1.0 | 3.9 ± 1.0 | 0.064 |
| Hemoglobin, g/L | 129.8 ± 19.8 | 127.1 ± 20.9 | 131.1 ± 19.2 | 0.001 |
| White blood cell, 109/L | 6.5 ± 2.2 | 6.5 ± 2.2 | 6.5 ± 2.2 | 0.618 |
| Platelets, 109/L | 185.7 ± 56.5 | 180.1 ± 54.7 | 188.3 ± 57.2 | 0.013 |
| Mean platelet volume, fL | 10.7 ± 1.2 | 10.7 ± 1.3 | 10.7 ± 1.2 | 0.903 |
| NT-proBNP, pg/mL | 3199.0 ± 5299.7 | 4196.0 ± 5837.9 | 2748.0 ± 4975.6 | < 0.001 |
| Neutrophil/lymphocyte | 3.2 ± 3.1 | 3.6 ± 3.1 | 3.0 ± 3.0 | 0.004 |
| Left ventricular ejection fraction, % | 48.1 ± 11.7 | 45.7 ± 12.0 | 49.1 ± 11.4 | < 0.001 |
| ACEIs | 30.8% | 29.9% | 31.2% | 0.623 |
| Angiotensin II receptor blockers | 33.0% | 34.1% | 32.5% | 0.550 |
| Calcium-channel blockers | 44.5% | 43.6% | 44.9% | 0.654 |
| Beta-blockers | 74.0% | 76.8% | 72.8% | 0.120 |
| Spironolactone | 45.9% | 56.9% | 40.9% | < 0.001 |
| Other diuretics | 45.5% | 56.9% | 40.3% | < 0.001 |
| Digoxin | 28.7% | 37.0% | 25.0% | < 0.001 |
| Nitrates | 63.8% | 68.0% | 61.8% | 0.029 |
| Aspirin | 70.7% | 71.8% | 70.2% | 0.550 |
| Clopidogrel | 50.2% | 50.7% | 49.9% | 0.794 |
| Warfarin | 15.4% | 12.3% | 16.8% | 0.033 |
| Statins | 78.4% | 78.2% | 78.5% | 0.915 |
| Length of stay, days | 11.3 ± 8.6 | 12.3 ± 7.8 | 10.9 ± 8.9 | 0.004 |
| NYHA class III/IV | 36.3% | 51.9% | 29.2% | < 0.001 |
Data are expressed as mean ± SD or %. ACEIs: angiotensin-converting enzyme inhibitors; eGFR: estimated glomerular filtration rate; MCE: major cardiovascular events; NT-proBNP: N-terminal pro-brain natriuretic peptide; NYHA class III/IV: New York Heart Association heart failure class III/IV.
Patients with MCE were more likely to be older and male and to have lower diastolic blood pressure, CAD, atrial fibrillation (AF), and renal failure. Albumin, sodium, hemoglobin (Hb), and platelet levels and the estimated glomerular filtration rate (eGFR) were lower in patients who experienced MCE, whereas blood urea nitrogen (BUN) and creatinine levels were higher. More patients who experienced MCE were receiving spironolactone, other diuretics, digoxin, and nitrates, but only 12.3% of the patients with MCE were on a warfarin, compared with 16.8% of the patients without MCE. In addition, patients who experienced MCE had worse CHF at baseline, as indicated by a lower LVEF, a higher HYHA class, and longer hospital stays.
NT-proBNP levels and the N/L ratio were significantly higher in patients who had MCE. ROC curves examining the power of NT-proBNP and the N/L ratio to predict MCE are shown in Figure 1. The areas under the curve were 0.628 (P < 0.001, 95% CI: 0.596–0.660) and 0.582 (P < 0.001, 95% CI: 0.549–0.615), respectively.
Figure 1. ROC curves based on a univariate model examining the power of N-terminal pro-brain natriuretic peptide and N/L ratio to predict major cardiovascular events.
