Abstract
Leishmania parasites have evolved a number of strategies to cope with the harsh environmental changes during mammalian infection. One of these mechanisms involves the functional gain that allows mitochondrial 2-Cys peroxiredoxins to act as molecular chaperones when forming decamers. This function is critical for parasite infectivity in mammals, and its activation has been considered to be controlled exclusively by the enzyme redox state under physiological conditions. Herein, we have revealed that magnesium and calcium ions play a major role in modulating the ability of these enzymes to act as molecular chaperones, surpassing the redox effect. These ions are directly involved in mitochondrial metabolism and participate in a novel mechanism to stabilize the decameric form of 2-Cys peroxiredoxins in Leishmania mitochondria. Moreover, we have demonstrated that a constitutively dimeric Prx1m mutant impairs the survival of Leishmania under heat stress, supporting the central role of the chaperone function of Prx1m for Leishmania parasites during the transition from insect to mammalian hosts.
Keywords: calcium, Leishmania, molecular chaperone, oligomerization, peroxiredoxin
Introduction
The Leishmania parasites, causative agents of human and canine leishmaniasis, are exposed to different growth conditions during their life cycle because of the migration from the insect to the mammalian host environment. Among the differences are the elevation of temperature, exposure to oxidants produced by the macrophages, pH acidification, and lower availability of oxygen and nutrients (1). In this context, parasite survival as well as the establishment of a successful intracellular infection relies on the development of adaptive mechanisms for hostile conditions. In Leishmania infantum, for instance, one such mechanism involves mitochondrial 2-Cys peroxiredoxin (LiPrx1m)4 (2), also named tryparedoxin peroxidase, which allows the parasite to cope with heat stress during the transition from the insect (25 °C) to the mammalian host (37 °C) (2, 3).
Prx1 subfamily members are peroxide-scavenging enzymes that display a 2-Cys catalytic mechanism and can assume distinct oligomeric states (dimers, decamers, and higher-order oligomers) (4). A dual function of peroxidase and molecular chaperone has been reported for several Prx1 enzymes and seems to be modulated by changes in quaternary structure (5–7). Factors such as the pH (8–10), ionic strength (8, 11, 12), protein concentration (3, 12, 13), and protein redox state (14, 15) can affect the dimer-decamer equilibrium of Prx1 members, but how these factors modulate the peroxidase and chaperone activities is still poorly understood. Overoxidation of peroxidasic cysteine, in particular, has been demonstrated to shut down the peroxidase function and to enhance the chaperone activity by stabilizing oligomers larger than decamers (5, 6). However, this functional switch appears to be relevant for only some members of Prx1 subfamily (16).
In Leishmania, the mitochondrial Prx1 can act as molecular chaperone and as a peroxidase, but only its role as chaperone is crucial for parasite infectivity in mammals (2, 3). Nonetheless, in vitro studies show that the peroxidase catalytic cycle modulates the chaperone reservoir of LiPrx1m, favoring chaperone-active decamers when reactive cysteine is reduced (Cp–SH) and chaperone-inactive dimers when Cp is oxidized into CpS–SCr (3). On the other hand, the enzyme from Leishmania mitochondrion (2) seems to be resistant to the over-oxidation mechanism that inactivates the peroxidase function, stabilizes high-order oligomers, and enhances the chaperone activity of fungal (5) and mammalian (6) 2-Cys Prx.
Recently, we have demonstrated that pH variations also affect the dimer-decamer equilibrium of Leishmania braziliensis Prx1m (LbPrx1m), a close orthologue of the L. infantum enzyme, indicating that the chaperone function of these proteins might not be exclusively modulated by their redox state (9). A pH shift from 8.0 to 7.0, commonly observed in the mitochondria of nutrient-deprived cells (17), is sufficient to stabilize oxidized decamers of LbPrx1m (9). Furthermore, it is unclear whether other factors from the mitochondrial environment affect the dimer-decamer interconversion of Leishmania Prx1m and its dual function. For 2-Cys Prx from distantly related species, it has been demonstrated that, at least in vitro, such equilibrium can be affected by ionic strength variations (11, 18) and post-translational modifications (19, 20).
Herein, we have demonstrated that the divalent cations Ca2+ and Mg2+, which are important co-factors of mitochondrial enzymes involved in cellular respiration (21–23), activate the chaperone function of oxidized LbPrx1m and enhance that of the reduced enzyme via a novel mechanism of decamer stabilization. Using an LbPrx1m mutant unable to decamerize, we showed that decamer formation is crucial for both the chaperone and peroxidase activities of LbPrx1m as well as for the protective role of this protein against heat stress in the parasite context. Together, our findings unveil an exclusive and redundant system in Leishmania that uses Ca2+ and Mg2+, in addition to pH and redox mechanisms, to maintain most of Prx1m in the decameric form and hence support parasite survival and infectivity in the mammalian host.
Results
Ca2+/Mg2+ ions induce LbPrx1m decamerization
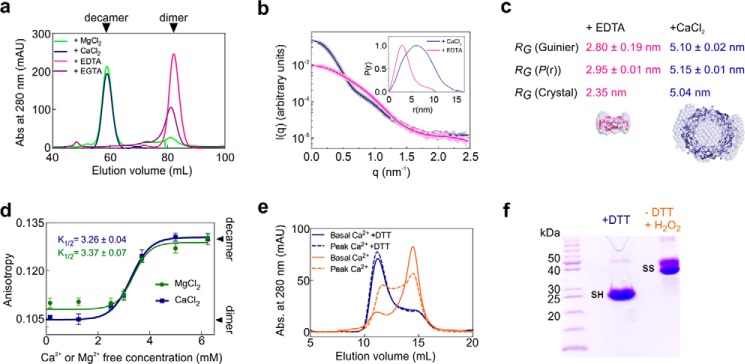
Analytical size-exclusion chromatography (aSEC) and small-angle X-ray scattering (SAXS) experiments at physiological pH revealed that oxidized (disulfide-bonded) LbPrx1m assumes a dimeric structure in the presence of chelating agents, whereas it assembles into decamers upon the addition of Ca2+ or Mg2+ (Fig. 1, a–c). Post-treatment of Ca2+ decamers with EDTA induced their disassembly into dimers, showing that the cation effect is a reversible process (Table 1). Upon comparison of several divalent cations, only Mg2+ and Ca2+ showed similar effects on LbPrx1m oligomerization (Table 2), indicating a specific role for these ions in stabilizing LbPrx1m decamers. The hypothesis that the Ca2+/Mg2+ effect would be due to ionic strength variations in the medium was discarded, as LbPrx1m presented the same SEC elution profile in the presence or absence of 150 mm NaCl (data not shown). As the effects of Mg2+ and Ca2+ were indistinguishable from each other, and because LbPrx1m eluted as dimers when incubated with EGTA (Fig. 1a), which chelates Ca2+ with a higher selectivity (>105) (24) than Mg2+, some of the in vitro and in silico assays described here were performed with Ca2+ only.
Figure 1.
Ca2+ and Mg2+ ions induce the decamerization of LbPrx1m. a, aSEC chromatograms of oxidized LbPrx1m at 130 μm (2 ml) in a HiLoad Superdex 200 16/600 column pre-equilibrated with Tris buffer (pH 7.5) containing 25 mm CaCl2, 25 mm MgCl2, 5 mm EDTA, or 5 mm EGTA. mAU, milliabsorbance units. b, experimental SAXS curves (open circles with error bars) and theoretical scattering profiles (lines) computed from the P(r) function (inset) of oxidized LbPrx1m (108 μm) in Tris buffer (pH 7.5) containing 25 mm CaCl2 (blue) or 5 mm EDTA (pink). c, radius of gyration (RG) for LbPrx1m incubated with EDTA or CaCl2, calculated independently from SAXS curves (graph in b), P(r) function (inset in b), or crystallographic data. The RG value (crystal) was calculated from the scattering profiles computed for the crystal structures (PDB accession no. 4KB3 and 4KCE) using CRYSOL. The crystal structures of the dimer (pink) and the decamer (blue) are fitted into the respective envelopes calculated from SAXS data. d, fluorescence anisotropy data of oxidized LbPrx1m preincubated at 80 μm with increasing concentrations of MgCl2 or CaCl2. The lines represent the nonlinear data fitting (Boltzmann sigmoid function) used to estimate K½, i.e. the Mg2+ or Ca2+ concentration at which 50% of the dimers are converted to decamers. All measurements were performed in triplicate. e, aSEC chromatograms of LbPrx1m at 100 μm (200 μl) in Tris buffer (pH 7.5) containing 700 μm MgCl2 and 200 nm CaCl2 (solid lines) or 90 μm CaCl2 (dashed lines). The protein was incubated with 2 mm DTT (blue lines) or submitted to DTT treatment followed by DTT removal and protein oxidation using 100 μm H2O2 (orange lines). f, nonreducing SDS-PAGE confirming the reduced state (SH monomers) of DTT-treated samples and the oxidized state (S–S dimers) of samples pretreated with DTT and then oxidized with H2O2.
Table 1.
DLS analysis of LbPrx1m in the presence of CaCl2
Samples were pretreated with CaCl2 (I) and then incubated for 10 min with EDTA (II) to illustrate the reversibility of the decamerization process. Note that the hydrodynamic radius (RH) of the protein decreases upon the addition of EDTA in samples pretreated with CaCl2.
| Condition | RH | Pda | Mass |
|---|---|---|---|
| nm | % | % | |
| I (25 mm CaCl2) | 6.5 | 9.7 | 83.6 |
| II (I + 50 mm EDTA) | 3.1 | 11.9 | 99.6 |
a Pd, polydispersity.
Table 2.
DLS analysis of LbPrx1m pretreated with several divalent cations
Samples were pretreated with several divalent cations (at 20 mm) in 25 mm Tris-HCl (pH 7.5) and 5 mm EDTA. Note that only the additives CaCl2 and MgCl2 led to a monodisperse protein size distribution (Pd < 20%) in which most of the enzyme population (≥80% mass) displayed an average RH (∼6 nm) compatible with the decameric structure (RG = 5 nm). Zn2+, in particular, induced the formation of larger aggregates besides the species of RH ∼ 9 nm, probably because of non-specific interactions with the enzyme.
| Salt | RH | Pda | Mass |
|---|---|---|---|
| nm | % | % | |
| CaCl2 | 5.9 | 8.0 | 90 |
| MgCl2 | 5.6 | 10.1 | 80 |
| NiCl2 | 6.0 | 30.9 | 55 |
| FeCl2 | 4.6 | 29.3 | 50 |
| MnCl2 | 4.6 | 86.4 | 25 |
| ZnCl2 | 9.2 | 11.9 | 10 |
a Pd, polydispersity.
To study the effect of increasing concentrations of Ca2+ and Mg2+ in the dimer-decamer equilibrium of LbPrx1m, we monitored changes in the oligomerization state of oxidized samples titrated with CaCl2 or MgCl2 by measuring the anisotropy of intrinsic protein fluorescence (Fig. 1d). Based on these data, we estimated a K½ near 3 mm for both ions, indicating a low-affinity system. During these assays, we noticed that the cation effect was dependent on a critical protein concentration (∼80 μm) below which oxidized LbPrx1m dimers became less sensitive to the presence of cation (data not shown). However, after the formation of cation-stabilized decamers, the protein dilution to levels below the critical concentration did not induce decamer disassembly, indicating that cation binding to LbPrx1m involves the formation of “transitional” decamers followed by the binding of Ca2+ or Mg2+ to yield stable cation-decamer complexes.
As a first approach to evaluating the physiological relevance of the Ca2+/Mg2+ effect in the quaternary structure of LbPrx1m, we performed aSEC assays under two conditions: first by simulating mitochondrial basal concentrations of free Mg2+ and Ca2+ ions (25, 26) and then by mimicking a Ca2+ increase to levels already reported for the L. braziliensis mitochondrion (27). Basal concentrations of Mg2+/Ca2+ were sufficient to maintain most of the reduced enzyme in the decameric form, indicating that physiological levels of Mg2+/Ca2+ stabilize reduced decamers (Fig. 1, e and f). We next exposed the cation-stabilized, reduced decamers to a low concentration of H2O2 and evaluated the aSEC profile of the oxidized (S–S-bonded) enzyme. In basal concentrations of Mg2+/Ca2+, most of the decamers dissociate into dimers upon Cp oxidation/resolution (Fig. 1, e and f), which correlates with the low affinity of these cations to the oxidized enzyme (Fig. 1d). However, under the condition simulating a calcium overload, almost half of the population remained decameric, indicating that supraphysiological Ca2+ concentration already reported for Leishmania mitochondria can increase the level of oxidized decamers (Fig. 1e).
