INTRODUCTION
Cutaneous squamous cell carcinoma (SCC) is the second most common human malignancy, with more than 700,000 cases expected in 2016.1–4 Although typically curable by excision with clear margins, SCC can behave aggressively and metastasize to local lymph nodes despite treatment.5–7 Cutaneous SCC is characterized by the malignant proliferation of keratinocytes that typically occurs on sun-damaged skin and tends to develop within areas of noninvasive actinic keratosis (AK). Progression from AK to SCC is thought to occur as a result of ultraviolet (UV)-induced mutations in proto-oncogene genes, with as few as 2 mutations being sufficient to drive SCC development.8,9 Additional factors from the tumor microenvironment likely influence SCC progression.
THE TUMOR MICROENVIRONMENT OF SQUAMOUS CELL CARCINOMA
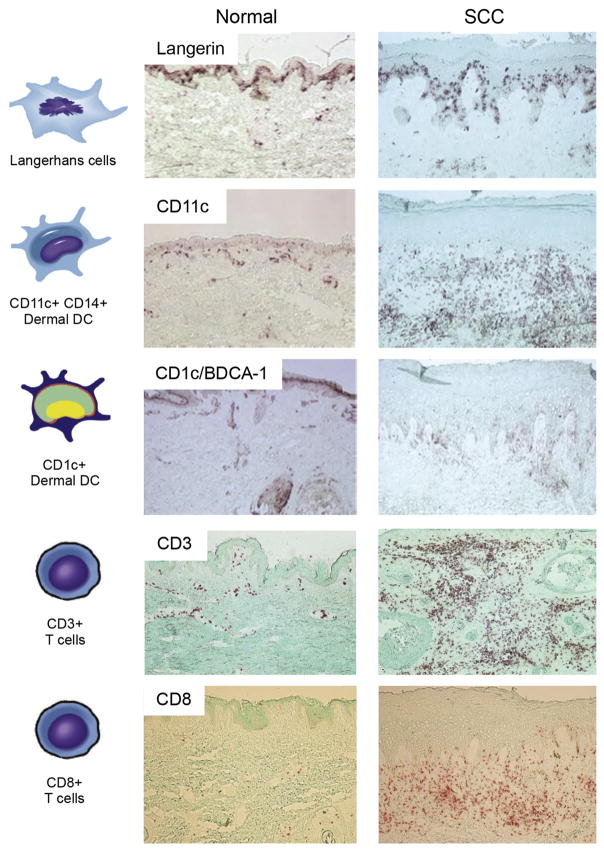
Gene expression profiling of invasive SCC has revealed the presence of a large inflammatory response.10 On a broad level, immune cells are thought to play a role in the antitumor response. These immune cells include Langerhans cells (LCs), dermal dendritic cells (DCs), CD4+ and CD8+ T cells, T regulatory cells (Treg), macrophages, and natural killer (NK) cells. For SCC, some cell types typically implicated in tumor eradication, NK, B cells, and monocytes, are rarely detected surrounding and infiltrating tumors.11 However, CD4+ and CD8+ T cells, DCs, and tumor-associated macrophages (TAMs) are often found infiltrating SCC tumors (Fig. 1).11,12 These cells are thought to stunt malignancy and promote regression of primary tumors, but in the context of SCC some of these cell types may be functionally impaired or may exert protumor effects.
Fig. 1.
Distribution of immune cells in normal skin versus cutaneous SCC. Representative immunohistochemistry showing the relative abundance of Langerhans cells (Langerin), Dermal DCs (CD11c+ and CD1c+), and T cells (CD3+ and CD8+). Frozen tissue sections of normal skin and SCCs were stained with the following antibodies: Langerin (Immunotech), CD11C (BD Pharmigen), CD1C/BDCA-1 (Biosource), and CD3 and CD8 (BD Pharmingen). Biotin-labeled horse anti-mouse antibody (Vector Laboratories) was then amplified with avidin-biotin complex (Vector Laboratories) and developed with a chromogen 3-amino-9-ethylcarbazole (Sigma Aldrich). Counterstaining was then carried out with light green (Sigma-Aldrich). With each immunohistochemistry experiment the appropriate isotype controls were performed. (10× magnification). (Adapted from Yanofsky VR, Mitsui H, Felsen D, et al. Understanding dendritic cells and their role in cutaneous carcinoma and cancer immunotherapy. Clin Dev Immunol 2013;2013:624123; and Zhang S, Fujita H, Mitsui H, et al. Increased Tc22 and Treg/CD8 ratio contribute to aggressive growth of transplant associated squamous cell carcinoma. PLoS One 2013;8(5):e62154; with permission.)
T cells are a significant component of the SCC microenvironment.13–17 Gene expression profiling has shown that T-cell activity is decreased in SCC, as shown by downregulation of activation maker CD69 and impaired secretion of granzyme B and inducible nitric oxide synthase (iNOS).10 In addition, myeloid-derived suppressor cells are present in SCC and function to impair T cell–mediated immunity by secreting nitric oxide in the tumor microenvironment. The consequence of this is dampening E-selectin expression on epithelial cells and impairment of T-cell entry into tumors.18 In contrast, human T cells have been reported to secrete interleukin (IL)-24 in response to antigen stimulation, secretion of which has been shown to increase matrix metalloproteinase (MMP)-7 gene expression by SCC cells in culture, potentially contributing to SCC invasion.19,20 Antibody blocking of SCC cells with MMP-7–specific antibody has been shown to significantly delay SCC migration.19
Antigen-presenting cells (APCs) in the skin include epidermal LCs and dermal myeloid DCs. Myeloid DCs from SCC do not stimulate T-cell proliferation, even when cultured in the presence of maturation promoting cytokines such as IL-1β, IL-6, tumor necrosis factor alpha (TNFα) and pros-taglandin E2.21 These data might indicate a significant gap in defense against SCC. This possibility is further supported by the presence of IL-10 and transforming growth factor beta (TGFβ) in the SCC microenvironment and expression of CD200 receptor by SCC-associated DCs.21,22 Each of these has been shown to decrease DC-mediated T-cell responses.23 LCs, located in the epidermal layer, should be the first APCs to encounter SCC tumor antigens generated by transformed keratinocytes. These cells are primarily found in tissues adjacent to and including the interface with the tumor.24 Moreover, LCs from SCC have been shown to stimulate CD4+ and CD8+ T-cell proliferation at rates higher than those from normal skin eliciting a type 1 T-cell response.24 In addition, they express higher levels of LC maturation markers, CD40, CD80, CD83, and CD86. This finding might indicate an antitumor role for LCs in human SCC. In contrast, diphtheria toxin depletion of LCs in mice decreases ultraviolet B (UVB)–induced SCC tumor growth.25 These data suggest that LC responses somehow contribute to SCC tumor growth. LCs from SCC have been reported to express immune-activating signal transducer and activator of transcription (STAT) 4, IL-15, and CD80, and immune tolerizing CD200 genes.24 Therefore, it is possible that LCs can have protumor roles in the context of SCC, despite their ability to elicit T-cell responses.
