Abstract
Objectives
To assess a novel physical rehabilitation intervention in older patients hospitalized for acute decompensated heart failure (ADHF).
Background
Following ADHF, older patients, who are frequently frail with multiple co-morbidities, have prolonged and incomplete recovery of physical function and remain at high risk for poor outcomes.
Methods
This was a 3-site, randomized, attention-controlled pilot study of a tailored, progressive, multi-domain physical rehabilitation intervention beginning in the hospital and continuing for 12 weeks post-discharge in patients ≥60 years hospitalized with ADHF. The primary purpose was to assess feasibility and reasonableness of the hypothesis that the novel rehabilitation intervention would improve physical function (Short Physical Performance Battery (SPPB)) over 3 months and reduce all-cause rehospitalizations over 6 months.
Results
We enrolled 27 ADHF patients aged 60–98 years: 59% women, 56% African-American, 41% preserved ejection fraction (>45%). At baseline, participants had marked impairments in physical function, multiple co-morbidities, and frailty. Study retention (89%) and intervention adherence (93%) were excellent. At 3 months, we measured an intervention effect size for the SPPB score of +1.1 units (7.4±0.5 vs 6.3±0.5) and at 6 months observed an effect size for all-cause rehospitalization rate of −0.48 (1.16±0.35 vs. 1.64±0.39). The change in SPPB score was strongly related to all-cause rehospitalizations, explaining 91% of change.
Conclusions
These findings support the feasibility and rationale for a recently-launched, NIH-funded trial to test the safety and efficacy of this novel multi-domain physical rehabilitation intervention to improve physical function and reduce rehospitalizations in older, frail ADHF patients with multiple comorbidities.
Keywords: Rehabilitation, Physical Function, Frailty, Hospitalization, Exercise
Introduction
Acute decompensated heart failure (ADHF) is the leading cause of hospitalization in older adults and is associated with high rates of morbidity, mortality, and health care expenditures.(1) Improving outcomes following ADHF hospitalization is a national health care priority. However, even with optimal adherence to heart failure (HF) management guidelines, outcomes following hospitalization remain poor(2,3) and >50% experience readmission or death within 6 months.(4)
We and others have recently shown that older patients hospitalized with ADHF are frequently frail with severe impairments in multiple domains of physical function, including strength, balance, mobility, and endurance.(5–7) These impairments may help explain persistently high rehospitalization rates, the majority of which are not due to recurrent ADHF.(4,8) However, current ADHF management strategies do not account for such impairments and prior exercise training trials in HF have systematically excluded patients with current or recent ADHF.(9–11) Furthermore, conventional cardiac rehabilitation programs are not designed to address the multi-domain functional impairments common in older ADHF patients, particularly balance impairments and commencing rehabilitation without doing so can increase injuries.(12) Consequently, the role of physical rehabilitation intervention in patients with ADHF and older, frail HF patients with severe, widespread functional impairments has been identified as a critical evidence gap.(11)
To begin to address this gap, we conducted a prospective, randomized, pilot study of a novel intervention designed to address the severe and widespread physical function impairments in older patients with ADHF. We hypothesize that a tailored, progressive, physical rehabilitation intervention addressing deficits in balance, mobility, strength, and endurance that begins during hospitalization and continues for 3 months following discharge will improve physical function and reduce rehospitalizations in this vulnerable population. Because there were no prior studies of such an intervention in this frail, elderly, acutely hospitalized population and considerable equipoise existed, the present pilot study was designed to determine feasibility and support the rationale and design of a future, large, definitive trial.
Methods
Study Design and Population
This was a 3-site, randomized, attention-controlled pilot study of a novel, multi-domain physical rehabilitation intervention for older patients with ADHF beginning in the hospital and continuing for 12 weeks post-discharge with blinded assessment of outcome measures. As a pilot, the study was specifically not powered to definitively test the hypothesis. Rather, the study was designed to determine the feasibility of enrollment, retention, adherence, and follow-up, as well as the potential safety and potential for efficacy of the intervention, including estimate the intervention effect size. Such data are critical to determine the reasonableness of the hypotheses and to guide the design of a larger, definitive trial.
