Abstract
Background
It is uncertain whether improvements in primary care high-risk prescribing seen in research trials can be realised in the real-world setting.
Aim
To evaluate the impact of a 1-year system-wide phase IV prescribing safety improvement initiative, which included education, feedback, support to identify patients to review, and small financial incentives.
Design and setting
An interrupted time series analysis of targeted high-risk prescribing in all 56 general practices in NHS Forth Valley, Scotland, was performed. In 2013–2014, this focused on high-risk non-steroidal anti-inflammatory drugs (NSAIDs) in older people and NSAIDs with oral anticoagulants; in 2014–2015, it focused on antipsychotics in older people.
Method
The primary analysis used segmented regression analysis to estimate impact at the end of the intervention, and 12 months later. The secondary analysis used difference-in-difference methods to compare Forth Valley changes with those in NHS Greater Glasgow and Clyde (GGC).
Results
In the primary analysis, downward trends for all three NSAID measures that were existent before the intervention statistically significantly steepened following implementation of the intervention. At the end of the intervention period, 1221 fewer patients than expected were prescribed a high-risk NSAID. In contrast, antipsychotic prescribing in older people increased slowly over time, with no intervention-associated change. In the secondary analysis, reductions at the end of the intervention period in all three NSAID measures were statistically significantly greater in NHS Forth Valley than in NHS GGC, but only significantly greater for two of these measures 12 months after the intervention finished.
Conclusion
There were substantial and sustained reductions in the high-risk prescribing of NSAIDs, although with some waning of effect 12 months after the intervention ceased. The same intervention had no effect on antipsychotic prescribing in older people.
Keywords: antipsychotic drugs, general practice, high-risk prescribing, interrupted time series analysis, non-steroidal anti-inflammatory drugs, quality improvement
INTRODUCTION
High-risk prescribing is common in primary care,1,2 although it is not always inappropriate: expected benefit can outweigh expected harm in individual patients. Drugs commonly implicated in preventable adverse drug events (ADEs) that result in hospital admissions include non-steroidal anti-inflammatory drugs (NSAIDs) and aspirin, which are responsible for 30% of ADE-related hospital admissions, due to bleeding, stroke, and renal injury.3 Similar to primary care organisations elsewhere, health boards in Scotland use a variety of means to try to influence primary care prescribing, including education and feedback, pharmacist support, and small financial incentives.4,5 Historically, the focus of most of this work has been on controlling prescribing costs,6 but the growing availability of better prescribing data opens up new opportunities to target quality and safety.
A range of prescribing safety indicators have been developed,7–9 and interventions to improve subsets of them have been evaluated in phase III cluster randomised trials in primary care.10–13 The pharmacist-led information technology intervention for medication errors (PINCER) trial evaluated practitioner education, informatics tools to identify relevant patients, and intensive pharmacist support to review patients and improve prescribing systems.11 For the three primary high-risk prescribing outcome indicators — non-selective NSAIDs prescribed to those with a history of peptic ulcer without co-prescription of a proton-pump inhibitor; beta-blockers prescribed to those with a history of asthma; long-term prescription of angiotensin converting enzyme [ACE] inhibitor or loop diuretics to those aged ≥75 years without assessment of urea and electrolytes in the preceding 15 months — there was a reduction in the odds of each of 27–49% at 6 months, which diminished to 9–37% by 12 months.
The Data-driven Quality Improvement in Primary Care (DQIP) trial evaluated education, informatics to support patient identification, and financial incentives for patient review.12 There was a 41% reduction in the odds of the composite measure of targeted high-risk prescribing at 1 year, sustained in the following year. The lower-intensity Effective Feedback to Improve Primary Care Prescribing Safety (EFIPPS) intervention had a 14% reduction in the odds of six measures of high-risk NSAID and antipsychotic prescribing after five rounds of quarterly feedback.13 However, whether these improvements can be replicated in everyday practice is uncertain. The UK Medical Research Council recommends phase IV evaluation to ‘determine whether others can reliably replicate your intervention and results in uncontrolled settings over the long term’10 as:
‘... effects are likely to be smaller and more variable once the intervention becomes implemented more widely, and … long-term follow-up may be needed to determine whether short-term changes persist’.14
How this fits in
There is good evidence from phase III cluster randomised trials that a number of interventions reduce high-risk prescribing in primary care, but whether similar improvements can be realised in the real-world setting is less clear. A system-wide quality-improvement intervention combining education, feedback, support to identify patients to review, and small financial incentives resulted in large reductions in the high-risk prescribing of non-steroidal anti-inflammatory drugs (NSAIDs) of a similar magnitude to those seen in phase III trials. The effect on high-risk NSAID prescribing waned somewhat in the year after the intervention ended, highlighting the need for healthcare improvement to monitor impact over a longer term and consider interventions to sustain benefit. The same intervention had no effect on the high-risk prescribing of antipsychotics, highlighting that interventions may have differential effectiveness, depending on the wider context of prescribing.