An abundance of TAMs are found surrounding as well as penetrating SCC.26 TAMs may play a dual role in the tumor microenvironment, because they maintain the potential to eradicate tumor cells but have been shown to promote tumor growth. TAMs in the SCC microenvironment have been shown to be classically activated M1 marker, alternatively activated M2 marker, or biactivated M1 and M2 marker expressing (Fig. 2).27,28 M1 activation is driven through the type 1 T helper (Th1) cytokine interferon gamma (IFNγ),27,29 whereas M2 activation occurs as a result of type 2 T helper (Th2) cytokines, which include IL-10, IL-13, and IL-4.29 Although M1 macrophage responses are thought to prevent tumor growth, the predominant subtype found in SCC tumors are M2 macrophages, which have lower antigen-processing capacity.30 In addition, they have been shown to negatively correlate with tumor growth and progression. The authors found that SCC TAMs express MMP-11, MMP-9, STAT1, STAT6, and vascular endothelial growth factor C (VEGF-C), which have been shown to promote increased lymphatic vessel density that is correlated with increased metastasis.31–33 Hence, SCC TAMs may show poor antigen-presentation capacity in addition to secreting factors that may potentiate tumor progression.
Fig. 2.
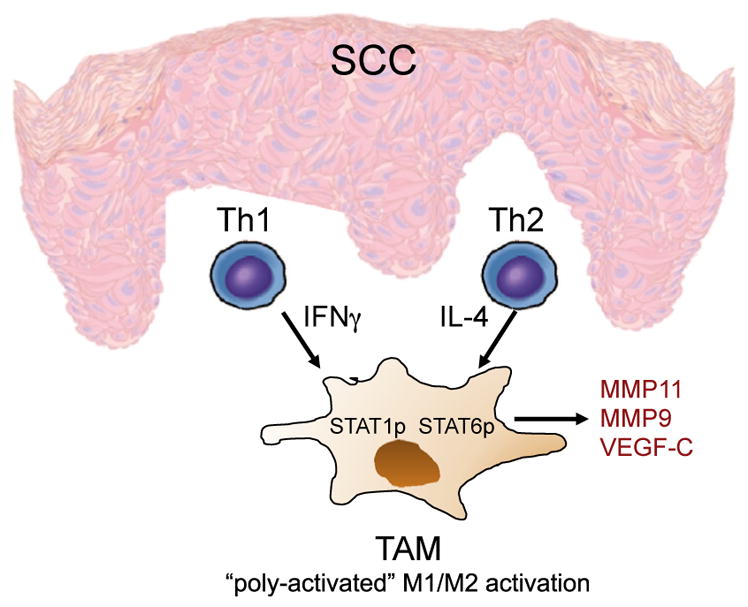
Model of cutaneous SCC macrophage polarization. Th1 and Th2 cells in the microenvironment produce interferon gamma (IFNγ) and IL-4, respectively. These cytokines stimulate M1 classic and M2 alternative phenotypes, resulting in poly activated TAMs. TAMs are characterized by the presence of phosphor-ylated STAT1 and STAT6 and expression of MMP-11, MMP-9, and vascular endothelial growth factor C (VEGF-C).
TRANSPLANT-ASSOCIATED SQUAMOUS CELL CARCINOMA
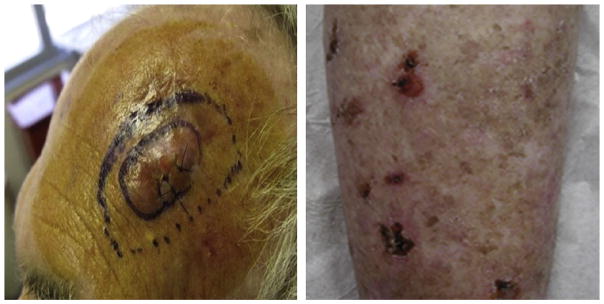
Solid organ transplant recipients (OTRs) represent a particularly high-risk population for the development of SCC. Immunosuppressive therapy used by OTRs to prevent allograft rejection predisposes patients to cutaneous infections and neoplasms.34 OTRs are 60 to 100 times more likely to develop transplant-associated SCC (TSCC) compared with age-matched immune-competent populations.4,35 Patients with TSCC may experience catastrophic body surface area involvement with metastatic rates as high as 8% (Fig. 3).3,36–39
Fig. 3.
TSCC. In transit metastatic SCC is defined by the presence of dermal or subcutaneous papules or nodules that occur between the site of the primary tumor and lymph node basin. This patient (left) presented with in transit metastases 6 weeks after resection of a high-risk, primary SCC. Some patients with catastrophic carcinomatosis develop extensive field disease with confluent AK, in situ SCCs involving more than 50% body surface area (right). (From Belkin D, Carucci JA. Mohs surgery for squamous cell carcinoma. Dermatol Clin 2011;29(2):169; with permission.)