The study was inclusive of patients with multiple comorbidities, heterogeneous functional performance, and both reduced (<45%) and preserved (≥45%) ejection fraction (EF). Criteria for ADHF included: acute worsening of at least one HF symptom (exertional dyspnea or fatigue, swelling of legs or abdomen, orthopnea or paroxysmal nocturnal dyspnea); at least one sign of HF (pulmonary congestion by exam or x-ray, elevated jugular or central venous pressure, peripheral edema, elevated B-type Natriuretic Peptide ( >100 pg/ml) or N-terminal prohormone of B-type Natriuretic Peptide (>220pg/ml)); and change in medical treatment consistent with HF (e.g. augmentation of diuretics). The diagnosis of ADHF was confirmed by a study cardiologist with expertise in HF. Additional inclusion criteria were age >60 years, independence with basic activities of daily living prior to hospitalization, achievement of clinical stability allowing study participation, able to ambulate at least 4 meters (assistive device allowed) at the time of enrollment, and planned return to home post-discharge.
Exclusion criteria included: acute coronary syndrome; severe aortic stenosis; end-stage HF requiring advanced therapies or home intravenous inotropic therapy; functional status limited by condition other than HF at the time of enrollment; advanced chronic kidney disease defined as estimated glomerular filtration rate <20 mL/min/1.73 m2; terminal illness other than HF; actively participating in supervised exercise training prior to hospitalization; or inability or unwillingness to adhere with the study protocol. All participants provided informed consent. The study was approved by Institutional Review Boards at each center and was registered at Clinicaltrials.gov (NCT01508650).
Prior to hospital discharge and following medical stabilization and acquisition of baseline measurements, participants were randomly assigned in 1:1 fashion to a novel, progressive, multi-domain, 12-week physical function intervention or attention control. Participants were randomization using a computer-generated list (SAS software, version 9.0) and stratified by enrolling site and HF category (EF <45% or >45%).
Study Intervention
The multi-domain rehabilitation intervention for this study was a novel application of established rehabilitation therapies selected and integrated specifically for older patients hospitalized for ADHF. The goal of the intervention program was to improve performance in 4 domains: balance, strength, mobility and endurance. Exercise prescription was adapted to individual functional deficits in each domain based on standardized protocols and administered by trained interventionists using specific milestones for progression.
The majority of the intervention consisted of 60-minute sessions three times per week for 12 weeks in the outpatient setting beginning immediately following discharge. When feasible, daily 30-minute sessions during the hospitalization were also conducted. During the first 2–4 weeks following hospital discharge, supervised home-based training was allowed for patients with particularly poor functional status. Target intensity was based on patient-reported rate of perceived exertion (6–20 scale) and was initially low (≤12); after 2 weeks increased to 13 (“somewhat hard”; range 11–15) for endurance training and 15–16 for strength training.
To guide exercise prescription, participants in the intervention arm were stratified into 1 of 4 levels for each functional domain (balance, mobility, strength and endurance). Exercises appropriate to the participant’s functional level in each domain were then selected from the intervention protocols. These included static and dynamic balance training (e.g., standing with narrow base of support; stand and reach); mobility training (e.g., dynamic start and stop and changing direction while walking); functional strength training focused on lower extremities (e.g., chair rise; step-ups); and endurance training (sustained walking preferred). During exercise sessions, rest breaks were allowed as needed and close one-on-one supervision was provided by study interventionists.
As performance improved, participants were advanced through a structured, gradual progression using specific small increments in each exercise. Standardized re-assessment of performance in each domain was conducted every 2 weeks to guide exercise progression.
In addition to these supervised, facility-based sessions, participants were given a brief home exercise prescription to be performed on non-program days. This included low intensity walking at usual pace for up to 30 minutes and simple functional strengthening exercises, such as repeated chair rise or supported calf raise. After completion of the 12-week outpatient intervention, participants transitioned to an unsupervised home-based maintenance exercise program utilizing an individualized exercise prescription developed by the intervention team.
The standardized protocol of exercises and progressions was designed to support consistent implementation of the intervention over time, between participants, and across sites. To further support intervention fidelity, in-person training of the study interventionists, who were experienced exercise physiologists and physical therapists, was conducted. An intervention leader provided ongoing oversite of study rehabilitation sessions at each site. Bi-weekly intervention teleconferences among all site intervention leaders and interventionists were conducted to provide continued monitoring and guidance.
Adherence and Retention
During the enrollment period and prior to randomization, participants underwent screening for potential barriers to adherence. The demands of study participation were discussed in detail with potential participants and, whenever possible, family and/or caregivers. A standardized assessment tool was used to query personal commitment to adhering to the study requirements, degree of support from family members, caregivers and outpatient physicians, and potential transportation barriers. Those unable or unwilling to fully commit to all aspects of study participation or who had a lack of support were considered high risk for non-adherence and were not randomized and were excluded from the study.
