Abstract
Extracellular vesicles are small lipid-based membrane-bound entities shed by cells under both physiological and pathological conditions. Their discovery as intercellular communicators through transfer of nucleic acid- and protein-based cargos between cells locally and at distance in a highly specific manner has created recent excitement. The information they transport and their composition may vary depending on the cell of origin as well as the eliciting stimulus. Such sensitive changes in vesicle characteristics hold significant promise for the improved diagnosis of pathological conditions, including infections and neoplastic lesions in a minimally invasive way. Similarly, these cell-derived vesicles exhibit promising characteristics that could enhance drug targeting efficiencies. Recent developments in the field have aimed at studying EVs as novel drug carriers due to their natural composition, biological function and selective cell interaction. In this review, we discuss new research avenues in diagnostics and drug therapy based on extracellular vesicles. We show how cell-derived vesicles can be harvested and engineered to meet application-specific design requirements. We finally discuss potential risks encountered when translating extracellular vesicle based approaches into (pre)clinical applications.
Extracellular vesicles (EVs) are small membrane particles that are shed by cells under both physiological and pathological conditions [1]. EVs, which are sometimes referred to as natural liposomes, consist of a lipid bilayer membrane (e.g., various phospholipids, cholesterol) decorated with surface and membrane proteins (Figure 1), and have been shown to transport protein-, RNA- and DNA-based cargoes [2, 3]. In addition to soluble factors and direct cell-cell interactions, these cell-derived particles are pivotal mediators of intercellular communication and transfer information within complex multicellular systems [4]. While EVs were initially described as platelet dust almost five decades ago [5], it is nowadays known that EVs are produced by (almost) all cell types both in vitro and in vivo. Importantly, the secretion of EVs is not only limited to eukaryotic cells but they are also present in bacteria and other prokaryotes [6, 7]. It has also become increasingly evident that EVs play a major role in a multitude of physiological processes and pathologies, which has triggered new developments in the fields of diagnostics and targeted drug delivery.
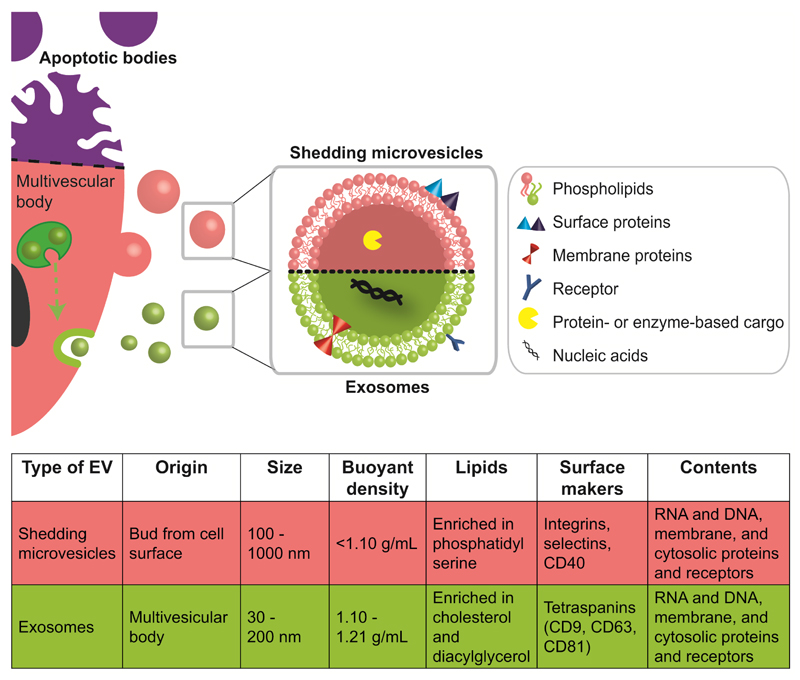
Figure 1. Biogenesis and characteristics of major classes of EVs.
EVs are cell-derived particles composed of a phospholipid bilayer membrane, decorated with surface and membrane proteins, and carrying nucleic acid or protein-based cargos. There are three major classes of EVs: apoptotic bodies, shedding microvesicles (SMVs) and exosomes. Apoptotic bodies are released when cells die (indicated by purple upper part of cell). These bodies contain DNA, histone and organelle fragments, are up to 5 μm in size, and possess a buoyant density of 1.16-1.28 g/mL [10]. SMV originate from the budding of cytoplasmic protrusions and the fission of their stalks. Thus, their lipid content is often closely related to their mother cell’s membrane composition. Exosomes are usually generated by invagination of the multivesicular bodies’ (MVB) membrane. Fusion of the MVB membrane with the outer cell membrane leads to release of exosomes into the extracellular space. The endosomal sorting complex required for transport (ESCRT) is involved in cargo and (membrane) protein integration into exosomes. Both SMVs and exosomes can have a size in the lower nanometre scale but they feature significant differences in their physico-chemical characteristics (i.e., size, density) and their lipid and protein (i.e., surface markers) composition.
Here review recent developments in the EV field, in particular EV-based diagnostics and the use of EVs as targeted drug delivery vectors. We discuss the advantages and drawbacks of natural EVs compared to synthetic liposomes and highlight potential strategies by which to engineer EVs to meet application-specific design requirements (Box 1). This review also includes a short tutorial on the isolation and characterisation of EVs that can be used as an initial parameter set to explore new diagnostic and drug delivery strategies. We hope such a tutorial makes EV-based approaches more accessible to a broader scientific community. Finally, we critically discuss strategies to overcome the hurdles associated with the use of these cell-derived vesicles in preclinical and clinical environments.
Box 1. Advantages (+) and limitations (-) of state of the art approaches for diagnostics and drug delivery compared with the potential of extracellular vesicles.
| State of the art | Extracellular vesicles | |
|---|---|---|
| Diagnostics |
Body fluid diagnostics + established procedures + clinical data available - can have limited specificity Biopsies + established procedures + histological information + genetic information - invasive - risk of dissemination, collateral damage - painful - restricted to well-accessible tissue sites |
+ potentially increased specificity + access to cell-specific information + minimally invasive + information is spatially stored (cargo, membrane, size, etc) + monitoring of drug efficacy (e.g., chemotherapy) - sample collection and processing not standardized - long-term sample stability unknown - little clinical data available |
| Theranostics | - few to no theranostic systems available |
+ allow integrated approach combining diagnostics, therapy and therapy efficacy monitoring - no systems have reached clinical stage yet |
| Drug Delivery |
Nanoparticulate carrier (e.g., liposomes, micelles, polymer nanoparticles, etc) + size 10-200 nm + (high) drug loading efficiency + various loading methods + targeting ligands can be attached + large scale production + uptake/drug release well studied + (bio)chemically well defined - activation immune system - circulation time often limited upon repeated administration - potential toxicity - limited (pre)clinical success |
+ size 50-200 nm + naturally-derived composition + stability in biological fluids + potentially reduced immunogenicity + cell-cell communicators + (unidirectional) targeting + inherent biological activity + various drug encapsulation methods underway + clinical trials for cancer vaccines ongoing - cell-derived isolation - scale-up production difficult - detailed in vivo data lacking - production/uptake mechanism yet poorly described - analysis of biochemical composition ongoing - (pre)clinical evaluation for drug delivery lacking |
Exosomes, shedding microvesicles and apoptotic bodies
There are three major categories of EVs: (1) apoptotic bodies; (2) shedding microvesicles (SMVs); and (3) exosomes. Each of these show a different mechanism of release and are morphologically and biochemically different (Figure 1).
Apoptotic bodies are released when cells die and represent a heterogeneous population of vesicles with a wide size range of 50-5000 nm. They usually carry extensive amounts of phosphatidylserine in their membranes because this lipid is enriched in the outer membrane of apoptotic cells. Both SMVs and exosomes serve for the intercellular transfer of lipids, RNA, and cytosolic proteins [8]. However, a very recent study suggested that SMVs and exosomes have distinct differences in the functional transfer of loaded reporter molecules to target cells [9].
