Abstract
Background
Many chronic fatigue syndrome (CFS) and fibromyalgia syndrome (FMS) patients (35–68%) use nutritional supplements, while it is unclear whether deficiencies in vitamins and minerals contribute to symptoms in these patients. Objectives were (1) to determine vitamin and mineral status in CFS and FMS patients as compared to healthy controls; (2) to investigate the association between vitamin and mineral status and clinical parameters, including symptom severity and quality of life; and (3) to determine the effect of supplementation on clinical parameters.
Methods
The databases PubMed, EMBASE, Web of Knowledge, and PsycINFO were searched for eligible studies. Articles published from January 1st 1994 for CFS patients and 1990 for FMS patients till March 1st 2017 were included. Articles were included if the status of one or more vitamins or minerals were reported, or an intervention concerning vitamins or minerals was performed. Two reviewers independently extracted data and assessed the risk of bias.
Results
A total of 5 RCTs and 40 observational studies were included in the qualitative synthesis, of which 27 studies were included in the meta-analyses. Circulating concentrations of vitamin E were lower in patients compared to controls (pooled standardized mean difference (SMD): -1.57, 95%CI: -3.09, -0.05; p = .042). However, this difference was not present when restricting the analyses to the subgroup of studies with high quality scores. Poor study quality and a substantial heterogeneity in most studies was found. No vitamins or minerals have been repeatedly or consistently linked to clinical parameters. In addition, RCTs testing supplements containing these vitamins and/or minerals did not result in clinical improvements.
Discussion
Little evidence was found to support the hypothesis that vitamin and mineral deficiencies play a role in the pathophysiology of CFS and FMS, and that the use of supplements is effective in these patients.
Registration
Study methods were documented in an international prospective register of systematic reviews (PROSPERO) protocol, registration number: http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42015032528.
Introduction
Chronic fatigue syndrome (CFS) and fibromyalgia syndrome (FMS) are syndromes of unknown origin. The core symptom of CFS is profound disabling fatigue [1], whereas FMS is characterized by chronic widespread pain [2,3]. CFS and FMS are known for substantial clinical and diagnostic overlap, for example, chronic pain and fatigue are common in both patient groups. The two syndromes are often comorbid; up to 80% of CFS patients reported a history of clinician-diagnosed FMS [4,5]. This has resulted in the hypothesis that these syndromes share etiological pathways [6].
Vitamin and mineral deficiencies may play a role in the pathophysiology of both CFS and FMS, although mechanisms behind this hypothesis are not entirely clear [7,8]. In addition, results of studies investigating the effects of nutritional supplementation or dietary intake on, for example, symptom severity in these patient groups, are conflicting [9–12]. Nevertheless, a large proportion of CFS and FMS patients indicate they use nutritional supplements (35%-68%) [10,13–15], compared to the Dutch general population (27–56%) [16]. The higher nutritional supplement use among patients may be due to encouragements by specialty stores, the internet or (complementary medicine) clinics. Vitamins and minerals in these products are sometimes supplemented in doses high enough to cause health problems, for example gastric discomfort, insomnia, dizziness and weakness [17]. More information is needed on the evidence for (marginal) vitamin and mineral deficiencies in CFS and FM, and the potential benefits in taking nutritional supplements.
Recently, a review investigating hypovitaminosis D in both chronic pain and FMS patients showed that these patients were at significantly higher risk of hypovitaminosis D than healthy controls [18]. Unfortunately, further reviews on vitamin and mineral deficiencies among CFS and FMS patients are lacking. We therefore carried out this first systematic review on vitamin and mineral status in CFS and FMS. We explored the following research questions: first, what is the evidence for deficiencies in vitamin and mineral status in CFS and FMS patients as compared to healthy controls? Second, is vitamin and mineral status associated with clinical parameters, including symptom severity and quality of life, in CFS and FMS? Third, what is the evidence for an effect of vitamin and mineral supplementation, as compared to placebo, on clinical parameters in CFS and FMS patients? Because it is currently unknown whether CFS and FMS result from the same etiology, we analyzed results both for the combined and for the separate syndromes.
Methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (S1 Table) [19]. Prior to start of article inclusion, we documented study methods in an international prospective register of systematic reviews (PROSPERO) protocol, registration number: CRD42015032528, http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42015032528.
Data sources and searches
The databases PubMed, EMBASE, Web of Knowledge, and PsycINFO were systematically searched. Articles published between January 1st 1994 and 1990, for CFS and FMS respectively, and March 1st 2017 were included. We focused on the most recent diagnostic guidelines, namely the International Center of Disease Control (CDC) diagnostic criteria for CFS that was established in 1994 [1], and the American College of Rheumatology (ACR) criteria for FMS in 1990 [2]. To retrieve relevant articles from PubMed, we formulated a search string (S1 Appendix) that consisted of CFS, FMS, and synonyms, vitamins, minerals, micronutrients and synonyms, while excluding systematic reviews or animal studies. This search string was adapted according to the thesaurus of the databases EMBASE, Web of Knowledge, and PsycINFO. All included studies were screened for potential references that were not included in the first search. Duplicates were removed, as well as studies including pediatric participants. There were no language restrictions; included non-English articles were translated (French, Italian, Polish, and Turkish articles) by native speakers.
Study selection
Title and abstract were screened by two independent reviewers (M.L.J. and I.M.) for the following criteria: (1) CFS or FMS patients; (2) vitamin or mineral status; and (3) study design. Studies which were in agreement with the eligibility criteria were retrieved as full text. Discrepancies between the two researchers were resolved by consensus, and when needed a third assessor was consulted (J.G.M.R.). Reasons for exclusion and percentage of agreement, as Cohen’s kappa, between the assessors were documented.
Participants of the included studies had to be adults (i.e. ≥18 years) suffering from CFS or FMS according to the official diagnostic criteria [1–3]. Studies that involved patients with a combination of CFS and FMS or other comorbid medical conditions were excluded. Furthermore, the vitamin or mineral status had to be assessed or reported in the article, or there had to be an intervention concerning vitamins or minerals. Patients were compared with healthy controls in observational studies, or vitamin and mineral supplementation were compared with placebo in intervention studies. Lastly, cross-sectional studies comparing cases and controls, cohort studies and randomized controlled trials (RCTs) were included. Case reports, clinical cohorts without appropriate controls (e.g. controls with musculoskeletal pain or fatigue), (systematic) reviews, expert opinion, and other study designs were excluded.
Data extraction
Two reviewers (M.L.J. and I.M.) independently extracted data and assessed the risk of bias for each study. The first ten articles were screened together to pilot the data extraction and risk of bias form. Reasons for exclusion and percentage of agreement between the assessors were documented.
From the included articles, the following information was extracted: name first author, publication year, type FSS, number and age of the participants, and vitamin or mineral status. In addition, data on smoking habits or alcohol use, diet (and assessment tool used), BMI (or waist circumference, waist-hip ratio), physical activity (assessment tool), socioeconomic status, ethnicity, severity of illness (assessment tool), duration of illness, co-morbidities (somatic and psychiatric), medication use, clinical parameters including symptom severity and quality of life, and in case of RCTs the relevant co-intervention(s) were also extracted.
Quality assessment
To assess quality of RCTs, the Cochrane Collaboration’s tool for assessing risk of bias was employed [20]. For observational studies, literature indicates lack of a single methodological assessment tool [21,22]. Therefore, we adjusted a previously developed quality tool for observational studies in this field [23], for use in studies that focus specifically on the association between vitamin and mineral status and CFS or FMS. Eight of the nine items in this original quality tool originated from guidelines or tools for either reporting or appraising observational research [24–26]. These items were adjusted to the specific question on vitamins and minerals and classified into three key domains: appropriate selection of participants (validated disorder, representative controls, in- and exclusion criteria, disease characteristics), appropriate quantification of vitamin and mineral status (duplicate quantification, appropriate outcome), and appropriate control for confounding (assessed confounders, analyses adjusted). The item: “Is the assessor blind for disease status”, was excluded since from the original quality tool since it is not applicable in the current review. Furthermore, we added the item “Are methods for assessment of vitamin and mineral status clearly stated”, based on the adapted Newcastle Ottawa scale for cross-sectional studies (S2 Appendix) [27]. RCTs that contained relevant observational data (n = 4/5), were assessed with both the Cochrane tool and the observational studies quality tool. For both quality tools, items were rated as (0) low risk, (1) medium risk, and (2) high risk of bias. The maximum attainable quality score was 14 for RCTs, and 18 for observational studies.
Data synthesis and analysis
We first constructed an overview of available data on the different vitamins and minerals. Characteristics of the included studies were systematically listed to generate a clear overview of the current literature on vitamins and minerals in CFS and FMS patients. For those vitamins and minerals with more than five studies available, we did quantitative syntheses on aggregated data. For these syntheses, data was pooled with the random effects model of meta-analysis, using Stata statistical software, version 14 (Statacorp LP, Texas). To allow pooling across studies that used different outcomes of vitamin or mineral plasma or serum levels, we calculated the standardized mean difference (SMD). For proportions of deficiencies, the odds ratio (OR) was calculated and pooled. Subsequently, the SMD and OR for each study were weighted by their inverse variance and the corresponding 95%CI were calculated. The existence of heterogeneity among studies was assessed by Q-tests, and the degree of the heterogeneity was quantified by calculating the I-squared (I2) value. Publication bias was inspected visually by a funnel plot, and an Egger’s test was conducted to quantify funnel plot asymmetry [28]. The Tweedie’s Trim and Fill test was performed as an additional sensitivity analysis to identify and correct for funnel plot asymmetry arising from publication bias [29]. When the Trim and Fill test was performed, and additional studies were added to the analyses, contour-enhanced funnel plots were used instead of regular funnel plots to examine whether asymmetry in the funnel plots was due to publication bias [30]. Subgroup analyses were performed including studies with more than half of the maximum study quality score (>9 quality points), if more than three studies with a sufficient quality score were available. Furthermore, vitamin and mineral status of CFS and FMS patients were investigated separately if more than three studies were available. Findings were considered statistically significant if P<0.05.
