Abstract
Purpose of review
Therapeutic exposure to high doses of radiation can severely impair organ function due to ablation of stem cells. Normal tissue injury is a dose-limiting toxicity for radiation therapy (RT). Although advances in the delivery of high precision conformal RT has increased normal tissue sparing, mitigating and therapeutic strategies that could alleviate early and chronic radiation effects are urgently needed in order to deliver curative doses of RT, especially in abdominal, pelvic and thoracic malignancies. Radiation-induced gastrointestinal injury is also a major cause of lethality from accidental or intentional exposure to whole body irradiation in the case of nuclear accidents or terrorism. This review examines the therapeutic options for mitigation of non-hematopoietic radiation injuries.
Recent findings
We have developed stem cell based therapies for the mitigation of acute radiation syndrome (ARS) and radiation-induced gastrointestinal syndrome (RIGS). This is a promising option because of the robustness of standardized isolation and transplantation of stromal cells protocols, and their ability to support and replace radiation-damaged stem cells and stem cell niche. Stromal progenitor cells (SPC) represent a unique multipotent and heterogeneous cell population with regenerative, immunosuppressive, anti-inflammatory, and wound healing properties. SPC are also known to secrete various key cytokines and growth factors such as platelet derived growth factors (PDGF), keratinocyte growth factor (KGF), R-spondins (Rspo), and may consequently exert their regenerative effects via paracrine function. Additionally, secretory vesicles such as exosomes or microparticles can potentially be a cell-free alternative replacing the cell transplant in some cases.
Summary
This review highlights the beneficial effects of SPC on tissue regeneration with their ability to (a) target the irradiated tissues, (b) recruit host stromal cells, (c) regenerate endothelium and epithelium, (d) and secrete regenerative and immunomodulatory paracrine signals to control inflammation, ulceration, wound healing and fibrosis.
Keywords: Stromal Progenitor Cells, Growth factors, Radiation Induced Gastrointestinal Diseases, Intestinal Stem Cells, Epithelial Regeneration, Vascularization, Exosomes, Inflammation
INTRODUCTION
Currently 50% of all cancer patients receive some form of radiation therapy (RT), including curative and palliative therapy with about 40% patients receiving curative treatment, making it one of the most efficient and popular treatment modalities against cancer (1). However despite the obvious benefits of curing neoplastic diseases and extending survival significantly, RT tends to lead to acute and chronic effects in normal tissue (2). Most RT studies indicate that treatment modalities associated with better tumor control and survival more often lead to normal tissue toxicity; therefore, adjuvant mitigators of normal tissue injury are needed for improving local tumor control and overall survival with RT (3). Radiation injury occurring within days after exposure is classified as “acute injury” and often associated with direct damage to the stem cell and its niche, including the stroma and microvasculature. Late injury, which manifests months to years after exposure, is due to persistent oxidative and inflammatory signaling, aberrant stem cell regeneration, damage to the vascular stroma and scaring. Among various pharmacological approaches used, cell transplantation is one of the most effective strategies in treating radiation injury (4–6).
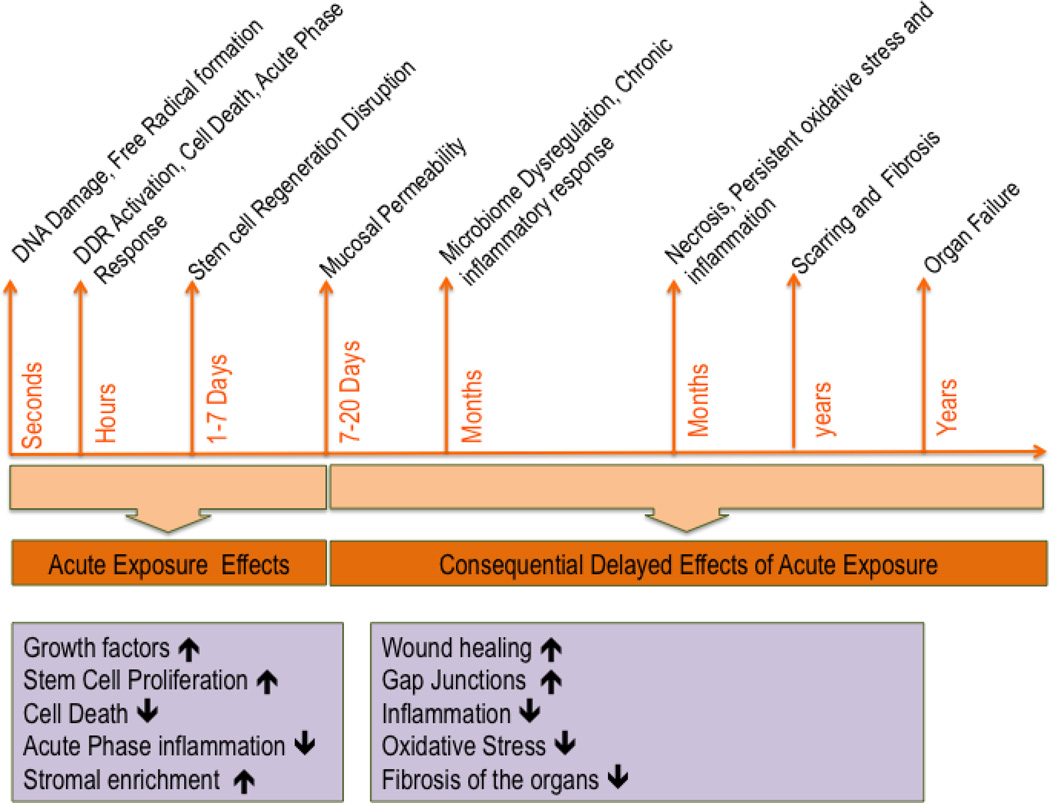
Bone marrow transplantation (BMT) has been an attractive treatment option for various pathologies including radiation injury. Traditionally, BMT is used for bone marrow injury that involves loss of hematopoietic stem cells. Although bone marrow derived stromal progenitor cells (SPC) were initially assumed to contribute only to the HSC niche, it has become increasingly evident that the stromal progenitors derived from bone marrow (BM-SPC) or adipose tissue (Ad-SPC) contribute to extramedullary regeneration and repair, including recovery from radiation-induced gastrointestinal, pulmonary, and renal injury (7–9). This review briefly covers the beneficial role of SPCs or BM derived stromal cells in mitigating radiation injury, especially that pertaining to radiation-induced gastrointestinal syndrome (RIGS). The progression of radiation toxicity over time and the manifestation of acute and consequential delayed injuries from acute exposure, along with the mechanisms involved, are depicted in Figure 1. The figure also describes the beneficial role of SPC or MSC at various stages of acute and delayed injury of the GI tract.
