Abstract
Cell-surface cytokine receptors are regulated by their cis-interacting stimulatory and inhibitory co-receptors. We previously showed that the Ig-like cell-adhesion molecule nectin-4 cis-interacts with the prolactin receptor through the extracellular region and stimulates prolactin-induced prolactin receptor activation and signaling, resulting in alveolar development in the mouse mammary gland. However, it remains unknown how this interaction stimulates these effects. We show here that the cis-interaction of the extracellular region of nectin-4 with the prolactin receptor was not sufficient for eliciting these effects and that the cytoplasmic region of nectin-4 was also required for this interaction. The cytoplasmic region of nectin-4 directly interacted with suppressor of cytokine signaling 1 (SOCS1), but not SOCS3, JAK2, or STAT5a, and inhibited the interaction of SOCS1 with JAK2, eventually resulting in the increased phosphorylation of STAT5a. The juxtamembrane region of nectin-4 interacted with the Src homology 2 domain of SOCS1. Both the interaction of nectin-4 with the extracellular region of the prolactin receptor and the interaction of SOCS1 with the cytoplasmic region of nectin-4 were required for the stimulatory effect of nectin-4 on the prolactin-induced prolactin receptor activation. The third Ig-like domain of nectin-4 and the second fibronectin type III domain of the prolactin receptor were involved in this cis-interaction, and both the extracellular and transmembrane regions of nectin-4 and the prolactin receptor were required for this direct interaction. These results indicate that nectin-4 serves as a stimulatory co-receptor for the prolactin receptor by regulating the feedback inhibition of SOCS1 in the JAK2-STAT5a signaling pathway.
Keywords: cell adhesion, cell surface receptor, cell-cell interaction, Janus kinase (JAK), prolactin, STAT transcription factor, nectin, suppressor of cytokine signaling 1 (SOCS1)
Introduction
Cell-surface receptors are activated by interacting with their specific ligands. In addition, activity of many receptors is regulated by their specific transmembrane proteins, which are called co-receptors or accessory receptors (1, 2). Of these co-receptors, some of them cis-interact with the receptors on the same cell membrane. Integrins are one of the co-receptors modulating the activity of cis-interacting receptors (3–5). E-Cadherin also cis-interacts with the EGF receptor and modulates EGF-stimulated EGF receptor signaling (6). Thus, several cell-adhesion molecules play a role as cis-interacting co-receptors, and the cis-interactions of cell-adhesion molecules with cell-surface receptors are generally important for their proper localization, activation, and signaling.
It has previously been shown that the Ig-like cell-adhesion molecules with three Ig-like domains, nectins and nectin-like molecules (Necls),3 cis-interact with cell-surface membrane receptors and integrins and regulate their activation and signaling (7–9). Nectins comprise a family with four members (nectin-1, nectin-2, nectin-3, and nectin-4), whereas Necls comprise a family with five members (Necl-1, Necl-2, Necl-3, Necl-4, and Necl-5) (7–9). Nectins and Necls are defined on the basis of their ability to interact with afadin, an actin filament-interacting protein; nectins interact with afadin, whereas Necls do not. Although Necl-5 belongs to the Necl family, its functional and structural properties are more similar to those of nectins except that it does not interact with afadin (10). Nectin-1 cis-interacts with the FGF receptor (11) and integrin αvβ3 (12); nectin-3 cis-interacts with the PDGF receptor (13) and integrin αvβ3 (12, 14); and Necl-5 cis-interacts with the PDGF receptor (15–17), the VEGF receptor (18), and integrin αvβ3 (19). These nectins and Necl-5 show the stimulatory effects on these receptors and the integrin. In contrast, Necl-2 cis-interacts with ErbB3 (20), ErbB4 (21), and integrin α6β4 (22), and Necl-4 cis-interacts with ErbB3 and integrin α6β4 (23). These Necls show inhibitory effects on these receptors and the integrin. By cis-interacting with these membrane receptors and integrins, nectins and Necls play roles not only in cell adhesion, but also in many other cell functions, such as cell polarization, morphogenesis, movement, proliferation, differentiation, and survival (24).
In our prior study (25), we showed that nectin-4 served as a stimulatory co-receptor for the prolactin receptor in the mouse mammary gland. Mammary gland development is induced by the actions of various hormones to construct the structure consisting of collecting ducts and milk-secreting alveoli, which comprise two types of epithelial cells, known as luminal and basal cells (26). These cells adhere to each other by cell adhesion apparatuses, whose roles in hormone-dependent mammary gland development remained largely unknown. We identified a novel cell adhesion apparatus at the boundary between the luminal and basal cells, in addition to desmosomes (25). This apparatus was formed by the trans-interaction between nectin-4 and nectin-1, which were expressed in the luminal and basal cells, respectively. Nectin-4 of this apparatus further cis-interacted with the prolactin receptor through their extracellular regions in the luminal cells to stimulate the prolactin-induced prolactin receptor activation and signaling for alveolar development with lactogenic differentiation. However, it remained unresolved how this interaction stimulates the prolactin-induced prolactin receptor activation and signaling.
The prolactin receptor is a member of the cytokine receptors that lacks an intrinsic kinase domain but possesses a JAK2-associating region. Upon prolactin stimulation, the prolactin receptor transduces signals through the activation of JAK2, causing tyrosine 1007 phosphorylation in the activation loop of JAK2 (27). The cytoplasmic region of the prolactin receptor is phosphorylated by activated JAK2 and recruits the STAT family proteins, mainly STAT1, STAT3, and STAT5 (28). STAT5 has two isoforms, STAT5a and STAT5b (29). Stat5a-deficient mice are unable to lactate during pregnancy, suggesting an essential role for the development of the mammary gland (30). During the prolactin-induced prolactin receptor activation, STAT5a and STAT5b are also phosphorylated by JAK2 and thereby translocated from the cytosol into the nucleus and induce the transcription of many genes, including β-casein and whey protein, which are necessary for alveolar development (31, 32). The tyrosine-phosphorylated STAT5 further induces the transcription of suppressor of cytokine signaling 1 (SOCS1). SOCS1 interacts with the phosphorylated tyrosine at 1007 on JAK2 through the Src homology 2 (SH2) domain and then inhibits the JAK2 kinase activity through the kinase-inhibitory region of SOCS1 (33–35), thus serving as a negative feedback regulator for the prolactin-induced prolactin receptor activation and signaling.
In the present study, we show here that nectin-4 cis-interacts directly with the prolactin receptor through their extracellular and transmembrane regions and interacts with SOCS1 through the cytoplasmic region, blocking SOCS1 from interacting with JAK2, eventually stimulating the prolactin-induced prolactin receptor activation and signaling.
Results
Stimulation of the prolactin-induced prolactin receptor activation and signaling by the nectin-4 mutant lacking the extracellular region
We previously showed that nectin-4 cis-interacted with the prolactin receptor through their extracellular regions and stimulated the prolactin-induced tyrosine phosphorylation of the prolactin receptor and STAT5a in cultured EpH4 cells (25). However, we have not examined the role of the cytoplasmic region of nectin-4. We therefore first examined the effect of the nectin-4 mutant lacking the extracellular region (Nectin-4-ΔEC) on the prolactin-induced prolactin receptor activation and signaling by measuring the phosphorylation of STAT5a. When FLAG-tagged Nectin-4-ΔEC (FLAG-Nectin-4-ΔEC) or the empty vector (control) was stably expressed in EpH4 cells and the cells were stimulated with prolactin, STAT5a was phosphorylated in a time-dependent manner. This phosphorylation of STAT5a was enhanced in the cells stably expressing FLAG-Nectin-4-ΔEC, compared with that in the control cells (Fig. 1). These results indicate that the cytoplasmic region of nectin-4 alone has the activity to stimulate the prolactin-induced prolactin receptor activation and signaling.
Figure 1.
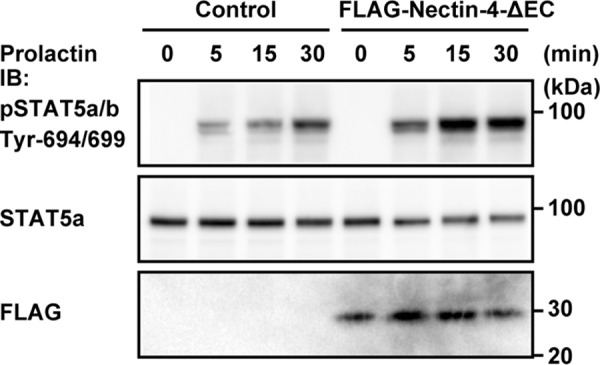
Stimulatory effect of the nectin-4 mutant lacking the extracellular region on the prolactin-induced phosphorylation of STAT5a. EpH4 cells stably expressing the FLAG-tagged nectin-4 mutant lacking the extracellular region (FLAG-Nectin-4-ΔEC) or an empty vector (Control) were cultured on Matrigel-coated dishes and stimulated with 3 μg/ml prolactin for the indicated periods of time. The samples (25 μg of protein each, including Matrigel) were subjected to Western blotting using the indicated Abs. The results are representative of four independent experiments. IB, immunoblotting; pSTAT5, phospho-STAT5.
Interaction of SOCS1 with the cytoplasmic region of nectin-4
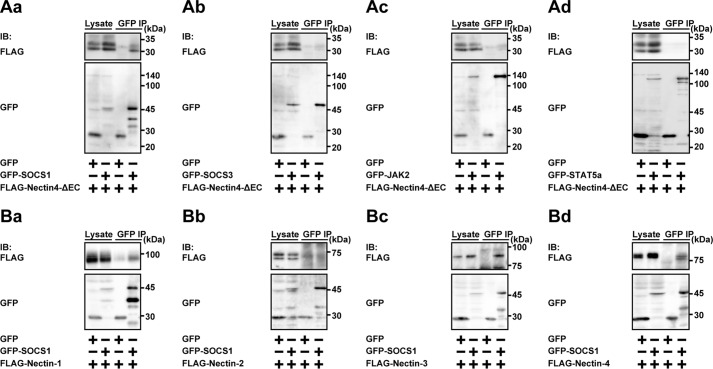
We previously showed that the cytoplasmic region of nectin-4 did not interact with the prolactin receptor (25). However, the above results raised the possibility that the cytoplasmic region of nectin-4 interacts with the prolactin receptor signaling molecules, such as JAK2, STAT5a, SOCS1, and/or SOCS3, and modulates its or their signaling. To identify the protein interacting with the cytoplasmic region of nectin-4, either GFP-tagged SOCS1 (GFP-SOCS1), GFP-tagged SOCS3 (GFP-SOCS3), GFP-tagged JAK2 (GFP-JAK2), or GFP-tagged STAT5a (GFP-STAT5a) was co-expressed with FLAG-Nectin-4-ΔEC in HEK293E cells. When GFP or GFP-SOCS1 was immunoprecipitated using the anti-GFP antibody (Ab), FLAG-Nectin-4-ΔEC was co-immunoprecipitated with GFP-SOCS1 but not with GFP (Fig. 2Aa). FLAG-Nectin-4-ΔEC was not co-immunoprecipitated with GFP-SOCS3 (Fig. 2Ab), GFP-JAK2 (Fig. 2Ac), or GFP-STAT5a (Fig. 2Ad). These results indicate that nectin-4 specifically interacts with SOCS1 through the cytoplasmic region. It was noted that the interaction of SOCS1 with nectin-4 was specific for SOCS1, because FLAG-Nectin-4-ΔEC was not co-immunoprecipitated with GFP-SOCS3 (Fig. 2, Aa and Ab).
Figure 2.