Flexible scheduling, ongoing engagement of the participant, participant’s family and caregivers, and transportation support were provided to promote study adherence and retention. In the event of an interruption due to illness, which was anticipated in this frail, high-risk population, participants in the intervention group were allowed up to two additional weeks to complete 36 multi-domain intervention sessions. Participants in both arms were allowed up to 2 additional weeks to complete the 3-month assessments.
Attention control group
Participants randomized to attention control received at least monthly contact from study personnel with scheduled phone calls (months 2, 4, 5 and 6) and follow-up assessments (months 1 and 3).
Usual care
Participants in both arms could receive standard therapies, including home health and outpatient rehabilitation, as directed by their clinical providers. In the event of scheduling conflict, priority was given to usual care therapies. Medical therapy and HF management was at the discretion of the participant’s treating physician and was specifically not addressed by the study protocol for either study arm.
Outcome Measures
The primary outcome measure was change in the Short Physical Performance Battery (SPPB) at 3 months.(13) The SPPB is composed of 3 components, standing balance, gait speed, and timed repeated chair rise, each scored from 0–4 and combined for a total score of 0–12. The secondary outcome measure was all-cause rehospitalizations through 6 months following discharge from index hospitalization. Other measures included 6-month all-cause rehospitalization days, and 3 month change in Six Minute Walk Distance (6MWD), frailty status based on the Fried phenotype(14), health-related quality of life (HR-QOL) based on the Kansas City Cardiomyopathy Questionnaire (KCCQ)(15), cognitive function based on the Montreal Cognitive Assessment (MoCA)(16) and depression symptoms based on the 15-item Geriatric Depression Score (GDS-15).(17) All baseline measures were collected during the index hospitalization prior to randomization. Follow-up assessments were collected by trained, blinded assessors according to standardized protocols. Collection of clinical events was based on patient and caregiver report as well as review of medical records.
Statistical Analysis
Comparison of change in SPPB at 3 months between the attention control and intervention arms was made with analysis of covariance, with the 3-month value as the outcome and the baseline value as the covariate. Similar analyses were done for change in SPPB components, 6MWD, frailty status, KCCQ, MoCA score and GDS-15 score.
Re-hospitalizations were tracked and analyzed for all 27 participants (intention-to-treat analysis) for the entire 6-month study period. The number of all-cause re-hospitalizations per participant in each study arm was compared using analysis of covariance with HF category and baseline SPPB score as covariates. Secondary analysis of total number of all-cause rehospitalization days was also performed.
Spearman rank correlations between the effect on SPPB or 6MWD and the number of all-cause 6-month rehospitalizations were calculated. The extent the effect of group assignment (intervention vs attention control) on all-cause 6-month hospitalization was mediated by SPPB score was assessed. A two-tailed p value of <0.05 was used for significance.
Results
Of the 65 patients who met inclusion and exclusion criteria and were offered participation, 30 consented. Three patients were excluded by adherence risk screening, as per study protocol, such that 27 were randomized (15 intervention arm; 12 attention control). Baseline characteristics were balanced between the study arms with regards to older age, gender, race, number of comorbid conditions (approximately 5), markedly impaired functional status across multiple domains of physical function, high rates of frailty (≥50%), mild cognitive impairment (≥80%) and symptoms of depression (≥25%) (Table 1). Baseline functional status was very poor with approximately 40% of participants initially at the lowest stratification for most domains, 33% were at level 2, and 27% were at level 3. For example, nearly half the participants were unable to stand from a chair even once without the use of arms (strength level 1), stand unsupported with feet together for 10 seconds (balance level 1), tolerate >2 minutes of continuous walking (endurance level 1), and had a gait speed of ≤0.4 m/s (mobility level 1).
Table 1.
