Abstract
Objective:
To report the presentation, main syndromes, human leukocyte antigen (HLA) association, and immunoglobulin G (IgG) subclass in the anti-IgLON5 disease: a disorder with parasomnias, sleep apnea, and IgLON5 antibodies.
Methods:
This was a retrospective clinical analysis of 22 patients. The IgG subclass was determined using reported techniques.
Results:
Patients' median age was 64 years (range 46–83). Symptoms that led to initial consultation included sleep problems (8 patients; 36%), gait abnormalities (8; 36%), bulbar dysfunction (3; 14%), chorea (2; 9%), and cognitive decline (1; 5%). By the time of diagnosis of the disorder, 4 syndromes were identified: (1) a sleep disorder with parasomnia and sleep breathing difficulty in 8 (36%) patients; (2) a bulbar syndrome including dysphagia, sialorrhea, stridor, or acute respiratory insufficiency in 6 (27%); (3) a syndrome resembling progressive supranuclear palsy (PSP-like) in 5 (23%); and (4) cognitive decline with or without chorea in 3 (14%). All patients eventually developed parasomnia, sleep apnea, insomnia, or excessive daytime sleepiness. HLA-DRB1*10:01 and HLA-DQB1*05:01 were positive in 13/15 (87%) patients; the DRB1*10:01 allele was 36 times more prevalent than in the general population. Among 16 patients with paired serum and CSF samples, 14 had IgLON5 antibodies in both, and 2 only in serum (both had a PSP-like syndrome). Twenty of 21 patients had IgG1 and IgG4 antibodies; the latter predominated in 16.
Conclusions:
Patients with IgLON5 antibodies develop a characteristic sleep disorder preceded or accompanied by bulbar symptoms, gait abnormalities, oculomotor problems, and, less frequently, cognitive decline. IgG4 subclass antibodies predominate over IgG1; we confirm a strong association with the HLA-DRB1*10:01 allele.
We recently identified a new disorder characterized by non-REM (NREM) and REM parasomnias, obstructive sleep apnea (OSA), and stridor that occur in association with antibodies against extracellular epitopes of IgLON5, a neuronal cell adhesion protein of unknown function. Symptoms are progressive and can lead to life-threatening respiratory problems such as central hypoventilation. This clinical picture frequently suggests a chronic sleep disorder or neurodegenerative disease and unless the physician is familiar with it, the presence of an underlying autoantibody is not considered. Additional features include association with human leukocyte antigen (HLA)–DRB1*10:01 and HLA-DQB1*05:01 alleles and postmortem findings consistent with a novel neuronal tauopathy predominantly involving the hypothalamus and tegmentum of the brainstem.1,2 These findings place this disorder (named anti-IgLON5 disease) at the convergence of neurodegenerative and autoimmune mechanisms.
In addition to the distinctive sleep disorder, there are other symptoms that have not been reported or described in detail. The importance of these symptoms is that they may surpass or precede the development of the sleep disorder and therefore lead to the initial visit to the physician. We reasoned that early diagnosis is important not only to avoid unnecessary tests, but to prevent complications (acute respiratory failure), and may have the potential to improve outcome if immunotherapy is implemented early. With the aim to increase recognition of this disease, we describe 22 patients, focusing on the presenting symptoms, main syndromes, outcome, HLA genotyping, and antibody immunoglobulin G (IgG) subclass.
METHODS
Patients and data collection.
We reviewed the clinical data of all patients with IgLON5 antibodies identified in the Neuroimmunology Laboratory of the Institute of Biomedical Research August Pi i Sunyer (IDIBAPS), Hospital Clinic (Barcelona, Spain). Clinical information was provided by the referring physician through a structured written questionnaire obtained at the time of diagnosis and subsequent follow-up. The information was included in a database that was returned to the referring physician who validated the data.
Clinical information included the reason the patient sought medical attention, predominant neurologic syndrome, and temporal progression of the disease. We also investigated if sleep symptoms were present at the initial visit and if they were spontaneously reported by the patient or bed partner, or only reported after direct questioning. This focused on the presence of vocalizations and abnormal behaviors during sleep, sleep breathing difficulty, and complaints of insomnia and excessive daytime sleepiness. Other symptoms assessed included cognitive deterioration, gait disturbance, disequilibrium (impaired balance, unexpected falls), cerebellar symptoms, parkinsonism, chorea and other movement disorders, supranuclear gaze dysfunction and other oculomotor abnormalities, bulbar symptoms (dysphagia, dysarthria, central hypoventilation, stridor), and dysautonomic features (urinary problems, orthostatic hypotension). The diagnosis of stridor during sleep required confirmation by polysomnography (PSG) with audio recording. The diagnosis of vocal cord palsy required confirmation by laryngoscopy. If a patient was deceased, information on the cause and circumstances of death was obtained. The neurologic disability at diagnosis, after treatment, and at the last visit was evaluated using the modified Rankin Scale.3
The main syndrome of each patient at the time of demonstration of IgLON5 antibodies was independently assessed by 3 investigators (C. Gaig, F.G., and Y.C.) using their own data or information provided by the referring physician. Four syndromes were identified and each patient was allocated to one of these. Possible discrepancies were resolved by discussion of the case among investigators. Finally, for each patient, we asked the referring physicians if they were in agreement with the syndrome allocation of their specific patient.
