Abstract
Sinonasal teratocarcinosarcoma (SNTCS) is one of the rarest, aggressive malignant neoplasms of sinonasal tract, consisting of primitive neuroepithelial elements with various malignant epithelial and mesenchymal components. Previously described as teratoid carcinosarcoma, malignant teratoma, or blastoma, SNTCS constitutes less than 1% of all cancers and approximately 3% of all malignancies of head and neck region, which is mainly located in the nasal cavity and paranasal sinuses, although tumors occurring in other locations including the nasopharynx and oral cavity have been described. Here, we are presenting a 22-year-old patient with SNTCS involving the nasal cavity, nasopharynx, and all paranasal sinuses with bilateral orbital and intracranial extension treated with surgery followed by radiotherapy and chemotherapy.
Keywords: Intracranial, sinonasal, teratocarcinosarcoma
Introduction
Sinonasal teratocarcinosarcoma (SNTCS) is a very unusual and aggressive neoplasm characterized by a histologic combination of malignant teratoma and carcinosarcoma with a triphasic growth pattern including epithelial, mesenchymal, and primitive neuroectodermal components[1] and occurs mainly in the nasal cavity and paranasal sinuses, although tumors occurring in other locations including the nasopharynx and oral cavity have been described.[2,3,4] SNTCS is a highly malignant neoplasm and has a higher rate of recurrence even after gross total resection and is treated with surgery, with or without adjuvant therapies which include radiotherapy and chemotherapy, used alone or in combination.[5,6] Here, we are presenting a case with SNTCS involving the nasal cavity, nasopharynx, and all paranasal sinuses with bilateral orbital and intracranial extension, whose diagnosis was confirmed by histopathologic examination (HPE) of resected tumor tissues and immunohistochemical examination, treated with surgery followed by radiotherapy and chemotherapy.
Case Report
A 22-year-old male patient presented with complaints of blocked nose and epistaxis for 1½ years, and headache and diminished vision for 20 days. Patient was previously seen by an ear, nose and throat surgeon 1 year back when he had only nose block and epistaxis and was diagnosed as having a left nasal mass, which was removed by lateral rhinotomy approach. HPE of the resected mass suggested an inverted papilloma (sinonasal type). After the surgery, patient was symptom free for about 3 months and then again developed the same symptoms plus headache and diminished vision later on. When the patient presented to us, on examination, he had bilateral proptosis (right >left) with absent perception of light (PL) in right eye and finger counting was present at 3 feets in left eye. Papilledema was present bilaterally. There was a polypoidal, vascular soft tissue mass filling whole of the nasal cavity, going posteriorly into the nasopharynx. Lymph nodes, thyroid, salivary glands, and liver were non-palpable.
Plain and contrast computed tomography (CT) scanning of paranasal sinuses revealed a soft tissue mass filling whole of the nasal cavity and nasopharynx, causing destruction of all the turbinates with deviation as well as erosion of bony nasal septum, medial walls of bilateral ethmoid sinuses, bilateral maxillary sinuses and both orbits, floor of the sphenoid sinus, bilateral frontal sinuses, cribriform plates, bilateral orbital (right > left) and intracranial extension with heterogeneous enhancement on contrast scans [Figures 1–4]. CT angiography showed multiple prominent vessels within the mass, supplied mainly from branches of bilateral maxillary arteries [Figures 5 and 6]. A near-total removal of the mass was done through pyriform aperture using modified Weber-Ferguson incision [Figure 7]. The intracranial extension was extradural, bony skull base defect was not large, and no dural defect was made during surgery, and therefore we did not do repaired of skull base.
Figure 1.
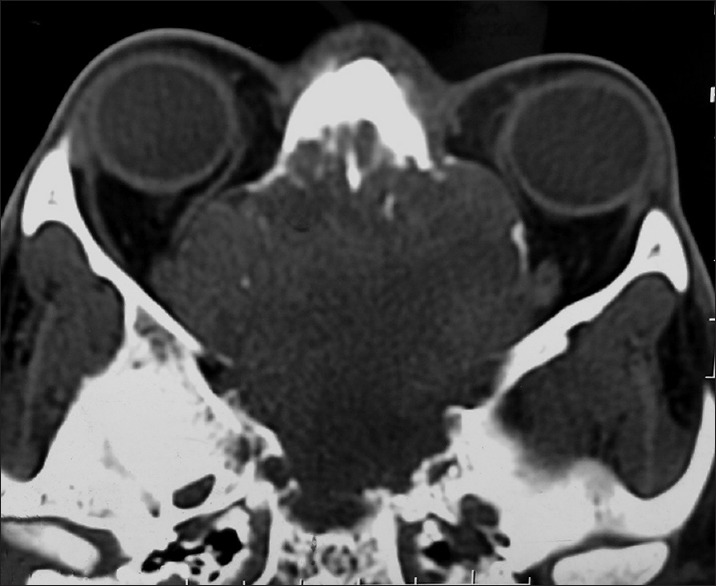
Computed tomography scan of nose and paranasal sinuses: Plain
Figure 4.
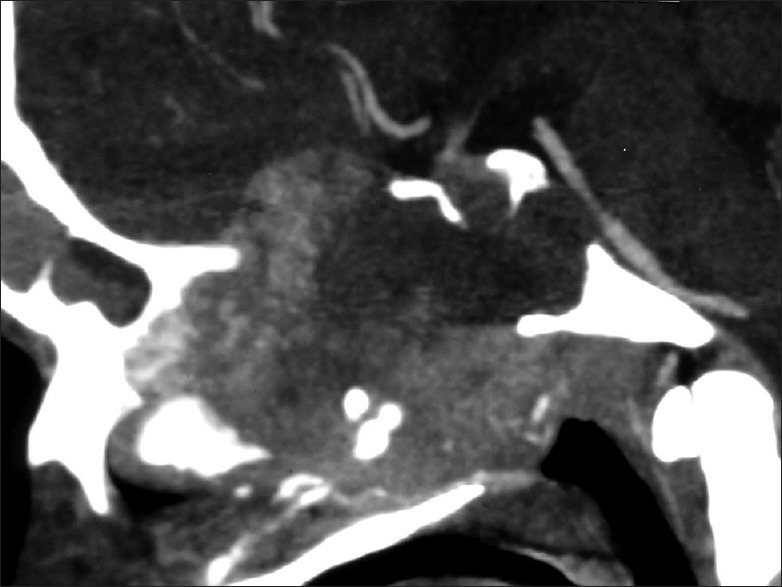
Contrast computed tomography scan: Sagittal view
Figure 5.