Similar to SCC in immune-competent patients, TSCC tumors were shown to have heightened levels of inflammatory gene expression measured using gene set enrichment analysis.40 More recently, the authors showed that TSCC tumors contain a higher percentage of IL-22–producing CD8+ T cells and increased expression of the IL-22 receptor.41 IL-22 has been shown to promote SCC proliferation in a dose-dependent manner in vitro. In addition, IL-22 drives inflammation and suppresses keratinocyte apoptosis; this may explain the increased proliferation and poorer outcomes observed in patients with TSCC.41,42
GENERAL OVERVIEW OF INTERLEUKIN-22 SIGNALING
Interleukin (IL) 22 is a cytokine that was discovered in 2000 after being cloned from activated T cells.43–45 IL-22 targets cell types comprising outer-body barriers such as respiratory and intestinal epithelial cells, hepatocytes, and keratinocytes, but not cells of hematopoietic origin. The cytokine plays an important role in tissue homeostasis, tissue repair, and wound healing.43 Moreover, its expression has been linked to the development and progression of multiple carcinomas.46–51
IL-22 is characterized as an IL-10 cytokine family member along with IL-10, IL-20, IL-24, and IL-26.43,52,53 The cytokine signals via the heterodimeric transmembrane IL22 receptor (IL-22R) 1 and IL-10R2, and shares IL-22R1 with 2 other IL-10 family members, IL-20 and IL-24.45,54 Signaling through the IL-22 receptor primarily induces Janus kinase activity and STAT transcription factors. In primary cells, STAT3 activation is the main event observed, along with weak activation of STAT1 and/or STAT5.53 In addition, activation of mitogen-activated protein kinase (MAPK), phosphoinositide 3-kinase (PI3K), and AKT–mammalian target of rapamycin (mTOR) also occurs.42,55–59
The cellular sources of IL-22 are CD4+ T cells (Th17, Th22), CD8+ T cells (Tc17, Tc22), γδ T cells, NK T cells, and lymphoid tissue inducer cells.60,61 Although a wide variety of innate and adaptive cell types have been shown to secrete IL-22, CD4+ T cells (Th17 and Th22) and innate lymphoid cells are considered the predominant source of IL-22 in humans. As mentioned earlier, CD4+ and CD8+T cells, often found infiltrating tumors, play an important role in SCC progression.11
ROLE OF INTERLEUKIN-22 IN SQUAMOUS CELL CARCINOMA
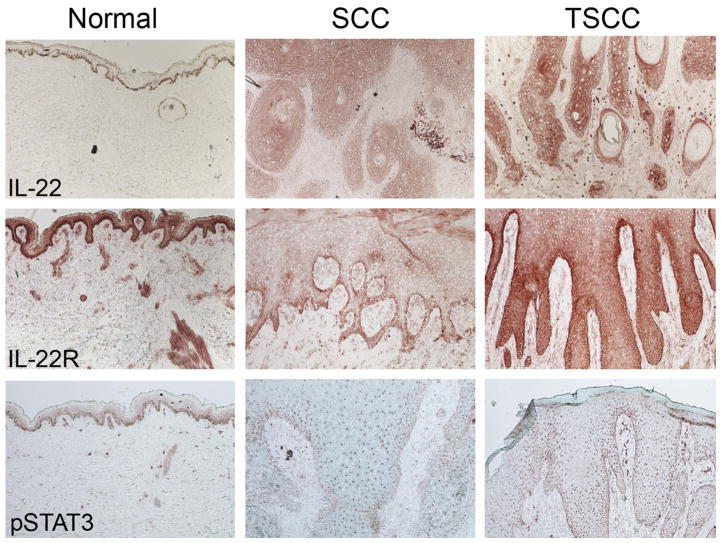
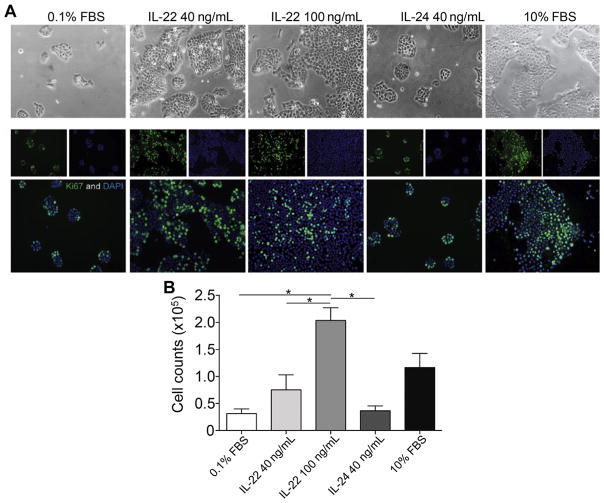
The role of IL-22 in skin cancer is an area of active research. It was reported that cutaneous SCCs contain high numbers of infiltrating IL-22–producing CD4+ T cells, whose recruitment to the tumor partially depended on matrix metalloproteinase 10 (MMP-10) and S100A15 production.62 The authors found IL-22, IL-22R, and downstream mediator phosphorylated STAT3 (pSTAT3) to be highly expressed in SCC (Fig. 4).41 Further, IL-22 treatment of SCC results in enhanced proliferation in vitro (Fig. 5).41 IL-22 has been shown to increase iNOS expression and activity, contributing to proinflammatory as well as proangiogenic properties in colon carcinoma cells.51 In SCC, iNOS expression has been reported and correlates with enhanced tumorigenic potential.63,64 SCC-associated DCs contain a subset of TNFα-positive, iNOS-positive cells.21 Dermal keratinocyte iNOS expression is enhanced by UVB irradiation, which is an environmental factor linked to SCC development.65–69 Further studies are necessary to determine whether IL-22 treatment coupled with UVB irradiation could promote increased SCC tumor growth as a result of iNOS expression. Importantly, administration of IL-22 antibody to SKH-1 animals during UVB-induced cutaneous carcinoma resulted in a decrease in the number and size of SCC tumors.70 This result further confirms the role of IL-22 in promoting SCC tumorigenesis.
Fig. 4.
IL-22 cytokine, receptor, and downstream signaling is enhanced in TSCC. Immunohistochemistry performed on normal, SCC and TSCC shows enhanced expression of IL-22, IL-22R, and pSTAT3. Frozen tissue sections of normal skin and SCCs and TSCCs were stained with the following antibodies: IL-22 (R&D systems), IL-22 Receptor (Prosci), and pSTAT3 (Santa Cruz Biotechnology). Biotin-labeled horse anti-mouse antibody (Vector Laboratories) was then amplified with avidin-biotin complex (Vector Laboratories) and developed with a chromogen 3-amino-9-ethylcarbazole (Sigma Aldrich). Counterstaining was then carried out with light green (Sigma-Aldrich). With each immunohistochemistry experiment the appropriate isotype controls were performed. (10× magnification). (Adapted from Zhang S, Fujita H, Mitsui H, et al. Increased Tc22 and Treg/CD8 ratio contribute to aggressive growth of transplant associated squamous cell carcinoma. PLoS One 2013;8(5):e62154; with permission.)
Fig. 5.
IL-22 treatment enhances human cutaneous SCC proliferation. (A) A431 cells were cultured in 0.1% fetal bovine serum (FBS) serum starvation conditions with or without the indicated cytokines for 72 hours. Cells cultured in the presence of IL-22 cytokine or 10% FBS showed increased colony formation compared with those grown under serum starvation conditions or treated with IL-24. The phase images (below) are representative immunofluorescence images staining for the Ki-67 proliferation marker (green) and nuclear 4,6-diamidino-2-phenylindole (DAPI) (blue). (B) Cell counts were performed after 72 hours in the indicated conditions and show enhanced cell numbers after treatment with control 10% FBS and IL-22 conditions (1-way analysis of variance [ANOVA], P<.001). *p< 0.05, determined by one-way ANOVA. (From Zhang S, Fujita H, Mitsui H, et al. Increased Tc22 and Treg/CD8 ratio contribute to aggressive growth of transplant associated squamous cell carcinoma. PLoS One 2013;8(5):e62154; with permission.)
