Abstract
Background:
Extradural removal of the anterior clinoid process (ACP) is a crucial step in the proper surgical exposure of various pathologies in and around the central skull base. Since the pioneering description by Dolenc, the technique of extradural clinoidectomy has undergone several refinements in the light of improved understanding of microsurgical anatomy and maturation of neurosurgical techniques. Mastery of the surgical nuances involved in performing this surgical exercise will allow the young neurosurgeon to execute this step without undue reluctance and trepidation.
Objective:
This paper is an attempt to describe in detail, from a learner's viewpoint, the sequence of maneuvers involved in extradural removal of the ACP.
Materials and Methods:
The standard pterional approach and extradural anterior clinoidectomy was performed on four sides of two formalin fixed and latex injected cadaver heads. Important steps were photographed through the surgical microscope.
Conclusion:
An accurate understanding of the microsurgical anatomy of this region and the surgical nuances relevant to extradural clinoidectomy helps simplify the complexity of this surgical step.
Keywords: Anterior clinoid, extradural clinoidectomy, nuances
Introduction
Extradural removal of the anterior clinoid process (ACP) is an important step in surgical access to the lesions involving the central skull base, especially, sellar and parasellar pathologies. Clinoidectomy was initially described intradurally by Drake et al.[1] Vinco Dolenc pioneered the extradural removal of the clinoid process in his path-breaking papers on the exposure of vascular lesions within and around the cavernous sinus.[2] Ever since the importance of clinoidectomy in enhancing surgical access in this region was realized, the proper side of the dura from which to perform this step has been debated upon. Extradural anterior clinoidectomy is often perceived as difficult and risky due to the constricted working space and the vulnerability of critical anatomical structures encountered, which includes the clinoidal carotid segment, oculomotor nerve and the optic nerve. However, with proper extradural exposure of the ACP and accurate knowledge of the anatomical relationships in this region, extradural anterior clinoidectomy can be safely accomplished. Illustrating through cadaveric prosection, we intend to describe in this paper the proper technique for extradural clinoidectomy, emphasizing the key steps for optimum exposure and safe removal of the anterior clinoid.
Surgical anatomy of the anterior clinoid process
The anterior clinoid process is conical structure with a narrow tip and a wide base. When viewed from above, the tip of the ACP is directed postero-medially. Anteriorly, the base of the clinoid process is continuous with the lesser wing of the sphenoid bone. Medially, two bony elements, namely the anterior and posterior roots form a bridge between the base of ACP and the body of the sphenoid bone. The anterior root forms the roof the optic canal. The posterior root is the optic strut, which separates the optic canal from the superior orbital fissure (SOF). In cross-section through the mid-portion of the ACP, we can recognize a cortical shell enclosing a trabeculated medullary space, which is pneumatized to a variable degree. The sphenoid sinus commonly extends to the optic strut but only occasionally extends to the ACP. All the three connections of the ACP need to be divided before it can be removed. A controlled and safe removal of the clinoid requires the exposure of the clinoid process almost up to its tip,[3] which needs certain crucial maneuvers described in the following sections.
Positioning, incision and craniotomy
The head is held in skull clamp and positioned with neck slightly flexed and the head extended. The head is also rotated to the contralateral side up to 30 degrees. Extensive head rotation kinks the veins in the neck and should be avoided. We prefer a fronto-temporal (FT) craniotomy to the cranio-orbito zygomatic (COZ) approach for extradural removal of the clinoid, as the former exposure is quicker and avoids extensive soft tissue dissection. Unless dictated by the pathology, COZ does not confer an advantage over FT for extradural clinoidectomy per se.
A curvilinear incision is then marked beginning within 1 cm of the tragus from the superior border of the zygomatic arch and extended towards the midline behind the hairline. Care is taken to ensure that the straight line joining the two ends of the incision is within 1 cm of the junction of superior temporal line and zygomatic process (which is the surface marking for the McCarty's “key” burrhole) [Figure 1a]. A sub-galeal scalp flap is raised preserving the parietal branch of the superficial temporal artery. A sub-fascial dissection is carried out over the temporal fat pad to preserve the frontal branch of the facial nerve. Preserving a cuff of temporalis muscle for reattachment, the temporalis muscle is dissected subperiosteally as illustrated [Figure 1b and c]. A retrograde dissection technique described by Oikawa et al.[4] helps to maintain the intended plane of dissection. Moreover the periosteum is most strongly adherent to the bone at the squamosal suture and must be carefully dissected in this region. Use of monopolar cautery should be avoided while elevating the temporalis muscle in order to minimize postoperative atrophy. It is important to strip the muscle attachment from the frontal process of the zygomatic bone until the fronto-zygomatic suture and the zygomatic tubercle are exposed. If this tubercle is prominent it may be drilled to render it flush with the lateral orbital wall. The temporalis muscle is then strongly retracted inferiorly with loops of full-thickness sutures and elastic bands. This low profile retraction of the muscle optimizes the working space. In patients having a thick temporalis muscle the zygomatic notch may be deepened by exposing the zygomatic process fully and drilling the notch.[5]
Figure 1.