Baseline characteristics by randomization arm
| Characteristics | Intervention (N=15) | Attention Control (N=12) | P-value |
|---|---|---|---|
| Age (years), mean ± SD | 72.7 ± 10.8 | 71.8 ± 9.1 | 0.83 |
| Women | 53% | 67% | 0.70 |
| Race (Black) | 53% | 58% | 1.0 |
| Body mass index (mean ± SD) | 30.8 ± 7.1 | 27.3 ± 6.0 | 0.19 |
| Days Hospitalized, median (IQR) | 5 (2) | 6 (4) | 0.49 |
| Left ventricular ejection fraction (mean ± SD) | 40 ± 13 | 34 ± 18 | 0.33 |
| Preserved ejection fraction (> 45%) | 42% | 40% | 1.0 |
| Prior hospitalization within 6 months | 20% | 42% | 0.40 |
| Comorbidities | |||
| Diabetes mellitus | 60% | 33% | 0.25 |
| Hyperlipidemia | 47% | 50% | 1.0 |
| Chronic obstructive pulmonary disease | 13% | 33% | 0.36 |
| Kidney disease (estimated GFR < 60) | 67% | 58% | 0.71 |
| Anemia (Hgb <14 males, <12 females) | 60% | 58% | 1.0 |
| Number of comorbidities (mean ± SD) | 4.6 ± 1.8 | 5.3 ± 2.1 | 0.39 |
| Heart Failure Therapies | |||
| Loop diuretic | 93% | 100% | 1.0 |
| Beta-blocker | 93% | 75% | 0.29 |
| ACE-I or ARB | 53% | 67% | 0.70 |
| Aldosterone antagonist | 27% | 17% | 0.66 |
| Implantable cardioverter-defibrillator | 14% | 17% | 1.0 |
| Study Measures | |||
| Short Physical Performance Battery | 4.7 ± 2.7 | 6.0 ± 3.0 | 0.23 |
| 6-Minute Walk Distance (meters) | 160 ± 85 | 201 ± 120 | 0.32 |
| Frailty (≥3 of 5 Fried criteria) | 53% | 58% | 1.0 |
| Gait Speed (meters/second) | 0.55 ± 0.19 | 0.61 ± 0.28 | 0.50 |
| Kansas City Cardiomyopathy Questionnaire | 43 ± 19 | 45 ± 17 | 0.79 |
| Cognitive Impairment (MoCA < 26) | 80% | 83% | 1.0 |
| Depression (GDS-15 > 5) | 33% | 25% | 0.70 |
Variables presented as number (percent) unless otherwise noted. P-values determined by t-test or Fisher’s exact test. Abbreviations: SD, standard deviation; IQR, interquartile range; ACE-I, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor blocker; GFR, glomerular filtration rate; Hgb, hemoglobin; MoCA, Montreal Cognitive Assessment.
Twenty-four patients completed follow-up (3 dropouts; 89% retention rate). Participants who completed the intervention attended 92% of scheduled sessions.
At three months following hospital discharge the change in the SPPB score in the intervention group (4.8±2.8 to 6.9±3.0 units) compared to attention control (6.0±3.0 to 6.8±3.3 units) showed an intervention effect size of +1.1 units (7.4±0.5 vs. 6.3±0.5) (Table 2; Figure 1). For perspective on this magnitude of effect size, a clinically meaningful change in SPPB score is ≥0.6 units.(18) All individual component scores of the SPPB increased with the strongest trend seen with chair rise (lower extremity functional strength) (Table 2). At 3-month follow-up, there was an intervention effect size for 6MWD of +23 meters (247±22 vs 224±22 meters, intervention vs control, respectively) (Table 2; Figure 1). A clinically meaningful change in 6MWD is ≥20 meters.(18)
Table 2.
| Intervention (N=12) | Attention Control (N=12) | |||||
|---|---|---|---|---|---|---|
| Baseline | 3-months | LS mean ± SE | Baseline | 3-months | LS mean ± SE | |
| SPPB | 4.8±2.8 | 6.9±3.0 | 7.4±0.5 | 6.0±3.0 | 6.8±3.3 | 6.3±0.5 |
| SPPB Components | ||||||
| Balance | 2.0±1.5 | 2.8±1.1 | 3.1±0.2 | 2.8±1.3 | 2.9±1.4 | 2.7±0.2 |
| Gait Speed | 2.2±1.0 | 2.6±1.0 | 2.6±0.2 | 2.3±1.2 | 2.8±1.3 | 2.7±0.2 |
| Chair Rise | 0.7±0.7 | 1.5±1.4 | 1.6±0.3 | 0.9±0.8 | 1.2±1.2 | 1.0±0.3 |
| 6MWD | 170±83 | 232±113 | 247±22 | 201±120 | 239±129 | 224±22 |
| Frailty | ||||||
| Fried criteria* | 2.58±0.90 | 2.00±1.21 | 1.97±0.28 | 2.50±1.31 | 2.33±1.44 | 2.37±0.28 |
| Gait Speed (m/s) | 0.59±0.19 | 0.73±0.28 | 0.75±0.05 | 0.61±0.28 | 0.67±0.27 | 0.65±0.05 |
| KCCQ | ||||||
| Overall | 40±18 | 65±19 | 67±4 | 45± 17 | 63±22 | 61±4 |
| Physical | 40±27 | 57±19 | 59±5 | 48± 24 | 52±21 | 50±5 |
| Social | 43±28 | 64±25 | 66±7 | 49± 22 | 64±27 | 62±7 |
| QOL | 32±22 | 62±32 | 65±7 | 47± 28 | 68±21 | 64±7 |
| Symptoms | 30±24 | 66±25 | 66±6 | 30± 18 | 65±31 | 65±6 |
| MoCA | 20.5±5.0 | 22.0±4.7 | 22.1±1.1 | 20.8±4.9 | 20.4±6.5 | 20.3±1.2 |
| GDS-15 | 5.8±4.6 | 4.4±4.1 | 3.7±0.8 | 3.9±3.2 | 3.0±3.4 | 3.7±0.8 |
Analysis included all participants who completed baseline and 3-month assessments. Change between groups was determined by analysis of covariance, with the 3-month value as the outcome and the baseline value as the covariate. Variables presented as mean ± SD unless otherwise noted.