Prx1m decamer stabilization by Ca2+/Mg2+ is redox-independent and a unique feature of the mitochondrial Prx1 from Leishmania parasites
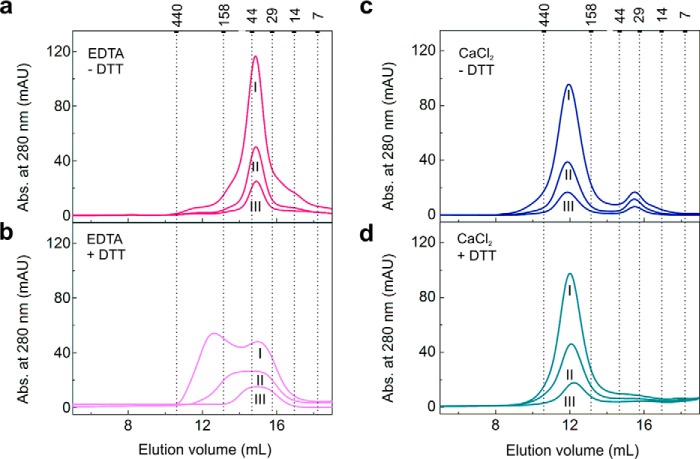
To further investigate how the redox state affects the oligomerization of LbPrx1m, the hydrodynamic behavior of oxidized and reduced proteins was assessed under chelating conditions or in the presence of Ca2+ (Fig. 2). The aSEC results indicate that when the cation is absent the dimer-decamer equilibrium becomes more responsive to the protein redox state; the oxidized enzyme remains dimeric, regardless the protein concentration (Fig. 2a), whereas the reduced enzyme gets into a dimer-decamer equilibrium that is shifted to the decamer by increasing protein concentrations (Fig. 2b). In contrast, 25 mm CaCl2 stabilizes a major population of oxidized and reduced decamers even when they are diluted to low protein concentrations (Fig. 2, c and d), supporting the conclusion that the Ca2+ effect surpasses the redox state in stabilizing LbPrx1m decamers.
Figure 2.
Ca2+ stabilizes both oxidized and reduced LbPrx1m decamers. aSEC chromatograms of LbPrx1m at different concentrations in Tris buffer (pH 7.5) containing 5 mm EDTA (a), 5 mm EDTA with 10 mm DTT (b), 25 mm CaCl2 (c), or 25 mm CaCl2 with 10 mm DTT (d). Roman numerals represent the protein concentration of the input samples (250 μl): I, 94 μm; II, 23 μm; III, 9 μm. Numbers above the graphs represent the molecular mass (kDa) of standard proteins used for column calibration. For this assay, the His tag was removed, using TEV protease to show that the untagged protein behaves similar to the His-tagged samples upon CaCl2 and EDTA treatments (see Fig. 1a). Note that the Ca2+-stabilized decamers, preformed at 94 μm protein, did not dissociate upon protein dilution even in the absence of DTT. Samples not treated with DTT are air-oxidized (S–S-bonded). mAU, milliabsorbance units.
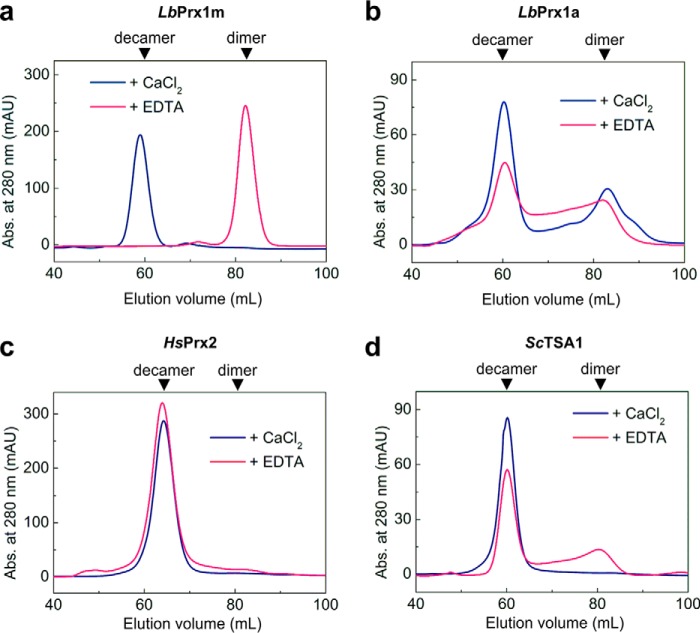
Unlike LbPrx1m, the cytoplasmic 2-Cys Prx from the same pathogen, as well as from two evolutionarily distant organisms, were not as dependent as LbPrx1m on the decamer-stabilizing effect of Ca2+, eluting mainly as decamers regardless the presence of this ion or EDTA (Fig. 3). These findings indicate that the high sensitivity of dimer-decamer equilibrium to Ca2+/Mg2+ ions is a unique feature of LbPrx1m and possibly of other mitochondrial orthologues from Leishmania spp, according to the comparative structural and sequence analyses described below.
Figure 3.
Decamer stabilization of cytoplasmic 2-Cys Prx from Leishmania and some distantly related homologues is Ca2+-independent. SEC chromatograms of air-oxidized LbPrx1m at 130 μm (a), cytoplasmic Prx1 from L. braziliensis (LbPrx1a) at 43 μm (b), human Prx2 at 130 μm (c), and yeast TSA1 at 43 μm (d) loaded (2 ml) into a HiLoad Superdex 200 16/600 column pre-equilibrated with Tris buffer (pH 7.5) containing 25 mm CaCl2 or 5 mm EDTA. mAU, milliabsorbance units.
Structural basis for the cation-dependent mechanism of decamer stabilization
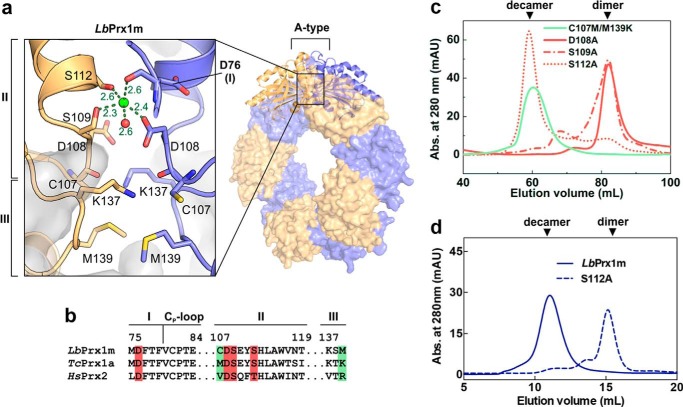
Despite extensive efforts, we were unable to crystallize LbPrx1m in complex with Ca2+. Crystals of LbPrx1m decamers prestabilized with CaCl2 were observed only under acidic conditions (pH 4.4), known to enhance decamer stability in a cation-independent manner (9) and to decrease the Ca2+-binding affinity to proteins (28, 29). Thus, to determine the Ca2+/Mg2+-binding site in LbPrx1m, we analyzed the crystal structure of the pH-stabilized decamer (PDB accession no. 4KB3 (9)) and used in in silico approaches combined with site-directed mutagenesis to validate the predicted site.
Like other members of the AhpC/Prx1 subfamily (30), the LbPrx1m decamer is formed when five dimers bind to each other via the A-type interface (9). Because the formation of this interface depends on conformational changes in region I (residues 75–79) preceding the Cp-loop (residues 80–84) (9), we hypothesized that the decamer-stabilizing effect of Ca2+/Mg2+ might be related to their binding to the A-type interface and the concomitant stabilization of region I in a conformation that favors the decameric assembly.
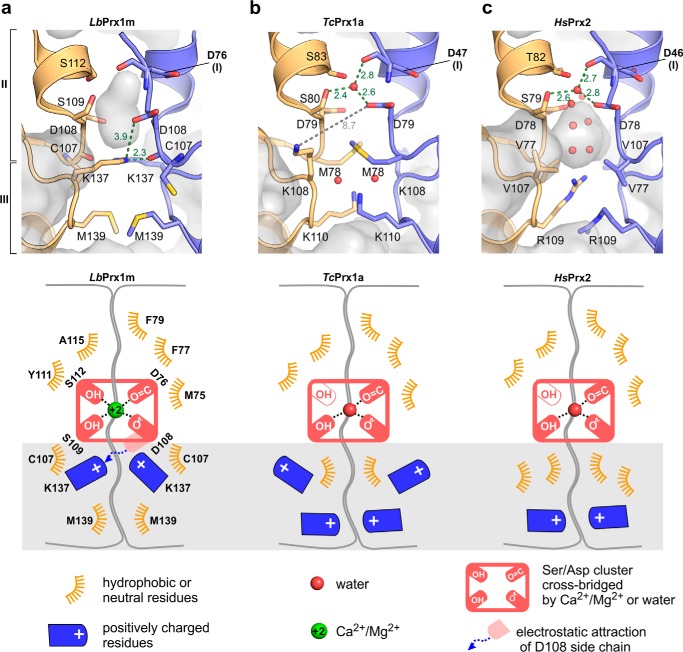
To test this hypothesis, we searched in the A-type interface of LbPrx1m decamer (9) for negatively charged cavities in which a positive ion such as Ca2+ could bind and maintain the closed conformation of region I required for decamer stabilization (9). As expected, we found a site in which Ca2+ could be coordinated by residues from both interfacing subunits, assuming a distorted trigonal bipyramidal geometry according to molecular dynamics simulations (Fig. 4a). This site includes the main chain of Asp-76, a residue from region I, and is duplicated at each A-type interface, implying a stoichiometry of 10 cations/decamer.
Figure 4.
Structural basis for the Ca2+/Mg2+-dependent decamerization of LbPrx1m. a, magnified view of the A-type interface from LbPrx1m crystal structure (PDB accession no. 4KB3) after molecular dynamics simulations in the presence of Ca2+ (green sphere). Interfacing subunits are shown in orange and violet. For purposes of clarity, we named the A-type interface regions according to the nomenclature proposed by Wood et al. (15) (regions I–III). Shown as sticks are the residues involved in the cation-dependent mechanism of decamer stabilization: Asp-76, region I; Cys-107, Asp-108, Ser-109, and Ser-112, region II; Lys-137 and Met-139, region III. b, sequence alignment of A-type interface regions from LbPrx1m and two cation-independent 2-Cys Prx, highlighting the residues predicted to play a role in cation binding (red boxes) and those predicted to determine the cation dependence of decamer stabilization (green boxes). c, aSEC chromatograms of air-oxidized LbPrx1m D108A, S109A, S112A, and C107M/M139K mutants at 86 μm (500 μl) in Tris buffer (pH 7.5) containing 25 mm CaCl2 (red lines) or 5 mm EDTA (green line). The single mutant C107M was unstable in solution, and thus it was not included in our analyses. mAU, milliabsorbance units. d, aSEC chromatogram of air-oxidized LbPrx1m WT and S112A mutant at 13 μm (200 μl). Note that WT protein eluted as decamer, whereas the S112A mutant eluted mainly as dimer under the same conditions. aSEC assays were carried out in Tris buffer (pH 7.5) containing 25 mm CaCl2.
We next evaluated the Ca2+ effect on the aSEC profile of mutants lacking one of the side chains predicted to coordinate this cation. These side chains belong to the residues Asp-108, Ser-109, and Ser-112, located in region II (residues 107–120) of the A-type interface (Fig. 4). The mutants D108A and S109A eluted essentially as dimers in the presence of Ca2+ at pH 7.5, whereas mutant S112A showed a concentration-dependent behavior, eluting as a decamer at 86 μm and as a dimer at 13 μm (Fig. 4, c and d). These results indicate that Asp-108 and Ser-109 are essential for the Ca2+-dependent stabilization of the LbPrx1m decamer, whereas Ser-112 plays a facultative role in this mechanism.
