Abstract
Autotaxin (ATX) or ectonucleotide pyrophosphatase/phosphodiesterase 2 (ENPP2) is a secretory glycoprotein and functions as the key enzyme for lysophosphatidic acid generation. The mechanism of ATX protein trafficking is largely unknown. Here, we demonstrated that p23, a member of the p24 protein family, was the protein-sorting receptor required for endoplasmic reticulum (ER) export of ATX. A di-phenylalanine (Phe-838/Phe-839) motif in the human ATX C-terminal region was identified as a transport signal essential for the ATX-p23 interaction. Knockdown of individual Sec24 isoforms by siRNA revealed that ER export of ATX was impaired only if Sec24C was down-regulated. These results suggest that ATX is selectively exported from the ER through a p23, Sec24C-dependent pathway. In addition, it was found that AKT signaling played a role in ATX secretion regulation to facilitate ATX ER export by enhancing the nuclear factor of activated T cell-mediated p23 expression. Furthermore, the di-hydrophobic amino acid motifs (FY) also existed in the C-terminal regions of human ENPP1 and ENPP3. Such a p23, Sec24C-dependent selective ER export mechanism is conserved among these ENPP family members.
Keywords: COPII, endoplasmic reticulum (ER), lysophospholipid, protein sorting, receptor, autotaxin, ENPP family, ER export, Sec24C, p23
Introduction
Autotaxin (ATX),4 also known as ecto-nucleotide pyrophosphatase/phosphodiesterase 2 (ENPP2), belongs to the ENPP family. ATX functions as the key enzyme in the generation of lysophosphatidic acid (LPA), through its lysophospholipase D (lyso-PLD) activity (1). LPA is a bioactive phospholipid with diverse functions and is involved in the regulation of various cellular functions such as cell proliferation, migration, survival, and cell-cell interaction. LPA exerts its function through binding to its specific receptors on cellular membranes (2, 3). So far, six G protein-coupled receptors (GPCRs) have been identified as LPA receptors termed as LPA1–6 (4, 5). Most biological functions of ATX are performed via the LPA-LPA receptor signaling, although it has been reported that ATX can stimulate cell migration through direct interaction with integrin, independent of its lyso-PLD activity (6). Increased ATX expression has been detected in several cancers, and the effects of the ATX-LPA axis in cancer development have been extensively studied (7). Additionally, emerging data indicate that the ATX-LPA axis plays an important role in immunity (8). ATX is highly expressed in adipose tissue and is involved in obesity-related diseases such as atherosclerosis and diabetes (9, 10).
ATX is a secretory glycoprotein. Matured ATX protein consists of two N-terminal somatomedin B-like domains, a central catalytic phosphodiesterase (PDE) domain, and a C-terminal nuclease-like (NUC) domain (11, 12). As a secretory protein, the process of ATX secretion is an important part of its biological generation. Based on its overall structural similarity to the better characterized ecto-nucleotide pyrophosphatase/phosphodiesterase 1 (ENPP1), ATX has been assumed as a type II transmembrane protein that is anchored at the plasma membrane and then released into the extracellular space via proteolytic cleavage. However, the N-terminal hydrophobic sequence of ATX functions as a signal peptide and not as a transmembrane segment. ATX is actually synthesized as a secreted pre-pro-enzyme that is proteolytically cleaved in the endomembrane system before secretion (13–15). In addition, ATX contains several glycosylation sites, which are required for its function and secretion (15, 16). It has been reported that an essential disulfide bridge between Cys-413 and Cys-805, which links the PDE and NUC domains, and the residues 829–850 within the NUC domain are involved in ATX secretion, indicating that the NUC domain, which can fold and interact with the PDE domain, may be associated with ATX stability and secretion (17). However, the detailed mechanism of ATX transport during its secretion process is largely unknown.
In eukaryotic cells, endoplasmic reticulum (ER), Golgi apparatus, and ER-Golgi intermediate compartment (ERGIC) constitute the protein secretory pathway. Newly synthesized and properly folded proteins are selectively exited from the ER and then transported to the Golgi apparatus for further processing and maturation (18, 19). Protein transport is a very precise and well controlled process, and each in-process transport vesicle has to be highly selective to maintain the high degree of organization and efficiency. The coatomer protein complex II (COPII), which is known to associate with the cytosolic surface of the ER membrane, mediates the budding and the formation of transport vesicles, and it participates in protein transport from the ER to Golgi or ERGIC (20–22). The COPII coat is composed of the small G-protein Sar1, Sec23-Sec24 complex, and Sec13-Sec31 complex. Mammalian cells express four Sec24 isoforms termed as Sec24A–D, which are responsible for the selective export of proteins from the ER (23, 24). ER export of some cargo proteins is determined by direct interaction of the cargo proteins with coat subunits, through ER export motifs in their cytoplasmic region (22). However, adaptor proteins or transmembrane receptors are required in ER export of other proteins, to mediate efficient linkage of the cargo to the coat complex. Several transmembrane protein sorting receptor families such as the ERGIC-53 family, p24 family, and different ER vesicle proteins have been characterized (25, 26). The p24 family proteins are type I membrane proteins, recycled between ER and Golgi apparatus, and act as cargo receptors responsible for exit of selective cargoes from the ER. All p24 family proteins, such as p23, p24, p25, and p28, have conserved motifs in their cytoplasmic domains that can bind to COPI and COPII (27). It has been reported that p24 family proteins can recognize ER lumen-localized glycosylphosphatidylinositol-anchored proteins (GPI-APs) and act as cargo receptors for correctly remodeled GPI-APs to be sorted into COPII vesicles efficiently (28) in a Sec24C/D-dependent manner (29). p24 family proteins are also required for secretion of Wnt proteins in Drosophila and mammalian cells (30, 31) and for the transport of some GPCRs such as protease-activated receptor 2 (PAR2) (32).
So far, five different ATX isoforms have been identified, termed as ATX α, β, γ, ϵ, and δ. The ATX referred to in this study was ATX β, which consists of 863 amino acid residues in its full length and is the most abundant ATX isoform in plasma. In this study, we demonstrated that p23, a member of the p24 family, functioned as the cargo receptor for ER export of ATX. A di-hydrophobic (Phe-838/Phe-839) motif in the C-terminal region of human ATX was identified as the protein sorting signal to meditate the interaction between ATX and p23. Using siRNA-based silencing, it was found that knockdown of Sec24C, but not other Sec24 isoforms, significantly impaired ER export of ATX, suggesting that Sec24C is required for the selective ER export of ATX. In addition, we found that AKT signaling was involved in ATX secretion regulation. ATX secretion was significantly suppressed by AKT knockdown or AKT inhibitor treatment. AKT could activate nuclear factor of activated T cells (NFAT) via the inhibition of glycogen synthase kinase 3β (GSK3β) to facilitate ATX ER export by promoting the NFAT-mediated p23 expression. Furthermore, the di-hydrophobic amino acid motifs (FY) also existed in the C-terminal regions of ENPP1 and ENPP3 and were essential for the ER exit of ENPP1 and ENPP3. The p23, Sec24C-dependent early secretory pathway was conserved among these ENPP family members.
