Key Points
Bleeding in US hemophilia patients has decreased dramatically in parallel with increased use of prophylaxis.
Only prophylaxis started before age 4 years preserves normal joint function.
Abstract
This analysis of the US Hemophilia Treatment Center Network and the Centers for Disease Control and Prevention surveillance registry assessed trends in prophylaxis use and its impact on key indicators of arthropathy across the life-span among participants with severe hemophilia A. Data on demographics, clinical characteristics, and outcomes were collected prospectively between 1999 and 2010 at annual clinical visits to 134 hemophilia treatment centers. Trends in treatment and outcomes were evaluated using cross-sectional and longitudinal analyses. Data analyzed included 26 614 visits for 6196 males; mean age at first registry visit was 17.7 years; and median was 14 (range, 2 to 69). During this time, prophylaxis use increased from 31% to 59% overall, and by 2010, 75% of children and youths <20 years were on prophylaxis. On cross-sectional analysis, bleeding rates decreased dramatically for the entire population (P < .001) in parallel with increased prophylaxis usage, possibly because frequent bleeders adopted prophylaxis. Joint bleeding decreased proportionately with prophylaxis (22%) and nonprophylaxis (23%), and target joints decreased more with prophylaxis (80% vs 61%). Joint, total, and target joint bleeding on prophylaxis were 33%, 41%, and 27%, respectively, compared with nonprophylaxis. On longitudinal analysis of individuals over time, prophylaxis predicted decreased bleeding at any age (P < .001), but only prophylaxis initiation prior to age 4 years and nonobesity predicted preservation of joint motion (P < .001 for each). Using a national registry, care providers in a specialized health care network for a rare disorder were able to detect and track trends in outcomes over time.
Introduction
Prior to the widespread availability and adoption of prophylaxis, arthropathy has been the most prevalent and costly complication of hemophilia. Hemophilic arthropathy is caused by recurrent hemorrhage into joints and results in an arthritis, characterized by soft tissue changes of proliferation of hemosiderin-laden synovium and osteochondral changes of subchondral erosions, cyst formation, and cartilage loss.1 Synovial inflammation promotes frequent recurrent joint hemorrhage in an affected joint, commonly called a target joint, which accelerates the destructive process. Ultimately, bones and joints affected by hemophilic arthropathy develop osteoporosis, osteophytic growths, and fibrous contractures, severely limiting and distorting mobility. Historically, hemophilic arthropathy has affected 90% of adults with severe hemophilia and involved 1 to 6 joints.2 Hemophilic arthropathy causes chronic pain and functional limitation often necessitating chronic opioid dependence and multiple orthopedic procedures, including joint replacements and fusions. In addition to the enormous expense of replacement factor concentrate, hospitalizations, and joint replacements, the human cost of hemophilic arthropathy is loss of employment opportunities, less favorable insurance access, decreased social participation, and a high prevalence of depression.3,4
Prophylaxis, a therapy that seeks to prevent bleeding events in hemophilia by routine replacement of deficient clotting factor, has been shown in a randomized clinical trial to prevent both joint hemorrhage and arthropathy when started prior to 30 months of age and prior to the occurrence of 3 hemorrhages into any single joint.5 To date, clinical trial evidence supports the efficacy of initiation of prophylaxis after exceeding these thresholds in reducing the rate of joint hemorrhage in adolescents and adults with preexisting arthropathy, but has not provided definitive data on joint outcomes other than bleeding in patients beyond early childhood.6,7
Factors previously determined to predict joint disease and poor physical function in boys with hemophilia include joint hemorrhage, increasing age, obesity, African American race, and inhibitor formation.8-10 In 1997, the Centers for Disease Control and Prevention (CDC) in collaboration with the US Hemophilia Treatment Center Network (USHTCN), a specialty health care network primarily housed in academic centers, initiated a prospective surveillance system called the Universal Data Collection (UDC) system for outcomes of hemophilia, directed and funded by a US Congressional mandate, with a goal of developing preventive strategies; the structure, organization, and participants of the UDC have been previously described.11 The outcomes of interest included bloodborne pathogens, mortality, joint disease, inhibitor formation, health insurance and employment status, obesity, and bleeding rate. This analysis of the surveillance registry was performed to assess trends in key indicators of arthropathy in participants with severe hemophilia A across the life span.