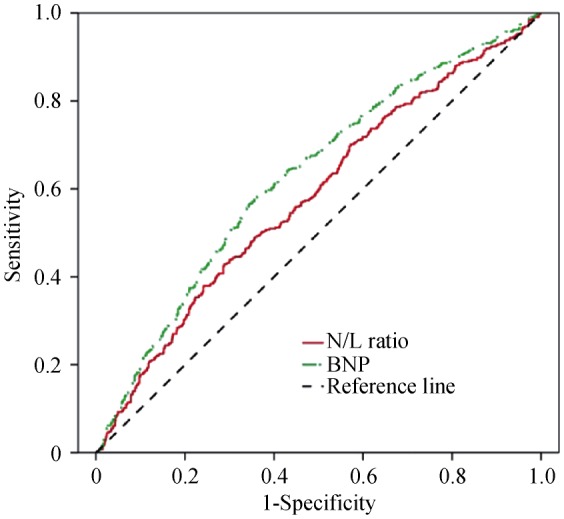
The areas under the curve were 0.628 (P < 0.001, 95% CI: 0.596–0.660) and 0.582 (P < 0.001, 95% CI: 0.549–0.615). BNP: brain natriuretic peptide; N/L ratio: neutrophil-to-lymphocyte ratio; ROC: receiver operating characteristic.
The prognoses of heart failure with reduced, mid-range and preserved ejection fraction are shown in Table 2. Patients with HFmrEF and HFpEF had a better prognosis than those with HFrEF.
Table 2. Prognoses of heart failure with reduced, mid-range and preserved ejection fraction.
| HFrEF | HFmrEF | HFpEF | P value | |
| MCE | 45.1% | 26.7% | 26.4% | < 0.001 |
| Non-MCE | 54.9% | 73.3% | 73.6% | < 0.001 |
HFmrEF: heart failure with mid-range ejection fraction; HFpEF: heart failure with preserved ejection fraction; HFrEF: heart failure with reduced ejection fraction; MCE: major cardiovascular events.
3.2. The results of the Spearman correlation test
The results of the Spearman correlation test are shown in Table 3. The N/L ratio and NT-proBNP exhibited a positive correlation (r = 0.274, P < 0.001). The N/L ratio was positively correlated with age, heart rate, BUN, creatinine, WBC and length of stay. This measurement was also positively correlated with being male; cigarette smoking; a history of diabetes mellitus or renal failure; the use of spironolactone, other diuretics or digoxin; and HYHA class. The N/L ratio was negatively correlated with body mass index (BMI), total protein, albumin, eGFR, sodium, cholesterol, Hb and LVEF. The N/L ratio was also negatively correlated with the use of aspirin and statins. Higher NT-proBNP levels were associated with increased age, heart rate, BUN, creatinine, WBC, mean platelet volume, and length of stay; worse HYHA status; a history of diabetes mellitus or renal failure; and the use of spironolactone, other diuretics or digoxin. The peptide's levels were lower in patients with a higher BMI, systolic blood pressure, total protein, albumin, eGFR, sodium, cholesterol, Hb, platelets and LVEF; subject history of AF; and the use of angiotensin II receptor blockers, calcium-channel blockers, aspirin or statins.
Table 3. Spearman correlation coefficients for N-terminal pro-brain natriuretic peptide and N/L ratio.