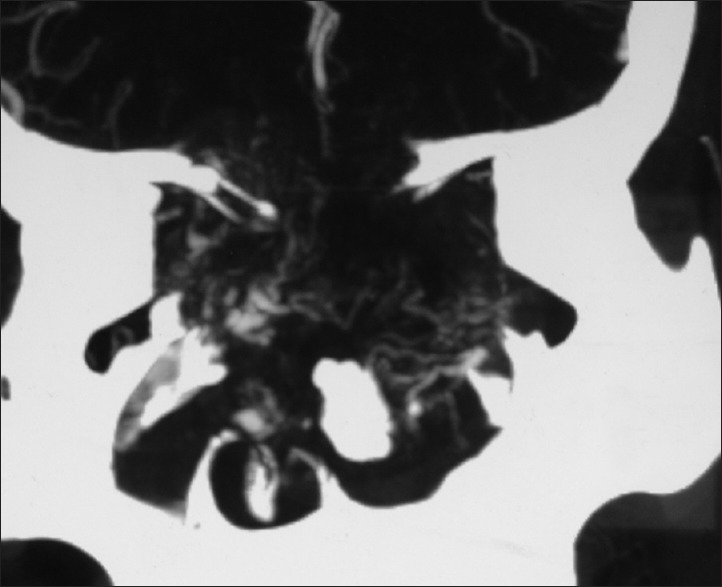
Computed tomography angiogram: Coronal view showing multiple prominent vessels within the mass supplied mainly from branches of bilateral maxillary arteries
Figure 6.
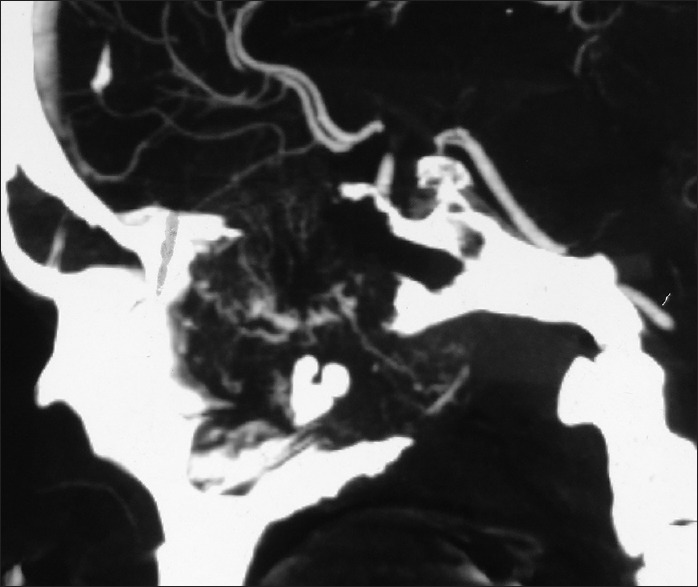
Computed tomography angiogram: Sagittal view showing multiple prominent vessels within the mass supplied mainly from branches of bilateral maxillary arteries
Figure 7.
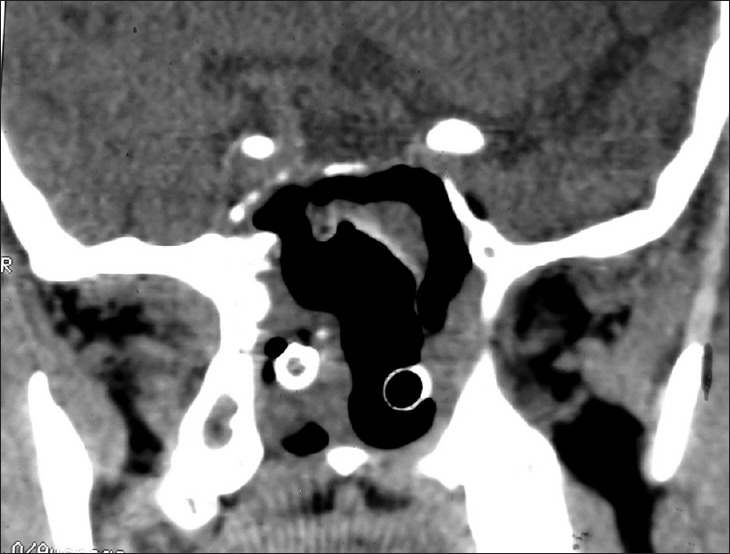
Plain Computed tomography scan of nose and paranasal sinuses done on post-op day 2
Figure 2.
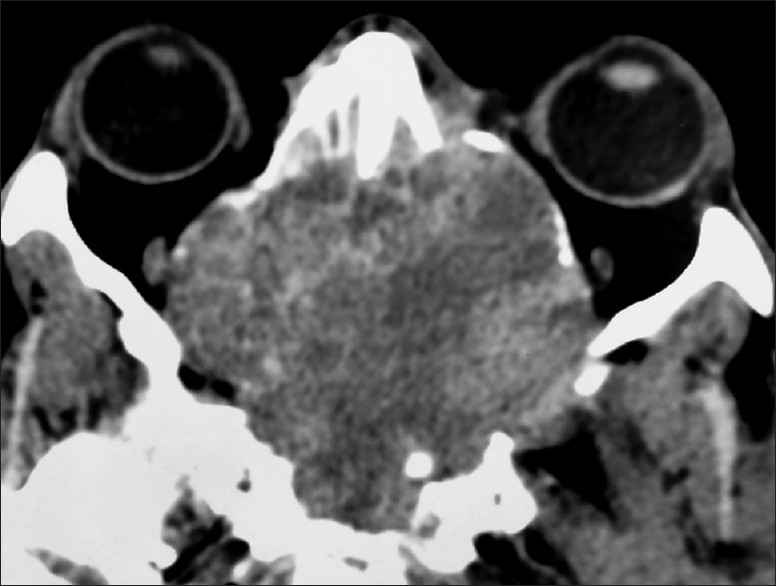
Contrast computed tomography scan of nose and paranasal sinuses showing heterogeneous enhancement
Figure 3.
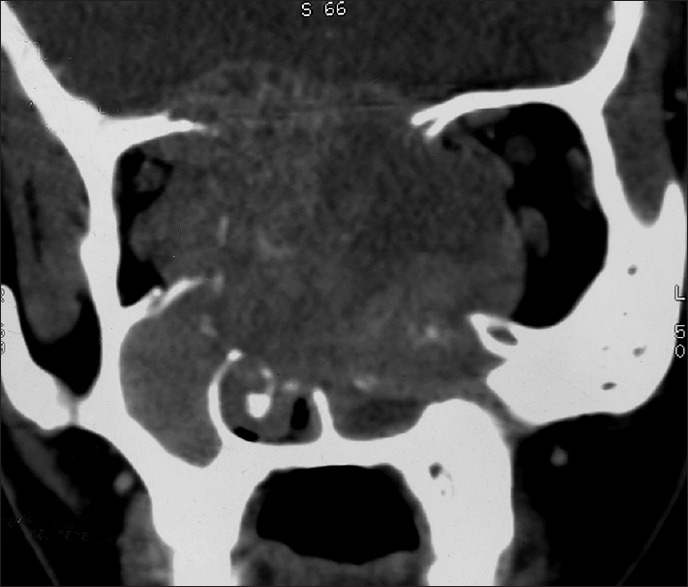
Contrast computed tomography scan: Coronal view
On HPE, the tumor tissues showed wide variety of cellular components of variable maturity and pleomorphism which contained neuroepithelial tissue, squamous cell nests, round cells, and smooth muscles. These elements ranged from malignant to virtual normal in appearance. Immunohistochemistry showed strong neuron specific enolase (NSE) positivity in small round cells [Figure 8], focal positivity for synaptophysin [Figure 9] and chromogranin in small round cells [Figure 10], and strong S-100 positivity in both epithelial and stromal components [Figure 11]. CD99 was positive in vacuolated squamous epithelial cells [Figure 12], pan – cytokeratin (CK-PAN) was strongly positive in gland-like structures, clear cell squamous epithelium, and focal positive in clusters of small round cells [Figure 13]. Vimentin was positive in connective tissue and small round cell components [Figure 14], smooth muscle actin (SMA) was focal positive in spindle cell component [Figure 15], and high molecular weight-cytokeratin (HMW-CK) was negative in small round cells and positive in basal lining of few of the glands and also in the basal cells of the small round cell clusters and in the squamous epithelial cells [Figure 16]. Desmin, HMB-45, glial fibrillary acidic protein (GFAP), human chorionic gonadotrophin (HCG), placental alkaline phosphatase (PALP), alpha-feto protein (AFP), epithelial membrane antigen (EMA), and CD-30 were negative in tumor cells. Ki67/MIB1 proliferative index was variable (15-30%) and was very high in focal clusters of round cells.