Results
Study inclusion
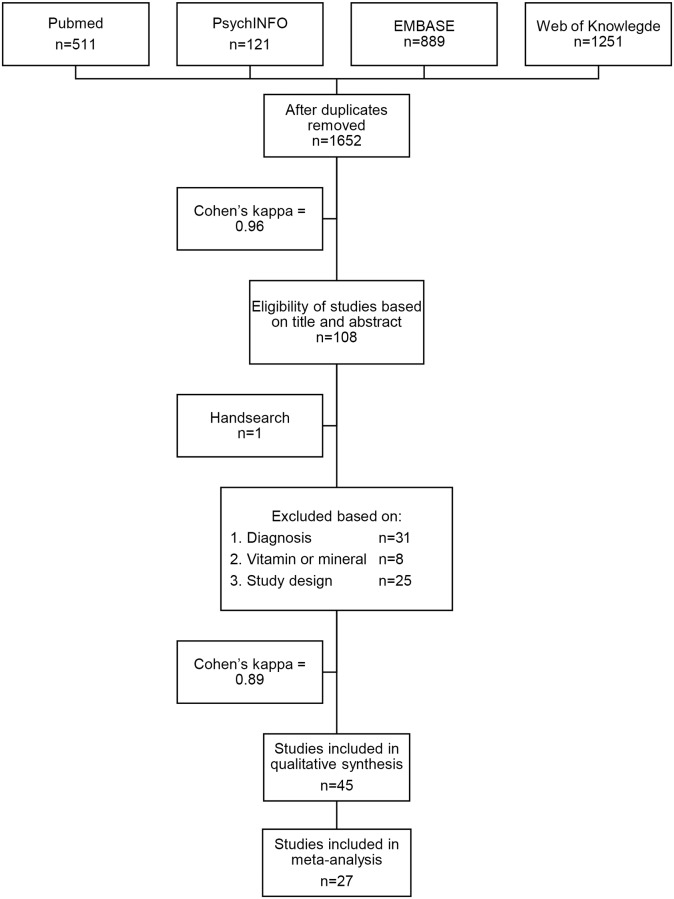
Results of the systematic review and meta-analysis are presented in a flow diagram (Fig 1). Cohen’s kappa’s for the abstract and full text selection were 0.96 and 0.89 respectively, indicating very good consistency of agreement [31]. Out of 108 studies included for the full text review, 45 studies were included in the current review.
Fig 1. Flow diagram.
Characteristics of the included studies are presented in Table 1, and results of the quality assessment in Tables 2 and 3. Most studies involved FMS patients (n = 35/45); 4 of the 5 RCTs also contained relevant observational data. Vitamin and mineral status was mainly assessed in plasma or serum (n = 40/45). Furthermore, quality scores revealed poor study quality (i.e. equal or less than half of the maximum study quality score) in the vast majority of observational studies (n = 27/44; range 4–14 points) and RCTs (n = 3/5; range 5–12 points). Only few observational studies defined all described in- and exclusion criteria for the investigated population, including medication use, somatic morbidity, and psychiatric morbidity (n = 10/44). The CFS or FMS diagnostic criteria were often described in observational studies, but researchers failed to state whether or not the syndromes were diagnosed by a physician (n = 40/44). Disease characteristics were frequently not fully presented (n = 15/44), or were completely absent (n = 18/44) in observational studies. Almost all observational studies did not assess vitamin or mineral in duplicate (n = 38/44). Most studies that assessed vitamin or mineral status did not clearly state the methods for assessment of vitamin and mineral status (n = 27/44). Furthermore, most observational studies did not adjust their analyses for any potential confounders (n = 43/44). Lastly, most RCTs had a medium to high risk of bias for random sequence generation (n = 3/5), allocation concealment (n = 3/5), blinding of outcome assessment (n = 4/5), incomplete data (n = 4/5), selective reporting quantification (n = 3/5), and other bias (n = 5/5).
Table 1. Characteristics of included studies.
| Study | Setting | Type of FSS | N of cases | Study design | Mean age in years (SD) | Mean FSS severity (SD) and/or mean duration in months (SD) | Comparison group (n) | Vitamin and/or mineral | Material |
| Akkus et al, 2009 [32] | Secondary care | FMS | 30 | Case-control | 40.1 (5.2) | FIQ: 59.8 (7.9) 68.8 |
Healthy controls (30) | Vitamin A, C, E | Plasma |
| Al-Allaf et al, 2003 [33] | Secondary care | FMS | 40 | Case-control | 42.5 (3.6) | FIQ (score out of 10): 6.5 (2.2) 48 (31) |
Healthy controls (37) | Vitamin D, calcium | Serum |
| Bagis et al, 2013 [34] | Secondary care | FMS | 60 | RCT and case-control | 40.7 (5.2) | FIQ: 38.8 (10.4) |
Healthy controls (20) | Magnesium | Serum, erythrocytes |
| Baygutalp et al, 2014 [35] | Secondary care | FMS | 19 | Case-control | 35 (7.5) | FIQ: 19.3 (21.5) 4.4 (1.2) |
Healthy controls (21) | Vitamin D | Serum |
| Bazzichi et al, 2008 [36] | Secondary care | FMS | 25 | Case-control | 48.8 (9.3) | FIQ: 57.9 (17.3) | Secondary care patients without FM or musculo-skeletal pain (25) | Calcium, magnesium | Platelets |
| Brouwers et al, 2002 [37] | Tertiary care | CFS | 24 | RCT | 40.0 (9.9) | CIS: 51.4 (4.2) Disease duration (years, median (IQR)) 8.0 (2–15) | Placebo, CFS patients (25) | Polynutrient supplement | NA |
| Costa et al, 2016 [38] | Secondary care | FMS | 100 | Case-control | 42.4 (8.4) | NR | Healthy controls (57) | Calcium | Serum |
| Eisinger et al, 1997 [39] | NR | FMS | 25 | Case-control | 40 | NR | Healthy controls (20) | Vitamin A, E, magnesium, selenium, zinc | Plasma |
| Eisinger et al, 1996 [40] | NR | FMS | 25 | Case-control | 40 | NR | Healthy controls (20) | Magnesium | Serum, erythrocytes, lencocytes |
| Study | Setting | Type of FSS | N of cases | Study design | Mean age in years (SD) | Mean FSS severity (SD) and/or mean duration in months (SD) | Comparison group (n) | Vitamin and/or mineral | Material |
| Heidari et al, 2010 [41] | Secondary care | FMS | 17 | Case-control | 40.6 (8.3) | NR | Secondary care patients without FM or musculoskeletal pain (202) | Vitamin D | Serum |
| Jammes et al, 2011 [42] | NR | CFS | 5 | Case-control | 39 (8) | 72 (12) | Healthy controls (23) | Vitamin C, potassium, sodium | Plasma |
| Jammes et al, 2009 [43] | Secondary care | CFS | 18 | Case-control | 38 (5) | NR | Medical checkup patients (9) | Vitamin C | Plasma |
| Kasapoğlu Aksoy et al, 2016 [44] | Secondary care | FMS | 53 | Case-control | 48.2 (9.6) | VAS pain (0–10) median, min-max: 8.0 (4.0–10.0) | Healthy controls (47) | Vitamin D | Serum |
| Khalifa et al, 2016 [45] | Secondary care | FMS | 31 | Case-control | 40.2 (13.3) | FIQR mean: 32.4 | Healthy controls (21) | Calcium, copper, magnesium, zinc | Serum |
| Kim et al, 2011 [46] | Secondary care | FMS | 44 | Case-control | 42.5 (6.9) | NR | Healthy controls (122) | Calcium, copper, ferritin, magnesium, manganese, phosphorus, potassium, selenium, sodium, zinc | Hair |
| Kurup et al, 2003 [47] | Secondary care | CFS | 15 | Case-control | 30–40 range | NR | Healthy controls (15) | Vitamin E, magnesium | Plasma, RBC |
| La Rubia et al, 2013 [48] | NA | FMS | 45 | Case-control | 52.2 (7.5) | FIQ: 61.4 (13.1) | Healthy controls (25) | Copper, ferritin, iron, zinc | Serum |
| Maafi et al, 2016 [49] | Tertiary care | FMS | 74 | Case-control | 37.9 (9.8) | FIQR: 51.8 (17.2) 13.2 (6.2) |
Healthy controls (68) | Vitamin D, calcium, phosphorus | Serum |
| Mader et al, 2012 [50] | Secondary care | FMS | 84 | Case-control | 52 (12) | FIQ: 57.1 (20.2) | Healthy controls (87) | Ferritin, iron | Serum |
| Maes et al, 2006 [51] | Secondary care | CFS | 12 | Case-control | 41.9 (13.2) | NR | Healthy controls (12) | Zinc | Serum |
| Study | Setting | Type of FSS | N of cases | Study design | Mean age in years (SD) | Mean FSS severity (SD) and/or mean duration in months (SD) | Comparison group (n) | Vitamin and/or mineral | Material |
| Mateos et al, 2014 [52] | Secondary care | FMS | 205 | Case-control | 51.5 (9.6) | NR | Healthy controls (205) | Vitamin D, calcium | Serum |
| McCully et al, 2005 [53] | NR | CFS | 20 | Case-control | NR | NR | Healthy sedentary controls (11) | Magnesium | Skeletal muscle |
| Mechtouf et al, 1998 [54] | NR | FMS | 54 | Case-control | Min-max: 20–75 | NR | Healthy controls (36) | Vitamin B1 | Plasma |
| Miwa et al, 2010 [55] | Secondary care | CFS | 27 | Case-control | 29 (6) | NR | Secondary care patients free from fatigue for at least a month (27) | Vitamin E | Serum |
| Miwa et al, 2008 [56] | NR | CFS | 50 | Case-control | NR | NR | Healthy controls (40) | Vitamin E | Serum |
| Nazıroğlu et al, 2010 [57] | Secondary care | FMS | 31 | RCT and case-control | 40.1 (5.2) | Number tender points: 15 (2) | Healthy controls (30) | Vitamin A, C, E | Plasma |
| Ng et al, 1999 [58] | Secondary care | FMS | 12 | Case-control | 44.6 | NR | Healthy controls (12) | Calcium, magnesium | Hair |
| Norregaard et al, 1994 [59] | NR | FMS | 15 | Case-control | 49 | NR | Healthy controls (15) | Potassium | Plasma |