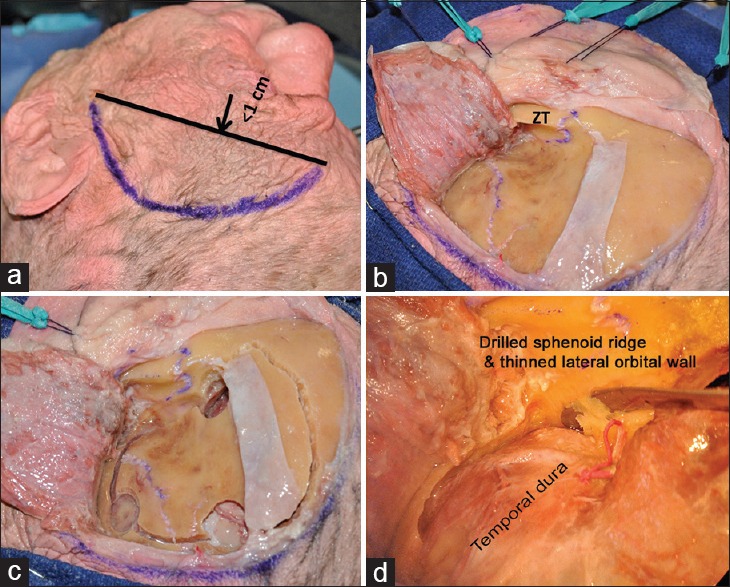
(a) Marking the skin incision, (b) the scalp flap and the temporalis muscle elevated preserving the shiny pericranium underlying the temporalis muscle (zygomatic tubercle), (c) burrholes and demarcation of bone flap, (d) dissecting the periorbita from its bony confines after drilling the sphenoid ridge
The FT free bone flap is elevated in routine manner and the sphenoid ridge drilled extensively till the posterior portion of lateral orbital wall and the orbital roof are reduced to egg shell thickness. This thin shell is then fractured with a sharp curved dissector to create a window. Through this fenestration the periorbita is dissected away from the remaining bony enclosure [Figure 1d], keeping it intact. The posterior half of the orbital wall and roof are then removed with a small rongeur, which unroofs the SOF and also exposes the orbitotemporal periosteal fold (OTPF).[6] It must be noted that at this step the continuity of the ACP with the lesser wing of sphenoid has been interrupted.
Dividing the orbitotemporal periosteal fold and exposure of anterior clinoid process
The meningio-orbital artery running in the OTPF is then coagulated and cut. This is a crucial step. Although the OTPF is being divided (preferably with a Beaver® miniblade), the anterior root of the ACP, medial to this fold, must constantly be felt with the advancing blade of the instrument. Proceeding beyond the “bony feel,” is likely to lead the instrument to the SOF resulting in damage to the lacrimal nerve, which runs most superficially in the fissure. The orientation of the “dural groove” marking the junction of periorbita and temporal dura is followed for orientation while dividing the OTPF [Figure 2a]. This dural groove also marks the starting point for next step; the peeling of the dura propria from the lateral wall of the cavernous sinus progressively exposing the third nerve, fourth, V1 (ophthalmic), V2 (maxillary) nerves. Sectioning the OTPF actually facilitates the identification and establishment of the proper plane for this dissection. The fourth nerve is first to be identified in this dissection, as the ACP often hides the third nerve for the most distal part of its course in the lateral wall of cavernous sinus. If the plane of dissection is lost somewhere during this step, it is advisable to return to point where it is relatively well-established and proceed from there. The purpose of this dissection is to reveal the whole length of the ACP, almost up to its tip. As a result, the surgical working cone becomes larger.
Figure 2.