Intriguingly, the cation-binding site identified in LbPrx1m is highly conserved in some 2-Cys Prx, where the decamers remain stable without Ca2+/Mg2+ ions, such as the cytoplasmic peroxiredoxin from Trypanosoma cruzi (TcPrx1a) in which the decamer is crystallized in the presence of EDTA (31) and human Prx2 (HsPrx2) (Fig. 4b). This finding prompted us to search for other structural elements that would possibly account for the Ca2+/Mg2+ effect on LbPrx1m.
A comparison of the A-type interfaces of the LbPrx1m, TcPrx1a, and HsPrx2 crystallographic decamers (Fig. 5) showed that residues Cys-107 and Met-139 are exclusive of LbPrx1m and expose Asp-108 to the electrostatic attraction of Lys-137 (Fig. 5a). However, in TcPrx1a, the Asp-108 counterpart (Asp-79) is shielded from the influence of such a lysine by a residue that is bulkier than Cys-107 (Met-78), which seems to favor the interaction between the Asp-79 side chain and a water molecule that occupies the predicted cation-binding site (Fig. 5b). The same water-mediated link occurs in HsPrx2, which lacks the corresponding Lys-137 (Fig. 5c). Thus, we hypothesized that the attraction of the Asp-108 side chain by Lys-137 prevents Asp-108 from coordinating a water molecule, but not a Ca2+ or Mg2+ ion, within the Ser/Asp cluster, destabilizing the A-type interface when Ca2+ or Mg2+ is absent and the medium is alkaline (9). To test this hypothesis, we mutated Cys-107 (region II) to a methionine and Met-139 (region III) to a lysine, thus mimicking the TcPrx1a Asp-79 (equivalent to Asp-108) microenvironment in which Met-78 blocks the access of the lysine to the aspartate (Fig. 5b). As envisioned, this double mutation rendered LbPrx1m decamer stabilization Ca2+-independent (Fig. 4c), demonstrating that changes in regions II and III of the A-type interface gave rise to the Ca2+/Mg2+ effect on LbPrx1m oligomerization (Fig. 5a).
Figure 5.
Structural comparison of the A-type interface between LbPrx1m (a), TcPrx1a (PDB accession no. 4LLR) (b), and human Prx2 (PDB accession no. 1QMV) (c) reveals the molecular determinants for calcium specificity in LbPrx1m. Green or gray dashed lines indicate interatomic distances labeled in Å, and red spheres represent water molecules. In b, note the long distance between the Asp-79 and Lys-108 side chains, indicating they do not interact in the TcPrx1a structure. Below each panel there is a schematic representation of the A-type interface of LbPrx1m, TcPrx1a, and human Prx2 that highlights the changes observed in regions II and III (gray box), which determine the Ca2+ dependence of LbPrx1m decamerization at physiological pH.
Ca2+/Mg2+ ions enhance the peroxidase activity of LbPrx1m
The dimer-decamer switch seems to play a role during the peroxidase catalytic cycle of AhpC/Prx1 subfamily members by influencing enzymatic efficiency (15, 32). We thus used the trypanothione-dependent enzyme cascade from Leishmania to evaluate in vitro how the decamer-stabilizing effect of Ca2+/Mg2+ influences the capability of LbPrx1m to reduce hydrogen peroxide.
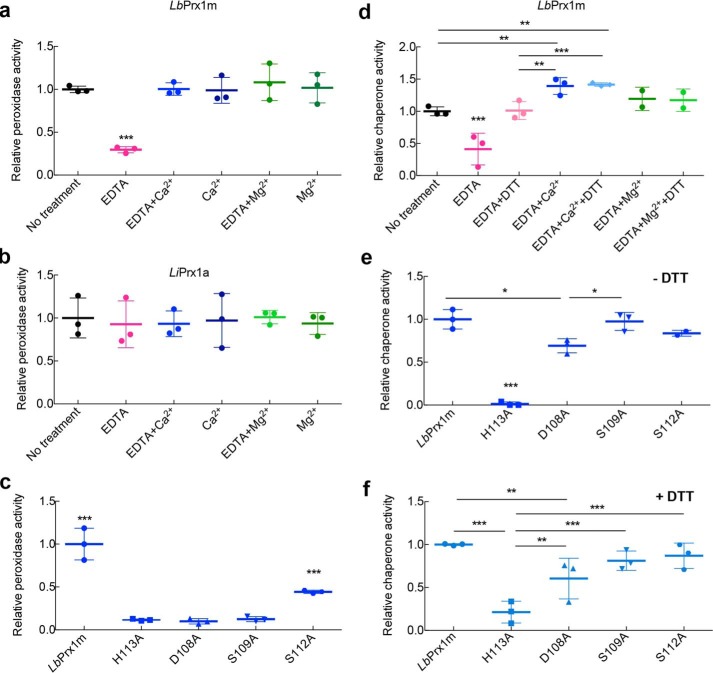
The chelation of metal ions drastically decreased the peroxidase activity of purified LbPrx1m (Fig. 6a). In samples pretreated with EDTA and then supplemented with Ca2+ or Mg2+, the catalytic activity was recovered, showing that these divalent cations play a role in the peroxidase function of LbPrx1m (Fig. 6a). The increment of Ca2+ in purified LbPrx1m did not alter its catalytic activity (Fig. 6a), an effect that can be ascribed to divalent cations from Escherichia coli that remained bound to LbPrx1m decamers during the purification process, as indicated by aSEC analysis of untreated samples (results not shown). The observed effects of EDTA and Ca2+ cannot be attributed to interference from the other molecules of the trypanothione cascade, because these additives did not alter the peroxidase activity of cytoplasmic Prx1 from L. infantum (LiPrx1a) (Fig. 6b). Importantly, the observation that the Ca2+/Mg2+ ions did not affect the peroxidase activity of LiPrx1a correlates with the fact that Ca2+/Mg2+ ions are not required to stabilize the decameric structure of its ortholog in L. braziliensis (Fig. 3), corroborating our view of the specific effect of Ca2+/Mg2+ on the peroxidase activity of the Leishmania mitochondrial enzyme.
Figure 6.
Ca2+ and Mg2+ ions enhance both peroxidase and chaperone activities of LbPrx1m. a, relative peroxidase activity of LbPrx1m left untreated, pretreated with 5 mm EDTA only, pretreated with 5 mm EDTA followed by the addition of 25 mm CaCl2 or MgCl2, and pretreated with 20 mm CaCl2 or MgCl2 alone. b, relative peroxidase activity of LiPrx1a prepared as described for LbPrx1m. The absolute peroxidase activity values (μmol NADPH·min−1·mg−1) were 0.18 ± 0.04 (for untreated LbPrx1m) and 5.12 ± 1.19 (for untreated LiPrx1a). c, comparison between the relative peroxidase activity of WT LbPrx1m and mutants pretreated with 5 mm EDTA followed by the addition of 25 mm CaCl2. d, relative chaperone activity of air-oxidized LbPrx1m left untreated and pretreated with 5 mm EDTA only or 5 mm EDTA followed by the addition of 25 mm CaCl2 or MgCl2. The same treatments were carried out with the protein reduced using 2 mm DTT. e and f, comparison between the relative chaperone activity of WT LbPrx1m and muteins pretreated with 5 mm EDTA followed by the addition of 25 mm CaCl2 without DTT (air-oxidized) (e) or with 2 mm DTT (reduced) (f). *, p < 0.1; **, p < 0.05; ***, p < 0.01. All experiments were performed in triplicate.
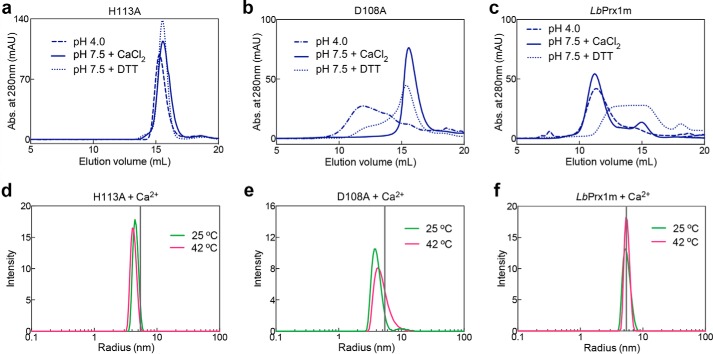
To demonstrate that the effect of Ca2+ on peroxidase activity is due to its binding at the A-type interface and consequent decamer stabilization, we compared the peroxidase activity of Ca2+-binding site mutants with that of the wild-type (WT) protein or the mutant H113A (Fig. 6, a–c), which preserves the dimeric structure but is unable to decamerize in response to pH (9), redox, and cation stimuli (Fig. 7a). Mutants D108A and S109A displayed only residual activity compared with the WT enzyme in the presence of Ca2+ (Fig. 6c). As expected, the mutation S112A was not as efficient as the D108A and S109A substitutions in decreasing the peroxidase activity of LbPrx1m, which correlates with the facultative role of Ser-112 in Ca2+ binding. The effects of the D108A and S109A mutations on peroxidase activity were equivalent to that caused by the decamer-disrupting H113A substitution, further supporting that Ca2+/Mg2+ ions enhance LbPrx1m peroxidase activity by stabilizing its decameric form.
Figure 7.
The influence of low pH, CaCl2, DTT, or temperature on the hydrodynamic behavior of the mutants H113A and D108A and WT LbPrx1m. aSEC assays of H113A (a), D108A (b), and WT LbPrx1m (c) were carried out with proteins at 48 μm (250 μl) in MMT buffer (pH 4.0) and 10 mm EDTA (dashed-dotted line), Tris buffer (pH 7.5) and 25 mm CaCl2 (solid line), or Tris buffer (pH 7.5) and 5 mm EDTA containing 2 mm DTT (dotted line). Chromatograms of mutant H113A and LbPrx1m at pH 4.0 as well as LbPrx1m at pH 7.5 plus 2 mm DTT were shown previously (9) and are represented here for comparison purposes. DLS analyses at 25 and 42 °C of H113A (d), D108A (e), and WT LbPrx1m (f) were performed with protein samples at 100 μm pretreated with 5 mm EDTA followed by the addition of 25 mm CaCl2 in Tris buffer (pH 7.5). The vertical gray lines represent the mean radius estimated for WT LbPrx1m decamers. Samples not treated with DTT are air-oxidized (S–S-bonded). mAU, milliabsorbance units.
Reduced and oxidized cation-stabilized decamers possess the required conformation to perform chaperone function
Our finding that Ca2+/Mg2+ ions stabilize oxidized decamers of LbPrx1m led us to investigate whether these decamers were able to suppress luciferase thermal aggregation similarly to the reduced decamers (3). Under non-reducing conditions, metal chelation inhibited the chaperone activity of LbPrx1m, indicating a role for Ca2+/Mg2+ in activating the chaperone function of oxidized LbPrx1m (Fig. 6d). When cation-free samples were supplemented with CaCl2 or MgCl2, the chaperone activity was recovered, reaching levels significantly higher than that of the untreated samples in the case of Ca2+ (Fig. 6d). Although LbPrx1m reduction also rescued the chaperone activity of cation-free LbPrx1m, the average activity of the reduced decamers was further stimulated by CaCl2 supplementation (Fig. 6d), which is in agreement with aSEC data showing the better performance of Ca2+ than protein reduction in stabilizing decamers (Fig. 2).