| Okyay et al, 2016 [60] | Tertiary care | FMS | 79 | Case-control | 37 (9) | NR | Healthy controls (80) | Vitamin D | Serum |
| Olama et al, 2013 [61] | Secondary care | FMS | 50 | Case-control | 32.3 (9.4) | 47 (24) | Healthy controls (50) | Vitamin D, calcium, phosphorus | Serum |
| Ortancil et al, 2010 [62] | Secondary care | FMS | 46 | Case-control | 46.9 (10.6) | FIQ: 60.0 (10.9) | Healthy controls (46) | Vitamin B12, ferritin, folic acid | Serum |
| Özcan et al, 2014 [63] | Secondary care | FMS | 60 | Case-control | 41.9 (9.8) | FIQ: 58.6 (10.3) 27.3 (17.3) |
Healthy controls (30) | Vitamin D | Serum |
| Study | Setting | Type of FSS | N of cases | Study design | Mean age in years (SD) | Mean FSS severity (SD) and/or mean duration in months (SD) | Comparison group (n) | Vitamin and/or mineral | Material |
| Reinhard et al, 1998 [64] | Secondary care | FMS | 68 | Case-control | 47 | NR | Blood donors without FM or musculoskeletal pain (97) | Selenium | Serum |
| Rezende Pena et al, 2010 [65] | Secondary care | FMS | 87 | Case-control | 44.9 (8.6) | Number tender points: 14 (5) | Secondary care patients without FM or musculoskeletal pain (92) | Vitamin D | Serum |
| Rosborg et al, 2007 [66] | Secondary care | FMS | 38 | Case-control | Median (min-max): 49 (31–71) | NR | Healthy controls (41) | Calcium, copper, ferritin, iodine, magnesium, molybdenum, potassium, selenium, sodium, zinc | Whole blood, fasting urine |
| Sakarya et al, 2011 [67] | NR | FMS | 40 | Case-control | 33.6 (7.6) | FIQ: 61.3 (9.2) | Healthy controls (40) | Vitamin A, C, E, magnesium | Plasma |
| Samborski et al, 1997 [68] | Secondary care | FMS | 60 | Case-control | 46,4 (9.8) | NR | Healthy controls (20) | Calcium | Plasma |
| Sendur et al, 2008 [69] | NR | FMS | 32 | Case-control | 42.9 (7.7) | FIQ: 53.3 (7.9) | Healthy controls (32) | Magnesium, selenium, zinc | Serum |
| Tandeter et al, 2009 [70] | Secondary care | FMS | 68 | Case-control | 43.8 (7.6) | NR | Regular periodic blood tests patients with no FM (82) | Vitamin D | Serum |
| Türkyilmaz et al, 2010 [71] | Secondary care | FMS | 30 | Case-control | 39.8 (6.2) | SF- 36: 47.4 (17.3) 72 (62.2) |
Healthy controls (30) | Vitamin D, calcium, phosphorus | Serum |
| Ulusoy et al, 2010 [72] | NR | FMS | 30 | Case-control | 32.2 (6.8) | FIQ: 64.7 (14.3) 32.7 (19.7) |
Healthy controls (30) | Vitamin D, calcium, phosphorus | Serum |
| Study | Setting | Type of FSS | N of cases | Study design | Mean age in years (SD) | Mean FSS severity (SD) and/or mean duration in months (SD) | Comparison group (n) | Vitamin and/or mineral | Material |
| Vecchiet et al, 2002 [73] | Secondary care | CFS | 21 | Case-control | 42 (8) | VAS muscle fatigue (0–100): 52.9 (4.9) 44.5 (27.6) |
Healthy controls (20) | Vitamin E | Plasma, LDL |
| Wepner et al, 2014 [74] | General population and secondary care | FMS | 15 | RCT and cross-sectional | Overall (n = 30) 48.3 (5.3) | Number tender points: 15 (2) | Placebo, FMS patients (15) | Vitamin D | Serum |
| Witham et al, 2015 [75] | Secondary care | CFS | 25 | RCT and case-control | 48.1 (12.0) | Piper fatigue scale: 6.3 (1.6) | Placebo, CFS patients (25) |
RCT: depending on serum levels 2400 or 1200 IU cholecalciferol Observational: Vitamin D |
Serum |
| Yildirim et al, 2016 [76] | NR | FMS | 99 | Case-control | 49.4 (9.2) | FIQ: 62.9 (17.7) | Healthy controls (99) | Vitamin D | Serum |
CFS = chronic fatigue syndrome, CIS = checklist individual strength (8–56), FIQ = fibromyalgia impact questionnaire (0–100), FIQR = revised fibromyalgia impact questionnaire (0–100), FMS = fibromyalgia syndrome, FSS = functional somatic syndrome, NR = not reported, RBC = red blood cells, RCT = randomised controlled trail, VAS = visual analogue scale.
Table 2. Results of the quality assessment of observational studies.
| Appro-priate selection of par-ticipants | Validated disorder | Repre-sentative controls | In- and exclusion criteria | Disease charac-teristics | Appro-priate quanti-fication | Validated methods | Duplicate quanti-fication | Appro-priate outcome | Appro-priate control for con-founding | Assessed con-founders | Analyses adjusted | Total score | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Akkus et al, 2009 [32] | 10 | ||||||||||||
| Al-Allaf et al, 2003 [33] | 9 | ||||||||||||
| Bagis et al, 2013 [34] | 7 | ||||||||||||
| Baygutalp et al, 2014 [35] | 14 | ||||||||||||
| Bazzichi et al, 2008 [36] | 10 | ||||||||||||
| Costa et al, 2016 [38] | 6 | ||||||||||||
| Eisinger et al, 1997 [39] | 8 | ||||||||||||
| Eisinger et al, 1996 [39] | 7 | ||||||||||||
| Heidari et al, 2010 [41] | 8 | ||||||||||||
| Jammes et al, 2011 [42] | 10 | ||||||||||||
| Jammes et al, 2009 [43] | 11 | ||||||||||||
| Kasapoğlu Aksoy et al, 2016 [44] | 8 | ||||||||||||
| Khalifa et al, 2016 [45] | 6 | ||||||||||||
| Kim et al, 2011 [46] | 9 | ||||||||||||
| Kurup et al, 2003 [47] | 8 | ||||||||||||
| La Rubia et al, 2013 [48] | 9 | ||||||||||||
| Maafi et al, 2016 [49] | 11 | ||||||||||||
| Mader et al, 2012 [50] | 9 | ||||||||||||
| Maes et al, 2006 [51] | 8 | ||||||||||||
| Mateos et al, 2014 [52] | 7 | ||||||||||||
| McCully et al, 2005 [53] | 4 | ||||||||||||
| Mechtouf et al, 1998 [54] | 6 | ||||||||||||
| Miwa et al, 2010 [55] | 9 | ||||||||||||
| Miwa et al, 2008 [56] | 6 | ||||||||||||
| Nazıroğlu et al, 2010 [57] | 9 | ||||||||||||
| Ng et al, 1999 [58] | 6 | ||||||||||||
| Norregaard et al, 1994 [59] | 5 | ||||||||||||
| Okyay et al, 2016 [60] | 8 | ||||||||||||
| Olama et al, 2013 [61] | 11 | ||||||||||||
| Ortancil et al, 2010 [62] | 10 | ||||||||||||
| Özcan et al, 2014 [63] | 9 | ||||||||||||
| Reinhard et al, 1998 [64] | 7 | ||||||||||||
| Rezende Pena et al, 2010 [65] | 11 | ||||||||||||
| Rosborg et al, 2007 [66] | 9 | ||||||||||||
| Sakarya et al, 2011 [67] | 10 | ||||||||||||
| Samborski et al, 1997 [68] | 4 | ||||||||||||
| Sendur et al, 2008 [69] | 10 | ||||||||||||
| Tandeter et al, 2009 [70] | 11 | ||||||||||||
| Türkyilmaz et al, 2010 [71] | 10 | ||||||||||||
| Ulusoy et al, 2010 [71] | 10 | ||||||||||||
| Vecchiet et al, 2002 [73] | 10 | ||||||||||||
| Wepner et al, 2014 [74] | 10 | ||||||||||||
| Witham et al, 2015 [75] | 14 | ||||||||||||
| Yildirim et al, 2016 [76] | 8 | ||||||||||||
| Total score mean (SD): 8.7 (2.2) | |||||||||||||
According to the quality tool to assess methodological quality of vitamin and mineral studies in CFS and FM (S2 Appendix).
white = low risk, light gray = medium risk, dark gray = high risk
Table 3. Results of the quality assessment of randomized controlled trials.
| Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete data | Selective reporting quantification | Other bias | Total score | |
|---|---|---|---|---|---|---|---|---|
| Bagis et al, 2013 [34] | 5 | |||||||
| Brouwers et al, 2002 [37] | 6 | |||||||
| Nazıroğlu et al, 2010 [57] | 6 | |||||||
| Wepner et al, 2014 [74] | 8 | |||||||
| Witham et al, 2015 [75] | 12 | |||||||
| Total score mean (SD): 10.0 (2.6) | ||||||||
According to the Cochrane Collaboration’s tool.
white = low risk, light gray = medium risk, dark gray = high risk
Systematic review
Studies that were not included in the meta-analyses are presented in Table 4.
Table 4. Vitamin and mineral status in the included studies.