| NT-proBNP | P value | N/L ratio | P value | |
| N/L ratio | 0.274 | < 0.001 | − | − |
| Age, yrs | 0.114 | < 0.001 | 0.156 | < 0.001 |
| Male | −0.018 | 0.505 | 0.185 | < 0.001 |
| BMI, kg/m2 | −0.200 | < 0.001 | −0.085 | 0.002 |
| Systolic blood pressure, mmHg | −0.160 | < 0.001 | 0.010 | 0.723 |
| Heart rate, beats/min | 0.238 | < 0.001 | 0.093 | 0.001 |
| Diabetes mellitus | 0.086 | 0.002 | 0.084 | 0.002 |
| Atrial fibrillation | −0.057 | 0.037 | 0.001 | 0.968 |
| Renal failure | 0.327 | < 0.001 | 0.190 | < 0.001 |
| Total protein, g/L | −0.123 | < 0.001 | −0.108 | < 0.001 |
| Albumin, g/L | −0.318 | < 0.001 | −0.202 | < 0.001 |
| Blood urea nitrogen, mmol/L | 0.308 | < 0.001 | 0.190 | < 0.001 |
| Creatinine, μmol/L | 0.315 | < 0.001 | 0.193 | < 0.001 |
| eGFR, mL·min−1 per 1.73m−2 | −0.347 | < 0.001 | −0.161 | < 0.001 |
| Sodium, mmol/L | −0.166 | < 0.001 | −0.244 | < 0.001 |
| Cholesterol, mmol/L | −0.109 | < 0.001 | −0.090 | 0.001 |
| Hemoglobin, g/L | −0.214 | < 0.001 | −0.108 | < 0.001 |
| White blood cell, 109/L | 0.065 | 0.017 | 0.312 | < 0.001 |
| Platelets, 109/L | −0.069 | 0.011 | −0.052 | 0.056 |
| Mean platelet volume, fL | 0.102 | < 0.001 | −0.023 | 0.387 |
| Left ventricular ejection fraction, % | −0.362 | < 0.001 | −0.071 | 0.009 |
| Angiotensin II receptor blockers | −0.069 | 0.011 | −0.018 | 0.505 |
| Calcium-channel blockers | −0.113 | < 0.001 | 0.027 | 0.326 |
| Spironolactone | 0.342 | < 0.001 | 0.142 | < 0.001 |
| Other diuretics | 0.315 | < 0.001 | 0.146 | < 0.001 |
| Digoxin | 0.287 | < 0.001 | 0.095 | < 0.001 |
| Aspirin | −0.076 | 0.005 | −0.068 | 0.012 |
| Statins | −0.158 | < 0.001 | −0.083 | 0.002 |
| Length of stay, days | 0.204 | < 0.001 | 0.163 | < 0.001 |
| NYHA class | 0.419 | < 0.001 | 0.190 | < 0.001 |
BMI: body mass index; eGFR: estimated glomerular filtration rate; N/L ratio: neutrophil-to-lymphocyte ratio; NT-proBNP: N-terminal pro-brain natriuretic peptide; NYHA class: New York Heart Association heart failure class.
3.3. Risk factor of AF
Variables associated with AF were analyzed using multivariate logistic regression (Table 4). On multivariate regression, NLR (OR: 1.079, 95% CI: 1.027–1.134, P = 0.003) was independently associated with AF in the elderly with CHF.
Table 4. Effects of multiple variables on atrial fibrillation in multivariate logistic regression analysis.
| Variable | OR | 95% CI | P value |
| Age | 1.056 | 1.035–1.077 | < 0.001 |
| Diastolic blood pressure, mmHg | 1.013 | 1.000–1.026 | 0.047 |
| Heart rate, beats/min | 1.015 | 1.004–1.025 | 0.005 |
| Coronary artery disease | 0.489 | 0.325–0.735 | 0.001 |
| Diabetes mellitus | 0.640 | 0.475–0.864 | 0.004 |
| Neutrophil/lymphocyte | 1.079 | 1.027–1.134 | 0.003 |
| Cholesterol, mmol/L | 0.728 | 0.624–0.849 | < 0.001 |
| Hemoglobin, g/L | 1.018 | 1.010–1.026 | < 0.001 |
| Left ventricular ejection fraction, % | 1.125 | 1.109–1.142 | < 0.001 |
| Clopidogrel | 0.548 | 0.400–0.752 | < 0.001 |
| Warfarin | 12.030 | 6.453–22.426 | < 0.001 |
Adjusted for: age, gender, body mass index, diastolic blood pressure, heart rate, hypertension, coronary artery disease, diabetes mellitus, neutrophil-to-lymphocyte ratio, N-terminal pro-brain natriuretic peptide, creatinine, cholesterol, hemoglobin, platelets, left ventricular ejection fraction, length of stay, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, calcium-channel blockers, spironolactone, other diuretics, nitrates, aspirin, clopidogrel, warfarin, statins, and New York Heart Association heart failure class.