Interaction of SOCS1 with the cytoplasmic region of nectin-4. A, interaction of SOCS1 with the nectin-4 mutant lacking the extracellular region. HEK293E cells were transfected with various combinations of the indicated plasmids. GFP and the GFP-tagged proteins were immunoprecipitated from each lysate using the anti-GFP Ab, and each sample was subjected to Western blotting using the indicated Abs. Aa, GFP-tagged SOCS1 (GFP-SOCS1); Ab, GFP-tagged SOCS3 (GFP-SOCS3); Ac, GFP-tagged JAK2 (GFP-JAK2); Ad, GFP-tagged STAT5a (GFP-STAT5a). B, interaction of SOCS1 with the nectin family members. GFP or GFP-SOCS1 was co-expressed with FLAG-tagged nectin-1 (FLAG-Nectin-1), FLAG-tagged nectin-2 (FLAG-Nectin-2), FLAG-tagged nectin-3 (FLAG-Nectin-3), or FLAG-tagged nectin-4 (FLAG-Nectin-4) in HEK293E cells. GFP and the GFP-tagged proteins were immunoprecipitated from each lysate using the anti-GFP Ab, and each sample was subjected to Western blotting using the indicated Abs. Ba, FLAG-Nectin-1; Bb, FLAG-Nectin-2; Bc, FLAG-Nectin-3; Bd, FLAG-Nectin-4. The results are representative of two independent experiments. IB, immunoblotting; IP, immunoprecipitation.
We then tested whether the nectin family members other than nectin-4 interact with SOCS1. GFP or GFP-SOCS1 was co-expressed with FLAG-tagged nectin-1 (FLAG-Nectin-1), nectin-2 (FLAG-Nectin-2), nectin-3 (FLAG-Nectin-3), or nectin-4 (FLAG-Nectin-4) in HEK293E cells. When GFP-SOCS1 was immunoprecipitated from each cell lysate using the anti-GFP Ab, FLAG-Nectin-3 and FLAG-Nectin-4 were co-immunoprecipitated with GFP-SOCS1 to similar extents (Fig. 2, Bc and Bd), and FLAG-Nectin-1 was immunoprecipitated with GFP-SOCS1 to a lesser extent than observed for FLAG-Nectin-3 and FLAG-Nectin-4 (Fig. 2Ba). FLAG-Nectin-2 was not co-immunoprecipitated with GFP-SOCS1 (Fig. 2Bb). These results indicate that SOCS1 interacts with nectin-1, nectin-3, and nectin-4 but not nectin-2.
Involvement of SOCS1 in the stimulatory effect of nectin-4 on the prolactin-induced prolactin receptor activation and signaling
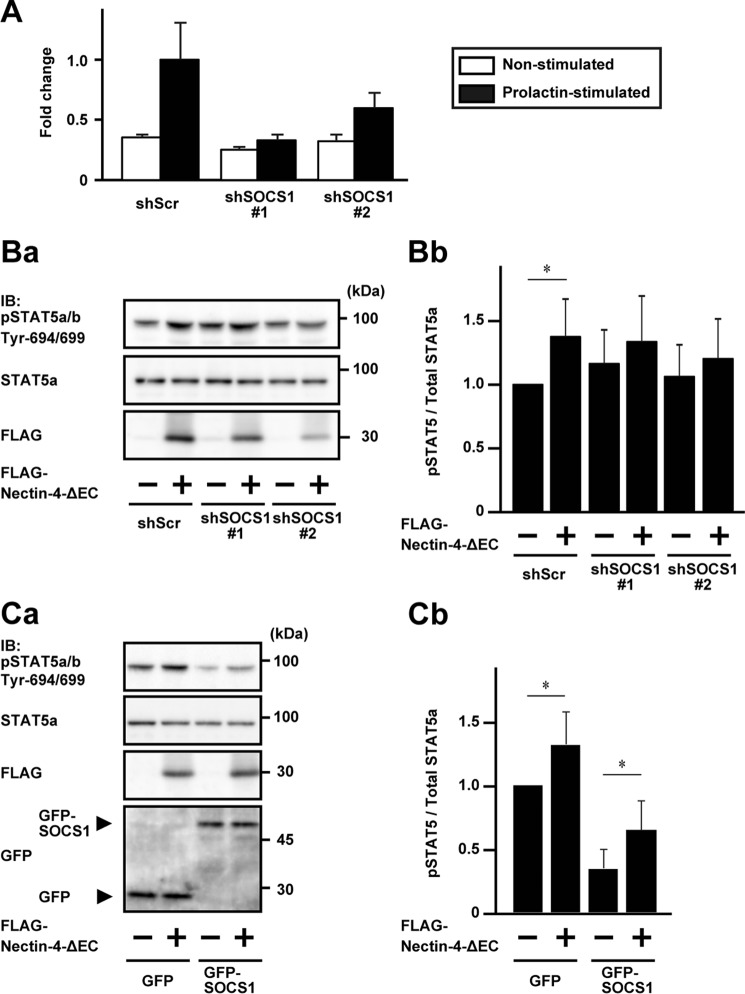
We then examined whether SOCS1 is indeed involved in this stimulatory effect of nectin-4 on the prolactin-induced prolactin receptor activation and signaling. For this purpose, SOCS1 was knocked down by expressing two different shRNAs (shSOCS1#1 and shSOCS1#2) in EpH4 cells. The knockdown efficiency was then estimated by the semiquantitative real-time PCR analysis before and after the prolactin stimulation. The increased expression of SOCS1 after the prolactin stimulation was reduced to about 33 and 60% levels by the stable expression of shSOCS1#1 and shSOCS1#2, respectively, compared with that of the scramble shRNA (shScr) control (Fig. 3A). The ratios of the levels of the prolactin-induced increases in the phosphorylation of STAT5a were further increased in the EpH4 cells stably expressing either of these shRNAs for SOCS1, compared with those in the cells stably expressing shScr, and the phosphorylation of STAT5a was increased in accordance with the knockdown efficiency of SOCS1 (Fig. 3, Ba and Bb).
Figure 3.
Involvement of SOCS1 in the stimulatory effect of nectin-4 on the prolactin-induced phosphorylation of STAT5a. A, knockdown validation of SOCS1. EpH4 cells stably expressing an shRNA for SOCS1 (shSOCS1#1 or shSOCS1#2) and shScr were stimulated with or without 3 μg/ml prolactin for 30 min, and the total RNA was extracted. The relative expression levels of the SOCS1 mRNA were determined by semiquantitative real-time PCR using the GAPDH mRNA as a reference mRNA. The value of the SOCS1 mRNA in the prolactin-stimulated shScr was set at 1.0. Error bars, S.D. of triplicates. B, increased phosphorylation of STAT5a by the knockdown of SOCS1. Ba, Western blotting of the phosphorylation of STAT5a. EpH4 cells stably expressing shSOCS1#1, shSOCS1#2, or shScr were co-expressed stably with FLAG-Nectin-4-ΔEC or the vector control. The cells were cultured on Matrigel-coated dishes and stimulated with 3 μg/ml prolactin for 15 min. Each sample (25 μg of protein each, including Matrigel) was subjected to Western blotting using the indicated Abs. The results are representative of four independent experiments. Bb, quantification of the phosphorylation of STAT5a. The intensity of the phosphorylation of STAT5a was normalized to the total amount of the STAT5a protein, and the value of the cells that expressed shScr, but not FLAG-Nectin-4-ΔEC, was set at 1.0 as a reference in each blotting. Error bars, S.D. of quadruplicates. C, enhancement of the phosphorylation of STAT5a by the expression of Nectin-4-ΔEC in the cells expressing SOCS1. Ca, Western blotting of the phosphorylation of STAT5a. EpH4 cells stably expressing GFP or GFP-SOCS1 were co-expressed stably with FLAG-Nectin-4-ΔEC or the vector control. The cells were cultured on Matrigel-coated dishes and stimulated with 3 μg/ml prolactin for 15 min. Each sample (25 μg of protein each, including Matrigel) was subjected to Western blotting using the indicated Abs. Cb, quantification of the phosphorylation of STAT5a. The intensity of the phosphorylation of STAT5a was normalized to the total amount of STAT5a protein, and the value of the cells that expressed GFP, but not FLAG-Nectin-4-ΔEC, was set at 1.0 as a reference in each blotting. Error bars, S.D. of quadruplicates. *, p < 0.05. IB, immunoblotting; IP, immunoprecipitation; pSTAT5, phospho-STAT5.
We then examined the effect of FLAG-Nectin-4-ΔEC on the prolactin-induced phosphorylation of STAT5a in the EpH4 cells expressing either of these two shRNAs for SOCS1. The phosphorylation of STAT5a was significantly enhanced by stably expressing FLAG-Nectin-4-ΔEC in the cells stably expressing shScr, whereas the phosphorylation of STAT5a was slightly, but not significantly, enhanced in the cells stably expressing FLAG-Nectin-4-ΔEC in the SOCS1-knockdown cells (Fig. 3, Ba and Bb). The reason why the phosphorylation of STAT5a was slightly increased by the stable expression of FLAG-Nectin-4-ΔEC in the SOCS1-knockdown cells is not known, but it may be due to incomplete abrogation of the expression of SOCS1 and to a remaining small amount of SOCS1. In addition, the expression level of FLAG-Nectin-4-ΔEC in the cells stably expressing shSOCS1#2 was lower than that in the cells stably expressing shScr or shSOCS1#1. The slight enhancement of the phosphorylation of STAT5a might be also due to this lower expression of FLAG-Nectin-4-ΔEC.
We further established the EpH4 cells stably expressing GFP-SOCS1 to examine the effect of the Nectin-4-ΔEC on the prolactin-induced phosphorylation of STAT5a. The strong reduction of the phosphorylation of STAT5a after the prolactin stimulation was observed in the cells stably expressing GFP-SOCS1, but not in the cells stably expressing GFP. The phosphorylation of STAT5a was significantly enhanced by the stable expression of Nectin-4-ΔEC (Fig. 3, Ca and Cb). These results indicate that SOCS1 is involved in the stimulatory effect of nectin-4 on the prolactin-induced prolactin receptor activation and signaling.
Identification of the interacting regions of nectin-4 and SOCS1
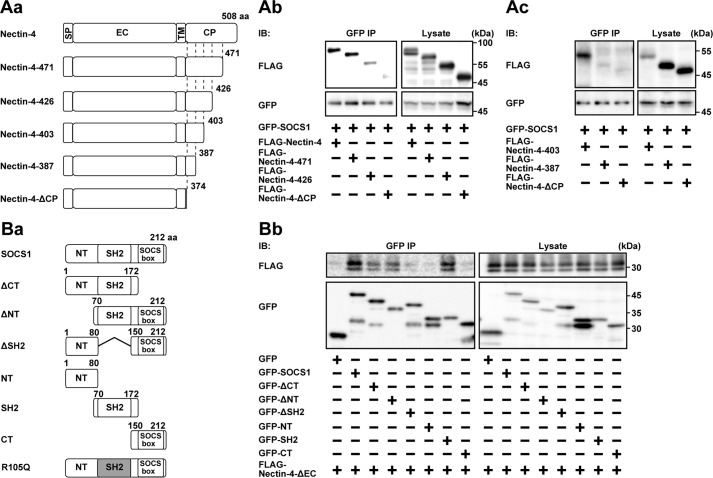
The domain structures of FLAG-Nectin-4 and GFP-SOCS1 and their mutants to be tested are shown in Fig. 4 (Aa and Ba). When GFP-SOCS1 was co-expressed with FLAG-Nectin-4 in HEK293E cells and was immunoprecipitated from each lysate using the anti-GFP Ab, FLAG-Nectin-4 was co-immunoprecipitated with GFP-SOCS1 (Fig. 4Ab). When similar experiments were performed using the FLAG-tagged nectin-4 mutant encoding amino acids (aa) 30–471 (FLAG-Nectin-4-471), aa 30–426 (FLAG-Nectin-4-426), aa 30–403 (FLAG-Nectin-4-403), aa 30–387 (FLAG-Nectin-4-387), or the FLAG-tagged nectin-4 mutant lacking the cytoplasmic region (FLAG-Nectin-4-ΔCP; encoding aa 30–374), the mutants such as FLAG-Nectin-4-471, FLAG-Nectin-4-426, and FLAG-Nectin-4-403, but not FLAG-Nectin-4-387 and FLAG-Nectin-4-ΔCP, were co-immunoprecipitated with GFP-SOCS1 (Fig. 4, Ab and Ac). However, the amount of FLAG-Nectin-4-426 co-immunoprecipitated with GFP-SOCS1 was markedly reduced, compared with that of full-length FLAG-Nectin-4 or FLAG-Nectin-4-471. Taken together, these results suggest that the juxtamembrane region of nectin-4 spanning from aa 388 to 403 is essential for the interaction with SOCS1 and that the region from aa 427 to 471 in nectin-4 may be additionally required for the efficient interaction with SOCS1.