Figure 8.
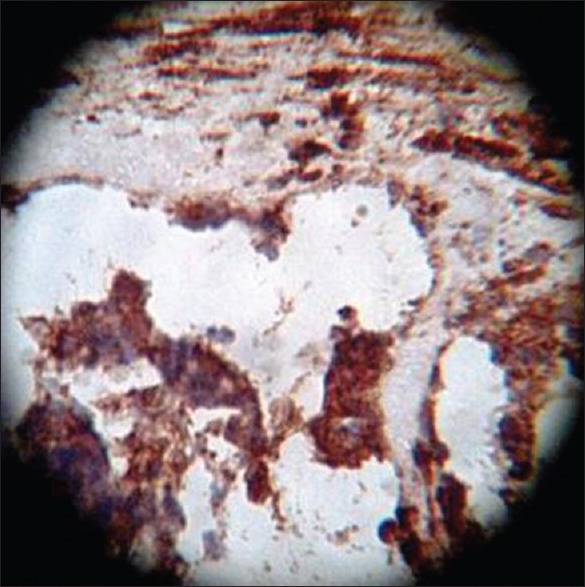
Neuron specific enolase, ×40 high power
Figure 9.
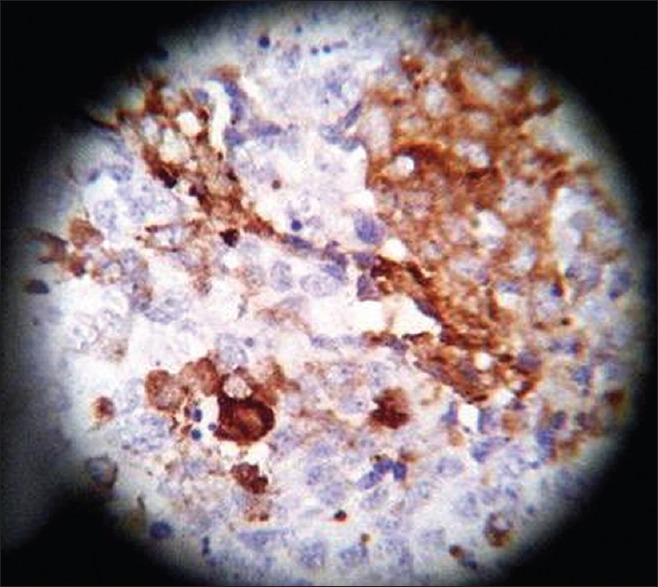
Synaptophysin, ×40 high power
Figure 10.
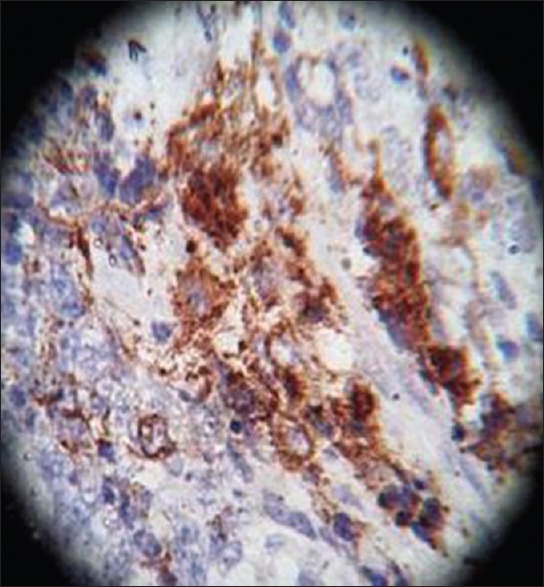
Chromogranin, ×40 high power
Figure 11.
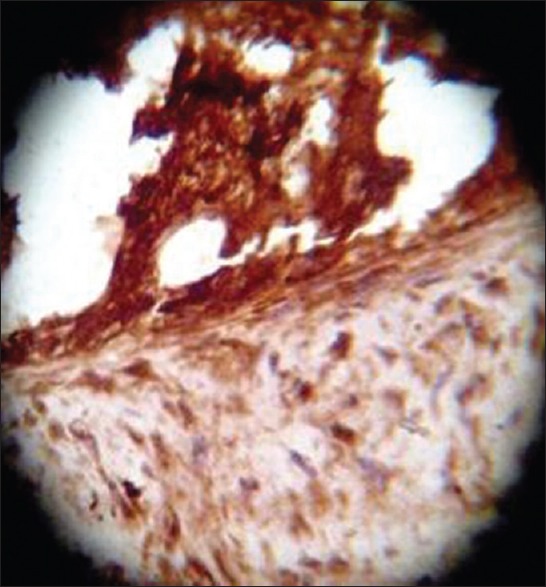
S-100, ×40 high power
Figure 12.
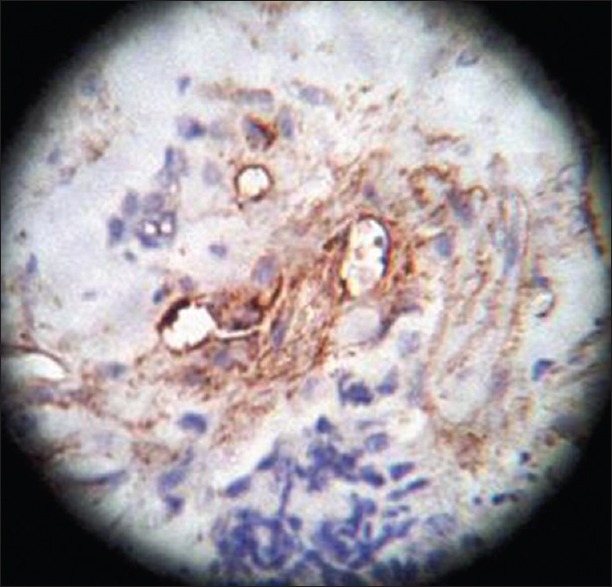
CD99, ×40 high power
Figure 13.