SMVs bud directly off the cell membrane following a stimulus and are reported to have sizes of 80-1000 nm depending on the cell source and separation method [11]. Although SMVs generally appear to be a heterogeneous population, common biochemical features have been indentified between SMVs from different sources such as surface markers (CD40 ligands, integrins and selectins) and the enrichment of phosphatidylserine in their membrane. Interestingly, the general membrane composition does not always reflect the one of the mother cell, although the mechanism for this phenomenon is not fully clarified [12]. The membrane shedding of SMVs can be influenced through various triggers such as Ca2+ influx, phorbol esters, ATP, or through a P2Y receptor [13]. Exosomes are reported to be a more homogeneous population of vesicles and their size range is around 50-120 nm [14]. Their biogenesis occurs within a multivesicular body (MVB) by invagination of the endosomal membrane. After transit of the MVB to the cell membrane, exosomes are relased upon MVB-membrane fusion with the cellular membrane. Exosomes often comprise of a specific set of membrane and surface proteins (e.g., tetraspanin CD9, CD63, CD81 [15]) and a nucleic acid based cargo. The endosomal cell sorting complex is mainly responsible for the biogenesis of exosomes and the incorporation of these cargos [16], although other pathways independent of this sorting mechanism have also been found [17]. In general, exosomes are considered to be transporters of miRNA which regulate specific intracellular mRNA activity [18]. A recent study reported that miRNA may not be as abundant in exosomes as previously estimated [19]. It was shown that most exosomes from standard preparations contained on average less than one copy of miRNA per vesicle. Although efforts for the harmonisation of EV nomenclature are currently underway (http://www.exocarta.org), the use of the terms SMVs and exosomes in already published studies is still not consistent. Reasons for this are limited knowledge on EV biogenesis, variations in isolation protocols and methods, and insufficient EV characterisation. In the following discussion, SMVs and exosomes will be uniformly termed as EVs.
Cell-derived vesicles in physiological and pathological conditions
EVs are shed by most cells constitutively as well as in response to endogenous and exogenous triggers. EVs are involved in the orchestration of intercellular communication under both physiological and pathophysiological conditions [20]. They have been isolated from numerous body fluids including blood, urine, saliva, amniotic fluid, ascites fluid, bronochoalveolar lavage fluid, cerebrospinal fluid, bile, breast milk, and semen [8]. Under physiological conditions, EVs are vital for cell-to-cell and inter-tissural communication and exhibit important regulatory functions in homeostasis by mediating phenotype adjustments in a variety of conditions [8, 11]. The EV cargo can be transmitted to target cells which then modify the cell’s physiology, e.g., the horizontal transfer of RNA is then translated by the recipient cell [21]. While this process is essential for tissue homeostasis, lateral information transfer may have a detrimental impact in pathological conditions.
EVs have recently been shown to substantially contribute to various disease states including tumorigenesis and metastasis [22], inflammation [23], and activation of the immune system [24]. It is now increasingly being recognised that EVs are key mediators in pathological processes and can act locally as well at a systemic level. While a few proteins (e.g., integrin β1) and lipids have so far been found in all EVs investigated, EVs derived from immune and tumour cells have been shown to be enriched in proteolytic enzymes. Such enzymes are capable of digesting the extracellular matrix, which in turn enables downstream processes in inflammation (tissue migration of immune cells) and tumour progression (angiogenesis, tumour growth, and metastasis). EVs shed by cancer cells have a critical yet poorly understood role in remodelling the extracellular matrix, the tumour microenvironment, and metastatic processes.
Tumour-derived EVs may interfere with the host immune system by activating apoptosis in activated anti-tumour T cells and impair the differentiation of monocytes into dendritic cells or induce myeloid suppressive cells. EVs with immunosuppressive and angiogenic properties have been detected in the body fluids of cancer patients, a finding which suggests a critical involvement of EVs in disease progression [25]. Skog et al. [26] showed that glioblastoma-derived EVs are taken up by normal host cells such as brain microvascular endothelial cells, and that messages delivered by (tumour-derived) microvesicles are translated by recipient cells. Additionally, pro-angiogenic factors contained in these vesicles have been shown to promote endothelial tubule formation, which assists in the remodelling of the tumour-microenvironment. Similarly, it has been demonstrated in a mouse cancer cell model that EVs activate and chemoattract stroma fibroblasts and endothelial cells, and induce expression of pro-angiopoietic factors in stromal cells [27]. Taken together, these findings demonstrate a pivotal involvement of tumour-derived EVs in cancer progression.
Another EV subclass with known pathological relevance is platelet-derived EVs with procoagulant activity. Platelet-derived EVs are involved in pathological (inflammatory) processes including stroke [11], cardiovascular diseases [28], acute respiratory distress syndrome (ARDS) [29, 30], disseminated intravascular coagulation [31], and (meningococcal) sepsis [32]. This creates an environment that potentially aggravates the patient’s clinical condition by interfering with the coagulation cascade [31].
While cell-derived EVs induced during pathological conditions may have detrimental effects on a patient’s health, the detection of vesicles with disease-specific properties in body fluids is intriguing and may allow for the early, non-invasive detection of neoplastic lesions and inflammation-mediated pathologies. In order to become early diagnostic markers in clinical practice, pathology-induced changes of EV populations need to be carefully evaluated in the clinical setting. This relies heavily on standardised sample collection and EV characterisation as to their origin, cargo, and functional properties.
Extracellular vesicles as diagnostic probes
There is growing evidence that EVs contain a unique signature and that their size, (membrane) composition, and cargo depend on both the cellular origin but also the eliciting stimulus (Figure 2A,B and Table 1). As a result, EV characterisation are an attractive candidate as a non-invasive diagnostic probe for hard-to-reach tissues, neoplastic lesions too small for detection, or screening approaches where invasive procedures are not justifiable. An analysis of EV concentrations and properties has been shown to give access to characteristic fingerprint information about the donor cell. This holds great potential for novel diagnostic approaches where microvesicle populations diagnose the presence or absence of pathological conditions, or may form the basis for further testing. The most likely diagnostic applications include the rapid probing of (distant) tissue sites without involving invasive (and potentially painful and risky) procedures such as biopsies. Shao et al. have demonstrated that glioblastoma can be diagnosed and therapy can be real-time monitored based on circulating tumour-derived EV signatures [33]. For an overview on EVs as potential cancer biomarkers, we refer readers to a comprehensive recent review [34].
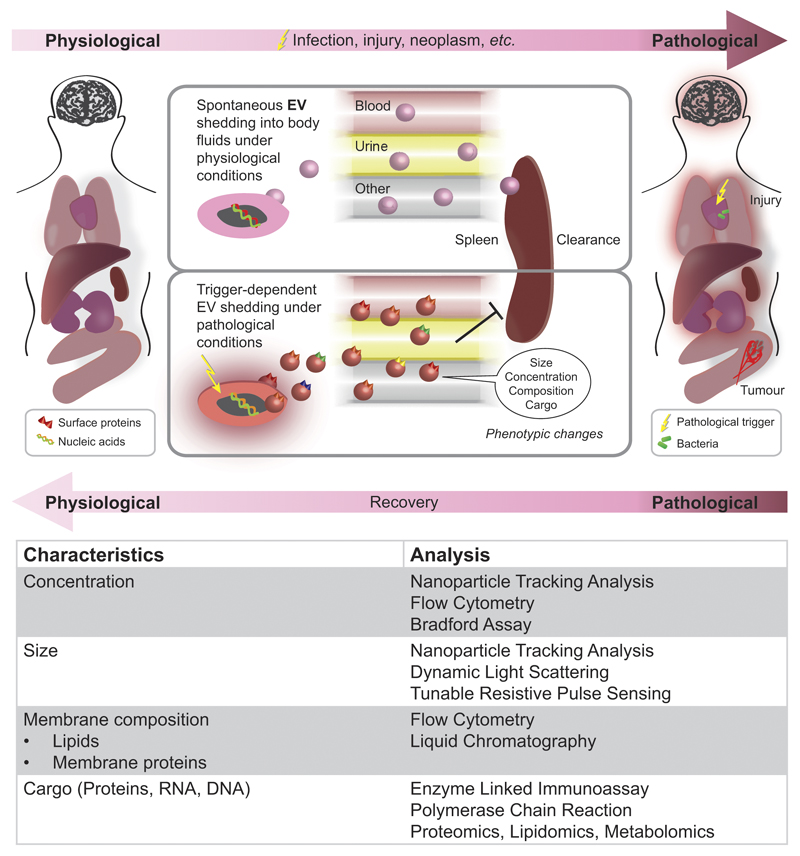
Figure 2. EV characteristics under physiological and pathological conditions and methods of characterisation.