Results
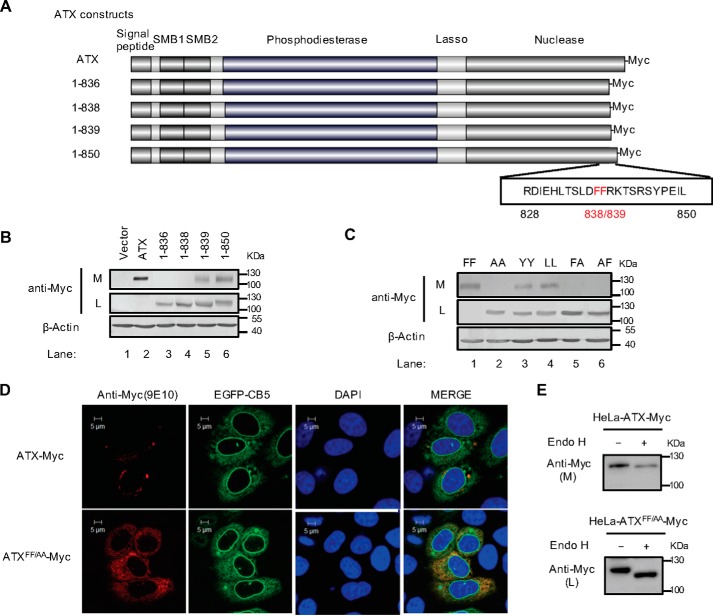
Di-phenylalanine (Phe-838/Phe-839) motif is critical for ER export of ATX
ATX is a secreted lysophospholipase-D converting lysophosphatidycholine (LPC) to lysophosphatidic acid (LPA). ATX is synthesized as a pre-pro-enzyme and is secreted after proteolytic cleavage. It has been reported that the residues 829–850 are involved in the secretion of ATX (17). To further clarify the amino acid residues responsible for ATX secretion, we constructed the plasmids to express the Myc-tagged wild-type ATX and ATX mutants with progressive C-terminal truncations as indicated in Fig. 1A. These plasmids were respectively transfected into HeLa cells, in which the endogenous ATX expression is undetectable, and then the intracellular ATX and extracellular secreted ATX levels were detected, respectively, by Western blotting. As shown in Fig. 1B, wild-type ATX was almost totally secreted; ATX(1–850) and ATX(1–839) were partially secreted; and ATX(1–838) and ATX(1–836) were restrained in cells. The 838/839 amino acids of ATX are phenylalanines, which together constitute a hydrophobic FF motif. To demonstrate the role of this FF motif in ATX secretion, we constructed a series of ATX mutants with point mutation on these two residues. When these ATX mutants were expressed in HeLa cells, it was found that ATX was not secreted into the culture medium if either one or both of the phenylalanine residues of the ATX 838FF839 motif were mutated to alanine (AA, FA, or AF) (Fig. 1C). Meanwhile, ATX could be partially secreted when the FF motif was replaced by YY or LL (Fig. 1C), suggesting that the hydrophobic character of this FF motif is important for its biological function.
Figure 1.
Identification of a di-phenylalanine (838FF839) motif essential for ATX secretion. A, schematic representation of the wild-type ATX and the truncated ATX mutants with Myc tag at the C terminus. The number of amino acid residues in each ATX protein is indicated, and the di-phenylalanine (838FF839) motif is labeled in red. B, wild-type ATX and the truncated ATX mutants with C-terminal Myc tag were expressed in HeLa cells as indicated. The levels of ATX-Myc or ATX mutant with Myc tag in cell lysates (L) and culture medium (M) were detected by immunoblotting with anti-Myc antibody. C, wild-type ATX (FF) and the indicated ATX mutants, in which the FF motif was replaced by two alanines (AA), two tyrosines (YY), two leucines (LL), or AF, were expressed in HeLa cells as indicated. The levels of ATX-Myc or ATX mutant with Myc tag in cell lysates and culture medium were detected by immunoblotting with anti-Myc antibody. D, plasmid pcDNA3-ATX-Myc or pcDNA3-ATXFF/AA-Myc, in which the FF motif was replaced by AA, was co-transfected with pEGFP-N1-CB5 into HeLa cells. Cells were fixed and permeabilized 48 h after transfection. ATX-Myc and ATXFF/AA-Myc were visualized by confocal microscopy with anti-Myc (9E10) monoclonal antibody (red). Endoplasmic reticulum was labeled by EGFP-CB5 (green). Nuclei were counterstained with DAPI (blue). E, Endo H treatment of secreted ATX-Myc and intracellular ATXFF/AA-Myc. The concentrated (∼30-fold) serum-free conditional culture medium of the cells transfected with pcDNA3-ATX-Myc and the lysate of the cells transfected with pcDNA3-ATXFF/AA-Myc were treated with Endo H and then analyzed by Western blotting with anti-Myc antibody. Data are representative of three independent experiments.
To clarify the function of the FF motif in the ATX secretion process, we examined the effect of the FF motif mutation on ATX cellular localization. When transiently expressed in HeLa cells, ATXFF/AA was retained within the cells and co-localized with ER marker cytochrome b5 (CB5). In contrast and consistent with its role as a secretory protein, only a small amount of wild-type ATX was detected within the cells (Fig. 1D). It has been reported that ATX is a secretory protein with N-glycosylation (15). Endoglycosidase H (Endo H) cleaves mannose and oligosaccharides from N-linked glycoproteins. Glycoproteins that undergo modifications in the Golgi complex become Endo H-resistant. It was found that the ATX-Myc secreted into culture medium was resistant to Endo H, whereas the ATXFF/AA-Myc retained within the cells was sensitive to Endo H, consistent with its ER location (Fig. 1E). These results indicate that the FF motif is essential for ATX ER export.
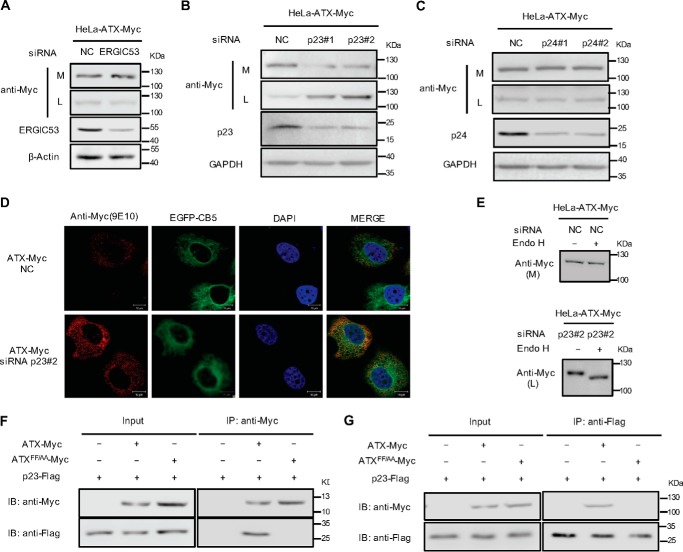
p23 functions as cargo receptor for ER export of ATX
It has been reported that ATX is synthesized as a secreted protein but not a type II transmembrane protein. With its N-terminal hydrophobic sequence as a signal peptide, newly synthesized ATX is translocated into the ER lumen, which suggests that ATX cannot directly interact with the COPII coat subunits through the FF motif in its C-terminal region. Therefore, we proposed that the ER export of ATX is dependent on the assistance of a protein-sorting receptor. The siRNA-based silencing of individual protein-sorting receptor expression was performed to identify the receptor involved in ATX ER export. It was found that knockdown of ERGIC-53 did not affect the efficiency of ATX secretion (Fig. 2A), suggesting that ERGIC-53 is not involved in the ATX secretion process. To detect the potential role of p24 family proteins in ATX secretion, p23 and p24 were down-regulated using specific siRNA in the HeLa cells expressing ATX-Myc. Knockdown of p23, but not p24, significantly suppressed the secretion of ATX (Fig. 2, B and C). To further confirm the role of p23 in ATX secretion, we observed the cellular localization of ATX when p23 was down-regulated by siRNA. It was found that the knockdown of p23 resulted in the retention of ATX in the ER (Fig. 2D). The retained ATX in the cells is sensitive to Endo H, consistent with the blocking of its ER export by p23 knockdown (Fig. 2E). Furthermore, the direct interaction between ATX and p23 was detected by immunoprecipitation. It was interesting to note that ATX could interact with p23, whereas the ATX mutant with the FF motif replaced by AA (ATXFF/AA) could not (Fig. 2, F and G), indicating that the FF motif in the ATX C-terminal region functions as a transport signal essential for ATX-p23 interaction.
Figure 2.