3.4. Prognosis of CHF
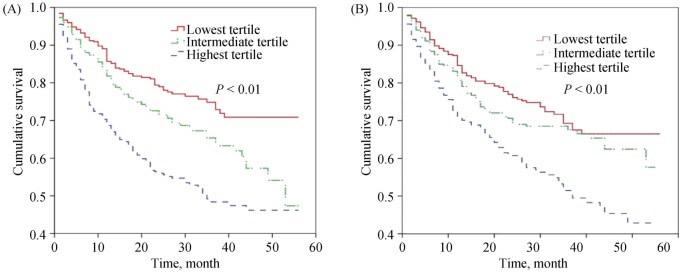
During a median follow-up period of 18 (interquartile range 12 to 29) months, MCE occurred in 422 patients (31.1%). Kaplan-Meier plots for NT-proBNP levels and the N/L ratio by tertiles are shown in Figure 2A and 2B, respectively. In univariate analyses using the lowest tertile of the N/L ratio as a reference, the patients in the highest tertile demonstrated a higher risk of MCE (HR: 1.586, 95% CI: 1.243–2.025, P < 0.001). The highest NT-proBNP tertile was also significantly associated with MCE (HR: 2.152, 95% CI: 1.671–2.772, P < 0.001), compared with the lowest tertile. In a multivariable model using tertile of both variable, age; cholesterol and platelets levels; HYHA class; and a history of CAD, AF, and renal failure were also independent predictors of outcome. The highest tertile of the N/L ratio was significantly associated with MCE (HR: 1.407, 95% CI: 1.098–1.802, P = 0.007), compared with the lowest tertile. Similarly, the highest NT-proBNP tertile was also significantly associated with MCE (HR: 1.461, 95% CI: 1.104–1.934, P = 0.008), compared with the lowest tertile. No differences were observed between the intermediate and lowest tertiles in terms of MCE risk for either variable (Table 5).
Figure 2. Kaplan–Meier plot for N-terminal pro-brain natriuretic peptide (A) and neutrophil-to-lymphocyte ratio by tertiles (B).
(A): The highest N-terminal pro-brain natriuretic peptide tertile was significantly associated with MCE (HR: 2.152, 95% CI: 1.671–2.772, P < 0.001); (B): The highest neutrophil-to-lymphocyte ratio tertile was significantly associated with MCE (HR: 1.586, 95% CI: 1.243–2.025, P < 0.001). MCE: major cardiovascular events.
Table 5. Univariate and multivariate Cox models using tertiles of N-terminal pro-brain natriuretic peptide and N/L ratio for major cardiovascular events.