| Vitamin A | ||||||
| Study | Patients | Controls | Statistically significant | Linked to clinical parameter | ||
| Mean | SD | Mean | SD | |||
| Akkus et al, 2009 [32] | 0.30 μmol/l | 0.10 | 0.45 | 0.16 | p<.01 | NR |
| Eisinger et al, 1997 [39] | 2.7 μmol/l | 1.5 | 2.3 | 0.9 | NS | NR |
| Nazıroğlu et al, 2010 [57] | 1.5 μmol/l | 0.5 | 2.4 | 0.2 | p<.05 | NR |
| Sakarya et al, 2011 [67] | 1.46 mmol/l | 0.47 | 1.25 | 0.26 | NS | FIQ Pearson’s correlation coefficient: -0.083 (NS) |
| Vitamin B1 | ||||||
| Mechtouf et al, 1998 [54] | 58 ng/ml | 38.9 | 49.6 | 14.8 | p<.05 | NR |
| Vitamin B12 | ||||||
| Ortancil et al, 2010 [62] | 297.6 pg/ml | 120.7 | 295.7 | 113.0 | NS | NR |
| Vitamin C | ||||||
| Sakarya et al, 2011 [67] | x | x | x | x | x | FIQ Pearson’s correlation coefficient: -0.115 (NS) |
| Vitamin D | ||||||
| Al-Allaf et al, 2003 [33] | <20nmol/l (n (%)): | 18 (45) | n (%): | 7 (18.9%) | p<0.015 | NR |
| Baygutalp et al, 2014 [35] | x | x | x | x | x | FIQ Spearman correlation: 0.231 (NS) |
| Kasapoğlu Aksoy et al, 2016 [44] | x | x | x | x | x |
<30 ng/ml vs >30 ng/ml in FMS: VAS pain: 8.4 (1.6) vs 6.7 (2.0) p = .002 FIQ: 65.4 (12.0) vs 57.2 (16.1) p = .088 |
| Maafi et al, 2016 [49] | x | x | x | x | x |
FIQR
Spearman correlation: -0.093 (NS) Number of tender points: -0.194 (NS) VAS pain: -0.097 (NS) |
| Okyay et al, 2016 [60] | x | x | x | x | x |
<20 ngl/ml vs 20–30 vs >30 ng/ml in FMS: FIQ: 56.6 (8.9) vs 48.8 (2.8) vs 41.4 (8.2) p = .000 VAS pain: 7.4 (1.4) vs 6.4 (0.5) vs 5.1 (1.0) p = .000 FIQ Spearman correlation: -0.621 (p = .000) VAS pain Spearman correlation: -0.578 (p = .000) |
| Study | Patients | Controls | Statistically significant | Linked to clinical parameter | ||
| Mean | SD | Mean | SD | |||
| Rezende Pena et al, 2010 [65] | x | x | x | x | x |
Number of tender points
Pearson’s correlation coefficient: -0.160 (NS) VAS pain: -0.196 (NS) |
| Ulusoy et al, 2010 [72] | <20ng/l (n (%)): | 26 (86.7) | n (%): | 29 (96.7) | NS | FIQ Pearson’s correlation coefficient: 0.071 (NS) |
| Wepner et al, 2014 [74] | 19.94 ng/ml | 6.066 | NR | NR | NR | NR |
| Witham et al, 2015 [75] | 44 and 48 nmol/l | 15 and 20 | NR | NR | NR | Piper fatigue scale: no improvement after vitamin D3 treatment |
| Yildirim et al, 2016 [76] | x | x | x | x | x |
FIQ
Pearson’s correlation coefficient: r = 0.112 (NS) VAS pain: r = 0.104 (NS) |
| Vitamin E | ||||||
| Kurup et al, 2003 [47] | 5.22 μg/ml RBC | 0.31 | 5.25 | 0.33 | NS | NR |
| Miwa et al, 2010 [55] | 2.81 mg/g lipids | 0.73 | 3.88 | 0.65 | p<.001 | NR |
| Miwa et al, 2008 [56] | 3.03 mg/g lipids | 0.72 | 3.78 | 0.66 | p<.001 | NR |
| Sakarya et al, 2011 [67] | x | x | x | x | x | FIQ Pearson’s correlation coefficient: −0.171 (NS) |
| Vecchiet et al, 2002 [73] | 9.5 μmol/mg LDL | 1.0 | 18.0 | 1.5 | p<.001 |
Linear regression analyses
fatigue
versus vitamin E in plasma: Y = 56.674–0.4467X r = -0.6098 (p < 0.004) |
| Calcium | ||||||
| Bazzichi et al, 2008 [36] | 231.0 nM platelet | 13.75 (SEM) | 198.3 | 10.40 | NS | NR |
| Kim et al, 2011 [46] | 775 μg/g | 439–1,366 (95%CI) | 1,093 | 591–2,020 | p = .001 | NR |
| Ng et al, 1999 [58] | 2288.4 μg/g hair | 1486.2 | 846.3 | 645.7 | p = .025 | NR |
| Rosborg et al, 2007 [66] | 49 mg/l (median whole blood) 72.8 mg/l (median urine) |
28.5–62.2 <29–258 (range) |
48.0 74.5 |
39.7–58.5 <29–519 |
NS | NR |
| Copper | ||||||
| Khalifa et al, 2016 [45] | 145.8 μg/dl | 17.34 | 116.50 | 14.35 | p<.05 | NR |
| Kim et al, 2011 [46] | 28.3 μg/g | 11.8–68.1 (95%CI) |
40.2 | 16.1–100.0 | p = .029 | NR |
| La Rubia et al, 2013 [48] | 105.99 mg/dl | 17.03 | 83.55 | 9.20 | p<.001 | NR |
| Study | Patients | Controls | Statistically significant | Linked to clinical parameter | ||
| Mean | SD | Mean | SD | |||
| Rosborg et al, 2007 [66] | 971 μg/l (median whole blood) 28.1 μg/l (median urine) |
620–1740 6.7–186 (range) |
855 34.7 |
690–1475 8.6–92.2 |
p = .002 NS |
NR |
| Ferritin | ||||||
| Kim et al, 2011 [46] | 5.90 μg/g | 4.21–8.26 (95%CI) |
7.10 | 4.73–10.66 | p = .007 | NR |
| La Rubia et al, 2013 [48] | 52.33 g/dl | 15.07 | 57.42 | 17.01 | NS | NR |
| Mader et al, 2012 [50] | 63.68 ng/ml ≤30 ng/mL n (%): 23 (27.4) |
49.72 | 53.70 n (%): 38 (43.7) |
46.24 | p = .18 p<.04 |
FIQ Spearman correlation: NS |
| Ortancil et al, 2010 [62] | 27.3 ng/ml <50 ng/mL n (%): 40 (87.0) |
20.9 | 43.8 n (%): 26 (56.5) |
30.8 | p = .035 p = .001 |
FIQ Spearman correlation: NS |
| Rosborg et al, 2007 [66] | 422 mg/l (median) | 245–585 (range) | 400 | 273–465 | p = .046 | NR |
| Folic acid | ||||||
| Ortancil et al, 2010 [62] | 9.2 ng/ml | 3.1 | 8.9 | 2.5 | NS | NR |
| Iodine | ||||||
| Rosborg et al, 2007 [66] | <650 μg/l (median whole blood) 788 μg/l (median urine) |
<650–1900 <130–5395 (range) |
<650 2000 |
<650–693 <130–12145 |
NS p = .001 |
NR |
| Iron | ||||||
| La Rubia et al, 2013 [48] | 81.82 mg/dl | 34.64 | 83 | 30.07 | NS | NR |
| Mader et al, 2012 [50] | 82.32 μg/dl | 32.75 | 75.31 | 29.13 | NS | FIQ Spearman correlation: NS |
| Magnesium | ||||||
| Bagis et al, 2013 [34] | Erythrocyte: 2.27/2.70/2.91 mmol/l |
0.41/0.47/0.42 | 3.22 mmol/l | 0.36 | p<.001 |
FIQ
Pearson’s correlation serum Mg: -0.426 (p<.001) Erythrocyte Mg: -0.309 (p = .013) |
| Bazzichi et al, 2008 [36] | 1.30 mM platelet | 0.079 (SEM) | 1.07 | 0.056 | p = .02 | NR |
| Eisinger et al, 1997 [39] | 2.36 mmol/l erythrocyte | 0.24 | 2.39 | 0.24 | NS | NR |
| Eisinger et al, 1996 [40] | 4.9 fmol/cell lencocyte | 1.7 | 3.9 | 1.3 | NS | NR |
| Kim et al, 2011 [46] | 52 μg/g | 25–107 (95%CI) | 72 | 36–147 | p = .008 | NR |
| Study | Patients | Controls | Statistically significant | Linked to clinical parameter | ||
| Mean | SD | Mean | SD | |||
| McCully et al, 2005 [53] | 0.47 mM muscle | 0.07 | 0.36 | 0.06 | p<.01 | NR |
| Ng et al, 1999 [58] | 84.7 μg/g hair | 73.3 | 46.8 | 28.9 | p = .05 | NR |
| Rosborg et al, 2007 [66] | 28.6 mg/l (median whole blood) 47.1 mg/l (median urine) |
24.5–37.8 <25–189 (range) |
28.2 60.5 |
23.2–37.2 <25–171 |
NS | NR |
| Sakarya et al, 2011 [67] | x | x | x | x | x | FIQ Pearson’s correlation coefficient: 0.014 (NS) |
| Sendur et al, 2008 [69] | x | x | x | x | x | FIQ Pearson’s correlation coefficient: -0.040 (NS) |
| Manganese | ||||||
| Kim et al, 2011 [46] | 140 ng/g | 80–260 (95%CI) | 190 | 80–480 | p = .029 | NR |
| Molybdenum | ||||||
| Rosborg et al, 2007 [66] | 0.6 μg/l (median) | <0.25–4.4 (range) | 0.6 | <0.25–5.7 | NS | NR |
| Phosphorus | ||||||
| Kim et al, 2011 [46] | 146 μg/g | 116–183 (95%CI) | 143 | 116–176 | NS | NR |
| Maafi et al, 2016 [49] | 3.6 mg/dl | 0.47 | 3.66 | 0.54 | NS | NR |
| Olama et al, 2013 [61] | 3.55 mg/dl | 0.12 | 3.6 | 0.16 | NS | NR |
| Türkyilmaz et al, 2010 [71] | 3.2 mg/dl | 0.4 | 3.3 | 0.5 | NS | NR |
| Ulusoy et al, 2010 [72] | 3.54 mg/dl | 0.56 | 3.57 | 0.46 | NS | NR |
| Polynutrient supplement | ||||||
| Brouwers et al, 2002 [37] | Baseline CIS: 51.4 Follow up CIS: 48.6 |
4.2 7.4 |
51.3 48.2 |
3.6 7.6 |
NS | NR |
| Potassium | ||||||
| Jammes et al, 2011 [42] | 3.92 mmol/l | 0.12 | 3.99 | 0.08 | NS | NR |
| Kim et al, 2011 [46] | 75 μg/g | 25–219 (95%CI) | 56 | 23–138 | NS | NR |
| Norregaard et al, 1994 [59] | 3.25 mmol/l (median) | NR | 3.9 | NR | NS | NR |
| Rosborg et al, 2007 [66] | 926 mg/l (median urine) | 205–3300 (range) | 1410 | 378–5200 | p = .013 | NR |
| Selenium | ||||||
| Eisinger et al, 1997 [39] | 83 ng/ml | 17 | 87 | 12 | NS | NR |
| Kim et al, 2011 [46] | 75 μg/g | 25–219 (95%CI) | 56 | 23–138 | NS | NR |
| Reinhard et al, 1998 [64] | Median: 70.8 μg/l | 67.7–75.3 (95%CI) | 76.8 | 73.4–81.6 | p<.05 | NR |
| Study | Patients | Controls | Statistically significant | Linked to clinical parameter | ||
| Mean | SD | Mean | SD | |||
| Rosborg et al, 2007 [66] | 117 μg/l (median whole blood) 18.4 μg/l (median urine) |
77.6–207 5.5–55.7 (range) |
105 23.5 |
66.4–137 2.3–52.2 |
p = .015 NS |
NR |
| Sendur et al, 2008 [69] | 44.4 μg/dl | 12.1 | 38.7 | 13.9 | NS | FIQ Pearson’s correlation coefficient: 0.011 (NS) |
| Sodium | ||||||
| Jammes et al, 2011 [42] | 138 mmol/l | 0.5 | 140 | 0.4 | NS | NR |
| Kim et al, 2011 [46] | 78 μg/g | 31–195 (95%CI) | 72 | 27–195 | NS | NR |
| Rosborg et al, 2007 [66] | 1560 mg/l (median urine) | 90.8–3705 (range) | 1700 | 510–4790 | NS | NR |
| Zinc | ||||||
| Eisinger et al, 1997 [39] | 16.9 mmol/l | 1.8 | 16.1 | 1.9 | NS | NR |
| Khalifa et al, 2016 [45] | 75.87 μg/dL | 5.5 | 93.21 | 11.94 | p<.05 | NR |
| Kim et al, 2011 [46] | 167 μg/g | 120–232 (95%CI) | 165 | 125–217 | NS | NR |
| La Rubia et al, 2013 [48] | 66.48 ng/ml | 18.82 | 106.8 | 22.41 | p<.001 | PCS-12 Pearson’s correlation coefficient: 0.402 (p = .017) |
| Maes et al, 2006 [51] | 73.5 mg/dl | NR | 87 | NR | p = .0001 | Fibrofatigue scale Pearson’s correlation coefficient: -0.039 (NS) |
| Rosborg et al, 2007 [66] | 6000 μg/l (median whole blood) 294 μg/l (median urine) |
3720–9400 35.8–1230 (range) |
5450 290 |
3900–7300 35.0–66.5 |
p = .026 NS |
NR |
| Sendur et al, 2008 [69] | 102.8 μg/dl | 24.7 | 77.2 | 31 | p = .001 | FIQ Pearson’s correlation coefficient: -0.106 (NS) |
CI = confidence interval, CIS = checklist individual strength, FIQ = fibromyalgia impact questionnaire, FIQR = revised fibromyalgia impact questionnaire, NR = not reported, NS = not significant, PCS = physical component summary, SD = standard deviation, VAS = visual analogue scale, x = reported in meta-analyses.