Figure 1.
Beneficial effects of stromal progenitor cells in various stages of acute and chronic radiation injuries
RIGS and stromal injury
Radiation-induced gastrointestinal syndrome (RIGS) results from a combination of direct cytocidal effects on intestinal crypt and endothelial cells and subsequent loss of the mucosal barrier, resulting in microbial infection, septic shock and systemic inflammatory response syndrome (SIRS) (10). The intestine is also a model for an “early responding” tissue that is frequently targeted by DNA damaging agents, such as ionizing radiation (IR), resulting in RIGS. It is due in part to ISC depletion and signaling defects of IR-damaged ISC niche, thus providing an ideal model system to study intestinal stem cell (ISC) growth and differentiation in response to radiation-induced intestinal injury.
The mammalian intestinal mucosa is a rapidly proliferating tissue and is a model for tissue parenchymal cells originating via hierarchical cell proliferation and differentiation from stem cells, in this case the ISC located in the intestinal crypt. The intestinal crypts are surrounded by a variety of supporting cells, including mesenchymal-derived cells, microvascular endothelial cells, macrophages and lymphocytes. These stromal cells provide the niche and supply critical growth factor/signals for ISC regeneration; thus, acute injury to the stroma is also highly detrimental to the ISC function. While IR induces apoptosis of ISCs, crypt endothelial cells and enterocytes within hours in a dose dependent manner, stromal components play critical role in preserving the ISC function. For instance, recruitment of host myelomonocytic cells and mesenchymal stem cell to irradiated GI region has been shown to improve GI regenerative capacity and maintain function when the stroma is damaged by irradiation (5, 11).
Intestinal Stem Cells homeostasis and radiation sensitivity
ISCs proliferate and migrate either toward the villus while differentiating into enterocytes, goblet, and enteroendocrine cells, or toward the crypt base while differentiating into Paneth cells. The severe gastrointestinal side effects of radiotherapy (RT) have been attributed to radiation-induced death of ISCs. The early mitotic arrest and cell death occurringin these stem cells, coupled with continued migration of differentiated daughter cells toward the extrusion zone at the villus apex, results in cellular depletionof the crypt while attempting to maintain the villus mass (12). Shrinkage of the villus commences after crypt depletion is completed, leading to involution of the mucosa and formation of frank ulcers with consequential metabolite and electrolyte imbalance, diarrhea and sepsis secondary to the entry of microbial agents in the systemic circulation. Within 3–4 day post-RT, epithelial regeneration proceeds from a few surviving cells out of a larger clonogenic population, seen as microcolonies of 10 or more crypt cells (12). By day 7 post-RT, the process of crypt budding provides new crypts with their own stem cells. Thus, after a cytotoxic insult induced by RT or drugs, ISCs and their immediate progeny are capable of dividing to regenerate an entire functional intestinal epithelium.
Differential sensitivity of critical tissue elements of the GI tract to radiation-induced cell death is observed in a dose dependent fashion. ISCs are heterogeneous, and comprise both active cycling stem cells as well as quiescent reserve stem cells that mediate regeneration after injury (13). The relative radiosensitivity and growth requirements of various intestinal stem cell pools are poorly understood. However, genetic inducible fate mapping studies have demonstrated that small intestinal epithelial cells are replaced from at least two principal stem cell pools: active, recycling, crypt based columnar cells (CBCs) expressing Lgr5, and more quiescent cells situated at position +4 above the crypt base which are often Hopx- and Bmi1-expressing (14–17)(18). Yan et al (17) reported that in mice exposed to 12 Gy whole body irradiation (WBI) and treated with BMT, Bmi1-ISC were able to regenerate intestinal epithelium whereas, Lgr5− ISC were depleted up to 7 days post-WBI, thus demonstrating that Lgr5+ cells are largely dispensable while other ISC populations including Bmi1+ cells are capable of intestinal epithelial regeneration. This could be possibly due to interconversion between active and quiescent stem cell populations following intestinal injury, which could not be demonstrated by the linage tracing models used in this study. Alternatively, Lgr5+ cells may not be of major importance in regenerative conditions.
Using a similar Cre-dependent lineage tracing model, Asfaha et al (19) demonstrated that Krt19+ labels a unique ISC, distinct from Lgr5+ CBCs and located above the +4 region. Under normal homeostasis, K19+ cells can give rise to Lgr5+ cells and the entire intestinal and colonic epithelium. Furthermore, interconversion between Krt19+ and Lgr5+ ISCs occurs readily in vivo (19). Importantly, in contrast to Lgr5+ and Dll1+ cells that are radiosensitive, Krt19+ stem cells are radioresistant, and thus in response to 12 Gy WBI, robust intestinal regeneration occurs from Krt19+ cells. These two studies used 12 Gy WBI and BMT as a model, and found that BMT was able to extend the survival in mice and allowed for completeion linage tracing experiments for up to 60 days post-WBI. In contrast, studies by Saha et al (4) indicated that at higher radiation doses (>12 Gy), BMT alone was not efficient and mice succumbed to RIGS within 2 weeks post-AIR. This latter study also showed that the SPC population was essential for mitigating RIGS. Taken together, these studies also emphasized the important role of both meyloid and stromal progenitor cells in intestinal epithelial regenratio and post-irradiation survival.
Microvascular endothelial cell injury in Radiation-Induced Gastrointestinal Syndrome (RIGS)
Irradiation induces apoptosis in endothelial cells primarily via the ceramide pathway (20). Previous studies have demonstrated that microvascular endothelial apoptosis represents a critical response to tissue damage in the irradiated lungs of C3H/HeJ mice, and intravenous administration of an endothelial growth factor, such as basic fibroblast growth factor (bFGF), partially abrogated the radiation injury (21). In a similar study, angiogenic growth factors, such as bFGF and VEGF, were found to be radioprotective for RIGS when administered 24 h before or 1 h after irradiation (22). Since these factors did not stimulate crypt proliferation, the mechanism of radioprotection remained elusive. Paris et al. reported that a single large dose of radiation administered to the mouse gastrointestinal tract primarily damages the endothelial cells of the gut microvasculature (23). Since ISCs reside in the crypts of Lieberkühn and are separated from the microvasculature by a very short distance (~100 um), this close apposition enables endothelial cells and epithelial progenitors to communicate with each other by release of growth factors and hormones, in addition to diffusion of nutrients and oxygen from blood vessels to the ISC niche. Thus, these investigators concluded that the death of ISCs might be a secondary event, resulting from the demise of the niche endothelial cells upon which stem cells depend.