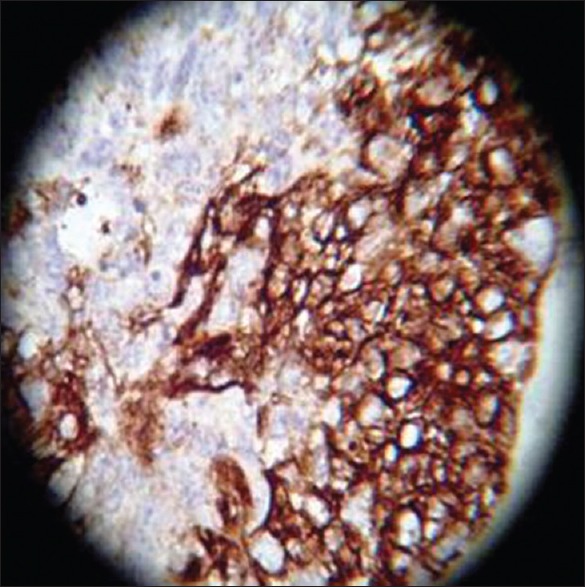
CK-PAN, ×40 high power
Figure 14.
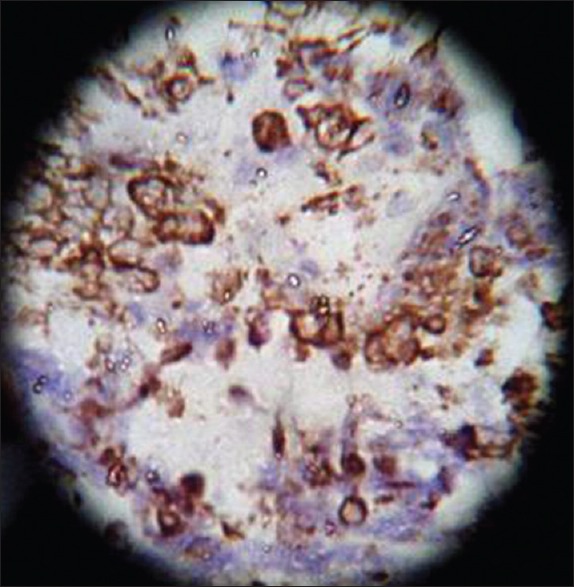
Vimentin, ×40 high power
Figure 15.
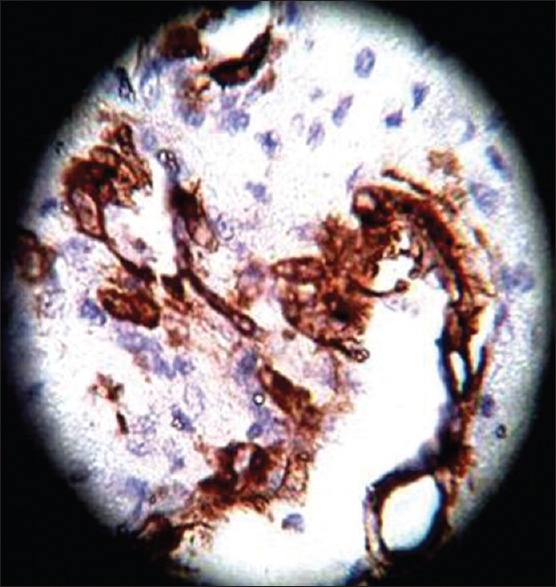
SMA, ×40 high power
Figure 16.
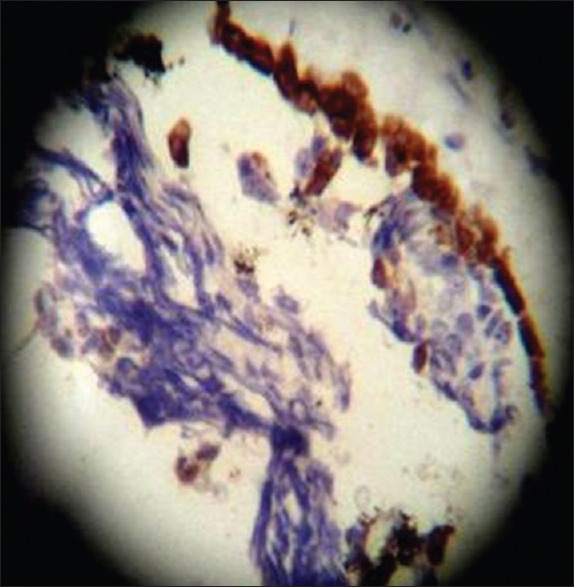
HMW-CK, ×40 high power
Immediately following surgery, the patient had transient deterioration of vision in left eye, which gradually improved after 1 week, and at present, the patient's vision in left eye is 6/60 and in right eye is PL negative. Postoperative adjuvant conventional radiotherapy followed by chemotherapy with cisplatin and paclitaxel were started, and he recently received third cycle of chemotherapy. Patient was reviewed regularly and a follow-up CT scan [Figure 17] was done 4 months after surgery, which did not reveal any recurrence. Till date, after 6 months of surgery, patient is doing well without recurrence or metastasis.
Figure 17.
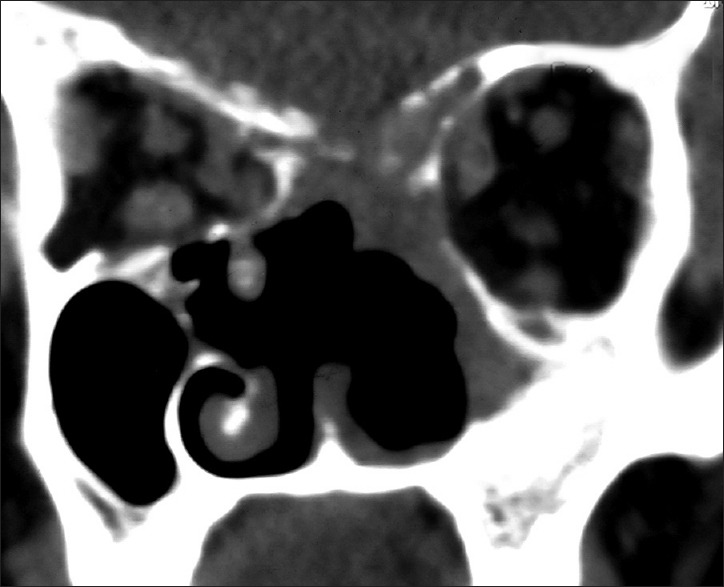
Follow-up computed tomography scan of nose and paranasal sinuses: Coronal view, done 4 months following surgery
Discussion
SNTCS is one of the rarest, aggressive malignant neoplasms of sinonasal tract, consisting of primitive neuroepithelial elements with various malignant epithelial and mesenchymal components.[7] Henfer and Hyams were the first to coin the term “teratocarcinosarcoma” after the clinicopathologic study of 20 patients with sinonasal tract neoplasms.[8] Previously described as teratoid carcinosarcoma, malignat teratoma, or blastoma, SNTCS constitutes less than 1% of all cancers and approximately 3% of all malignancies of head and neck region.[9] One study revealed only 1 (0.5%) case of SNTCS among 200 malignant sinonasal tumors.[9] SNTCS is mainly located in the nasal cavity and paranasal sinuses, although tumors occurring in other locations including the nasopharynx and oral cavity have been described.[2,3,4] This neoplasm is mostly seen in adults, with a reported age range from 18 to 79 years with a mean age of 55 years, and a strong male predominance with a 7:1 male to female ratio.[10] The most common presentation of SNTCS is nasal obstruction and epistaxis with average duration of symptoms reported as about 3.5 months.[5,8,11] Rare symptoms include eye and/or facial pain, epiphora, headache, vision loss, exophthalmos, and anosmia.[5] Neurological symptoms are rare with SNTCS as they do not usually invade the intracranial space[11] and there are only few reports of neurological symptoms from SNTCS with intracranial extension.[7,12,13]
SNTCS is characterized by a histologic combination of malignant teratoma and carcinosarcoma with a triphasic growth pattern including epithelial, mesenchymal, and primitive neuroectodermal components.[1] As this neoplasm constitutes various histopathologic characteristics, small samples from the biopsy are often inadequate to achieve the correct diagnosis. The carcinoma may be either squamous or adenocarcinoma, and the mesenchymal component may manifest spindle, smooth, skeletal muscle, cartilage, and bone features. Tissue heterogeneity and a variegated architectural pattern are characteristic of these tumors. The epithelial components include malignant squamous epithelium, glandular differentiated malignant cells, and clear cell squamous epithelium. Fetal appearing clear cell squamous epithelium is a diagnostic factor reported in some studies.[1,8] These components are also malignant with prominent nucleoli and abundant mitoses. In addition to these structures, poorly differentiated neuroepithelial tissues with neural rosettes are present in most cases.[8,14,15,16,17] Immunohistochemistry usually shows expression of vimentin, CD99 (MIC2), and neuron-specific enolase in most cells, and focal expression of cytokeratin, epithelial membrane antigen (EMA), GFAP, chromogranin, and synaptophysin, and is consistently negative for beta-HCG, neurofilament protein, and leukocyte common antigen.[18]
The histogenesis of SNTCS is still controversial despite many studies conducted on it. The origin of SNTCS was postulated to be related to the olfactory membrane due to the presence of neural tissue.[8] Alternatively, this tumor was thought to be originated from primitive embryonic tissues or immature pluripotential cells that have remained sequestrated in the sinonasal tract during development.[8,19] By finding trisomy 12 (a well-known cytogenetic abnormality occurring in majority of malignant germ cell tumors) in their case of SNTCS, Vranic et al.[20] proposed the germ cell origin of these tumors in 2008, but Salem et al.[21] recently reported three cases of SNTCS with no amplification of chromosome 12 p, thus questioning the germ cell origins of SNTCS. Thus, from all these hypotheses, a stem or pluripotential progenitor cell with multidirectional differentiation is a likely candidate.[22]
Surgical removal, as complete as possible, and postoperative adjuvant therapy seem to be the treatments of choice for patients with SNTCS.[5] There are various surgical approaches described in literature for removal of sinonasal masses with intracranial extension. These are transcranial, subcranial, transfacial, endonasal, endoscopic, and combination of transcranial-transfascial, subcranial-transfacial, or any of the open approaches combined with endoscopic surgery.[23,24]
Transcranial approach for removal of skull base lesions with intracranial extensions provides better visualization and a good possibility for total removal of anterior skull base tumors and also permits reconstruction of the skull base damaged by the tumor.[25] The greatest disadvantage of this approach is that it requires to be combined with other transfacial or endoscopic approaches for successful resection of large tumor mass with nose and paranasal sinus involvement.[25,26,27] Other disadvantages include more chances of brain parenchymal injuries as it involves frontal lobe retraction, large external scar, and more chances of cerebrospinal fluid (CSF) leak.[27]