| Variable | Univariate HR (95% CI) | P value | Multivariable HR (95% CI) | P value |
| NT-proBNP (high vs. low tertile) | 2.152 (1.671–2.772) | < 0.001 | 1.461 (1.104–1.934) | 0.008 |
| NT-proBNP (Intermediate vs. low tertile) | 1.399 (1.073–1.824) | 0.013 | 1.232 (0.940–1.616) | 0.130 |
| N/L ratio (high vs. low tertile) | 1.586 (1.243–2.025) | < 0.001 | 1.407 (1.098–1.802) | 0.007 |
| N/L ratio (Intermediate vs. low tertile) | 1.178 (0.913–1.520) | 0.207 | 1.051 (0.813–1.360) | 0.704 |
| Age, yrs | 1.028 (1.015–1.040) | < 0.001 | 1.015 (1.002–1.028) | 0.021 |
| Coronary artery disease | 1.433 (1.117–1.837) | 0.005 | 1.435 (1.108–1.859) | 0.006 |
| Atrial fibrillation | 0.743 (0.613–0.900) | 0.002 | 0.772 (0.629–0.947) | 0.013 |
| Renal failure | 2.073 (1.713–2.510) | < 0.001 | 1.525 (1.237–1.881) | < 0.001 |
| Platelets, 109/L | 0.998 (0.996–0.999) | 0.008 | 0.998 (0.996–1.000) | 0.013 |
| NYHA class | 1.698 (1.494–1.930) | < 0.001 | 1.369 (1.182–1.586) | < 0.001 |
| left ventricular ejection fraction | 0.981 (0.973–0.989) | < 0.001 | 0.995 (0.984–1.006) | 0.346 |
Adjusted for: age, gender, coronary artery disease, atrial fibrillation, renal failure, albumin, blood urea nitrogen, creatinine, estimated glomerular filtration rate, sodium, cholesterol, hemoglobin, platelets, left ventricular ejection fraction, diastolic blood pressure, length of stay, other diuretics, digoxin, warfarin, and New York Heart Association heart failure class. N/L ratio: neutrophil-to-lymphocyte ratio; NT-proBNP: N-terminal pro-brain natriuretic peptide; NYHA class: New York Heart Association heart failure class.
4. Discussion
In our study, patients with HFpEF had a better prognosis than those with HFrEF. But there are conflicting reports regarding the long-term outcomes of HFpEF. Some have shown better event rates in HFpEF,[10] while others have shown similar event rates between HFpEF or HFrEF.[11],[12] The multitude of concurrent issues also renders it more difficult to pinpoint heart failure as the cause of patients' symptoms or mortality in HFpEF patients. Indeed, although classifying the mode of death is highly subjective, HFpEF patients are less likely than HFrEF to die from definite heart failure or cardiovascular disease in general.[13] The contribution of non-cardiovascular modes of death may also explain the inconsistent mortality rate found in different HFpEF studies in different populations with different comorbidities.
We found that in elderly patients with CHF, the N/L ratio is one of the important risk factors for AF, and it is independent of NT-proBNP as a prognostic biomarker and that the risk of MCE increases 1.407-fold when the patient N/L ratio is elevated to the highest tertile (median 4.10, interquartile range: 3.39–6.01).
We discovered that the N/L ratio was one of the important risk factors for AF in elderly patients with CHF. The prevalence of AF is increasing as the population ages. Inflammation, which is related to the initiation and maintenance of AF, plays a key role in AF.[14] Both the pre- and post-operative N/L ratios are associated with an increased risk of developing AF after CABG,[15] and the N/L ratio is predictor of AF recurrence after cardioversion with amiodarone.[16] Heart failure and AF often coexist and each condition can promote the other, with an associated increase in overall morbidity and mortality.[17] So a higher N/L ratio was associated with an increased risk of AF which may make the prognosis of heart failure become worse in the elderly with CHF.
Previous studies examining the effect of the N/L ratio on heart failure prognosis did not include comparisons with NT-proBNP. The predictive ability of BNP or NT-proBNP values has long been reported in patients with heart failure.[18],[19] One study confirmed that NT-proBNP values provided independent prognostic information in Chinese elderly and very elderly patients with CHF.[20] Therefore, an evaluation of the N/L ratio's performance as a prognostic biomarker in relation to that of BNP or NT-proBNP in elderly patients with CHF is necessary.