In support of this postulate, these investigators prevented RIGS by inhibiting endothelial cell apoptosis pharmacologically with intravenous bFGF, or genetically in mice by deletion of the acid sphingomyelinase gene that encodes the enzyme necessary for the production of the second messenger, ceramide, a proapoptotic lipid that facilitates endothelial cell death (23). Microvascular endothelial cells express the receptor for bFGF, whereas epithelial stem cells of the intestinal crypts do not, suggesting that bFGF protects the gut mucosa from radiation damage through its effects on endothelial cells. The relative contribution of endothelial, as opposed to epithelial, injury in the ARS syndrome remains to be established, and remains controversial. For example, Coderre and colleagues applied a selective microvasculature radiation technique using intravenous administration of 10Boronated liposomes (24). In this model, the threshold dose for death from RIGS after neutron-beam-only irradiation was 9.0 +/− 0.6Gy, whereas there were no deaths from RIGS, despite calculated absorbed doses to endothelial cells as high as 27.7 Gy from boronated liposomes. This suggests that endothelial cell damage is not causative in the loss of intestinal crypt stem cells and the eventual development of RIGS. Therefore, a two-compartment model has been proposed (25), where radiation-induced cell death in both intestinal endothelial and crypt stem cells contribute to RIGS. Thus, when mouse intestines were protected against microvascular apoptosis by bFGF administration, higher radiation doses (>16 Gy) are needed to activate the ceramide synthase-mediated crypt stem cell apoptosis program required to initiate intestinal damage.
Therapeutic application of Stromal Progenitor Cells (SPC) in Radiation injuries of the gastrointestinal tract
Mitigation of RIGS
Radiation-induced gastrointestinal syndrome (RIGS) results from stem cell and villi depletion, loss of mucosal barrier, infiltration of enteric bacteria into circulation, endotoxemia, and acute inflammatory response. The response of pathogen-free mice to whole body irradiation (WBI), where rapidly proliferating hematopoietic progenitor cells are also damaged, is quite different from that observed with the abdominal irradiation (AIR) model, where the hematopoietic progenitors are not damaged (26). Gastrointestinal injury related to RT is similar to the AIR model, where as the injury observed in a WBI model is more typical of mutliorgan failure. In addition to the GI toxicity related to RT, RIGS is a critical concern for a mass casualty scenario, where a large population is exposed to a high dose of ionizing radiation, likely involving both whole body (WBI) or partial body (PBI) exposure, with a high risk of gastrointestinal toxicity and related injuries. To date, there are no treatment options available to remedy RIGS in humans. Studies by Saha et al (4) demonstrated that bone marrow adherent stromal cells (BMASC) given 24 and 72 h post-lethal doses of WBI mitigated acute radiation injury to the gastrointestinal tract, and improved post-irradiation survival dramatically by stimulating stem cell proliferation and crypt regeneration, thereby reducing mucosa disruption and permeability. The bone marrow adherent cell population used in the studies was characterized as a combination of mesenchymal stem cells (MSC), endothelial (EC) and myeloid cells. Depletion of either the myeloid or non-myeloid fraction led to significant loss of post-irradiation survival, indicating that the mitigating mechanisms involved both MSC and myeloid cells. These effects were observed in a WBI model where host bone marrow was also exposed to a lethal dose of radiation, and in an abdominal irradiation model (AIR), where most of the BM was spared; thus, the effect of BMASC was primarily from donor derived cells. Interestingly, depletion of host myeloid cells by clodronate liposomes resulted in reduction in post-irradiation survival by BMASC treatment, implying a critical supporting role for host-derived macrophages (4). This study indicated that donor BMASC and host cells are equally important in the mitigation of radiation injury.
Similar results were obtained in independent studies reported by Chang et al (27), where a different source of MSC - human adipose derived mesenchymal stem cells (Ad-MSC) - were used to mitigate acute injury from lethal dose of AIR in rats. The authors described the mechanism of action as a combination of (i) reduction in inflammation as hallmarked by reduced myeloperoxidase (MPO) activity and increased IL10 levels, (ii) increased neovascualization in the irradiated tissue, and (iii) increase proliferation in Bmi1- positive intestinal stem cells. Studies by Ch’ang et al (5) reported that BMT in lethally irradiated mice rescued intestinal mucosa, increased stromal cell proliferation and improved post-irradiation survival. Although these studies utilized a conventional BMT strategy and not the stromal or adherent population, the results indicated that BMT increased the recruitment of host stromal and myelomonocytic cells in the irradiated intestines, despite poor engraftment of donor cells in the intestine. Depletion of host myelmonocytic cells by carrageenan abolished the increase in post-irradiation survival in mice by BMT. It is important to note that the efficacy of BMT was somewhat limited, and beneficial only to at specific radiation doses. Garg et al (28) also reported that BMT was able to rescue intestinal mucosa in mice exposed to 8–10 Gy WBI, a dose where hematopoietic damage is the major causative factor leading to death. The author showed increases in peripheral blood cells as a result of BMT, but also a subsequent increase in the recruitment of donor macrophages to the intestine and a reduction in intestinal permeability. Beneficial effects of SPC in mitigating radiation induced gastrointestinal injuries are summarized in Table 1.
Table 1.