The subcranial approach is an effective technique for the resection of nasal masses with intracranial extension. Osteotomy of the frontonasoorbital external skeletal frame provides optimum anterior access to the orbital and sphenoethmoidal planes as well as to the nasal and paranasal cavities, while avoiding frontal lobe retraction and the external facial incisions characteristic of transcranial and transfacial approaches. The improved visualization of the anterior skull base and clival-sphenoidal region facilitates en bloc tumor removal, optic nerve decompression, exposure of the medial aspect of the cavernous sinus, and watertight realignment of the anterior cranial base dura.[28] A combined subcranial-transfacial approach may be utilized for tumors involving the skull base and the lower or lateral segments of the maxilla, combined subcranial-transorbital approach may be used for tumors invading the orbit, and an extended subcranial-orbitozygomatic approach may be used for tumors invading the middle cranial fossa or involving the cavernous sinus.[29]
Transfacial approach is the mainstay surgical approach to all lesions of nose and sinonasal region; however, it provides inadequate access to those tumors which have large intracranial extension. Transfacial approaches either utilize incisions placed in favorable lines of facial skin or incisions confined to the mouth and nasal cavity, which minimizes facial scarring. In midface degloving approach, sublabial and intranasal incisions are given to lift the facial skin superiorly and off of the midface bony structures. This approach provides access to bilateral nasal cavity, middle third of face, and central skull base. All these areas are also accessible with Weber-Ferguson and lateral rhinotomy approaches, but these produce facial scarring. Midface degloving approach provides better access to the lateral sphenopalatine and the infratemporal fossa. There is also potential for easy extension and modification of midface degloving technique.[30] The main limitations of midface degloving technique include reduced access to frontal sinuses and anterior skull base. Transfacial approaches that involve facial incisions include lateral rhinotomy approach, Weber-Ferguson approach, and its different modifications. Lateral rhinotomy incision is useful for smaller lesions of nasal cavity and medial maxilla. Weber-Ferguson incisions, on the other hand, provide wide access to most of the sinonasal tumors without much intracranial extension. All these techniques can be combined with transcranial or subcranial approach for sinonasal tumors with intracranial extensions,[26] when they are not accessible through transfacial approach.
The expanded endoscopic endonasal approach is a promising, minimally invasive alternative to open transcranial or transfacial approaches for selective midline anterior cranial base and sinonasal tumors.[31] This technique involves two co-surgeons, usually an otolaryngologist and a neurosurgeon, working simultaneously and side by side throughout the procedure.[32] Tumors that extend laterally across midorbit cannot be entirely resected endonasally. Tumors that demand complete maxillectomy, orbital exenteration, or skin removal to obtain safe margin are also not a candidate for this approach.[33] The golden rule of endonasal surgery is to address the pathology without displacement of critical neural or vascular structures. If a tumor extends lateral or deep to the cranial nerves or major vascular structure, a lateral conventional approach may be preferable. Extensive tumors often require combination of an endonasal corridor and other traditional open surgical approaches to access various components of the tumor.[23,32,34] Principles of tumor dissection for endoscopic approaches are identical to those of other traditional open surgical methods. Critical neural and vascular structures are identified early and, when possible, blood supply to the tumor is controlled. Conventional open approaches utilize facial or scalp incisions combined with a craniotomy and/or maxillofacial osteotomies,[32] and so postoperative pain after conventional approaches is significant and convalescence may take weeks. Surgical scars and complications, such as wound infection, bone malunion, and loss of cranio-orbital bone grafts, sometimes lead to substantial disfigurement. In addition, transcranial approaches often require brain retraction and manipulation, which may result in cognitive or memory impairment.[32] On the other hand, use of expanded endonasal approach provides a caudal approach to the ventral skull base, obviating brain manipulation, facial incisions, and osteotomies, decreasing postoperative pain and hospital stay, and eliminating chances of facial scarring and disfigurement.[32] Through experience and innovation, the expanded endonasal approach currently provides a nasal corridor for the surgical management of various benign and malignant pathologies in any area of ventral skull base. Recent refinements in endoscopic techniques together with the development of related surgical instruments allow complete radical resection of complex anatomic structures through combined transcranial and endonasal approaches without compromising any oncological principles.[23]
The application of image-guidance technology to sinonasal and skull base procedures has ushered in a new era of surgical approaches to conditions affecting this region.[35] Image guidance during surgical procedure is helpful in this type of lesions, providing better anatomic orientation, and delineating tumor margins and their relation to critical neurovascular structures, and provides a valuable aid for safe and complete removal of these lesions with fewer intraoperative complications and improved clinical outcomes.[35,36] However, we do not have personal experience of navigation system.