Methods
Data collection
At the time of an annual visit to one of 134 USHTCN clinics, following informed consent, predefined data were collected and transferred on standardized clinical research forms without personal identifiers to a central CDC database. The study was approved by the CDC’s Central Institutional Review Board and the local board of each participating site. This analysis reports data covering serial USHTCN visits for participants ≥2 years of age from 1999 through 2010. Data for all elements related to bleeding were collected for the preceding 6 months. Predictors or indicators of arthropathy extracted for this analysis included the following: rates of total and joint bleeding, number of target joints, proportion of total normal joint range of motion (ROM), race/ethnicity, and obesity.
Case definitions were as follows:
Severe hemophilia A: <1% factor VIII (FVIII) activity by local laboratory assay.
Race and ethnicity: defined as white non-Hispanic, black non-Hispanic, Hispanic (any race), Asian/Pacific Islander, or Other by self-report.
Joint hemorrhage: clinically assessed with one or more of the following: joint pain, stiffness, limitation of motion, and/or visible or palpable swelling.
Any hemorrhage: any bleeding into a joint, muscle, soft tissue, or organ that would generally prompt treatment with FVIII replacement.
Target joint: a single joint that experienced 4 or more hemorrhages within 6 consecutive months; this definition was chosen to align with the definition used in the Joint Outcomes Study of prophylaxis.5
Proportion of normal joint ROM: joint ROM of 10 joints (ankles, knees, hips, elbows, and shoulders), measured by a staff trained in use of the examination tool, as a proportion of the mean normal arc measured in a reference population of healthy adults without known disorders affecting joints.12
Nonprophylaxis or episodic therapy: FVIII replaced only following the onset of bleeding or prior to an activity with a high risk of bleeding.
Continuous prophylaxis therapy: FVIII replaced on a regular schedule to prevent bleeding and expected to continue for an indefinite period of time.
Primary prophylaxis <4 years: first documentation of routine FVIII replacement therapy at a UDC visit prior to the fourth birthday. This age was chosen to align as closely as possible with the Joint Outcomes Study definition, as well as with previous European definitions of primary prophylaxis.5,13-15
Primary prophylaxis 4 to 6 years: first documentation of routine FVIII replacement therapy at a UDC visit on or after the fourth birthday but before the sixth birthday.
Secondary prophylaxis: first documentation of prophylaxis use at a UDC visit at age ≥6 years.
Overweight/obese: overweight: body mass index (BMI) ≥85th percentile for age and sex in pediatric participants 2 to 19 years; BMI ≥25 in adults ≥20 years. Obese: BMI ≥95th percentile for age and sex in pediatric patients; BMI ≥30 in adults.
FVIII inhibitor: a participant was defined to have an inhibitor if he was recorded to have a measured titer >0.5 BU in a local laboratory, or to use bypassing agents, or to be on immune tolerance therapy at any UDC visit.
Statistical analysis
Descriptive statistics were used to describe demographic and clinical characteristics, and outcomes. The cross-sectional prevalence of prophylaxis for each study year was the proportion of all participants in each age group whose treatment of that year was categorized as prophylaxis. Outcomes analyses also stratified prophylaxis as primary (initiation, <4 years), primary 4 or 5 years, or secondary.
The mean values for each outcome measure were calculated for each age group and by prophylaxis status for each year of the study. To assess linear trend over time in the mean values within groups of participants, linear regression analysis was used.
Longitudinal analysis was performed using all data collected on those participants whose first visit occurred before their 20th birthday and who had data collection on more than one occasion. The younger age cutoff was chosen so that each person’s current exposures would be more likely to reflect their exposures early in life and, therefore, more likely to have an effect on the ROM outcomes that we measured. The analysis was performed using a mixed linear regression model to estimate the random effects of the studied risk factors on ROM, while accounting for the correlation between repeated measures over time using a first-order autoregressive covariance structure. This structure assumed that repeated measurements on the same person that occurred closest in time to each other were more correlated than measurements taken later in the follow-up period. Two effect estimates were generated by this model: (1) the effect of the covariate (risk factor) on the initial status of the outcome at the first measurement time; and (2) the effect of the covariate on the rate of change of the outcome over the period of follow up. The sign of the parameter estimate indicates whether the indicated covariate level causes a decreased (+) or an increased (−) rate of ROM loss relative to the reference.
All analyses were conducted using SAS statistical software (SAS Institute, Cary, NC) and test values of P ≤ .05 were considered to represent statistically significant differences.