Identification of cargo receptor involved in ER export of ATX. A, HeLa cells were transfected with pcDNA3-ATX-Myc. Twenty four hours after transfection, cells were transfected with siRNA against ERGIC53. Forty eight hours after siRNA transfection, ATX-Myc protein levels in cell lysates (L) and culture medium (M) were detected by immunoblotting with anti-Myc antibody. B and C, HeLa cells were transfected with pcDNA3-ATX-Myc. Twenty four hours after transfection, cells were transfected with nonspecific control siRNA (NC) or siRNAs (#1 and #2) against p23 (B) or siRNAs (#1 and #2) against p24 (C). Forty eight hours after siRNA transfection, ATX-Myc protein levels in cell lysates (L) and culture medium (M) were detected by immunoblotting with anti-Myc antibody. D, HeLa cells were co-transfected with pcDNA3-ATX-Myc and pEGFP-N1-CB5 and then treated with nonspecific control siRNA (NC) or p23 siRNA (#2) for 48 h. ATX-Myc was visualized by confocal microscopy with anti-Myc (9E10) monoclonal antibody (red). Endoplasmic reticulum was labeled by EGFP-CB5 (green). Nuclei were counterstained with DAPI (blue). E, HeLa cells were transfected with pcDNA3-ATX-Myc. Twenty four hours after plasmid transfection, cells were transfected with nonspecific control siRNA (NC) or p23 siRNA (#2) for 48 h. The concentrated (∼30-fold) serum-free conditional culture medium of the control siRNA-treated cells and the lysate of p23 siRNA-treated cells were treated with Endo H and then analyzed by Western blotting with anti-Myc antibody. F, HeLa cells were co-transfected with the plasmid driving the expression of p23-FLAG together with pcDNA3-ATX-Myc or pcDNA3-ATXFF/AA-Myc as indicated. Cells were collected 48 h after transfection. The whole-cell lysates were immunoprecipitated with anti-Myc antibody attached to agarose, and then the immunocomplex was detected by Western blotting using anti-Myc and anti-FLAG as indicated. G, whole-cell lysates mentioned in F were immunoprecipitated with anti-FLAG antibody attached to agarose, and then the immunocomplex was detected by Western blotting using anti-Myc and anti-FLAG antibody as indicated. Data are representative of three independent experiments.
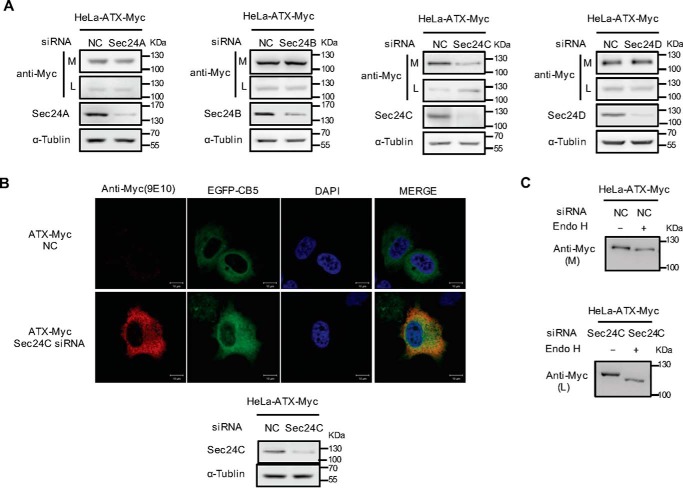
Sec24C is involved in the selective export of ATX from ER
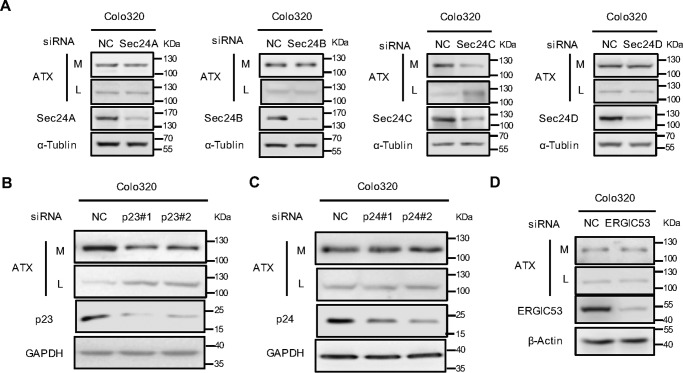
Mammalian cells express four Sec24 isoforms termed as Sec24A–D, which are responsible for the selective export of proteins from ER by interacting either with cargo proteins directly or with protein-sorting receptors. The p24 proteins are type I transmembrane proteins with a short cytosolic C-terminal tail, which contains signals for binding the Sec24 subunit in COPII to selectively transport target cargo protein from ER to Golgi apparatus. To determine the Sec24 subunit involved in ATX secretion, four Sec24 isoforms were silenced, respectively, with their specific siRNAs in HeLa cells with exogenous ATX expression. The levels of ATX retained in cytoplasm or secreted in culture medium were detected by Western blotting. The siRNA-induced knockdown of Sec24C resulted in intracellular retention of ATX, whereas the knockdowns of other Sec24 isoforms had no significant effects on ATX secretion (Fig. 3A). The role of Sec24C in ATX secretion was further confirmed by immunohistochemical analysis. When Sec24C was down-regulated using its specific siRNA in HeLa cells expressing ATX-Myc, ATX-Myc was retained in the cells to be co-localized with the ER marker CB5 and to be sensitive to Endo H treatment (Fig. 3, B and C). These results suggest that the ER export of ATX is dependent on Sec24C to mediate the physical association of the ATX-p23 complex with the COPII vesicles. Moreover, we analyzed the role of p23 and Sec24C in the secretion of endogenous ATX in Colo320 cells, which have high levels of endogenous ATX. ATX secretion was suppressed by Sec24C knockdown but not by the knockdown of other Sec24 isoforms (Fig. 4A). When p23 was down-regulated by siRNA, the level of ATX retained within cells was significantly increased (Fig. 4B), whereas knockdown p24 or ERGIC-53 did not affect the efficiency of endogenous ATX secretion (Fig. 4, C and D). These data further confirm that p23 and Sec24C play important roles in the secretion of ATX.
Figure 3.
Identification of Sec24 isoform involved in ER export of ATX. A, HeLa cells were transfected with pcDNA3-ATX-Myc. Twenty four hours after transfection, cells were transfected with nonspecific control siRNA (NC) or siRNA against the indicated Sec24 isoform. Forty eight hours after siRNA transfection, the ATX-Myc protein levels in cell lysates (L) and culture medium (M) were detected by immunoblotting with anti-Myc antibody. B, HeLa cells were co-transfected with pcDNA3-ATX-Myc and pEGFP-N1-CB5 and then treated with nonspecific control siRNA or Sec24C siRNA for 48 h. ATX-Myc was visualized by confocal microscopy with anti-Myc (9E10) monoclonal antibody (red). Endoplasmic reticulum was labeled by EGFP-CB5 (green). Nuclei were counterstained with DAPI (blue). C, HeLa cells were transfected with pcDNA3-ATX-Myc. Twenty four hours after transfection, cells were transfected with nonspecific control siRNA or Sec24C siRNA for 48 h. The concentrated (∼30-fold) serum-free conditional culture medium of the control siRNA-treated cells and the lysate of Sec24C siRNA-treated cells were treated with Endo H and then analyzed by Western blotting with anti-Myc antibody. Data are representative of three independent experiments.
Figure 4.
Effects of Sec24 isoform, p23, or ERGI53 knockdown on the secretion of endogenous ATX. A, Colo320 cells were transfected with nonspecific control siRNA (NC) or siRNA against the indicated Sec24 isoform. Forty eight hours after transfection, ATX protein levels in cell lysates (L) and culture medium (M) were detected by immunoblotting with anti-ATX antibody. B and C, Colo320 cells were transfected with nonspecific control siRNA or siRNAs (#1 and #2) against p23 (B) or siRNAs (#1 and #2) against p24 (C). Forty eight hours after siRNA transfection, ATX protein levels in cell lysates and culture medium were detected by immunoblotting with anti-ATX antibody. D, Colo320 cells were transfected with the nonspecific control siRNA or siRNA against ERGIC53. Forty eight hours after transfection, ATX protein levels in cell lysates and culture medium were detected by immunoblotting with anti-ATX antibody. Data are representative of three independent experiments.