Immunologic studies.
IgLON5 antibodies were screened by immunohistochemistry on frozen sections of rat brain and confirmed by cell-based assay (CBA) using HEK 293 cells transfected with IgLON5 (Origene, Rockville, MD).1 Determination of IgLON5-IgG subclass was performed by CBA with specific fluorescein isothiocyanate–labeled secondary antibodies against the 4 IgG subclasses (The Binding Site, Birmingham, England). The relative percentage of each IgG subclass in an individual serum was calculated by flow cytometry as described in appendix e-1 at Neurology.org. To calculate the risk ratio of the HLA-DRB1*10:01 and HLA-DQB1*05:01 alleles and the development of the anti-IgLON5 disease we compared the frequency of these alleles in the 15 HLA typed patients with the anti-IgLON5 disease and in the Spanish population that presents a similar allele frequency to other Caucasian populations (allelefrequencies.net).4
Standard protocol approvals, registrations, and patient consents.
The ethics committee of the Hospital Clinic approved the study. All patients or proxies gave written informed consent for the storage and use of serum, CSF, and clinical information for research purposes.
RESULTS
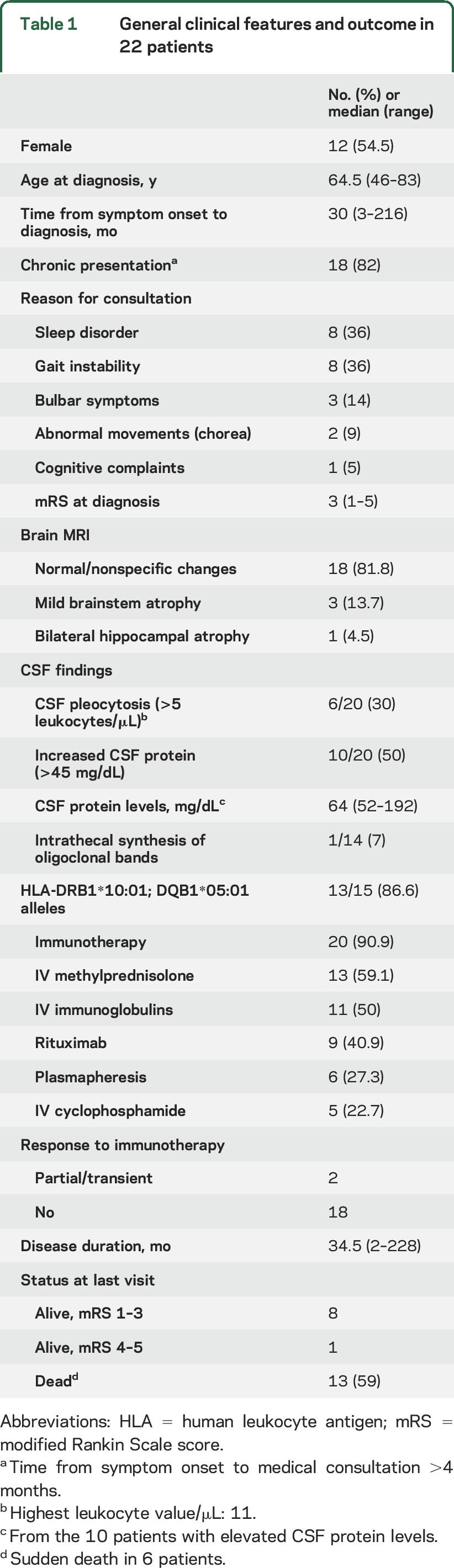
We identified 22 patients with IgLON5 antibodies; 12 of them have been reported in studies more focused on sleep dysfunction, or isolated case reports.1,5–8 The median age at diagnosis was 64 years (range 46–83) and the median time to diagnosis was 30 months (range 3–216). All patients were seen by one or more of the authors with a geographic distribution including Europe (20), Australia (1), and Brazil (1). One patient had autoimmune factor VIII deficiency, and another developed a hypernephroma; none of the other patients had a history of autoimmunity or cancer (table 1).
Table 1.
General clinical features and outcome in 22 patients
Reasons for neurologic consultation.