Fried criteria = number of the 5 Fried criteria met.
Abbreviations: LS, least square; SE, standard error; GDS-15, 15-item Geriatric Depression Scale; KCCQ, Kansas City Cardiomyopathy Questionnaire; MoCA, Montreal Cognitive Assessment; QOL, Quality of life; SPPB, Short Physical Performance Battery
Figure 1.
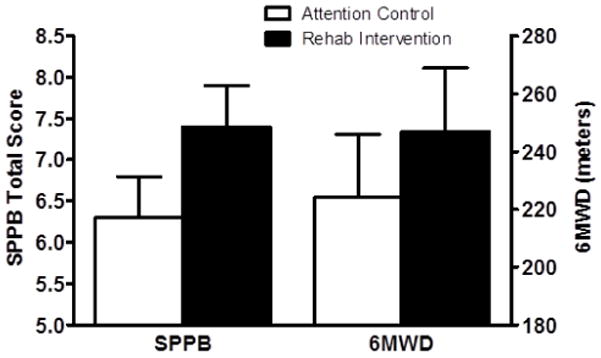
Comparison of the Short Physical Performance Battery (SPPB) and 6-Minute Walk distance (6MWD). At three months following hospital discharge the intervention effect size was +1.1 units for the SPPB score (7.4±0.5 vs 6.3±0.5 units) and +23 meters for the 6MWD (247±22 vs 224±22 meters). Comparisons made with analysis of covariance, with the 3-month value as the outcome and the baseline value as the covariate.
As expected during recovery from an ADHF hospitalization, at 3-month follow-up both groups had higher KCCQ score, with an intervention effect size of +5.4 points (+5 points is considered clinically meaningful).(19) Changes at 3-month follow-up in other variables, including in frailty, cognitive function, and depression are shown in Table 2.
There were 37 all-cause rehospitalizations during the 6-month follow-up, including 22 for ADHF, with 18 (67%) of the 27 participants experiencing at least one rehospitalization. The rate of 6-month all-cause rehospitalization was 29% lower in the intervention group (1.16±0.35 vs 1.64±0.39) and 6-month all-cause rehospitalization days were 47% lower per participant (6.0±2.5 vs 11.4±2.8), yielding an intervention effect size of −0.48 hospitalizations and −5.4 days, respectively (Figure 2). The change in the SPPB score was inversely correlated with the change in 6-month all-cause rehospitalizations (−0.60; p<0.01). Change in in 6MWD did not correlate with change in hospitalizations (−0.17; p=0.42). The change in the SPPB score explained 91% of the change in all-cause rehospitalizations by mediation analysis.
Figure 2.
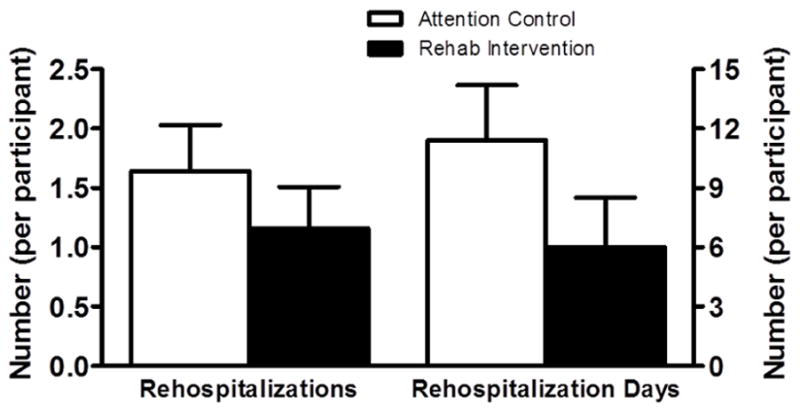
Comparison of 6-month all-cause rehospitalizations and rehospitalization days. The 6-month all-cause rehospitalization rate was 29% lower in the intervention group (1.16±0.35 vs 1.64±0.39), yielding an effect size −0.48 hospitalizations. The number of 6-month all-cause rehospitalization days were 47% lower per participant (6.0±2.5 vs 11.4±2.8), yielding an effect size of −5.4 days. Rehospitalization outcomes were tracked and analyzed for all 27 participants (Rehab n=15; Control n=12). Comparisons made with analysis of covariance with HF category (ejection fraction <45% or >45%) and baseline SPPB score as covariates.