Interventions
Five RCTs were included. The first RCT determined the effect of magnesium citrate treatment in combination with amitriptyline versus amitriptyline only, on FMS symptoms, over a period of 8 weeks [34]. They found that amitriptyline and magnesium supplementation was more effective on all measured outcomes than amitriptyline alone. The second RCT investigated the effect of a polynutrient supplement (containing several vitamins (including A, B, C, D, E), minerals (including calcium, magnesium) and (co)enzymes), on fatigue and physical activity of patients with CFS, over a period of 10 weeks [37]. They found no significant difference between the placebo and treatment group on any of the outcome measures. A third RCT examined vitamin C and E treatment combined with exercise versus exercise only, in FMS patients, over a period of 12 weeks [57]. Although both interventions lead to significantly higher vitamin A, C, and E serum levels, the FMS symptoms did not improve in both groups. Furthermore, the most recent RCT investigated the effect of vitamin D, on symptoms in CFS patients, over a period of 6 months [75]. Despite a statistically significant increase in vitamin D, they found no evidence of improvement in symptoms of fatigue or depression. Lastly, in the fifth RCT, cholecalciferol was administered for 20 weeks in FMS patients, with the dosage depending on patients calcifediol levels [74]. A significant treatment effect on intensity of pain was found in the treatment group versus placebo. No changes in somatization, depression and anxiety, physical and mental health, and FMS symptom severity were observed in both the treatment and placebo group.
Clinical parameters
All studies investigating vitamin A (n = 1) [67], vitamin C (n = 1) [67], ferritin (n = 2) [50,62], iron (n = 1) [50], and selenium (n = 1) [69], found no significant associations between vitamin and mineral status and clinical parameters in FMS patients (Table 3). Most studies investigating vitamin D (n = 6) found no significant associations between vitamin D and clinical parameters in CFS [75] and FMS [35,49,65,72,76] patients. However, two studies found significantly higher VAS-score for pain in patients with vitamin D levels <30 ng/ml compared to FMS patients with vitamin D levels of >30ng/ml [44,60]. Significant negative associations were found for vitamin E in plasma and fatigue in CFS patients (n = 1/2) [73], and serum and erythrocyte magnesium and fibromyalgia symptoms (n = 1/3) [34]. A significant positive association was found for serum zinc and somatic symptoms in fibromyalgia patients (n = 1/3) [48].
Vitamin and mineral status
All studies that investigated vitamin B12 (n = 1) [62], folic acid (n = 1) [62], iron (n = 2) [48,50], molybdenum (n = 1) [66], phosphorus (n = 4) [46,49,61,71,72] sodium (n = 3) [42,46,66], and iodine (n = 1) [66], and the majority of studies that investigated potassium (n = 3/4) [42,46,59], and selenium status (n = 4/5) [39,46,66,69] found no statistically significant difference between patients and controls (Table 3). In contrast, all studies that investigated vitamin B1 (n = 1/1) [54], and manganese (n = 1/1) [46], and the majority of studies that investigated vitamin A (n = 2/4) [39,67], found statistically significant lower serum values in patients versus controls. The majority of the studies that were not suitable for inclusion in the meta-analyses reported significantly lower vitamin E in patients versus controls (n = 3/4) [55,56,73]. Statistically significant results were found in the majority of the included studies investigating copper (n = 3/4) [46,48,66], ferritin (n = 4/5) [46,50,62,66], and zinc (n = 5/7) status [48,51,66,69]. However, the direction of the differences was equivocal for all three minerals: levels of copper were higher among patients in 3 studies and lower in 1, levels ferritin were higher among patients in 2 studies and lower in 2, and levels of zinc were lower in 3 studies and higher in 2.
Meta-analysis
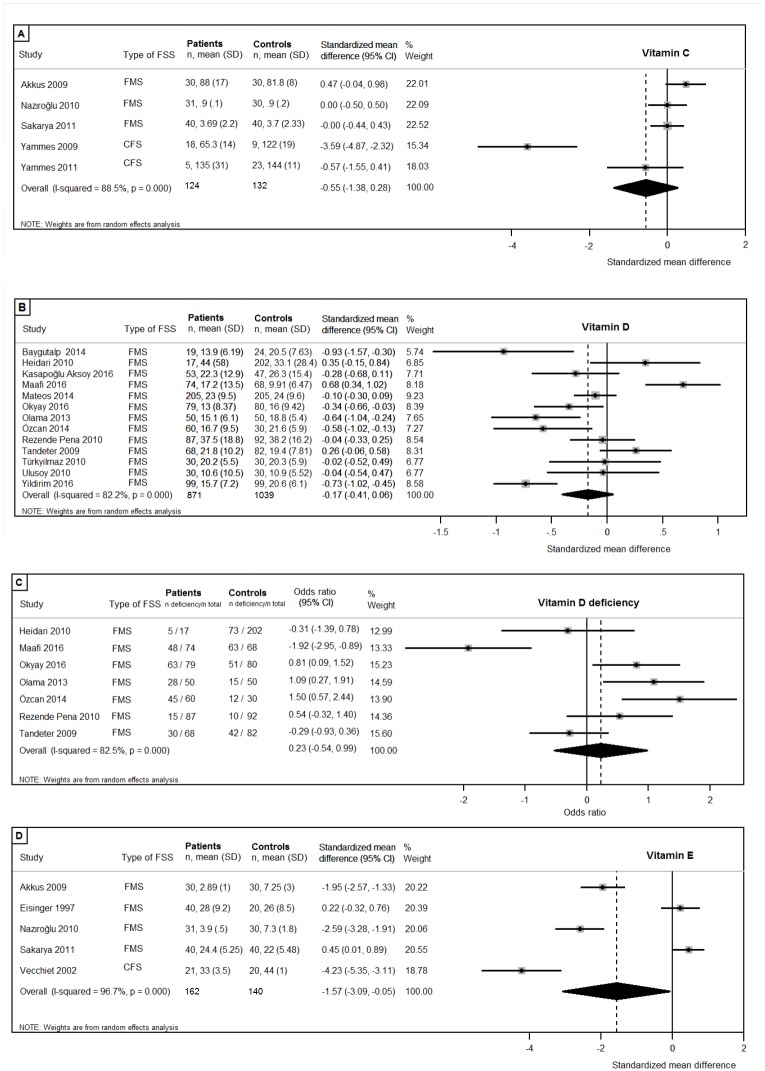
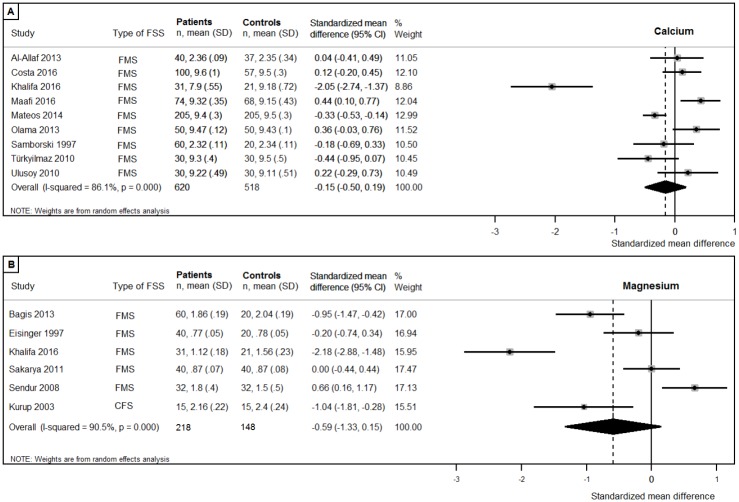
Vitamin C, vitamin D, vitamin D deficiency (<20ng/ml), vitamin E (Fig 2), and the minerals calcium, and magnesium status, and were reported in more than five studies and were therefore investigated using meta-analysis (Fig 3). Meta-analysis revealed that circulating concentrations of vitamin E were lower in patients compared to controls (patients n = 162, controls n = 140; pooled SMD:-1.57, 95%CI:-3.09,-0.05; p = .042). No differences were found in patients compared to controls in circulating concentrations of vitamin C (patients n = 124, controls n = 132; pooled SMD:-0.55, 95%CI:-1.38,0.28; p = .19), vitamin D (patients n = 871, controls n = 1039; pooled SMD:-0.17, 95%CI:-0.41,0.06; p = .15), and vitamin D deficiency (patients n = 435, controls n = 604; pooled OR:0.23, 95%CI:-0.54,0.99; p = .17). There were no differences between patients and controls in circulating concentrations of the minerals calcium (patients n = 620, controls n = 518; pooled SMD:-0.15, 95%CI:-0.50,0.19; p = .38), and magnesium (patients n = 218, controls n = 148; pooled SMD:-0.59, 95%CI:-1.33,0.15; p = .12). All analyses revealed substantial to considerable heterogeneity in the effect sizes, as can be found in Fig 2.
Fig 2. Forest plots of studies investigating vitamins.