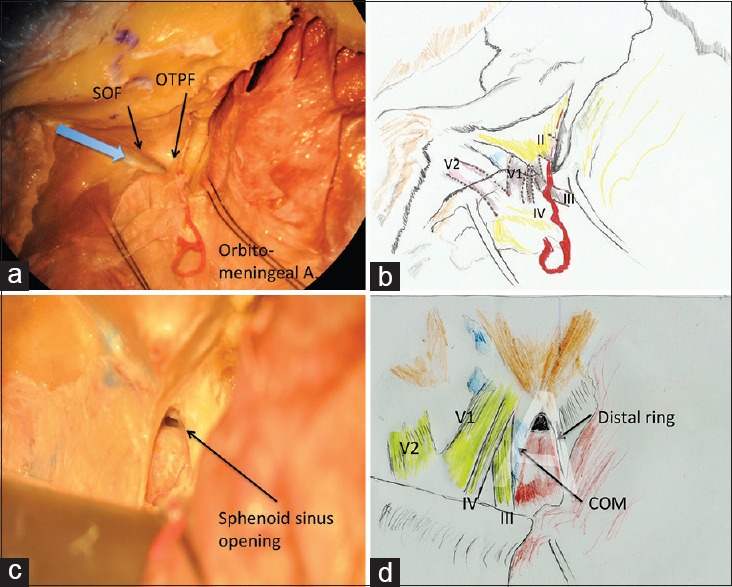
(a) The orbitotemporal periosteal fold (OTPF) and superior orbital fissure, the blue arrow shows the direction in which OTPF should be incised, (b) schematic drawing showing the expected positions of neural structures around the OTPF, (c) the clinoidal space following clinoidectomy, (d) schematic drawing showing the important structures in clinoidal space
To check the bleeding from cavernous sinus, fibrin glue may be injected into the angle between the V1 and V2.[7] In certain cases where the dural compartment is tense, a small dural nick given over the sylvian fissure permits release of cerebrospinal fluid (CSF) from the sylvian cistern and relaxes the dura. To maintain dural retraction while peeling the dura propria, epidural traction stitches are preferred.
Unroofing of the optic canal
Next, attention is directed to the anterior root of the ACP, which is nothing but the roof of the optic canal. The roof of the canal is then drilled long its entire length with a diamond 3 mm drill bit, under constant irrigation. The drill must work intermittently to prevent excessive increase in temperature, which may inflict thermal damage on the nerve. After thinning the bone to eggshell thickness, it is removed with a periosteal dissector. In cases where there is tumor extension into the optic canal or significant manipulation of the optic nerve is anticipated during the course of surgery, it is advisable to drill away the medial wall of the canal also. This step increases the mobility of the nerve, but often violates the sphenoid sinus. The sinus opening must then be cautiously obliterated with muscle piece and fat, at an appropriate stage during the operation.
Drilling the clinoid process and its removal
Only one attachment of the clinoid process remains now, which is the posterior root (optic strut). The clinoid remnant is cored out uniformly with a diamond drill (3 mm caliber). It is important to remember that the clinoidal carotid is immediately deep to the clinoid process. Schematically, if the optic nerve and the oculomotor nerve are assumed to form an “A” then the clinoidal carotid is the short horizontal arm of A [Figure 2b, c and d]. The apex of this “A” is formed by the convergence of the optic nerve and oculomotor nerve. The optic strut occupies the small triangle above the short horizontal arm. This “A” is classically known as the Dolenc's triangle or the clinoidal triangle. Once the clinoidal remnant has been cored out thoroughly, the remaining shell is manipulated inwards towards the hollowed core and removed piecemeal. Temptation to extract the clinoidal remnant in one piece should be punctiliously avoided as the tip of the clinoid process may be sharp (almost sickle shaped) and may lacerate the carotid if pulled out forcefully. Sometimes, the middle clinoid process may additionally connect the ACP tip to the sphenoid body. To obtain the surgical advantage of clinoidectomy in these cases, it is necessary to denude the middle clinoid process as extensively as possible, after completing the steps of a regular clinoidectomy.
Further steps with regards to different surgical objectives:
Circumferential exposure of the clinoidal segment
Specific to clinoidal segment aneurysms, circumferential exposure and mobilization of the clinoidal segment is needed. In these cases, further reduction of the optic strut is needed which almost always opens the sphenoid sinus. Carefully, the residual bony shell of the strut is further abraded with a 1.5 mm diamond burr, in frequent starts and stops. The space available at the apex of clinoidal triangle is extremely narrow and a blunt-tipped strong microscissors is often the ideal instrument to remove the last bit of bone.