The aim of this phase IV study, therefore, was to evaluate the impact of a complex, whole-system, real-world intervention to improve prescribing safety implemented in all practices in a Scottish health board region with a population about 300 000, including whether impact was sustained post intervention.
METHOD
The overall design is segmented regression analysis of interrupted time series (ITS) data from a Scottish health board that implemented the intervention and a comparator Scottish health board that did not. There are about 300 000 registered patients in the intervention health board (NHS Forth Valley) and approximately 1 200 000 registered patients in the neighbouring comparator health Board (NHS Greater Glasgow and Clyde [GGC]), which was chosen because it had not implemented any specific improvement projects focusing on these particular prescribing measures in the time period examined.
Data source
Data on prescriptions dispensed by community pharmacies between April 2009 and September 2015 were extracted from NHS Scotland’s Prescribing Information System. In total, 94.7% of dispensed prescriptions in NHS Forth Valley and 94.5% in NHS GGC have an associated unique patient identifier, allowing the construction of patient-level prescribing histories and the identification of co-prescribing in individuals.
Interventions and outcomes
In the financial year 2013–2014, NHS Forth Valley implemented a prescribing improvement intervention targeting three high-risk NSAID prescribing measures as part of its annual whole system working (WSW) primary care improvement programme (Box 1). The WSW intervention included:
education;
feedback;
searches and pharmacist support to identify relevant patients from electronic health records; and
financial incentives for practices to report any changes in the high-risk prescribing rates to the health board at year end.
Box 1. The NHS Forth Valley intervention to improve primary care prescribing safety.
| Context |
| From April 2010 to March 2017 NHS Forth Valley contracted an enhanced service with GP practices called whole system working (WSW). In the financial periods of 2013–2014 and 2014–2015, WSW included an intervention that — alongside other activities to improve patient safety, to improve communication between practices, hospital consultants and out-of-hours services, and increase engagement with locality improvement activity — aimed to improve primary care prescribing safety by focusing on reducing the use of unsafe drug combinations. In each financial year, practices were paid £0.80 per registered patient (approximately £4000 for an average-sized practice of 5000 patients) for completing all work related to WSW. |
| Intervention specifics |
Targeted high-risk prescribing (2013–2014)
|
Targeted high-risk prescribing (2014–2015)
|
| Educational workshop |
| A brief educational intervention focusing on NSAID risks (quarter two, 2013) and antipsychotic risks in older people (quarter two, 2014) lasting approximately 45 minutes was delivered each year in June, during a 2.5-hour long educational session on patient safety in primary care, which the majority of GPs attended. The 45-minute educational session included providing comparative data on practice rates for that year’s measures, and what was expected of practices. |
| Feedback and written educational material |
| During each year, around the same time as the educational workshop was held, each practice was given written educational material summarising the educational outreach workshop information. Accompanying this was a single round of feedback showing practice rates of targeted high-risk prescribing compared with the average for the health board and practices’ ranking within the health board. The same written material and feedback were given directly to all participants at the educational workshop. |
| Financial incentive |
| Within each year practices qualified for the WSW payment only when they provided evidence of completing all WSW elements. The evidence required for the high-risk prescribing component was simply to report to the health board the number of patients triggering the measures at baseline and 6 months later, rather than to provide evidence of change in high-risk prescribing rates. |
| Patient identification support |
| In both years, practices identified patients for review using search tools supplied by NHS Forth Valley to run in their own electronic medical record systems. Pharmacists employed by the health board reviewed the output of these searches to produce a clean list of patients for GPs to focus on (for example, by checking that the patient had actually received the targeted drug combinations). GPs were asked to review identified patients’ records, and then take whatever action they judged appropriate (for example, continuing, amending or stopping medication without further review, or contacting the patient to discuss). There was no WSW requirement to report to the health board the actual action taken. |
ACEI = angiotensin converting enzyme inhibitor. ARB = angiotensin receptor blocker. NSAID = non-steroidal anti-inflammatory drug. WSW = whole system working.