(A) Vitamin C; (B) Vitamin D; (C) Vitamin D deficiency (<20ng/ml); (D) Vitamin E.
Fig 3. Forest plots of studies investigating minerals.
(A) Calcium; (B) Magnesium.
Subgroup analyses
Subgroup analyses were performed including studies with more than half the maximum study quality score (>9 quality points), if more than three studies with a sufficient quality score were available. The additional analysis was not possible for magnesium, since only two studies achieved more than half of the maximum quality score. No differences in circulating concentrations of vitamin C (patients n = 93, controls n = 102, pooled SMD:-0.78, 95CI:-1.95, 0.39; p = .19) [32,42,43,67], vitamin D (patients n = 358, controls n = 376, pooled SMD:-0.07, 95%CI:-0.44,0.30; p = .71) [35,49,61,65,70–72], vitamin D deficiency (patients n = 121, controls n = 130; pooled OR:-0.12, 95%CI:-1.24,1.01; p = .84) [49,61,65,70], and calcium = (patients n = 184, controls n = 178; pooled SMD:0.18 95%CI:-0.18,0.54; p = .34) [49,61,71,72] were found. The significant difference in circulating concentrations of vitamin E between patients and controls disappeared when studies with low quality score were excluded (patients n = 91, controls n = 90, pooled SMD: -1.86, 95%CI:-4.28, 0.56; p = .13) [32,67,73].
Subgroup analyses were performed separately for the syndromes, when more than three studies were available per syndrome. Since vitamin D, vitamin D deficiency and calcium were only determined in FMS patients, additional subgroup analyses were possible for vitamin C, vitamin E and magnesium. No statistically significant difference between patients and controls was found in the three studies investigating circulating concentrations of vitamin C in FMS patients (patients n = 101, controls n = 100; pooled SMD:0.14, 95%CI:-0.16,0.44; p = .32). However, the heterogeneity was substantially lower (I2 = 13.3% versus 88.5% in the overall analysis including CFS patients), indicating a high consistency of studies’ results. The significant difference in circulating concentrations of vitamin E between patients and controls disappeared when the single CFS study was excluded (patients n = 141, controls n = 120; pooled SMD:-0.95, 95%CI:-2.41,0.50; p = .20. Lastly, no considerable differences were found in analyses of the five studies investigating circulating concentrations of magnesium in FMS patients (patients n = 203, controls n = 133; pooled SMD:-0.51, 95%CI:-1.34,0.32; p = .23).
Publication bias
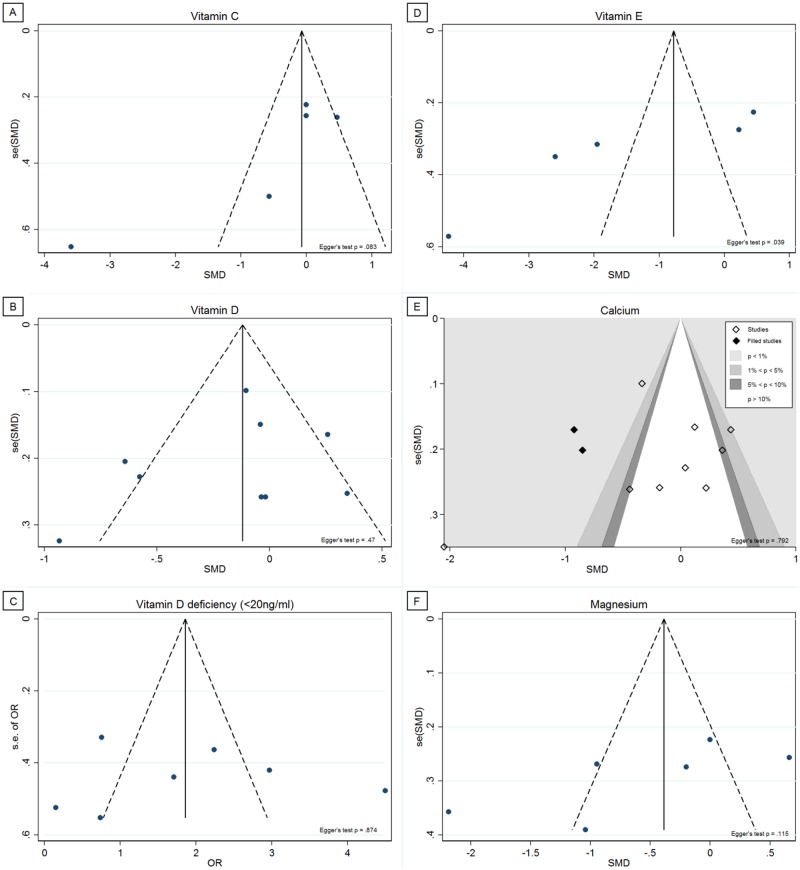
Finally, we tested whether publication bias could have affected the results. Corresponding funnel plots can be found in Fig 4. Egger's test showed that there was significant funnel plot asymmetry in vitamin E (p = .039), with no significant asymmetry among the other analyses. Trimming was performed in the calcium studies using the Trim and Fill test, and the contour-enhanced funnel plot revealed two added studies in the statistically significant areas. No studies were trimmed or filled among the vitamin C, vitamin D, vitamin D deficiency, vitamin E, and magnesium studies, indicating absence of substantial publication bias.
Fig 4. Funnel plots.
(A) Vitamin C; (B) Vitamin D; (C) Vitamin D deficiency (<20ng/ml); (D) Vitamin E; (E) Calcium; (F) Magnesium.
Discussion
We found little evidence to support our hypothesis that vitamin and mineral deficiencies play a role in the pathophysiology of both CFS and FMS, or that the use of nutritional supplements is effective in these patients. Poor study quality and considerable heterogeneity in most studies was found, which makes it difficult to reach a final conclusion. Consistent significant lower circulating concentrations were found repeatedly and in the majority of studies for vitamin A and vitamin E in patients compared to controls. However, the significant difference in circulating concentrations of vitamin E between patients and controls disappeared when excluding low quality studies. None of these or other vitamins and minerals have been repeatedly or consistently linked to clinical parameters. In addition, RCTs testing supplements containing these vitamins and/or minerals did not result in clinical improvements.
This review has several strengths. First, this is the first review focusing on vitamin and mineral deficiencies among CFS and FMS patients. We were able to give a clear overview of the current knowledge existing in literature. Second, we included only studies that examined CFS and FMS patients according to the official diagnostic criteria. We therefore have included relatively homogeneous groups of patients. Third, because we defined strict in- and exclusion criteria, e.g. patients should meet the official diagnostic criteria, or clinical cohorts must have an appropriate control group, poor quality studies were filtered out. Nevertheless, the vast majority of the included studies scored a quality score below a reasonable study quality. Fourth, enough studies that investigated similar vitamins or minerals were available, which made it possible to conduct six meta-analyses. Lastly, we had no language restrictions for the included abstracts or full text articles, which enabled us to include all relevant articles.
We must acknowledge that this study also has its limitations, which are mostly due to limitations in original studies on which this review was based. First, most studies were observational in nature. In general, observational studies have a lower validity than RCTs, and they are more susceptible to bias (e.g. selection and information bias) and confounding factors. Potential confounders were assessed in about half of the studies, but almost no studies adjusted their analyses for potential confounders. Consequently, the results of the current review may be affected by the methodological weaknesses that are accompanied by the observational study designs. Second, quality assessment revealed a poor study quality in the majority of studies. This demonstrates that substantial improvements can be made in terms of study quality, especially in specification of in- and exclusion criteria, presenting disease characteristics of the participants, making use of validated methods to assess vitamin and mineral status, to perform the vitamin and mineral assessments in duplicate, and, as mentioned earlier, to adjust analyses for potential confounders. Furthermore, a quality issue in research on CFS and FMS patients is that of careful selection of control groups. Our quality assessment showed that many included studies fell short because of the selection of the controls, which could result in inaccurate study results. Third, a problem that affects the validity of meta-analyses is the presence of publication bias. Funnel plots indicated the absence of publication bias in the majority of the meta-analyses. Trimming was performed among the calcium studies, and two “missing” studies were added, while no significant funnel plot asymmetry was present. However, trimming was performed in the statistically significant areas, which argues against the presence of publication bias. Although Egger’s test is preferred for more than 10 studies, it revealed significant funnel plot asymmetry in vitamin E, while no trimming was performed. It is therefore possible that the significant outcomes of vitamin E in patients are influenced by publication bias. Lastly, a substantial to considerable heterogeneity in most studies was found, which makes it difficult to reach a final conclusion about vitamin status in CFS and FMS patients.
This review reveals that very few RCTs have investigated the effect of vitamin and mineral supplementation versus placebo in CFS and FMS patients. Most published RCTs found no treatment effect of vitamin and mineral supplementation on clinical parameters. So, the evidence for beneficial effects of supplementation in CFS and FMS patients is not proportional to the large quantity of supplements that are used by these patients. Nevertheless, the industry of vitamin and minerals supplements is increasing, for example, Americans spend an estimated $36.7 billion each year on supplements [77]. This is important information, since the vitamins and minerals in these products are sometimes supplemented in doses high enough to cause side effects, for example gastric discomfort, insomnia, dizziness or weakness [17]. The vast majority of available studies concerned FMS patients. Several FMS studies investigated vitamin D, whereas most CFS studies have focused on vitamin E. Only one CFS study that investigated vitamin E was suitable for inclusion in the meta-analysis. It is remarkable that the significant difference of vitamin E between patients and controls disappeared when the single CFS study was excluded in the sensitivity analysis, while the studies that were not suitable for inclusion in the meta-analysis reported significant lower vitamin E concentrations in particularly CFS patients versus controls. Further research is needed to determine whether this may indicate that vitamin E levels are lower in CFS patients, but not in FMS patients. This systematic review and meta-analysis provides no further insights in whether the remaining vitamins and minerals differ between these two medical conditions.
We conclude that there is little evidence to support the hypothesis that vitamin and mineral deficiencies play a role in the pathophysiology of both CFS and FMS. Furthermore, the current literature on vitamins and minerals in CFS and FMS is of poor quality and stresses the need for well-performed intervention research, and large population-based and age-matched prospective studies in CFS and FMS, in order to gain more insight in the role of vitamins and minerals in the pathophysiology of CFS and FMS. According to our results, potential vitamins and minerals that should be further examined include vitamin A and vitamin E.
Supporting information
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
Contributors: The authors wish to acknowledge the translators of the non-English articles (Léopold Brunet, Jurek Cislo, Anne-Marie Daubigney, Michele Eisenga, Giulia Iozzia, Akin Ozyilmaz, Mehmet Suludere), which made it possible to include all the articles in the current review.