To demonstrate that protection against luciferase aggregation depends on the formation of decamers and to evaluate the effect of Ca2+/Mg2+-binding site mutations on chaperone function, we measured the chaperone activity of LbPrx1m mutants H113A, D108A, S109A, and S112A. The H113A substitution, which prevents the decameric assembly of dimers, completely abolished the chaperone activity of oxidized LbPrx1m and drastically decreased that of the reduced protein, demonstrating that decamer formation is a prerequisite for chaperone activity (Fig. 6, e and f). Unexpectedly, of the four Ca2+-binding site mutations, only D108A significantly decreased the chaperone activity under both non-reducing and reducing conditions (Fig. 6, e and f). However, the mutant D108A was more active than H113A, which prompted us to investigate whether the high temperature of the chaperone assay favors the formation of D108A decamers. In agreement with the chaperone activity data, mutant D108A entered into a dimer-decamer equilibrium at 42 °C (Fig. 7, b and e), whereas mutant H113A remained in the dimeric state at this temperature (Fig. 7, a and d). Under the same conditions, the WT protein was decameric, indicating that decamer formation is necessary and sufficient to trigger the chaperone function of LbPrx1m (Fig. 7, c and f).
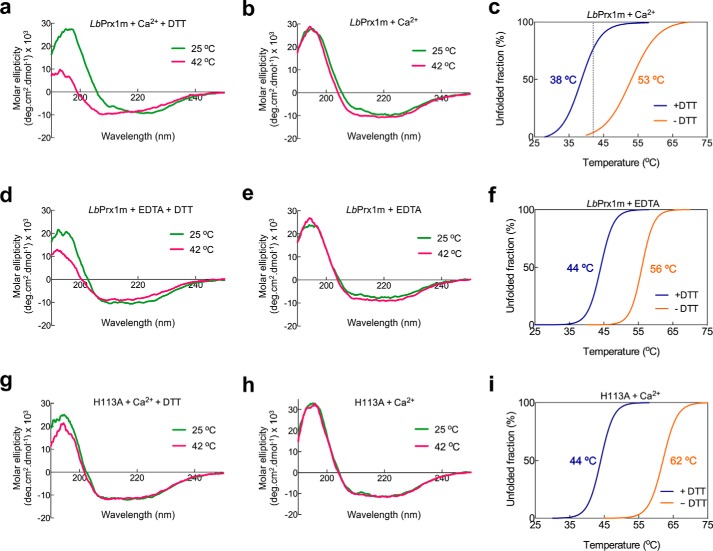
Based on studies of reduced decamers, it has been proposed that the chaperone function of Leishmania Prx1m is activated by an increase in temperature, which induces conformational rearrangements that expose hydrophobic regions (3). As our data showed that oxidized decamers also display chaperone activity, we investigated whether their behavior upon temperature increase supports the model in which thermo-induced conformational changes activate the chaperone function of Leishmania Prx1m. For this purpose, circular dichroism (CD) spectra of WT LbPrx1m were collected under reducing and non-reducing conditions at 25 and 42 °C in the presence of Ca2+. Interestingly, at 25 °C, the CD spectra of the reduced and oxidized decamers were virtually identical, showing that the conformational differences between their respective fully folded and locally unfolded active sites were undetectable by this technique (Fig. 8, a and b). Upon a temperature increase from 25 to 42 °C, the CD spectrum of the reduced decamers showed a decrease in the absolute values at 193 and 222 nm and presented a shift of the minimum at 208 nm toward smaller wavelengths, indicating the loss of α-helical structures (Fig. 8a). In agreement with this result, differential scanning fluorimetry (DSF) data showed the exposure of hydrophobic surfaces in reduced decamers heated up to 42 °C (Fig. 8c). However, no significant changes in CD spectra or exposure of hydrophobic regions in DSF analyses were observed for the oxidized decamers in the same temperature range (Fig. 8, b and c). This enhanced thermostability of the oxidized species correlates with the presence of the disulfide bond linking Cp, located in the loop connecting strand β3 to helix α2, and Cr, placed at the C-terminal extension downstream the helix α6. Because this is the only disulfide bond present in oxidized LbPrx1m (9), the loss of structure indicated by CD and DSF analyses of reduced decamers likely reflects, among other events, the unfolding of helices α2 and/or α6, which is favored when Cp and Cr are reduced.
Figure 8.
Biophysical analyses of LbPrx1m dimers and decamers. Circular dichroism spectra of WT LbPrx1m or mutant H113A pretreated with 5 mm EDTA followed by the addition of 25 mm CaCl2 and 2 mm DTT (a and g), 25 mm CaCl2 only (S–S-bonded) (b and h), 2 mm DTT only (d), or without further additives (air-oxidized) (e) at 25 °C (green) and 42 °C (pink). Shown are DSF data of reduced (+DTT) or air-oxidized (−DTT) LbPrx1m (c and f) as well as mutant H113A (i) treated as described for the CD experiments. In these assays, WT LbPrx1m data in the presence of Ca2+ reflect the CD and DSF profiles of decamers, whereas those of WT LbPrx1m in the presence of EDTA or those of the mutant H113A represent the behavior of the dimers. The calculated melting temperatures are color-coded according to the respective curves. In c, note that the dashed line indicates the temperature of the chaperone assay in which the reduced decamers expose hydrophobic patches, whereas the oxidized decamers remain almost completely folded.
Considering that the helices α2 and α6 are located at the external surface of the decamer, i.e. far from the luciferase-binding site (3), their unfolding might not interfere with the chaperone function of reduced decamers (Fig. 9). In support of this hypothesis, the Ca2+-stabilized reduced species preserved the decameric structure at 42 °C (data not shown) and were as effective as the oxidized samples in suppressing the thermal aggregation of luciferase in vitro (Fig. 6d). Together, these analyses suggest that the thermo-induced conformational changes observed for the reduced decamer might not be required to activate the chaperone function of Leishmania Prx1m.
Figure 9.
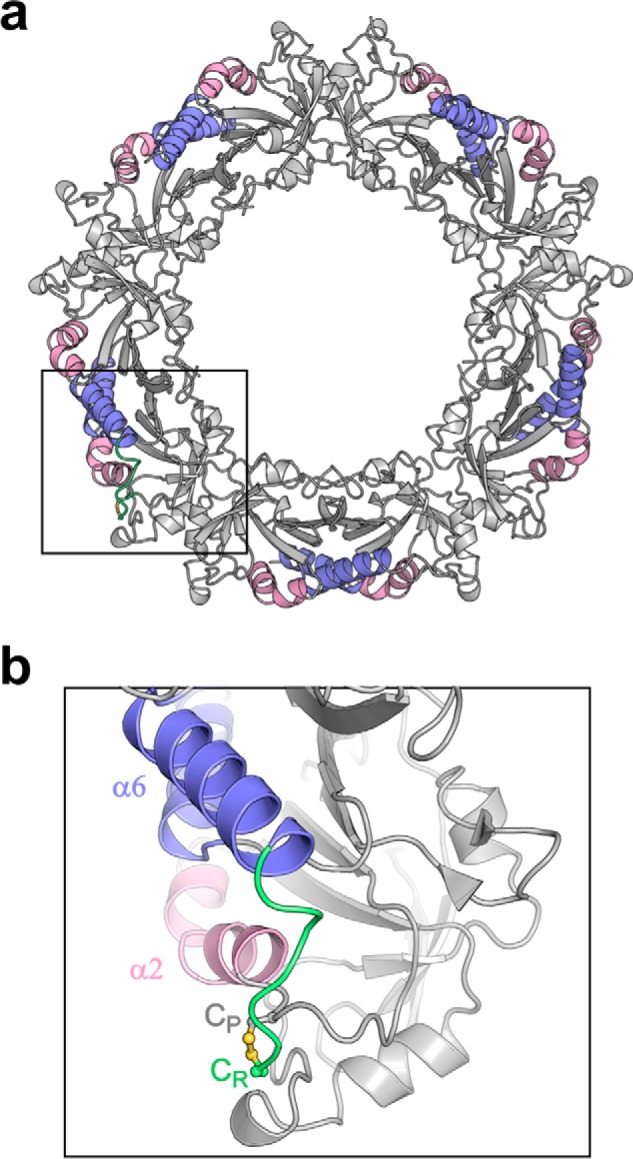
The disulfide bond between Cp and Cr probably suppresses the unfolding of helices α2 and or α6 at 42 °C. a, schematic representation of LbPrx1m crystallographic decamer highlighting helices α2 (pink) and α6 (violet). b, magnified view of the boxed region in a, showing the disulfide bond between Cp (gray, carbon atoms) and Cr (green, carbon atoms). The C-terminal extension harboring Cr is shown in green.
For comparison purposes, we also performed CD and DSF analyses for the WT LbPrx1m in presence of EDTA, a condition that favors the dimeric state, and the mutant H113A, which is fully dimeric in solution regardless of its redox state (Fig. 8, d–i). These experiments confirmed that the mutant H113A is properly folded (Fig. 8, g and h) and revealed that the CpS–SCr disulfide bond has a higher impact in thermostability than variations in the oligomeric state, leading to an increase of at least 12 °C in the melting temperature of both the dimers and the decamers (Fig. 8, c, f, and i).
Preventing Prx1m decamer formation impairs leishmanial survival at 37 °C
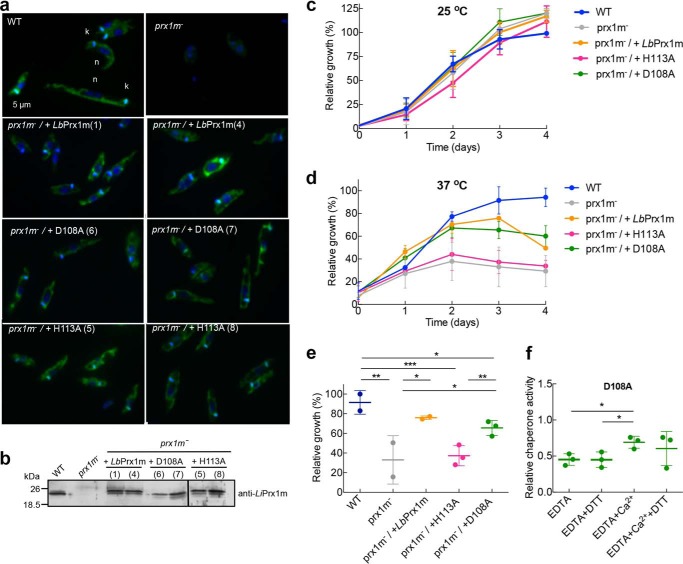
Previous work has shown that L. infantum parasites devoid of LiPrx1m are thermosensitive when exposed to 37 °C, a phenotype that is partially reverted upon reintroduction of the enzyme (2). To investigate the phenotypic implications of Prx1m mutants unable to decamerize or with a lesser tendency to form Ca2+/Mg2+-stabilized decamers, L. infantum knock-out parasites for LiPrx1m (prx1m−) were transfected with WT LbPrx1m and the corresponding H113A and D108A muteins. Using indirect immunofluorescence (Fig. 10a), Western blotting (Fig. 10b), and PCR (data not shown) analyses, it was verified that knock-out parasites lacked the expression of LiPrx1m, whereas the transfected parasites expressed the WT protein and mutants into the mitochondrion.
Figure 10.
Leishmania growth at 37 °C depends on Prx1m decamerization. a, indirect immunofluorescence of L. infantum (WT), L. infantum knock-out for LiPrx1m (prx1m−), and prx1m− complemented with LbPrx1mH113A (clones 5 and 8), LbPrx1mD108A (clones 6 and 7), and WT LbPrx1m (clones 1 and 4). Parasites were incubated with anti-Prx1m antibody (green) and merged with DAPI (blue) (n, nucleus; k, kinetoplast). b, Western blotting analysis using anti-LiPrx1m antibody (53) of L. infantum WT, prx1m−, and the transfectants. A total of 20 μg of protein extracts were loaded per lane. Leishmania growth curves at 25 °C (c) and 37 °C (d) for WT, prx1m−, and transfectants. The experiments were performed in duplicate for L. infantum WT and prx1m− and in triplicate for the transfectants. e, statistical analysis of Leishmania relative growth at 37 °C considering the day 3 representation in d. *, p < 0.1; **, p < 0.05; ***, p < 0.01. f, in vitro chaperone activity of mutant D108A in the presence of 5 mm EDTA or 5 mm EDTA followed by the addition of 25 mm CaCl2, with or without 2 mm DTT, in Tris buffer (pH 7.5) (*, p < 0.1). All experiments were performed in triplicate. Relative activities were calculated with respect to the activity of WT protein in the presence of Ca2+ or Ca2+ plus DTT according to the absence or presence of DTT in the tested condition. Samples not treated with DTT are air-oxidized (S–S-bonded).