Therapeutic applications of SPC and SPC derived growth factors against radiation induced intestinal injuries
| A. Therapeutic application of SPC in various radiation induced pathologies | |||
|---|---|---|---|
| Cells | Targets/Mechanisms | Application (disease model) | Ref |
| Bone marrow stromal progenitors, epithelial progenitors, myeloid progenitors |
Lgr5 positive ISC proliferation Crypt maintenance Mucosal integrity |
Radiation induced gastrointestinal syndrome (mouse) |
4 |
| Bone marrow transplant |
Recruitment of host myelomonocytic and MSC to the irradiated tissue |
Radiation induced gastrointestinal syndrome (mouse) |
5, 11 |
| Adipose derived mesenchymal stem cells |
Increase in Bmi-1 positive ISC proliferation Reduction in inflammation Increase in anti-inflammatory Increase in vascularization |
Radiation induced gastrointestinal syndrome (mouse) |
26 |
| Bone marrow transplant |
Recruitment of host myeloid cells Reduction in GI permeability |
Radiation induced mucosal injury (mouse) |
27 |
| Bone marrow derived mesenchymal stem cells |
Increase in intestinal epithelial regeneration |
Radiation Enteritis (dogs) | 30 |
| Bone marrow derived mesenchymal stem cells |
Activation of non-canonical signaling Increase colonic epithelial regeneration Reduction in ulceration Recruitment of host mesenchymal stem cells to colon |
Radiation induced Pelvic Disease (moues) |
35 |
| Bone marrow derived mesenchymal stem cells |
Reduction in acute inflammation Reduction in infiltration of leukocytes Reduction in fibrosis and vascular remodeling |
Radiation Proctitis (pigs) | 38 |
| B. SPC derived cytokines and growth factors | |||
| Growth Factors | Targets/Mechanisms | Application (disease model) | Ref |
| Vascular endothelial growth factor |
Vascularization | Radiation induced gastrointestinal syndrome (mouse) |
4, 41, 40, 45 |
| Rspondin1 | Lgr5-ISC proliferation | Radiation induced gastrointestinal syndrome (mouse) |
4 |
| Platelet derived growth factor |
Stromal proenitor cell proliferation | Radiation induced gastrointestinal syndrome (mouse) |
4, 40, 45 |
| Fibroblast growth factor |
Intestinal Sub-epithelial myofibroblasts proliferation |
Radiation induced gastrointestinal syndrome (mouse) |
4 |
| Keratinocyte growth factor |
ISC and Transit amplifying cell growth factor |
Radiation induced gastrointestinal syndrome (mouse) |
4 |
| Interleukin10 | Anti-inflammatory | Radiation Proctitis (pigs) | 40 |
| Hepatocyte growth factor |
Growth factor for epithelial and endothelial cells |
Radiation induced epithelial and endothelial injury |
45 |
| Interleukin 17 | Anti-inflammatory | Radiation induced inflammatory response (ulcers, proctitis, cystitis) |
44 |
| Stromal cell derived factor 1 |
Stem cell proliferation Recruitment of host MSC |
Acute radiation injury | 40, 45 |
| Interleukin 6 | Proliferation Hematopoiesis |
Acute radiation injury | 40, 45 |
| Angiopoietin 1, 2 | Vascular growth factor | Radiation Proctitis (pigs) | 40 |
Radiation Enteritis (RE)
RE is the most prevalent complication from RT of abdominal and kidney cancers, and ~75% of cancer patients undergoing abdominal RT treatment suffer from side effects related to RE. Recent studies (29) show a positive correlation between systemic inflammation, nutritional status and chronic RE. Acute RE occurs around 2 weeks post-treatment and is associated with mucosal barrier loss, epithelial denudation, nutrient loss, infiltration of leukocytes into the lamina propria and inflammatory responses such as mucosal ulceration. Ischemia, necrosis and fibrosis, on the other hand, are the classical features of chronic enteritis, which typically manifests around 8–12 months post-treatment (30). Currently there is no standard evaluation system for assessing radiation enteritis, other than symptoms, and the use of pharmacological intervention is not approved. Albeit several mouse studies show promising effects of BMSC in radiation injury, few reports show their therapeutic efficacy in large animal models. Xu et al (31) demonstrated that BMSCT significantly increased intestinal epithelial regeneration, reduced the clinical symptoms associated with chronic enteritis, and improved survival in lethally irradiated dogs. BMSCT significantly reduced the frequency of nausea and vomiting, a prognostic indicator of radiation injury and mortality.
Pelvic Radiation Disease (PRD)
Cancers in the pelvic region are one of the most frequently diagnosed cancers and the treatment often requires RT (32, 33). The incidence of radiation toxicity from pelvic RT depends on the volume and duration of the treatment course, about 4–10% patients experience life threatening effects of pelvic RT (34). As survival is prolonged following successful treatment, the incidence of pelvic radiation disease continue to rise, with the number of patients developing PVD now twice the number of that with Crohn’s disease (32). Because the symptoms of PRD are similar to many other bowel disorders, comprehensive care and management of symptoms tailored to RT toxicity are not currently available, with limited interventional strategies including hyperbaric chamber and surgery (30). Additionally, the toxicity also depends on several risk factors such as diabetes, inflammatory bowel disease, body mass index (BMI) and smoking (35). Acute toxicity develops during the second week of radiation treatment, and peaks by the 5th week, where histological changes are most prominent. Delayed toxicity (after the 5th week) is more common, with almost 90% patients developing permanent changes in the GI tract, and 50% of patients end up with a significant decline in quality of life (33). The most frequently reported symptoms of toxicity from pelvic RT include rectal bleeding, bloating, vomiting, abdominal cramps, constipation, diarrhea, fecal incontinence, lactose intolerance, abdominal, rectal and anal pain. Use of probiotics or antibiotics relieves some of the symptoms such as diarrhea, and motility changes; however most of the symptoms remain refractory to treatment. Delayed toxicity effects such as ulceration, stricture formation, and obstruction are even more critical and newer approaches are needed to prevent these complications (33).
Several interventional strategies are used to reduce the extent of PRD, including antioxidants such as amifostine, and probiotics (35), Amifostine was shown to be beneficial for reducing acute toxicity, but did not improve chronic PRD, while probiotics had similar effect with no significant benefit in delayed toxicity. Studies by Semont et al (36) used a rat model to establish PRD histologically comparable to human disease. The authors demonstrated that infusion of BM-SPC (devoid of hematopoietic cells) reduced radiation-induced colonic ulceration, increased recruitment of host MSC to the irradiated tissue, and induced colonic epithelial regeneration by non-canonical Wnt activation, consequently leading to improvement in post-irradiation survival. Additionally the authors also analyzed other functional endpoints, including improvement in colonic mucosal damage, extent of fibrosis, vascular sclerosis and muscular dystrophy, leading to overall improvement in late injury in presence of MSC with the exception of the fibrosis scoring This model did not analyze other organs in the pelvic cavity that may have shown radiation-induced acute and delayed injury. Feasibility studies using systemic administration of MSC in patients with intestinal lesions showed substantial benefit in hemorrhage reduction (34). The treatment was well tolerated and the MSC cultures exhibited genomic stability. Further studies are being currently undertaken based on these results.
