Abstract
Venous air embolism (VAE) is a well-known complication of sitting position neurosurgery which most characteristically manifests as mild to severe hemodynamic alterations. Development of pulmonary edema is a known, though infrequent, manifestation of VAE. We report here the occurrence of acute pulmonary edema without accompanying hemodynamic changes in a patient undergoing retromastoid craniotomy and tumor decompression in the sitting position. The patient required supportive treatment and elective ventilation for 11 days before the edema resolved. He also developed significant postoperative thrombocytopenia which though, was self-limiting. Thus, VAE may manifest with atypical manifestations like pulmonary edema and thrombocytopenia that can significantly contribute to postoperative patient morbidity.
Keywords: Pulmonary edema, sitting position, thrombocytopenia, venous air embolism
Introduction
Venous air embolism (VAE) is a well-recognized but dreaded complication of surgery in sitting position for posterior cranial and cervical spine lesions, with hemodynamic alterations being the most characteristic consequence.[1] However, equally serious, though infrequently reported adverse effects of VAE are acute pulmonary edema without cardiovascular instability[2,3,4,5,6,7,8] and post-VAE thrombocytopenia.[9,10] We report here the rare occurrence of both these problems after VAE in a patient undergoing suboccipital craniotomy and tumor decompression in the sitting position.
Case Report
A 32-year-old male patient, with no other obvious abnormality, underwent a retromastoid craniotomy and decompression of a left cerebellopontine angle epidermoid in the sitting position. A standard anesthesia and intraoperative monitoring regimen was deployed and the patient was ventilated using an oxygen–air mixture. Just after the start of tumor resection, the patient's end-tidal carbon-dioxide(EtCO2) suddenly decreased from 30 mmHg to 18 mmHg, followed immediately by a decrease in his oxygen saturation (SpO2) from 100% to 95%; there was no change in his arterial blood pressure (ABP) of 136/84 mmHg, heart rate (HR) of 86 beats/minute, heart rhythm, and central venous pressure (CVP) of 1 cm H2O at aural level, and no murmur was heard through the precordial stethoscope. Arterial blood gas (ABG) analysis revealed a pH of 7.281, PaO2 of 73.5 mmHg, PaCO2 of 60 mmHg, and a base deficit of 6.4. VAE was suspected; the surgical field was flooded with saline, inspired oxygen concentration (FiO2) was increased to 1.0, and rapid infusion of intravenous (IV) fluids and correction of metabolic acidosis was done; aspiration from the CVP line in the right internal jugular vein revealed a few milliliters of air. The patient's EtCO2 and SpO2 soon normalized and surgery was resumed. Ten minutes later, the patient had another transient episode of VAE manifesting as an isolated, abrupt fall in his EtCO2 to 22 mmHg, which promptly responded to treatment. However, within half an hour, the patient's SpO2 suddenly decreased to 92%, copious frothy secretions filled his endotracheal tube, and chest auscultation revealed bilateral diffuse crepitations; there was no change in his EtCO2, ABP, HR, and heart rhythm. His CVP was 3 cm H2O and ABG revealed a PaO2 of 58 mmHg, PaO2/FiO2 of 145, PaCO2 of 57 mmHg, pH of 7.187, and base deficit of 10.2. No rise of intracranial pressure or excessive surgical bleeding was seen. Pulmonary edema was suspected and managed with IV morphine (6 mg), furosemide (40 mg), and sodium bicarbonate (80 mEq), and controlled ventilation with 100% oxygen and positive end-expiratory pressure of 10 cm H2O; supportive treatment was continued in the intensive care unit (ICU) after surgery. The patient's postoperative X-ray chest revealed bilateral interstitial infiltrates, normal cardiothoracic ratio, and absent Kerley lines [Figure 1]; computed tomography (CT) scan of the brain and echocardiogram were normal (left ventricular ejection fraction ~60%). Over the next 3 days, the patient also developed progressive thrombocytopenia (lowest platelet count ~64,000/mm3), not associated with any bleeding episodes. The pulmonary edema resolved gradually over a week and the arterial oxygenation improved (PaO2/FiO2 ratio of 292); the platelet count also normalized on its own. The patient was extubated on the 11th postoperative day, following which he was conscious with no new sensorimotor deficit and had a normal hemodynamic and oxygenation status. His remaining hospital stay was uneventful.