Mobilization of the clinoidal carotid segment
The clinoidal carotid segment is tethered by the proximal (carotico-oculomotor membrane) and the distal dural rings. Hence the mobilization of this segment necessitates the division of these rings. The clinoidal segment is invested by the carotid collar, a derivative of the proximal ring, which fuses with the carotid surface at the level of the upper ring.[8] The venous channels contained between the carotid collar and the clinoidal carotid are in communication with the cavernous venous space and often the fibrin glue injected into the cavernous sinus seeps into these channels.
The carotico-oculomotor membrane is then incised parallel to the oculomotor nerve and the oculomotor nerve mobilized laterally [Figure 3a]. Brisk bleeding encountered at this step can be controlled with fibrin glue injection.
Figure 3.
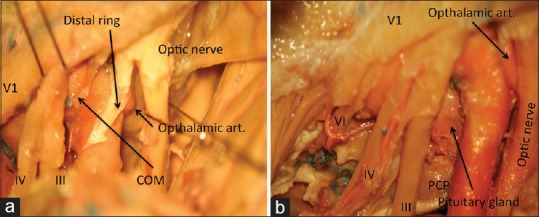
(a) The proximal and distal dural rings, (b) a combination of extradural and subdural dissections showing the approach to sella (PCP = Posterior clinoid process)
The distal dural ring is densely adherent to the carotid adventitia and must be divided circumferentially even anterior and deep to the carotid. Aggressive removal of the optic strut greatly facilitates this step. While incising the distal ring care must be taken to avoid the ophthalmic artery, which arises just distal to this ring.
Lateral transcavernous approach to the sella
The mobilization of the oculomotor nerve as described above directly leads the surgeon into the sella, which is separated from the cavernous sinus by a thin, unilayered and often fenestrated membrane [Figure 3b].[9] This surgical step grants convenient access to the cavernous sinus extensions of sellar tumors, chiefly, pituitary adenoma.
Discussion
When initially described by Drake in 1968,[1] the surgical advantage conferred by the removal of ACP was unclear. Drake himself concluded that it was unnecessary for carotico-opthalmic aneurysms. However, with the maturation of microanatomical knowledge and neurosurgical techniques anterior clinoidectomy again found favour. The role of intradural removal of clinoid in treatment of paraclinoid aneurysms was established by Yasargil et al.[10] and others, such as Sundt and Piepgras.[11] When Dolenc introduced the extradural clinoidectomy in 1985, it was recognized as an exercise technically challenging to master. Nevertheless, due to the pioneering efforts of Parkinson,[12] Umansky and Nathan,[13] Harris and Rhoton,[14] the microsurgical anatomy of the cavernous sinus walls and their methodical surgical dissection was standardized. The crucial role of this anatomic understanding in enhancing the exposure of ACP is now acknowledged.[6,15]
Several papers[16,17,18] have examined in detail the surgical benefit of clinoidectomy. Most importantly, the area of carotid-oculomotor triangle and the available length of optic nerve increases almost two-fold following clinoidectomy, which critically enhances the pliability and tolerance of the nerve to surgical handling. Hence, ACP removal is increasingly included in the standard exposures for even supposedly straightforward pathologies such as posterior communicating artery aneurysms[19] and tuberculum sellae meningioma.[20,21]
The relative advantages and drawbacks of intradural and extradural routes to anterior clinoidectomy are contentious. Extradural clinoidectomy offers the protection of dura to critical neurovascular structures during drilling and handling of the ACP. On the other hand, intradural removal of the clinoid presents a possibility of tailoring the clinoidectomy to the demands of the intradural pathology directly under view. Theoretically, extradural clinidectomy is expected to have a higher incidence of CSF leaks in view of more extensive bone removal when compared with the intradural procedure. However, recent evidence[22] suggests that incidence of iatrogenic CSF leak is similar for extradural or intradural ACP removal, especially when the potential sites for leak are carefully identified and sealed intraoperatively. It is also important to take into consideration the anatomical variations, identifiable on preoperative imaging, which may demand a modification of the standard technique for extradural anterior clinoidectomy. The most important elemental variations include the presence of a middle clinoid process and extensive pneumatisation of the ACP. When the former situation is encountered, the ACP cannot be extracted completely and the clinoid is carefully denuded till the clinoidal carotid is exposed circumferentially. When the clinoid process is pneumatised extensively, its removal may be easier but the violated sinus cavity warrants careful packing with voluminous fat and glue to prevent CSF rhinorrhea.