Funders: The authors received no funding for this work.
Prior presentations: the European Association for Psychosomatic Medicine (EAPM), 17 June 2016.
Abbreviations
- CFS
chronic fatigue syndrome
- FMS
fibromyalgia syndrome
- RCT
randomized controlled trial
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
S.J.L.B. obtained grants from DSM and Friesland Campina, and S.J.L.B. and I.M. obtained funding from the Top Institute Food and Nutrition (TIFN, grants CH-001 and CH-003, respectively). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A. The chronic fatigue syndrome: A comprehensive approach to its definition and study. Ann Intern Med. 1994;121(12):953–9. [DOI] [PubMed] [Google Scholar]
- 2.Wolfe F, Smythe HA, Yunus MB, Bennet RM, Bombardier C, Goldenberg DL, et al. The american college of rheumatology 1990 criteria for the classification of fibromyalgia. Arthritis & Rheumatism. 1990;33(2):160–72. [DOI] [PubMed] [Google Scholar]
- 3.Wolfe F, Clauw DJ, Fitzcharles M, Goldenberg DL, Katz RS, Mease P, et al. The american college of rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis care & research. 2010;62(5):600–10. [DOI] [PubMed] [Google Scholar]
- 4.Aaron LA, Burke MM, Buchwald D. Overlapping conditions among patients with chronic fatigue syndrome, fibromyalgia, and temporomandibular disorder. Arch Intern Med. 2000;160(2):221 [DOI] [PubMed] [Google Scholar]
- 5.Janssens KA, Zijlema WL, Joustra ML, Rosmalen JG. Mood and anxiety disorders in chronic fatigue syndrome, fibromyalgia, and irritable bowel syndrome: Results from the LifeLines cohort study. Psychosom Med. 2015;77(4), 449–457. 10.1097/PSY.0000000000000161 [DOI] [PubMed] [Google Scholar]
- 6.Wessely S, Nimnuan C, Sharpe M. Functional somatic syndromes: One or many? Lancet 1999;354(9182):936–9. 10.1016/S0140-6736(98)08320-2 [DOI] [PubMed] [Google Scholar]
- 7.Werbach MR. Nutritional strategies for treating chronic fatigue syndrome. Altern Med Rev. 2000;5(2):93–108. [PubMed] [Google Scholar]
- 8.Arranz L, Canela M, Rafecas M. Fibromyalgia and nutrition, what do we know? Rheumatol Int. 2010;30(11):1417–27. 10.1007/s00296-010-1443-0 [DOI] [PubMed] [Google Scholar]
- 9.Lauche R, Cramer H, Häuser W, Dobos G, Langhorst J. A systematic overview of reviews for complementary and alternative therapies in the treatment of the fibromyalgia syndrome. Evid Based Complement Alternat Med. 2015; vol. 2015, Article ID 610615, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grant JE, Veldee MS, Buchwald D. Analysis of dietary intake and selected nutrient concentrations in patients with chronic fatigue syndrome. J Am Diet Assoc. 1996;96(4):383–6. 10.1016/S0002-8223(96)00104-6 [DOI] [PubMed] [Google Scholar]
- 11.Batista ED, Andretta A, de Miranda RC, Nehring J, dos Santos Paiva E, Schieferdecker MEM. Food intake assessment and quality of life in women with fibromyalgia. Rev Bras Reumatol (English Edition). 2016;562:105–110. [DOI] [PubMed] [Google Scholar]
- 12.Dykman KD, Tone C, Ford C, Dykman RA. The effects of nutritional supplements on the symptoms of fibromyalgia and chronic fatigue syndrome. Integr Physiol Behav Sci. 1998;33(1):61–71. [DOI] [PubMed] [Google Scholar]
- 13.Bennett RM, Jones J, Turk DC, Russell I, Matallana L. An internet survey of 2,596 people with fibromyalgia. BMC Musculoskelet Disord. 2008;8(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wahner-Roedler DL, Elkin PL, Vincent A, Thompson JM, Oh TH, Loehrer LL, et al. Use of complementary and alternative medical therapies by patients referred to a fibromyalgia treatment program at a tertiary care center. Mayo Clin Proc. 2005;80(1):55–60. 10.1016/S0025-6196(11)62958-3 [DOI] [PubMed] [Google Scholar]
- 15.van’t Leven M, Zielhuis GA, van der Meer Jos W, Verbeek AL, Bleijenberg G. Fatigue and chronic fatigue syndrome-like complaints in the general population. Eur J Public Health. 2010;20(3):251–7. 10.1093/eurpub/ckp113 [DOI] [PubMed] [Google Scholar]
- 16.Van Rossum C, Fransen H, Verkaik-Kloosterman J, Buurma-Rethans E, Ocké M. Dutch national food consumption survey 2007–2010: Diet of children and adults aged 7 to 69 years. 2011;RIVM rapport 350050006.
- 17.Halsted CH. Dietary supplements and functional foods: 2 sides of a coin? Am J Clin Nutr. 2003;77(4 Suppl):1001S–7S. [DOI] [PubMed] [Google Scholar]
- 18.Chang K. Is serum hypovitaminosis D associated with chronic widespread pain including fibromyalgia? A meta-analysis of observational studies. Pain physicia 2015;18:E877–87. [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann Intern Med. 2009;151(4):264–9. [DOI] [PubMed] [Google Scholar]
- 20.Higgins JP, Altman DG, Gotzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanderson S, Tatt ID, Higgins JP. Tools for assessing quality and susceptibility to bias in observational studies in epidemiology: A systematic review and annotated bibliography. Int J Epidemiol. 2007;36(3):666–76. 10.1093/ije/dym018 [DOI] [PubMed] [Google Scholar]
- 22.Deeks JJ, Dinnes J, D’amico R, Sowden AJ, Sakarovitch C, Song F, et al. Evaluating non-randomised intervention studies. Health Technol Assess. 2003;7(27):1–179. [DOI] [PubMed] [Google Scholar]
- 23.Tak LM, Riese H, de Bock GH, Manoharan A, Kok IC, Rosmalen JG. As good as it gets? A meta-analysis and systematic review of methodological quality of heart rate variability studies in functional somatic disorders. Biol Psychol. 2009;82(2):101–10. 10.1016/j.biopsycho.2009.05.002 [DOI] [PubMed] [Google Scholar]
- 24.Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Prev Med. 2007;45(4):247–51. 10.1016/j.ypmed.2007.08.012 [DOI] [PubMed] [Google Scholar]
- 25.Altman DG, Lyman GH. Methodological challenges in the evaluation of prognostic factors in breast cancer. Breast Cancer Res Treat. 1998;52(1–3):289–303. [DOI] [PubMed] [Google Scholar]
- 26.Siegfried N, Muller M, Deeks JJ, Volmink J. Male circumcision for prevention of heterosexual acquisition of HIV in men. Cochrane Database Syst Rev. 2009;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patra J, Bhatia M, Suraweera W, Morris SK, Patra C, Gupta PC, et al. Exposure to second-hand smoke and the risk of tuberculosis in children and adults: A systematic review and meta-analysis of 18 observational studies. PLoS Med. 2015;12(6):e1001835 10.1371/journal.pmed.1001835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duval S, Tweedie R. Trim and fill: A simple funnel‐plot—based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–63. [DOI] [PubMed] [Google Scholar]
- 30.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol. 2008;61(10):991–6. 10.1016/j.jclinepi.2007.11.010 [DOI] [PubMed] [Google Scholar]
- 31.Brennan P, Silman A. Statistical methods for assessing observer variability in clinical measures. BMJ. 1992;304(6840):1491–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akkuş S, Nazıroğlu M, Eriş S, Yalman K, Yılmaz N, Yener M. Levels of lipid peroxidation, nitric oxide, and antioxidant vitamins in plasma of patients with fibromyalgia. Cell Biochem Funct. 2009;27(4):181–5. 10.1002/cbf.1548 [DOI] [PubMed] [Google Scholar]
- 33.Al-Allaf AW, Mole PA, Paterson CR, Pullar T. Bone health in patients with fibromyalgia. Rheumatology (Oxford). 2003;42(10):1202–6. [DOI] [PubMed] [Google Scholar]
- 34.Bagis S, Karabiber M, As I, Tamer L, Erdogan C, Atalay A. Is magnesium citrate treatment effective on pain, clinical parameters and functional status in patients with fibromyalgia? Rheumatol Int. 2013;33(1):167–72. 10.1007/s00296-011-2334-8 [DOI] [PubMed] [Google Scholar]
- 35.Baygutalp NK, Baygutalp F, Seferoğlu B, Bakan E. Serum vitamin D seviyelerinin fibromiyalji sendromunun klinik bulguları ile ilişkisi (The relation between serum vitamin D levels and clinical findings of fibromyalgia syndrome). Dicle Tıp Dergisi. 2014;41(3):446–450. [Google Scholar]
- 36.Bazzichi L, Giannaccini G, Betti L, Fabbrini L, Schmid L, Palego L. ATP, calcium and magnesium levels in platelets of patients with primary fibromyalgia. Clin Biochem. 2008;41(13):1084–90. 10.1016/j.clinbiochem.2008.06.012 [DOI] [PubMed] [Google Scholar]
- 37.Brouwers FM, Van Der Werf S, Bleijenberg G, Van Der Zee L, Van Der Meer JW. The effect of a polynutrient supplement on fatigue and physical activity of patients with chronic fatigue syndrome: A double-blind randomized controlled trial. QJM. 2002;95(10):677–83. [DOI] [PubMed] [Google Scholar]
- 38.Costa JM, Ranzolin A, Costa Neto CA, Marques CD, Duarte AL. High frequency of asymptomatic hyperparathyroidism in patients with fibromyalgia: random association or misdiagnosis? Rev Bras Reumatol. 2016;56(5):391–397. [DOI] [PubMed] [Google Scholar]
- 39.Eisinger J, Gandolfo C, Zakarian H, Ayavou T. Reactive oxygen species, antioxidant status and fibromyalgia. J Musculoskeletal Pain. 1997;5(4):5–15. [Google Scholar]
- 40.Eisinger J, Zakarian H, Pouly E, Plantamura A, Ayavou T. Protein peroxidation, magnesium deficiency and fibromyalgia. Magnes Res. 1996;9(4):313–6. [PubMed] [Google Scholar]