The recent development is robotic endoscopic skull base surgery which is still in an experimental stage. Transantral robotic surgery provides adequate endoscopic access to the anterior and central skull base. This novel approach also allows for three-dimensional, two-handed, tremor-free endoscopic dissection, suturing, and precise closure of dural defects.[37] These advantages may expand the indications of minimally invasive robotic assisted endoscopic approaches to the skull base and may be utilized for removal of this type of sinonasal tumors in the near future.
Resection of large sinonasal tumors with intracranial extensions may destroy skull base bone, dura mater, pia mater, and can involve cranial cavity and the extracranial structures. Resection of such tumor can result in extensive skull base defects and produce a free conduit between the paranasal sinuses and the intracranial space. Following tumor resection, such skull base defects require reconstruction to create a secure barrier between these two compartments and to prevent complications such as intracranial infection, brain herniation, pulsatile exopthalmos, and leakage of CSF. Therefore, the reconstructive surgery should close the dura, reconstruct a permanent and reliable barrier between cranial cavity and the nasal cavity, paranasal sinuses, and oral cavity.[38] A wide variety of options, ranging from grafts and alloplastic implants to local, regional, and distant revascularized free flaps, are available for skull base reconstruction. The principal grafts used in skull base reconstruction are: (1) fascial grafts used as dural patches, (2) fat or dermal fat grafts used to obliterate relatively small, well-contained cavities and restore lost soft tissue bulk, and (3) bone grafts used to restore resected osseous structure in critical locations. Fascial grafts are usually available within the local field by harvesting from the deep temporal fascia or from pericranium. Both materials can be successfully used to patch holes in the dural cover, but the superficial layer of deep temporal fascia provides a thicker, more durable membrane than the pericranium. In cases of recurrent tumor with depletion of local fascial stock during previous surgery or in cases of extremely large dural defects, harvest of readily obtainable distant fascia (e.g. fascia latae of the lateral thigh) may be necessary.[39] Fat and dermal fat grafts are most commonly taken from the abdomen through a periumbilical incision. Fat grafts are typically used in small-to-moderate sterile cavities as commonly occurs following lateral skull base surgery of the middle and posterior cranial fossae. Disadvantages include a volume reduction of up to 50% over time and an inability to heal in the setting of wound infection, necessitating removal of the fat. Communication of the resection cavity with mucosal surfaces is a contraindication to fat grafting; prior radiation is a relative contraindication. Bone grafts are readily obtained as split-thickness calvaria, which can be harvested from the inner table of bone segments removed during the craniotomy access or separately as outer table grafts from intact portions of the parietal bones. If the skull has been depleted previously or very large quantities of bone are required, the iliac crest is a suitable second-choice source for bone grafts. Note that many osseous defects of the skull base can go unrepaired without significant deformity or dysfunction. Allografts are tissues that are obtained from human sources and are treated appropriately before use in patients to eliminate or greatly reduce the risk of microbial transmission. Various allograft materials, such as lyophilized dura, freeze-dried bone, chemically treated acellular dermis, and fibrin glue, may be used when autogenous tissues are otherwise lacking. In general, allografts tend to survive less well than autografts. Local flaps involve the transfer of vascularized tissue located adjacent to the defect under reconstruction. The flap tissue is mobilized and repositioned into the defect using various techniques such as rotation, transposition, or advancement. The blood supply to local flaps is via the preservation of a vascular pedicle at the base of the flap, which maintains continuity with the donor site following transfer. Advantages of local flaps are: (1) location within the operative field of skull base procedures, (2) relative ease of dissection, and (3) generally, little donor site morbidity. Unfortunately, well-vascularized tissue with sufficient bulk for use as a reliable local flap in the cranial base region is generally in short supply. This situation is particularly true for larger defects located deep within the skull base. Local flaps that can be used are forehead and scalp flaps, galeal-pericranial fascial flaps,[40] temporal based flap system that can incorporate any combination of the temporoparietal fascia (superficial temporal fascia), the deep temporal fascia, or the temporalis muscle. Use of regional flaps also has been described, but when applied to skull base reconstruction, these flaps are significantly limited in their ability to reach into the various defects, and tethering by the muscular pedicle restricts their mobility.[41] Successful reconstructions of the cranial base defect after endoscopic skull base surgery have been described and most successful flap for reconstruction is vascularized pedicled nasoseptal flap (PNSF).[42,43]
Individualized therapy with surgery followed by radiotherapy and a histology-specific multidrug chemotherapy has been tried and showed no recurrence after 36 months of follow-up.[44]
Wei et al.[10] reviewed 54 reported cases of SNTCS and found that 67% of patients with initial single surgical resection and 80% of patients primarily treated with radiotherapy had recurrence, or metastasis, or unresponsiveness to treatment, and almost half of the patients died of tumor within 3 years of diagnosis, despite aggressive therapy. Seventy percent of the patients who survived more than 1 year had the initial therapeutic regimen of combined surgery and adjuvant therapies, suggesting that aggressive therapeutic approaches may improve the treatment outcome. The high rate of local recurrence and distant metastasis is indicative of its highly aggressive biologic behavior. Distant metastasis has been reported in cervical lymph nodes, craniospinal axis, and respiratory tract.[8,12,45]
Mean survival for this neoplasm has been reported by Hefner et al.[8] as 1.7 years with a 60% mortality rate within 3 years of their 15 cases who came for follow-up, of which 8 patients were treated with combination of surgery and radiotherapy, 3 patients had initial surgery alone, 3 were treated with radiotherapy alone, and 1 with radiotherapy plus chemotherapy, and 35% of their patients had developed metastasis, all to the cervical lymph nodes.
We are presenting this unique case of a young adult with SNTCS involving whole of the nasal cavity, nasopharynx, and all paranasal sinuses with bilateral orbital and intracranial extension. Our patient had initially blocked nose and epistaxis, for which he attended an ENT surgeon and was diagnosed as having left nasal mass. The mass was removed first by an ENT surgeon by lateral rhinotomy approach and was diagnosed as having inverted papilloma of sinonasal type; however, the tumor recurred and patient presented to us after 1 year of first surgery with blocked nose, epistasis, vision loss, and headache. Preoperatively we thought it to be a nasopharyngeal carcinoma or angiofibroma as evidenced by CT scans and angiogram; however, on HPE and immunohistochemical study, it came out to be SNTCS. Postoperative adjuvant radiotherapy followed by six cycles of chemotherapy with cisplatin and paclitaxel was planned. Now after 6 months following surgery after receiving adjuvant radiotherapy and fifth cycle of chemotherapy, patient is doing well without recurrence or metastasis.