As expected, at 25 °C, all parasites had the same growth rates (Fig. 10c). However, at 37 °C, the H113A dimers were incapable of rescuing the thermosensitive phenotype of Prx1m− promastigotes, demonstrating that the decameric state of Prx1m is crucial for Leishmania survival at temperatures akin to those encountered in the mammalian host (Fig. 10, d and e).
When exposed to 37 °C, knock-out parasites expressing the D108A mutein, which is less prone to form Ca2+/Mg2+-stabilized decamers, presented a behavior similar to those complemented with the WT protein (Fig. 10, d and e). This result is likely explained by the fact that the chaperone activity of this mutant retains a residual response to Ca2+ (Fig. 10f). Furthermore, mutant D108A is susceptible to the influence of medium acidification, which stabilizes a subpopulation of D108A as decamers in vitro (Fig. 7b) and probably counterbalances the lower responsiveness of D108A decamers to Ca2+/Mg2+ in vivo. Interestingly, the chaperone activity of the mutant D108A in vitro is unresponsive to DTT treatment even under cation-free conditions (Fig. 10f), indicating that decamer-stabilizing factors other than protein reduction support the chaperone function of this mutein in vivo.
Discussion
Ca2+/Mg2+ ions stabilize decamers and activate the dual function of mitochondrial 2-Cys Prx from Leishmania parasites
In this work, we have revealed that Ca2+ and Mg2+ ions affect the quaternary structure and the dual function of mitochondrial 2-Cys peroxiredoxins from Leishmania parasites. Our data show that these divalent cations stabilize LbPrx1m decamers and thereby stimulate peroxidase and chaperone activities. The mechanism involves the binding of Ca2+ or Mg2+ at transitional A-type interfaces, stabilizing dimer-dimer interactions. Although the K½ estimated for Ca2+ and Mg2+ binding was near 3 mm for the oxidized enzyme, our analyses suggest that this affinity can be enhanced by protein reduction, which stabilizes transitional decamers and favors the formation of cation-bound decamers. Supporting this hypothesis, air-oxidized samples treated with EDTA and then incubated with 1 mm Ca2+ behaved essentially as dimers (Fig. 1d); however, when these EDTA-treated samples were reduced with DTT, incubated with similar amounts of cation, and then reoxidized, about 50% of the enzyme remained in the decameric form (Fig. 1, e and f). In other words, the amount of cation-bound oxidized decamers increases when these complexes are formed under reducing conditions prior to enzyme oxidation. In summary, our data indicate that physiological concentrations of free Ca2+/Mg2+ stabilize reduced decamers, stimulating the peroxidase function and contributing to the maintenance of a basal pool of chaperone-active LbPrx1m at the alkaline environment of mitochondria (pH 7.5–8.0) (33). When mitochondrial Ca2+ uptake is stimulated (23), the level of oxidized decamers increases, contributing to an enlarged chaperone reservoir of Prx1m in Leishmania.
The closed conformation of region I is required to stabilize the fully folded conformation of the reduced Cp-loop
According to data from the literature, the decameric structure of 2-Cys Prx contributes to stabilizing the fully folded conformation of the active site, allowing the optimal orientation of substrate and the activation of the catalytic Cp to reduce the peroxide oxygen (15, 32). Our data support the conception that for the Cp-loop to adopt the fully folded conformation, the adjacent region I might be stabilized in a closed conformation via interactions involving residue Asp-76 (Fig. 11). The disruption of such interactions by point mutations severely decreases the peroxidase activity of LbPrx1m (Fig. 6c). Although the link between the Asp-76 and His-113 side chains is intramolecular and direct, those involving the Asp-76 main chain require decamer assembly and are mediated by Ca2+ or Mg2+ ions in the case of LbPrx1m (Fig. 11). Our data revealed that Mg2+ or Ca2+ ions increase the peroxidase activity by binding at the A-type interface of LbPrx1m, thus holding the Asp-76 main chain with the assistance of Asp-108, Ser-109, and optionally Ser-112 side chains. Noticeably, mutations at positions 108 and 109 displayed a higher impact on peroxidase activity in comparison with EDTA treatment, suggesting that, in the absence of divalent cations, a solvent molecule could link this Ser/Asp cluster allowing a suboptimal activity. Indeed, in several members of the AhpC/Prx1 subfamily, from bacteria to mammals, a water molecule plays the role of linking the highly conserved Ser/Asp cluster at the A-type interface (Fig. 12).
Figure 11.
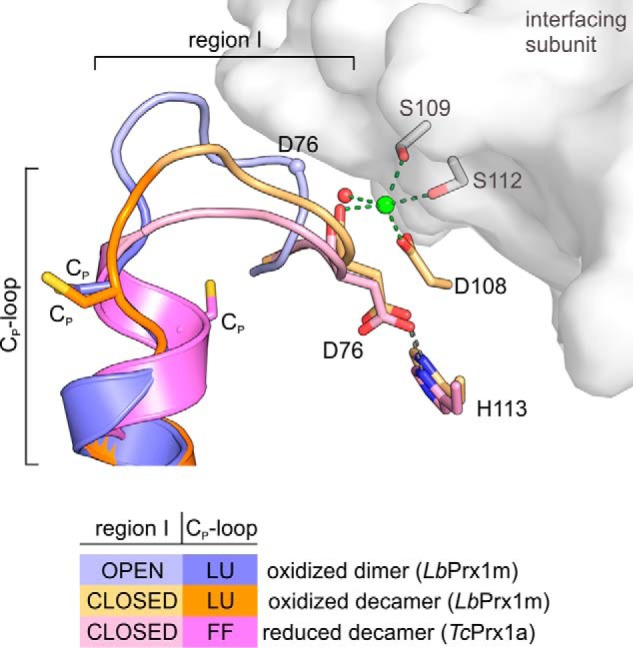
Stabilization of region I in the closed conformation is required to maintain the fully folded conformation of the Cp-loop upon Cp reduction. Shown is a 3D alignment of the loop-helix active-site motif (region I + Cp-loop) of LbPrx1m-oxidized dimer (shades of violet; PDB accession no. 4KCE), LbPrx1m-oxidized decamer (shades of orange; PDB accession no. 4KB3) after molecular dynamics simulations with Ca2+ bound (green sphere) at the A-type interface (gray surface), and reduced decamer TcPrx1a (shades of pink; PDB accession no. 4LLR). Note that region I adopts an open conformation in dimers, but it assumes a closed conformation in the oxidized and reduced decamers. According to our data, the stabilization of region I in the closed conformation requires at least an H-bond between Asp-76 and His-113 as well as Ca2+ (green sphere), Mg2+, or (less efficiently) the water molecule at the A-type interface. The establishment of such interactions is crucial to maintain the fully folded (FF) form of the reduced Cp-loop. In the LbPrx1m dimer, the high entropy of the region I, trapped in an open conformation in chain B but disordered in chain A, might propagate to the adjacent Cp-loop, hampering the stabilization of fully folded conformation required for substrate binding and catalysis. LU, locally unfolded.
Figure 12.

Stabilization of region I in the closed conformation is mediated by Ca2+/Mg2+ in mitochondrial Prx1 from Leishmania species and by a water molecule in other AhpC/Prx1 subfamily members. a, sequence alignment of regions I, II, and III from the A-type interface as well as the Cp-loop from mitochondrial (Prx1m) and cytoplasmic (Prx1a) 2-Cys Prx from trypanosomatids with known crystallographic decamers. Note that residues involved in cation binding in LbPrx1m are highly conserved in the AhpC/Prx1 subfamily (red boxes). However, only the mitochondrial enzymes from Leishmania (black outlined area) conserve residues Cys-107, Lys-137, and Met-139 (green boxes) that render decamer stabilization of LbPrx1m highly sensitive to Ca2+ and Mg2+ ions (green circle). In other 2-Cys Prx, Lys-137 is replaced by neutral polar or hydrophobic residues or kept away from Asp-108 by residues bulkier than Cys-107. In most of the analyzed structures of AhpC/Prx1 subfamily members (blue circles), a water molecule mediates the link between the Asp-76 main chain and Asp-108, Ser-109, and eventually Ser-112 side chains. In a few cases, solvent molecules are not observed in the crystallographic structures due to the low resolution of data (open circles). Lb, L. braziliensis; Li, L. infantum; Lp, Leishmania panamensis; Ld, Leishmania donovani; La, L. amazonensis; Lm, Leishmania major; Tv, Trypanosoma vivax; Tb, Trypanosoma brucei; Tc, T. cruzi; Tco, Trypanosoma congolense; Ac, Ancylostoma ceylanicum; Hs, Homo sapiens; Bt, Bos taurus; Pc, Pseudosciaena crocea; Mm, Mus musculus; Sm, Schistosoma mansoni; Pv, Plasmodium vivax; Sc, S. cerevisiae; Hp, Helicobacter pylori; Mt, Mycobacterium tuberculosis; Se, Salmonella enterica. b, 3D alignment of cation-independent 2-Cys Prx showing the highly conserved water that links the Asp/Ser cluster at the A-type interface. Note the high conservation at positions 108 and 109 in contrast with the higher variability at position 112 (facultative role in cation or water coordination) and position 76 (the main chain involved in cation/water binding). PDB accession codes are in parentheses.
By converting LbPrx1m into a cation-independent 2-Cys Prx, we have provided strong evidence that the microenvironment of Asp-108 selects LbPrx1m as a Ca2+/Mg2+-sensitive Prx1. According to our model, LbPrx1m requires Ca2+/Mg2+ to surpass the electrostatic attraction caused by Lys-137 on Asp-108 and to restore the solvent-mediated link that holds the main chain of Asp-76 and contributes to maintain the fully folded conformation of the Cp-loop, which is mandatory for substrate binding and catalysis. Comparative sequence analysis suggests that the high Ca2+/Mg2+ sensitivity observed for LbPRx1m extends exclusively to mitochondrial 2-Cys Prx from the Leishmania species (Fig. 12).
Ca2+/Mg2+ ions compose a redundant molecular system that supports the chaperone function in vivo
Our studies have demonstrated the similar efficiency of oxidized and reduced cation-stabilized decamers in preventing luciferase aggregation under heat stress conditions. This finding suggests that the Leishmania reservoir of Prx1m chaperones is not only formed by reduced proteins, as envisaged previously (3), but can also comprise oxidized Prx1m (S–S-bonded). As the S–S-bonded species represent about half of the Prx1m population present in the parasite (2), their capability to form chaperone-active decamers may be of great relevance for Leishmania virulence, considering that the chaperone function of Prx1m is crucial for the parasite survival in the mammalian host (3). Moreover, the resistance of Leishmania Prx1m to Cp overoxidation (2) and the lack of the sulfiredoxin protein in these parasites further supports the importance of an alternative mechanism to modulate the chaperone activity of Prx1m in Leishmania species.
When LbPrx1m dimers fail to respond to the three components of the decamer-stabilizing system (pH, redox state, and Ca2+/Mg2+), they lose their capacity to rescue the temperature-sensitive phenotype of prx1m− promastigotes, as indicated by our studies with mutant H113A. Based on our results, we suggest that the residue His-113 is not directly involved in cation binding but is necessary for the formation of a transitional A-type interface that is then stabilized by cation binding, His-113 protonation (9), or less effectively, by Cp reduction. The crystal structure of oxidized LbPrx1m dimers shows that region I preceding the Cp-loop is highly flexible and can transit between an open and a closed conformation, which favors dimers and decamers, respectively (9). The prerequisite to form transitional A-type interfaces likely involves the H-bond between His-113 and Asp-76 when region I accesses the closed conformation. Because this interaction is insufficient to lock region I in the closed conformation, decamer stabilization requires a second stimulus.