EVs are shed by most cells in the body and are subject to endogenous and exogenous changes. Most body fluids (e.g., blood, urine, saliva, amniotic fluid, ascites fluid, bronochoalveolar lavage fluid, cerebrospinal fluid, bile, breast milk, semen) contain characteristic EV populations. Injuries such as trauma, inflammatory or infectious processes, as well as neoplastic lesions can affect EV properties (e.g., concentration, size, composition, cargo) and clearance. Changes in EV properties can be characterised by means of various analytical techniques including light scattering, flow cytometry, and biochemical assays.
Table 1. Extracellular vesicles for diagnostics and drug delivery applications.
| Type/source of EV | Application | Reference | |
|---|---|---|---|
| Diagnostic applications EVs | |||
| Diagnostic application of EVs for cancer and other dispositions | Glioblastoma-derived microvesicles | Microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers | Skog (2008) [82] |
| Lung adenocarcinoma-derived exosomes | Total exosome and miRNA levels in lung cancer patients employed as diagnostic measure | Rabinowits (2008) [83] | |
| Ovarian cancer-derived exosomes | MicroRNA signatures of tumor-derived exosomes are diagnostic biomarkers of ovarian cancer | Taylor (2008) [84] | |
| Prostate cancer-derived urine exosomes | Prostate cancer biomarkers in exosomes isolated from urine as diagnostic measure | Skog (2009) [85] | |
| Melanoma-derived exosomes | Plasma levels of exosomes (CD63 and Caveolin-1) are increased in tumour patients | Logozzi (2009) [86] | |
| Glioblastoma-derived microvesicles | Circulating microvesicles allow profiling and real-time monitoring of glioblastoma therapy | Shao (2012) [33] | |
| Hepatocyte-derived exosomes | Circulating microRNAs in exosomes for detection of inflammation in alcoholic, drug-induced, and inflammatory liver diseases | Bala (2012) [87] | |
| Therapeutic applications EVs | |||
| Therapeutic applications by inhibition of EV release | Platelets | Calcium channel blocker (nifedipine) reduce platelet microparticle production in patients with transient ischemic attack | Lee (1993) [39] |
| Platelets | Murine/human chimeric monoclonal antibody fragment blocks integrin GPIIb/IIIa and decreases platelet microparticle production in ischemic high risk patients | Reverter (1996) [38] | |
| Mouse oligodendroglial cell (Oli-neu) | Endosomal membrane sorting and exosome release require sphingolipid ceramide | Trajkovic (2008) [17] | |
| Mouse dendritic cells (DC) and various tumor cell lines (B16F10, MCA101, TS/A, 4T1, MB49) | Blockage of GTPase Rab27a in mammary carcinoma cells decreases exosome secretion, primary tumor growth and dissemination of metastatic carcinoma (4T1) | Bobrie (2012) [42], Ostrowski (2010) [41] | |
| Therapeutic application using EV as drug carrier – endogenous loading and transfer of nucleic acid cargo | Mouse and human mast cell lines (MC/9 and HMC-1) | EVs carry mRNA which can be delivered to human mast cells and translated into new proteins (“exosomal shuttle RNA” (esRNA)) | Valadi (2007) [18] |
| Human blood cells and human monocytic leukemia cells (THP-1) | Functional miRNA secreted into EVs are delivered to human microvascular endothelial cells (HMEC-1) | Zhuang (2010) [88] | |
| Dendritic cells | Loading of siRNA into exosomes improves brain delivery in mice | Alvarez-Erviti (2011) [53] | |
| THP-1 cells | RNA-transfected cells shed microvesicles containing miRNA (in vitro and in vivo) | Akao (2011) [72] | |
| Human and mouse liver cells, primary human B cells | Transfer of RNA interference (RNAi) is independent of cell-cell contact and partially mediated by exosomes | Pan (2012) [89] | |
| HEK-293T cells | Engineered microvesicles carry suicide gene for treatment of nerve sheath tumors (schwannomas) in mice | Mizrak (2013) [90] | |
| HEK-293 cells | Exosomes can deliver microRNA to epidermal growth factor receptor-expressing breast cancer cells | Ohno (2013) [91] | |
| Therapeutic applications using EV as drug carrier – exogenous drug loading | Mouse lymphoma cell line (EL-4) | Loading of cumin into exosomes improves its pharmacokinetic and anti-inflammatory properties in mice | Sun (2010) [77], Zhuang (2011) [78] |
| Human embryonic kidney HEK cells (293T) | Incorporation of Adeno-associated virus vectors into EVs (vexosomes) display improved transduction efficiency | Maguire (2012) [92] | |
| Human peripheral blood mononuclear cells | Exosomes loaded with siRNA using electroporation deliver cargo to monocytes and lymphocytes | Wahlgren (2012) [73] | |
| Cancer cells (HeLa) and ascites | Exosomes are loaded with siRNA using electroporation and lipofectamine | Shtam (2013) [74] | |
| Mouse immature dendritic cells | Loading of doxorubicin into RGD-harbouring exosomes inhibit integrin-positive breast cancer growth in vitro and in vivo | Tian (2014) [79] | |
| Human umbilical vein endothelial cells (HUVEC), breast cancer cells (MDA-MB231), human mesenchymal and embryonic stem cells (hMSC and hESC) | Loading of photoactive porphyrins improves their uptake and therapeutic efficiency in cancer cells | Fuhrmann (2014) [54] | |
| Therapeutic applications using EV-mimetics | Bacterial outer membrane vesicles | Engineered low immunogenicity vesicles improve siRNA delivery in a mouse cancer model | Gujrati (2014) [56] |
| Exosome-mimetics from human monocytes and macrophages | Extrusion of cells in presence of doxorubicin creates exosome-like nanoparticles with improved cancer targeting | Jang (2013 and 2014) [55, 93] | |
| Apoptotic bodies from mouse hepatocarcinoma tumour cells (H22) | Packaging of methotrexate into apoptotic bodies improves drug activity in a murine tumour model | Tang (2012) [57] |
Isolation and profiling of EVs from easily accessible patient body fluids (urine, blood, bronchoalveolar lavage [BAL], cerebrospinal fluid) has repeatedly been shown to give access to previously unobtainable information. In order for EVs to be a reliable diagnostic tool, they must be accessed in a consistent manner since the collection, processing, and storage of body fluids has been reported to affect EV concentration and properties. Recent efforts have focussed on establishing standardised processing protocols for clinical sample collection (anticoagulant, needle size) and processing (centrifugation speed, storage conditions) [35]. Following adequate sample collection, EV analysis and characterisation is performed (Figure 2C). Dynamic Light Scattering (DLS), Nanoparticle Tracking Analysis (NTA) and Tuneable Resistive Pulse Sensing (e.g., qNano) can be employed to study EV size and concentration. The mean size of EVs, particularly in blood sera, is typically at the lower nm scale and difficult to measure by standard flow cytometry protocols. Van der Vlist et al. recently published a protocol for a more standardised analysis of EVs by flow cytometry [36] . Liquid chromatography and mass spectrometry allow for the analysis of EV lipid composition. However, >90% of plasma-derived EV are platelet-derived and cannot be easily separated, which makes screening and detection of other EV-populations challenging. Evaluating EV formation under uniform in vitro conditions and involving a minimal number of cell types has been shown to be a valid approach for the reduction of matrix complexity and the validation of clinically observed findings.