The oculomotor nerve may be more vulnerable to injury in the intradural procedure as compared to the extradural clinoid removal.[15,23,24] The exposure for extradural clinoidectomy uncovers the cavernous portion of the third nerve which in this region is invested with a thick epineurial sheath making it tolerant to manipulation. To avoid injury to the nerve, it is important to respect the anatomical plain of cleavage between the lamina propria and lateral wall of cavernous sinus during the epidural cavernous sinus dissection.
Similarly, extradural removal of ACP is safe even in the case of paraclinoid aneurysms.[25,26] Most of the aneurysms of the paraclinoid region are well protected by a layer of dura when the clinoid process is being drilled extradurally, except for those which are pointing toward the ACP and tend to erode into it. These aneurysms are fortunately rare. Moreover, Krisht and Hsu[27] have mentioned excellent microsurgical outcomes with extradural clinoidectomy even in these aneurysms.
Conclusion
Extradural anterior clinoidectomy is an important technique for addressing pathologies in and around the central cranial base. An accurate understanding of the microsurgical anatomy of this region and the relevant surgical nuances helps simplify the complexity of this surgical step.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors are thankful to Prof. Ali Krisht, world renowned cerebrovascular and skull base surgeon, for his microsurgical demonstrations, which inspired this paper. The cadaver dissections were performed in the microsurgical laboratory at the Arkansas Neurosciences Institute, St. Vincent's Infirmary, Little Rock.
References
- 1.Drake CG, Vanderlinden RG, Amacher AL. Carotid-ophthalmic aneurysms. J Neurosurg. 1968;29:24–31. doi: 10.3171/jns.1968.29.1.0024. [DOI] [PubMed] [Google Scholar]
- 2.Dolenc V. Direct microsurgical repair of intracavernous vascular lesions. J Neurosurg. 1983;58:824–31. doi: 10.3171/jns.1983.58.6.0824. [DOI] [PubMed] [Google Scholar]
- 3.Krisht AF. The clinoidal cone: Indications and surgical techniques for its removal. Contemp Neurosurg. 2004;26:1–5. [Google Scholar]
- 4.Oikawa S, Mizuno M, Muraoka S, Kobayashi S. Retrograde dissection of the temporalis muscle preventing muscle atrophy for pterional craniotomy. Technical note. J Neurosurg. 1996;84:297–9. doi: 10.3171/jns.1996.84.2.0297. [DOI] [PubMed] [Google Scholar]
- 5.Krisht AF, Kadri PA. Surgical clipping of complex basilar apex aneurysms: A strategy for successful outcome using the pretemporal transzygomatic transcavernous approach. Neurosurgery. 2005;56:261–73. doi: 10.1227/01.neu.0000156785.63530.4e. [DOI] [PubMed] [Google Scholar]
- 6.Froelich SC, Aziz KM, Levine NB, Theodosopoulos PV, van Loveren HR, Keller JT. Refinement of the extradural anterior clinoidectomy: Surgical anatomy of the orbitotemporal periosteal fold. Neurosurgery. 2007;61:179–85. doi: 10.1227/01.neu.0000303215.76477.cd. [DOI] [PubMed] [Google Scholar]
- 7.Krayenbühl N, Hafez A, Hernesniemi JA, Krisht AF. Taming the cavernous sinus: Technique of hemostasis using fibrin glue. Neurosurgery. 2007;61:E52. doi: 10.1227/01.neu.0000289712.72555.9c. [DOI] [PubMed] [Google Scholar]
- 8.Yasuda A, Campero A, Martins C, Rhoton AL, Jr, de Oliveira E, Ribas GC. Microsurgical anatomy and approaches to the cavernous sinus. Neurosurgery. 2005;56:4–27. doi: 10.1227/01.neu.0000144208.42171.02. [DOI] [PubMed] [Google Scholar]
- 9.Yasuda A, Campero A, Martins C, Rhoton AL, Jr, Ribas GC. The medial wall of the cavernous sinus: Microsurgical anatomy. Neurosurgery. 2004;55:179–89. doi: 10.1227/01.neu.0000126953.59406.77. [DOI] [PubMed] [Google Scholar]
- 10.Yasargil MG, Gasser JC, Hodosh RM, Rankin TV. Carotid-ophthalmic aneurysms: Direct microsurgical approach. Surg Neurol. 1977;8:155–65. [PubMed] [Google Scholar]