In 2014–2015, a new measure (antipsychotic use in people aged ≥75 years) replaced the NSAID measures targeted in the previous year. The prescribing measures used in the intervention and evaluation were ones used in the EFIPPS trial13 (in which neither NHS Forth Valley nor NHS GGC participated), and are shown in Table 1.
Table 1.
Definitions of targeted prescribing and outcome measures
| Measure short name | Measure definition (patients in NHS Forth Valley immediately before intervention) | Associated harm | NHS Forth Valley intervention period | Prevalence of high-risk prescribing in NHS Forth Valley immediately before intervention, rate per 1000 (95% CI) |
|---|---|---|---|---|
| ‘Triple whammy’ | Patients aged ≥65 years (n= 51 595) prescribed diuretic + ACEI or ARB + NSAID (n= 596) | Acute kidney injury15,16 | April 2013–March 2014 | 11.6 (10.7 to 12.6)a |
| NSAIDs in older people | Patients aged ≥65 years (n= 51 595) prescribed NSAID without gastroprotection (n= 1 832) | Gastrointestinal bleeding17 | April 2013–March 2014 | 35.5 (33.9 to 37.1)a |
| NSAIDs with OAC | Patients prescribed OAC (n= 3 423), then prescribed NSAID without gastroprotection (n= 23) | Gastrointestinal bleeding17 | April 2013–March 2014 | 6.7 (4.5 to 10.0)b |
| Antipsychotics in older peoplec | Patients aged ≥75 years (n= 22 980) prescribed oral antipsychotic (n= 512) | Stroke and death18 | April 2014–March 2015 | 22.3 (20.5 to 24.3)d |
Rate per 1000 population aged ≥65 years.
Rate per 1000 prescribed OAC.
As a proxy for older people with dementia.
Rate per 1000 population aged ≥75 years. ACEI = angiotensin converting enzyme inhibitor. ARB = angiotensin receptor blocker. NSAID = non-steroidal anti-inflammatory drug. OAC = oral anticoagulant.
Over the same period, NHS GGC chose to focus on other areas of prescribing improvement, including medication review in older people with polypharmacy at risk of re-admission to hospital. High-risk prescribing of NSAIDs and oral antipsychotics were included in the medication review but no specific targeting of the measures which were used in NHS Forth Valley.19 Within each health board, more than 95% of practices participated in these activities.
Statistical analysis
The primary analysis used segmented regression of ITS data to examine the impact of the implementation and withdrawal after 1 year of the WSW intervention in NHS Forth Valley. The intervention period started at the beginning of quarter two (April) of the relevant year, and finished at the end of quarter one of the next year (March).
For each measure in NHS Forth Valley, the following were estimated:
trend before each interruption;
step changes immediately after the start and end of the intervention period; and
changes in trend following the start and end of the intervention period.
Model estimates were used to calculate the intervention effect (by subtracting the observed value from the predicted value if prior trends had continued) at the end of the 12-month intervention period, and at 12 months after the intervention period ended.20,21
The overall intervention effect of the three measures of high-risk NSAID prescribing was estimated using a composite of all three that accounted for some patients having multiple risk factors. Secondary analyses compared changes in NHS Forth Valley with changes in the same prescribing measures in the same period in NHS GGC. For this analysis, a segmented regression model was fitted for the difference between rates in the two health boards (NHS Forth Valley minus NHS GGC), allowing estimation of the difference-in-differences of change in NHS Forth Valley relative to change in NHS GGC (details are available from the author on request).22
For the three NSAID measures there were 16 quarterly time points before the intervention start, four during the intervention period, and eight after the intervention period. For the antipsychotics in older people measure, there were 20 quarterly time points before, four during, and four after the intervention period. Modelling accounted for autocorrelation by using the Cumby-Huizinga general test and fitting lag terms to models as required. Newey-West standard errors were estimated to account for autocorrelation and possible heteroscedasticity.23 Statistical analysis was undertaken using Stata (version 13.1).