Figure 1.
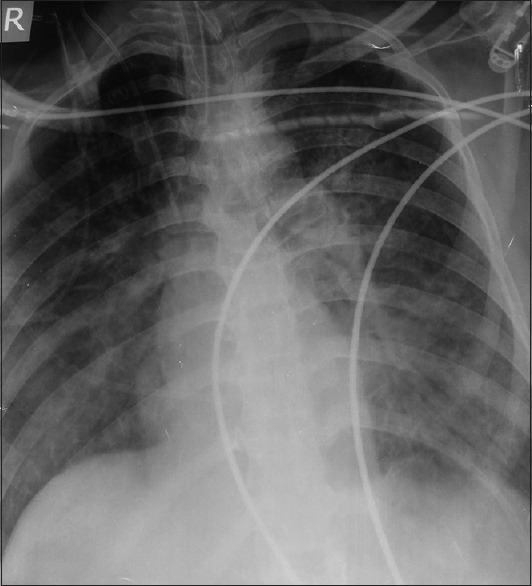
Postoperative chest X-ray showing bilateral infiltrates
Discussion
VAE is a known complication of neurosurgery in the sitting position with a reported incidence of 9.3-43%.[1] It has diverse intraoperative manifestations, ranging from transient sudden decreases in EtCO2 and/or SpO2 to more serious cardiovascular sequelae like hypotension, tachycardia, arrhythmias, right heart failure, shock, and even cardiac arrest.[1] In our patient, VAE was diagnosed on the basis of the typical decreases in his EtCO2 and a positive air aspiration; confirmatory monitors like precordial Doppler ultrasound or transesophageal echocardiograph were not available to us.
Our patient developed acute pulmonary edema following two episodes of VAE. A cardiogenic cause of pulmonary edema appears unlikely with no prior evidence of cardiac disease, no associated hemodynamic manifestations or overt signs of heart failure, and a normal ECG and echocardiography; use of a well-titrated intraoperative fluid regimen rules out fluid overload as the cause. Neurogenic pulmonary edema is usually associated with brain stem traction or injury, raised intracranial pressures, or massive bleeding; absence of these factors here and the patient's normal postoperative neurological recovery and normal CT scan do not support a neurogenic cause of edema. The pulmonary edema in this patient was most probably a direct consequence of VAE.
VAE-induced pulmonary edema has been reported earlier in patients undergoing sitting position craniotomy[2,3,4] wherein the VAE manifested as transient decreases in the EtCO2 without accompanying hemodynamic instability; slow resolution of the pulmonary edema necessitated prolonged elective ventilation, and in one patient, inotropic support and 5 L of plasma were required to replace the massive fluid lost in the lungs.[2] Post-VAE edema usually occurs only after multiple episodes of air trapping[2,5,6] and may manifest rapidly[2,3,4,5] or several hours later.[7] Among the various theories postulating the pathogenesis of post-VAE pulmonary edema, those implicating pulmonary hypertension or local hypoxia as the likely causes are not supported by animal studies.[2] The favored hypothesis is a direct toxic effect of the luminal air bubbles on the pulmonary endothelium; neutrophil aggregation around the air bubbles and subsequent release of toxic oxygen metabolites creates gaps between pulmonary arterial and arteriolar endothelial cells and consequent increase in microvascular permeability and pulmonary exudation.[2] The supportive management of post-VAE pulmonary edema is similar to that in other forms of edema, but its resolution is mostly gradual because of delayed reversal of the underlying microvascular injury.[2]
Our patient also developed sudden thrombocytopenia in the immediate postoperative period which gradually self-corrected. He had no commonly known thrombocytopenia-inducing conditions like sepsis, intake of recognized causative drugs and antibiotics, or definitive heparin therapy. Though heparin-induced thrombocytopenia (HIT) may also occur following use of small amounts of heparin in the monitoring line flushes,[11] this cause appears unlikely here as it manifests late, usually following longer use of heparin flushes, and resolves only after the flushes are discontinued, unlike the situation in this patient. The low platelet counts in this case were most likely secondary to VAE which is known to induce both thrombocytopenia and decrease in platelet function.[9,10] These effects are postulated to be due to a direct platelet to air bubble binding and consequent release of mediators like endothelin and serotonin and complement activation, which further induce platelet aggregation and sequestration in the lungs.[9] Development of significant thrombocytopenia following VAE is also reported in patients undergoing craniotomy in the semi-sitting position.[10] The decreased platelet count and function can cause excessive perioperative bleeding, and the platelet–air bubble binding can result in slow reabsorption of the entrained air and consequent aggravation of the hemodynamic complications of VAE;[9] however, VAE-induced thrombocytopenia is self-limiting and usually does not necessitate platelet transfusion.