Taken together, EV-mediated intercellular communication is clearly important for tissue homeostasis. However, numerous pathological conditions have been shown to involve intercellular information transport mediated by EVs. While phenotypic shifts in EV populations may serve as early diagnostic markers, the alteration of paracrine signalling mediated by EVs and due to underlying pathological conditions is likely to have detrimental effects on a patient’s health. As a result, controlling EV-based intercellular communication will be important to halt disease progression. Additionally, EVs have shown to have high tissue specificity and home to target preferential tissues [14, 37]. This opens the possibility of using EVs as Trojan-horse-like drug delivery vehicles, as outlined in the second part of this review.
Inhibition and interaction with EVs as a therapeutic avenue
Due to their extensive involvement in disease onset and progression, EVs are very attractive therapeutic targets. Inhibition of their biogenesis and release and hindrance of their cellular uptake are the most straightforward ways of drug interference (Figure 3A and Table 1). No universal mechanisms of secretion and cellular interaction of EVs have yet been observed. Differences in cell types will make it necessary to target the biogenesis of EVs differently. Some of the first reports summarising potential ways of modulating the secretion of EVs mentioned various treatments such as abciximab (a monoclonal antibody antagonising integrin αIIbβ3) [38], nifedipine (calcium channel blocker) [39], high doses of vitamin C [40], or inhibitors of membrane lipid raft formation [20]. However, those drugs are unspecific and/or are being used for other indications, which is a severe drawback in a therapeutic setting due to potential side effects. Sphingolipid ceramide is involved in the biogenesis of EVs and thus it has been suggested that blocking sphingomyelinase (the enzyme that generates ceramide from sphingomyelin) could be a viable treatment strategy [17]. Indeed, inhibition or depletion of sphingomyelinase using an inhibitor or RNAi has significantly reduced exosome biogenesis and release [17]. Other potential targets are the GTPase Rab27a and Rab27b which affect intracellular trafficking and induce exosome secretion in HeLa (cervical cancer) cells [41]. Silencing of these Rab27 isoforms has been shown to inhibit the release of exosomes [41] and tumour growth in mouse models of mammary carcinoma [42]. In addition, Rab27 RNA interference induced the reduction of exosome secretion and was associated with reduced tumour metastasis [42, 43]. Given these findings, interference with Rab27 appears to be a promising target for preclinical assessments. However, as for many other therapeutic systems, the success of this approach will also depend on the selection of a suitable drug delivery carrier to deliver the RNA-based drug to the desired tissue [44].
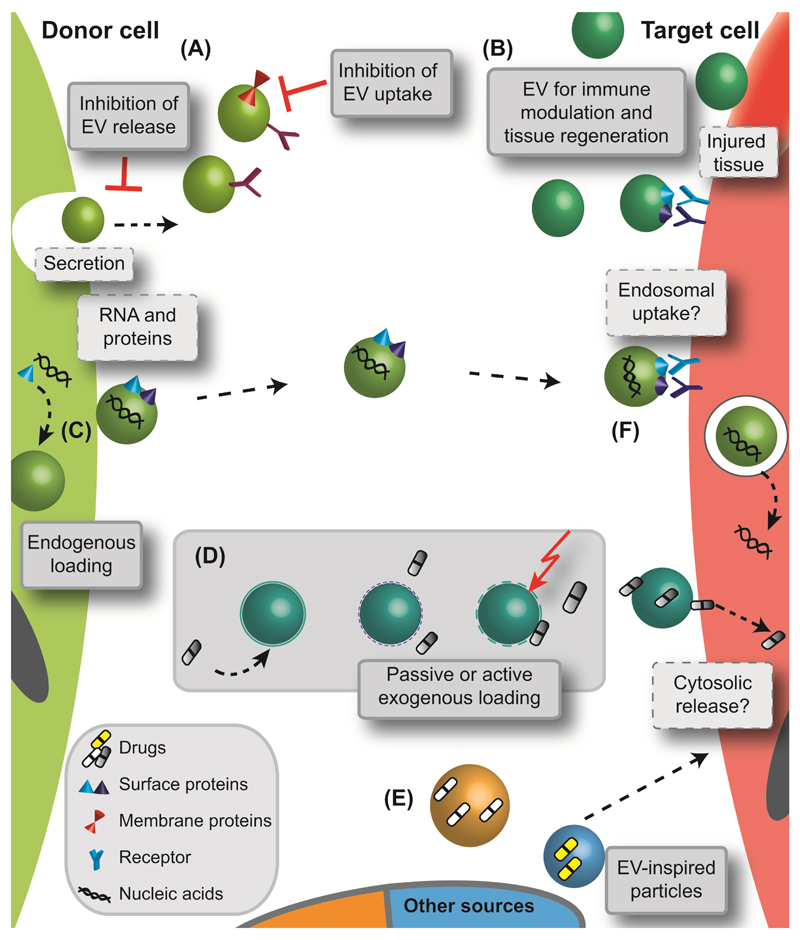
Figure 3. Current concepts employing EVs and EV-inspired systems for drug therapy.
There are several ways by which to interact with EVs or harness their delivery potential in a therapeutic setting. (A) EVs could serve as a therapeutic target by inhibition of their biogenesis, release or cellular uptake. (B) The use of EVs for immune modulatory purposes is currently being exploited. Depending on their cellular origin (e.g., B cells or dendritic cells), EVs may carry molecules from the major histocompatibility complex which enables innate immunomodulatory ability [45, 46]. Exosomes could be employed for vaccination because they are transporters of antigens and MHC molecules [24], and they have been shown to selectively transfer information between T cells and antigen presenting cells (APCs) [37]. Vaccination strategies have proven successful for tumour rejection [47, 48], mycobaterial [49], and parasite [50] infections. EVs with highly immunogenic potential are ideal cell-free options for vaccination and particularly for antigens with low immunogenicity. Exosomes can transfer and amplify interferon-induced antiviral activity [51]. The potential of EVs to enhance tissue regeneration has also been studied in detail both in vitro and in vivo. Due to their bioactive payload and their specific cellular interaction, EVs have improved kidney function in a mouse model of chronic kidney disease [52] and enhanced the survival of mouse haematopoetic stem/progenitor cells [21]. (C,D) On the other hand, EVs are being used as drug carriers with advantageous biological properties and delivery functionalities. For drug loading, (C) endogenous (i.e., incorporation of therapeutic entity by cell [53]) and (D) exogenous (i.e., encapsulation after purification of native EVs [54]) strategies are being investigated including passive loading, pretreatment of EVs with surfactants or electroporation (indicated by dashed EV membrane and lightning). (E) In addition to cell-derived vesicles, EV-inspired carriers are being developed. These include exosome-mimetics [55], bacterial derived vesicles [56], and drug loaded apoptotic bodies [57]. Following transfer to the target cell, EVs are taken up after direct interaction with the cell membrane and fusion or via receptor-mediated internalisation. However, it is not known whether EVs deliver their payload directly into the cytosol [58, 59] or whether vesicles follow the endosomal pathway [60].
Although several potential targets of interference with EV biogenesis, secretion or uptake have been identified, none of these approaches have yet progressed to the preclinical status. It is important to remember that any treatment interacting with basic biological signalling pathways bears a significant risk of unwanted side effects. Therefore, a strict and comprehensive risk and safety analysis is necessary when developing drugs interacting with EVs in a therapeutic setting.