RESULTS
NHS Forth Valley NSAIDs (2013–2014)
All three NSAID measures had a statistically significant downward trend before the implementation of WSW between April 2013 and March 2014. Following the start of the implementation in April 2013, there was no immediate change in the rate of prescribing for the ‘triple whammy’ and NSAIDs in older people measures, but the existing downward trends significantly steepened (Figure 1 and Table 2). For NSAIDs with OAC, there was a statistically significant immediate decrease in prescribing, but no significant change in trend (Figure 1). At the end of the intervention period in April 2014, there was a statistically significant immediate increase in both ‘triple whammy’ and NSAID prescribing in older people, but not for NSAIDs with OAC. After the end of the intervention period, there were statistically significant changes in trend leading either to a reversion to pre-intervention downward trends (triple whammy) or a diminished intervention effect (NSAIDs in older people) and a reversal of pre-intervention trends (NSAIDs with OAC).
Figure 1.
Change in NSAID outcome measures before, during, and after intervention. Vertical dotted lines indicate the start and finish times for the intervention. ACE = angiotensin converting enzyme. ARB = angiotensin receptor blocker. NSAID = non- steroidal anti-inflammatory drug. OAC = oral anticoagulant.
Table 2.
NHS Forth Valley segmented regression analysis
| Prescribing measures | Baseline rate, patients prescribed per 1000 at risk (95% CI) | Pre-intervention period trend, change in rate per quarter (95% CI) | Immediate step change in rate at start of intervention period (95% CI) | Change in trend after start of intervention period, change in rate per quarter (95% CI) | Immediate step change in rate at end of intervention period (95% CI) | Change in trend after end of intervention period, change in rate per quarter (95% CI) | Absolute difference at end of intervention period, patients prescribed per 1000 at risk (95% CI) | Absolute difference 12 months post end of intervention period, patients prescribed per 100 00 at risk (95% CI) |
|---|---|---|---|---|---|---|---|---|
| 2013–2014 outcome measures | ||||||||
| ‘Triple whammy’: patients aged ≥65 years prescribed NSAID + ACE/ARB + diuretica | 13.1 (12.4 to 13.8) | −0.2 (−0.2 to −0.1) | −0.5 (−1.3 to 0.3) | −1.2 (−1.4 to −1.1) | 1.5 (1.1 to 1.9) | 1.3 (1.2 to 1.3) | −5.5 (−6.7 to −4.3) | −4.0 (−5.4 to −2.6) |
|
| ||||||||
| NSAIDs in older people: patients aged ≥65 years prescribed NSAID without gastroprotectiona | 44.1 (42.9 to 45.4) | −0.7 (−0.9 to −0.6) | −1.6 (−3.8 to 0.6) | −4.6 (−4.95 to −4.3) | 7.4 (6.2 to 8.5) | 5.2 (4.9 to 5.6) | −20.1 (−23.0 to −17.1) | −10.3 (−13.9 to −6.8) |
|
| ||||||||
| NSAIDs with OAC: patients prescribed OAC + NSAID without gastroprotectionb | 9.1 (8.1 to 10.1) | −0.2 (−0.3 to −0.04) | −2.0 (−3.6 to −0.3) | −0.3 (−0.8 to 0.2) | 0.6 (−0.8 to 2.0) | 0.6 (0.1 to 1.1) | −3.3 (−4.9 to −1.6) | −1.5 (−3.5 to 0.6) |
|
| ||||||||
| 2014–2015 outcome measure | ||||||||
| Antipsychotics in older people: patients aged ≥75 years prescribed oral antipsychoticc | 19.6 (18.7 to 20.5) | 0.1 (−0.01 to 0.1) | −0.1 (−1.1 to 0.8) | 0.1 (−0.1 to 0.3) | −0.6 (−1.4 to 0.2) | 0.3 (0.1 to 0.6) | 0.2 (−1.2 to 1.5) | 1.2 (−0.5 to 2.9) |
Rate per 1000 population aged ≥65 years.
Rate per 1000 prescribed OAC.
Rate per 1000 population aged ≥75 years. ACE = angiotensin converting enzyme. ARB = angiotensin receptor blocker. NSAID = non-steroidal anti-inflammatory drug. OAC = oral anticoagulant.