The reasons for neurologic consultation included sleep disturbances in 8 patients, gait dysfunction in 8, and other symptoms in 6 (3 bulbar, 2 chorea, 1 cognitive decline) (table 1). Among the 14 patients who initially sought medical attention for symptoms other than sleep dysfunction, 7 acknowledged sleep problems suggestive of parasomnia and sleep breathing difficulty. Therefore, 15 (68%) patients had evidence of sleep dysfunction (predominant or overshadowed by other symptoms) at the initial visit. Among the 7 patients without evidence of sleep dysfunction at the initial visit, 4 developed these symptoms over the ensuing years (range 1–8 years).
Frequency of symptoms and main syndromes during the course of the disease.
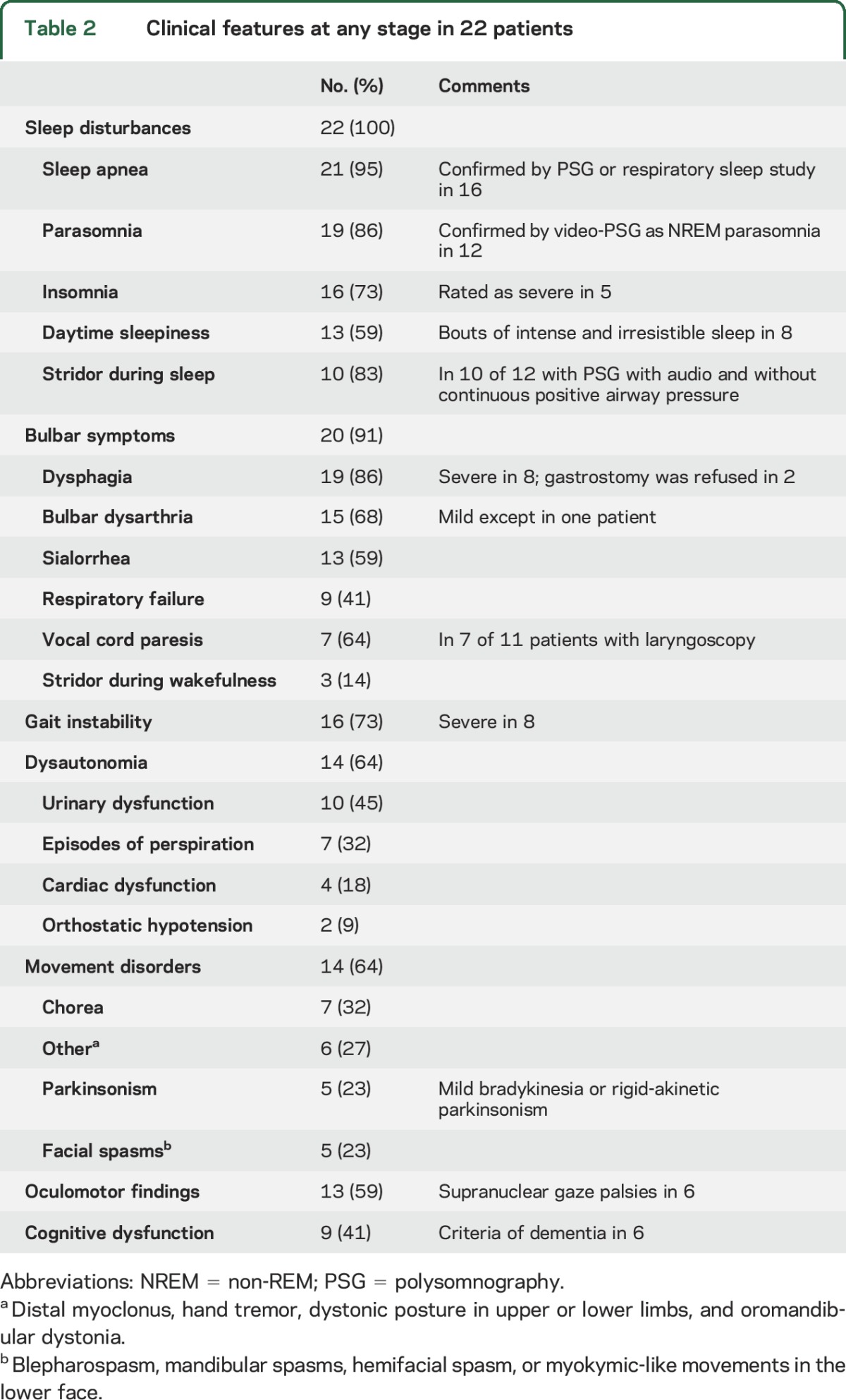
The frequency of neurologic symptoms is shown in table 2 and table e-1. All 22 patients had sleep problems. In 19 (86%), bed partners described episodes of vocalizations, limb movements, and purposeful-looking gestures during sleep as well as snoring and apneas. Insomnia with fragmented and nonrestorative sleep was present in 14 of these 19 patients and mild to irresistible bouts of excessive daytime sleepiness occurred in 13. Three patients had no history of parasomnia or sleep breathing difficulty; 2 of them slept alone and sleep studies demonstrated frequent OSA in both. In the remaining patient, mild insomnia was present but abnormal behaviors and breathing difficulties during sleep were not reported. Symptoms of bulbar dysfunction occurred in 20 (91%) patients, including dysarthria, dysphagia, laryngeal stridor, sialorrhea, and episodes of respiratory failure. Nine (41%) patients with respiratory failure required intensive care unit admission; the cause of respiratory failure was central hypoventilation in 7 and severe stridor in the other 2. Complaints of gait dysfunction were reported by 16 (73%) patients. Eight of these patients were able to walk alone but they felt unsteady, with the subjective feeling of either lateropulsion or retropulsion, or dizziness; 3 had gait ignition failure; in 3 of these patients, the symptoms were initially attributed to cerebellar dysfunction and in 1 to leg stiffness and dystonia. In the other 8 patients the gait disorder became severe, with frequent falls and inability to walk. At examination, all 16 patients had abnormal postural reflexes, suggesting that disequilibrium and impaired balance were the main reasons for the gait