Extracellular vesicles as smart drug carriers
In recent years, EVs have attracted significant attention in regenerative medicine and for immune modulation due to their inherent biological activity (Figure 3B and Table 1). A detailed discussion of these properties is beyond the scope of this review. In the field of drug delivery, excitement was created because of EVs’ natural role in transporting bioactive entities between cells. This has formed the concept of using EVs as a “Trojan horse” through harnessing their natural targeting properties for selective drug delivery [12] (Figure 3C). The composition of EVs’ surface and membrane proteins is one of the key factors for their specific cell interactions. For example, EVs from T cells are transferred unidirectional to antigen-presenting cells [37] while platelet-derived EVs interact with endothelial cells and macrophages, but not neutrophils [61]. EVs have been reported to be less immunogenic than artificial nanoparticulate carriers (particularly if harvested from autologous cells) due to their natural composition [62, 63]. In addition, EVs can potentially bypass complement activation and interactions with coagulation factors, which leads to better stability in blood circulation [64]. EVs have also been proposed to specifically recognise their target cells [37, 65], a feature that would reduce off-target effects. Moreover, they have been proposed to cross the blood-brain-barrier following systemic injection [66]. Finally, their small size of 100-200 nm would allow EVs to pass the fenestration in leaky blood vessels of cancer tissue and may take advantage of the enhanced permeation and retention (EPR) effect [67]. In order to employ EV-mediated delivery, drugs have to be loaded into these particles. Drug encapsulation can be performed endogenously or exogenously. For endogenous loading, cells are transfected or engineered to shed the desired drug/molecule directly into EVs (Figure 3C). This method is convenient and requires very few manipulation steps. However, this approach only works for protein- and nucleic acid-based drugs as these molecules can be produced by cells. Exogenous loading techniques require the encapsulation of drugs into pre-assembled EVs (Figure 3D). They are usually labour-intensive but offer a wider choice of drugs. Independent of the loading method, EVs need to be harvested and purified first. A variety of isolation and purification methods exist including filtration, ultracentrifugation, density gradients, immunoaffinity, gel chromatography and commercially available kits (e.g., ExoQuick™, Total Exosome Isolation reagent) [62]. Although some of these techniques are evaluated in more detail (e.g., ultracentrifugation) it remains often unclear what effect on EV size, morphology or biological activity the different methods may have [68, 69]. There is nevertheless an ongoing effort for standardisation of these isolation and purification avenues [70].
Some of the first examples of exosome-mediated delivery of therapeutic RNA indicated that microRNA (miRNA) was secreted into human blood cell derived EVs [71]. MiRNA is a class of non-coding RNA which is involved in regulation of posttranscriptional gene expression. These RNA loaded vesicles were able to transfer their payload to human microvascular endothelial cells. In a similar study, monocytes were transfected with RNA or chemically modified RNA. Subsequent shedding of microvesicles with RNA cargo was observed both in vitro and after systemic administration of transfected cells in nude mice [72]. Both these studies established a basis for the first successful application of exosomes in a therapeutic context in vivo [53]. Mouse dendritic cells were transfected to express a neuronal targeting protein. Purified exosomes were loaded with siRNA by electroporation and assessed in wild-type mice. Due to their targeting surface protein, EVs were able to migrate across the blood-brain-barrier and they decreased expression levels of an Alzheimer associated gene (BACE1) by 60%. Moreover, exosomes appeared to be non-immunogenic as indicated by unchanged levels of serum interleukin-6, interferon gamma-induced protein 10, tumour necrosis factor alpha, and interferon alpha concentrations.
Other studies have since shown that efficient gene silencing can be obtained after loading nucleic acid-based drugs into EVs by means of electroporation [73, 74]. A protocol on nucleic acid loading using electroporation has been published [75]. However, recently another mechanism was put forward, suggesting that this technique may induce possible side-effects [76]. It has been shown that precipitate formation can occur when RNA drugs are electroporated without EVs. Even though the drug delivery efficiency of electroporated EVs has been shown in a number of studies, these findings should be interpreted with caution due to potential electroporation by-products which may influence the exosomal delivery. In early studies, passive co-incubation was employed to load small molecules into EVs [77]. EV loading by this technique has been proven with therapeutically relevant drugs [78], but passive incubation would likely only work for rather hydrophobic entities. As a consequence, electroporation has been proposed as a means by which to load small molecule drugs into EVs for improved cancer and photodynamic therapy [54, 79]. We showed that depending on the hydrophobicity of the drug, electroporation can improve its encapsulation into EVs [54]. Moreover, drug loaded vesicles significantly improved the cellular uptake compared to free drug and even liposomal formulations [54]. This same study also investigated other active loading methods and found that saponin-assisted loading of EVs increased the encapsulation efficiency of hydrophilic drugs 11-fold compared to passive loading and without compromising the EVs’ delivery activity [54].
Recently, strategies have been developed using EVs from sources other than mammalian cells and have been called “EV-mimetics” (Figure 3E). One of these strategies employed bacterial outer membrane vesicles (OMVs) as therapeutic carriers in a mouse cancer model [56]. OMVs were derived from a mutant E. coli strain and shed with a human epidermal growth factor receptor 2 (HER2)-specific affibody in the membrane to enable active targeting. Upon systemic administration of OMVs loaded with siRNA, efficient tumour growth inhibition was observed. Although the bacterial vesicles were shown to be of low immunogenicity and reduced endotoxicity towards human cells, safety concerns may still arise due to the bacterial origin of the particles. Preclinical evaluation in an in vivo setting would be beneficial to help clarify this question. Another strategy of EV-inspired drug delivery systems was evaluating exosome-mimetic nanoparticles prepared by extrusion/disintegration of human monocytes and macrophages [55]. In this study, vesicles were loaded with chemotherapeutics (doxorubicin) and injected into tumour-bearing mice. Both accumulations in the tumour and inhibition of cancer growth were observed. Another advantage of the mimetics strategy is the high yield with which these particles can be obtained. The authors claimed that exosome-mimetics helps preserve the targeting properties from their cellular origin. However, it is worth speculating whether membrane proteins and receptors rearrange correctly following the harsh extrusion process. The immunogeniceity aspects of these particles should also be considered for future applications. It has further been suggested that exosome-mimetics could be prepared artificially [80]. Such biotechnologically-tailored particles consist of all the necessary EV components for stability and targeting (proteins and nucleic acids) and are incorporated into synthetic vesicles [12, 80]. Nevertheless, it appears to be rather complex to identify all required elements and it is supposedly difficult to incorporate them in their natural conformation (in particular membrane proteins). Not only exosomes and SMVs for drug delivery, a recent study reported the use of cytostatic drugs loaded into apoptotic bodies [57]. Tumour cells were incubated with the drug and irradiated with UV light to induce shedding of apoptotic bodies. These vesicles induced tumour killing both in vitro and in vivo. However, cancer cell origin and the apoptotic nature of these vesicles are likely to preclude further clinical testing.
Together, these studies demonstrate that the preclinical evaluation of EVs and their loading is well underway. Another important issue is the uptake mechanism of EVs by target cells (Figure 3F). Current studies discuss endosomal uptake or direct fusion and cargo release into the cytosol. Further evaluation may clarify these questions and be instrumental when developing EVs as drug carriers. Appropriate safety and efficiency analyses under in vivo conditions will further indicate whether the “Trojan horse” paradigm is valid and whether EVs can act as smart drug carriers for future treatment options.
Perspectives
In summary, the field of EV-based diagnostics and therapeutics holds significant promise to enable earlier diagnosis and targeted drug delivery with superior efficiency. However, many aspects of EV-based systems, particularly the off-target effects, remain unknown. In order to make the field of EV-based systems more accessible to a broader research community and trigger interdisciplinary approaches, this review contains a brief road map that outlines the key steps involved in the evaluation and design of EV-based diagnostic and therapeutic systems. Figure 4 includes a typical set of parameters that can be used as an initial setting for the isolation of EVs from either clinical samples or cell culture media and may then be further optimized depending on the application. Sample collection and storage procedures need to be standardised to prevent the occurrence of artefacts (e.g., contaminations by apoptotic bodies) due to inadequate sample processing.