Compared with rates predicted based on pre-intervention trends, the estimated relative effect at the end of the intervention period in April 2014 was a 55.4% (95% confidence interval [CI] = 43.6 to 67.2) reduction for the triple whammy measure, a 69.9% (95% CI = 59.6 to 80.2) reduction for NSAIDs in older people, and a 55.1% (95% CI = 27.0 to 83.2) reduction for NSAIDs with OAC (Table 3). The relative impact 12 months after the intervention period ended in April 2015 was still substantial but somewhat smaller: reductions of 42.7% (95% CI = 27.4 to 58.0), 40.1% (95% CI = 26.4 to 53.8), and 27.6% (95% CI = −11.4 to 66.7, not statistically significant) respectively (Table 3).
Table 3.
Relative effect size in changed rates of high-risk prescribing in NHS Forth Valley, and for the difference between NHS Forth Valley and NHS GGC
| Prescribing measures | NHS Forth Valley | Difference between NHS Forth Valley and NHS GGC | ||
|---|---|---|---|---|
|
|
|
|||
| Relative difference, %, from predicted at end of intervention period (95% CI) | Relative difference, %, from predicted 12 months after end of intervention period (95% CI) | Relative difference, %, compared with NHS GGC at end of intervention period (95% CI) | Relative difference, %, compared with NHS GGC 12 months after period (95% CI) end of intervention | |
| 2013–2014 outcome measures | ||||
| ‘Triple whammy’: patients aged ≥65 years prescribed NSAID + ACE/ARB + diuretica | −55.4 (−67.2 to −43.6) | −42.7 (−58.0 to −27.4) | −34.3 (−42.3 to −26.4) | −25.7 (−36.3 to −15.2) |
|
| ||||
| NSAIDs in older people: patients aged ≥65 years prescribed NSAID without gastroprotectiona | −69.9 (−80.2 to −59.6) | −40.1 (−53.8 to −26.4) | −59.4 (−66.3 to −52.5) | −28.6 (−38.3 to −18.8) |
|
| ||||
| NSAIDs with OAC: patients prescribed OAC + NSAID without gastroprotectionb | −55.1 (−83.2 to −27.0) | −27.6 (−66.7 to 11.4) | −85.0 (−100 to −45.5) | −69.0 (−100 to −27.2) |
|
| ||||
| 2014–2015 outcome measure | ||||
| Antipsychotics in older people: patients aged ≥75 years prescribed oral antipsychoticc | 0.8 (−5.7 to 7.2) | 5.6 (−2.3 to 13.5) | −11.3 (−18.9 to −3.6) | −2.0 (−10.4 to 6.5) |
Rate per 1000 population aged ≥65 years.
Rate per 1000 prescribed OAC.
Rate per 1000 population aged ≥75 years. ACE = angiotensin converting enzyme. ARB = angiotensin receptor blocker. NHS GGC = NHS Greater Glasgow and Clyde. NSAID = non-steroidal anti-inflammatory drug. OAC = oral anticoagulant.
Pre-intervention trends in NHS GGC were not significantly different from those in NHS Forth Valley for the ‘triple whammy’ and NSAIDs with OAC measures. For NSAIDs in older people there was a small but statistically significant more rapid decline in the pre-intervention trend in NHS Forth Valley than NHS GGC (Figure 1, more data available from the authors on request). For the ‘triple whammy’ measure, at the end of the intervention period in April 2014, there was a reduction of 34.3% (95% CI = 26.4 to 42.3) relative to NHS GGC, which diminished over the 12 months after the intervention finished in April 2015 to a reduction of 25.7% (95% CI = 15.2 to 36.3) (Table 3). For NSAIDs in older people, at the end of the intervention period in April 2014, there was a reduction of 59.4% (95% CI = 52.5 to 66.3) relative to NHS GGC, reducing to 28.6% (95% CI = 18.8 to 38.3) by April 2015 (Table 3). For NSAIDs with OAC, at the end of the intervention period there was a reduction of 85.0% (95% CI = 45.5 to 100) relative to NHS GGC, reducing to 69.0% (95% CI = 27.2 to 100) by April 2015 (Table 3).
The total reduction in the number of patients prescribed ‘triple whammy’, NSAIDs in older people, or NSAIDs with OAC, accounting for some patients having multiple risk factors, featured 1221 fewer patients than expected at the end of the intervention period in April 2014. At April 2015, 12 months after the intervention ended, there were 751 fewer patients than expected triggering one or more of the three indicators.