disorder. Dysautonomic features occurred in 14 (64%) patients, including urinary urgency, incontinence, nocturnal enuresis (10 patients), and episodes of spontaneous intense perspiration (7) that occurred without an identified trigger. Only 2 patients had documented orthostatic hypotension. Autonomic cardiac dysfunction was suspected in 4 patients: 2 had an episode of ventricular tachycardia, 1 had episodes of symptomatic bradycardia requiring placement of a pacemaker, and the third patient developed an episode of Takotsubo cardiomyopathy in the setting of nocturnal stridor. Movement disorders during daytime were identified in 14 (64%) patients, 7 developed chorea, 6 had distal myoclonus, hand tremor, limb, or oromandibular dystonia, and 5 spasms in facial muscles. Other less frequent symptoms included cognitive impairment in 9 (41%) patients, with attention and episodic memory difficulties and executive dysfunction. Six of the 9 patients fulfilled criteria for dementia. Supranuclear gaze paresis occurred in 6 patients (27%), and mild bradykinesia in 5 (23%).
Table 2.
Clinical features at any stage in 22 patients
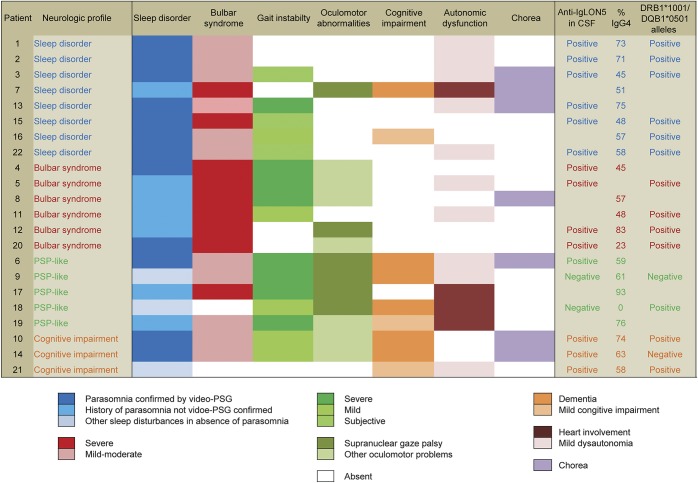
Overall, 20 (91%) patients had a sleep disorder and bulbar dysfunction, and 15 (68%) had gait impairment in addition to these 2 main symptoms. Based on the clinical picture by the time of detection of IgLON5 antibodies, 4 syndromes were identified (figure 1): (1) predominant sleep disorder, as described above, in 8 (36.4%) patients; (2) bulbar syndrome, predominating over the sleep disorder or gait dysfunction, in 6 (27.3%) patients; (3) a syndrome with gait instability and various combinations of vertical and horizontal supranuclear gaze palsies resembling progressive supranuclear palsy (PSP-like syndrome) in 5 (22.7%) patients. Only one of these patients fulfilled criteria of PSP9; this patient was identified among a series of 32 patients with the clinical diagnosis of PSP1 who were found to be antibody-negative; and (4) cognitive impairment in 3 (13.6%) patients; 2 of them fulfilled criteria of dementia with impairment of attention, episodic memory, and executive function, and also developed chorea resembling Huntington disease (figure 1).
Figure 1. Neurologic profile at diagnosis.
Clinical symptoms and immunologic features in the 22 patients with anti-IgLON5 disease classified according to the neurologic profile at diagnosis. PSG = polysomnography; PSP = progressive supranuclear palsy.