- 41.Heidari B, Shirvani JS, Firouzjahi A, Heidari P, Hajian-Tilaki KO. Association between nonspecific skeletal pain and vitamin D deficiency. Int J Rheum Dis. 2010;13(4):340–6. 10.1111/j.1756-185X.2010.01561.x [DOI] [PubMed] [Google Scholar]
- 42.Jammes Y, Steinberg J, Delliaux S. Chronic fatigue syndrome: Acute infection and history of physical activity affect resting levels and response to exercise of plasma oxidant/antioxidant status and heat shock proteins. J Intern Med. 2012;272(1):74–84. 10.1111/j.1365-2796.2011.02488.x [DOI] [PubMed] [Google Scholar]
- 43.Jammes Y, Steinberg J, Delliaux S, Brégeon F. Chronic fatigue syndrome combines increased exercise‐induced oxidative stress and reduced cytokine and hsp responses. J Intern Med. 2009;266(2):196–206. 10.1111/j.1365-2796.2009.02079.x [DOI] [PubMed] [Google Scholar]
- 44.Kasapoğlu Aksoy M, Altan L, Ökmen Metin B. The relationship between balance and vitamin 25 (OH) D in fibromyalgia patients. Mod Rheumatol. 2016: 1–7. [DOI] [PubMed] [Google Scholar]
- 45.Khalifa II, Hassan MF, AL-Deri SM, Gorial FI. Determination of Some Essential & Non-Essential Metals in Patients with Fibromyalgia Syndrome (FMS). IJPSR. 2016;8(5):306–311. [Google Scholar]
- 46.Kim Y, Kim K, Lee D, Kim BT, Park SB, Cho DY, et al. Women with fibromyalgia have lower levels of calcium, magnesium, iron and manganese in hair mineral analysis. J Korean Med Sci. 2011;26(10):1253–7. 10.3346/jkms.2011.26.10.1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kurup RK, Kurup PA. Isoprenoid pathway dysfunction in chronic fatigue syndrome. Acta Neuropsychiatr. 2003;15(5):266–73. 10.1034/j.1601-5215.2003.00045.x [DOI] [PubMed] [Google Scholar]
- 48.La Rubia M, Rus A, Molina F, Del Moral ML. Is fibromyalgia-related oxidative stress implicated in the decline of physical and mental health status? Clin Exp Rheumatol. 2013;31(6 Suppl 79):S121–7. [PubMed] [Google Scholar]
- 49.Maafi AA, Ghavidel-Parsa B, Haghdoost A, Aarabi Y, Hajiabbasi A, Shenavar Masooleh I, et al. Serum Vitamin D Status in Iranian Fibromyalgia Patients: according to the Symptom Severity and Illness Invalidation. Korean J Pain. 2016;29(3):172–178. 10.3344/kjp.2016.29.3.172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mader R, Koton Y, Buskila D, Herer P, Elias M. Serum iron and iron stores in non-anemic patients with fibromyalgia. Clin Rheumatol. 2012;31(4):595–9. 10.1007/s10067-011-1888-x [DOI] [PubMed] [Google Scholar]
- 51.Maes M, Mihaylova I, De Ruyter M. Lower serum zinc in chronic fatigue syndrome (CFS): Relationships to immune dysfunctions and relevance for the oxidative stress status in CFS. J Affect Disord. 2006;90(2):141–7. [DOI] [PubMed] [Google Scholar]
- 52.Mateos F, Valero C, Olmos J, Casanueva B, Castillo J, Martínez J, et al. Bone mass and vitamin D levels in women with a diagnosis of fibromyalgia. Osteoporosis Int. 2014;25(2):525–33. [DOI] [PubMed] [Google Scholar]
- 53.McCully KK, Malucelli E, Iotti S. Increase of free Mg2+ in the skeletal muscle of chronic fatigue syndrome patients. Dyn Med. 2006;5(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mechtouf A, Jacob L, Zakarian H, Ayavou T, Eisinger J. Vitamin B1 abnormalities in persons with fibromyalgia, myofascial pain syndrome and chronic alcoholism. Lyon Mediterr Med Med Sud-Est. 1998;34(3–4):28–31. [Google Scholar]
- 55.Miwa K, Fujita M. Fluctuation of serum vitamin E (α-tocopherol) concentrations during exacerbation and remission phases in patients with chronic fatigue syndrome. Heart Vessels. 2010;25(4):319–23. 10.1007/s00380-009-1206-6 [DOI] [PubMed] [Google Scholar]
- 56.Miwa K, Fujita M. Increased oxidative stress suggested by low serum vitamin E concentrations in patients with chronic fatigue syndrome. Int J Cardiol. 2008;136(2):238–9. 10.1016/j.ijcard.2008.04.051 [DOI] [PubMed] [Google Scholar]
- 57.Nazıroğlu M, Akkuş S, Soyupek F, Yalman K, Çelik Ö, Eriş S, et al. Vitamins C and E treatment combined with exercise modulates oxidative stress markers in blood of patients with fibromyalgia: A controlled clinical pilot study. Stress. 2010;13(6):498–505. 10.3109/10253890.2010.486064 [DOI] [PubMed] [Google Scholar]
- 58.Ng SY. Hair calcium and magnesium levels in patients with fibromyalgia: A case center study. J Manipulative Physiol Ther. 1999;22(9):586–93. [DOI] [PubMed] [Google Scholar]
- 59.Nørregaard J, Btilow P, Mehlsen J, Danneskiold‐Samsøe B. Biochemical changes in relation to a maximal exercise test in patients with fibromyalgia. Clin Physiol. 1994;14(2):159–67. [DOI] [PubMed] [Google Scholar]
- 60.Okyay R, Koçyiğit B, Gürsoy S. Vitamin D Levels in Women with Fibromyalgia and Relationship between Pain, Tender Point Count and Disease Activity. Acta Medica Mediterranea. 2016;32(1): 243–247. [Google Scholar]
- 61.Olama SM, Senna MK, Elarman MM, Elhawary G. Serum vitamin D level and bone mineral density in premenopausal egyptian women with fibromyalgia. Rheumatol Int. 2013;33(1):185–92. 10.1007/s00296-012-2361-0 [DOI] [PubMed] [Google Scholar]
- 62.Ortancil O, Sanli A, Eryuksel R, Basaran A, Ankarali H. Association between serum ferritin level and fibromyalgia syndrome. Eur J Clin Nutr. 2010;64(3):308–12. 10.1038/ejcn.2009.149 [DOI] [PubMed] [Google Scholar]
- 63.Özcan DS, Oken O, Aras M, Koseoglu BF. Fibromiyaljili kadin hastalarda vitamin D duzeyleri ve agri, depresyon, uyku ile iliskisi (Vitamin D levels in women with fibromyalgia and relationship between pain, depression, and sleep). Turkish J Phys Med and Rehab. 2014;60(4):329–335. [Google Scholar]
- 64.Reinhard P, Schweinsberg F, Wernet D, Kötter I. Selenium status in fibromyalgia. Toxicol Lett. 1998;96:177–80. [DOI] [PubMed] [Google Scholar]
- 65.Rezende Pena C, Grillo LP, das Chagas Medeiros MM. Evaluation of 25-hydroxyvitamin D serum levels in patients with fibromyalgia. J Clin Rheumatol. 2010;16(8):365–9. 10.1097/RHU.0b013e3181fe8a90 [DOI] [PubMed] [Google Scholar]
- 66.Rosborg I, Hyllén E, Lidbeck J, Nihlgård B, Gerhardsson L. Trace element pattern in patients with fibromyalgia. Sci Total Environ. 2007;385(1):20–7. [DOI] [PubMed] [Google Scholar]
- 67.Sakarya ST, Akyol Y, Bedir A, Canturk F. The relationship between serum antioxidant vitamins, magnesium levels, and clinical parameters in patients with primary fibromyalgia syndrome. Clin Rheumatol. 2011;30(8):1039–43. 10.1007/s10067-011-1697-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Samborski W, Stratz T, Lacki JK, Mackiewicz SH, Mueller W. The serum concentration of calcitonin in patients with fibromyalgia—new therapeutic approach? Reumatologia (Warsaw). 1997;35(2):160–5. [Google Scholar]
- 69.Sendur OF, Tastaban E, Turan Y, Ulman C. The relationship between serum trace element levels and clinical parameters in patients with fibromyalgia. Rheumatol Int. 2008;28(11):1117–21. 10.1007/s00296-008-0593-9 [DOI] [PubMed] [Google Scholar]
- 70.Tandeter H, Grynbaum M, Zuili I, Shany S, Shvartzman P. Serum 25-OH vitamin D levels in patients with fibromyalgia. Isr Med Assoc J. 2009;11(6):339–42. [PubMed] [Google Scholar]
- 71.Turkyilmaz AK, Yalcinkaya EY, Ones K. The effects of bone mineral density and level of serum vitamin-D on pain and quality of life in fibromialgia patients (Fibromiyalji hastalarinda kemik mineral yogunlugu ile serum D vitamini duzeyinin agri ve yasam kalitesi uzerine etkisi). From the Osteoporosis World. 2010; 53–58. [Google Scholar]
- 72.Ulusoy H, Sarica N, Arslan S, Ozyurt H, Cetin I, Birgul OE, et al. Serum vitamin D status and bone mineral density in fibromyalgia. Bratisl Lek Listy. 2010;111(11):604–9. [PubMed] [Google Scholar]
- 73.Vecchiet J, Cipollone F, Falasca K, Mezzetti A, Pizzigallo E, Bucciarelli T, et al. Relationship between musculoskeletal symptoms and blood markers of oxidative stress in patients with chronic fatigue syndrome. Neurosci Lett. 2003;335(3):151–4. [DOI] [PubMed] [Google Scholar]
- 74.Wepner F, Scheuer R, Schuetz-Wieser B, Machacek P, Pieler-Bruha E, Cross HS, et al. Effects of vitamin D on patients with fibromyalgia syndrome: A randomized placebo-controlled trial. PAIN®. 2014;155(2):261–8. [DOI] [PubMed] [Google Scholar]
- 75.Witham M, Adams F, McSwiggan S, Kennedy G, Kabir G, Belch JJF, et al. Effect of intermittent vitamin D3 on vascular function and symptoms in chronic fatigue syndrome—A randomised controlled trial. Nutr Metab Cardiovasc Dis. 2015;25(3):287–94. 10.1016/j.numecd.2014.10.007 [DOI] [PubMed] [Google Scholar]
- 76.Yildirim T, Solmaz D, Akgol G, Ersoy Y. Relationship between mean platelet volume and vitamin D deficiency in fibromyalgia. Biomed Res. 2016;27(4): 1265–1270. [Google Scholar]
- 77.Nutrition Business Journal. NBJ's supplement business report: An analysis of markets, trends, competition and strategy in the U.S. dietary supplement industry. New York: Penton Media, 2011.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.