There was one adverse event judged to be possibly related to the study intervention. The participant had completed a study intervention session uneventfully and returned home. Several hours later the participant had chest pain and was subsequently hospitalized with a non-ST elevation myocardial infarction. There were no other adverse events judged to be related to study participation and the intervention was otherwise well tolerated without injury or falls, even among those at the lowest level of functioning. There were no deaths.
Discussion
The REHAB-HF Pilot study was a prospective, multi-center, pilot clinical trial evaluating a novel rehabilitation intervention in hospitalized older HF patients. The study successfully randomized 27 patients ≥60 years old hospitalized with ADHF to either a novel multi-domain physical rehabilitation intervention or attention control. In both study arms baseline physical function impairments were severe, involving all physical function domains (balance, mobility, strength, and endurance), and frailty, severely reduced quality-of-life, cognitive dysfunction, and depression were common.(6) The intervention, which began during the index hospitalization and continued for 12-weeks in the outpatient setting, was generally well tolerated and there was good retention and adherence. At 3-month follow-up, we observed an intervention effect size of +1.1 units in the SPPB score, a well-accepted, standardized measure of physical function in frail older persons that is known to be strongly correlated with important clinical outcomes, including hospitalization, disability, and death.(7,13) The established threshold for clinically meaningful change in SPPB score is +0.6 units.(18) At 6-month follow-up, we observed an intervention effect size on all-cause re-hospitalizations of −0.48 and on re-hospitalization days of −5.4 days. The change in SPPB score was inversely related to the change in rehospitalization rate. By mediation analysis, change in SPPB score explained 91% of the change in rehospitalization rate, supporting a potential mechanistic link between physical function and rehospitalizations in frail, older ADHF patients.
These findings support the reasonableness of our overall study hypothesis that a novel, tailored, progressive, multi-domain physical rehabilitation intervention is feasible in older patients with ADHF who have high rates of frailty and co-morbidities and has the potential to improve physical function and reduce rehospitalization rates. The results of this pilot study informed the design of a subsequent multi-center clinical trial that was funded by the National Institutes of Health (NIH) and is designed to definitively test this hypothesis. The recently launched trial (NCT# NCT01508650) will enroll 360 patients, a sample size supported by the present data.
To our knowledge, this is the first randomized controlled trial of a physical rehabilitation intervention in older, hospitalized ADHF patients. Prior trials of exercise training in HF systematically excluded such patients(10) and evidence regarding the safety and efficacy of exercise intervention within 4–6 weeks following an ADHF hospitalization is very limited.(11) Consequently, the most recent national society consensus statement recommends against enrolling recently hospitalized HF patients in cardiac rehabilitation and such patients were excluded from the U.S. Centers for Medicare and Medicaid Services (CMS) decision memo expanding coverage for cardiac rehabilitation for patients with HF.(20,21) Addressing the critical evidence gap regarding the role of physical rehabilitation in patients recently hospitalized with AHDF has been designated as a high research priority by the NIH.(11)
Our findings suggest these severe functional impairments in older patients with ADHF may be modifiable with a sustained, targeted and progressive multi-domain rehabilitation intervention. The favorable trend towards reduced hospitalization observed in the intervention arm, which was almost entirely mediated by the change in physical function, provides encouraging evidence that clinical outcomes in older ADHF patients may be improved by successfully addressing these severe functional impairments.