Paraclinical investigations and outcome.
Brain MRI, EEG, CSF, and EMG findings were unremarkable in most patients (table 1). On brain MRI, 3 patients showed mild brainstem and cerebellar atrophy and 1 had bilateral hippocampal atrophy with increased fluid-attenuated inversion recovery signal. Video-PSG in 12 patients showed a complex parasomnia with vocalizations, frequent limb movements, and purposeful-looking gestures during undifferentiated NREM sleep or less often in stage N2, and in 8 of 9 patients in whom findings typical of REM sleep behavior disorder could be recorded. All these patients and 4 additional ones who had a simplified respiratory sleep study had OSA (16 out of 16; median apnea-hypopnea index of 33/hour, range 15–97). Stridor was recorded in 10 of 12 patients with a PSG study with audio recording and performed without continuous positive airway pressure.
Twenty patients were treated with immunotherapy, which usually included monthly cycles of IV steroids or immunoglobulins (table 1). Only 2 patients had mild and transient improvement of symptoms. Thirteen patients (59%) died: 6 died suddenly when they were out of the hospital (2 while sleeping, 2 during wakefulness, and for the other 2 the time of death was not available), 6 died of aspiration pneumonia, and 1 as a result of hypernephroma progression.
Immunologic findings.
All patients had IgG1 or IgG4 IgLON5 antibodies. IgG4 antibodies were present in 20/21 patients and this was the predominant IgG subclass in 16 (72.7%). One patient, with PSP-like syndrome, only had IgLON5 IgG1 antibodies (figure 2). The percentage of specific IgG4 over total IgLON5 antibodies per sample ranged from 0% to 93% (mean 58%, 95% confidence interval [CI] 49–67), and for specific IgG1 from 7% to 100% (mean 38%; 95% CI 29–48). Among the 16 patients with paired serum and CSF samples, 14 had IgLON5 antibodies in both samples and 2, both with a PSP-like syndrome, only in serum.
Figure 2. Anti-IgLON5 immunoglobulin G (IgG) subclass distribution.
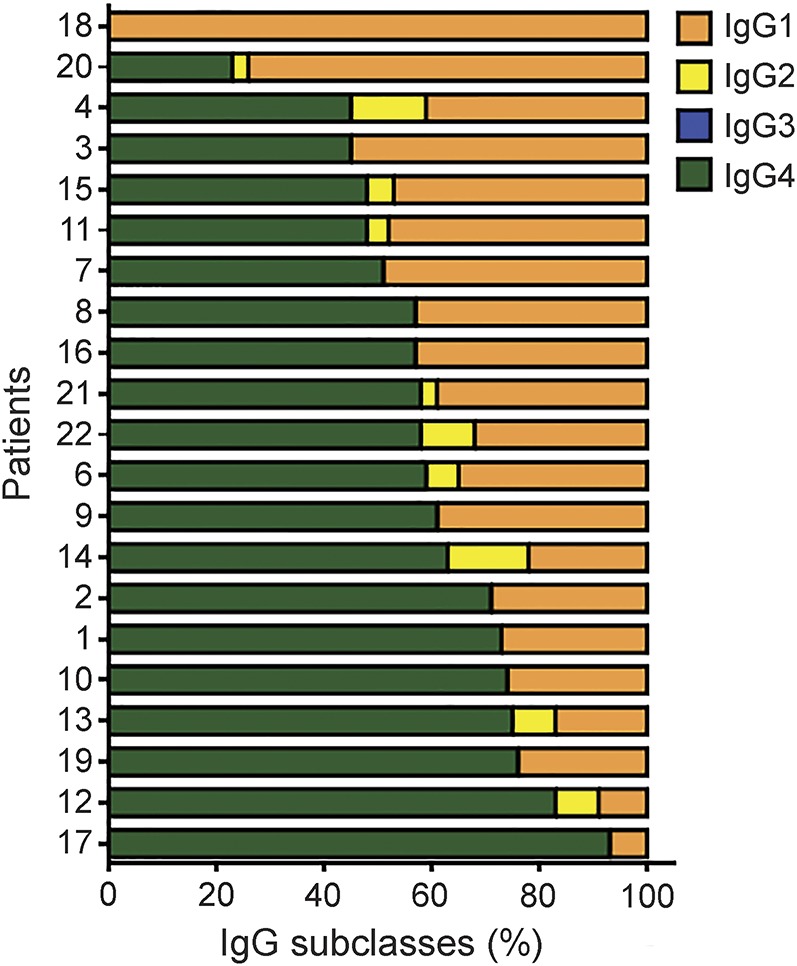
Subclasses of anti-IgLON5 IgG in the serum samples of 21 patients with anti-IgLON5 disease (IgG subclass analysis was not done in one patient due to lack of sample).