The N/L ratio is a composite inflammatory biomarker and has been associated with various cardiac and noncardiac diseases.[6],[21] Many studies in acute coronary syndromes have reported that increased outcome risk was observed in patients with higher N/L values.[22],[23] In recent years, the N/L ratio has also been studied in heart failure, especially in acute heart failure. The N/L ratio has been found to be a better predictor of long-term mortality in patients with acute decompensated heart failure, which was independent of the LVEF.[7] A higher N/L ratio has been found to be associated with an increased risk of in-hospital mortality in patients with acute heart failure.[24],[25] Additionally, in patients with advanced heart failure, elevated N/L ratios were also associated with increased mortality or heart transplantation risk.[8] However, little attention has been paid to the importance of the N/L ratio in the context of CHF. The N/L ratio has not yet been investigated in elderly patients with CHF and has not been compared with NT-proBNP levels.
As a systemic syndrome, CHF is known to activate the immune system and inflammatory responses and is thus characterized by elevated levels of pro-inflammatory cytokines in the circulation.[26],[27] Inflammation is important in the pathogenesis, progression, severity and prognosis of CHF, and inflammatory mediators participate in its pathophysiology in various ways such as by exerting a direct impact on cardiac myocytes, fibroblasts and β-adrenergic receptors, leading to hypertrophy, fibrosis and impaired cardiac contractility, respectively, and by inducing apoptosis through stimulation of the proper genes.[28],[29] Inflammatory markers such as IL-6 and TNF are associated with heart failure risk among elderly patients and may improve heart failure risk stratification.[30] The N/L ratio is a composite inflammatory biomarker that combines two different immune pathways: neutrophils are involved in active nonspecific inflammation, and lymphopenia is associated with physiological stress.[6] Our study also found that age, heart rate, smoking, creatinine levels and albumin levels were correlated with the N/L ratio; these factors are among the nine routinely available variables used in the Health Aging and Body Composition heart failure model for incident heart failure in the elderly.[31] Thus, the N/L ratio appears to be a potent risk marker for CHF in elderly patients.
4.1. Study limitations
This study included some limitations. We estimated only the predictive power of variables at a single time point and cannot assess changes in these variables over time or the impact of these changes on CHF outcomes. Our study was based on a single center and restricted to hospitalized patients, which may have introduced bias. Therefore, our results may be difficult to generalize to all elderly patients with CHF. We did not have information on the components of MCE, including cardiac death and rehospitalization for heart failure. Because neutrophilia and lymphopenia are reported to be associated with increased mortality in acute coronary syndromes and heart failure,[32],[33] other works are needed to compare their prognostic power with that of the N/L ratio.
4.2. Conclusions
Our study demonstrated for the first time that the N/L ratio was one of the important risk factors for AF and it was an inexpensive and readily available marker in elderly patients with CHF. This measurement provides good prognostic value, even when compared with a relatively expensive NT-proBNP measurement. We believe that the N/L ratio has a strong potential as a useful future marker of CHF.
Acknowledgments
This work was supported by the International Science and Technology Cooperation Program of China (2013DFA31170) and the Science and Technology Program of Beijing (Z151100003915075). The authors have no conflicts of interest to disclose.