- 11.Sundt TM, Jr, Piepgras DG. Surgical approach to giant intracranial aneurysms. Operative experience with 80 cases. J Neurosurg. 1979;51:731–42. doi: 10.3171/jns.1979.51.6.0731. [DOI] [PubMed] [Google Scholar]
- 12.Parkinson D. Lateral sellar compartment: History and anatomy. J Craniofac Surg. 1995;6:55–68. doi: 10.1097/00001665-199501000-00016. [DOI] [PubMed] [Google Scholar]
- 13.Umansky F, Nathan H. The lateral wall of the cavernous sinus. With special reference to the nerves related to it. J Neurosurg. 1982;56:228–34. doi: 10.3171/jns.1982.56.2.0228. [DOI] [PubMed] [Google Scholar]
- 14.Harris FS, Rhoton AL. Anatomy of the cavernous sinus. A microsurgical study. J Neurosurg. 1976;45:169–80. doi: 10.3171/jns.1976.45.2.0169. [DOI] [PubMed] [Google Scholar]
- 15.Yonekawa Y, Ogata N, Imhof HG, Olivecrona M, Strommer K, Kwak TE, et al. Selective extradural anterior clinoidectomy for supra- and parasellar processes. Technical note. J Neurosurg. 1997;87:636–42. doi: 10.3171/jns.1997.87.4.0636. [DOI] [PubMed] [Google Scholar]
- 16.Evans JJ, Hwang YS, Lee JH. Pre- versus post-anterior clinoidectomy measurements of the optic nerve, internal carotid artery, and opticocarotid triangle: A cadaveric morphometric study. Neurosurgery. 2000;46:1018–21. [PubMed] [Google Scholar]
- 17.Sade B, Kweon CY, Evans JJ, Lee JH. Enhanced exposure of carotico-oculomotor triangle following extradural anterior clinoidectomy: A comparative anatomical study. Skull Base. 2005;15:157–61. doi: 10.1055/s-2005-871523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andaluz N, Beretta F, Bernucci C, Keller JT, Zuccarello M. Evidence for the improved exposure of the ophthalmic segment of the internal carotid artery after anterior clinoidectomy: Morphometric analysis. Acta Neurochir (Wien) 2006;148:971–5. doi: 10.1007/s00701-006-0862-x. [DOI] [PubMed] [Google Scholar]
- 19.Park SK, Shin YS, Lim YC, Chung J. Preoperative predictive value of the necessity for anterior clinoidectomy in posterior communicating artery aneurysm clipping. Neurosurgery. 2009;65:281–5. doi: 10.1227/01.NEU.0000348296.09722.2F. [DOI] [PubMed] [Google Scholar]
- 20.Otani N, Muroi C, Yano H, Khan N, Pangalu A, Yonekawa Y. Surgical management of tuberculum sellae meningioma: Role of selective extradural anterior clinoidectomy. Br J Neurosurg. 2006;20:129–38. doi: 10.1080/02688690600776747. [DOI] [PubMed] [Google Scholar]
- 21.Mathiesen T, Kihlström L. Visual outcome of tuberculum sellae meningiomas after extradural optic nerve decompression. Neurosurgery. 2006;59:570–6. doi: 10.1227/01.NEU.0000228683.79123.F9. [DOI] [PubMed] [Google Scholar]
- 22.Chi JH, Sughrue M, Kunwar S, Lawton MT. The “yo-yo” technique to prevent cerebrospinal fluid rhinorrhea after anterior clinoidectomy for proximal internal carotid artery aneurysms. Neurosurgery. 2006;59:ONS101–7. doi: 10.1227/01.NEU.0000219962.15984.34. [DOI] [PubMed] [Google Scholar]
- 23.Dolenc VV. Transcranial epidural approach to pituitary tumors extending beyond the sella. Neurosurgery. 1997;41:542–50. doi: 10.1097/00006123-199709000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Kulwin C, Tubbs RS, Cohen-Gadol AA. Anterior clinoidectomy: Description of an alternative hybrid method and a review of the current techniques with an emphasis on complication avoidance. Surg Neurol Int. 2011;2:140. doi: 10.4103/2152-7806.85981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krisht AF, Hsu SP. Paraclinoid aneurysms: Part 1: Superior (True Ophthalmic) aneurysms. Contemp Neurosurg. 2008;30:1–5. [Google Scholar]
- 26.Krisht AF, Hsu SP. Paraclinoid aneurysms: Part II: Inferior paraclinoid. Contemp Neurosurg. 2008;30:1–6. [Google Scholar]
- 27.Krisht AF, Hsu SP. Paraclinoid aneurysms: Part III: Lateral aneurysms. Contemp Neurosurg. 2008;30:1–5. [Google Scholar]