HLA typing was performed in 15 patients (all Caucasian except for 1 from the Philippines) and 13 (86.6%) had the HLA-DRB1*10:01 and HLA-DQB1*05:01 alleles. Calculation of the risk ratio indicated that DRB1*10:01 was 36 times (95% CI 19.5–67.0) more frequent in patients who develop the anti-IgLON5 disease than in the general population, whereas that for DQB1*05:01 was 3.5 times more frequent (95% CI 2.7–4.5).
DISCUSSION
In our initial description of the anti-IgLON5 disease, we focused on the characterization of the sleep disorder and identification of the antigen.1 Here we provide evidence that the anti-IgLON5 disease can be readily suspected if one considers the combination of a distinctive sleep disorder in association with one or more of the following symptoms: bulbar dysfunction, gait difficulties, oculomotor abnormalities, chorea, or cognitive deterioration. As occurs in other neurologic disorders (e.g., multiple system atrophy [MSA] or PSP), the presentation of the anti-IgLON5 disease is heterogeneous. The main symptoms of the disease (sleep disturbances, bulbar symptoms, and gait abnormalities) may each occur with different severity and appear in different combinations and time periods or sequence, leading to distinct clinical subtypes of the disease. These diverse clinical presentations likely reflect the variable degree of involvement by the neuropathologic process of the different brain areas. At symptom presentation, 4 clinical profiles were recognized: (1) predominant sleep disorder, (2) a bulbar syndrome, (3) a PSP-like syndrome, and (4) cognitive impairment that may associate with chorea. In the last 3 presentations, the sleep disorder may appear later or be present but surpassed by the other symptoms. Therefore, it is important to question patients and families about sleep disturbances and if present, this should raise the suspicion of the anti-IgLON5 disease. Finally, our study confirms the robust association of this disease with the HLA-DRB1*10:01 genotype. In all studied populations, the frequency of the HLA-DRB1*10:01 allele is very low, and when present strongly segregates with DQB1*05:01 (allelefrequencies.net).
The anti-IgLON5 disease is an intriguing CNS disorder with a clinical profile and pathologic features suggestive of a neurodegenerative disease that associates with a highly specific antibody and with particular HLA alleles. The exact pathogenesis of this disorder is unclear and we cannot rule out that the autoimmune response is a secondary event of a primary neurodegenerative process.10 However, an alternative mechanism that could explain the neuronal tau accumulation could be antibody interference of the interaction of IgLON5 with the internal cytoskeletal network, leading to neuronal dysfunction and ultimately neurodegeneration.11 To support this hypothesis, we recently demonstrated that IgLON5 antibodies cause an irreversible antibody-mediated internalization of surface IgLON5 in cultures of hippocampal neurons.12 In this scenario, early diagnosis and treatment would be crucial to prevent irreversible neuronal damage and the detection of the antibody will help to make a correct diagnosis. Although most of our patients did not improve with immunotherapy, the prolonged delay between symptom onset and diagnosis (median of 2 years) suggests that further studies focusing on early immunotherapy are critically important.
Up to 68% of patients with the anti-IgLON5 disease described sleep problems at the initial visit. Video-PSG is essential to identify and define the complex sleep disorder and confirm the characteristic features of the disorder.1 Other than the sleep disorder, the most common signs or symptoms at the initial visit included gait difficulty and bulbar dysfunction. The cause of gait problems is probably not uniform. For example, in one patient, it was considered secondary to a mixture of leg stiffness and dystonia.8 However, in about half of the patients, the gait abnormality was severe, accompanied by impaired balance and abnormal postural reflexes highly suggestive of a subcortical origin.13 The pathophysiology underlying this type of gait dysfunction is not well known but has been associated with dysfunction of mesencephalic nuclei, which are involved in the control of gait and balance.14 Although routine MRI studies did not demonstrate clear atrophy in our patients, the midbrain tegmentum was affected in the autopsy studies.2
In some patients, the gait disorder combined with supranuclear gaze palsy suggested PSP. However, the sleep disorder associated with the anti-IgLON5 disease does not occur in patients with PSP, the oculomotor abnormalities are different, and the parkinsonian features of PSP are rarely present.15 Even with these differences, one of our patients, an 83-year-old man, fulfilled clinical criteria for PSP. This patient had an unusual prolonged course (18 years) for PSP16 and was also atypical for the anti-IgLON5 disease because he did not have parasomnia (only insomnia), he was HLA-DRB1*10:01–negative, and IgLON5 antibodies were absent in the CSF.1
Dysautonomia, stridor, and sleep apnea are frequent but they may also occur in MSA. IgLON5 antibodies, however, were not found in 18 patients with clinical diagnosis of probable MSA-P.1 Compared with patients with MSA, those with the anti-IgLON5 disease rarely develop predominant parkinsonian features or cerebellar ataxia, the autonomic dysfunction is milder, and very few had severe orthostatic hypotension, a hallmark of MSA.17 In contrast, we identified several potentially serious heart complications in 4 patients with the anti-IgLON5 disease that may explain the frequent sudden death of patients with this disorder.18 Takotsubo cardiomyopathy is rarely reported in patients with neurologic disorders other than stroke,19 and the possible association with the anti-IgLON5 disease needs further studies.