Although encouraging, our findings also support the need for caution and further study regarding the role and design of physical rehabilitation in older patients with ADHF. The functional impairments in older patients following an ADHF hospitalization are far more severe and widespread than those reported in chronic stable HF patients and targeted by conventional cardiac rehabilitation.(6,9) For example, the average baseline 6MWD in the present cohort (178m) was half of that observed in similarly aged patients in the HF-ACTION trial (~350m).(22) Severe lower extremity weakness prevented nearly half of older, frail ADHF patients from standing even once from a seated position without the use of arms.(6) Importantly, older patients with ADHF also had severe deficits in balance and mobility which are not typically seen in chronic, stable HF patients and which are not addressed by conventional, endurance-based cardiac rehabilitation.(6,23) Initiating standard endurance exercise training in such patients without first addressing deficits in balance and mobility may limit efficacy(24) and increase the risk of injuries and falls.(12) To safely address these deficits, one-on-one training is often required(25), a feature incorporated into our intervention but not typically supported under current cardiac rehabilitation reimbursement models.
The present study was intended to address a critical evidence gap regarding physical function intervention in recently hospitalized, older frail HF patients. In HF-ACTION, the largest trial of physical rehabilitation in HF, mean age was <60 years and patients were mandated to be chronic, stable, with no medication changes or hospitalization within the prior 6 weeks. However, in reality, few HF-ACTION participants had been hospitalized for HF within even 6 months of enrollment.(9) Furthermore, functional impairments in older patients recovering from an ADHF hospitalization are dramatically more severe and widespread than those observed in chronic stable HF.(6) Recovery from this precipitous loss of function may be delayed and incomplete following hospital discharge.(26) Indeed, the attention control arm of this study continued to demonstrate severe physical dysfunction even three months following discharge. If successful, novel physical rehabilitation interventions like the one investigated in this study could serve as a “bridge” to more conventional cardiac rehabilitation interventions once functional impairments improve to the level for which cardiac rehabilitation has proven benefit.(9,10)
Limitations
This was a pilot study and was not designed or powered to definitively assess the efficacy or safety of the physical rehabilitation intervention. The findings should be considered preliminary and encouraging trends require confirmation in a larger, adequately powered clinical trial. There was considerable focus on retention and exercise adherence and similar rates may not be achieved outside the clinical research setting. Because they were excluded from the study, the results may not apply to patients who were not independent prior to admission, or who were expected to be discharged to a nursing home.
Future directions
Based on these pilot data, a larger, adequately powered NIH-funded multicenter randomized clinical trial, Rehabilitation and Exercise Training After Hospitalization (REHAB-HF) (ClinicalTrials.gov Identifier: NCT01508650) was recently launched to determine if this novel physical rehabilitation intervention will improve physical function and reduce all-cause hospitalizations in older ADHF patients. Until results from adequately powered trials such as REHAB-HF are available, and consistent with CMS policy(21) and the most recent society consensus statement(20) we recommend caution regarding immediate or early rehabilitation in unselected older hospitalized ADHF patients.
Conclusions
The findings from this pilot study, while preliminary, support the feasibility and safety of a novel, tailored multi-domain physical rehabilitation intervention starting in the hospital and continuing for 12 weeks immediately following discharge in older, frail ADHF patients, who may often have severe impairments in multiple physical domains (balance, mobility, strength and endurance). They also support the underlying hypothesis that in older ADHF patients, severe impairments in physical function are modifiable with a targeted intervention and that doing so may improve clinical outcomes. This pilot study informed the design of an ongoing clinical trial adequately powered to test if this novel intervention can improve physical function and reduce all-cause rehospitalization in older patients hospitalized with ADHF.
Clinical Perspectives.
Clinical Relevance
The present findings support that older patients hospitalized with ADHF have severe and widespread impairments in physical function that persist despite conventional HF treatment and contribute to adverse clinical outcomes.
Translational Outlook
Further study is needed to determine if sustained and gradually progressive multi-domain physical rehabilitation interventions targeting deficits in balance, mobility, strength and endurance can improve physical function and clinical outcomes in older patients with ADHF.
Acknowledgments
This study was supported by the following research grants: NIH Grants R01AG045551 and R01AG18915; The Claude D. Pepper Older Americans Independence Center of Wake Forest School of Medicine Winston-Salem, NC NIH Grant P30AG021332; the Kermit Glenn Phillips II Endowed Chair in Cardiology; Dean’s Faculty Achievement Award, Jefferson College of Health Professions, Philadelphia, PA; and the Oristano Family Research Fund.