ATX ER export is regulated by AKT-signaling pathway
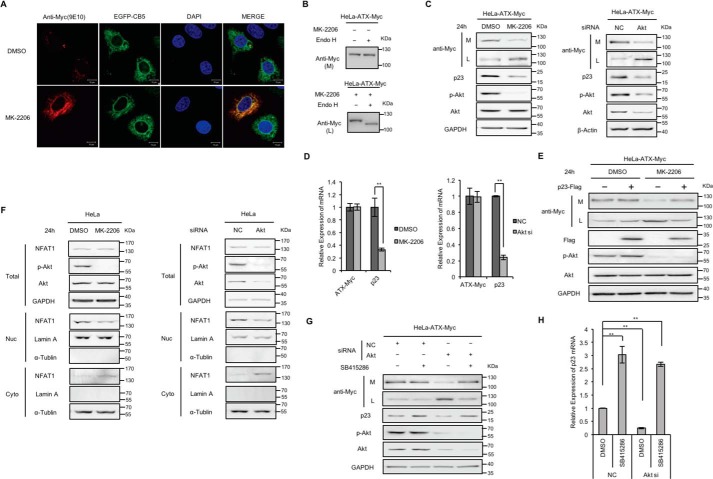
When HeLa cells were transfected with the vector driving the expression of ATX-Myc and subsequently treated with MK-2206 (5 μm), a selective allosteric inhibitor of AKT (33), it was found that ATX-Myc was trapped within the cells and co-localized with the ER marker CB5. In the control cells without MK-2206 treatment, as a secreted protein, ATX-Myc was predominantly detected in culture medium and barely in cells (Fig. 5, A and C). ATX-Myc secreted into the culture medium was resistant to Endo H, whereas the ATX-Myc restrained within cells by the treatment of AKT inhibitor was sensitive to Endo H (Fig. 5B). In addition, secretion of ATX-Myc was significantly suppressed when AKT was down-regulated by siRNA, leading to the retention of ATX-Myc within cells (Fig. 5C). These results suggest that AKT signaling is involved in the regulation of ATX secretion. It was interesting to note that, in the HeLa cells transfected with the vector driving the expression of ATX-Myc, AKT inhibitor MK-2206 or AKT siRNA treatment did not affect the ATX-Myc expression but resulted in the down-regulation of p23 expression at the transcriptional level (Fig. 5D). The suppression of ATX secretion by AKT inhibitor was released by the exogenous expression of p23 (Fig. 5E). It has been demonstrated that the expression of human p23 gene is transcriptionally regulated by NFAT (34). We found that the AKT inhibitor MK-2206 or AKT siRNA treatment enhanced the nuclear export of NFAT (Fig. 5F). It is well known that inhibition of AKT activation is able to activate GSK-3β, a protein kinase that has been reported to enhance the nuclear export of NFAT (35, 36). In fact, the inhibition of GSK-3β with its specific inhibitor SB415286 increased p23 expression and reversed the p23 expression suppression by AKT siRNA (Fig. 5, G and H). Collectively, the activation of AKT signaling, which leads to GSK-3β inhibition, can facilitate ATX ER export by enhancing the NFAT-mediated p23 expression at transcriptional level.
Figure 5.
Effects of AKT inhibitor treatment and AKT knockdown on ATX secretion. A, HeLa cells were co-transfected with pcDNA3-ATX-Myc and pEGFP-N1-CB5 and then treated with or without AKT inhibitor MK-2206 (5 μm) for 24 h. ATX-Myc was visualized by confocal microscopy with anti-Myc (9E10) monoclonal antibody (red). Endoplasmic reticulum was labeled by EGFP-CB5 (green). Nuclei were counterstained with DAPI (blue). B, HeLa cells were transfected with pcDNA3-ATX-Myc and then treated with or without AKT inhibitor MK-2206 (5 μm) for 24 h. The concentrated (∼30-fold) serum-free conditional culture medium of the control cells and the lysate of AKT inhibitor-treated cells were treated with Endo H and then analyzed by Western blotting with anti-Myc antibody. C, HeLa cells with exogenous expression of ATX-Myc were treated with MK-2206 (5 μm) for 24 h or treated with siRNA against AKT for 48 h. Then ATX-Myc protein levels in cell lysates (L) and culture medium (M) were detected by immunoblotting with anti-Myc antibody. p23, AKT, and phosphorylated AKT (p-AKT) levels in cell lysates were detected by immunoblotting. D, HeLa cells with exogenous expression of ATX-Myc were treated with MK-2206 (5 μm) for 24 h or treated with siRNA against AKT for 48 h. ATX-Myc and p23 mRNA levels were detected by quantitative reverse transcription PCR (qRT-PCR). E, HeLa cells with exogenous expression of ATX-Myc and FLAG-p23 were treated with or without AKT inhibitor MK-2206 (5 μm) for 24 h. ATX-Myc protein levels in cell lysates and culture medium were detected by immunoblotting with anti-Myc antibody. FLAG-p23 levels in cell lysates were detected by immunoblotting with anti-FLAG antibody. F, HeLa cells were treated with MK-2206 (5 μm) for 24 h or treated with siRNA against AKT for 48 h. Then, NFAT1 levels in nuclear fraction (Nuc), cytoplasmic fraction (Cyto), and total cell lysates (Total) were detected by Western blotting. Lamin A and α-tubulin were used as the nuclear and cytoplasm markers, as indicated. G and H, HeLa cells with exogenous expression of ATX-Myc were transfected with nonspecific control siRNA (NC) or siRNA against AKT. Thirty two hours after transfection, cells were treated with or without GSK3β inhibitor SB415286 (25 μm) for 16 h. Then, ATX-Myc protein levels in cell lysates and culture medium, and p23 levels in cell lysates were detected by immunoblotting (G). p23 mRNA levels were detected by qRT-PCR (H). The p value was derived from analysis of variance. **, p < 0.005. Data are representative of three independent experiments.
Selective ER export of ENPP1 and ENPP3
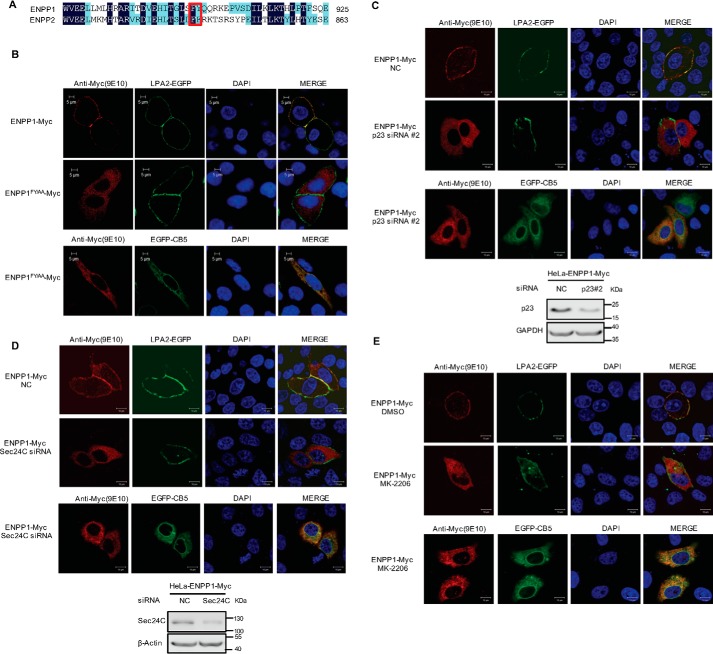
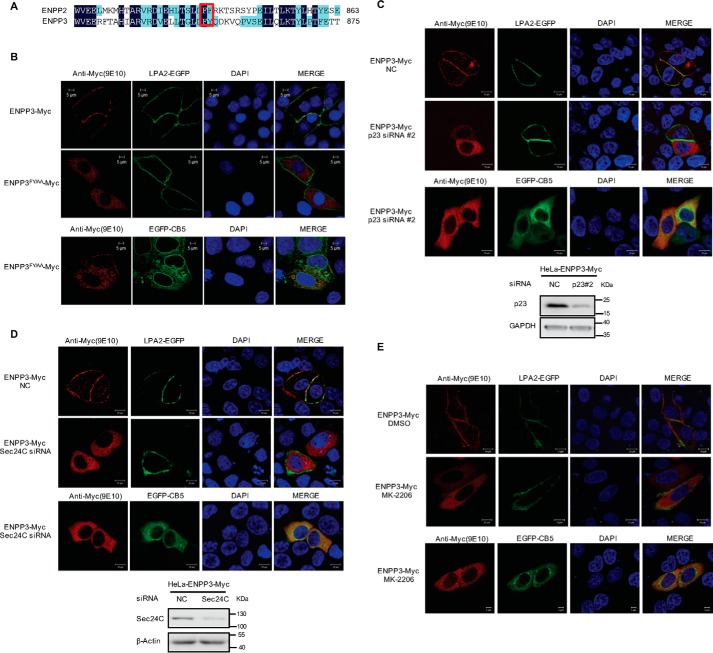
ATX, also known as ENPP2, belongs to the ENPP family. ENPP1 and ENPP3, akin to the structure of ATX, are type II transmembrane proteins located at the cell membrane through their N-terminal transmembrane region. Sequence homology analysis showed that there is a di-hydrophobic FY motif in the C-terminal regions of human ENPP1 and ENPP3, corresponding to the position of FF motif in ATX (Figs. 6A and 7A). We constructed the plasmid expressing LPA receptor 2 (LPA2)-GFP fusion protein as a molecular marker of cell membrane. When LPA2-GFP was co-expressed with ENPP1-Myc, ENPP1FY/AA-Myc, ENPP3-Myc, or ENPP3FY/AA-Myc, respectively, in HeLa cells, it was found that ENPP1-Myc and ENPP3-Myc were co-localized with membrane marker LPA2-EGFP, whereas ENPP1FY/AA-Myc and ENPP3FY/AA-Myc were retained within cells to be co-localized with the ER marker CB5 (Figs. 6B and 7B). These results suggest that the FY motifs in the C-terminal region of ENPP1 and ENPP3 are required for their ER export. When p23 or Sec24C was down-regulated by siRNA, the translocation of ENPP1/3 to the cytomembrane was disrupted, and ENPP1/3 was retained within cells (Figs. 6, C and D, and 7, C and D). When the cells were treated with AKT inhibitor MK-2206, ENPP1/3 translocation was also inhibited, and both of them were retained within cells to be co-localized with the ER marker CB5 (Figs. 6E and 7E). Therefore, the di-hydrophobic motifs (FF or FY) in the nuclease-like domain of ENPP1, ENPP2 (ATX), and ENPP3 are functionally conserved, participating in their secretion/translocation, particularly in the process of ER export. ENPP1, ENPP2 (ATX), and ENPP3 may have a common ER export mechanism with the involvement of p23, Sec24C, and AKT.