Results
Between 1999 and 2010, registry data were collected from 26 614 USHTCN visits for 6196 males with severe hemophilia A, ≥2 years of age, all of whom are included in the cross-sectional results. The number of visits per year ranged from 1560 in 1999 to 2498 in 2006, and averaged 2218 visits per year. At their first registry visit, participants ranged in age from 2 to 69 years, and the average (median) age at first visit was 17.7 (14) years. Longitudinal data were available for 3078 participants who have complete data for 2 or more registry visits. Table 1 displays demographics of the participants.
Table 1.
Baseline characteristics of 6196 males with severe hemophilia A receiving care in US hemophilia treatment centers, 1999 to 2010
| Characteristic | N | % |
|---|---|---|
| Age at first visit, y | ||
| 2-9 | 2333 | 37.6 |
| 10-19 | 1650 | 26.6 |
| 20-29 | 933 | 15.1 |
| 30-39 | 624 | 10.1 |
| 40-49 | 423 | 6.8 |
| 50-69 | 233 | 3.8 |
| Race/ethnicity | ||
| White, non-Hispanic | 3888 | 62.8 |
| Black, non-Hispanic | 963 | 15.5 |
| Hispanic* | 840 | 13.5 |
| Asian | 242 | 3.9 |
| Other | 263 | 4.3 |
| BMI | ||
| Normal/underweight | 3897 | 62.9 |
| Overweight | 1262 | 20.4 |
| Obese | 1037 | 16.7 |
| Treatment type at first visit | ||
| Prophylaxis | 2309 | 37.3 |
| On demand | 3887 | 62.7 |
| Inhibitor | ||
| Yes | 688 | 11.1 |
| No | 5508 | 88.9 |
Hispanic ethnicity of either black or white race.
Cross-sectional data analyses (data across participants within each year of study)
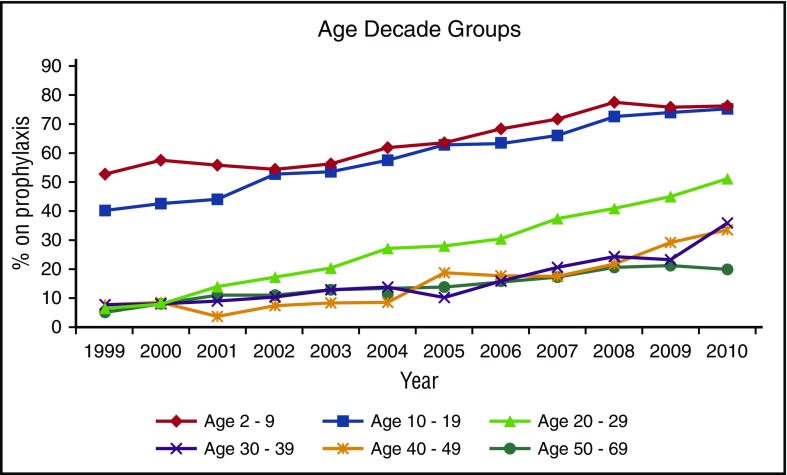
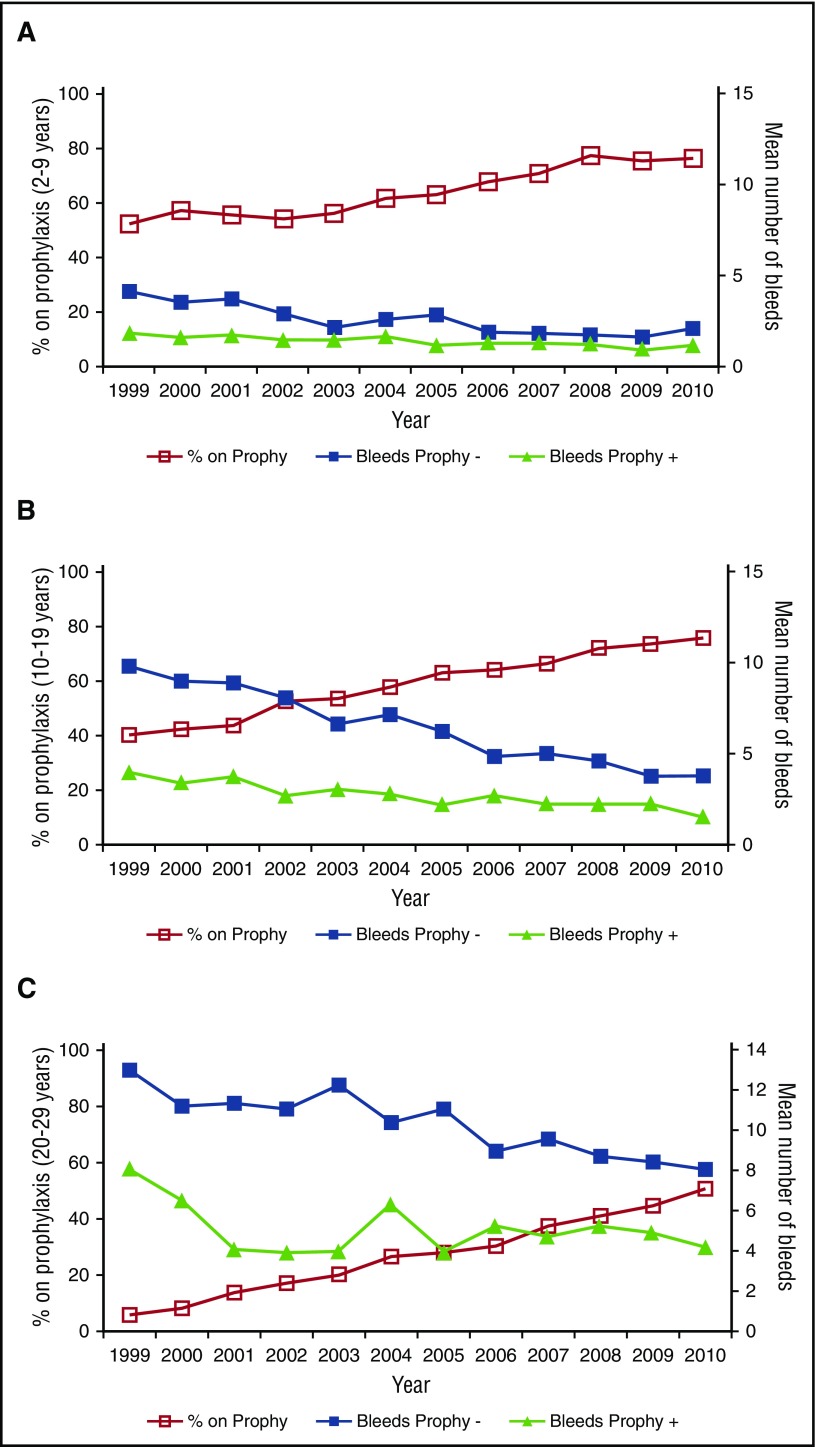
Over the 12 years of study, the overall rate of prophylaxis use increased from 31% to 59%. As demonstrated in Figure 1, the increases in prophylaxis have been steady in all age groups, with younger participants adopting prophylaxis earlier than older participants. In 2010, 75% of children and