Most patients with the anti-IgLON5 disease had IgG1 and IgG4 antibodies with the IgG4 subclass representing a mean of 58% of the total IgG reactive with IgLON5. The pathogenic mechanism of IgG1 and IgG4 antibodies are different: IgG1 antibodies induce crosslinking and internalization of the antigen whereas IgG4 antibodies more often interfere with the normal protein–protein interactions of the target antigen.20 In vivo studies injecting IgLON5 antibodies to experimental animals will clarify whether the antibodies are pathogenic and the predominant IgG subclass–related mechanism.
We did not find any correlation between the IgG subclass of the antibodies and the main neurologic symptoms. However, patients with the PSP-like phenotype were more likely to have IgLON5 antibodies only in serum, IgG1 subclass predominance, and negative HLA-DRB1*10:01. Whether patients with these atypical immunologic features represent true cases of anti-IgLON5 disease or have other neurologic disorders is currently unclear but this observation emphasizes the need for comprehensive clinical descriptions of patients with IgLON5 antibodies, pathologic confirmation whenever possible, and studies examining the prevalence of IgLON5 antibodies in a larger cohort of patients with PSP.
A potential limitation of our study is that the accrual of patients with IgLON5 antibodies could be influenced by our original description of the disorder that highlighted the novel sleep disorder.1 Although this possibility may be partially correct, our laboratory receives approximately 4,500 samples every year from patients with suspected autoimmune or paraneoplastic syndromes, as well as cases of rapidly progressive dementia (the laboratory is reference center for the study of prion diseases in Catalonia, Spain)21 and all are investigated for neuronal surface antibodies, including anti-IgLON5. Should the neurologic spectrum of the anti-IgLON5 disease be much wider than that described here, it is likely we would have been able to identify it. Despite this limitation and the retrospective collection of data, the findings of this study should improve the clinical recognition of the anti-IgLON5 disease.
ACKNOWLEDGMENT
The authors thank Mercè Alba, Esther Aguilar, and Eva Caballero for technical assistance in the CBA and flow cytometry studies.
GLOSSARY
- CBA
cell-based assay
- CI
confidence interval
- HLA
human leukocyte antigen
- IgG
immunoglobulin G
- MSA
multiple system atrophy
- NREM
non-REM
- OSA
obstructive sleep apnea
- PSG
polysomnography
- PSP
progressive supranuclear palsy
Footnotes
Supplemental data at Neurology.org
Editorial, page 1688
AUTHOR CONTRIBUTIONS
Design/conceptualization of the study: C. Gaig, F.G., J.D.; analysis/interpretation of the data: all authors; HLA studies: G.E.; IgG subclass analysis: L.S., F.G.; figure development: C. Gaig, L.S., F.G.; polysomnogram analysis: C. Gaig, A.I., J.S.; drafting the manuscript: C. Gaig, F.G., J.D.; revising the manuscript: all authors.
STUDY FUNDING
This study was supported in part by Fondo de Investigaciones Sanitarias, FEDER, Spain (FIS 15/00377, FG; FIS 14/00203, J.D.), NIH RO1NS077851 (J.D.), and Fundació Cellex (J.D.).