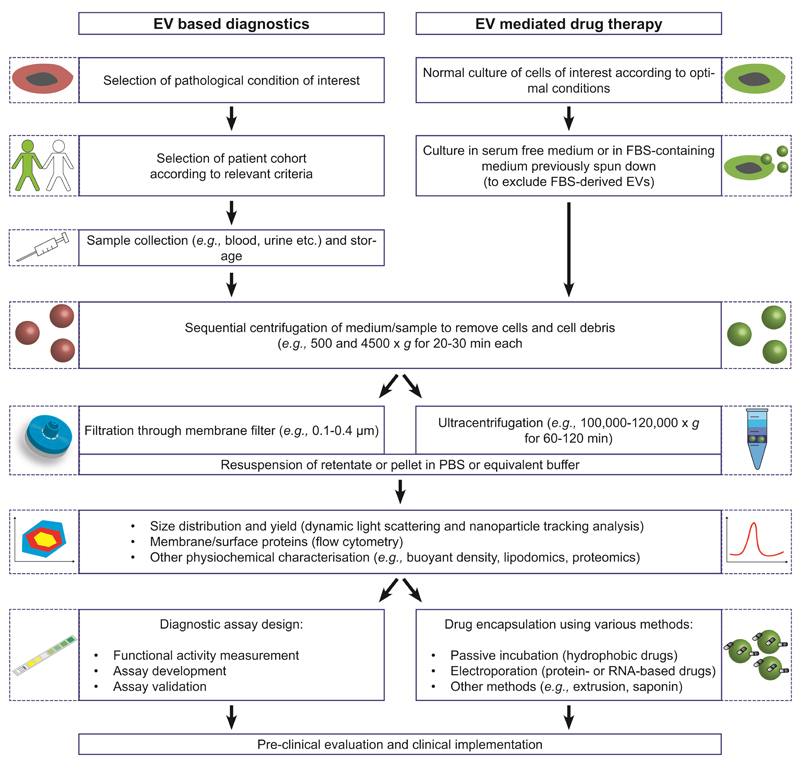
Figure 4. EV isolation and processing for diagnostic and therapeutic applications.
The tutorial gives a general overview on the isolation and characterisation of EVs for both diagnostic and therapeutic purposes. Experimental details (e.g., time and speed of centrifugation, etc.) are recommendations from broadly established protocols but conditions can be adjusted to fulfil the experimental needs of individual studies.
Before EV-based strategies can be translated to the clinic, several major hurdles need to be overcome. These include the reproducibility required to meet good manufacturing practice (GMP) standards (e.g., cell-based material, batch-to-batch variations) along with a scaling-up of the production process to produce EVs at a pharmaceutically relevant scale that meet quality requirements and are stable for storage (e.g., shelf life). Once a GMP-compliant manufacturing process has been established, the approval for clinical evaluations will largely depend on the risk profile of the pharmaceutical formulation. It is expected that a better understanding of both on-target and off-target effects and the mechanism of action is vital before the first clinical evaluations of EVs can be initiated. Sensitive disease detection and selective therapy with reduced side-effects are major challenges in the field of diagnostics and drug delivery. The earliest and most accurate detection of a disease state followed by efficient pharmacotherapy with as little drug as necessary is the most desirable scenario. EVs have created excitement in the research field of theranostics (combining therapy and diagnostics) because of their potential to achieve this goal. EV-based diagnostics constitute a rapidly evolving field, particularly for early cancer diagnosis and recent studies suggest a promising future for EV-based diagnostic systems. In the area of drug delivery, EVs also show great promise. Studies have shown that EVs can be loaded with molecules of choice and are at least equally efficient at delivering their cargo than artificial carriers, including through physiological barriers. However, it remains to be determined whether cell-derived EVs can be made safe for widespread clinical use. It will be a challenge to control the exact composition of cell-derived natural EVs and then understand their complex interactions inside whole organisms. While immunological and toxicological responses can be evaluated using state-of-the-art tests, more studies are needed before cell-derived microvesicles can be translated to the clinic. Even though autologous EVs may be considered as a safer alternative, a lack of suitable risk assessment strategies to evaluate difficult-to-assess pleiotropic effects currently precludes the clinical evaluation of cell-derived vesicles. However, recent studies indicated that EVs from human mesenchymal stem cells may be harnessed as a safe and new avenue for the treatment of severe forms of graft-versus-host disease [81]. These results using natural EVs in preclinical settings indicate that these vesicles constitute a very promising approach for the development of bio-inspired drug delivery and therapy systems. Such preclinical investigations will allow a better understanding of natural targeted delivery mechanisms and homing, and will enable sophisticated bio-inspired drug delivery systems with high specificity.
Acknowledgements
The authors acknowledge financial support from the European Commission (Marie Curie Intra European Fellowship for GF, grant number 326961, acronym SMART) and the Swiss National Science Foundation (Postdoctoral Fellowship for IH, grant number 145756). MMS holds a Wellcome Trust Senior Investigator Award.
References
- [1].Camussi G, Deregibus MC, Bruno S, Cantaluppi V, Biancone L. Kidney Int. 2010;78:838–848. doi: 10.1038/ki.2010.278. [DOI] [PubMed] [Google Scholar]
- [2].Waldenstrom A, Genneback N, Hellman U, Ronquist G. Plos One. 2012;7:e34653. doi: 10.1371/journal.pone.0034653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chen X, Liang HW, Zhang JF, Zen K, Zhang CY. Protein Cell. 2012;3:28–37. doi: 10.1007/s13238-012-2003-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Fevrier B, Raposo G. Curr Opin Cell Biol. 2004;16:415–421. doi: 10.1016/j.ceb.2004.06.003. [DOI] [PubMed] [Google Scholar]
- [5].Harding C, Heuser J, Stahl P. Eur J Cell Biol. 1984;35:256–263. [PubMed] [Google Scholar]
- [6].Mashburn LM, Whiteley M. Nature. 2005;437:422–425. doi: 10.1038/nature03925. [DOI] [PubMed] [Google Scholar]
- [7].Kulp A, Kuehn MJ. Annu Rev Microbiol. 2010;64:163–184. doi: 10.1146/annurev.micro.091208.073413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Raposo G, Stoorvogel W. The Journal of Cell Biology. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kanada M, Bachmann MH, Hardy JW, Frimannson DO, Bronsart L, Wang A, Sylvester MD, Schmidt TL, Kaspar RL, Butte MJ, Matin AC, et al. Proceedings of the National Academy of Sciences. 2015 doi: 10.1073/pnas.1418401112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Théry C, Boussac M, Véron P, Ricciardi-Castagnoli P, Raposo G, Garin J, Amigorena S. The Journal of Immunology. 2001;166:7309–7318. doi: 10.4049/jimmunol.166.12.7309. [DOI] [PubMed] [Google Scholar]
- [11].Cocucci E, Racchetti G, Meldolesi J. Trends Cell Biol. 2009;19:43–51. doi: 10.1016/j.tcb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- [12].van Dommelen SM, Vader P, Lakhal S, Kooijmans SAA, van Solinge WW, Wood MJA, Schiffelers RM. J Control Release. 2012;161:635–644. doi: 10.1016/j.jconrel.2011.11.021. [DOI] [PubMed] [Google Scholar]
- [13].Cocucci E, Racchetti G, Podini P, Meldolesi J. Traffic. 2007;8:742–757. doi: 10.1111/j.1600-0854.2007.00566.x. [DOI] [PubMed] [Google Scholar]
- [14].El Andaloussi S, Mager I, Breakefield XO, Wood MJA. Nat Rev Drug Discov. 2013;12:347–357. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- [15].Thery C, Zitvogel L, Amigorena S. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- [16].Hurley JH, Hanson PI. Nature Reviews Molecular Cell Biology. 2010;11:556–566. doi: 10.1038/nrm2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brügger B, Simons M. Science. 2008;319:1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- [18].Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- [19].Chevillet JR, Kang Q, Ruf IK, Briggs HA, Vojtech LN, Hughes SM, Cheng HH, Arroyo JD, Meredith EK, Gallichotte EN, Pogosova-Agadjanyan EL, et al. Proceedings of the National Academy of Sciences. 2014;111:14888–14893. doi: 10.1073/pnas.1408301111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak MZ. Leukemia. 2006;20:1487–1495. doi: 10.1038/sj.leu.2404296. [DOI] [PubMed] [Google Scholar]