adolescents ≤19 years were treated with prophylaxis in the United States; the rate of prophylaxis use in adolescents 10 to 19 years approximated that of children by 2002. Figure 2 shows trends in the mean number of hemarthroses in participants <30 years, and demonstrates lower rates of joint hemorrhage in participants on prophylaxis in all age groups, and across the 12 years of observation, with rates on prophylaxis approximately half that of persons not receiving prophylaxis. Additionally, Figure 2 shows that as rates of prophylaxis usage increased (from 46% to 76% in children 2 to <10 years, 30% to 75% in adolescents 10 to 19 years, and 11% to 51% in adults 20 to 29 years), and as those participants in the nonprophylaxis cohort with higher rates of joint hemorrhage increasingly crossed over to the prophylaxis group, the rates of hemarthroses fell for the remaining cohort not using prophylaxis, whereas it continued to decrease for the prophylaxis cohort.
Figure 1.
Trends in prophylaxis use for children and adults with severe hemophilia A from 1999 to 2010. The proportion of each participant age cohort (in decades) using continuous prophylaxis is displayed for each year of the study from 1999 to 2010.
Figure 2.
Trends in mean number of joint hemorrhages in the preceding 6 months by prophylaxis status, 1999 to 2010. (A-C) The vertical axis on the right displays the mean number of joint hemorrhages per 6 months during each study year for participants using (▲) or not using (▪) continuous prophylaxis. The vertical axis on the left displays the proportion of the entire age cohort using prophylaxis for each study year (□). (A-C) Displays results for children ages 2 to 9 years (A), adolescents 10 to 19 years (B), and adults 20 to 29 years (C), respectively. Prophy, prophylaxis; Prophy +, data from patients on prophylaxis; Prophy −, data from patients not on prophylaxis.