Figure 6.
Roles of di-hydrophobic amino acid motif (FY), p23, Sec24C, and AKT in the cellular translocation of ENPP1. A, amino acid sequence alignment of ENPP1 and ENPP2 (ATX) C-terminal regions. The FF/FY motif is indicated in a red rectangular box. B, di-hydrophobic amino acid motif (FY) is essential for the translocation of ENPP1. pEGFP-C1-LPA2 or pEGFP-N1-CB5 was co-transfected with pcDNA3-ENPP1-Myc or pcDNA3-ENPP1FY/AA-Myc as indicated into HeLa cells. Cells were fixed and permeabilized 48 h after transfection. C, effects of p23 knockdown on the translocation of ENPP1. HeLa cells were treated with nonspecific control siRNA (NC) or p23 siRNA#2 for 24 h and then transfected with pEGFP-C1-LPA2 or pEGFP-N1-CB5 together with pcDNA3-ENPP1-Myc. Cells were fixed and permeabilized 24 h after transfection. D, effects of Sec24C knockdown on the translocation of ENPP1. HeLa cells were treated with nonspecific control siRNA (NC) or Sec24C siRNA for 24 h and then transfected with pEGFP-C1-LPA2 or pEGFP-N1-CB5 together with pcDNA3-ENPP1-Myc. Cells were fixed and permeabilized 24 h after transfection. E, HeLa cells were transfected with pEGFP-C1-LPA2 or pEGFP-N1-CB5 together with pcDNA3-ENPP1-Myc and then treated with or without AKT inhibitor MK-2206 (5 μm). Cells were fixed and permeabilized 24 h after inhibitor treatment. ENPP1-Myc and ENPP1FY/AA-Myc were visualized by confocal microscopy with anti-Myc (9E10) monoclonal antibody (red). LPA2-EGFP was used as a marker of cell membrane (green). Endoplasmic reticulum was labeled by EGFP-CB5 (green). The nuclei were counterstained with DAPI (blue). Data are representative of three independent experiments.
Figure 7.
Roles of di-hydrophobic amino acid motif (FY), p23, Sec24C, and AKT in the cellular translocation of ENPP3. A, amino acid sequence alignment of ENPP3 and ENPP2 (ATX) C-terminal regions. The FF/FY motif is indicated in red rectangular box. B, di-hydrophobic amino acid motif (FY) is essential for the translocation of ENPP3. pEGFP-C1-LPA2 or pEGFP-N1-CB5 was co-transfected with pcDNA3-ENPP3-Myc or pcDNA3-ENPP3 FY/AA-Myc as indicated into HeLa cells. Cells were fixed and permeabilized 48 h after transfection. C, effects of p23 knockdown on the translocation of ENPP3. HeLa cells were treated with nonspecific control siRNA (NC) or p23 siRNA#2 for 24 h and then transfected with pEGFP-C1-LPA2 or pEGFP-N1-CB5 together with pcDNA3-ENPP3-Myc. Cells were fixed and permeabilized 24 h after transfection. D, effects of Sec24C knockdown on the translocation of ENPP3. HeLa cells were treated with nonspecific control siRNA (NC) or Sec24C siRNA for 24 h, and then transfected with pEGFP-C1-LPA2 or pEGFP-N1-CB5 together with pcDNA3-ENPP3-Myc. Cells were fixed and permeabilized 24 h after transfection. E, HeLa cells were transfected with pEGFP-C1-LPA2 or pEGFP-N1-CB5 together with pcDNA3-ENPP3-Myc and then treated with or without AKT inhibitor MK-2206 (5 μm). Cells were fixed and permeabilized 24 h after inhibitor treatment. ENPP3-Myc and ENPP3FY/AA-Myc were visualized by confocal microscopy with anti-Myc (9E10) monoclonal antibody (red). LPA2-EGFP was used as a marker of cell membrane (green). Endoplasmic reticulum was labeled by EGFP-CB5 (green). The nuclei were counterstained with DAPI (blue). Data are representative of three independent experiments.
Discussion
ATX is a secretory glycoprotein with lyso-PLD activity and functions as the key enzyme in the generation of LPA. LPA is a bioactive lysophospholipid mediator that regulates cell proliferation, differentiation, apoptosis, and migration through its specific receptor on cell membranes. The ATX-LPA axis is involved in the regulation of various physiological and pathological processes especially in inflammation and cancer development. Although the mechanisms that control ATX gene expression have been extensively studied, the process and regulatory mechanism of ATX secretion are still not well understood.
In this study, we demonstrated that a di-phenylalanine (Phe-838/Phe-839) motif in the ATX C-terminal region functioned as an ER export signal. The ATXFF/AA mutant was trapped in the ER, whereas wild-type ATX was exported from the ER efficiently, indicating that the FF motif is essential for the ER export of ATX. It has been reported that the di-hydrophobic motif (FF, LL, VV, etc.) in the C-terminal cytosolic domain of transmembrane protein can interact with Sec24 in COPII to mediate the export of the membrane protein from the ER (37). However, it has been reported that ATX is not a transmembrane protein, which means that the FF motif in the C-terminal region of ATX cannot directly bind to COPII subunits. In the structure of ATX, the C-terminal region is sandwiched between the PDE and NUC domain with the FF motif (FY in mouse and rat ATX) exposed at the surface of the molecule (38). This structural arrangement is consistent with its role in supporting the secretion of ATX.
The export of cargo protein from the ER can be mediated by transmembrane cargo receptors such as ERGIC-53 protein, p24 proteins, and a set of ER vesicle (Erv) proteins, which link lumenal cargo to COPII subunits (39). In this study, for the first time we demonstrated that p23, a member of the p24 protein family, participated in ER export of ATX as a cargo receptor. ATX could interact with p23, and the FF motif in ATX C-terminal region was essential for the ATX-p23 interaction. It has been reported that p23 can interact with other members of the p24 subfamily to form an oligomeric complex, especially to form the functional p23-p24 complex (29). However, ER export of ATX was significantly suppressed by the knockdown of p23, but not p24, suggesting that p23 is specifically required for ATX transport. The homozygous knock-out of p23 in mice results in early embryonic lethality, indicating that p23 plays an essential and non-redundant role in the early stage of mammalian development (40). Because ATX knock-out in mice is also lethal in the embryonic stage (41), the identification of ATX as the cargo substrate of p23 will improve our understanding of p23 biological function. It is worth investigating the physiological importance of p23-mediated selective secretion of ATX in embryonic development in a future study.
The cytosolic C-terminal tail of p23 protein contains a sorting signal for binding COPII allowing the p23-bound cargo protein to be efficiently sorted into COPII vesicles (42). Cells are endowed with four Sec24 isoforms termed as Sec24A–D, which bind to specific sorting signals to mediate selective export of proteins from the ER (23, 24). The ER export of ATX was significantly suppressed by the knockdown of Sec24C, but not other Sec24 subunits, indicating Sec24C is specifically required for ATX ER export.