- [21].Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P, Ratajczak MZ. Leukemia. 2006;20:847–856. doi: 10.1038/sj.leu.2404132. [DOI] [PubMed] [Google Scholar]
- [22].Azmi AS, Bao B, Sarkar FH. Cancer Metastasis Rev. 2013;32:623–642. doi: 10.1007/s10555-013-9441-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Buzas EI, Gyorgy B, Nagy G, Falus A, Gay S. Nat Rev Rheumatol. 2014;10:356–364. doi: 10.1038/nrrheum.2014.19. [DOI] [PubMed] [Google Scholar]
- [24].Robbins PD, Morelli AE. Nature Reviews Immunology. 2014;14:195–208. doi: 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Iero M, Valenti R, Huber V, Filipazzi P, Parmiani G, Fais S, Rivoltini L. Cell Death Differ. 2007;15:80–88. doi: 10.1038/sj.cdd.4402237. [DOI] [PubMed] [Google Scholar]
- [26].Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Curry WT, Carter BS, Krichevsky AM, Breakefield XO. Nat Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wysoczynski M, Ratajczak MZ. International Journal of Cancer. 2009;125:1595–1603. doi: 10.1002/ijc.24479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Xiong J, Miller VM, Li Y, Jayachandran M. Journal of Cardiovascular Pharmacology. 2012;59:124–132. doi: 10.1097/FJC.0b013e31820c6254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Bastarache JA, Fremont RD, Kropski JA, Bossert FR, Ware LB. American Journal of Physiology - Lung Cellular and Molecular Physiology. 2009;297:L1035–L1041. doi: 10.1152/ajplung.00214.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Curry N, Raja A, Beavis J, Stanworth S, Harrison P. Journal of Extracellular Vesicles. 2014;3 doi: 10.3402/jev.v3403.25625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Solum NO. Arteriosclerosis, Thrombosis, and Vascular Biology. 1999;19:2841–2846. doi: 10.1161/01.atv.19.12.2841. [DOI] [PubMed] [Google Scholar]
- [32].Nieuwland R, Berckmans RJ, McGregor S, Böing AN, Romijn FPHThM, Westendorp RGJ, Hack CE, Sturk A. Blood. 2000;95:930–935. [PubMed] [Google Scholar]
- [33].Shao H, Chung J, Balaj L, Charest A, Bigner DD, Carter BS, Hochberg FH, Breakefield XO, Weissleder R, Lee H. Nat Med. 2012;18:1835–1840. doi: 10.1038/nm.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ohno S, Ishikawa A, Kuroda M. Advanced Drug Delivery Reviews. 2013;65:398–401. doi: 10.1016/j.addr.2012.07.019. [DOI] [PubMed] [Google Scholar]
- [35].K.W. Witwer, E.I. Buzás, L.T. Bemis, A. Bora, C. Lässer, J. Lötvall, E.N. Nolte-‘t Hoen, M.G. Piper, S. Sivaraman, J. Skog, C. Théry, M.H. Wauben, F. Hochberg, 2013, (2013).
- [36].van der Vlist EJ, Nolte-'t Hoen ENM, Stoorvogel W, Arkesteijn GJA, Wauben MHM. Nat Protocols. 2012;7:1311–1326. doi: 10.1038/nprot.2012.065. [DOI] [PubMed] [Google Scholar]
- [37].Mittelbrunn M, Gutierrez-Vazquez C, Villarroya-Beltri C, Gonzalez S, Sanchez-Cabo F, Gonzalez MA, Bernad A, Sanchez-Madrid F. Nat Commun. 2011;2:282. doi: 10.1038/ncomms1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Reverter JC, Beguin S, Kessels H, Kumar R, Hemker HC, Coller BS. J Clin Invest. 1996;98:863–874. doi: 10.1172/JCI118859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Lee YJ, Jy W, Horstman LL, Janania J, Reyes Y, Kelley RE, Ahn YS. Thrombosis Research. 1993;72:295–304. doi: 10.1016/0049-3848(93)90138-e. [DOI] [PubMed] [Google Scholar]
- [40].Rössig L, Hoffmann J, Hugel B, Mallat Z, Haase A, Freyssinet J-M, Tedgui A, Aicher A, Zeiher AM, Dimmeler S. Circulation. 2001;104:2182–2187. doi: 10.1161/hc4301.098284. [DOI] [PubMed] [Google Scholar]
- [41].Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, Moita CF, Schauer K, Hume AN, Freitas RP, Goud B, et al. Nat Cell Biol. 2010;12:19–30. doi: 10.1038/ncb2000. [DOI] [PubMed] [Google Scholar]
- [42].Bobrie A, Krumeich S, Reyal F, Recchi C, Moita LF, Seabra MC, Ostrowski M, Thery C. Cancer Res. 2012;72:4920–4930. doi: 10.1158/0008-5472.CAN-12-0925. [DOI] [PubMed] [Google Scholar]
- [43].Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, Garcia-Santos G, Ghajar CM, Nitadori-Hoshino A, et al. Nat Med. 2012;18:883–+. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Yin H, Kanasty RL, Eltoukhy AA, Vegas AJ, Dorkin JR, Anderson DG. Nature Reviews Genetics. 2014;15:541–555. doi: 10.1038/nrg3763. [DOI] [PubMed] [Google Scholar]
- [45].Raposo G, Nijman HW, Stoorvogel W, Leijendekker R, Harding CV, Melief CJM, Geuze HJ. The Journal of Experimental Medicine. 1996;183:1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Nat Med. 1998;4:594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]
- [47].Wolfers J, Lozier A, Raposo G, Regnault A, Thery C, Masurier C, Flament C, Pouzieux S, Faure F, Tursz T, Angevin E, et al. Nat Med. 2001;7:297–303. doi: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- [48].Escudier B, Dorval T, Chaput N, Andre F, Caby M-P, Novault S, Flament C, Leboulaire C, Borg C, Amigorena S, Boccaccio C, et al. Journal of Translational Medicine. 2005;3:10. doi: 10.1186/1479-5876-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Cheng Y, Schorey JS. Eur J Immunol. 2013;43:3279–3290. doi: 10.1002/eji.201343727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Beauvillain C, Ruiz S, Guiton R, Bout D, Dimier-Poisson I. Microb Infect. 2007;9:1614–1622. doi: 10.1016/j.micinf.2007.07.002. [DOI] [PubMed] [Google Scholar]
- [51].Li J, Liu K, Liu Y, Xu Y, Zhang F, Yang H, Liu J, Pan T, Chen J, Wu M, Zhou X, et al. Nat Immunol. 2013;14:793–803. doi: 10.1038/ni.2647. [DOI] [PubMed] [Google Scholar]
- [52].van Koppen A, Joles JA, van Balkom BWM, Lim SK, de Kleijn D, Giles RH, Verhaar MC. PLoS ONE. 2012;7:e38746. doi: 10.1371/journal.pone.0038746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJA. Nat Biotech. 2011;29:341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- [54].*Fuhrmann G, Serio A, Mazo M, Nair R, Stevens MM. J Control Release. 2014 doi: 10.1016/j.jconrel.2014.11.029. [DOI] [PubMed] [Google Scholar]
- [55].Jang SC, Kim OY, Yoon CM, Choi D-S, Roh T-Y, Park J, Nilsson J, Lötvall J, Kim Y-K, Gho YS. ACS Nano. 2013;7:7698–7710. doi: 10.1021/nn402232g. [DOI] [PubMed] [Google Scholar]
- [56].Gujrati V, Kim S, Kim SH, Min JJ, Choy HE, Kim SC, Jon S. ACS Nano. 2014;8:1525–1537. doi: 10.1021/nn405724x. [DOI] [PubMed] [Google Scholar]
- [57].Tang K, Zhang Y, Zhang H, Xu P, Liu J, Ma J, Lv M, Li D, Katirai F, Shen G-X, Zhang G, et al. Nat Commun. 2012;3:1282–1288. doi: 10.1038/ncomms2282. [DOI] [PubMed] [Google Scholar]