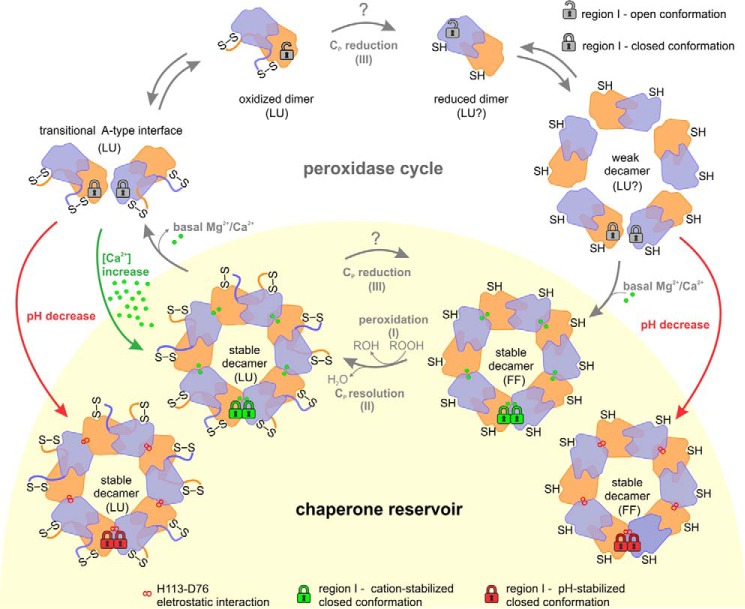
According to our model (Fig. 13), in basal concentrations of enzyme, Mg2+, and Ca2+, most of Prx1m enzymes are decameric when reduced and dimeric when oxidized. During heat shock, which stimulates Ca2+ uptake in Leishmania promastigotes (34), higher levels of Ca2+ at the mitochondrion (27) feed the chaperone reservoir with oxidized decamers. Besides stimulating mitochondrial Ca2+ uptake, cytosolic Ca2+ elevations can also lead to a mitochondrial pH decrease of about 0.2 pH units (35), providing an extra stimulus to enlarge the reservoir of Prx1m decamers (9). At pH 7, for example, we detected in vitro a cooperative effect between pH and Ca2+ in stabilizing oxidized decamers (data not shown).
Figure 13.
Physiological role of Mg2+/Ca2+, pH, and redox state in the maintenance of the chaperone reservoir of mitochondrial Prx1 in Leishmania. During the peroxidase cycle, Leishmania Prx1m transits between the dimeric (chaperone-inactive) and the decameric (chaperone-active) forms. This oligomeric shift is regulated by the conformational changes of two regions from the loop-helix active-site motif: the Cp-loop (LU ⇔ FF), which is redox-sensitive, and the region I (open ⇔ closed), which is Ca2+/Mg2+/pH-sensitive. When dimers are reduced (SH), they tend to form weak decamers in which free Mg2+ and Ca2+ ions bind to enhance their peroxidase and chaperone functions. In basal conditions, after the peroxidation (I) and resolution (II) steps, oxidized (S–S) decamers tend to release Mg2+ and Ca2+ ions and dissociate into dimers, a process that involves an intermediate state, here named the transitional A-type interface. However, under heat-stress conditions, two stimuli can boost the chaperone reservoir of Prx1m, mainly by stabilizing the oxidized decamers: red arrows, small pH decreases; green arrows, Ca2+ overload. Because our data could not discriminate the preferable substrate for tryparedoxin (Prx1m dimers or decamers), we labeled the Cp reduction step with a question mark. The same is applicable for the conformational state proposed for the active site in reduced dimers and reduced weak decamers. For purposes of clarity, the padlocks illustrating the conformational state of region I are shown only for a pair of dimers at the decamers. LU, locally unfolded; FF, fully folded.
Together, our studies show that basal concentrations of Mg2+/Ca2+ ions support the dual function of mitochondrial Prx1 from Leishmania and reveal a molecular mechanism that may help explain why calcium uptake is crucial for Leshimania thermotolerance and differentiation in the mammalian host (34). Furthermore, we have demonstrated that the decameric structure, independently of its redox state, is both necessary and sufficient for the protective effect of Prx1m against heat stress in Leishmania, a vital attribute for the establishment of a successful infection in the mammalian host (2, 3). This finding implies that the search for compounds that prevent Prx1m decamerization represents the best strategy for inhibiting the crucial chaperone function of this attractive therapeutic target (36). Zhao et al. (37) already have identified chaperone inhibitors for the human Prx I, demonstrating the feasibility of such an approach.
Experimental procedures
Molecular cloning and site-directed mutagenesis
The LbPrx1m gene (RefSeq accession no. XM_001562186.1) was cloned into a pET28a-His-TEV vector as described previously (38). Human gene PRX2 (RefSeq accession no. NM_005809.5) was amplified by PCR and cloned into the pET28a vector between the NdeI and SalI restriction sites. The TSA1 gene from Saccharomyces cerevisiae was cloned into the pET15b vector as described previously (39). The pET28a construct containing the DNA sequence of the LbPrx1a gene (RefSeq accession no. XM_001563506.1) between the NdeI and SalI restriction sites was purchased from GenScript (Piscataway, NJ). All LbPrx1m mutants were produced using the QuikChangeTM site-directed mutagenesis kit (Stratagene).
Protein expression and purification
The protein LbPrx1m and corresponding muteins were expressed and purified as described previously (9). LbPrx1a and HsPrx2 were produced in E. coli BL21(DE3)ΔSlyD cells containing the plasmid pRARE2, whereas TSA1 was produced in BL21(DE3) cells. After the cell culture reached A600 nm ∼ 0.6 in LB medium, protein expression was induced with 0.5 mm isopropyl 1-thio-β-d-galactopyranoside at 30 °C for 4 h at 200 rpm (HsPrx2 and LbPrx1a) or with 1 mm isopropyl 1-thio-β-d-galactopyranoside at 37 °C for 3 h at 200 rpm (TSA1). Protein extraction and affinity purification followed that described for LbPrx1m (9). All purified proteins were dialyzed against the buffer used in the analytical size-exclusion chromatography step and concentrated using Amicon Ultra devices (Millipore). The oxidized (S–S-bonded) state of the purified proteins was confirmed by SDS-PAGE analysis under non-reducing conditions, whereas the reduced state of proteins treated with DTT was confirmed by quantification of free thiol groups using 5,5′-dithiobis(nitrobenzoic acid) as described previously (9).
Analytical size-exclusion chromatography
A total of 2 ml of LbPrx1m at 130 μm, LbPrx1a at 43 μm, yeast TSA1 at 43 μm, and HsPrx2 at 130 μm were loaded onto a HiLoad 16/600 Superdex 200 column (GE Healthcare) pre-equilibrated with 25 mm Tris-HCl (pH 7.5) containing 25 mm CaCl2, 25 mm MgCl2, 5 mm EDTA, or 5 mm EGTA. aSEC experiments for the LbPrx1m mutants D108A, S109A, and S112A at 86 μm (500 μl input) were carried out using the same column pre-equilibrated with buffer T-Ca (25 mm Tris-HCl, 150 mm NaCl, and 25 mm CaCl2, pH 7.5). The same assay was performed using the mutant C107M/M139K at 86 μm (500 μl input) in buffer T-EDTA (25 mm Tris-HCl, 150 mm NaCl, and 5 mm EDTA, pH 7.5). Additionally, WT LbPrx1m was cleaved with TEV protease to remove the His tag, preincubated at 94 μm with or without 10 mm DTT in buffer T-Ca or T-EDTA, and divided into three samples at different protein concentrations (94, 23, and 9 μm) that were loaded (250 μl) onto a Superdex 200 10/300 GL column (GE Healthcare) pre-equilibrated with the sample buffer.
aSEC assays mimicking in vivo conditions (3, 25–27, 40, 41) were performed with TEV-cleaved LbPrx1m at 100 μm (200 μl input) in buffer T-EDTA plus the following additives: 5.7 mm MgCl2 (free Mg2+ = 0.7 mm) and 200 nm CaCl2 (condition I); and the same as described in condition I but with 90 μm CaCl2 (condition II). These assays were carried out in a Superdex 200 10/300 GL column (GE Healthcare). Samples were pretreated and eluted in the presence of 2 mm DTT or pretreated with 2 mm DTT, re-oxidized, and eluted in buffer without DTT. To obtain re-oxidized samples, DTT was removed as described by Morais et al. (9), and the concentrated protein was incubated with H2O2 in a 1:1 (protein:H2O2) molar ratio prior injection. The redox state of the samples was analyzed by non-reducing SDS-PAGE.
aSEC assays of LbPrx1m WT and S112A mutant at 13 μm (200 μl input) were performed in buffer T-Ca using a Superdex 200 10/300 GL column (GE Healthcare). Additional aSEC assays comparing WT LbPrx1m and H113A and D108A mutants at 48 μm (250 μl input) were performed at pH 4.0, as described in Morais et al. (9), in buffer T-EDTA plus 2 mm DTT or in buffer T-Ca. For comparative purposes, the molar concentrations estimated for all analyzed proteins refer to monomers. Columns were calibrated using the gel filtration calibration kits LMW and HMW (GE Healthcare).
Dynamic light scattering (DLS)
DLS measurements were performed on a Dynapro molecular sizing instrument at 25 or 42 °C. Protein samples at 100 μm were centrifuged previously for 20 min at 20,000 × g. Data were collected at intervals of 10 s with at least 100 acquisitions. The diffusion coefficient (D) was determined from the analysis of measured time-dependent fluctuations in the scattering intensity and used to calculate the hydrodynamic radius (RH) of the protein according to the Stokes-Einstein equation. Data analysis was performed using the software Dynamics V6.3.40.
Small angle X-ray scattering
SAXS data were collected at the D02A/SAXS2 beamline (Brazilian Synchrotron Light Laboratory, Campinas, Brazil). The radiation wavelength was set to 1.48 Å and a 165-mm MarCCD detector was used to record the scattering patterns. The sample-to-detector distance was set to 1534.5 mm to give a scattering vector range from 0.25 to 2.5 nm−1. Protein samples at 108 μm were prepared in 25 mm Tris-HCl (pH 7.5) with 5 mm EDTA or 25 mm CaCl2. Frames with an exposure time of 600 s were recorded, and buffer baselines were collected under identical conditions. Background scattering was subtracted from the protein-scattering pattern, which was then normalized and corrected. Experimental data fitting and evaluation of the pair-distance distribution function P(r) were performed using the program GNOM (42). The low-resolution envelopes were determined using ab initio modeling as implemented in the program DAMMIN (43). An averaged model was generated using the package DAMAVER (44) The low-resolution model and the crystal structure were superimposed using the program SUPCOMB (45).
Fluorescence anisotropy measurements
Fluorescence anisotropy data were collected in a PC-1 fluorimeter (ISS Instruments) coupled to a thermal bath at 25 °C using an excitation wavelength of 280 nm (46). Samples of LbPrx1m at 80 μm were preincubated in buffer at pH 7.5 containing 25 mm Tris-HCl, 150 mm NaCl, and 5 mm EDTA and increasing amounts of CaCl2 or MgCl2. For data acquisition, samples were diluted in buffer consisting of 25 mm Tris-HCl and 150 mm NaCl (pH 7.5) to a final protein concentration of 2 μm. Free cation concentrations were defined by subtracting the added Ca(Mg)Cl2 concentration from the EDTA concentration of each sample. The mean data of three independent experiments were fitted to a nonlinear regression to estimate the constant K½ using GraphPad Prism v.6.0.
Molecular dynamics simulations
The most favorable geometric coordination of Ca2+ by the residues Ser-109(chain A), Ser-112(chain A), Asp-76(chain J) and Asp-108(chain J) of the LbPrx1m decamer (PDB accession no. 4KB3) was evaluated using molecular dynamics simulations. The system was submitted to an explicit solvent simulation with a water density of 1 g cm−3 and neutralized using a 0.9% NaCl solvent (mass fraction) at 298 K. The protonation states for ionizable groups were set according to pH 7.0 using an empirical equation derived from experimental data that considers electrostatic potential, hydrogen bonds, and accessible surface area (47). The simulation was carried out for 10 ns using the YAMBER3 force field (48), which includes the cation parameters, on the program YASARA. The calcium coordination sphere for every 25-ps snapshot was analyzed using a customized script implemented in FindGeo (49).