The role of AKT in ATX secretion regulation was explored in this study. In the HeLa cells transfected with plasmid expressing ATX, ATX secretion was significantly suppressed when AKT-signaling pathway was blocked by the AKT inhibitor or by AKT knockdown, indicating that AKT is involved in the regulation of ATX secretion. It has been reported that p23 gene expression is transcriptionally regulated by NFAT signaling (34). Further study demonstrated that inhibition of the AKT-signaling pathway resulted in the down-regulation of p23 expression by promoting NFAT export from the nucleus and that the exogenous expression of p23 could release the suppression of ATX secretion by the AKT inhibitor. GSK-3β, which is activated when AKT signaling is inhibited, functions as one of the NFAT kinases to promote the nuclear export of NFAT (35, 36) and suppresses NFAT-mediated gene expression (36). The down-regulation of p23 expression by AKT knockdown was reversed by treatment with the GSK-3β inhibitor. Therefore, our findings and work by others suggest that ATX ER export can be regulated by the AKT-GSK-3β pathway through modulating the NFAT-mediated p23 expression. It has been reported previously that NFATs induce the transcription of the ATX in breast epithelial cells (43). Actually, in Colo320 cells, which have high levels of endogenous ATX, the AKT inhibitor not only suppressed ATX secretion but also inhibited endogenous ATX expression at the transcriptional level (data not show). Therefore, AKT signaling may be involved in both ATX expression and secretion regulation. ATX is highly expressed in several cancer cells and contributes to their tumorigenesis, invasion, and metastases (44). AKT is often hyperactivated in aggressive cancers and regarded as a key target of anti-cancer therapeutics (45–47). The findings in this study provide a relationship between the AKT-signaling pathway and ATX, which could be a potential target for tumor therapy.
ATX, also named as ENPP2, belongs to the ENPP family, and it shares high homology with ENPP1 and ENPP3. We uncovered an FY motif in the C-terminal regions of ENPP1 and ENPP3, at the corresponding location of the FF motif in ATX. The FY motif was required for the intracellular transport of ENPP1 and ENPP3, and the translocation of ENPP1/3 to cell membrane was disrupted when either p23 or Sec24C was down-regulated by specific siRNA. Upon treatment with the AKT inhibitor, ER retention of ENPP1/3 was observed in the HeLa cells. These data indicate that the selective ER export mediated by p23 and Sec24C and its regulation by AKT signaling are conserved among these ENPP family members.
In conclusion, we have demonstrated the mechanism of ATX ER export in this study. p23 was identified as the cargo receptor for ATX and an FF motif in ATX C-terminal region as an ER export signal. The selective export of ATX from the ER is mediated by Sec24C and regulated by the AKT-signaling pathway by modulating the NFAT-mediated p23 expression. The di-hydrophobic motif also exists in the C-terminal region of ENPP1 and ENPP3, and the p23, Sec24C, and AKT-involved ER export mechanism is conserved among these ENPP family members. Our work not only reveals the molecular mechanism of the selective ER export of ATX but also puts insight into the potential novel biological function for the p23-mediated protein secretion.
Experimental procedures
Reagents and antibodies
Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum (FBS) were purchased from Thermo Fisher Scientific (Bremen, Germany). l-Glutamine and penicillin/streptomycin were obtained from Gibco Invitrogen. Primary monoclonal antibody (mAb) against the Myc epitope was purchased from Sigma (Buchs, Switzerland). Anti-FLAG (M185-3L) antibody was purchased from MBL (Nagoya, Japan). Anti-autotaxin(58–157) polyclonal antibody was obtained as described previously (48). Rabbit antibody against human Sec24C, anti-p23 antibody, anti-ERGIC53 antibody, and the anti-NFAT1 antibody were purchased from Abcam (Cambridge, UK). Anti-AKT antibody and anti-AKT (Ser-473) antibody were purchased from Cell Signaling Technology (Danvers, MA). Anti-p24 antibody, anti-lamin A/C antibody, and anti-α-tubulin antibody were purchased from Santa Cruz Biotechnology. AKT inhibitor MK-2206 (S1078) and GSK3β inhibitor SB415286 were purchased from Selleck Chemicals (Houston, TX).
Plasmids
pEGFP-N1-CB5 was provided by Dr. Peng Li (Tsinghua University). pEGFP-C1-LPA2 was obtained from Dr. Yan Xu (Indiana University). cDNAs encoding wild-type ATX, ENPP1, and ENPP3 were cloned into the pcDNA3 vector, creating the pcDNA3-ATX-Myc, pcDNA3-ENPP1-Myc, and pcDNA3-ENPP3-Myc, respectively, to express ENPPs fused with Myc tag at their C terminus. Mutagenesis of ATXFF/FA-Myc, ATXFF/AF-Myc, ATXFF/AA-Myc, ATXFF/LL-Myc, ATXFF/YY-Myc, ENPP1FY/AA-Myc, and ENPP3FY/AA-Myc were generated by site-directed mutagenesis from pcDNA3-ATX-Myc, pcDNA3-ENPP1-Myc, and pcDNA3-ENPP3-Myc, respectively. The cDNA encoding p23 was cloned into a pCMV-tag4 vector, creating the plasmid pCMV-p23-FLAG to express p23 protein fused with FLAG tag at its C terminus.
Cell culture and transfection
HeLa cells were maintained in DMEM containing 10% FBS, 100 units/ml penicillin, and 100 mg/liter streptomycin in 5% CO2 at 37 °C. Transient transfection was performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. The target sequences of siRNAs used in this study were as follows: si-NC, 5′-GGCTGCTGTGTAGATCTCT-3′; si-Sec24A, 5′-GAGTCAGTGAGCCAAGGAT-3′; si-Sec24B, 5′-GCCGATCCTGTCGAACGTATA-3′; si-Sec24C, 5′-CATAAACAAGACCTCACAG-3′; si-Sec24D, 5′-CTGTCTTACCCAGGAGGCT-3′; si-AKT, 5′-CAGCATGAGGTTCTCCAGC-3′; si-p23#1, 5′-ATACCTGACCAACTCGTGA-3′; si-p23#2, 5′-GCCATATTCTCTACTCCAA-3′; si-p24#1, 5′-AAGCATTTACTAATTGGAAA-3′; si-p24#2, 5′-CCAAACACCTTGGTCATAA-3′ and si-ERGIC53, 5′-GGACAGAATCGTATTCAT-3′.
RNA isolation and quantitative reverse transcription PCR
Total cellular RNA was extracted from cells with TRIzol regent (Sigma, Buchs, Switzerland). RNA (1 μg) from cells was reverse-transcribed using an anchored oligo(dT) primer and a reverse transcription system (Promega, Madison, WI). The levels of ATX mRNA, GAPDH mRNA, and p23 mRNA were detected using the following primer pairs: 5′-TATGCTTCGGAAAGAAATGGAG-3′ and 5′-ATGTTCAATGTCACGCACCCT-3′ for ATX mRNA; 5′-TTAGCACCCCTGTCCAAGG-3′ and 5′-CCTACTCCTTGGAGGCCATG-3′ for GAPDH mRNA; and 5′-GCGACGCCTAGAAGACCTTT-3′ and 5′-GAAGCGTCGCAGGTAGAAGA-3′ for p23 mRNA. Each RT-PCR experiment was repeated at least three times with three replicate samples. Real time RT-PCR was performed using SYBR Green Supermix (Thermo Fisher Scientific, Bremen, Germany) with an iCycler iQ real time RT-PCR detection system (Bio-Rad). Relative expression of each target gene was determined by normalization to the expression of GAPDH. Standard curves were generated using relative concentration versus the threshold cycle (Ct). Based on the slopes of the standard curves, the amplification efficiencies of the standards ranged from 93 to 103%, (derived from the formula E = 101/−slope −1). The not normalized Ct values of all the genes in all the samples were within 14.5 to 30 cycles, covered by the range of the standard curves.
Western blotting analysis
Cells were lysed with RIPA lysis buffer (150 mm NaCl, 1% Nonidet P-40), 50 mm Tris-HCl, 1 mm phenylmethylsulfonyl fluoride, 1 μg/ml leupeptin, 1 mm deoxycholic acid, and 1 mm EDTA) at 4 °C and then subjected to centrifugation at 12,000 × g for 10 min. After centrifugation, the supernatant was quantified by the bicinchoninic acid assay (Micro BCA; Pierce). For experiments on detection of secreted ATX protein, the culture medium was concentrated (∼30-fold) using Amicon Ultra 30,000 (Millipore). Protein samples were resolved by 10% SDS-PAGE, transferred to polyvinylidene fluoride transfer membranes (RPN303F, Amersham Biosciences, Freiburg, Germany), and blocked with skim milk. Membranes were then incubated with primary antibodies, using the manufacturer's protocol, followed by incubation with the appropriate secondary antibody conjugated with horseradish peroxidase. Blot signals were exposed using enhanced chemiluminescence detection system.