The popularity and usage of sitting position in neurosurgery appears to be in decline primarily because of the potential risk of VAE and its negative effects on cardiovascular hemodynamics. Even seemingly minor episodes of VAE can have atypical but serious perioperative consequences like pulmonary edema and thrombocytopenia, resulting in prolonged elective ventilation and duration of ICU stay of the affected patients. We believe that recognition of these adverse effects of VAE and their appropriate management can significantly help in reducing the morbidity and mortality associated with the sitting patient position for neurosurgery.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Mirski MA, Lele AV, Fitzsimmons L, Toung TJK. Diagnosis and treatment of vascular air embolism. Anesthesiology. 2007;106:164–77. doi: 10.1097/00000542-200701000-00026. [DOI] [PubMed] [Google Scholar]
- 2.Lam KK, Hutchinson RC, Gin T. Severe pulmonary oedema after venous air embolism. Can J Anaesth. 1993;40:964–7. doi: 10.1007/BF03010100. [DOI] [PubMed] [Google Scholar]
- 3.Chandler WF, Dimcheff DG, Taren JA. Acute pulmonary edema following venous air embolism during a neurosurgical procedure. J Neurosurg. 1974;40:400–4. doi: 10.3171/jns.1974.40.3.0400. [DOI] [PubMed] [Google Scholar]
- 4.Ishida K, Hishinuma M, Miyazawa M, Tanaka T, Iwasawa K, Kitoh T. Pulmonary edema due to venous air embolism during craniotomy: A case report. Masui. 2008;57:1257–60. [PubMed] [Google Scholar]
- 5.Frim DM, Wolmann LM, Evans AB, Ojemann RG. Acute pulmonary edema after low level air embolism during craniotomy. Case report. J Neurosurg. 1996;85:937–40. doi: 10.3171/jns.1996.85.5.0937. [DOI] [PubMed] [Google Scholar]
- 6.Arora R, Chablani D, Rath GP, Prabhakar H. Pulmonary oedema following venous air embolism during transsphenoidal pituitary surgery. Acta Neurochir (Wien) 2007;149:1177–8. doi: 10.1007/s00701-007-1286-y. [DOI] [PubMed] [Google Scholar]
- 7.Smelt WL, Baerts WD, de Langhe JJ, Booij LH. Pulmonary edema following air embolism. Acta Anaesthesiol Belg. 1987;38:201–5. [PubMed] [Google Scholar]
- 8.El Kettani C, Badaoui R, Fikri M, Jeanjean P, Montpellier D, Tchaoussoff J. Pulmonary oedema after venous air embolism during craniotomy. Eur J Anaesthesiol. 2002;19:846–8. doi: 10.1017/s0265021502291358. [DOI] [PubMed] [Google Scholar]
- 9.Schafer ST, Neumann A, Lindemann J, Gorlinger K, Peters J. Venous air embolism induces both platelet dysfunction and thrombocytopenia. Acta Anaesthesiol Scand. 2009;53:736–41. doi: 10.1111/j.1399-6576.2009.01947.x. [DOI] [PubMed] [Google Scholar]
- 10.Schafer ST, Sandalcioglu IE, Stegen B, Neumann A, Asgari S, Peters J. Venous air embolism during semi-sitting craniotomy evokes thrombocytopenia. Anaesthesia. 2011;66:25–30. doi: 10.1111/j.1365-2044.2010.06584.x. [DOI] [PubMed] [Google Scholar]
- 11.Brieger DB, Mak K, Kottke-Marchant K, Topol EJ. Heparin-induced thrombocytopenia. J Am Coll Cardiol. 1998;31:1449–59. doi: 10.1016/s0735-1097(98)00134-x. [DOI] [PubMed] [Google Scholar]