CHANGES IN THE SQUAMOUS CELL CARCINOMA TUMOR MICROENVIRONMENT INFLUENCED BY CYCLOSPORINE A
OTRs require immune-suppressive drugs to prevent allograft rejection. These drugs include cyclosporine A (CSA), which was considered the mainstay of therapy until recently. CSA prevents T-cell activation by binding cyclophilin molecules, which are needed for calcineurin phosphatase activity. Calcineurin phosphatase activity induces nuclear factor of activated T cell (NFAT) transcription of immune-related cytokine genes, such as IL-2 and IFNγ, expression of which is detrimental to allograft maintenance in OTRs.71–73
Effects of Cyclosporine A on Immune Cell Infiltrates
Differences in immune infiltrates are found in SCC compared with TSCC tumors and have revealed a shift in the microenvironment that favors tumor occurrence and malignancy. The authors have shown that TSCCs have an increased ratio of Treg to cytotoxic CD8+ T cells and a lower percentage of IFNγ-producing CD4+ T cells compared with immune-competent SCC.41 In addition, the Tregs are immune-suppressive cells that are present at a higher proportion in TSCC tumors and may contribute to poor prognosis by suppressing anti-tumor activity and aiding in immune evasion.74–76 CD4+ helper T cells infiltrating TSCC tumors are reduced for IL17A mRNA, a Th17-specific cytokine involved in inflammation that promotes recruitment of innate immune cells.41,77,78
CD8+ T cells producing IFNγ play an important role in combatting aggressive tumor behavior and were shown to be decreased in TSCC compared with SCC.41 Despite decreased proportions of CD8+ T-cell infiltrate, there was an increase in the Tc22 polarization in TSCC tumors.41 The levels of IL-22 cytokine, IL-22R, and phosphorylated STAT3 were dramatically increased in TSCC compared with SCC (see Fig. 4).41 Treatment of peripheral blood mononuclear cell (PBMC)-derived T cells from healthy volunteers cultured with CSA resulted in a decrease in the number of CD4+ and CD8+ IFNγ-producing and IL-17–producing cells but had no effect on those producing IL-4 and IL-22.70 As a result, CSA treatment increases the proportion of T22 cells compared with other T-cell subtypes.
Effects of Cyclosporine A on Squamous Cell Carcinoma
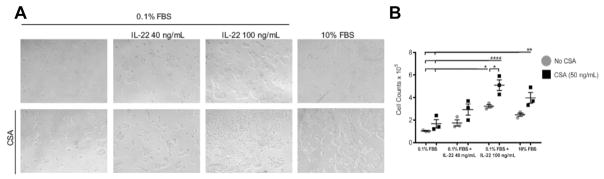
CSA has been implicated in promoting SCC. For instance, CSA treatment has been shown to induce phenotypic changes in SCC cells by promoting tumor growth and increasing invasive potential.79,80 CSA treatment was shown to counteract p53-dependent SCC tumor cell senescence, thereby increasing tumorigenesis.80 In addition, CSA treatment of nude mice, which lack a functional immune system, has been shown to promote the growth of SCC keratinocytes in vivo.81 Moreover, CSA treatment inhibits keratinocyte UVB-induced DNA damage repair and apoptosis by inhibiting NFAT expression.82 CSA is a calcineurin inhibitor that inhibits NFAT expression, a transcription factor that has been shown to promote phosphatase and tensin homolog (PTEN) transcription.83 UV-irradiated keratinocyte growth and survival were increased by CSA treatment through enhancement of AKT activation by suppressing PTEN transcription.81 The mechanism by which CSA inhibits PTEN transcription to promote keratinocyte survival is unknown, because there are no canonical NFAT binding sites in the PTEN promoter. In addition, CSA enhanced IL-22 promotion of SCC growth, resulting in increased cell proliferation, enhanced colony formation, and increased cell numbers (Fig. 6).70 Moreover, CSA and IL-22, alone or in combination, enhanced SCC migratory and invasive potential in vitro.70
Fig. 6.
Cyclosporine treatment potentiates IL-22–mediated human cutaneous SCC proliferation. (A) A431 cells were cultured in 0.1% FBS serum starvation conditions with or without the indicated cytokines for 72 hours. CSA treatment increased growth and colony formation either alone in serum starvation conditions or in the presence of IL-22 cytokine or 10% FBS. (B) Cell counts performed on A431 cells treated in the indicated conditions. CSA treatment increased proliferation alone, in serum starvation conditions, or in the presence of IL-22 cytokine. Data are representative of 3 experiments ± standard error of the mean (1-way ANOVA, P<.001). *p< 0.05, **p< 0.01, ****p< 0.0001 determined by one-way ANOVA with Dunnett’s multiple comparisons test. (From Abikhair M, Mitsui H, Yanofsky V, et al. Cyclosporine A immunosuppression drives catastrophic squamous cell carcinoma through IL-22. JCI Insight 2016;1(8):e86434; with permission.)
SUMMARY
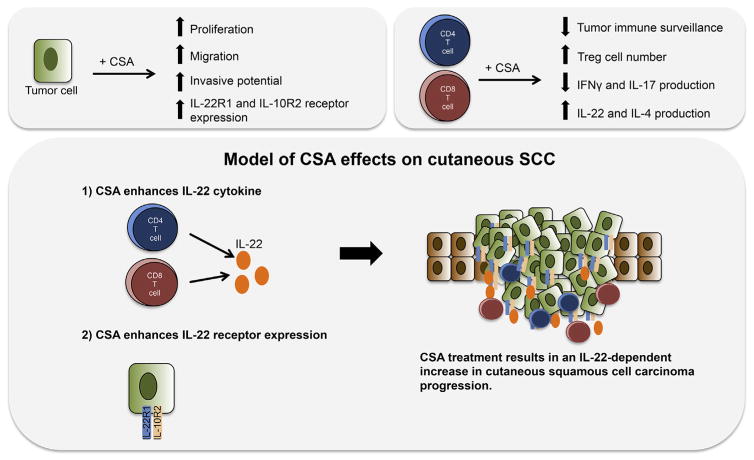
CSA is an immunosuppressive agent widely used to support organ transplant. Until recently, immunosuppression was considered the mechanism by which CSA causes SCC. However, recent studies suggest that CSA treatment favors malignant progression in more ways than previously thought. First, CSA treatment had been shown to increase SCC tumor growth in mice that have no immune system for CSA to suppress.81 CSA functions to enhance the survival and growth of transformed keratinocytes, even promoting invasive capacity.79,80 Mechanistic investigations have shown that CSA treatment reduces PTEN transcription, resulting in enhanced AKT activity and cell survival that depends on mTOR signaling. Further delineating the mechanism by which CSA promotes keratinocyte invasive potential and growth may provide insight into the mechanisms of SCC metastasis. Importantly, CSA treatment modulates the repertoire of immune cells in the SCC tumor microenvironment, promoting increased numbers of IL-22–producing immune infiltrates, and treatment of PBMCs with CSA results in enhanced IL-22 production from CD4+ and CD8+ T cells.70 Because IL-22 has been shown to promote SCC cell growth, migration, and proinflammatory cytokine release, enhanced amounts of IL-22 in the tumor microenvironment of CSA-treated patients could have negative effects (Fig. 7). It is possible that CSA acts through the IL-22 axis to drive aggressive behavior by SCC. Further elucidation of the IL-22 axis and its effects on SCC proliferation, migration, and invasion may provide novel rational targets for therapeutic intervention.