Immunoprecipitation
HeLa cells were co-transfected with the plasmid driving the expression of p23-FLAG and pcDNA3-ATX-Myc or pcDNA3-ATXFF/AA-Myc. At 48 h after transfection, cells were lysed in 500 μl of lysis buffer (50 mm Tris-HCl (pH 7.6), 150 mm NaCl, 5 mm MgCl2, 0.1% Nonidet P-40, 1 mm dithiothreitol, 0.1 mm sodium orthovanadate, 1 mm phenylmethylsulfonyl fluoride (PMSF), and 10 units/ml proteinase inhibitor tablet (Roche Diagnostics, Mannheim, Germany)) on ice for 30 min and then centrifuged at 12,000 × g for 10 min. Each lysate was immunoprecipitated with 30 μl of anti-Myc agarose affinity gel (Sigma, Buchs, Switzerland) or 30 μl of anti-FLAG M2 Affinity Gel (Macgene, Beijing, China) for 3 h at 4 °C. Precipitated samples were washed once with 1 ml of lysis buffer and three times with 1 ml of 20 mm Tris-HCl (pH 7.6), 100 mm NaCl, 1 mm EDTA, and 0.1% Nonidet P-40 for 10 min at 4 °C. Proteins bound to the beads were eluted with SDS-loading buffer and separated by SDS-PAGE followed by immunoblotting.
Immunofluorescence microscopy
HeLa cells were transfected with plasmid driving the expression of the Myc-tagged protein as indicated. Forty eight hours after transfection, the cells were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) for 15–30 min. Fixed cells were blocked with skim milk for 1 h and incubated with anti-Myc antibody overnight at 4 °C. Following incubation with secondary goat anti-mouse antibody-FITC in PBS (with Tween 20), 5% skim milk for 1 h, the fluorescence images were detected with a confocal laser-scanning microscope LSM-510-Meta (Carl Zeiss, Jena, Germany).
Nuclear and cytosolic extract preparation
Cells were washed with cold PBS, and cellular fractions were isolated using a nuclear and cytoplasmic extraction kit (CW0199S and CWBIO, Beijing, China). Nuclear and cytoplasm fractions were analyzed for NFAT1 protein levels by Western blotting. Lamin A and α-tubulin were used as the nuclear and cytoplasm markers, respectively.
Deglycosylation assay
The concentrated (∼30-fold) serum-free conditional culture medium (for the secreted ATX) and cell lysate (for the ATX within cells) were treated with Endo H (New England Biolabs, P0702) following the manufacturer's protocol. The deglycosylated samples were subjected to Western blotting analysis.
Author contributions
J. Z. conceived and designed the experiments. J. Z. and L. L. wrote the manuscript. L. L. performed the experiments to identify the role of p23, Sec24C, AKT, and the di-hydrophobic motif (FF or FY) in the ER export of ATX and ENPP1/3. B. W. contributed partly to demonstrate the requirement di-hydrophobic motif (FF or FY) in the ER export of ENPP1/2/3. C. X. contributed partly to identify the role of FF motif in ATX ER export. Xiaotian Zhang and Xiaoyan Zhang provided technical and material support.
This work was supported by Grants 31470765 and 31500619 from the National Natural Science Foundation of China. The authors declare that they have no conflicts of interest with the contents of this article.
- ATX
- autotaxin
- LPA
- lysophosphatidic acid
- ER
- endoplasmic reticulum
- COPII
- coatomer protein complex II
- ENPP
- ectonucleotide pyrophosphatase/phosphodiesterase
- NFAT
- nuclear factor of activated T cell
- GSK3β
- glycogen synthase kinase 3β
- CB5
- cytochrome b5
- EGFP
- enhanced green fluorescent protein
- Endo H
- endoglycosidase H
- GPCR
- G protein-coupled receptor
- GPI-AP
- glycosylphosphatidylinositol-anchored protein
- ERGIC
- ER-Golgi intermediate compartment
- lyso-PLD
- lysophospholipase D
- PDE
- phosphodiesterase
- NUC
- nuclease-like.
References
- 1. Yuelling L. M., and Fuss B. (2008) Autotaxin (ATX): a multi-functional and multi-modular protein possessing enzymatic lyso-PLD activity and matricellular properties. Biochim. Biophys. Acta 1781, 525–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pua T. L., Wang F. Q., and Fishman D. A. (2009) Roles of LPA in ovarian cancer development and progression. Future Oncol. 5, 1659–1673 [DOI] [PubMed] [Google Scholar]
- 3. Willier S., Butt E., and Grunewald T. G. (2013) Lysophosphatidic acid (LPA) signalling in cell migration and cancer invasion: a focussed review and analysis of LPA receptor gene expression on the basis of more than 1700 cancer microarrays. Biol. Cell 105, 317–333 [DOI] [PubMed] [Google Scholar]
- 4. Zaslavsky A., Singh L. S., Tan H., Ding H., Liang Z., and Xu Y. (2006) Homo- and hetero-dimerization of LPA/S1P receptors, OGR1 and GPR4. Biochim. Biophys. Acta 1761, 1200–1212 [DOI] [PubMed] [Google Scholar]
- 5. Im D. S. (2010) Pharmacological tools for lysophospholipid GPCRs: development of agonists and antagonists for LPA and S1P receptors. Acta Pharmacol. Sin. 31, 1213–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wu T., Kooi C. V., Shah P., Charnigo R., Huang C., Smyth S. S., and Morris A. J. (2014) Integrin-mediated cell surface recruitment of autotaxin promotes persistent directional cell migration. FASEB J. 28, 861–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Houben A. J., and Moolenaar W. H. (2011) Autotaxin and LPA receptor signaling in cancer. Cancer Metastasis Rev. 30, 557–565 [DOI] [PubMed] [Google Scholar]
- 8. Knowlden S., and Georas S. N. (2014) The autotaxin-LPA axis emerges as a novel regulator of lymphocyte homing and inflammation. J. Immunol. 192, 851–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rancoule C., Dusaulcy R., Tréguer K., Grès S., Attané C., and Saulnier-Blache J. S. (2014) Involvement of autotaxin/lysophosphatidic acid signaling in obesity and impaired glucose homeostasis. Biochimie 96, 140–143 [DOI] [PubMed] [Google Scholar]
- 10. Nishimura S., Nagasaki M., Okudaira S., Aoki J., Ohmori T., Ohkawa R., Nakamura K., Igarashi K., Yamashita H., Eto K., Uno K., Hayashi N., Kadowaki T., Komuro I., Yatomi Y., and Nagai R. (2014) ENPP2 contributes to adipose tissue expansion and insulin resistance in diet-induced obesity. Diabetes 63, 4154–4164 [DOI] [PubMed] [Google Scholar]
- 11. Tabchy A., Tigyi G., and Mills G. B. (2011) Location, location, location: a crystal-clear view of autotaxin saturating LPA receptors. Nat. Struct. Mol. Biol. 18, 117–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nishimasu H., Okudaira S., Hama K., Mihara E., Dohmae N., Inoue A., Ishitani R., Takagi J., Aoki J., and Nureki O. (2011) Crystal structure of autotaxin and insight into GPCR activation by lipid mediators. Nat. Struct. Mol. Biol. 18, 205–212 [DOI] [PubMed] [Google Scholar]
- 13. Koike S., Keino-Masu K., Ohto T., and Masu M. (2006) The N-terminal hydrophobic sequence of autotaxin (ENPP2) functions as a signal peptide. Genes Cells 11, 133–142 [DOI] [PubMed] [Google Scholar]
- 14. Jansen S., Stefan C., Creemers J. W., Waelkens E., Van Eynde A., Stalmans W., and Bollen M. (2005) Proteolytic maturation and activation of autotaxin (NPP2), a secreted metastasis-enhancing lysophospholipase D. J. Cell Sci. 118, 3081–3089 [DOI] [PubMed] [Google Scholar]
- 15. Pradère J. P., Tarnus E., Grès S., Valet P., and Saulnier-Blache J. S. (2007) Secretion and lysophospholipase D activity of autotaxin by adipocytes are controlled by N-glycosylation and signal peptidase. Biochim. Biophys. Acta 1771, 93–102 [DOI] [PubMed] [Google Scholar]