References
- 1.Abete P, Testa G, Della-Morte D, et al. Treatment for chronic heart failure in the elderly: current practice and problems. Heart Fail Rev. 2013;18:529–551. doi: 10.1007/s10741-012-9363-6. [DOI] [PubMed] [Google Scholar]
- 2.Ruskoaho H. Cardiac hormones as diagnostic tools in heart failure. Endocr Rev. 2003;24:341–356. doi: 10.1210/er.2003-0006. [DOI] [PubMed] [Google Scholar]
- 3.Masson S, Latini R, Anand IS, et al. Prognostic value of changes in N-terminal pro-brain natriuretic peptide in Val-HeFT (Valsartan Heart Failure Trial) J Am Coll Cardiol. 2008;52:997–1003. doi: 10.1016/j.jacc.2008.04.069. [DOI] [PubMed] [Google Scholar]
- 4.Naffaa M, Makhoul BF, Tobia A, et al. Brain natriuretic peptide at discharge as a predictor of 6-month mortality in acute decompensated heart failure. Am J Emerg Med. 2014;32:44–49. doi: 10.1016/j.ajem.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Berin R, Zafrir B, Salman N, et al. Single measurement of serum N-terminal pro-brain natriuretic peptide: the best predictor of long-term mortality in patients with chronic systolic heart failure. Eur J Intern Med. 2014;25:458–462. doi: 10.1016/j.ejim.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Bhat T, Teli S, Rijal J, et al. Neutrophil to lymphocyte ratio and cardiovascular diseases: a review. Expert Rev Cardiovasc Ther. 2013;11:55–59. doi: 10.1586/erc.12.159. [DOI] [PubMed] [Google Scholar]
- 7.Uthamalingam S, Patvardhan EA, Subramanian S, et al. Utility of the neutrophil to lymphocyte ratio in predicting long-term outcomes in acute decompensated heart failure. Am J Cardiol. 2011;107:433–438. doi: 10.1016/j.amjcard.2010.09.039. [DOI] [PubMed] [Google Scholar]
- 8.Benites-Zapata VA, Hernandez AV, Nagarajan V, et al. Usefulness of neutrophil-to-lymphocyte ratio in risk stratification of patients with advanced heart failure. Am J Cardiol. 2015;115:57–61. doi: 10.1016/j.amjcard.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 10.Meta-analysis Global Group in Chronic Heart Failure (MAGGIC) The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: an individual patient data meta-analysis. Eur Heart J. 2012;33:1750–1757. doi: 10.1093/eurheartj/ehr254. [DOI] [PubMed] [Google Scholar]
- 11.Bhatia RS, Tu JV, Lee DS, et al. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–269. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 12.Rickenbacher P, Pfisterer M, Burkard T, et al. Why and how do elderly patients with heart failure die? Insights from the TIME-CHF study. Eur J Heart Fail. 2012;14:1218–1229. doi: 10.1093/eurjhf/hfs113. [DOI] [PubMed] [Google Scholar]
- 13.Hamaguchi S, Kinugawa S, Sobirin MA, et al. Mode of death in patients with heart failure and reduced vs. preserved ejection fraction: report from the registry of hospitalized heart failure patients. Circ J. 2012;76:1662–1669. doi: 10.1253/circj.cj-11-1355. [DOI] [PubMed] [Google Scholar]
- 14.Aviles RJ, Martin DO, Apperson-Hansen C, et al. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108:3006–3010. doi: 10.1161/01.CIR.0000103131.70301.4F. [DOI] [PubMed] [Google Scholar]
- 15.Gibson PH, Cuthbertson BH, Croal BL, et al. Usefulness of neutrophil/lymphocyte ratio as predictor of new-onset atrial fibrillation after coronary artery bypass grafting. Am J Cardiol. 2010;105:186–191. doi: 10.1016/j.amjcard.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Karavelioğlu Y, Karapınar H, Yüksel M, et al. Neutrophil to lymphocyte ratio is predictor of atrial fibrillation recurrence after cardioversion with amiodarone. Clin Appl Thromb Hemost. 2015;21:5–9. doi: 10.1177/1076029613518368. [DOI] [PubMed] [Google Scholar]
- 17.Thihalolipavan S, Morin DP. Atrial fibrillation and heart failure: update 2015. Prog Cardiovasc Dis. 2015;58:126–135. doi: 10.1016/j.pcad.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Hartmann F, Packer M, Coats AJ, et al. Prognostic impact of plasma N-terminal pro-brain natriuretic peptide in severe chronic congestive heart failure: a substudy of the Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) trial. Circulation. 2004;110:1780–1786. doi: 10.1161/01.CIR.0000143059.68996.A7. [DOI] [PubMed] [Google Scholar]