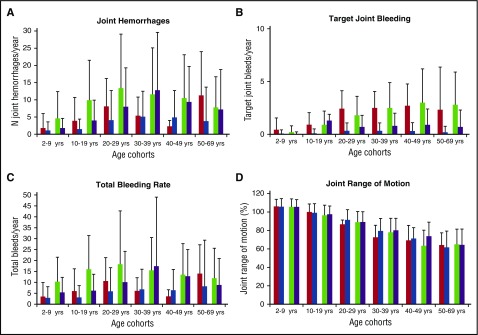
Trends in the 2010 data compared with 1999 are shown in Figure 3. Rates of joint bleeding in participants on prophylaxis fell 22% from a mean of 3.03/6 months in 1999 to 2.36 in 2010. Over the same time, joint bleeding on nonprophylaxis decreased 23% from 9.42 events/6 months to 7.25. Total bleeding rates fell 17% for prophylaxis (4.91 to 4.07/6 months) and 30% for nonprophylaxis (14.2 to 9.87/6 months). Target joints decreased 80% on prophylaxis (mean, 0.8 to 0.16) and 61% on nonprophylaxis (1.52 to 0.59).
Figure 3.
Cross-sectional analyses: mean changes in bleeding rates and joint range of motion from 1999 to 2010. (A-D) Shows changes in outcome rates from 1999 to 2010 stratified by age cohort and prophylaxis usage. (A) Rates of joint hemorrhages; (B) rates of target joints; (C) total bleeding rates; and (D) percentage of normal joint ROM in participants. Patients on prophylaxis in 1999 (red); patients on prophylaxis in 2010 (blue); patients not on prophylaxis in 1999 (green); and patients not on prophylaxis in 2010 (purple).
Rates of joint and total bleeding events in participants not using prophylaxis were approximately twice that of participants using prophylaxis (Figure 3A,C; P < .01 for each). Joint hemorrhage rates within the population using prophylaxis continued to decrease in age groups 2 to 9 years (P < .05) and 10 to 19 (P < .001); rates in participants >19 years using prophylaxis showed nonsignificant decreases. Decreases in rates of joint hemorrhage were observed in participants not using prophylaxis in cohorts 2 to 9, 10 to 19, and 20 to 29 years over the 12 years of observation (P < .01 for each), although rates were still twice that for the corresponding prophylaxis cohort. Data on the older cohorts (≥40 years) were skewed, both by the very small number of participants in these age cohorts on prophylaxis in 1999 (in some cases fewer than 10) and the fact that early adopters of prophylaxis among older patients tended to have high rates of joint hemorrhage.
The number of target joints is an indicator of a severe bleeding outcome and usually reflects inflammatory synovitis. Over the 12 years of observation, target joints were almost eliminated in USHTCN participants. Numbers of target joints decreased from 1999 to 2010 for every age cohort, both using and not using prophylaxis (Figure 3B; P < .01 for each). Specifically, target joint bleeding was reduced by 80% in participants using prophylaxis (from mean 0.80 target joints; standard deviation [SD] 1.32 to 0.16 target joints; SD 0.55) and was reduced by 61% in participants not using prophylaxis (from mean 1.52 target joints; SD, 2.16 to 0.59 target joints; SD, 1.2), with the number of target joints four to fivefold higher in the cohort not using prophylaxis. Similar to rates of joint hemorrhage and target joints, the rates of total bleeding for participants on prophylaxis were approximately half that of those not using prophylaxis.
Limited joint ROM is a long-term indicator of hemophilic arthropathy. It can be noted that children <10 years physiologically have joint ROM greater than that of healthy adults (ie, the proportion of normal ROM is >100%). Decreased overall joint ROM was noted in adolescents with severe hemophilia A and worsened progressively with age (Figure 3D). Data for the upper extremity ROM was similar but with less loss of motion than for the lower extremity (data not shown). Overall, on cross-sectional analysis, joint ROM decreased with age regardless of prophylaxis usage.
Longitudinal analyses (analyses of repeated measurements on individual participants over time)
For the longitudinal analyses, there were 3078 eligible subjects, of whom 509 (16.5%) had primary <4 years, 331 (10.8%) had primary 4 or 5 years, and 1358 (44.1%) had secondary prophylaxis. Data were collected at 14 130 visits during the study period; 23.5% had 2 or 3 visits, 26% had 4 or 5 visits, and 35.7% had 6 to 12 visits. In Table 2, factors significantly associated with decreased overall joint ROM at the initial visit included advancing age, non-white race, and obesity. Having an inhibitor was also negatively associated with ROM, but fell just short of statistical significance. The rate of joint ROM loss over the serial visits was decreased with primary prophylaxis, begun before the fourth birthday, and increased by obesity (P < .05 for each). The decreased rate of loss with primary prophylaxis started at age 4 or 5 years or with secondary prophylaxis (≥6 years) was not statistically significant.