Radiation proctitis
Radiation proctitis is defined as a radiation injury to the lining of the rectum primarily as a result of prostate radiation (37, 38). The onset of acute toxicity is observed as early as 2 weeks into the radiation treatment, and observed in ~13% of patients with intermittent bleeding, cramps, constipation and mucoid discharge. Currently there are no medical interventional strategies to treat radiation proctitis and alleviate the symptoms other than surgery., Studies by Linard et al showed successful administration of autologous bone marrow-derived MSC for mitigation of radiation proctitis in pig model (39). Endoscopic and histological evaluation of pigs treated with MSC showed significant reduction in acute inflammation, infiltration of leukocyte at the site of injury, and delayed effects such as vascular injury, remodeling and fibrosis.
Effects of SPC on intestinal stem cells and the niche factors
Histological evidence suggests that SPC treatment mitigates RIGS via accelerated regeneration of irradiated host intestinal stem cells, rather than simply replacement of host stroma with donor derived cells (4, 27, 40). Saha et al (4) demonstrated that bone marrow stromal transplant increased Lgr5-GFP cells in irradiated mice, indicating direct effect of SPC on ISC proliferation, Semont el al also independently demonstrated reduction in apoptotic cells, increased ki67 staining in the crypt region, and increased miRNA levels of Lgr5, mTERT and Sox9 in response to Ad-MSC in irradiated mice (36, 40), indicative of elevated ISC activity in response to stromal cell transplant. Studies by Saha et (4) also demonstrated several-fold increases in mRNA levels in cells isolated from the crypt region of intestinal growth factors and inflammatory cytokines, such as fibroblasts growth factor 10 (FGF10), keratinocyte growth factor (KGF), epidermal growth factor (EGF), fibroblast growth factor 2 (FGF2), vascular endotheial growth factor (VEGF), and anti-inflammatory cytokine interleukin 10 (IL-10) with BMSC treatment., These changes were not observed with a conventional BMT devoid of stromal progenitor cells. Recent studies by Kamprom et al (41) also reported elevated mRNA levels of stromal cell-derived factor 1 (SDF1), insulin-like growth factor 1 (IGF1), placental growth factor (PlGF), fibroblast growth factor 2 (FGF2), angiopoietin 1 (ANGPT1), angiopoietin 2 (ANGPT2), vascular endothelial growth factor (VEGEF), interleukin 6 (IL6), interleukin 8 (IL8) in MSC obtained from bone marrow and placenta derived MSC, indicating direct role of MSC in angiogenesis. Independent studies involving use of R-spondin-1 (42), KGF (43), VEGF (22) have shown that these growth factors have beneficial effects in regeneration of intestinal epithelium after radiation injury. Together, these results suggested that stromal progenitor cells can modulate the regenerative signals in the intestinal microenvironment.
Paracrine functions of SPC: Secreted microvesicles as a new therapeutic regimen
The most well documented properties of SPC include (i) targeting to the injured site, (ii) recruiting host MSC to the injured site, (iii) supplying growth factors to the injured stromal and stem cells, and (iv) controlling inflammation at the local level. However, it is now recognized that several of these functions by SPC are exerted via paracrine mechanisms involving secreted factors, most likely via extracellular vesicles (4, 27,31, 44–48). Several of the studies have reported that SPC secrete stromal growth factors such as basic fibroblast growth factor (FGF2), platelet derived growth factor (PDGF), R-spondins, Wnts (Wnt 4, 5) (4, 45), anti-inflammatory cytokines such as Interleukin-17, glucocorticoids (49), and angiogenic factors such as VEGEF (4, 45). These studies demonstrated that the culture medium from SPC was equally effective in exerting beneficial effects of SPC as an alternative to cell transplant. Growth factors and cytokines secreted by SPC that are responsible for various biological activities of SPC are summarized in Table 1. Additionally, reports also indicate that SPC exosomes increase ATP levels in cardiomyocytes, preventing ischemia injury associated (50).
Potential role of stromal progenitor cells in Mitochondrial replacement therapy
More recently, mitochondrial replacement therapy is being viewed as a new paradigm shift in regenerative molecular medicine. In addition to mitochondrial genetic disease (51), altered biogenesis and mitochondrial exhaustion is the leading cause of several oxidative disorders, including Chronic obstructive pulmonary disease (COPD) and aging related degenerative pathologies (52). Beneficial effects of mitochondrial transfer are observed in ischemic perfusion model and in metastatic cancer cells (53). An interesting study by Islam et al (54) showed that bone marrow-derived stromal progenitor cells were able to transfer mitochondria containing microvesicles to the epithelial cells of a rat exposed to lipopolysaccharide (LPS) injection in an acute lung injury model; this transfer was also associated with increased ATP activity. Thus, MSC are also emerging as a source of healthy mitochondria for treatment of diseases associated with mitochondrial mutation and defects in bioenergetics.
CONCLUSION AND FUTURE PERSPECTIVES
Overall, SPC exert their effects by repairing, replacing or reprograming damaged cells. Although the mechanisms by which they exert these various effects remain incompletely defined, recent studies have emphasized the role of secreted extracellular vesicles as a dominant pathway in their mechanism of action. The development of stromal progenitor cells (SPC) or MSC into a highly evolved regenerative cell-based therapy for a variety of diseases - ranging from inflammatory (colitis, enteritis, inflammatory bowel disease, pulmonary fibrosis, bladder fibrosis), autoimmune (graft versus host disease in transplants), regenerative (wounds, burns, scars, radiation injury) - is striking but not surprising. However, transition from preclinical studies into clinical studies has been somewhat limited due to uncertainties regarding the multipotent nature of SPC and technical difficulties in their expansion. BM-derived SPC are poorly proliferative compared to the adipose-derived SPC, and the difference in their proliferative capacity has been attributed to higher expression of SDF-1 in Ad-SPC (27). The stability of transplanted and engrafted SPC remains one of the critical concerns in future clinical studies with MSCs; however, MSC have been shown to be highly immune-tolerant and genomically stable, and thus they continue to be highly promising. Use of secreted vesicles from SPC is another emerging area that could in theory render whole cell transplants obsolete, since all of the beneficial effects of MSC might be transferred to patients in the form of their secreted vesicles. In the context of radiation injury, especially of the GI where epithelial, mucosal, endothelial and immune components are simultaneously destabilized and damaged, SPC therapy offers great therapeutic promise.