Fig. 7.
Model of cyclosporine effects on cutaneous SCC. It is possible that CSA acts through the IL-22 axis to drive aggressive behavior by SCC. CSA treatment promotes SCC growth, invasive capacity, and IL-22 receptor expression. The authors have previously shown that IL-22 treatment promotes SCC growth. In addition to decreased tumor immune surveillance, CSA treatment enhances Treg numbers and IL-22 production by CD4+ and CD8+ T cells. Therefore, higher IL-22 receptor expression on SCC tumor cells and increased levels of IL-22 cytokine produced by CD8+ and CD4+ T cells may contribute to tumor growth.
KEY POINTS.
Cutaneous squamous cell carcinoma (SCC) is frequently curable, but causes significant morbidity and mortality, particularly in immune-suppressed organ transplant recipients.
The SCC tumor microenvironment is rich and composed of multiple subsets of immune cells that exert antitumor and protumor effects.
Interleukin (IL)-22 and its receptor are highly expressed in SCC and more so in transplant-associated SCC.
IL-22 mediates SCC proliferation; an effect that is potentiated by cyclosporine.
IL-22 blockade results in decreased SCC burden in a murine model system in vivo and may represent a therapeutic target.
Abbreviations and acronyms
- AK
Actinic keratosis
- APC
Antigen-presenting cell
- CSA
Cyclosporine A
- DC
Dendritic cells
- IL
Interleukin
- iNOS
Inducible nitric oxide synthase
- LC
Langerhans cell
- mTOR
AKT-mammalian target of rapamycin
- NFAT
Nuclear factor of activated T cells
- NK
Natural killer cell
- OTR
Organ transplant recipient
- MMP
Matrix metalloproteinase
- SCC
Squamous cell carcinoma
- STAT
Signal transduced and activator of transcription
- TAM
Tumor-associated macrophage
- TNF
Tumor necrosis factor
- Treg
Regulatory T cell
- TSCC
Transplant-associated squamous cell carcinoma
- VEGF
Vascular endothelial growth factor
Footnotes
Conflict of Interest: The authors have no conflicts of interest.
References
- 1.Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. J Am Acad Dermatol. 2013;68(6):957–66. doi: 10.1016/j.jaad.2012.11.037. [DOI] [PubMed] [Google Scholar]
- 2.Weinberg AS, Ogle CA, Shim EK. Metastatic cutaneous squamous cell carcinoma: an update. Dermatol Surg. 2007;33(8):885–99. doi: 10.1111/j.1524-4725.2007.33190.x. [DOI] [PubMed] [Google Scholar]
- 3.Berg D, Otley CC. Skin cancer in organ transplant recipients: Epidemiology, pathogenesis, and management. J Am Acad Dermatol. 2002;47(1):1–17. doi: 10.1067/mjd.2002.125579. quiz: 18–20. [DOI] [PubMed] [Google Scholar]
- 4.Lindelof B, Sigurgeirsson B, Gabel H, et al. Incidence of skin cancer in 5356 patients following organ transplantation. Br J Dermatol. 2000;143(3):513–9. [PubMed] [Google Scholar]
- 5.Brantsch KD, Meisner C, Schonfisch B, et al. Analysis of risk factors determining prognosis of cutaneous squamous-cell carcinoma: a prospective study. Lancet Oncol. 2008;9(8):713–20. doi: 10.1016/S1470-2045(08)70178-5. [DOI] [PubMed] [Google Scholar]
- 6.Czarnecki D, Staples M, Mar A, et al. Metastases from squamous cell carcinoma of the skin in southern Australia. Dermatology. 1994;189(1):52–4. doi: 10.1159/000246783. [DOI] [PubMed] [Google Scholar]
- 7.Samarasinghe V, Madan V. Nonmelanoma skin cancer. J Cutan Aesthet Surg. 2012;5(1):3–10. doi: 10.4103/0974-2077.94323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dajee M, Lazarov M, Zhang JY, et al. NF-kappaB blockade and oncogenic Ras trigger invasive human epidermal neoplasia. Nature. 2003;421(6923):639–43. doi: 10.1038/nature01283. [DOI] [PubMed] [Google Scholar]
- 9.Lazarov M, Kubo Y, Cai T, et al. CDK4 coexpression with Ras generates malignant human epidermal tumorigenesis. Nat Med. 2002;8(10):1105–14. doi: 10.1038/nm779. [DOI] [PubMed] [Google Scholar]
- 10.Haider AS, Peters SB, Kaporis H, et al. Genomic analysis defines a cancer-specific gene expression signature for human squamous cell carcinoma and distinguishes malignant hyperproliferation from benign hyperplasia. J Invest Dermatol. 2006;126(4):869–81. doi: 10.1038/sj.jid.5700157. [DOI] [PubMed] [Google Scholar]
- 11.Terao H, Nakayama J, Urabe A, et al. Immunohistochemical characterization of cellular infiltrates in squamous cell carcinoma and Bowen’s disease occurring in one patient. J Dermatol. 1992;19(7):408–13. doi: 10.1111/j.1346-8138.1992.tb03250.x. [DOI] [PubMed] [Google Scholar]
- 12.Yanofsky VR, Mitsui H, Felsen D, et al. Understanding dendritic cells and their role in cutaneous carcinoma and cancer immunotherapy. Clin Dev Immunol. 2013;2013:624123. doi: 10.1155/2013/624123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho Y, Miyamoto M, Kato K, et al. CD4+ and CD8+ T cells cooperate to improve prognosis of patients with esophageal squamous cell carcinoma. Cancer Res. 2003;63(7):1555–9. [PubMed] [Google Scholar]
- 14.Claudatus JC, Jr, d’Ovidio R, Lospalluti M, et al. Skin tumors and reactive cellular infiltrate: further studies. Acta Derm Venereol. 1986;66(1):29–34. [PubMed] [Google Scholar]
- 15.De Panfilis G, Colli V, Manfredi G, et al. In situ identification of mononuclear cells infiltrating cutaneous carcinoma: an immunohistochemical study. Acta Derm Venereol. 1979;59(3):219–22. [PubMed] [Google Scholar]
- 16.Gatter KC, Morris HB, Roach B, et al. Langerhans’ cells and T cells in human skin tumours: an immunohistological study. Histopathology. 1984;8(2):229–44. doi: 10.1111/j.1365-2559.1984.tb02338.x. [DOI] [PubMed] [Google Scholar]