Enzymatic assays
NADPH consumption (ɛ340 nm = 6220 m−1 cm−1) by the Leishmania trypanothione system was monitored at 340 nm in a Shimatzu UV-2401 spectrophotometer (Shimatzu Corp.) with temperature set to 25 °C. The reactions were carried out with 280 μm NADPH, 0.4 μm trypanothione reductase LiTR (50), 75 μm trypanothione (Bachem), 4 μm tryparedoxin LiTXN1 (50), and 4.5 μm LbPrx1m (WT or mutants) or 0.4 μm LiPrx1a (50) in a buffer consisting of 50 mm Tris-HCl (pH 7.5). LiTR and trypanothione were used in excess. The Prx enzyme was either untreated or pretreated with 5 mm EDTA or 5 mm EDTA followed by the addition of 25 mm CaCl2 or MgCl2. The others components were incubated previously at 25 °C for 15 min. The treated Prx samples were diluted in the reaction medium, and the reaction was started with the addition of 70 μm H2O2. All experiments were performed with TEV-cleaved LbPrx1m in triplicate. Relative activities were calculated as mean values considering the untreated samples as a reference for the cation and EDTA treatments or the WT activity of LbPrx1m in the presence of CaCl2 as a reference for the muteins assayed under the same conditions.
Chaperone activity assays
To investigate the chaperone activity of reduced and oxidized LbPrx1m (WT or mutants), 100 nm luciferase (Promega) was incubated in 40 mm HEPES (pH 7.5) at 42 °C with a molar ratio of 1:10 (Luciferase:LbPrx1m). LbPrx1m was pretreated with 20 mm CaCl2 or MgCl2, 5 mm EDTA, or 5 mm EDTA plus 25 mm CaCl2 or MgCl2. The reduced samples were incubated with 2 mm DTT (final concentration). The reactions were kept at 42 °C, and luciferase aggregation was monitored in a Fluoromax-4 spectrofluorometer (Horiba) for 900 s using a wavelength of 360 nm for excitation and emission. Relative activities were calculated as mean values considering the activity of untreated samples as reference for those treated with additives (Ca2+, EDTA, and DTT) or the activity of WT protein as a reference for the muteins assayed in the same condition, unless stated otherwise. To exclude the effect that some additives have on luciferase aggregation, the relative activities were calculated according to the formula (NT − T)/(NR − R), where NT, T, NR, and R refer to light-scattering values recorded at 900 s of NT, the negative control reaction of the test condition (luciferase + additives); T is the test condition (NT + WT or mutant LbPrx1m); NR is the negative control reaction of the reference condition (luciferase + additives); and R is the reference condition (NR+ WT LbPrx1m). All assays were performed with TEV-cleaved LbPrx1m in triplicate.
Circular dichroism
CD measurements were acquired at 25 or 42 °C on a JASCO J-815 spectropolarimeter equipped with a Peltier temperature controller (Jasco Analytical Instruments). TEV-cleaved LbPrx1m samples (WT and H113A mutant) at 80 μm were pretreated with 5 mm EDTA plus (or not) 25 mm CaCl2 and diluted to a final concentration of 2 μm in 10 mm sodium phosphate (pH 7.5) with or without 2 mm DTT. Far-UV CD spectra were recorded between 190 and 260 nm at a speed of 50 nm/min with a total of 16 accumulations. The CD data were buffer-subtracted and normalized to molar residual ellipticity allowing the comparison between different treatments.
Differential scanning fluorimetry
DSF assays were performed in triplicate using a real-time PCR machine 7300 (Applied Biosystems). Samples of TEV-cleaved LbPrx1m (WT and H113A mutant) were pretreated as described above and diluted to a final concentration of 2 μm in buffer consisting of 20 mm HEPES (pH 7.5), 150 mm NaCl, with or without 2 mm DTT, and containing 5× SYPRO Orange fluorescent dye (Invitrogen-Molecular Probes). The 96-well plates were heated from 25 to 95 °C, increasing 1 °C/cycle, and the fluorescence emission was measured at 580 nm. The DSF melting curves were analyzed using GraphPad Prism software version 6.0.
Generation of L. infantum transfectants
To construct the pSSU-PHLEO-infantum-LbPrx1m plasmids, a DNA fragment corresponding to the mitochondrial targeting sequence of Prx1m was PCR-amplified with PfuTurbo from genomic DNA of Leishmania amazonensis with primers P1 (5′-cgcggatccATGCTCCGTCGTCTTGCTA-3′) and P2 (5′-tgctctagagctagcaggcctGACAGTCGCCGTACGGTA-3′) and cloned into the BamHI and XbaI sites of pSSU-PHLEO-infantum-LiPrx1m vector (2). Clamp sequences are indicated in lower case, and restriction sites are in italics. The resulting plasmid was subsequently digested with StuI and NheI and ligated to the rest of the LbPrx1m ORF (either the WT or mutated versions of the gene) obtained by PCR amplification with PfuTurbo and primers P3 (5′-GATCCTGCGCCGCAGTTT-3′) and P4 (5′-ctagctagcTCACATATTCTTCTCAAAAAATT-3′) from the plasmids pET28a-His-TEV-LbPrx1m WT or mutants. The accuracy of all constructs was verified by DNA sequencing at GATC Biotech (Konstanz, Germany). Prior to transfection of L. infantum, the pSSU-PHLEO-infantum-LbPrx1m constructs were linearized by digestion with NdeI and PmeI and purified from agarose gels.
Transfection of L. infantum and isolation of mutants
Transfections were carried out on L. infantum promastigotes (MHOM MA67ITMAP263) missing both Prx1m alleles (i.e. Prx1m null mutants or Prx1m−) produced previously (2). Parasites were grown to the logarithmic phase and electroporated at 450 V and 350–400 μF with 5 μg of DNA as described elsewhere (51). Transfectants were allowed to recover in culture medium without selective drug for 24 h prior to being plated onto agar plates containing 17.5 μg ml−1 bleomycin (Sigma-Aldrich). Upon 1 to 2 weeks of growth on agar, colonies were picked up, transferred to liquid medium, and analyzed by PCR, Western blotting, and indirect immunofluorescence to confirm LbPrx1m expression in the transfectants according to previously described procedures (2, 52).
Thermotolerance assays
L. infantum promastigotes, synchronized previously by three to four daily changes of culture medium, were seeded at 106 cells ml−1 in 24-well plates containing RPMI 1640-GlutaMAX medium supplemented with 10% inactivated fetal bovine serum, 50 units ml−1 penicillin, 50 mg ml−1 streptomycin (all from Gibco), and 20 mm HEPES sodium salt (pH 7.4) (Sigma). Parasites were allowed to grow for 4 days at either 25 or 37 °C. Every 24 h, cell densities were determined with a Neubauer counting chamber for growth curve determination. Two independent clones were analyzed for each transfectant.
Author contributions
M. A. B. M., P. O. G., T. A. C. B. S., H. C., R. V. H., P. S. L. O., L. E. S. N., A. M. T., and M. T. M. conceived and designed the experiments. M. A. B. M., P. O. G., T. A. C. B. S., H. C., and R. V. H. performed the experiments. M. A. B. M., P. O. G., T. A. C. B. S., H. C., R. V. H., P. S. L. O., L. E. S. N., A. M. T., and M. T. M. analyzed the data. M. A. B. M., P. O. G., and M. T. M. wrote the paper, and M. A. B. M., P. O. G., T. A. C. B. S., H. C., R. V. H., P. S. L. O., L. E. S. N., A. M. T., and M. T. M. revised the paper.
Acknowledgments
We are grateful to the Brazilian Synchrotron Light Laboratory (LNLS) and Brazilian Biosciences National Laboratory (LNBio) for the provision of time on MX2 and SAXS1 beamlines, ROBOLAB, LPP, and LEC.
This work was supported by Grant 2010/51730-0 from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and by grants from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (to M. T. M.). This work was also supported by grants from Project “NORTE-07-0124-FEDER-000002” (co-funded by the Norte Portugal Regional Operational Programme (NORTE 2020) under the Quadro de Referência Estratégico Nacional through the European Regional Development Fund (ERDF) and by the the Portuguese Foundation for Science and Technology) and from Project “NORTE-01-0145-FEDER-000012” (supported by NORTE 2020 under the PORTUGAL 2020 Partnership Agreement through ERDF) (to A. M. T.). The authors declare that they have no conflicts of interest with the contents of this article.
- LiPrx1m
- mitochondrial 2-Cys peroxiredoxin from Leishmania infantum
- aSEC
- analytical size-exclusion chromatography
- Cp
- peroxidatic cysteine
- Cr
- resolving cysteine
- DLS
- dynamic light scattering
- DSF
- differential scanning fluorimetry
- HsPrx2
- human Prx2
- LbPrx1m
- mitochondrial 2-Cys peroxiredoxin from Leishmania braziliensis
- LiPrx1a
- cytoplasmic 2-Cys peroxiredoxin from Leishmania infantum
- PDB
- Protein Data Bank
- Prx
- peroxiredoxin
- SAXS
- small angle X-ray scattering
- SEC
- size-exclusion chromatography
- TEV
- tobacco etch virus
- TcPrx1a
- cytoplasmic 2-Cys peroxiredoxin from Trypanosoma cruzi.