- [58].Parolini I, Federici C, Raggi C, Lugini L, Palleschi S, De Milito A, Coscia C, Iessi E, Logozzi M, Molinari A, Colone M, et al. J Biol Chem. 2009;284:34211–34222. doi: 10.1074/jbc.M109.041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].van den Boorn JG, Schlee M, Coch C, Hartmann G. Nat Biotech. 2011;29:325–326. doi: 10.1038/nbt.1830. [DOI] [PubMed] [Google Scholar]
- [60].Tian T, Wang Y, Wang H, Zhu Z, Xiao Z. J Cell Biochem. 2010;111:488–496. doi: 10.1002/jcb.22733. [DOI] [PubMed] [Google Scholar]
- [61].Lösche W, Scholz T, Temmler U, Oberle V, Claus RA. Platelets. 2004;15:109–115. doi: 10.1080/09537100310001649885. [DOI] [PubMed] [Google Scholar]
- [62].Lai RC, Yeo RWY, Tan KH, Lim SK. Biotechnol Adv. 2013;31:543–551. doi: 10.1016/j.biotechadv.2012.08.008. [DOI] [PubMed] [Google Scholar]
- [63].Yeo RWY, Lai RC, Zhang B, Tan SS, Yin Y, Teh BJ, Lim SK. Adv Drug Deliv Rev. 2012;65:336–341. doi: 10.1016/j.addr.2012.07.001. [DOI] [PubMed] [Google Scholar]
- [64].Clayton A, Harris CL, Court J, Mason MD, Morgan BP. Eur J Immunol. 2003;33:522–531. doi: 10.1002/immu.200310028. [DOI] [PubMed] [Google Scholar]
- [65].Pluskota E, Woody NM, Szpak D, Ballantyne CM, Soloviev DA, Simon DI, Plow EF. Blood. 2008;112:2327–2335. doi: 10.1182/blood-2007-12-127183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Aronin N, Moore M. New Engl J Med. 2012;367:1753–1754. doi: 10.1056/NEJMcibr1209595. [DOI] [PubMed] [Google Scholar]
- [67].Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nat Nano. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- [68].Van Deun J, Mestdagh P, Sormunen R, Cocquyt V, Vermaelen K, Vandesompele J, Bracke M, De Wever O, Hendrix A. J Extracell Vesicles. 2014;3 doi: 10.3402/jev.v3.24858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Jeppesen DK, Hvam ML, Primdahl-Bengtson B, Boysen AT, Whitehead B, Dyrskjot L, Orntoft TF, Howard KA, Ostenfeld MS. J Extracell Vesicles. 2014;3:25011. doi: 10.3402/jev.v3.25011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Witwer KW, Buzás EI, Bemis LT, Bora A, Lässer C, Lötvall J, Nolte-‘t Hoen EN, Piper MG, Sivaraman S, Skog J, Théry C, et al. J Extracell Vesicles. 2013;2:20360–20375. doi: 10.3402/jev.v2i0.20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Dong H, Lei J, Ding L, Wen Y, Ju H, Zhang X. Chemical Reviews. 2013;113:6207–6233. doi: 10.1021/cr300362f. [DOI] [PubMed] [Google Scholar]
- [72].Akao Y, Iio A, Itoh T, Noguchi S, Itoh Y, Ohtsuki Y, Naoe T. Mol Ther. 2011;19:395–399. doi: 10.1038/mt.2010.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Wahlgren J, Karlson TDL, Brisslert M, Vaziri Sani F, Telemo E, Sunnerhagen P, Valadi H. Nucleic Acids Res. 2012;40:e130. doi: 10.1093/nar/gks463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Shtam T, Kovalev R, Varfolomeeva E, Makarov E, Kil Y, Filatov M. Cell Communication and Signaling. 2013;11:88. doi: 10.1186/1478-811X-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].El-Andaloussi S, Lee Y, Lakhal-Littleton S, Li J, Seow Y, Gardiner C, Alvarez-Erviti L, Sargent IL, Wood MJA. Nat Protoc. 2012;7:2112–2126. doi: 10.1038/nprot.2012.131. [DOI] [PubMed] [Google Scholar]
- [76].Kooijmans SAA, Stremersch S, Braeckmans K, de Smedt SC, Hendrix A, Wood MJA, Schiffelers RM, Raemdonck K, Vader P. J Control Release. 2013;172:229–238. doi: 10.1016/j.jconrel.2013.08.014. [DOI] [PubMed] [Google Scholar]
- [77].Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, Liu C, Barnes S, Grizzle W, Miller D, Zhang H-G. Mol Ther. 2010;18:1606–1614. doi: 10.1038/mt.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Zhuang X, Xiang X, Grizzle W, Sun D, Zhang S, Axtell RC, Ju S, Mu J, Zhang L, Steinman L, Miller D, et al. Mol Ther. 2011;19:1769–1779. doi: 10.1038/mt.2011.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Tian YH, Li SP, Song J, Ji TJ, Zhu MT, Anderson GJ, Wei JY, Nie GJ. Biomaterials. 2014;35:2383–2390. doi: 10.1016/j.biomaterials.2013.11.083. [DOI] [PubMed] [Google Scholar]
- [80].Kooijmans SAA, Vader P, van Dommelen SM, van Solinge WW, Schiffelers RM. Int J Nanomed. 2012:1525–1541. doi: 10.2147/IJN.S29661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Kordelas L, Rebmann V, Ludwig AK, Radtke S, Ruesing J, Doeppner TR, Epple M, Horn PA, Beelen DW, Giebel B. Leukemia. 2014;28:970–973. doi: 10.1038/leu.2014.41. [DOI] [PubMed] [Google Scholar]
- [82].Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Curry WT, Carter BS, Krichevsky AM, Breakefield XO. Nat Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Rabinowits G, Gerçel-Taylor C, Day JM, Taylor DD, Kloecker GH. Clinical Lung Cancer. 2009;10:42–46. doi: 10.3816/CLC.2009.n.006. [DOI] [PubMed] [Google Scholar]
- [84].Taylor DD, Gercel-Taylor C. Gynecologic Oncology. 2008;110:13–21. doi: 10.1016/j.ygyno.2008.04.033. [DOI] [PubMed] [Google Scholar]
- [85].Nilsson J, Skog J, Nordstrand A, Baranov V, Mincheva-Nilsson L, Breakefield XO, Widmark A. Br J Cancer. 2009;100:1603–1607. doi: 10.1038/sj.bjc.6605058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Logozzi M, De Milito A, Lugini L, Borghi M, Calabrò L, Spada M, Perdicchio M, Marino ML, Federici C, Iessi E, Brambilla D, et al. PLoS One. 2009;4:e5219. doi: 10.1371/journal.pone.0005219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Bala S, Petrasek J, Mundkur S, Catalano D, Levin I, Ward J, Alao H, Kodys K, Szabo G. Hepatology. 2012;56:1946–1957. doi: 10.1002/hep.25873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Zhang Y, Liu D, Chen X, Li J, Li L, Bian Z, Sun F, Lu J, Yin Y, Cai X, Sun Q, et al. Mol Cell. 2010;39:133–144. doi: 10.1016/j.molcel.2010.06.010. [DOI] [PubMed] [Google Scholar]
- [89].Pan Q, Ramakrishnaiah V, Henry S, Fouraschen S, de Ruiter PE, Kwekkeboom J, Tilanus HW, Janssen HLA, van der Laan LJW. Gut. 2012;61:1330–1339. doi: 10.1136/gutjnl-2011-300449. [DOI] [PubMed] [Google Scholar]
- [90].Mizrak A, Bolukbasi MF, Ozdener GB, Brenner GJ, Madlener S, Erkan EP, Strobel T, Breakefield XO, Saydam O. Mol Ther. 2013;21:101–108. doi: 10.1038/mt.2012.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Ohno S-i, Takanashi M, Sudo K, Ueda S, Ishikawa A, Matsuyama N, Fujita K, Mizutani T, Ohgi T, Ochiya T, Gotoh N, et al. Mol Ther. 2013;21:185–191. doi: 10.1038/mt.2012.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Maguire CA, Balaj L, Sivaraman S, Crommentuijn MHW, Ericsson M, Mincheva-Nilsson L, Baranov V, Gianni D, Tannous BA, Sena-Esteves M, Breakefield XO, et al. Mol Ther. 2012;20:960–971. doi: 10.1038/mt.2011.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Jang SC, Gho YS. Nanomedicine. 2014;9:177–180. doi: 10.2217/nnm.13.206. [DOI] [PubMed] [Google Scholar]