- 17.Sznurkowski JJ, Zawrocki A, Emerich J, et al. Prognostic significance of CD4+ and CD8+ T cell infiltration within cancer cell nests in vulvar squamous cell carcinoma. Int J Gynecol Cancer. 2011;21(4):717–21. doi: 10.1097/IGC.0b013e3182131f36. [DOI] [PubMed] [Google Scholar]
- 18.Gehad AE, Lichtman MK, Schmults CD, et al. Nitric oxide-producing myeloid-derived suppressor cells inhibit vascular E-selectin expression in human squamous cell carcinomas. J Invest Dermatol. 2012;132(11):2642–51. doi: 10.1038/jid.2012.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitsui H, Suarez-Farinas M, Gulati N, et al. Gene expression profiling of the leading edge of cutaneous squamous cell carcinoma: IL-24-driven MMP-7. J Invest Dermatol. 2014;134(5):1418–27. doi: 10.1038/jid.2013.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poindexter NJ, Walch ET, Chada S, et al. Cytokine induction of interleukin-24 in human peripheral blood mononuclear cells. J Leukoc Biol. 2005;78(3):745–52. doi: 10.1189/jlb.0205116. [DOI] [PubMed] [Google Scholar]
- 21.Bluth MJ, Zaba LC, Moussai D, et al. Myeloid dendritic cells from human cutaneous squamous cell carcinoma are poor stimulators of T-cell proliferation. J Invest Dermatol. 2009;129(10):2451–62. doi: 10.1038/jid.2009.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belkin DA, Mitsui H, Wang CQ, et al. CD200 upregulation in vascular endothelium surrounding cutaneous squamous cell carcinoma. JAMA Dermatol. 2013;149(2):178–86. doi: 10.1001/jamadermatol.2013.1609. [DOI] [PubMed] [Google Scholar]
- 23.Wallet MA, Sen P, Tisch R. Immunoregulation of dendritic cells. Clin Med Res. 2005;3(3):166–75. doi: 10.3121/cmr.3.3.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujita H, Suarez-Farinas M, Mitsui H, et al. Langerhans cells from human cutaneous squamous cell carcinoma induce strong type 1 immunity. J Invest Dermatol. 2012;132(6):1645–55. doi: 10.1038/jid.2012.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewis JM, Burgler CD, Freudzon M, et al. Langerhans cells facilitate UVB-Induced epidermal carcinogenesis. J Invest Dermatol. 2015;135(11):2824–33. doi: 10.1038/jid.2015.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pettersen JS, Fuentes-Duculan J, Suarez-Farinas M, et al. Tumor-associated macrophages in the cutaneous SCC microenvironment are heterogeneously activated. J Invest Dermatol. 2011;131(6):1322–30. doi: 10.103/jid.2011.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mantovani A, Sica A, Sozzani S, et al. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25(12):677–86. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 28.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8(12):958–69. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Romagnani S. T-cell subsets (Th1 versus Th2) Ann Allergy Asthma Immunol. 2000;85(1):9–18. doi: 10.1016/S1081-1206(10)62426-X. quiz 18, 21. [DOI] [PubMed] [Google Scholar]
- 30.Allavena P, Sica A, Garlanda C, et al. The Yin-Yang of tumor-associated macrophages in neoplastic progression and immune surveillance. Immunol Rev. 2008;222:155–61. doi: 10.1111/j.1600-065X.2008.00607.x. [DOI] [PubMed] [Google Scholar]
- 31.Boone B, Blokx W, De Bacquer D, et al. The role of VEGF-C staining in predicting regional metastasis in melanoma. Virchows Arch. 2008;453(3):257–65. doi: 10.1007/s00428-008-0641-6. [DOI] [PubMed] [Google Scholar]
- 32.Moussai D, Mitsui H, Pettersen JS, et al. The human cutaneous squamous cell carcinoma microenvironment is characterized by increased lymphatic density and enhanced expression of macrophage-derived VEGF-C. J Invest Dermatol. 2011;131(1):229–36. doi: 10.1038/jid.2010.266. [DOI] [PubMed] [Google Scholar]
- 33.Sugiura T, Inoue Y, Matsuki R, et al. VEGF-C and VEGF-D expression is correlated with lymphatic vessel density and lymph node metastasis in oral squamous cell carcinoma: implications for use as a prognostic marker. Int J Oncol. 2009;34(3):673–80. doi: 10.3892/ijo_00000193. [DOI] [PubMed] [Google Scholar]
- 34.Euvrard S, Kanitakis J, Claudy A. Skin cancers after organ transplantation. N Engl J Med. 2003;348(17):1681–91. doi: 10.1056/NEJMra022137. [DOI] [PubMed] [Google Scholar]
- 35.Gerlini G, Romagnoli P, Pimpinelli N. Skin cancer and immunosuppression. Crit Rev Oncol Hematol. 2005;56(1):127–36. doi: 10.1016/j.critrevonc.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 36.Belkin D, Carucci JA. Mohs surgery for squamous cell carcinoma. Dermatol Clin. 2011;29(2):161–74. vii. doi: 10.1016/j.det.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 37.Campo E, Swerdlow SH, Harris NL, et al. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011;117(19):5019–32. doi: 10.1182/blood-2011-01-293050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carucci JA. Cutaneous oncology in organ transplant recipients: meeting the challenge of squamous cell carcinoma. J Invest Dermatol. 2004;123(5):809–16. doi: 10.1111/j.1523-1747.2004.23440.x. [DOI] [PubMed] [Google Scholar]
- 39.Ong CS, Keogh AM, Kossard S, et al. Skin cancer in Australian heart transplant recipients. J Am Acad Dermatol. 1999;40(1):27–34. doi: 10.1016/s0190-9622(99)70525-6. [DOI] [PubMed] [Google Scholar]