Table 2.
Multivariate analysis on the effect of prophylaxis on the repeated measures of overall joint ROM over time in 2908 US hemophilia participants aged 2 to 19 years
| Initial ROM status | Rate of ROM change | |||
|---|---|---|---|---|
| Covariates | Parameter estimate | P | Parameter estimate | P |
| Age | −0.85 | <.001 | — | — |
| Race: white vs others | 1.56 | <.001 | — | — |
| Inhibitor: yes vs no | −0.46 | .06 | — | — |
| BMI: ≥85th vs <85th percentile | −1.07 | .003 | −0.09 | .001 |
| Primary prophy <4 y: yes vs no | 0.96 | .24 | 0.20 | .03 |
| Primary prophy 4 or 5 y: yes vs no | 0.92 | .34 | 0.10 | .29 |
| Secondary prophy: yes vs no | 0.54 | .46 | 0.04 | .36 |
Prophy, prophylaxis.
Discussion
Arthropathy drives most adverse hemophilia outcomes including disability, chronic pain, and invasive surgeries. Total and joint hemorrhages, as well as target joint bleeding are predictors of hemophilic arthropathy. The organization of health care in the United States is decentralized and it has been difficult to obtain outcome data that are adequately specific to track health outcomes and trends, particularly in rare disorders. Prophylaxis therapy has been administered to persons with hemophilia, particularly in Europe, for 4 decades. However, evidence from a randomized clinical trial regarding the efficacy of this therapy to prevent joint damage in children was first reported in 2007.5 Randomized clinical data on the efficacy of prophylaxis to prevent bleeding and improve joint function has been more recently reported in adolescents and adults.16-18 Data from the CDC/USHTCN surveillance registry of over 6000 individuals shows that prophylaxis treatment increased from 31% overall in 1999 to 59% in 2010, including 75% of all children and adolescents. During the same time, joint and total hemorrhages were markedly reduced. Importantly, inflammatory synovitis expressed as target joint hemorrhage was greatly decreased. These data confirm substantial improvement in the bleeding outcome of hemophilia over a 12-year period, and furthermore demonstrate the capacity of a national network of specialized hemophilia centers to support the implementation of preventive health care strategies. In addition, the study demonstrates the capacity of CDC surveillance to document critical health outcomes in a rare disease.
An important finding of this study is that prophylaxis was effective in reducing joint bleeding rates, total bleeding rates, and target joint bleeding in all age groups regardless of the age of initiation, but on longitudinal analysis, prophylaxis was effective in preventing loss of joint motion only among those in whom prophylaxis was initiated prior to 4 years of age. Sensitivity analyses of longitudinal outcomes on children commencing prophylaxis at ages 4 or 5 years or ≥6 years failed to show a significant effect in decreasing the loss of joint ROM compared with no prophylaxis. These data in a large prospective cohort confirm earlier results of smaller retrospective analyses.13-15 Therefore, primary prophylaxis, or the prevention of joint damage by prevention of any joint hemorrhage is the only therapy effective to preserve joint structure and function. In addition, in over 12 years of observation, there was no observed benefit of prophylaxis to regain previously lost joint motion. Delayed initiation of prophylaxis has been shown to increase joint function and quality of life, but did not improve joint structure on magnetic resonance imaging.16 The current analysis suggests that continuous prophylaxis lowers the bleeding rate of participants with active joint bleeding, but may have little to offer to participants with advanced arthropathy and few acute hemarthroses in terms of reduction in bleeding. However, individuals with advanced arthropathy treated with prophylaxis may have decreased pain and increased mobility permitting physical therapy, increased exercise, improved fitness, and increased participation.