References
- 1. Pearson R. D., and Wilson M. E. (1989) Host defenses against prototypical intracellular protozoans, the Leishamania, in Parasitic Infections in the Compromised Host (Walzer P. D., and Genta R. M., eds) pp 31–81, Marcel Dekker, New York [Google Scholar]
- 2. Castro H., Teixeira F., Romao S., Santos M., Cruz T., Flórido M., Appelberg R., Oliveira P., Ferreira-da-Silva F., and Tomás A. M. (2011) Leishmania mitochondrial peroxiredoxin plays a crucial peroxidase-unrelated role during infection: insight into its novel chaperone activity. PLoS Pathog. 7, e1002325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Teixeira F., Castro H., Cruz T., Tse E., Koldewey P., Southworth D. R., Tomás A. M., and Jakob U. (2015) Mitochondrial peroxiredoxin functions as crucial chaperone reservoir in Leishmania infantum. Proc. Natl. Acad. Sci. U.S.A. 112, E616–E624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wood Z. A., Schröder E., Robin Harris J., and Poole L. B. (2003) Structure, mechanism and regulation of peroxiredoxins. Trends Biochem. Sci. 28, 32–40 [DOI] [PubMed] [Google Scholar]
- 5. Jang H. H., Lee K. O., Chi Y. H., Jung B. G., Park S. K., Park J. H., Lee J. R., Lee S. S., Moon J. C., Yun J. W., Choi Y. O., Kim W. Y., Kang J. S., Cheong G. W., Yun, et al. (2004) Two enzymes in one: two yeast peroxiredoxins display oxidative stress-dependent switching from a peroxidase to a molecular chaperone function. Cell 117, 625–635 [DOI] [PubMed] [Google Scholar]
- 6. Moon J. C., Hah Y. S., Kim W. Y., Jung B. G., Jang H. H., Lee J. R., Kim S. Y., Lee Y. M., Jeon M. G., Kim C. W., Cho M. J., and Lee S. Y. (2005) Oxidative stress-dependent structural and functional switching of a human 2-Cys peroxiredoxin isotype II that enhances HeLa cell resistance to H2O2-induced cell death. J. Biol. Chem. 280, 28775–28784 [DOI] [PubMed] [Google Scholar]
- 7. Chuang M. H., Wu M. S., Lo W. L., Lin J. T., Wong C. H., and Chiou S. H. (2006) The antioxidant protein alkylhydroperoxide reductase of Helicobacter pylori switches from a peroxide reductase to a molecular chaperone function. Proc. Natl. Acad. Sci. U.S.A. 103, 2552–2557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bernier-Villamor L., Navarro E., Sevilla F., and Lázaro J. J. (2004) Cloning and characterization of a 2-Cys peroxiredoxin from Pisum sativum. J. Exp. Bot. 55, 2191–2199 [DOI] [PubMed] [Google Scholar]
- 9. Morais M. A., Giuseppe P. O., Souza T. A., Alegria T. G., Oliveira M. A., Netto L. E., and Murakami M. T. (2015) How pH modulates the dimer-decamer interconversion of 2-Cys peroxiredoxins from the Prx1 subfamily. J. Biol. Chem. 290, 8582–8590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kristensen P., Rasmussen D. E., and Kristensen B. I. (1999) Properties of thiol-specific anti-oxidant protein or calpromotin in solution. Biochem. Biophys. Res. Commun. 262, 127–131 [DOI] [PubMed] [Google Scholar]
- 11. Kitano K., Niimura Y., Nishiyama Y., and Miki K. (1999) Stimulation of peroxidase activity by decamerization related to ionic strength: AhpC protein from Amphibacillus xylanus. J. Biochem. 126, 313–319 [DOI] [PubMed] [Google Scholar]
- 12. Matsumura T., Okamoto K., Iwahara S., Hori H., Takahashi Y., Nishino T., and Abe Y. (2008) Dimer-oligomer interconversion of wild-type and mutant rat 2-Cys peroxiredoxin: disulfide formation at dimer-dimer interfaces is not essential for decamerization. J. Biol. Chem. 283, 284–293 [DOI] [PubMed] [Google Scholar]
- 13. Barranco-Medina S., Kakorin S., Lázaro J. J., and Dietz K. J. (2008) Thermodynamics of the dimer-decamer transition of reduced human and plant 2-Cys peroxiredoxin. Biochemistry 47, 7196–7204 [DOI] [PubMed] [Google Scholar]
- 14. Guimarães B. G., Souchon H., Honoré N., Saint-Joanis B., Brosch R., Shepard W., Cole S. T., and Alzari P. M. (2005) Structure and mechanism of the alkyl hydroperoxidase AhpC, a key element of the Mycobacterium tuberculosis defense system against oxidative stress. J. Biol. Chem. 280, 25735–25742 [DOI] [PubMed] [Google Scholar]
- 15. Wood Z. A., Poole L. B., Hantgan R. R., and Karplus P. A. (2002) Dimers to doughnuts: redox-sensitive oligomerization of 2-cysteine peroxiredoxins. Biochemistry 41, 5493–5504 [DOI] [PubMed] [Google Scholar]
- 16. Wood Z. A., Poole L. B., and Karplus P. A. (2003) Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling. Science 300, 650–653 [DOI] [PubMed] [Google Scholar]
- 17. Sarkar A. R., Heo C. H., Xu L., Lee H. W., Si H. Y., Byun J. W., and Kim H. M. (2016) A ratiometric two-photon probe for quantitative imaging of mitochondrial pH values. Chem. Sci. 7, 766–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chauhan R., and Mande S. C. (2001) Characterization of the Mycobacterium tuberculosis H37Rv alkyl hydroperoxidase AhpC points to the importance of ionic interactions in oligomerization and activity. Biochem. J. 354, 209–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Park J. W., Piszczek G., Rhee S. G., and Chock P. B. (2011) Glutathionylation of peroxiredoxin I induces decamer to dimers dissociation with concomitant loss of chaperone activity. Biochemistry 50, 3204–3210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Engelman R., Weisman-Shomer P., Ziv T., Xu J., Arnér E. S., and Benhar M. (2013) Multilevel regulation of 2-Cys peroxiredoxin reaction cycle by S-nitrosylation. J. Biol. Chem. 288, 11312–11324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bui D. M., Gregan J., Jarosch E., Ragnini A., and Schweyen R. J. (1999) The bacterial magnesium transporter CorA can functionally substitute for its putative homologue Mrs2p in the yeast inner mitochondrial membrane. J. Biol. Chem. 274, 20438–20443 [DOI] [PubMed] [Google Scholar]
- 22. McCormack J. G., Halestrap A. P., and Denton R. M. (1990) Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol. Rev. 70, 391–425 [DOI] [PubMed] [Google Scholar]
- 23. Huang G., Vercesi A. E., and Docampo R. (2013) Essential regulation of cell bioenergetics in Trypanosoma brucei by the mitochondrial calcium uniporter. Nat. Commun. 4, 2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tsien R. Y. (1980) New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry 19, 2396–2404 [DOI] [PubMed] [Google Scholar]
- 25. Kolisek M., Zsurka G., Samaj J., Weghuber J., Schweyen R. J., and Schweigel M. (2003) Mrs2p is an essential component of the major electrophoretic Mg2+ influx system in mitochondria. EMBO J. 22, 1235–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rutter G. A., Burnett P., Rizzuto R., Brini M., Murgia M., Pozzan T., Tavaré J. M., and Denton R. M. (1996) Subcellular imaging of intramitochondrial Ca2+ with recombinant targeted aequorin: significance for the regulation of pyruvate dehydrogenase activity. Proc. Natl. Acad. Sci. U.S.A. 93, 5489–5494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Benaim G., Bermudez R., and Urbina J. A. (1990) Ca2+ transport in isolated mitochondrial vesicles from Leishmania braziliensis promastigotes. Mol. Biochem. Parasitol. 39, 61–68 [DOI] [PubMed] [Google Scholar]
- 28. Simonovic M., Dolmer K., Huang W., Strickland D. K., Volz K., and Gettins P. G. (2001) Calcium coordination and pH dependence of the calcium affinity of ligand-binding repeat CR7 from the LRP: comparison with related domains from the LRP and the LDL receptor. Biochemistry 40, 15127–15134 [DOI] [PubMed] [Google Scholar]
- 29. Suzuki N., Fujimoto Z., Morita T., Fukamizu A., and Mizuno H. (2005) pH-Dependent structural changes at Ca(2+)-binding sites of coagulation factor IX-binding protein. J. Mol. Biol. 353, 80–87 [DOI] [PubMed] [Google Scholar]
- 30. Nelson K. J., Knutson S. T., Soito L., Klomsiri C., Poole L. B., and Fetrow J. S. (2011) Analysis of the peroxiredoxin family: using active-site structure and sequence information for global classification and residue analysis. Proteins 79, 947–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Piñeyro M. D., Pizarro J. C., Lema F., Pritsch O., Cayota A., Bentley G. A., and Robello C. (2005) Crystal structure of the tryparedoxin peroxidase from the human parasite Trypanosoma cruzi. J. Struct. Biol. 150, 11–22 [DOI] [PubMed] [Google Scholar]
- 32. Parsonage D., Youngblood D. S., Sarma G. N., Wood Z. A., Karplus P. A., and Poole L. B. (2005) Analysis of the link between enzymatic activity and oligomeric state in AhpC, a bacterial peroxiredoxin. Biochemistry 44, 10583–10592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Casey J. R., Grinstein S., and Orlowski J. (2010) Sensors and regulators of intracellular pH. Nat. Rev. Mol. Cell Biol. 11, 50–61 [DOI] [PubMed] [Google Scholar]
- 34. Prasad A., Kaur S., Malla N., Ganguly N. K., and Mahajan R. C. (2001) Ca2+ signaling in the transformation of promastigotes to axenic amastigotes of Leishmania donovani. Mol. Cell. Biochem. 224, 39–44 [DOI] [PubMed] [Google Scholar]
- 35. Poburko D., Santo-Domingo J., and Demaurex N. (2011) Dynamic regulation of the mitochondrial proton gradient during cytosolic calcium elevations. J. Biol. Chem. 286, 11672–11684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Angelucci F., Miele A. E., Ardini M., Boumis G., Saccoccia F., and Bellelli A. (2016) Typical 2-Cys peroxiredoxins in human parasites: several physiological roles for a potential chemotherapy target. Mol. Biochem. Parasitol. 206, 2–12 [DOI] [PubMed] [Google Scholar]
- 37. Zhao Q., Ding Y., Deng Z., Lee O. Y., Gao P., Chen P., Rose R. J., Zhao H., Zhang Z., Tao X. P., Heck A. J., Kao R., and Yang D. (2015) Natural products triptolide, celastrol, and withaferin A inhibit the chaperone activity of peroxiredoxin I. Chem. Sci. 6, 4124–4130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. de Morais M. A., de Souza Tde A., and Murakami M. T. (2013) Cloning, expression, purification, crystallization and preliminary X-ray diffraction analysis of the mitochondrial tryparedoxin peroxidase from Leishmania braziliensis. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 69, 408–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. de Oliveira M. A., Genu V., Discola K. F., Alves S. V., Netto L. E., and Guimarães B. G. (2007) Crystallization and preliminary X-ray analysis of a decameric form of cytosolic thioredoxin peroxidase 1 (Tsa1), C47S mutant, from Saccharomyces cerevisiae. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 63, 665–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rutter G. A., Osbaldeston N. J., McCormack J. G., and Denton R. M. (1990) Measurement of matrix-free Mg2+ concentration in rat heart mitochondria by using entrapped fluorescent probes. Biochem. J. 271, 627–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Montero M., Alonso M. T., Carnicero E., Cuchillo-Ibáñez I., Albillos A., García A. G., García-Sancho J., and Alvarez J. (2000) Chromaffin-cell stimulation triggers fast millimolar mitochondrial Ca2+ transients that modulate secretion. Nat. Cell Biol. 2, 57–61 [DOI] [PubMed] [Google Scholar]
- 42. Svergun D. (1992) Determination of the regularization parameter in indirect-transform methods using perceptual criteria. J. Appl. Crystallogr. 25, 495–503 [Google Scholar]
- 43. Svergun D. I. (1999) Restoring low resolution structure of biological macromolecules from solution scattering using simulated annealing. Biophys. J. 76, 2879–2886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Volkov V., and Svergun D. (2003) Uniqueness of ab initio shape determination in small-angle scattering. J. Appl. Crystallogr. 36, 860–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kozin M. B., and Svergun D. I. (2001) Automated matching of high- and low-resolution structural models. J. Appl. Crystallogr. 34, 33–41 [Google Scholar]
- 46. LeTilly V., and Royer C. A. (1993) Fluorescence anisotropy assays implicate protein-protein interactions in regulating trp repressor DNA binding. Biochemistry 32, 7753–7758 [DOI] [PubMed] [Google Scholar]
- 47. Krieger E., Nielsen J. E., Spronk C. A., and Vriend G. (2006) Fast empirical pKa prediction by Ewald summation. J. Mol. Graph. Model. 25, 481–486 [DOI] [PubMed] [Google Scholar]
- 48. Krieger E., Darden T., Nabuurs S. B., Finkelstein A., and Vriend G. (2004) Making optimal use of empirical energy functions: force-field parameterization in crystal space. Proteins 57, 678–683 [DOI] [PubMed] [Google Scholar]
- 49. Andreini C., Cavallaro G., and Lorenzini S. (2012) FindGeo: a tool for determining metal coordination geometry. Bioinformatics 28, 1658–1660 [DOI] [PubMed] [Google Scholar]
- 50. Castro H., Sousa C., Novais M., Santos M., Budde H., Cordeiro-da-Silva A., Flohé L., and Tomás A. M. (2004) Two linked genes of Leishmania infantum encode tryparedoxins localised to cytosol and mitochondrion. Mol. Biochem. Parasitol. 136, 137–147 [DOI] [PubMed] [Google Scholar]
- 51. Beverley S. M., and Clayton C. E. (1993) Transfection of Leishmania and Trypanosoma brucei by electroporation. Methods Mol. Biol. 21, 333–348 [DOI] [PubMed] [Google Scholar]
- 52. Sousa A. F., Gomes-Alves A. G., Benítez D., Comini M. A., Flohé L., Jaeger T., Passos J., Stuhlmann F., Tomás A. M., and Castro H. (2014) Genetic and chemical analyses reveal that trypanothione synthetase but not glutathionylspermidine synthetase is essential for Leishmania infantum. Free Radic. Biol. Med. 73, 229–238 [DOI] [PubMed] [Google Scholar]
- 53. Castro H., Sousa C., Santos M., Cordeiro-da-Silva A., Flohé L., and Tomás A. M. (2002) Complementary antioxidant defense by cytoplasmic and mitochondrial peroxiredoxins in Leishmania infantum. Free Radic. Biol. Med. 33, 1552–1562 [DOI] [PubMed] [Google Scholar]