Conclusion
SNTCS is a very rare, aggressive malignant tumor of nasal cavity and paranasal sinuses, which may be encountered by a neurosurgeon following intracranial extension, and we, neurosurgeons, should be aware of this rare tumor's histogenesis, histology, and management protocols. Recent literatures shows decreased recurrences and increased survival following wide use of aggressive management protocols. Individualized therapy with surgery followed by radiotherapy and histology-specific multidrug chemotherapy seems to be better option.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Ferníndez PL, Cardesa A, Alós L, Pinto J, Traserra J. Sinonasal teratocarcinosarcoma: An unusual neoplasm. Pathol Res Pract. 1995;191:166–71. doi: 10.1016/S0344-0338(11)80567-4. [DOI] [PubMed] [Google Scholar]
- 2.Carrizo F, Pineda-Daboin K, Neto AG, Luna MA. Pharyngeal tera- tocarcinosarcoma: Review of the literature and report of two cases. Ann Diagn Pathol. 2006;10:339–42. doi: 10.1016/j.anndiagpath.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Rotenberg B, El-Hakim H, Lodha B, MacCormick A, Ngan BY, Forte V. Nasopharyngeal teratocarcinosarcoma. Int J Pediatr Otol Rhinol Aryngol. 2002;62:159–64. doi: 10.1016/s0165-5876(01)00575-4. [DOI] [PubMed] [Google Scholar]
- 4.Crazzolara R, Puelacher W, Ninkovic M, Zelger B, Buchberger W, Meister B, et al. Teratocarcinosar- coma of the oral cavity. Pediatr Blood Cancer. 2004;43:687–91. doi: 10.1002/pbc.20139. [DOI] [PubMed] [Google Scholar]
- 5.Smith SL, Hessel AC, Luna MA, Malpica A, Rosenthal DI, El-Naggar AK. Sinonasal teratocarcinosarcoma of the head and neck: A report of 10 patients treated at a single institution and comparison with reported series. Arch Otolaryngol Head Neck Surg. 2008;134:592–5. doi: 10.1001/archotol.134.6.592. [DOI] [PubMed] [Google Scholar]
- 6.Nitsche M, Hermann RM, Christiansen H, Berger J, Pradier O. Rationale for individualized therapy in sinonasal teratocarcinosarcoma (SNTC): Case report. Onkologie. 2005;28:653–6. doi: 10.1159/000089146. [DOI] [PubMed] [Google Scholar]
- 7.Terasaka S, Medary MB, Whiting DM, Fukushima T, Espejo EJ, Nathan G. Prolonged survival in a patient with sinonasal teratocarcinosarcoma with cranial extension. Case report. J Neurosurg. 1998;88:753–6. doi: 10.3171/jns.1998.88.4.0753. [DOI] [PubMed] [Google Scholar]
- 8.Heffner DK, Hyams VJ. Teratocarcinosarcoma (malignant teratoma?) of the nasal cavity and paranasal sinuses: A clinicopathologic study of 20 cases. Cancer. 1984;53:2140–54. doi: 10.1002/1097-0142(19840515)53:10<2140::aid-cncr2820531025>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 9.Cardesa A, Alos L. Franchi A: Nasal cavity and paranasal sinuses. In: Cardesa A, Slootweg PJ, editors. Pathology of the Head and Neck. Berlin, Heidelberg: Springer; 2006. pp. 50–64. [Google Scholar]
- 10.Wei S, Carroll W, Lazenby A, Bell W, Lopez R, Said-Al-Naief N. Sinonasal teratocarcinosarcoma: Report of a case with review of literature and treatment outcome. Ann Diagn Pathol. 2008;12:415–25. doi: 10.1016/j.anndiagpath.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Cedric S, Ali N, Marc D, Jaiyeola TO, Lian ts, Bharat G. Intracerebral metastasis of a sinonasal teratocarcinosarcoma: A case report. Skull Base. 2010;20:393–6. doi: 10.1055/s-0030-1254404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tchoyoson Lim CC, Thiagarajan A, Sim CS, Khoo ML, Shakespeare TP, Ng I. Craniospinal dissemination in teratocarcinosarcoma. J Neurosurg. 2008;109:321–4. doi: 10.3171/JNS/2008/109/8/0321. [DOI] [PubMed] [Google Scholar]
- 13.Szudek J, Bullock M, Taylor SM. Sinonasal teratocarcinosarcoma involving the cavernous sinus. J Otolaryngol. 2005;34:286–8. doi: 10.2310/7070.2005.34421. [DOI] [PubMed] [Google Scholar]
- 14.Patchefsky A, Sundmaker W, Marden PA. Malignant teratoma of ethmoid sinus. Cancer. 1968:714–21. doi: 10.1002/1097-0142(196804)21:4<714::aid-cncr2820210424>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 15.Dicke TE, Gates GA. Malignant teratoma of paranasal sinus. Arch Otolaryngol. 1970;91:391–4. doi: 10.1001/archotol.1970.00770040549020. [DOI] [PubMed] [Google Scholar]
- 16.Christensen EC, Fu YS, Wilson LJ, Hoover MD. Teratoid carcinosarcoma of nasal and paranasal cavities: A case report. Am J Rhinol. 1992:6:169–72. [Google Scholar]
- 17.Shemen L, Galantich P, Murali R. Malignant teratocarcinosarcoma of the sphenoid sinus. Otolaryngol Head Neck Surg. 1995;112:496–500. doi: 10.1016/S0194-59989570294-6. [DOI] [PubMed] [Google Scholar]
- 18.Pai SA, Naresh KN, Masih K, Ramarao C, Borges AM. Teratocarcinosarcoma of the paranasal sinuses: A clinicopathologic and immunohistochemical study. Hum Pathol. 1998;29:718–22. doi: 10.1016/s0046-8177(98)90281-7. [DOI] [PubMed] [Google Scholar]
- 19.Shanmugaratnam K, Kunaratnam N, Chia KB, Chiang GS, Sinniah R. Teratoid carcinosarcoma of paranasal sinuses. Pathology. 1983;15:413–9. doi: 10.3109/00313028309085168. [DOI] [PubMed] [Google Scholar]
- 20.Vranic S, Caughron SK, Djuricic S, Bilalovic N, Zaman S, Suljevic I, et al. Hamartomas, teratomas and teratocarcinosarcomas of the head and neck: Report of 3 new cases with clinico-pathologic correlation, cytogenetic analysis, and review of the literature. BMC Ear Nose Throat Disord. 2008;8:8. doi: 10.1186/1472-6815-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salem F, Rosenblum MK, Jhanwar SC, Kancherla P, Ghossein RA, Carlson DL. Teratocar. Cinosarcoma of the nasal cavity and paranasal sinuses: Report of three cases with assessment for chromosome 12p status. Hum Pathol. 2008;39:605–9. doi: 10.1016/j.humpath.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Batsakis JG, El-Naggar AK, Luna MA. Teratomas of the head and neck with emphasis on malignancy. Ann Otol Rhinol Laryngol. 1995;104:496–500. doi: 10.1177/000348949510400616. [DOI] [PubMed] [Google Scholar]