Over the 12 years of observation, joint outcomes of bleeding and target joints improved for all network participants with severe hemophilia, although improvements were more marked for participants on prophylaxis. The reasons why joint outcomes improved for participants not on prophylaxis is unknown. It is likely that participants with clinically severe bleeding phenotypes increasingly adopted prophylaxis, so that over time, the shrinking population of participants with severe FVIII deficiency not utilizing prophylaxis was over-represented by participants with milder bleeding phenotypes or advanced arthropathy with infrequent acute bleeding. Other factors may have contributed to improved joint outcomes in both treatment groups. Over the duration of this study, increasing education to hemophilia providers and consumers alike around the adverse consequences of joint hemorrhage, may have resulted in a progressively decreased tolerance for joint hemorrhage and earlier institution of preventive measures, including long- and short-term prophylaxis as well as more effective on-demand therapy, and possibly also the increased use of physical therapies. Finally, a therapeutic effect of the surveillance study itself must be considered. In the process of this project, participants and health care providers were directed to count the number of total and joint bleeding episodes, to measure joint ROM, and consider whether therapy given was preventive or reactive in nature. The attention directed to joint hemorrhage and its impact on joint mobility, by itself, may have motivated some participants to adopt a more preventive approach to their health care.
Some limitations of this study are noteworthy. First, the most significant limitation is in the cross-sectional nature of the data: declining trends in joint hemorrhages may not be causally related to trends of increasing prophylaxis use. Undoubtedly, there were many influences on the changes in hemophilic joint outcome that were documented. However, the longitudinal analysis results provide clear evidence that primary prophylaxis decreases the rate of ROM loss. These data provide strong evidence that prophylaxis should be started early in life in order to have the greatest benefit, as has been suggested by retrospective studies from Sweden, Germany, and the Netherlands.13-15
A second limitation is that the data on bleeding rates were generated by patient self-report, which is prone to recall bias. Third, the presence of synovitis, an important outcome, could only be approximated by target joint bleeding rate, because no imaging studies or other diagnostic investigations were performed for the purpose of surveillance. Fourth, the definitions of prophylaxis were very broad, and did not allow for any analysis of the effect of prophylaxis dose intensity, frequency, or duration.
In conclusion, these data demonstrate the power of a national public health surveillance program to detect and track trends in significant outcomes of a rare chronic disease, and the capacity of a national network of academic medical centers to collaborate in the collection of critical outcome data. A key use of the registry is to elucidate continuing unresolved outcome issues for development and conduct of prospective clinical trials. Future studies on rates of joint replacement surgeries and functional disability will be necessary to determine if these trends will result in improved quality of life for persons with hemophilia.
Acknowledgments
The authors acknowledge the other members of the Joint Outcomes Committee of the UDC project, including Robi Ingram-Rich, Heidi Lane, Meredith Oakley, Pattye Tobase, and Steven Humes, for their discussion and helpful comments related to this analytic project; all of the participating patients with severe hemophilia A and their hemophilia treatment center providers for the provision of data used in this analysis; and Donna DiMichele for enlightening discussions around the study data and for the critical review of the manuscript.
This study was funded by a grant from the CDC (U01DD000198).
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.J.M.-J. conceived and constructed the analytic project, interpreted data, wrote the first draft of the paper, and coordinated revisions of the manuscript; J.M.S. analyzed the study data, and read and edited the manuscript; and J.C.G. contributed to study data analysis plan and data interpretation, and read, edited, and approved the final manuscript. The findings and conclusions in this study are those of the authors and do not necessarily represent the official position of the CDC.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A list of additional members of the Joint Outcomes Committee of the UDC, USHTCN appears in “Appendix.”
Correspondence: Marilyn J. Manco-Johnson, Hemophilia and Thrombosis Center, University of Colorado Denver, 13199 E. Montview Blvd, Suite 100, Aurora, CO 80045; e-mail: marilyn.manco-johnson@ucdenver.edu.
Appendix: study group members
The members of the Joint Outcomes Committee of the Universal Data Collection, US Hemophilia Treatment Center Network are: M.J.M.-J., J.M.S., J.C.G., Robi Ingram-Rich, Heidi Lane, Meredith Oakley, Pattye Tobase, and Steven Humes.