Figure 4.
The interacting regions of nectin-4 and SOCS1. A, the region of nectin-4 interacting with SOCS1. Aa, schematics of the domain structures of nectin-4 and its mutants. Ab and Ac, identification of the SOCS1-interacting region of nectin-4. HEK293E cells were transfected with various combinations of the indicated plasmids. GFP-SOCS1 was immunoprecipitated from each lysate using the anti-GFP Ab, and each sample was subjected to Western blotting using the indicated Abs. B, the region of SOCS1 interacting with nectin-4. Ba, schematics of the domain structures of SOCS1 and its mutants. Bb, identification of the nectin-4-interacting region of SOCS1. HEK293E cells were transfected with various combinations of the indicated plasmids. GFP, GFP-SOCS1, and the mutants were immunoprecipitated from each lysate using the anti-GFP Ab, and each sample was subjected to Western blotting using the indicated Abs. The results are representative of two independent experiments. SP, signal peptide; EC, extracellular; TM, transmembrane; CP, cytoplasmic; IB, immunoblotting; IP, immunoprecipitation.
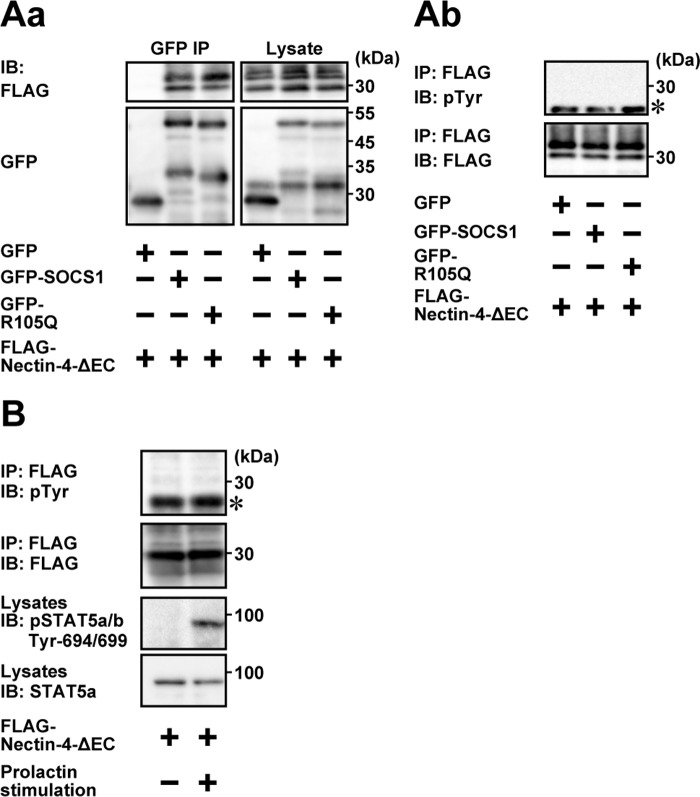
When GFP-SOCS1 was co-expressed with FLAG-Nectin-4-ΔEC in HEK293E cells and was immunoprecipitated from each lysate using the anti-GFP Ab, FLAG-Nectin-4-ΔEC was co-immunoprecipitated with GFP-tagged full-length SOCS1 (Fig. 4Bb). When similar experiments were performed using the mutant of SOCS1 encoding aa 1–172 (GFP-SOCS1-ΔCT), aa 70–212 (GFP-SOCS1-ΔNT), aa 1–80 and 150–212 (GFP-SOCS1-ΔSH2), aa 1–80 (GFP-SOCS1-NT), aa 70–172 (GFP-SOCS1-SH2), or aa 150–212 (GFP-SOCS1-CT), FLAG-Nectin-4-ΔEC was co-immunoprecipitated with GFP-SOCS1-ΔCT, GFP-SOCS1-ΔNT, and GFP-SOCS1-SH2, but not with GFP-SOCS1-ΔSH2, GFP-SOCS1-NT, or GFP-SOCS1-CT (Fig. 4Bb). The amount of Nectin-4-ΔEC co-immunoprecipitated with GFP-SOCS1-ΔCT or GFP-SOCS1-ΔNT was less than that of full-length SOCS1, suggesting that the N-terminal and C-terminal regions of SOCS1 have a cooperative role in this interaction. Taken together, these results indicate that the SH2 domain of SOCS1 is necessary for its interaction with nectin-4. When similar experiments were performed using the GFP-tagged SOCS1 mutant R105Q (GFP-R105Q), which is a defective mutant for the interaction with the phosphorylated tyrosine (36), it was also co-immunoprecipitated with FLAG-Nectin-4-ΔEC to an extent similar to that with wild-type GFP-SOCS1 (Fig. 5Aa). When FLAG-Nectin-4-ΔEC was immunoprecipitated using the anti-FLAG Ab and immunoblotted with the anti-phosphotyrosine Ab, the tyrosine phosphorylation of FLAG-Nectin-4-ΔEC was not detected in the condition where FLAG-Nectin-4-ΔEC interacted with GFP-R105Q (Fig. 5Ab). Furthermore, the tyrosine phosphorylation of FLAG-Nectin-4-ΔEC was not observed after the prolactin stimulation in EpH4 cells (Fig. 5B). Taken together, these results indicate that the interaction of Nectin-4-ΔEC with SOCS1 is not dependent on the phosphorylation of Nectin-4-ΔEC.
Figure 5.
Phosphotyrosine-independent interaction of SOCS1 with nectin-4. A, interaction of the SH2 domain mutant of SOCS1 R105Q with non-phosphorylated nectin-4. Aa, assay for co-immunoprecipitation of GFP-R105Q with FLAG-Nectin-4-ΔEC. HEK293E cells were transfected with various combinations of the indicated plasmids. GFP, GFP-SOCS1, and GFP-R105Q were immunoprecipitated from each lysate using the anti-GFP Ab, and each sample was subjected to Western blotting using the indicated Abs. Ab, no detectable phosphorylation of nectin-4. FLAG-Nectin-4-ΔEC was immunoprecipitated from each lysate in a using the anti-FLAG Ab, and each sample was subjected to Western blotting using the indicated Abs. The results are representative of two independent experiments. B, no detectable phosphorylation of nectin-4 by the prolactin stimulation. EpH4 cells stably expressing FLAG-Nectin-4-ΔEC were cultured on Matrigel-coated dishes and stimulated with or without 3 μg/ml prolactin for 15 min. FLAG-Nectin-4-ΔEC was immunoprecipitated from each lysate using the anti-FLAG Ab, and one-fifth of the immunoprecipitated protein sample as well as the total lysate sample (25 μg of protein each, including Matrigel) was subjected to Western blotting using the indicated Abs. The results are representative of two independent experiments. IB, immunoblotting; IP, immunoprecipitation; pTyr, phosphotyrosine. *, light chain of the FLAG Ab.
Inhibition of the interaction of SOCS1 to JAK2 by nectin-4
JAK2 has an N-terminal FERM domain, an SH2 domain, a pseudokinase domain (JH2), and a C-terminal kinase domain (JH1), as schematically shown in Fig. 6A. The SH2 domain of SOCS1 interacts with the phosphorylated tyrosine at 1007 in the JH1 domain of JAK2 and inhibits the activation of JAK2 (37). Since SOCS1 interacted with the cytoplasmic region of nectin-4 through the SH2 domain, we hypothesized that the cytoplasmic region of nectin-4 inhibits the interaction of JAK2 with SOCS1 in a competitive manner as schematically shown in Fig. 6B. To examine this hypothesis, we utilized the activated form of TEL-JAK2 in which the tyrosine at 1007 is constitutively phosphorylated (38). FLAG-tagged TEL-JAK2 (FLAG-TEL-JAK2) and the GST-tagged cytoplasmic region of nectin-4 (GST-Nectin-4-CP) were expressed in HEK293E cells and Escherichia coli, respectively, and were purified. When they were resolved by SDS-PAGE, followed by Coomassie Brilliant Blue (CBB) staining, FLAG-TEL-JAK2 showed a major band with several minor bands, whereas GST-Nectin-4-CP showed a major band with two minor bands (Fig. 6, Ca and Cb). GFP-SOCS1 was then captured on anti-GFP-Ab-conjugated beads, and the beads were incubated with a fixed amount of FLAG-TEL-JAK2 and various amounts of GST-Nectin-4-CP, followed by precipitation of the beads. The amounts of FLAG-TEL-JAK2 co-precipitated with GFP-SOCS1 were reduced in the presence of GST-Nectin-4-CP in a dose-dependent manner (Fig. 6D). These results indicate that the cytoplasmic region of nectin-4 inhibits the interaction of JAK2 with SOCS1 in a competitive manner.
Figure 6.
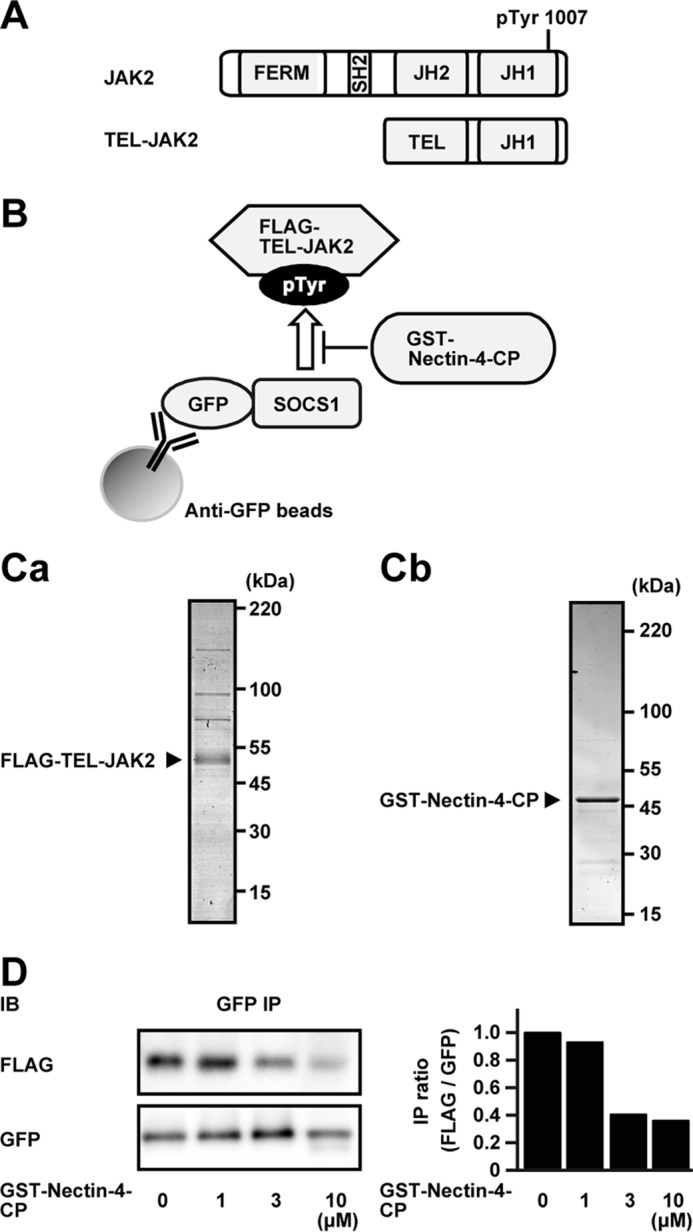
Inhibition of the interaction of SOCS1 with JAK2 by nectin-4. A, schematics of the domain structures of JAK2 and TEL-JAK2. B, schematics of the experimental design. C, purification of FLAG-TEL-JAK2 and GST-Nectin-4-CP. Ca, purity of FLAG-TEL-JAK2. FLAG-TEL-JAK2 was expressed in HEK293E cells and purified with anti-FLAG affinity chromatography. The protein (0.25 μg of protein) was resolved by SDS-PAGE and stained with CBB. Cb, purity of GST-Nectin-4-CP. GST-Nectin-4-CP was expressed in E. coli and purified with glutathione-Sepharose affinity chromatography. The protein (0.75 μg of protein) was resolved by SDS-PAGE and stained with CBB. D, inhibition of the interaction of SOCS1 to JAK2 by nectin-4. GFP-SOCS1 was immobilized on the anti-GFP-Ab-conjugated beads. The beads were mixed with 50 nm FLAG-TEL-JAK2 and increasing concentrations of GST-Nectin-4-CP, as indicated at the bottom, in a total volume of 30 μl at 4 °C for 2 h with gentle rotation. The beads were precipitated, and each protein was subjected to Western blotting using the indicated Abs. The results are representative of three independent experiments. The right panel indicates bar graphs of the immunoprecipitation ratio of FLAG/GFP (FLAG-TEL-JAK2/GFP-SOCS1). The ratio of FLAG/GFP at 0 μm GST-Nectin-4-CP was set at 1.0 as a reference. JH1, kinase domain; JH2, pseudokinase domain; IB, immunoblotting; IP, immunoprecipitation; pTyr, phosphorylated tyrosine.