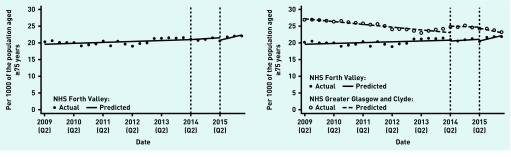
NHS Forth Valley antipsychotics (2014– 2015)
There was a non-significant increase in prescribing of antipsychotics in older people in NHS Forth Valley before the intervention, and no statistically significant changes immediately after the WSW intervention was introduced in April 2014. The upward trend significantly steepened at the time the intervention period ended in April 2015 (although in absolute terms the change is small) (Table 2, Figure 2). There was no significant estimated impact at the end of the intervention period or 12 months later (Table 3).
Figure 2.
Change in antipsychotic outcome measures before, during, and after intervention. Vertical dotted lines indicate the start and finish times for the intervention.
NHS GGC had a higher baseline rate than Forth Valley, which was falling, rather than rising. At the end of the intervention period in April 2015, NHS Forth Valley had an 11.3% (95% CI = 3.6 to 18.9) reduction relative to GGC; however, this difference was driven by an increase at NHS GGC (Figure 2) so is unrelated to the WSW intervention. There was no statistically significant difference 12 months after the end of the intervention period in April 2016 (Table 3, more details available from the authors on request).
DISCUSSION
Summary
The WSW intervention implemented in all NHS Forth Valley practices in 2013–2014 led to large (>55%) reductions in the three targeted measures of high-risk NSAID prescribing at the end of the intervention period. There was evidence of a diminished intervention effect 12 months later, but reductions were still substantial. Relative to NHS GGC these observed reductions remained significant, increasing confidence that the intervention was effective. In contrast, the same WSW intervention in 2014–2015 was not associated with any change in antipsychotic prescribing in older people. Although there were significant ‘reductions’ in NHS Forth Valley relative to GGC, the authors interpret the observed ‘reduction’ as being due to increased prescribing in NHS GGC rather than due to the intervention in NHS Forth Valley.
Strengths and limitations
ITS analysis is the most robust method available for evaluating non-randomised interventions. The analysis presented here used population-based, routine data to examine a system-wide prescribing safety intervention using ITS analysis. Secondary comparison with another health board (which did not implement specific improvement activity on the targeted prescribing) was consistent with the observed changes in NHS Forth Valley being attributable to the intervention.
Limitations include the risk of under-detection of high-risk prescribing, because not all prescriptions have a usable unique patient identifier; the authors, however, do not expect this to alter the interpretation, as there was no change in this over time and no difference between the health boards.
The assumptions of the difference-in-differences model were violated for the antipsychotics in older people measure, as there were different prior trends in the two health boards; the authors conclude that the intervention had no effect on targeted antipsychotic prescribing.
A further limitation is that the post-intervention period was relatively short, particularly for the 2014–2015 intervention. Finally, the possibility that the observed associations are due to some other intervention occurring at the same time cannot be excluded; likewise, the difference in NSAID and antipsychotic outcomes might also be due to changing pressures and priorities in primary care. However, there was no other intervention in NHS Forth Valley during the period examined, and the comparison with NHS GGC provides some reassurance that the NHS Forth Valley intervention caused the observed changes in prescribing.
Comparison with existing literature
The impact of this real-world intervention on the high-risk prescribing of NSAIDs at 1 year is similar to that observed in the DQIP trial of a more intensive, complex intervention.12 DQIP reduced ‘triple whammy’, NSAIDs in older people, and NSAIDs with OAC by 23%, 56%, and 69% respectively at the end of the 12-month intervention, compared with 55%, 70%, and 55% in NHS Forth Valley (although the measures used in the two studies are not identical in design).