Acknowledgments
This work was supported by NIH grant U19 AI091175 and U01DK103155.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
Shilpa Kulkarni, Timothy Wang and Chandan Guha declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Recently published papers of particular interest have been highlighted as:
• Of importance
•• Of major importance
- 1.Baskar R, Lee KA, Yeo R, Yeoh KW. Cancer and radiation therapy: current advances and future directions. International journal of medical sciences. 2012;9(3):193–199. doi: 10.7150/ijms.3635. PubMed PMID: 22408567. Pubmed Central PMCID: 3298009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnett GC, West CM, Dunning AM, Elliott RM, Coles CE, Pharoah PD, et al. Normal tissue reactions to radiotherapy: towards tailoring treatment dose by genotype. Nature reviews Cancer. 2009 Feb;9(2):134–142. doi: 10.1038/nrc2587. PubMed PMID: 19148183. Pubmed Central PMCID: 2670578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prasanna PG, Stone HB, Wong RS, Capala J, Bernhard EJ, Vikram B, et al. Normal tissue protection for improving radiotherapy: Where are the Gaps? Translational cancer research. 2012 Jun;1(1):35–48. PubMed PMID: 22866245. Pubmed Central PMCID: 3411185. [PMC free article] [PubMed] [Google Scholar]
- 4.Saha S, Bhanja P, Kabarriti R, Liu L, Alfieri AA, Guha C. Bone marrow stromal cell transplantation mitigates radiation-induced gastrointestinal syndrome in mice. PloS one. 2011;6(9):e24072. doi: 10.1371/journal.pone.0024072. PubMed PMID: 21935373. Pubmed Central PMCID: 3174150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ch'ang HJ, Lin LM, Chang PY, Luo CW, Chang YH, Chou CK, et al. Bone marrow transplantation enhances trafficking of host-derived myelomonocytic cells that rescue intestinal mucosa after whole body radiation. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2012 Sep;104(3):401–407. doi: 10.1016/j.radonc.2011.12.003. PubMed PMID: 22260849. [DOI] [PubMed] [Google Scholar]
- 6.Benderitter M, Caviggioli F, Chapel A, Coppes RP, Guha C, Klinger M, et al. Stem cell therapies for the treatment of radiation-induced normal tissue side effects. Antioxidants & redox signaling. 2014 Jul 10;21(2):338–355. doi: 10.1089/ars.2013.5652. PubMed PMID: 24147585. Pubmed Central PMCID: 4060814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y, Chen X, Cao W, Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol. 2014 Nov;15(11):1009–1016. doi: 10.1038/ni.3002. PubMed PMID: 25329189. [DOI] [PubMed] [Google Scholar]
- 8.Bianco P, Riminucci M, Gronthos S, Robey PG. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem cells. 2001;19(3):180–192. doi: 10.1634/stemcells.19-3-180. PubMed PMID: 11359943. [DOI] [PubMed] [Google Scholar]
- 9.Nicolay NH, Lopez Perez R, Debus J, Huber PE. Mesenchymal stem cells - A new hope for radiotherapy-induced tissue damage? Cancer letters. 2015 Oct 1;366(2):133–140. doi: 10.1016/j.canlet.2015.06.012. PubMed PMID: 26166559. [DOI] [PubMed] [Google Scholar]
- 10.Brown M. What causes the radiation gastrointestinal syndrome?: overview. Int J Radiat Oncol Biol Phys. 2008 Mar 1;70(3):799–800. doi: 10.1016/j.ijrobp.2007.12.001. PubMed PMID: 18262092. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Francois S, Bensidhoum M, Mouiseddine M, Mazurier C, Allenet B, Semont A, et al. Local irradiation not only induces homing of human mesenchymal stem cells at exposed sites but promotes their widespread engraftment to multiple organs: a study of their quantitative distribution after irradiation damage. Stem cells. 2006 Apr;24(4):1020–1029. doi: 10.1634/stemcells.2005-0260. PubMed PMID: 16339642. [DOI] [PubMed] [Google Scholar]
- 12.Withers HR, Elkind MM. Microcolony survival assay for cells of mouse intestinal mucosa exposed to radiation. Int J Radiat Biol Relat Stud Phys Chem Med. 1970;17(3):261–267. doi: 10.1080/09553007014550291. PubMed PMID: 4912514. Epub 1970/01/01. eng. [DOI] [PubMed] [Google Scholar]
- 13.Tian H, Biehs B, Warming S, Leong KG, Rangell L, Klein OD, et al. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 2011 Oct 13;478(7368):255–259. doi: 10.1038/nature10408. PubMed PMID: 21927002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007 Oct 25;449(7165):1003–1007. doi: 10.1038/nature06196. PubMed PMID: 17934449. eng. [DOI] [PubMed] [Google Scholar]
- 15.Barker N, van Es JH, Jaks V, Kasper M, Snippert H, Toftgard R, et al. Very Long-term Self-renewal of Small Intestine, Colon, and Hair Follicles from Cycling Lgr5+ve Stem Cells. Cold Spring Harb Symp Quant Biol. 2008 Nov 6; doi: 10.1101/sqb.2008.72.003. PubMed PMID: 19022755. Eng. [DOI] [PubMed] [Google Scholar]
- 16.Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet. 2008 Jul;40(7):915–920. doi: 10.1038/ng.165. PubMed PMID: 18536716. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yan KS, Chia LA, Li X, Ootani A, Su J, Lee JY, et al. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc Natl Acad Sci U S A. 2012 Jan 10;109(2):466–471. doi: 10.1073/pnas.1118857109. PubMed PMID: 22190486. Pubmed Central PMCID: 3258636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takeda N, Jain R, LeBoeuf MR, Wang Q, Lu MM, Epstein JA. Interconversion between intestinal stem cell populations in distinct niches. Science. 2011 Dec 9;334(6061):1420–1424. doi: 10.1126/science.1213214. PubMed PMID: 22075725. Pubmed Central PMCID: 3705713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asfaha S, Hayakawa Y, Muley A, Stokes S, Graham TA, Ericksen RE, et al. Krt19/Lgr5 Cells Are Radioresistant Cancer-Initiating Stem Cells in the Colon and Intestine. Cell Stem Cell. 2015 Jun 4;16(6):627–638. doi: 10.1016/j.stem.2015.04.013. PubMed PMID: 26046762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haimovitz-Friedman A, Kan CC, Ehleiter D, Persaud RS, McLoughlin M, Fuks Z, et al. Ionizing radiation acts on cellular membranes to generate ceramide and initiate apoptosis. J Exp Med. 1994 Aug 1;180(2):525–535. doi: 10.1084/jem.180.2.525. PubMed PMID: 8046331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fuks Z, Haimovitz-Friedman A, Kolesnick RN. The role of the sphingomyelin pathway and protein kinase C in radiation-induced cell kill. Important Adv Oncol. 1995:19–31. PubMed PMID: 7672806. [PubMed] [Google Scholar]