Direct cis-interaction of nectin-4 with the prolactin receptor and requirement of their transmembrane regions for this interaction
We have not examined whether the interaction of nectin-4 with the prolactin receptor is direct or indirect. To address this issue, we expressed the FLAG-tagged extracellular region of nectin-4 (FLAG-Nectin-4-EC), of which the N terminus was fused to the signal sequence to be secreted to the medium, in HEK293E cells (Fig. 7A, schematic representation). The extracellular region of the prolactin receptor, of which the C terminus was fused with the Fc portion of human IgG (PRLR-EC-Fc), was expressed in HEK293E cells. Each protein was affinity-purified and stained with CBB (Fig. 7Ba). When these two proteins were incubated and the PRLR-EC-Fc was precipitated by protein A-beads, FLAG-Nectin-4-EC was not co-precipitated (Fig. 7Bb). However, when similar experiments were performed using the extracellular and transmembrane regions of nectin-4 fused with FLAG tag (FLAG-Nectin-4-ΔCP) and the extracellular and transmembrane regions of the prolactin receptor fused with Fc tag (PRLR-ΔCP-Fc), FLAG-Nectin-4-ΔCP was co-precipitated with PRLR-ΔCP-Fc (Fig. 7Bb). When similar experiments were performed using the FLAG-Nectin-4-ΔCP and the PRLR-EC-Fc, the FLAG-Nectin-4-ΔCP was not co-precipitated with the PRLR-EC-Fc (Fig. 7Bb). Inversely, when the similar experiments were performed using the FLAG-Nectin-4-EC and the PRLR-ΔCP-Fc, FLAG-Nectin-4-EC was not co-precipitated with the PRLR-ΔCP-Fc (Fig. 7Bb). These results indicate that both the extracellular and transmembrane regions of nectin-4 and the prolactin receptor are required for their interaction and that this interaction is direct.
Figure 7.
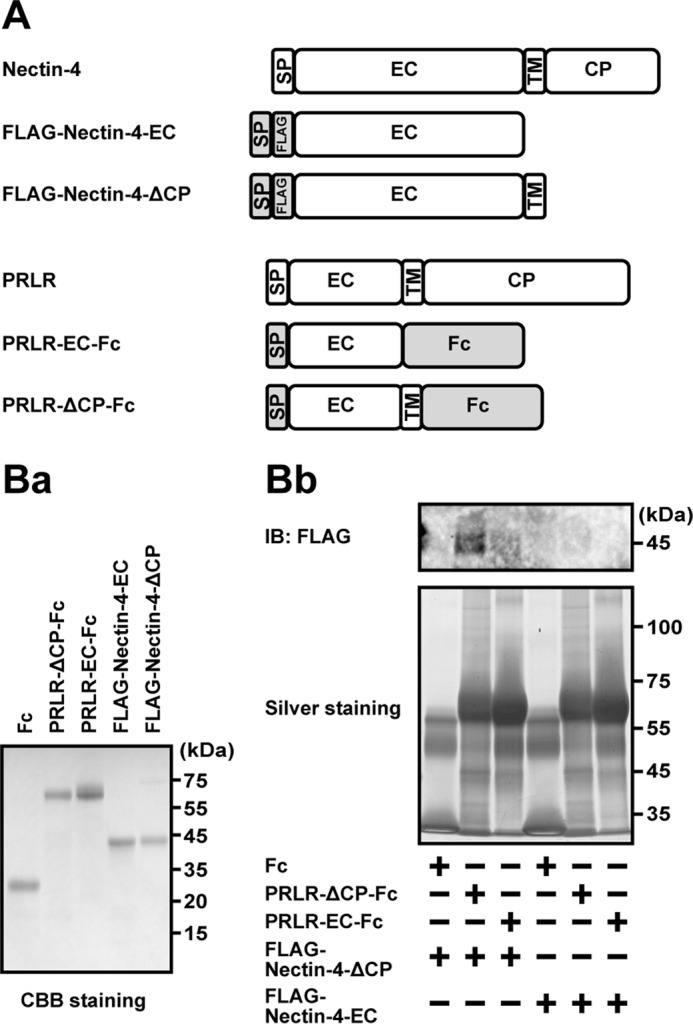
Direct interaction of nectin-4 with the prolactin receptor. A, schematics of the domain structures of nectin-4, the PRLR, and their mutants. B, interaction of nectin-4 with PRLR. Ba, purity of the recombinant proteins used in this assay. Each purified recombinant protein (1 μg of protein each) was resolved by SDS-PAGE and stained with CBB. Bb, direct interaction of FLAG-Nectin-4-ΔCP with PRLR-ΔCP-Fc. The protein A-beads were conjugated with 50 pmol of Fc tag, PRLR-EC-Fc, and PRLR-ΔCP-Fc and mixed with 5 μm FLAG-Nectin-4-ΔCP or FLAG-Nectin-4-EC in a total volume of 30 μl at 4 °C for 2 h with gentle rotation. Each Fc-fusion protein was precipitated, and each interacted protein was subjected to silver staining and Western blotting using the anti-FLAG Ab. The results are representative of three independent experiments. SP, signal peptide; EC, extracellular; TM, transmembrane; CP, cytoplasmic; IB, immunoblotting; IP, immunoprecipitation.
Identification of the interacting regions of nectin-4 and the prolactin receptor
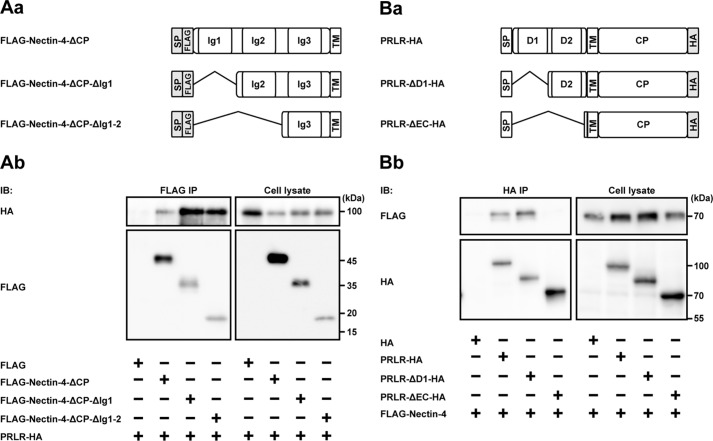
We next examined the interacting regions of nectin-4 and the prolactin receptor by expressing various mutants of each protein with different tags in HEK293E cells, followed by an immunoprecipitation assay. Domain structures of nectin-4 and the prolactin receptor and their truncated mutants to be tested are schematically shown in Fig. 8 (Aa and Ba, respectively). When the extracellular region of FLAG-tagged nectin-4 containing all three Ig-like domains (FLAG-Nectin-4-ΔCP) or the FLAG-tagged nectin-4 mutant lacking the first Ig-like domain (FLAG-Nectin-4-ΔCP-ΔIg1) or the first and second Ig-like domains (FLAG-Nectin-4-ΔCP-ΔIg1-2) was co-expressed with HA-tagged full-length prolactin receptor (PRLR-HA) in HEK293E cells and was immunoprecipitated from each lysate using the anti-FLAG Ab, all of these nectin-4 molecules were co-immunoprecipitated with PRLR-HA (Fig. 8Ab). Because the nectin-4 mutants lacking the extracellular region did not interact with the prolactin receptor (25), these results indicate that the third Ig-like domain of nectin-4, but not the first and second Ig-like domains, is required for its interaction with the prolactin receptor, although it could not be concluded from these results alone that the first or second Ig-like domain of nectin-4 does not interact with the prolactin receptor. However, the results shown in Fig. 9 excluded this possibility, and taking together these results, it is likely that the third Ig-like domain of nectin-4 interacts with the prolactin receptor.
Figure 8.
The interacting regions of nectin-4 and the prolactin receptor. A, the region of nectin-4 interacting with the PRLR. Aa, schematics of the domain structures of nectin-4 and its mutants. Ab, identification of the PRLR-interacting region of nectin-4. HEK293E cells were transfected with various combinations of the indicated plasmids and cultured in suspension. The FLAG-tagged nectin-4 or each of its mutants was immunoprecipitated from each lysate using the anti-FLAG Ab. Each sample was subjected to Western blotting using the indicated Abs. B, the region of the PRLR interacting with nectin-4. Ba, schematics of the domain structures of the PRLR and its mutants. Bb, identification of the nectin-4-interacting region of the PRLR. HEK293E cells were transfected with various combinations of the indicated plasmids and cultured in suspension. The HA-tagged PRLR or each of its mutants was immunoprecipitated from each lysate using the anti-HA Ab. Each sample was subjected to Western blotting using the indicated Abs. The results are representative of three independent experiments. SP, signal peptide; TM, transmembrane; CP, cytoplasmic; IB, immunoblotting; IP, immunoprecipitation.
Figure 9.