- 40.Kosmidis M, Dziunycz P, Suarez-Farinas M, et al. Immunosuppression affects CD4+ mRNA expression and induces Th2 dominance in the microenvironment of cutaneous squamous cell carcinoma in organ transplant recipients. J Immunother. 2010;33(5):538–46. doi: 10.1097/CJI.0b013e3181cc2615. [DOI] [PubMed] [Google Scholar]
- 41.Zhang S, Fujita H, Mitsui H, et al. Increased Tc22 and Treg/CD8 ratio contribute to aggressive growth of transplant associated squamous cell carcinoma. PLoS One. 2013;8(5):e62154. doi: 10.1371/journal.pone.0062154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolk K, Witte E, Wallace E, et al. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur J Immunol. 2006;36(5):1309–23. doi: 10.1002/eji.200535503. [DOI] [PubMed] [Google Scholar]
- 43.Dumoutier L, Louahed J, Renauld JC. Cloning and characterization of IL-10-related T cell-derived inducible factor (IL-TIF), a novel cytokine structurally related to IL-10 and inducible by IL-9. J Immunol. 2000;164(4):1814–9. doi: 10.4049/jimmunol.164.4.1814. [DOI] [PubMed] [Google Scholar]
- 44.Dumoutier L, Van Roost E, Colau D, et al. Human interleukin-10-related T cell-derived inducible factor: molecular cloning and functional characterization as an hepatocyte-stimulating factor. Proc Natl Acad Sci U S A. 2000;97(18):10144–9. doi: 10.1073/pnas.170291697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xie MH, Aggarwal S, Ho WH, et al. Interleukin (IL)-22, a novel human cytokine that signals through the interferon receptor-related proteins CRF2–4 and IL-22R. J Biol Chem. 2000;275(40):31335–9. doi: 10.1074/jbc.M005304200. [DOI] [PubMed] [Google Scholar]
- 46.Kim MJ, Jang JW, Oh BS, et al. Change in inflammatory cytokine profiles after transarterial chemotherapy in patients with hepatocellular carcinoma. Cytokine. 2013;64(2):516–22. doi: 10.1016/j.cyto.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 47.Qin S, Ma S, Huang X, et al. Th22 cells are associated with hepatocellular carcinoma development and progression. Chin J Cancer Res. 2014;26(2):135–41. doi: 10.3978/j.issn.1000-9604.2014.02.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Souza JM, Matias BF, Rodrigues CM, et al. IL-17 and IL-22 serum cytokine levels in patients with squamous intraepithelial lesion and invasive cervical carcinoma. Eur J Gynaecol Oncol. 2013;34(5):466–8. [PubMed] [Google Scholar]
- 49.Wen Z, Liao Q, Zhao J, et al. High expression of interleukin-22 and its receptor predicts poor prognosis in pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2014;21(1):125–32. doi: 10.1245/s10434-013-3322-x. [DOI] [PubMed] [Google Scholar]
- 50.Zhang W, Chen Y, Wei H, et al. Antiapoptotic activity of autocrine interleukin-22 and therapeutic effects of interleukin-22-small interfering RNA on human lung cancer xenografts. Clin Cancer Res. 2008;14(20):6432–9. doi: 10.1158/1078-0432.CCR-07-4401. [DOI] [PubMed] [Google Scholar]
- 51.Ziesche E, Bachmann M, Kleinert H, et al. The interleukin-22/STAT3 pathway potentiates expression of inducible nitric-oxide synthase in human colon carcinoma cells. J Biol Chem. 2007;282(22):16006–15. doi: 10.1074/jbc.M611040200. [DOI] [PubMed] [Google Scholar]
- 52.Ouyang W, Rutz S, Crellin NK, et al. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol. 2011;29:71–109. doi: 10.1146/annurev-immunol-031210-101312. [DOI] [PubMed] [Google Scholar]
- 53.Wolk K, Kunz S, Witte E, et al. IL-22 increases the innate immunity of tissues. Immunity. 2004;21(2):241–54. doi: 10.1016/j.immuni.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 54.Kotenko SV, Izotova LS, Mirochnitchenko OV, et al. Identification of the functional interleukin-22 (IL-22) receptor complex: the IL-10R2 chain (IL-10Rbeta) is a common chain of both the IL-10 and IL-22 (IL-10-related T cell-derived inducible factor, IL-TIF) receptor complexes. J Biol Chem. 2001;276(4):2725–32. doi: 10.1074/jbc.M007837200. [DOI] [PubMed] [Google Scholar]
- 55.Andoh A, Zhang Z, Inatomi O, et al. Interleukin-22, a member of the IL-10 subfamily, induces inflammatory responses in colonic subepithelial myofibroblasts. Gastroenterology. 2005;129(3):969–84. doi: 10.1053/j.gastro.2005.06.071. [DOI] [PubMed] [Google Scholar]
- 56.Ikeuchi H, Kuroiwa T, Hiramatsu N, et al. Expression of interleukin-22 in rheumatoid arthritis: potential role as a proinflammatory cytokine. Arthritis Rheum. 2005;52(4):1037–46. doi: 10.1002/art.20965. [DOI] [PubMed] [Google Scholar]
- 57.Lejeune D, Dumoutier L, Constantinescu S, et al. Interleukin-22 (IL-22) activates the JAK/STAT, ERK, JNK, and p38 MAP kinase pathways in a rat hepatoma cell line. Pathways that are shared with and distinct from IL-10. J Biol Chem. 2002;277(37):33676–82. doi: 10.1074/jbc.M204204200. [DOI] [PubMed] [Google Scholar]
- 58.Mitra A, Raychaudhuri SK, Raychaudhuri SP. IL-22 induced cell proliferation is regulated by PI3K/Akt/mTOR signaling cascade. Cytokine. 2012;60(1):38–42. doi: 10.1016/j.cyto.2012.06.316. [DOI] [PubMed] [Google Scholar]
- 59.Zhu X, Li Z, Pan W, et al. Participation of Gab1 and Gab2 in IL-22-mediated keratinocyte proliferation, migration, and differentiation. Mol Cell Biochem. 2012;369(1–2):255–66. doi: 10.1007/s11010-012-1389-5. [DOI] [PubMed] [Google Scholar]
- 60.Rutz S, Eidenschenk C, Ouyang W. IL-22, not simply a Th17 cytokine. Immunol Rev. 2013;252(1):116–32. doi: 10.1111/imr.12027. [DOI] [PubMed] [Google Scholar]