- 16. Jansen S., Callewaert N., Dewerte I., Andries M., Ceulemans H., and Bollen M. (2007) An essential oligomannosidic glycan chain in the catalytic domain of autotaxin, a secreted lysophospholipase-D. J. Biol. Chem. 282, 11084–11091 [DOI] [PubMed] [Google Scholar]
- 17. Jansen S., Andries M., Derua R., Waelkens E., and Bollen M. (2009) Domain interplay mediated by an essential disulfide linkage is critical for the activity and secretion of the metastasis-promoting enzyme autotaxin. J. Biol. Chem. 284, 14296–14302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bannykh S. I., Rowe T., and Balch W. E. (1996) The organization of endoplasmic reticulum export complexes. J. Cell Biol. 135, 19–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lippincott-Schwartz J., Roberts T. H., and Hirschberg K. (2000) Secretory protein trafficking and organelle dynamics in living cells. Annu. Rev. Cell Dev. Biol. 16, 557–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bonifacino J. S., and Glick B. S. (2004) The mechanisms of vesicle budding and fusion. Cell 116, 153–166 [DOI] [PubMed] [Google Scholar]
- 21. Ellgaard L., Molinari M., and Helenius A. (1999) Setting the standards: quality control in the secretory pathway. Science 286, 1882–1888 [DOI] [PubMed] [Google Scholar]
- 22. Miller E. A., and Barlowe C. (2010) Regulation of coat assembly–sorting things out at the ER. Curr. Opin. Cell Biol. 22, 447–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barlowe C. (2003) Signals for COPII-dependent export from the ER: what's the ticket out? Trends Cell Biol. 13, 295–300 [DOI] [PubMed] [Google Scholar]
- 24. Gürkan C., Stagg S. M., Lapointe P., and Balch W. E. (2006) The COPII cage: unifying principles of vesicle coat assembly. Nat. Rev. Mol. Cell Biol. 7, 727–738 [DOI] [PubMed] [Google Scholar]
- 25. Schweizer A., Fransen J. A., Bächi T., Ginsel L., and Hauri H. P. (1988) Identification, by a monoclonal-antibody, of a 53-Kd protein associated with a tubulo-vesicular compartment at the cis-side of the Golgi-apparatus. J. Cell Biol. 107, 1643–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Takida S., Maeda Y., and Kinoshita T. (2008) Mammalian GPI-anchored proteins require p24 proteins for their efficient transport from the ER to the plasma membrane. Biochem. J. 409, 555–562 [DOI] [PubMed] [Google Scholar]
- 27. Strating J. R., and Martens G. J. (2009) The p24 family and selective transport processes at the ER-Golgi interface. Biol. Cell 101, 495–509 [DOI] [PubMed] [Google Scholar]
- 28. Kinoshita T., Maeda Y., and Fujita M. (2013) Transport of glycosylphosphatidylinositol-anchored proteins from the endoplasmic reticulum. Biochim. Biophys. Acta 1833, 2473–2478 [DOI] [PubMed] [Google Scholar]
- 29. Bonnon C., Wendeler M. W., Paccaud J. P., and Hauri H. P. (2010) Selective export of human GPI-anchored proteins from the endoplasmic reticulum. J. Cell Sci. 123, 1705–1715 [DOI] [PubMed] [Google Scholar]
- 30. Li X., Wu Y., Shen C., Belenkaya T. Y., Ray L., and Lin X. (2015) Drosophila p24 and Sec22 regulate wingless trafficking in the early secretory pathway. Biochem. Biophys. Res. Commun. 463, 483–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Buechling T., Chaudhary V., Spirohn K., Weiss M., and Boutros M. (2011) p24 proteins are required for secretion of Wnt ligands. EMBO Rep. 12, 1265–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wu G. (2012) Regulation of post-Golgi traffic of G protein-coupled receptors. Subcell Biochem. 63, 83–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sangai T., Akcakanat A., Chen H. Q., Tarco E., Wu Y., Do K. A., Miller T. W., Arteaga C. L., Mills G. B., Gonzalez-Angulo A. M., and Meric-Bernstam F. (2012) Biomarkers of response to Akt inhibitor MK-2206 in breast cancer. Clin. Cancer Res. 18, 5816–5828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu S., Zhang S., Bromley-Brits K., Cai F., Zhou W., Xia K., Mittelholtz J., and Song W. (2011) Transcriptional regulation of TMP21 by NFAT. Mol. Neurodegener 6, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Beals C. R., Sheridan C. M., Turck C. W., Gardner P., and Crabtree G. R. (1997) Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science 275, 1930–1934 [DOI] [PubMed] [Google Scholar]
- 36. Kim M. S., Shutov L. P., Gnanasekaran A., Lin Z., Rysted J. E., Ulrich J. D., and Usachev Y. M. (2014) Nerve growth factor (NGF) regulates activity of nuclear factor of activated T-cells (NFAT) in neurons via the phosphatidylinositol 3-kinase (PI3K)-Akt-glycogen synthase kinase 3β (GSK3β) pathway. J. Biol. Chem. 289, 31349–31360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nufer O., Guldbrandsen S., Degen M., Kappeler F., Paccaud J. P., Tani K., and Hauri H. P. (2002) Role of cytoplasmic C-terminal amino acids of membrane proteins in ER export. J. Cell Sci. 115, 619–628 [DOI] [PubMed] [Google Scholar]
- 38. Nishimasu H., Ishitani R., Aoki J., and Nureki O. (2012) A 3D view of autotaxin. Trends Pharmacol. Sci. 33, 138–145 [DOI] [PubMed] [Google Scholar]
- 39. Dancourt J., and Barlowe C. (2010) Protein sorting receptors in the early secretory pathway. Annu. Rev. Biochem. 79, 777–802 [DOI] [PubMed] [Google Scholar]
- 40. Denzel A., Otto F., Girod A., Pepperkok R., Watson R., Rosewell I., Bergeron J. J., Solari R. C., and Owen M. J. (2000) The p24 family member p23 is required for early embryonic development. Curr. Biol. 10, 55–58 [DOI] [PubMed] [Google Scholar]
- 41. Fotopoulou S., Oikonomou N., Grigorieva E., Nikitopoulou I., Paparountas T., Thanassopoulou A., Zhao Z., Xu Y., Kontoyiannis D. L., Remboutsika E., and Aidinis V. (2010) ATX expression and LPA signalling are vital for the development of the nervous system. Dev. Biol. 339, 451–464 [DOI] [PubMed] [Google Scholar]
- 42. Contreras I., Yang Y., Robinson D. G., and Aniento F. (2004) Sorting signals in the cytosolic tail of plant p24 proteins involved in the interaction with the COPII coat. Plant Cell Physiol. 45, 1779–1786 [DOI] [PubMed] [Google Scholar]
- 43. Chen M., and O'Connor K. L. (2005) Integrin α6β4 promotes expression of autotaxin/ENPP2 autocrine motility factor in breast carcinoma cells. Oncogene 24, 5125–5130 [DOI] [PubMed] [Google Scholar]
- 44. Brindley D. N., Lin F. T., and Tigyi G. J. (2013) Role of the autotaxin-lysophosphatidate axis in cancer resistance to chemotherapy and radiotherapy. Biochim. Biophys. Acta 1831, 74–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mitsiades C. S., Mitsiades N., and Koutsilieris M. (2004) The Akt pathway: molecular targets for anti-cancer drug development. Curr. Cancer Drug Targets 4, 235–256 [DOI] [PubMed] [Google Scholar]
- 46. Morgensztern D., and McLeod H. L. (2005) PI3K/Akt/mTOR pathway as a target for cancer therapy. Anticancer Drugs 16, 797–803 [DOI] [PubMed] [Google Scholar]
- 47. Slomovitz B. M., and Coleman R. L. (2012) The PI3K/AKT/mTOR pathway as a therapeutic target in endometrial cancer. Clin. Cancer Res. 18, 5856–5864 [DOI] [PubMed] [Google Scholar]
- 48. Li S., and Zhang J. (2009) Lipopolysaccharide induces autotaxin expression in human monocytic THP-1 cells. Biochem. Biophys. Res. Commun. 378, 264–268 [DOI] [PubMed] [Google Scholar]