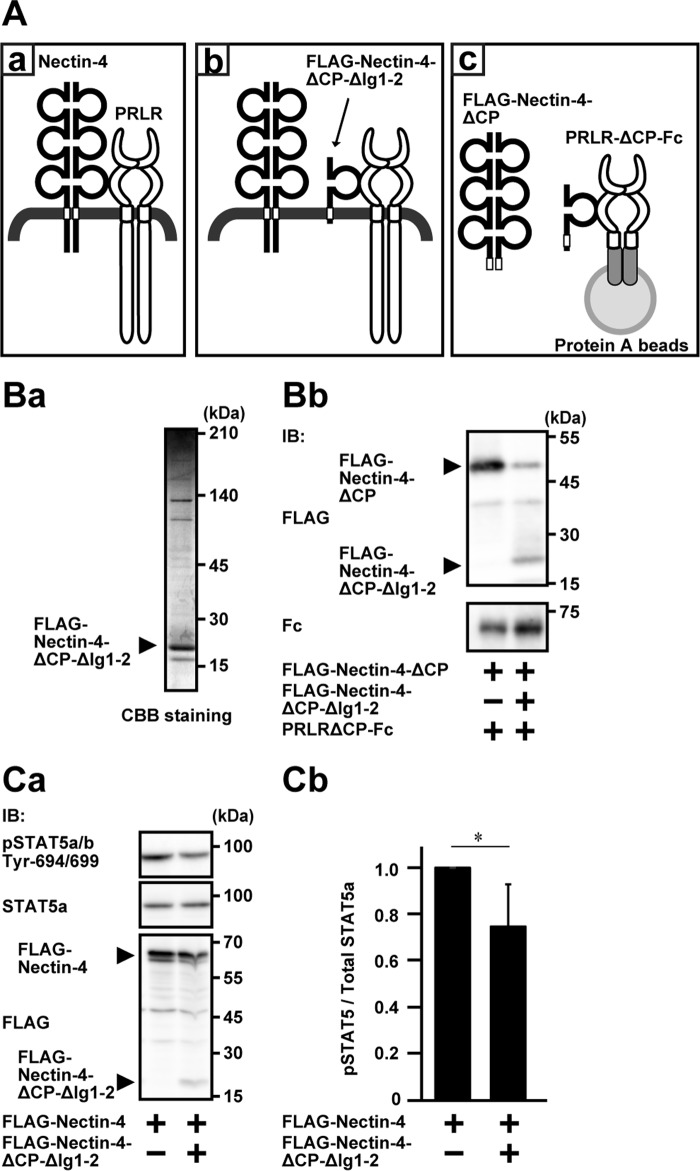
Requirement of the cis-interaction of the extracellular region of nectin-4 with the prolactin receptor for the prolactin-induced prolactin receptor activation and signaling. A, schematics of the interaction of nectin-4 with the PRLR and the experimental design. Aa, the interaction of full-length nectin-4 with the PRLR; Ab, inhibition of the interaction of full-length nectin-4 with the PRLR by the third Ig-like domain (FLAG-Nectin-4-ΔCP-ΔIg1-2); Ac, the experimental design. B, purification of FLAG-Nectin-4-ΔCP-ΔIg1-2 and inhibition of the interaction of full-length nectin-4 with the PRLR. Ba, purity of FLAG-Nectin-4-ΔCP-ΔIg1-2. FLAG-Nectin-4-ΔCP-ΔIg1-2 was expressed in HEK293E cells and purified with anti-FLAG affinity chromatography. The protein (0.75 μg of protein) was resolved by SDS-PAGE and stained with CBB. Bb, inhibition of the interaction of full-length nectin-4 with the PRLR. Five pmol of the PRLR mutant lacking the cytoplasmic region tagged with Fc (PRLR-ΔCP-Fc) was immobilized on protein A-Sepharose beads. The beads were resuspended in PBS containing 0.5 μm FLAG-tagged nectin-4 mutant lacking the cytoplasmic region (FLAG-Nectin-4-ΔCP) in the presence or absence of 5 μm FLAG-Nectin-4-ΔCP-ΔIg1-2 and incubated in a total volume of 30 μl at 4 °C for 2 h. Each interacted protein was resolved by SDS-PAGE and subjected to Western blotting using the indicated Abs. The results are representative of two independent experiments. C, inhibition by FLAG-Nectin-4-ΔCP-ΔIg1-2 of the stimulatory effect of nectin-4 on the prolactin-induced phosphorylation of STAT5a in EpH4 cells. Ca, Western blotting of the phosphorylation of STAT5a. EpH4 cells stably expressing shRNA for mouse nectin-1 were co-expressed stably with FLAG-Nectin-4 with or without FLAG-Nectin-4-ΔCP-ΔIg1-2. The cells were cultured on Matrigel-coated dishes and stimulated with 3 μg/ml prolactin for 15 min. The samples (25 μg of protein each, including Matrigel) were subjected to Western blotting using the indicated Abs. The results are representative of three independent experiments. Cb, quantification of the phosphorylation of STAT5a. The intensity of the phosphorylation of STAT5a was normalized to the total amount of STAT5a protein, and the value of the cells, which did not express FLAG-Nectin-4-ΔCP-ΔIg1-2, was set at 1.0 as a reference in each blotting. *, p < 0.05. Error bars, S.D. of triplicates. IB, immunoblotting; IP, immunoprecipitation; pSTAT5, phospho-STAT5.
The prolactin receptor contains two fibronectin type III domains at the extracellular region (39). To explore the nectin-4-interacting domain of the prolactin receptor, the mutant lacking the first fibronectin type III domain (PRLR-ΔD1-HA) and the mutant lacking the first and second fibronectin type III domains (PRLR-ΔEC-HA) were constructed (Fig. 8Ba). When the PRLR-HA or each of its various truncated mutants was co-expressed with FLAG-Nectin-4 and was immunoprecipitated from each lysate using the anti-HA Ab, FLAG-Nectin-4 was co-immunoprecipitated with PRLR-HA and the PRLR-ΔD1-HA, but not with the PRLR-ΔEC-HA (Fig. 8Bb). Since the extracellular region of nectin-4, but not the cytoplasmic region, was required for its interaction with the prolactin receptor, these results indicate that the second fibronectin type III domain, but not the first fibronectin type III domain, of the prolactin receptor is required for its interaction with nectin-4, although it could not be concluded from these results alone that the first fibronectin type III domain does not interact with nectin-4.
Requirement of the cis-interaction of the extracellular region of nectin-4 with the prolactin receptor for its stimulatory effect on the prolactin-induced prolactin receptor activation and signaling
We finally examined whether the cis-interaction of the nectin-4 with the prolactin receptor through their extracellular and transmembrane regions is required for the stimulatory effect of nectin-4 on the prolactin-induced prolactin receptor activation and signaling. For this purpose, we utilized the FLAG-Nectin-4-ΔCP-ΔIg1-2 as a competitive inhibitor for the cis-interaction of nectin-4 with the prolactin receptor, as schematically shown in Fig. 9 (Aa and Ab), because this region was a minimal interacting region with the prolactin receptor, as shown in Fig. 8Ab. We first confirmed that the FLAG-Nectin-4-ΔCP-ΔIg1-2 showed an inhibitory effect on the interaction of nectin-4 with the prolactin receptor. The PRLR-ΔCP-Fc and the FLAG-Nectin-4-ΔCP, which were used in the direct interaction assay, were used in this experiment as schematically shown in Fig. 9Ac. FLAG-Nectin-4-ΔCP-ΔIg1-2 was expressed in HEK293E cells and affinity-purified. When it was resolved by SDS-PAGE, followed by CBB staining, it showed a major band with several minor bands (Fig. 9Ba). PRLR-ΔCP-Fc was captured on protein A beads, and the fixed amount of FLAG-Nectin-4-ΔCP was incubated with or without FLAG-Nectin-4-ΔCP-ΔIg1-2. The amount of Nectin-4-ΔCP co-precipitated with PRLR-ΔCP-Fc was reduced in the presence of FLAG-Nectin-4-ΔCP-ΔIg1-2 (Fig. 9Bb). FLAG-Nectin-4-ΔCP-ΔIg1-2 was co-immunoprecipitated with PRLR-ΔCP-Fc (Fig. 9Bb). These results indicate that the third Ig-like domain of nectin-4 with the transmembrane region interacts with the prolactin receptor and inhibits the cis-interaction of nectin-4 with the prolactin receptor in a competitive manner.
We then examined by measuring the phosphorylation of STAT5a to determine whether the FLAG-Nectin-4-ΔCP-ΔIg1-2 inhibits the stimulatory effect of the prolactin-induced phosphorylation of STAT5a in cultured EpH4 cells (25). We first established cell lines stably expressing FLAG-Nectin-4 alone or FLAG-Nectin-4 plus FLAG-Nectin-4-ΔCP-ΔIg1-2 in EpH4 cells in which endogenous nectin-1 was knocked down as described previously (25). When these cells were stimulated with prolactin, the phosphorylation of STAT5a was reduced in the cells stably expressing the FLAG-Nectin-4 plus the FLAG-Nectin-4-ΔCP-ΔIg1-2, compared with that in the cells expressing the FLAG-Nectin-4 alone (Fig. 9, Ca and Cb). These results indicate that the cis-interaction of nectin-4 with the prolactin receptor through their extracellular and transmembrane regions is required for the stimulatory effect of nectin-4 on the prolactin-induced prolactin receptor activation and signaling.
Discussion
We previously showed that nectin-4 cis-interacted with the prolactin receptor through their extracellular and transmembrane regions and stimulated the prolactin-induced prolactin receptor activation and signaling, but it remained unresolved how this interaction stimulated these reactions. We first showed here that the cytoplasmic region of nectin-4 stimulated the prolactin-induced prolactin receptor activation and signaling. We then showed here that the cytoplasmic region of nectin-4 specifically interacted with SOCS1, but not SOCS3, JAK2, or STAT5, and inhibited the interaction of SOCS1 with JAK2. The juxtamembrane region of nectin-4 mapped from aa 388 to 403 interacted with the SH2 domain of SOCS1. Of the nectin family members, nectin-4, nectin-3, and nectin-1, but not nectin-2, showed this activity, although nectin-4 showed the stronger interacting activity than nectin-1, suggesting that the interaction of SOCS1 with nectin-4 is not nonspecific. It was shown that the FERM domain of willin also interacts with this juxtamembrane region of nectin-1 (40). Therefore, this region might generally serve as an interacting domain for signaling and cytoskeletal molecules.
We showed here that the interaction of SOCS1 with nectin-4 inhibited the interaction of SOCS1 with JAK2. Although it was shown that the tyrosine-phosphorylated JAK2 interacts with the SH2 domain of SOCS1 (35), we showed here that the interaction of the SH2 domain of SOCS1 with the juxtamembrane region of nectin-4 is independent of tyrosine phosphorylation, because the mutation of the interacting residue of phosphorylated tyrosine in the SH2 domain of SOCS1, arginine 105 to glutamine, also interacted with the cytoplasmic domain of nectin-4. This result was consistent with the earlier observations that VAV (41), p65 (42), and E7 (43) interacted with SOCS1 in a tyrosine phosphorylation-independent manner, although the interacting region of SOCS1 was not determined for these molecules.
It was suggested that when prolactin interacts with the prolactin receptor, the conformational change of the cytoplasmic domain of the prolactin receptor results in the activation of JAK2 and induces the tyrosine phosphorylation of the receptor and STAT5. The tyrosine-phosphorylated STAT5 is then dimerized and translocated from the cytosol to the nucleus and induces the transcriptional induction of SOCS1, which then interacts with JAK2 and inhibits its enzymatic activity on the tyrosine phosphorylation of the receptor and STAT5 in a feedback inhibition manner (44). Because the SOCS1 molecule interacted with the cytoplasmic region of nectin-4 was unable to interact with JAK2, this feedback inhibition mechanism does not operate, and thus nectin-4 enhances the prolactin-induced prolactin receptor activation and signaling in this way.
In our prior paper (25), we concluded that the extracellular and transmembrane regions of nectin-4, but not the cytoplasmic and transmembrane regions, cis-interacted with the extracellular and transmembrane regions of the prolactin receptor. However, we did not show whether the cis-interaction of nectin-4 with the prolactin receptor is direct or indirect. We showed here that this interaction was direct and that the transmembrane region, in addition to the extracellular region of both nectin-4 and the prolactin receptor, was required for this interaction. Of the extracellular regions of these molecules, the third Ig-like domain of nectin-4 and the second fibronectin type III region of the prolactin receptor were involved in this interaction. These extracellular regions and transmembrane regions of nectin-4 and the prolactin receptor may separately interact with each other, but it remains unknown how these two proteins interact with each other, and further studies by co-crystallographic analysis are needed for this issue.
We then showed here that the cis-interaction of the extracellular region of nectin-4 with the prolactin receptor, in addition to its cytoplasmic region, was required for its stimulatory effect on the prolactin-induced prolactin receptor activation and signaling, on the basis of the observation that the inhibition of the cis-interaction of full-length nectin-4 with the prolactin receptor by the third Ig-like domain of nectin-4 reduced the stimulatory effect of nectin-4 on the prolactin-induced prolactin receptor activation and signaling. Taken together, the present results indicate that both the extracellular and cytoplasmic regions of nectin-4 are involved in its stimulatory effect on the prolactin-induced prolactin receptor activation and signaling.
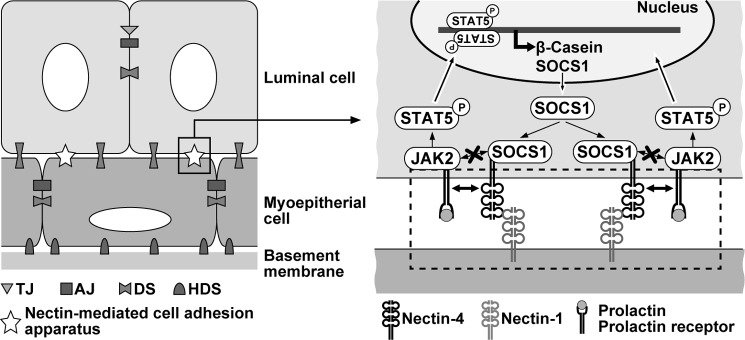
This mode of action of nectin-4 in the prolactin-induced prolactin receptor activation and signaling is analogous to that of Necl-2 and Necl-4 in the heregulin-induced ErbB2/ErbB3 activation and signaling, although nectin-4 shows a stimulatory effect, whereas Necl-2 and Necl-4 show an inhibitory effect. Necl-2 and Necl-4 interact with ErbB3 through the extracellular region and to the protein-tyrosine phosphatase PTPN13 through the cytoplasmic region (20, 23). When heregulin interacts with the extracellular region of ErbB3, it dimerizes with ErbB2 through their extracellular regions, and the cytoplasmic region of ErbB3 is tyrosine-phosphorylated by ErbB2, resulting in receptor activation and signaling through PI3K, Rac small G protein, and Akt (45). However, PTPN13 interacted with the cytoplasmic region of Necl-2 and Necl-4 is translocated nearby to the cytoplasmic region of the ErbB2/3 dimer and dephosphorylates the tyrosine-phosphorylated ErbB3, resulting in receptor inactivation and signal suppression. Similarly, nectin-4 interacts with the prolactin receptor through their extracellular region and transmembrane region and also interacts with SOCS1 through the cytoplasmic region as schematically shown in Fig. 10. When prolactin interacts with the extracellular region of the already dimerized receptor, it induces the activation of JAK2 associated with the prolactin receptor, but the cytoplasmic region of nectin-4 interacts with SOCS1 and inhibits its interaction with JAK2, resulting in the stimulation of the prolactin receptor activation and signaling. It could be speculated that nectin-4 cis-interacts with membrane receptors other than the prolactin receptor and regulates their activation and signaling in a similar way.