References
- 1.Valentino LA. Blood-induced joint disease: the pathophysiology of hemophilic arthropathy. J Thromb Haemost. 2010;8(9):1895-1902. [DOI] [PubMed] [Google Scholar]
- 2.Aledort LM, Haschmeyer RH, Pettersson H; The Orthopaedic Outcome Study Group. A longitudinal study of orthopaedic outcomes for severe factor-VIII-deficient haemophiliacs. J Intern Med. 1994;236(4):391-399. [DOI] [PubMed] [Google Scholar]
- 3.Iannone M, Pennick L, Tom A, et al. . Prevalence of depression in adults with haemophilia. Haemophilia. 2012;18(6):868-874. [DOI] [PubMed] [Google Scholar]
- 4.Cassis FRMY, Querol F, Forsyth A, Iorio A; HERO International Advisory Board. Psychosocial aspects of haemophilia: a systematic review of methodologies and findings. Haemophilia. 2012;18(3):e101-e114. [DOI] [PubMed] [Google Scholar]
- 5.Manco-Johnson MJ, Abshire TC, Shapiro AD, et al. . Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357(6):535-544. [DOI] [PubMed] [Google Scholar]
- 6.Manco-Johnson MJ, Kempton CL, Reding MT, et al. . Randomized, controlled, parallel-group trial of routine prophylaxis vs. on-demand treatment with sucrose-formulated recombinant factor VIII in adults with severe hemophilia A (SPINART). J Thromb Haemost. 2013;11(6):1119-1127. [DOI] [PubMed] [Google Scholar]
- 7.Manco-Johnson MJ, Nuss R, Geraghty S, Funk S, Kilcoyne R. Results of secondary prophylaxis in children with severe hemophilia. Am J Hematol. 1994;47(2):113-117. [DOI] [PubMed] [Google Scholar]
- 8.Monahan PE, Baker JR, Riske B, Soucie JM. Physical functioning in boys with hemophilia in the U.S. Am J Prev Med. 2011;41(6 suppl 4):S360-S368. [DOI] [PubMed] [Google Scholar]
- 9.Bladen M, Main E, Hubert N, Koutoumanou E, Liesner R, Khair K. Factors affecting the Haemophilia Joint Health Score in children with severe haemophilia. Haemophilia. 2013;19(4):626-631. [DOI] [PubMed] [Google Scholar]
- 10.Soucie JM, Wang C, Siddiqi A, Kulkarni R, Recht M, Konkle BA; Hemophilia Treatment Center Network. The longitudinal effect of body adiposity on joint mobility in young males with haemophilia A. Haemophilia. 2011;17(2):196-203. [DOI] [PubMed] [Google Scholar]
- 11.Soucie JM, McAlister S, McClellan A, Oakley M, Su Y. The universal data collection surveillance system for rare bleeding disorders. Am J Prev Med. 2010;38(suppl 4):S475-S481. [DOI] [PubMed] [Google Scholar]
- 12.Soucie JM, Wang C, Forsyth A, et al. ; Hemophilia Treatment Center Network. Range of motion measurements: reference values and a database for comparison studies. Haemophilia. 2011;17(3):500-507. [DOI] [PubMed] [Google Scholar]
- 13.Nilsson IM, Berntorp E, Löfqvist T, Pettersson H. Twenty-five years’ experience of prophylactic treatment in severe haemophilia A and B. J Intern Med. 1992;232(1):25-32. [DOI] [PubMed] [Google Scholar]
- 14.Kreuz W, Escuriola-Ettingshausen C, Funk M, Schmidt H, Kornhuber B. When should prophylactic treatment in patients with haemophilia A and B start?—The German experience. Haemophilia. 1998;4(4):413-417. [DOI] [PubMed] [Google Scholar]
- 15.Fischer K, van der Bom JG, Mauser-Bunschoten EP, et al. . The effects of postponing prophylactic treatment on long-term outcome in patients with severe hemophilia. Blood. 2002;99(7):2337-2341. [DOI] [PubMed] [Google Scholar]
- 16.Lundin B, Hong W, Raunig D, et al. . Effect on joint health of routine prophylaxis with Bayer's sucrose-formulated recombinant factor VIII (rFVIII-FS) in adolescents and adults previously treated on demand: MRI analyses from the 3-year Spinart study [abstract]. Blood. 2014;124(21). Abstract 2854. [Google Scholar]
- 17.Collins P, Faradji A, Morfini M, Enriquez MM, Schwartz L. Efficacy and safety of secondary prophylactic vs. on-demand sucrose-formulated recombinant factor VIII treatment in adults with severe hemophilia A: results from a 13-month crossover study. J Thromb Haemost. 2010;8(1):83-89. [DOI] [PubMed] [Google Scholar]
- 18.Valentino LA, Mamonov V, Hellmann A, et al. ; Prophylaxis Study Group. A randomized comparison of two prophylaxis regimens and a paired comparison of on-demand and prophylaxis treatments in hemophilia A management. J Thromb Haemost. 2012;10(3):359-367. [DOI] [PMC free article] [PubMed] [Google Scholar]