DISCLOSURE
C. Gaig reports no disclosures relevant to the manuscript. F. Graus receives royalties from Euroimmun for the use of IgLON5 as a diagnostic test. Y. Compta reports no disclosures relevant to the manuscript. B. Högl has received honoraria for speaking/advisory boards or consulting from AbbVie, Axovant, Lundbeck, Mundipharma, Otsuka, and UCB. L. Bataller reports no disclosures relevant to the manuscript. N. Brüggemann was funded by the Collaborative Center for X-linked Dystonia-Parkinsonism, received speaker's honoraria from the German Neurological Society, and received travel grants from UCB. C. Giordana and A. Heidbreder report no disclosures relevant to the manuscript. K. Kotschet has financial interest in Global Kinetics and research grant support from Ipsen. J. Lewerenz has received speaker's honoraria from Euroimmun and Teva. S. Macher, M.J. Marti, T. Montojo, J. Pérez, I. Puertas, C. Seitz, M. Simabukuro, N. Téllez, K. Wandinger, A. Iranzo, G. Ercilla, L. Sabater, and J. Santamaría report no disclosures relevant to the manuscript. J. Dalmau receives royalties from Athena Diagnostics for the use of Ma2 as autoantibody tests and from Euroimmun for the use of NMDAR and GABAB receptor as autoantibody tests and licensing fees from Euroimmun for the use of DPPX, GABAA receptor, and IgLON5 as diagnostic tests. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Sabater L, Gaig C, Gelpi E, et al. A novel non-rapid-eye movement and rapid-eye-movement parasomnia with sleep breathing disorder associated with antibodies to IgLON5: a case series, characterisation of the antigen, and post-mortem study. Lancet Neurol 2014;13:575–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gelpi E, Höftberger R, Graus F, et al. Neuropathological criteria of anti-IgLON5-related tauopathy. Acta Neuropathol 2016;132:531–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988;19:604–607. [DOI] [PubMed] [Google Scholar]
- 4.Balas A, García-Sánchez F, Vicario JL. Allelic and haplotypic HLA frequency distribution in Spanish hematopoietic patients: implications for unrelated donor searching. Tissue Antigens 2011;77:45–53. [DOI] [PubMed] [Google Scholar]
- 5.Högl B, Heidbreder A, Santamaria J, Graus F, Poewe W. IgLON5 autoimmunity and abnormal behaviours during sleep. Lancet 2015;385:1590. [DOI] [PubMed] [Google Scholar]
- 6.Simabukuro MM, Sabater L, Adoni T, et al. Sleep disorder, chorea, and dementia associated with IgLON5 antibodies. Neurol Neuroimmunol Neuroinflamm 2015;2:e136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montojo MT, Piren V, Benkhadra F, et al. Mimicking progressive supranuclear palsy and causing Tako-Tsubo syndrome: a case report on IgLON5 encephalopathy. Mov Disord 2015;30(suppl 1):710. Abstract. [Google Scholar]
- 8.Brüggemann N, Wandinger KP, Gaig C, et al. Dystonia, lower limb stiffness, and upward gaze palsy in a patient with IgLON5 antibodies. Mov Disord 2016;31:762–764. [DOI] [PubMed] [Google Scholar]
- 9.Litvan I, Agid Y, Calne D, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology 1996;47:1–9. [DOI] [PubMed] [Google Scholar]
- 10.Nogueras-Ortiz CJ, De Jesús-Cortes HJ, Vaquer-Alicea J, Vega IE. Novel autoimmune response in a tauopathy mouse model. Front Neurosci 2014;7:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leshchyns'ka I, Sytnyk V. Reciprocal interactions between cell adhesion molecules of the immunoglobulin superfamily and the cytoskeleton in neurons. Front Cell Dev Biol 2016;4:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sabater L, Planagumà J, Dalmau J, Graus F. Cellular investigations with human antibodies associated with the anti-IgLON5 syndrome. J Neuroinflammation 2016;13:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nutt JG, Marsden CD, Thompson PD. Human walking and higher-level gait disorders, particularly in the elderly. Neurology 1993;43:268–279. [DOI] [PubMed] [Google Scholar]
- 14.Demain A, Westby GW, Fernandez-Vidal S, et al. High-level gait and balance disorders in the elderly: a midbrain disease? J Neurol 2014;261:196–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Golbe LI. Progressive supranuclear palsy. Semin Neurol 2014;34:151–159. [DOI] [PubMed] [Google Scholar]
- 16.Williams DR, Lees AJ. Progressive supranuclear palsy: clinicopathological concepts and diagnostic challenges. Lancet Neurol 2009;8:270–279. [DOI] [PubMed] [Google Scholar]
- 17.Fanciulli A, Wenning GK. Multiple-system atrophy. N Engl J Med 2015;372:249–263. [DOI] [PubMed] [Google Scholar]
- 18.Pilgrim TM, Wyss TR. Takotsubo cardiomyopathy or transient left ventricular apical ballooning syndrome: a systematic review. Int J Cardiol 2008;124:283–292. [DOI] [PubMed] [Google Scholar]
- 19.Pelliccia F, Parodi G, Greco C, et al. Comorbidities frequency in Takotsubo syndrome: an international collaborative systematic review including 1109 patients. Am J Med 2015;128:654.e11–654.e119. [DOI] [PubMed] [Google Scholar]
- 20.Huijbers MG, Querol LA, Niks EH, et al. The expanding field of IgG4-mediated neurological autoimmune disorders. Eur J Neurol 2015;22:1151–1161. [DOI] [PubMed] [Google Scholar]
- 21.Grau-Rivera O, Sánchez-Valle R, Saiz A, et al. Determination of neuronal antibodies in suspected and definite Creutzfeldt-Jakob disease. JAMA Neurol 2014;71:74–78. [DOI] [PMC free article] [PubMed] [Google Scholar]