Figure 10.
A regulatory mechanism of nectin-4 for the regulation of the prolactin receptor activation and signaling. See “Discussion” for details. TJ, tight junction; AJ, adherens junction; DS, desmosome; HDS, hemidesmosome.
It has been shown that nectin-4 is up-regulated in many types of cancers, including breast, lung, ovarian, and colorectal cancers (46–48). In addition, cancer cells circulating in the blood express both nectin-1 and nectin-4, and the homophilic trans-interaction of nectin-4 and/or heterophilic trans-interaction of nectin-4 with nectin-1 confer on cancer cells an ability to survive in an anchorage-independent manner (49). This anchorage-independent cell growth is mediated by integrin β4 cis-interacting with nectin-4 and its downstream signaling molecule c-Src. Thus, up-regulated nectin-4 plays a crucial role in proliferation, invasion, and metastasis of a variety of cancer cells, and these results have been applied for development of a diagnosis marker and anti-cancer drugs (47, 50, 51). In many cancers in which the JAK-STAT pathway is up-regulated, SOCS1 is down-regulated by methylation of the promoter region of the SOCS1 gene (52–54). However, cancers in which nectin-4 is up-regulated and the JAK-STAT pathway is up-regulated, but SOCS1 is not down-regulated, may be found, and in these cancers, up-regulated nectin-4 may suppress the SOCS1 activity and thereby up-regulate the JAK-STAT pathway, conferring on the cancer cells tumorigenic, invasive, and metastatic properties.
Experimental procedures
Cell culture
Human embryonic kidney HEK293E cells were maintained in DMEM supplemented with 10% FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin and cultured at 5% CO2 at 37 °C. Mouse mammary epithelial EpH4 cells were maintained in DMEM/F-12 supplemented with 2% FBS, 5 μg/ml insulin, and 50 μg/ml gentamycin and cultured at 5% CO2 at 37 °C.
Plasmid constructions
C-terminal HA-tagged mouse prolactin receptor (pcDNA3.1-PRLR-HA) was constructed as described previously (25). The plasmid encoding C-terminally human IgG Fc-fused prolactin receptor mutant lacking the cytoplasmic region, corresponding to aa 265–608 (PRLR-ΔCP-Fc), or lacking the transmembrane and cytoplasmic regions, corresponding to aa 233–608 (PRLR-EC-Fc), was constructed by PCR. The plasmid encoding the prolactin receptor mutant lacking the extracellular region was also constructed. The extracellular region of the prolactin receptor consists of two fibronectin type III domains. The plasmid encoding the prolactin receptor mutant lacking the first fibronectin type III domain, corresponding to aa 23–107 (pcDNA3.1-PRLR-ΔD1), or lacking the extracellular region, corresponding to aa 23–209 (pcDNA3.1-PRLR-ΔEC), was constructed by inverse PCR using the pcDNA3.1-PRLR-HA as a template. The plasmids encoding N-terminally FLAG-tagged human nectin-1, mouse nectin-2, mouse nectin-3, and mouse nectin-4 were constructed as described previously (12). The plasmid encoding N-terminally FLAG-tagged nectin-4 mutant lacking the signal peptide and the cytoplasmic region, corresponding to aa 1–29 and 375–508, respectively (FLAG-Nectin-4-ΔCP), or lacking the extracellular region, corresponding to aa 1–332 (FLAG-Nectin-4-ΔEC), was constructed as described previously (25). The plasmid encoding N-terminally FLAG-tagged nectin-4 mutant either lacking the signal peptide and the cytoplasmic region, corresponding to aa 1–29 and 472–508, respectively (FLAG-Nectin-4-471); lacking the signal peptide and the cytoplasmic region, corresponding to aa 1–29 and 427–508, respectively (FLAG-Nectin-4-426); lacking the signal peptide and the cytoplasmic region, corresponding to aa 1–29 and 404–508, respectively (FLAG-Nectin-4-403); or lacking the signal peptide and the cytoplasmic region, corresponding to aa 1–29 and 388–508, respectively (FLAG-Nectin-4-387) were constructed. The plasmid encoding N-terminally FLAG-tagged nectin-4 mutant consisting of the extracellular region without signal peptide, corresponding to aa 30–342 (FLAG-Nectin-4-EC); lacking the signal peptide, the first Ig-like domain, and the cytoplasmic region, corresponding to aa 1–29, 30–149, and 375–508, respectively (FLAG-Nectin-4-ΔCP-ΔIg1); or lacking the signal peptide, the first and second Ig-like domain, and the cytoplasmic region, corresponding to aa 1–29, 30–239, and 375–508, respectively (FLAG-Nectin-4-ΔCP-ΔIg1-2), was also constructed. For the stable expression of each FLAG-tagged nectin and its mutant, each cDNA was introduced into the retrovirus vector pCX4-puro and pCX4-BSR. The plasmid of pGEX6P-1-Nectin-4-CP was constructed by introducing the cytoplasmic region of nectin-4, corresponding to aa 368–508, into pGEX6P-1. The cDNAs encoding JAK2, SOCS1, SOCS3, and the SH2 domain-defective mutant of SOCS1 (R105Q) were kindly provided by Dr. T. Naka (Kochi University), courtesy of Dr. T. Kishimoto (Osaka University). The cDNAs for TEL-JAK2 and STAT5a were kindly provided by Dr. A. Yoshimura (Keio University) and by Dr. T. Kitamura (University of Tokyo), respectively. The cDNAs were introduced into the pEGFP-C1 and FLAG-tagged expression vectors. The cDNAs for GFP and GFP-SOCS1 were introduced into pCX4-BSR. The SOCS1 mutants encoding aa 1–172 (ΔCT), aa 70–212 (ΔNT), aa 1–80 and 150–212 (ΔSH2), aa 1–80 (NT), aa 70–172 (SH2), and aa 150–212 (CT) were amplified by PCR and introduced into the pEGFP-C1 expression vector. For the stable expression of shRNA, the pSIREN retrovirus vector (Clontech) harboring the hygromycin B resistance gene (pSIREN-retroQ-hygro) was used as described previously (25). Targeting sequences were as follows 5′-GGCAGAGTACCAGGAGATC-3′ (for mouse nectin-1); 5′-GTGACTACCTGAGTTCCTT-3′ (mouse SOCS1#1), and 5′-GCATCCGCGTGCACTTCCA-3′ (mouse SOCS1#2). The scramble sequence (shScr) 5′-GACTAGAAGGCACAGAGGGAG-3′ was utilized for non-silencing control.
Antibodies
The following Abs were used: mouse anti-FLAG M2 mAb (for immunoprecipitation and immunoblotting; F3165, Sigma-Aldrich), rabbit anti-FLAG pAb (for immunoblotting; F7425, Sigma-Aldrich), rabbit anti-GFP pAb (for immunoprecipitation; 598, MBL, Nagoya, Japan), rat anti-GFP mAb (for immunoprecipitation; D153, MBL), chicken anti-GFP mAb (for immunoblotting; 600-901-215, Rockland Immunochemicals), mouse anti-HA mAb (for immunoblotting; MMS-101P, BioLegend, San Diego, CA), rabbit anti-HA pAb (for immunoprecipitation; H6908, Sigma-Aldrich), rabbit anti-STAT5a pAb (sc-1081, Santa Cruz Biotechnology), rabbit anti-phospho-STAT5 (Tyr-694) mAb (catalog no. 4322, Cell Signaling Technology), anti-phosphotyrosine (4G10, Merck Millipore), and goat anti-human IgG-Fcγ specific pAb (Jackson Laboratory). HRP-conjugated secondary Abs were purchased from GE Healthcare (Little Chalfont, UK).
Western blotting
Protein lysates were mixed with an SDS sample buffer (60 mm Tris-HCl, pH 6.7, 3% SDS, 2% 2-mercaptoethanol, and 5% glycerol) and boiled for 5 min. Then the samples were resolved on SDS-PAGE and transferred to PVDF membrane sheets (Merck Millipore). After being blocked with 2% skim milk or 5% BSA in Tris-buffered saline plus 0.05% Tween 20, the sheets were incubated with the indicated Abs. After being washed with Tris-buffered saline plus 0.05% Tween 20 three times, the sheets were incubated with HRP-conjugated anti-rabbit, anti-mouse, or anti-goat IgG Ab. The signals for the proteins were detected using Immobilon Western Chemiluminescent HRP substrate (Merck Millipore).
Coomassie Brilliant Blue staining and silver staining
Protein samples were resolved on SDS-PAGE, and the gels were stained with 0.1% (w/v) CBB R-250 in 20% methanol containing 7% (v/v) acetic acid for 30 min. The gels were destained with 20% methanol containing 7% (v/v) acetic acid overnight. For protein visualization with silver staining, SDS-polyacrylamide gels were stained using a silver stain kit (WAKO Pure Chemical Industries) as per the manufacturer's instructions.
Retrovirus production and infection
To generate the retroviral supernatant, HEK293E cells were co-transfected with pGP, pE-Ampho, and either pCX4-puro, pCX4-BSR, or pSIREN-retroQ-hygro and cultured for 48 h. The viral supernatant was then added to the EpH4 cells in the presence of 8 μg/ml Polybrene. The cells were further cultured in the presence of 5 μg/ml puromycin (Sigma-Aldrich), 5 μg/ml blasticidin S (Wako Pure Chemical Industries), and/or 500 μg/ml hygromycin B (InvivoGen) for a week and then used for the experiments.
Immunoprecipitation assay
HEK293E cells were transfected with various combinations of plasmids. After the transfection, the cells were cultured for 48 h and then lysed in a lysis buffer (20 mm Tris-HCl, pH 7.4, 150 mm NaCl, 1 mm EDTA, 10% glycerol, 1% Nonidet P-40, 10 mm NaF, 1 mm Na3VO4, 10 μg/ml leupeptin, 2 μg/ml aprotinin, and phosphatase inhibitor mixture 3 (Sigma-Aldrich)). The lysates were rotated for 30 min and subjected to centrifugation at 15,000 rpm for 15 min. The supernatant was precleared with protein A-Sepharose 4 Fast Flow beads (GE Healthcare) at 4 °C for 1 h. The precleared lysates were incubated with appropriate Abs and collected with protein A-Sepharose beads at 4 °C for 4 h. After the beads were extensively washed three times with the lysis buffer, each protein precipitated with the beads was eluted by boiling the beads in the SDS sample buffer for 5 min and subjected to SDS-PAGE, followed by Western blotting using the indicated Abs.
For the detection of the cis-interaction of nectin with the prolactin receptor, the same co-immunoprecipitation assay using HEK293E cells cultured in suspension was performed as described previously (55). Briefly, HEK293E cells transfected with various combinations of plasmids were detached with 0.05% trypsin and 0.53 mm EDTA and treated with a trypsin inhibitor (Sigma-Aldrich). Then the cells were cultured in suspension with DMEM containing 0.5% fatty acid-free BSA for 30 min, collected by centrifugation, washed once with PBS, and lysed with the lysis buffer. Immunoprecipitation was performed as described above.