- 22.Okunieff P, Mester M, Wang J, Maddox T, Gong X, Tang D, et al. In vivo radioprotective effects of angiogenic growth factors on the small bowel of C3H mice. Radiat Res. 1998 Aug;150(2):204–211. PubMed PMID: 9692366. eng. [PubMed] [Google Scholar]
- 23.Paris F, Fuks Z, Kang A, Capodieci P, Juan G, Ehleiter D, et al. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science. 2001 Jul 13;293(5528):293–297. doi: 10.1126/science.1060191. PubMed PMID: 11452123. eng. [DOI] [PubMed] [Google Scholar]
- 24.Schuller BW, Rogers AB, Cormier KS, Riley KJ, Binns PJ, Julius R, et al. No significant endothelial apoptosis in the radiation-induced gastrointestinal syndrome. International journal of radiation oncology, biology, physics. 2007 May 1;68(1):205–210. doi: 10.1016/j.ijrobp.2006.12.069. PubMed PMID: 17448874. [DOI] [PubMed] [Google Scholar]
- 25.Ch'ang HJ, Maj JG, Paris F, Xing HR, Zhang J, Truman JP, et al. ATM regulates target switching to escalating doses of radiation in the intestines. Nat Med. 2005 May;11(5):484–490. doi: 10.1038/nm1237. PubMed PMID: 15864314. eng. [DOI] [PubMed] [Google Scholar]
- 26.Mason KA, Withers HR, McBride WH, Davis CA, Smathers JB. Comparison of the gastrointestinal syndrome after total-body or total-abdominal irradiation. Radiation research. 1989 Mar;117(3):480–488. PubMed PMID: 2648450. [PubMed] [Google Scholar]
- 27.Chang P, Qu Y, Liu Y, Cui S, Zhu D, Wang H, et al. Multi-therapeutic effects of human adipose-derived mesenchymal stem cells on radiation-induced intestinal injury. Cell death & disease. 2013;4:e685. doi: 10.1038/cddis.2013.178. PubMed PMID: 23788042. Pubmed Central PMCID: 3698545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garg S, Wang W, Prabath BG, Boerma M, Wang J, Zhou D, et al. Bone marrow transplantation helps restore the intestinal mucosal barrier after total body irradiation in mice. Radiation research. 2014 Mar;181(3):229–239. doi: 10.1667/RR13548.1. PubMed PMID: 24568131. Pubmed Central PMCID: 4038129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cai Z, Cai D, Yao D, Chen Y, Wang J, Li Y. Associations between body composition and nutritional assessments and biochemical markers in patients with chronic radiation enteritis: a case-control study. Nutrition journal. 2016;15(1):57. doi: 10.1186/s12937-016-0177-6. PubMed PMID: 27233356. Pubmed Central PMCID: 4884391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodriguez ML, Martin MM, Padellano LC, Palomo AM, Puebla YI. Gastrointestinal toxicity associated to radiation therapy. Clinical & translational oncology : official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico. 2010 Aug;12(8):554–561. doi: 10.1007/s12094-010-0553-1. PubMed PMID: 20709653. [DOI] [PubMed] [Google Scholar]
- 31.Xu W, Chen J, Liu X, Li H, Qi X, Guo X. Autologous bone marrow stromal cell transplantation as a treatment for acute radiation enteritis induced by a moderate dose of radiation in dogs. Translational research : the journal of laboratory and clinical medicine. 2015 Dec 19; doi: 10.1016/j.trsl.2015.12.010. PubMed PMID: 26763584. [DOI] [PubMed] [Google Scholar]
- 32.Andreyev HJ, Wotherspoon A, Denham JW, Hauer-Jensen M. Defining pelvic-radiation disease for the survivorship era. The Lancet Oncology. 2010 Apr;11(4):310–312. doi: 10.1016/S1470-2045(10)70026-7. PubMed PMID: 20149738. [DOI] [PubMed] [Google Scholar]
- 33.Andreyev J. Gastrointestinal symptoms after pelvic radiotherapy: a new understanding to improve management of symptomatic patients. The Lancet Oncology. 2007 Nov;8(11):1007–1017. doi: 10.1016/S1470-2045(07)70341-8. PubMed PMID: 17976611. [DOI] [PubMed] [Google Scholar]
- 34.Chapel A, Francois S, Douay L, Benderitter M, Voswinkel J. New insights for pelvic radiation disease treatment: Multipotent stromal cell is a promise mainstay treatment for the restoration of abdominopelvic severe chronic damages induced by radiotherapy. World journal of stem cells. 2013 Oct 26;5(4):106–111. doi: 10.4252/wjsc.v5.i4.106. PubMed PMID: 24179599. Pubmed Central PMCID: 3812515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fuccio L, Frazzoni L, Guido A. Prevention of pelvic radiation disease. World journal of gastrointestinal pharmacology and therapeutics. 2015 Feb 6;6(1):1–9. doi: 10.4292/wjgpt.v6.i1.1. PubMed PMID: 25664197. Pubmed Central PMCID: 4318744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Semont A, Demarquay C, Bessout R, Durand C, Benderitter M, Mathieu N. Mesenchymal stem cell therapy stimulates endogenous host progenitor cells to improve colonic epithelial regeneration. PloS one. 2013;8(7):e70170. doi: 10.1371/journal.pone.0070170. PubMed PMID: 23922953. Pubmed Central PMCID: 3726425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Colwell JC, Goldberg M. A review of radiation proctitis in the treatment of prostate cancer. Journal of wound, ostomy, and continence nursing : official publication of The Wound, Ostomy and Continence Nurses Society / WOCN. 2000 May;27(3):179–187. doi: 10.1016/s1071-5754(00)90056-1. PubMed PMID: 10814951. [DOI] [PubMed] [Google Scholar]