- 23.Belli E, Rendine G, Mazzone N. Malignant ethmoidal neoplasms: A cranionasal endoscopy approach. J Craniofac Surg. 2009;20:1240–4. doi: 10.1097/SCS.0b013e3181acdfdc. [DOI] [PubMed] [Google Scholar]
- 24.Belli E, Longo B, Marini FB, Matteini C. Could transcranial endoscopy represent an alternative to craniotomy in skull base surgery? J Craniofac Surg. 2005;16:155–8. doi: 10.1097/00001665-200501000-00031. [DOI] [PubMed] [Google Scholar]
- 25.Hendryk S, Czecior E, Misiołek M, Namysłowski G, Mrówka R. Surgical strategies in the removal of malignant tumors and benign lesions of the anterior skull base. Neurosurg Rev. 2004;27:205–13. doi: 10.1007/s10143-004-0323-z. [DOI] [PubMed] [Google Scholar]
- 26.Iseh KR, Amutta SB, Shehu BB, Nasir J. Combined transfacial and transcranial approach for tumours of the nose and paranasal sinuses with intracranial extension. Niger J Med. 2011;20:333–6. [PubMed] [Google Scholar]
- 27.Goyal P, Kellman RM, Tatum SA., 3rd Transglabellar subcranial approach for the management of nasal masses with intracranial extension in pediatric patients. Arch Facial Plast Surg. 2007;9:314–7. doi: 10.1001/archfaci.9.5.314. [DOI] [PubMed] [Google Scholar]
- 28.Raveh J, Turk JB, Lädrach K, Seiler R, Godoy N, Chen J, et al. Extended anterior subcranial approach for skull base tumors: Long-term results. J Neurosurg. 1995;82:1002–10. doi: 10.3171/jns.1995.82.6.1002. [DOI] [PubMed] [Google Scholar]
- 29.Fliss DM, Abergel A, Cavel O, Margalit N, Gil Z. Combined subcranial approaches for excision of complex anterior skull base tumors. Arch Otolaryngol Head Neck Surg. 2007;133:888–96. doi: 10.1001/archotol.133.9.888. [DOI] [PubMed] [Google Scholar]
- 30.Eze NN, Wyatt ME, Bray D, Bailey CM, Hartley BE. The midfacial degloving approach to sinonasal tumors in children. Rhinology. 2006;44:36–8. [PubMed] [Google Scholar]
- 31.Dehdashti AR, Ganna A, Witterick I, Gentili F. Expanded endoscopic endonasal approach for anterior cranial base and suprasellar lesions: Indications and limitations. Neurosurgery. 2009;64:677–87. doi: 10.1227/01.NEU.0000339121.20101.85. [DOI] [PubMed] [Google Scholar]
- 32.Bhatki AM, Carrau RL, Snyderman CH, Prevedello DM, Gardner PA, Kassam AB. Endonasal surgery of the ventral skull base-endoscopic transcranial surgery. Oral Maxillofac Surg Clin North Am. 2010;22:157–68. doi: 10.1016/j.coms.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 33.Leo FS Ditzel Filho, Lara DD, Prevedello DM, Kasemsiri P, Otto BA, Old M, Kasam AB, Carrau RL. Expanded endonasal approaches to the anterior skull base. Otorhinolaryngology clinics. An Int J. 2011;3:176–83. [Google Scholar]
- 34.Galassi E, Pasquini E, Frank G, Marucci G. Combined endoscopy-assisted cranionasal approach for resection of infantile myofibromatosis of the ethmoid and anterior skull base. Case report. J Neurosurg Pediatr. 2008;2:58–62. doi: 10.3171/PED/2008/2/7/058. [DOI] [PubMed] [Google Scholar]
- 35.Sindwani R. Image-guided surgery of the paranasal sinuses and skull base. Mol Med. 2008;105:257–61. [PubMed] [Google Scholar]
- 36.Kurtsoy A, Menku A, Tucer B, Oktem IS, Akdemir H. Neuronavigation in skull base tumors. Minim Invasive Neurosurg. 2005;48:7–12. doi: 10.1055/s-2004-830151. [DOI] [PubMed] [Google Scholar]
- 37.Hanna EY, Holsinger C, DeMonte F, Kupferman M. Robotic endoscopic surgery of the skull base: A novel surgical approach. Arch Otolaryngol Head Neck Surg. 2007;133:1209–14. doi: 10.1001/archotol.133.12.1209. [DOI] [PubMed] [Google Scholar]
- 38.Wang B, Wu ST, Li Z, Liu PN. Anterior and middle skull base reconstruction after tumor resection. Chin Med J. 2010;123:281–5. [PubMed] [Google Scholar]
- 39.Hanasono MM, Sacks JM, Goel N, Ayad M, Skoracki RJ. The anterolateral thigh free flap for skull base reconstruction. Otolaryngol Head Neck Surg. 2009;140:855–60. doi: 10.1016/j.otohns.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 40.Zanation AM, Snyderman CH, Carrau RL, Kassam AB, Gardner PA, Prevedello DM. Minimally invasive endoscopic pericranial flap: A new method for endonasal skull base reconstruction. Laryngoscope. 2009;119:13–8. doi: 10.1002/lary.20022. [DOI] [PubMed] [Google Scholar]
- 41.Weizman N, Gil Z, Wasserzug O, Amir A, Gur E, Margalit N, et al. Surgical ablation and free flap reconstruction in children with malignant head and neck tumors. Skull Base. 2011;21:165–70. doi: 10.1055/s-0031-1275250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.El-Sayed IH, Roediger FC, Goldberg AN, Parsa AT, McDermott MW. Endoscopic reconstruction of skull base defects with the nasal septal flap. Skull Base. 2008;18:385–94. doi: 10.1055/s-0028-1096202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu JK, Schmidt RF, Choudhry OJ, Shukla PA, Eloy JA. Surgical nuances for nasoseptal flap reconstruction of cranial base defects with high-flow cerebrospinal fluid leaks after endoscopic skull base surgery. Neurosurg Focus. 2012;32:E7. doi: 10.3171/2012.5.FOCUS1255. [DOI] [PubMed] [Google Scholar]
- 44.Nitsche M, Hermann RM, Christiansen H, Berger J, Pradier O. Rationale for individualized therapy in sinonasal teratocarcinosarcoma (SNTC): Case report. Onkologie. 2005;28:653–6. doi: 10.1159/000089146. [DOI] [PubMed] [Google Scholar]
- 45.Carrizo F, Pineda-Daboin K, Neto AG, Luna MA. Pharyngeal teratocarcinosarcoma: Review of the literature and report of two cases. Ann Diagn Pathol. 2006;10:339–42. doi: 10.1016/j.anndiagpath.2006.03.006. [DOI] [PubMed] [Google Scholar]