The effect size is also similar in magnitude to those observed in the PINCER trial for a different NSAID measure (NSAIDs prescribed to people with a history of peptic ulcer).11 Like PINCER (but unlike DQIP), there was evidence of some waning of effect when the intervention ceased, although the impact at 12 months after the end of the intervention remained similar in magnitude to DQIP. Changes in NSAID prescribing were substantially larger than those observed in the simpler EFIPPS feedback intervention and, it is notable that, like this analysis, there was no evidence that the EFIPPS intervention reduced antipsychotic prescribing in older people.13
Whether organisational interventions shown to be effective in phase III trials will be effective in real-world, system-wide implementation is often uncertain, as trials are usually carried out by volunteers and often have higher intensity of intervention supported by research funding.10,14 This study shows that a phase IV intervention reduced high-risk primary care prescribing of NSAIDs to a similar degree as the two previous large, phase III trials of similar complex interventions in this field.
Consistent with the EFIPPS study,13 this analysis shows that impact may, at least partly, depend on the prescribing targeted. In NHS Forth Valley, the same intervention was highly effective at reducing high-risk NSAID prescribing, but ineffective at reducing antipsychotic prescribing in older people. Neither this study nor the EFIPPS trial can examine why this should be, but one possible explanation is that NSAID prescribing is largely initiated and managed by GPs;24 antipsychotic prescribing, however, is commonly initiated by specialists, plausibly reducing GP ownership of it, despite their being responsible for prescribing antipsychotics in the longer term. GPs have also reported that they continue antipsychotic prescribing in older people out of a sense of futility and concerns about harm if prescribing is stopped;25 therefore, there is unlikely to be a single ‘magic-bullet’ intervention that will be effective for all high-risk prescribing. However, the intervention presented here shows that high-risk prescribing rates can be reduced.
Implications for research and practice
Overall, the findings suggest that a blend of the intervention components used in trials —education, feedback, financial incentives — tailored to a local context are likely to be effective in system-wide implementation. The partial waning of effect in the year after the intervention ceased highlights that trials should, ideally, follow-up patients beyond the duration of the intervention, and that, at least some interventions, may need to be repeated to be sustainable. However, the lack of impact on antipsychotic prescribing in older people in this study and in the EFIPPS trial13 indicates the need for more research into how best to reduce this.
Although randomised trials will be helpful in addressing many of the uncertainties, further rigorous evaluations of phase IV system-wide implementations would be of great value. The growth of electronic prescribing makes interventions of this kind increasingly feasible internationally, and the time is ripe for wider implementation to improve prescribing safety.
Funding
There was no specific funding for this study; however, Sean MacBride-Stewart, Charis Marwick, Andrea Patton, and Bruce Guthrie received research grants from The Health Foundation for the submitted work.
Ethical approval
The analysis is a retrospective evaluation of an NHS improvement project, using routine NHS administrative data provided to health boards to support improvement and audit, and, as such, did not require ethical review.
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.Guthrie B, McCowan C, Davey P, et al. High risk prescribing in primary care patients particularly vulnerable to adverse drug events: cross sectional population database analysis in Scottish general practice. BMJ. 2011;342:d3514. doi: 10.1136/bmj.d3514. [DOI] [PubMed] [Google Scholar]
- 2.Stocks SJ, Kontopantelis E, Akbarov A, et al. Examining variations in prescribing safety in UK general practice: cross sectional study using the Clinical Practice Research Datalink. BMJ. 2015;351:h5501. doi: 10.1136/bmj.h5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pirmohamed M, James S, Meakin S, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ. 2004;329(7456):15–19. doi: 10.1136/bmj.329.7456.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scottish Government. NHS Circular: PCA(M)(2012)08. Health and Social Care Integration Directorate. Primary Care Division. Scottish quality prescribing Initiative. http://www.sehd.scot.nhs.uk/pca/PCA2012(M)08.pdf (accessed 16 Mar 2017). [Google Scholar]
- 5.Scottish Government, British Medical Association. Quality and Outcomes Framework (QOF) Guidance for NHS Boards and GP Practices 2014/15. http://www.sehd.scot.nhs.uk/publications/DC20140502QOFguidance.pdf (accessed 16 Mar 2017).
- 6.Audit Scotland. Prescribing in general practice in Scotland. http://www.audit-scotland.gov.uk/docs/health/2013/nr_130124_gp_prescribing.pdf (accessed 22 Feb 2017).