- 38.Babb RR. Radiation proctitis: a review. The American journal of gastroenterology. 1996 Jul;91(7):1309–1311. PubMed PMID: 8677984. [PubMed] [Google Scholar]
- 39.Linard C, Busson E, Holler V, Strup-Perrot C, Lacave-Lapalun JV, Lhomme B, et al. Repeated autologous bone marrow-derived mesenchymal stem cell injections improve radiation-induced proctitis in pigs. Stem Cells Transl Med. 2013 Nov;2(11):916–927. doi: 10.5966/sctm.2013-0030. PubMed PMID: 24068742. Pubmed Central PMCID: 3808206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Semont A, Mouiseddine M, Francois A, Demarquay C, Mathieu N, Chapel A, et al. Mesenchymal stem cells improve small intestinal integrity through regulation of endogenous epithelial cell homeostasis. Cell Death Differ. 2010 Jun;17(6):952–961. doi: 10.1038/cdd.2009.187. PubMed PMID: 20019749. [DOI] [PubMed] [Google Scholar]
- 41.Kamprom W, Kheolamai P, Y UP, Supokawej A, Wattanapanitch M, Laowtammathron C, et al. Effects of mesenchymal stem cell-derived cytokines on the functional properties of endothelial progenitor cells. European journal of cell biology. 2016 Mar-May;95(3–5):153–163. doi: 10.1016/j.ejcb.2016.02.001. PubMed PMID: 26899034. [DOI] [PubMed] [Google Scholar]
- 42.Bhanja P, Saha S, Kabarriti R, Liu L, Roy-Chowdhury N, Roy-Chowdhury J, et al. Protective role of R-spondin1, an intestinal stem cell growth factor, against radiation-induced gastrointestinal syndrome in mice. PLoS One. 2009;4(11):e8014. doi: 10.1371/journal.pone.0008014. PubMed PMID: 19956666. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Farrell CL, Bready JV, Rex KL, Chen JN, DiPalma CR, Whitcomb KL, et al. Keratinocyte growth factor protects mice from chemotherapy and radiation-induced gastrointestinal injury and mortality. Cancer Res. 1998 Mar 1;58(5):933–939. PubMed PMID: 9500453. eng. [PubMed] [Google Scholar]
- 44.Lee JW, Fang X, Krasnodembskaya A, Howard JP, Matthay MA. Concise review: Mesenchymal stem cells for acute lung injury: role of paracrine soluble factors. Stem cells. 2011 Jun;29(6):913–919. doi: 10.1002/stem.643. PubMed PMID: 21506195. Pubmed Central PMCID: 3293251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang YH, Lin LM, Lou CW, Chou CK, Ch'ang HJ. Bone marrow transplantation rescues intestinal mucosa after whole body radiation via paracrine mechanisms. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2012 Dec;105(3):371–377. doi: 10.1016/j.radonc.2012.10.005. PubMed PMID: 23146318. [DOI] [PubMed] [Google Scholar]
- 46.Conese M, Carbone A, Castellani S, Di Gioia S. Paracrine effects and heterogeneity of marrow-derived stem/progenitor cells: relevance for the treatment of respiratory diseases. Cells Tissues Organs. 2013;197(6):445–473. doi: 10.1159/000348831. PubMed PMID: 23652321. [DOI] [PubMed] [Google Scholar]
- 47.Au P, Tam J, Fukumura D, Jain RK. Bone marrow-derived mesenchymal stem cells facilitate engineering of long-lasting functional vasculature. Blood. 2008 May 1;111(9):4551–4558. doi: 10.1182/blood-2007-10-118273. PubMed PMID: 18256324. Pubmed Central PMCID: 2343592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ranganath SH, Levy O, Inamdar MS, Karp JM. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell. 2012 Mar 2;10(3):244–258. doi: 10.1016/j.stem.2012.02.005. PubMed PMID: 22385653. Pubmed Central PMCID: 3294273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bessout R, Semont A, Demarquay C, Charcosset A, Benderitter M, Mathieu N. Mesenchymal stem cell therapy induces glucocorticoid synthesis in colonic mucosa and suppresses radiation-activated T cells: new insights into MSC immunomodulation. Mucosal Immunol. 2014 May;7(3):656–669. doi: 10.1038/mi.2013.85. PubMed PMID: 24172849. [DOI] [PubMed] [Google Scholar]
- 50.Arslan F, Lai RC, Smeets MB, Akeroyd L, Choo A, Aguor EN, et al. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013 May;10(3):301–312. doi: 10.1016/j.scr.2013.01.002. PubMed PMID: 23399448. [DOI] [PubMed] [Google Scholar]
- 51.Khan SM, Bennett JP., Jr Development of mitochondrial gene replacement therapy. Journal of bioenergetics and biomembranes. 2004 Aug;36(4):387–393. doi: 10.1023/B:JOBB.0000041773.20072.9e. PubMed PMID: 15377877. [DOI] [PubMed] [Google Scholar]
- 52.Agrawal A, Mabalirajan U. Rejuvenating cellular respiration for optimizing respiratory function: targeting mitochondria. American journal of physiology Lung cellular and molecular physiology. 2016 Jan 15;310(2):L103–L113. doi: 10.1152/ajplung.00320.2015. PubMed PMID: 26566906. [DOI] [PubMed] [Google Scholar]
- 53.Liu CS, Chang JC, Kuo SJ, Liu KH, Lin TT, Cheng WL, et al. Delivering healthy mitochondria for the therapy of mitochondrial diseases and beyond. The international journal of biochemistry & cell biology. 2014 Aug;53:141–146. doi: 10.1016/j.biocel.2014.05.009. PubMed PMID: 24842105. [DOI] [PubMed] [Google Scholar]
- 54.Islam MN, Das SR, Emin MT, Wei M, Sun L, Westphalen K, et al. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012 May;18(5):759–765. doi: 10.1038/nm.2736. PubMed PMID: 22504485. Pubmed Central PMCID: 3727429. [DOI] [PMC free article] [PubMed] [Google Scholar]