Assay for the prolactin-induced phosphorylation of STAT5a
The phosphorylation of STAT5a in EpH4 cells was assayed as described previously (56). Briefly, EpH4 cells, plated at a density of 2 × 104 cells/cm2 on dishes coated with Matrigel (Corning), were cultured for 16–24 h, and the cells were stimulated with prolactin by exchanging with fresh DMEM/F-12 containing 2% Matrigel (v/v), 5 μg/ml insulin, 50 μg/ml gentamycin, 1 μg/ml hydrocortisone, and 3 μg/ml prolactin (Sigma-Aldrich) for the indicated periods of time. The cells were washed twice with ice-cold PBS and lysed with the lysis buffer. The lysates were then boiled in the SDS sample buffer for 5 min and subjected to SDS-PAGE, followed by Western blotting using the indicated Abs.
Semiquantitative real-time PCR
EpH4 cells were stimulated with or without 3 μg/ml prolactin for 30 min as described above, and the total RNAs were extracted using TRIzol (Thermo Fisher Scientific) according to the manufacturer's protocol. Reverse transcription was performed using SuperScript IV reverse transcriptase (Thermo Fisher Scientific). The abundance of each mRNA was measured using a StepOnePlus real-time PCR system (Thermo Fisher Scientific). The following primers were used for the semiquantitative real-time PCR: Socs1 (5′-ACCTTCTTGGTGCGCGAC-3′ and 5′-AAGCCATCTTCACGCTGAGC-3′) and Gapdh (5′-AACTTTGGCATTGTGGAAGG-3′ and 5′-CACATTGGGGGTAGGAACAC-3′).
Expression and purification of proteins
FLAG-Nectin-4-EC, FLAG-Nectin-4-ΔCP, FLAG-Nectin-4-ΔCP-ΔIg1-2, FLAG-TEL-JAK2, PRLR-EC-Fc, PRLR-ΔCP-Fc, or Fc portion was expressed in HEK293E cells. Each protein was purified from the cell lysate or the culture supernatant using anti-DDDDK beads (3327, MBL) or protein A-Sepharose 4 Fast Flow beads (GE Healthcare). The beads were extensively washed three times with the lysis buffer and once with ice-cold PBS. Each FLAG-tagged protein was eluted from the beads with DDDDK peptide and dialyzed against a dialysis buffer composed of PBS containing 1 mm DTT. Each Fc-fusion protein was coupled on protein A-Sepharose beads, and the beads were suspended in PBS containing 1 mm DTT at 4 °C until use.
GST-Nectin-4-CP was expressed using pGEX6P-1-Nectin-4-CP-transformed BL21 (DE3) pLysS. The E. coli was cultured in LB medium, and the protein expression was induced by adding 0.1 mm isopropyl β-d-1-thiogalactopyranoside at 25 °C for 16 h. After the induction, E. coli was collected and disrupted by sonication in PBS containing 1 mm DTT, 2 μg/ml aprotinin, and 1 mm PMSF. The lysate was clarified by centrifugation at 15,000 rpm for 15 min, and GST-Nectin-4-CP was purified from the supernatant using glutathione-Sepharose 4B (GE Healthcare). GST-Nectin-4-CP was eluted with 100 mm reduced glutathione and immediately dialyzed against the dialysis buffer. The purity of all purified proteins was confirmed by SDS-PAGE and CBB staining, and the amount of the purified proteins was estimated by BSA as a standard.
In vitro assay for the interaction of nectin-4 with the prolactin receptor
The direct interaction of the nectin-4 with the prolactin receptor was analyzed using the purified proteins described above. The protein A-Sepharose beads conjugated with the Fc-fusion proteins were mixed with the FLAG-tagged proteins and incubated in the lysis buffer for 2 h. The beads conjugated with the Fc-fusion proteins were precipitated by centrifugation and washed three times with the lysis buffer. Each protein precipitated with the beads was eluted by boiling the beads in the SDS sample buffer for 5 min and subjected to SDS-PAGE, followed by silver staining and Western blotting using the indicated Ab.
The competitive interaction assay of the nectin-4 and the minimal prolactin receptor-interacting region of the nectin-4 to the prolactin receptor was performed using FLAG-Nectin-4-ΔCP and PRLR-ΔCP-Fc by adding the minimal interacting region of the nectin-4 (FLAG-Nectin-4-ΔCP-ΔIg1-2) to the prolactin receptor. The protein A-Sepharose beads conjugated with PRLR-ΔCP-Fc were mixed with FLAG-Nectin-4-ΔCP in the presence or absence of FLAG-Nectin-4-ΔCP-ΔIg1-2 and incubated in the lysis buffer for 2 h. After the beads were washed three times with the lysis buffer, each protein precipitated with the beads was eluted by boiling the beads in the SDS sample buffer for 5 min and subjected to SDS-PAGE, followed by Western blotting using the indicated Abs.
The competitive interaction assay of the TEL-JAK2 and the cytoplasmic region of the nectin-4 to SOCS1 was performed by adding the GST-Nectin-4-CP. GFP-SOCS1 was captured on anti-GFP-Ab-conjugated beads by incubating with the lysates of the HEK293E cells expressing GFP-SOCS1. The beads were washed three times with the lysis buffer and once with the interaction buffer composed of PBS containing 0.1% Triton X-100, 1 mm DTT, 1 mm PMSF, and 1 mm sodium orthovanadate and then incubated with FLAG-TEL-JAK2 and the indicated concentrations of GST-Nectin-4-CP and incubated in the interaction buffer for 2 h. After the beads were washed three times with the interaction buffer, each protein precipitated with the beads was eluted by boiling the beads in the SDS sample buffer for 5 min and subjected to SDS-PAGE, followed by Western blotting using the indicated Abs.
Author contributions
Y. T. conceived the research project. M. M., K. M., and Y. T. designed the experiments. M. M., S. K., Y. U., and K. M. performed the experiments. M. M., K. M., and Y. T. wrote the paper.
Acknowledgments
We thank Drs. T. Kishimoto (Osaka University), T. Naka (Kochi University), A. Yoshimura (Keio University), and T. Kitamura (University of Tokyo) for generous gifts of reagents, and we thank T. Naka for helpful discussions.
This work was supported by Grants-in-Aid for Scientific Research 26251013 (to Y. T.), 26860190 (to K. M.), and 16K19035 (to M. M.) from the Japan Society for the Promotion of Science; Grant-in-Aid for Scientific Research on Innovative Areas 26114007 (to Y. T.) from the Ministry of Education, Culture, Sports, Science, and Technology; P-CREATE from the Japan Agency for Medical Research and Development (AMED) (to Y. T.); and the Japan Foundation for Applied Enzymology (to Y. T.). This work was also supported in part by the Research Complex Promotion Program of the Japan Science and Technology Agency. The authors declare that they have no conflicts of interest with the contents of this article.
- Necl
- nectin-like molecule
- SOCS
- suppressor of cytokine signaling
- SH2
- Src homology 2
- Ab
- antibody
- aa
- amino acids
- CBB
- Coomassie Brilliant Blue
- shScr
- scramble shRNA
- PRLR
- prolactin receptor.
References
- 1. Bezbradica J. S., and Medzhitov R. (2009) Integration of cytokine and heterologous receptor signaling pathways. Nat. Immunol. 10, 333–339 [DOI] [PubMed] [Google Scholar]
- 2. Kirkbride K. C., Ray B. N., and Blobe G. C. (2005) Cell-surface co-receptors: emerging roles in signaling and human disease. Trends Biochem. Sci. 30, 611–621 [DOI] [PubMed] [Google Scholar]
- 3. Yamada K. M., and Even-Ram S. (2002) Integrin regulation of growth factor receptors. Nat. Cell Biol. 4, E75–E76 [DOI] [PubMed] [Google Scholar]
- 4. Streuli C. H., and Akhtar N. (2009) Signal co-operation between integrins and other receptor systems. Biochem. J. 418, 491–506 [DOI] [PubMed] [Google Scholar]
- 5. Guo W., and Giancotti F. G. (2004) Integrin signalling during tumour progression. Nat. Rev. Mol. Cell Biol. 5, 816–826 [DOI] [PubMed] [Google Scholar]
- 6. Pece S., and Gutkind J. S. (2000) Signaling from E-cadherins to the MAPK pathway by the recruitment and activation of epidermal growth factor receptors upon cell-cell contact formation. J. Biol. Chem. 275, 41227–41233 [DOI] [PubMed] [Google Scholar]
- 7. Takai Y., and Nakanishi H. (2003) Nectin and afadin: novel organizers of intercellular junctions. J. Cell Sci. 116, 17–27 [DOI] [PubMed] [Google Scholar]
- 8. Takai Y., Ikeda W., Ogita H., and Rikitake Y. (2008) The immunoglobulin-like cell adhesion molecule nectin and its associated protein afadin. Annu. Rev. Cell Dev. Biol. 24, 309–342 [DOI] [PubMed] [Google Scholar]
- 9. Takai Y., Miyoshi J., Ikeda W., and Ogita H. (2008) Nectins and nectin-like molecules: roles in contact inhibition of cell movement and proliferation. Nat. Rev. Mol. Cell Biol. 9, 603–615 [DOI] [PubMed] [Google Scholar]
- 10. Ikeda W., Kakunaga S., Itoh S., Shingai T., Takekuni K., Satoh K., Inoue Y., Hamaguchi A., Morimoto K., Takeuchi M., Imai T., and Takai Y. (2003) Tage4/Nectin-like molecule-5 heterophilically trans-interacts with cell adhesion molecule Nectin-3 and enhances cell migration. J. Biol. Chem. 278, 28167–28172 [DOI] [PubMed] [Google Scholar]
- 11. Bojesen K. B., Clausen O., Rohde K., Christensen C., Zhang L., Li S., Køhler L., Nielbo S., Nielsen J., Gjørlund M. D., Poulsen F. M., Bock E., and Berezin V. (2012) Nectin-1 binds and signals through the fibroblast growth factor receptor. J. Biol. Chem. 287, 37420–37433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sakamoto Y., Ogita H., Hirota T., Kawakatsu T., Fukuyama T., Yasumi M., Kanzaki N., Ozaki M., and Takai Y. (2006) Interaction of integrin αvβ3 with nectin: implication in cross-talk between cell-matrix and cell-cell junctions. J. Biol. Chem. 281, 19631–19644 [DOI] [PubMed] [Google Scholar]
- 13. Kanzaki N., Ogita H., Komura H., Ozaki M., Sakamoto Y., Majima T., Ijuin T., Takenawa T., and Takai Y. (2008) Involvement of the nectin-afadin complex in PDGF-induced cell survival. J. Cell Sci. 121, 2008–2017 [DOI] [PubMed] [Google Scholar]
- 14. Sakamoto Y., Ogita H., Komura H., and Takai Y. (2008) Involvement of nectin in inactivation of integrin αvβ3 after the establishment of cell-cell adhesion. J. Biol. Chem. 283, 496–505 [DOI] [PubMed] [Google Scholar]
- 15. Miyata M., Ogita H., Komura H., Nakata S., Okamoto R., Ozaki M., Majima T., Matsuzawa N., Kawano S., Minami A., Waseda M., Fujita N., Mizutani K., Rikitake Y., and Takai Y. (2009) Localization of nectin-free afadin at the leading edge and its involvement in directional cell movement induced by platelet-derived growth factor. J. Cell Sci. 122, 4319–4329 [DOI] [PubMed] [Google Scholar]
- 16. Amano H., Ikeda W., Kawano S., Kajita M., Tamaru Y., Inoue N., Minami Y., Yamada A., and Takai Y. (2008) Interaction and localization of Necl-5 and PDGF receptor β at the leading edges of moving NIH3T3 cells: implications for directional cell movement. Genes Cells 13, 269–284 [DOI] [PubMed] [Google Scholar]