- 7.Avery AJ, Dex GM, Mulvaney C, et al. Development of prescribing-safety indicators for GPs using the RAND Appropriateness Method. Br J Gen Pract. 2011. DOI: https://doi.org/10.3399/bjgp11X588501. [DOI] [PMC free article] [PubMed]
- 8.Spencer R, Bell B, Avery AJ, et al. Identification of an updated set of prescribing-safety indicators for GPs. Br J Gen Pract. 2014. DOI: https://doi.org/10.3399/bjgp14X677806. [DOI] [PMC free article] [PubMed]
- 9.Dreischulte T, Grant AM, McCowan C, et al. Quality and safety of medication use in primary care: consensus validation of a new set of explicit medication assessment criteria and prioritisation of topics for improvement. BMC Clin Pharmacol. 2012;12:5. doi: 10.1186/1472-6904-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campbell M, Fitzpatrick R, Haines A, et al. Framework for design and evaluation of complex interventions to improve health. BMJ. 2000;321(7262):694–696. doi: 10.1136/bmj.321.7262.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Avery AJ, Rodgers S, Cantrill JA, et al. A pharmacist-led information technology intervention for medication errors (PINCER): a multicentre, cluster randomised, controlled trial and cost-effectiveness analysis. Lancet. 2012;379(9823):1310–1319. doi: 10.1016/S0140-6736(11)61817-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dreischulte T, Donnan P, Grant A, et al. Safer prescribing: a trial of education, informatics, and financial incentives. N Engl J Med. 2016;374(11):1053–1064. doi: 10.1056/NEJMsa1508955. [DOI] [PubMed] [Google Scholar]
- 13.Guthrie B, Kavanagh K, Robertson C, et al. Data feedback and behavioural change intervention to improve primary care prescribing safety (EFIPPS): multicentre, three arm, cluster randomised controlled trial. BMJ. 2016;354:i4079. doi: 10.1136/bmj.i4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Craig P, Dieppe P, Macintyre S, et al. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ. 2008;337:a1655. doi: 10.1136/bmj.a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas MC. Diuretics, ACE inhibitors and NSAIDs — the triple whammy. Med J Aust. 2000;172(4):184–185. doi: 10.5694/j.1326-5377.2000.tb125548.x. [DOI] [PubMed] [Google Scholar]
- 16.Loboz KK, Shenfield GM. Drug combinations and impaired renal function — the ‘triple whammy’. Br J Clin Pharmacol. 2005;59(2):239–243. doi: 10.1111/j.1365-2125.2004.02188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacDonald TM, Morant SV, Robinson GC, et al. Association of upper gastrointestinal toxicity of non-steroidal anti-inflammatory drugs with continued exposure: cohort study. BMJ. 1997;315(7119):1333–1337. doi: 10.1136/bmj.315.7119.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Medicines and Healthcare products Regulatory Agency. Antipsychotics: initiative to reduce prescribing to older people with dementia. Drug Safety Update 2012. 5(10):H1. [Google Scholar]
- 19.NHS Scotland Scottish Government. Polypharmacy guidance. October 2012. http://www.central.knowledge.scot.nhs.uk/upload/Polypharmacy%20full%20guidance%20v2.pdf (accessed 16 Mar 2017).
- 20.Effective Practice and Organisation of Care (EPOC) EPaOoC . EPOC resources for review authors. Oslo: Norwegian Knowledge Centre for the Health Services; 2013. Interrupted time series (ITS) analyses. [Google Scholar]
- 21.Newson R. Confidence intervals and p-values for delivery to the end user. Stata Journal. 2003;3(3):245–269. [Google Scholar]
- 22.Penfold RB, Zhang F. Use of interrupted time series analysis in evaluating health care quality improvements. Acad Pediatr. 2013;13(6 Suppl):S38–S44. doi: 10.1016/j.acap.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Linden A. Conducting interrupted time series analysis for single and multiple group comparisons. Stata Journal. 2015;15(2):480–500. [Google Scholar]
- 24.Duerden M, Millson D, Avery A, Smart S. The quality of GP prescribing. https://www.kingsfund.org.uk/sites/files/kf/field/field_document/quality-gp-prescribing-gp-inquiry-research-paper-mar11.pdf (accessed 16 Mar 2017).
- 25.Anderson K, Stowasser D, Freeman C, Scott I. Prescriber barriers and enablers to minimising potentially inappropriate medications in adults: a systematic review and thematic synthesis. BMJ Open. 2014;4(12):e006544. doi: 10.1136/bmjopen-2014-006544. [DOI] [PMC free article] [PubMed] [Google Scholar]