- 19.Doust JA, Pietrzak E, Dobson A, et al. How well does B-type natriuretic peptide predict death and cardiac events in patients with heart failure: systematic review. BMJ. 2005;330:625. doi: 10.1136/bmj.330.7492.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fu S, Xie L, Li D, et al. The predictive capacity and additional prognostic power of N-terminal pro-B-type natriuretic peptide in Chinese elderly with chronic heart failure. Clin Interv Aging. 2015;10:359–365. doi: 10.2147/CIA.S77417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Templeton AJ, McNamara MG, Šeruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:dju124. doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- 22.Akpek M, Kaya MG, Lam YY, et al. Relation of neutrophil/lymphocyte ratio to coronary flow to in-hospital major adverse cardiac events in patients with ST-elevated myocardial infarction undergoing primary coronary intervention. Am J Cardiol. 2012;110:621–627. doi: 10.1016/j.amjcard.2012.04.041. [DOI] [PubMed] [Google Scholar]
- 23.Park JJ, Jang HJ, Oh IY, et al. Prognostic value of neutrophil to lymphocyte ratio in patients presenting with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am J Cardiol. 2013;111:636–642. doi: 10.1016/j.amjcard.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 24.Tasal A, Erturk M, Uyarel H, et al. Utility of the neutrophil to lymphocyte ratio for predicting in-hospital mortality after levosimendan infusion in patients with acute decompensated heart failure. J Cardiol. 2014;63:418–423. doi: 10.1016/j.jjcc.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 25.Turfan M, Erdoğan E, Tasal A, et al. Neutrophil-to-lymphocyte ratio and in-hospital mortality in patients with acute heart failure. Clinics (Sao Paulo) 2014;69:190–193. doi: 10.6061/clinics/2014(03)08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diwan A, Tran T, Misra A, et al. Inflammatory mediators and the failing heart: a translational approach. Curr Mol Med. 2003;3:161–182. doi: 10.2174/1566524033361537. [DOI] [PubMed] [Google Scholar]
- 27.Yndestad A, Damås JK, Øie E, et al. Role of inflammation in the progression of heart failure. Curr Cardiol Rep. 2007;9:236–241. doi: 10.1007/BF02938356. [DOI] [PubMed] [Google Scholar]
- 28.Braunwald E. Biomarkers in heart failure. N Engl J Med. 2008;358:2148–2159. doi: 10.1056/NEJMra0800239. [DOI] [PubMed] [Google Scholar]
- 29.Bouras G, Giannopoulos G, Hatzis G, et al. Inflammation and chronic heart failure: from biomarkers to novel anti-inflammatory therapeutic strategies. Med Chem. 2014;10:682–699. doi: 10.2174/1573406410666140318113325. [DOI] [PubMed] [Google Scholar]
- 30.Kalogeropoulos A, Georgiopoulou V, Psaty BM, et al. Inflammatory markers and incident heart failure risk in older adults: the Health ABC (Health, Aging, and Body Composition) study. J Am Coll Cardiol. 2010;55:2129–2137. doi: 10.1016/j.jacc.2009.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Butler J, Kalogeropoulos A, Georgiopoulou V, et al. Incident heart failure prediction in the elderly: the health ABC heart failure score. Circ Heart Fail. 2008;1:125–133. doi: 10.1161/CIRCHEARTFAILURE.108.768457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arruda-Olson AM, Reeder GS, Bell MR, et al. Neutrophilia predicts death and heart failure after myocardial infarction: a community-based study. Circ Cardiovasc Qual Outcome. 2009;2:656–662. doi: 10.1161/CIRCOUTCOMES.108.831024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaduganathan M, Ambrosy AP, Greene SJ, et al. Predictive value of low relative lymphocyte count in patients hospitalized for heart failure with reduced ejection fraction: insights from the EVEREST trial. Circ Heart Fail. 2012;5:750–758. doi: 10.1161/CIRCHEARTFAILURE.112.970525. [DOI] [PubMed] [Google Scholar]