- 17. Minami A., Mizutani K., Waseda M., Kajita M., Miyata M., Ikeda W., and Takai Y. (2010) Necl-5/PVR enhances PDGF-induced attraction of growing microtubules to the plasma membrane of the leading edge of moving NIH3T3 cells. Genes Cells 15, 1123–1135 [DOI] [PubMed] [Google Scholar]
- 18. Kinugasa M., Amano H., Satomi-Kobayashi S., Nakayama K., Miyata M., Kubo Y., Nagamatsu Y., Kurogane Y., Kureha F., Yamana S., Hirata K., Miyoshi J., Takai Y., and Rikitake Y. (2012) Necl-5/poliovirus receptor interacts with VEGFR2 and regulates VEGF-induced angiogenesis. Circ. Res. 110, 716–726 [DOI] [PubMed] [Google Scholar]
- 19. Minami Y., Ikeda W., Kajita M., Fujito T., Amano H., Tamaru Y., Kuramitsu K., Sakamoto Y., Monden M., and Takai Y. (2007) Necl-5/poliovirus receptor interacts in cis with integrin αvβ3 and regulates its clustering and focal complex formation. J. Biol. Chem. 282, 18481–18496 [DOI] [PubMed] [Google Scholar]
- 20. Kawano S., Ikeda W., Kishimoto M., Ogita H., and Takai Y. (2009) Silencing of ErbB3/ErbB2 signaling by immunoglobulin-like Necl-2. J. Biol. Chem. 284, 23793–23805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yamada A., Inoue E., Deguchi-Tawarada M., Matsui C., Togawa A., Nakatani T., Ono Y., and Takai Y. (2013) Necl-2/CADM1 interacts with ErbB4 and regulates its activity in GABAergic neurons. Mol. Cell. Neurosci. 56, 234–243 [DOI] [PubMed] [Google Scholar]
- 22. Mizutani K., Kawano S., Minami A., Waseda M., Ikeda W., and Takai Y. (2011) Interaction of nectin-like molecule 2 with integrin α6β4 and inhibition of disassembly of integrin α6β4 from hemidesmosomes. J. Biol. Chem. 286, 36667–36676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sugiyama H., Mizutani K., Kurita S., Okimoto N., Shimono Y., and Takai Y. (2013) Interaction of Necl-4/CADM4 with ErbB3 and integrin α6β4 and inhibition of ErbB2/ErbB3 signaling and hemidesmosome disassembly. Genes Cells 18, 519–528 [DOI] [PubMed] [Google Scholar]
- 24. Mandai K., Rikitake Y., Mori M., and Takai Y. (2015) Nectins and nectin-like molecules in development and disease. Curr. Top. Dev. Biol. 112, 197–231 [DOI] [PubMed] [Google Scholar]
- 25. Kitayama M., Mizutani K., Maruoka M., Mandai K., Sakakibara S., Ueda Y., Komori T., Shimono Y., and Takai Y. (2016) A novel nectin-mediated cell adhesion apparatus that is implicated in prolactin receptor signaling for mammary gland development. J. Biol. Chem. 291, 5817–5831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Muschler J., and Streuli C. H. (2010) Cell-matrix interactions in mammary gland development and breast cancer. Cold Spring Harb. Perspect. Biol. 2, a003202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Feng J., Witthuhn B. A., Matsuda T., Kohlhuber F., Kerr I. M., and Ihle J. N. (1997) Activation of Jak2 catalytic activity requires phosphorylation of Y1007 in the kinase activation loop. Mol. Cell. Biol. 17, 2497–2501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. DaSilva L., Rui H., Erwin R. A., Howard O. M., Kirken R. A., Malabarba M. G., Hackett R. H., Larner A. C., and Farrar W. L. (1996) Prolactin recruits STAT1, STAT3 and STAT5 independent of conserved receptor tyrosines TYR402, TYR479, TYR515 and TYR580. Mol. Cell. Endocrinol. 117, 131–140 [DOI] [PubMed] [Google Scholar]
- 29. Liu X., Robinson G. W., Gouilleux F., Groner B., and Hennighausen L. (1995) Cloning and expression of Stat5 and an additional homologue (Stat5b) involved in prolactin signal transduction in mouse mammary tissue. Proc. Natl. Acad. Sci. U.S.A. 92, 8831–8835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu X., Robinson G. W., Wagner K. U., Garrett L., Wynshaw-Boris A., and Hennighausen L. (1997) Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes Dev. 11, 179–186 [DOI] [PubMed] [Google Scholar]
- 31. Matsumoto A., Seki Y., Kubo M., Ohtsuka S., Suzuki A., Hayashi I., Tsuji K., Nakahata T., Okabe M., Yamada S., and Yoshimura A. (1999) Suppression of STAT5 functions in liver, mammary glands, and T cells in cytokine-inducible SH2-containing protein 1 transgenic mice. Mol. Cell. Biol. 19, 6396–6407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu X., Robinson G. W., and Hennighausen L. (1996) Activation of Stat5a and Stat5b by tyrosine phosphorylation is tightly linked to mammary gland differentiation. Mol. Endocrinol. 10, 1496–1506 [DOI] [PubMed] [Google Scholar]
- 33. Tomic S., Chughtai N., and Ali S. (1999) SOCS-1, -2, -3: selective targets and functions downstream of the prolactin receptor. Mol. Cell. Endocrinol. 158, 45–54 [DOI] [PubMed] [Google Scholar]
- 34. Endo T. A., Masuhara M., Yokouchi M., Suzuki R., Sakamoto H., Mitsui K., Matsumoto A., Tanimura S., Ohtsubo M., Misawa H., Miyazaki T., Leonor N., Taniguchi T., Fujita T., Kanakura Y., et al. (1997) A new protein containing an SH2 domain that inhibits JAK kinases. Nature 387, 921–924 [DOI] [PubMed] [Google Scholar]
- 35. Yasukawa H., Misawa H., Sakamoto H., Masuhara M., Sasaki A., Wakioka T., Ohtsuka S., Imaizumi T., Matsuda T., Ihle J. N., and Yoshimura A. (1999) The JAK-binding protein JAB inhibits Janus tyrosine kinase activity through binding in the activation loop. EMBO J. 18, 1309–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Narazaki M., Fujimoto M., Matsumoto T., Morita Y., Saito H., Kajita T., Yoshizaki K., Naka T., and Kishimoto T. (1998) Three distinct domains of SSI-1/SOCS-1/JAB protein are required for its suppression of interleukin 6 signaling. Proc. Natl. Acad. Sci. U.S.A. 95, 13130–13134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ungureanu D., Saharinen P., Junttila I., Hilton D. J., and Silvennoinen O. (2002) Regulation of Jak2 through the ubiquitin-proteasome pathway involves phosphorylation of Jak2 on Y1007 and interaction with SOCS-1. Mol. Cell. Biol. 22, 3316–3326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kamizono S., Hanada T., Yasukawa H., Minoguchi S., Kato R., Minoguchi M., Hattori K., Hatakeyama S., Yada M., Morita S., Kitamura T., Kato H., Nakayama K., and Yoshimura A. (2001) The SOCS box of SOCS-1 accelerates ubiquitin-dependent proteolysis of TEL-JAK2. J. Biol. Chem. 276, 12530–12538 [DOI] [PubMed] [Google Scholar]
- 39. Bernard V., Young J., Chanson P., and Binart N. (2015) New insights in prolactin: pathological implications. Nat. Rev. Endocrinol. 11, 265–275 [DOI] [PubMed] [Google Scholar]
- 40. Ishiuchi T., and Takeichi M. (2012) Nectins localize Willin to cell-cell junctions. Genes Cells 17, 387–397 [DOI] [PubMed] [Google Scholar]
- 41. De Sepulveda P., Ilangumaran S., and Rottapel R. (2000) Suppressor of cytokine signaling-1 inhibits VAV function through protein degradation. J. Biol. Chem. 275, 14005–14008 [DOI] [PubMed] [Google Scholar]
- 42. Strebovsky J., Walker P., Lang R., and Dalpke A. H. (2011) Suppressor of cytokine signaling 1 (SOCS1) limits NFκB signaling by decreasing p65 stability within the cell nucleus. FASEB J. 25, 863–874 [DOI] [PubMed] [Google Scholar]
- 43. Kamio M., Yoshida T., Ogata H., Douchi T., Nagata Y., Inoue M., Hasegawa M., Yonemitsu Y., and Yoshimura A. (2004) SOCS1 inhibits HPV-E7-mediated transformation by inducing degradation of E7 protein. Oncogene 23, 3107–3115 [DOI] [PubMed] [Google Scholar]
- 44. Pezet A., Favre H., Kelly P. A., and Edery M. (1999) Inhibition and restoration of prolactin signal transduction by suppressors of cytokine signaling. J. Biol. Chem. 274, 24497–24502 [DOI] [PubMed] [Google Scholar]
- 45. Vivanco I., and Sawyers C. L. (2002) The phosphatidylinositol 3-kinase-AKT pathway in human cancer. Nat. Rev. Cancer 2, 489–501 [DOI] [PubMed] [Google Scholar]
- 46. Uhlen M., Oksvold P., Fagerberg L., Lundberg E., Jonasson K., Forsberg M., Zwahlen M., Kampf C., Wester K., Hober S., Wernerus H., Björling L., and Ponten F. (2010) Towards a knowledge-based Human Protein Atlas. Nat. Biotechnol. 28, 1248–1250 [DOI] [PubMed] [Google Scholar]
- 47. Fabre-Lafay S., Monville F., Garrido-Urbani S., Berruyer-Pouyet C., Ginestier C., Reymond N., Finetti P., Sauvan R., Adélaïde J., Geneix J., Lecocq E., Popovici C., Dubreuil P., Viens P., Gonçalves A., et al. (2007) Nectin-4 is a new histological and serological tumor associated marker for breast cancer. BMC Cancer 7, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Das D., Satapathy S. R., Siddharth S., Nayak A., and Kundu C. N. (2015) NECTIN-4 increased the 5-FU resistance in colon cancer cells by inducing the PI3K-AKT cascade. Cancer Chemother. Pharmacol. 76, 471–479 [DOI] [PubMed] [Google Scholar]
- 49. Pavlova N. N., Pallasch C., Elia A. E., Braun C. J., Westbrook T. F., Hemann M., and Elledge S. J. (2013) A role for PVRL4-driven cell-cell interactions in tumorigenesis. eLife 2, e00358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Msaouel P., Opyrchal M., Domingo Musibay E., and Galanis E. (2013) Oncolytic measles virus strains as novel anticancer agents. Expert Opin. Biol. Ther. 13, 483–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fabre-Lafay S., Garrido-Urbani S., Reymond N., Gonçalves A., Dubreuil P., and Lopez M. (2005) Nectin-4, a new serological breast cancer marker, is a substrate for tumor necrosis factor-α-converting enzyme (TACE)/ADAM-17. J. Biol. Chem. 280, 19543–19550 [DOI] [PubMed] [Google Scholar]
- 52. Galm O., Yoshikawa H., Esteller M., Osieka R., and Herman J. G. (2003) SOCS-1, a negative regulator of cytokine signaling, is frequently silenced by methylation in multiple myeloma. Blood 101, 2784–2788 [DOI] [PubMed] [Google Scholar]
- 53. Komazaki T., Nagai H., Emi M., Terada Y., Yabe A., Jin E., Kawanami O., Konishi N., Moriyama Y., Naka T., and Kishimoto T. (2004) Hypermethylation-associated inactivation of the SOCS-1 gene, a JAK/STAT inhibitor, in human pancreatic cancers. Jpn. J. Clin. Oncol. 34, 191–194 [DOI] [PubMed] [Google Scholar]
- 54. Chen C. Y., Tsay W., Tang J. L., Shen H. L., Lin S. W., Huang S. Y., Yao M., Chen Y. C., Shen M. C., Wang C. H., and Tien H. F. (2003) SOCS1 methylation in patients with newly diagnosed acute myeloid leukemia. Genes Chromosomes Cancer 37, 300–305 [DOI] [PubMed] [Google Scholar]
- 55. Kawano S., Mizutani K., Miyata M., Ikeda W., and Takai Y. (2010) Interaction of integrin α6β4 with ErbB3 and implication in heregulin-induced ErbB3/ErbB2-mediated DNA synthesis. Genes Cells 15, 995–1001 [DOI] [PubMed] [Google Scholar]
- 56. Xu R., Nelson C. M., Muschler J. L., Veiseh M., Vonderhaar B. K., and Bissell M. J. (2009) Sustained activation of STAT5 is essential for chromatin remodeling and maintenance of mammary-specific function. J. Cell Biol. 184, 57–66 [DOI] [PMC free article] [PubMed] [Google Scholar]