- 61.Wolk K, Kunz S, Asadullah K, et al. Cutting edge: immune cells as sources and targets of the IL-10 family members? J Immunol. 2002;168(11):5397–402. doi: 10.4049/jimmunol.168.11.5397. [DOI] [PubMed] [Google Scholar]
- 62.Briso EM, Guinea-Viniegra J, Bakiri L, et al. Inflammation-mediated skin tumorigenesis induced by epidermal c-Fos. Genes Dev. 2013;27(18):1959–73. doi: 10.1101/gad.223339.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen YK, Hsue SS, Lin LM. Increased expression of inducible nitric oxide synthase for human buccal squamous-cell carcinomas: immunohistochemical, reverse transcription-polymerase chain reaction (RT-PCR) and in situ RT-PCR studies. Head Neck. 2002;24(10):925–32. doi: 10.1002/hed.10131. [DOI] [PubMed] [Google Scholar]
- 64.Connelly ST, Macabeo-Ong M, Dekker N, et al. Increased nitric oxide levels and iNOS over-expression in oral squamous cell carcinoma. Oral Oncol. 2005;41(3):261–7. doi: 10.1016/j.oraloncology.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 65.Baudouin JE, Tachon P. Constitutive nitric oxide synthase is present in normal human keratinocytes. J Invest Dermatol. 1996;106(3):428–31. doi: 10.1111/1523-1747.ep12343523. [DOI] [PubMed] [Google Scholar]
- 66.Chang HR, Tsao DA, Wang SR, et al. Expression of nitric oxide synthases in keratinocytes after UVB irradiation. Arch Dermatol Res. 2003;295(7):293–6. doi: 10.1007/s00403-003-0433-4. [DOI] [PubMed] [Google Scholar]
- 67.Sasaki M, Yamaoka J, Miyachi Y. The effect of ultraviolet B irradiation on nitric oxide synthase expression in murine keratinocytes. Exp Dermatol. 2000;9(6):417–22. doi: 10.1034/j.1600-0625.2000.009006417.x. [DOI] [PubMed] [Google Scholar]
- 68.Seo SJ, Choi HG, Chung HJ, et al. Time course of expression of mRNA of inducible nitric oxide synthase and generation of nitric oxide by ultraviolet B in keratinocyte cell lines. Br J Dermatol. 2002;147(4):655–62. doi: 10.1046/j.1365-2133.2002.04849.x. [DOI] [PubMed] [Google Scholar]
- 69.Shimizu Y, Sakai M, Umemura Y, et al. Immunohistochemical localization of nitric oxide synthase in normal human skin: expression of endothelial-type and inducible-type nitric oxide synthase in keratinocytes. J Dermatol. 1997;24(2):80–7. doi: 10.1111/j.1346-8138.1997.tb02748.x. [DOI] [PubMed] [Google Scholar]
- 70.Abikhair M, Mitsui H, Yanofsky V, et al. Cyclosporine A immunosuppression drives catastrophic squamous cell carcinoma through IL-22. JCI Insight. 2016;1(8):1–12. doi: 10.1172/jci.insight.86434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Granelli-Piperno A. In situ hybridization for interleukin 2 and interleukin 2 receptor mRNA in T cells activated in the presence or absence of cyclosporin A. J Exp Med. 1988;168(5):1649–58. doi: 10.1084/jem.168.5.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Herold KC, Lancki DW, Moldwin RL, et al. Immunosuppressive effects of cyclosporin A on cloned T cells. J Immunol. 1986;136(4):1315–21. [PubMed] [Google Scholar]
- 73.Kronke M, Leonard WJ, Depper JM, et al. Cyclosporin A inhibits T-cell growth factor gene expression at the level of mRNA transcription. Proc Natl Acad Sci U S A. 1984;81(16):5214–8. doi: 10.1073/pnas.81.16.5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Beyer M, Kochanek M, Giese T, et al. In vivo peripheral expansion of naive CD4+CD25high FoxP3+ regulatory T cells in patients with multiple myeloma. Blood. 2006;107(10):3940–9. doi: 10.1182/blood-2005-09-3671. [DOI] [PubMed] [Google Scholar]
- 75.Mougiakakos D, Choudhury A, Lladser A, et al. Regulatory T cells in cancer. Adv Cancer Res. 2010;107:57–117. doi: 10.1016/S0065-230X(10)07003-X. [DOI] [PubMed] [Google Scholar]
- 76.Rutella S, Lemoli RM. Regulatory T cells and tolerogenic dendritic cells: from basic biology to clinical applications. Immunol Lett. 2004;94(1–2):11–26. doi: 10.1016/j.imlet.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 77.Oukka M. Th17 cells in immunity and autoimmunity. Ann Rheum Dis. 2008;67(Suppl 3):iii26–9. doi: 10.1136/ard.2008.098004. [DOI] [PubMed] [Google Scholar]
- 78.Wilson NJ, Boniface K, Chan JR, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8(9):950–7. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 79.Hojo M, Morimoto T, Maluccio M, et al. Cyclosporine induces cancer progression by a cell-autonomous mechanism. Nature. 1999;397(6719):530–4. doi: 10.1038/17401. [DOI] [PubMed] [Google Scholar]
- 80.Wu X, Nguyen BC, Dziunycz P, et al. Opposing roles for calcineurin and ATF3 in squamous skin cancer. Nature. 2010;465(7296):368–72. doi: 10.1038/nature08996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Han W, Ming M, He TC, et al. Immunosuppressive cyclosporin A activates AKT in keratinocytes through PTEN suppression: implications in skin carcinogenesis. J Biol Chem. 2010;285(15):11369–77. doi: 10.1074/jbc.M109.028142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yarosh DB, Pena AV, Nay SL, et al. Calcineurin inhibitors decrease DNA repair and apoptosis in human keratinocytes following ultraviolet B irradiation. J Invest Dermatol. 2005;125(5):1020–5. doi: 10.1111/j.0022-202X.2005.23858.x. [DOI] [PubMed] [Google Scholar]
- 83.Wang Q, Zhou Y, Jackson LN, et al. Nuclear factor of activated T cells (NFAT) signaling regulates PTEN expression and intestinal cell differentiation. Mol Biol Cell. 2011;22(3):412–20. doi: 10.1091/mbc.E10-07-0598. [DOI] [PMC free article] [PubMed] [Google Scholar]