Abbreviations List
- 6MWD
Six Minute Walk Distance
- ADHF
Acute Decompensate Heart Failure
- EF
Ejection Fraction
- GDS-15
Geriatric Depression Score
- HF
Heart Failure
- HR-QOL
Health-related Quality of Life
- KCCQ
Kansas City Cardiomyopathy Questionnaire
- MoCA
Montreal Cognitive Assessment
- SPPB
Short Physical Performance Battery
Footnotes
The authors have no relevant financial relationships with industry to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, et al. Executive summary: heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:948–54. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- 2.Kociol RD, Peterson ED, Hammill BG, et al. National Survey of Hospital Strategies to Reduce Heart Failure Readmissions: Findings From the Get With the Guidelines-Heart Failure Registry. Circ Heart Fail. 2012;5:680–687. doi: 10.1161/CIRCHEARTFAILURE.112.967406. [DOI] [PubMed] [Google Scholar]
- 3.Patterson ME, Hernandez AF, Hammill BG, et al. Process of care performance measures and long-term outcomes in patients hospitalized with heart failure. Med Care. 2010;48:210–6. doi: 10.1097/MLR.0b013e3181ca3eb4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng RK, Cox M, Neely ML, et al. Outcomes in patients with heart failure with preserved, borderline, and reduced ejection fraction in the Medicare population. Am Heart J. 2014;168:721–30. doi: 10.1016/j.ahj.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Sanchez E, Vidan MT, Serra JA, Fernandez-Aviles F, Bueno H. Prevalence of geriatric syndromes and impact on clinical and functional outcomes in older patients with acute cardiac diseases. Heart. 2011;97:1602–6. doi: 10.1136/hrt.2011.227504. [DOI] [PubMed] [Google Scholar]
- 6.Reeves GR, Whellan DJ, Patel MJ, et al. Comparison of Frequency of Frailty and Severely Impaired Physical Function in Patients >/=60 Years Hospitalized With Acute Decompensated Heart Failure Versus Chronic Stable Heart Failure With Reduced and Preserved Left Ventricular Ejection Fraction. Am J Cardiol. 2016 doi: 10.1016/j.amjcard.2016.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Volpato S, Cavalieri M, Sioulis F, et al. Predictive value of the Short Physical Performance Battery following hospitalization in older patients. J Gerontol A Biol Sci Med Sci. 2011;66:89–96. doi: 10.1093/gerona/glq167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunlay SM, Redfield MM, Weston SA, et al. Hospitalizations after heart failure diagnosis a community perspective. J Am Coll Cardiol. 2009;54:1695–702. doi: 10.1016/j.jacc.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Connor CM, Whellan DJ, Lee KL, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1439–50. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor RS, Sagar VA, Davies EJ, et al. Exercise-based rehabilitation for heart failure. Cochrane Database Syst Rev. 2014;4:CD003331. doi: 10.1002/14651858.CD003331.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fleg JL, Cooper LS, Borlaug BA, et al. Exercise training as therapy for heart failure: current status and future directions. Circ Heart Fail. 2015;8:209–20. doi: 10.1161/CIRCHEARTFAILURE.113.001420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tilson JK, Wu SS, Cen SY, et al. Characterizing and identifying risk for falls in the LEAPS study: a randomized clinical trial of interventions to improve walking poststroke. Stroke. 2012;43:446–52. doi: 10.1161/STROKEAHA.111.636258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–31. doi: 10.1093/gerona/55.4.m221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 15.Spertus JA, Jones PG, Kim J, Globe D. Validity, reliability, and responsiveness of the Kansas City Cardiomyopathy Questionnaire in anemic heart failure patients. Qual Life Res. 2008;17:291–8. doi: 10.1007/s11136-007-9302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–9. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 17.Almeida OP, Almeida SA. Short versions of the geriatric depression scale: a study of their validity for the diagnosis of a major depressive episode according to ICD-10 and DSM-IV. Int J Geriatr Psychiatry. 1999;14:858–65. doi: 10.1002/(sici)1099-1166(199910)14:10<858::aid-gps35>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 18.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743–9. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 19.Flynn KE, Pina IL, Whellan DJ, et al. Effects of exercise training on health status in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1451–9. doi: 10.1001/jama.2009.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ades PA, Keteyian SJ, Balady GJ, et al. Cardiac rehabilitation exercise and self-care for chronic heart failure. J Am Coll Cardiol HF. 2013;1:540–7. doi: 10.1016/j.jchf.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacques LJT, Schafer J, Chin J, Issa M. Services DoHaH, editor. Decision Memo for Cardiac Rehabilitation (CR) Programs - Chronic Heart Failure. Centers for Medicare & Medicaid Services; 2014. http://www.cms.gov/medicare-coverage-database/details/ncadecision-memo.aspx?NCAId=270. [Google Scholar]
- 22.Forman DE, Fleg JL, Kitzman DW, et al. 6-min walk test provides prognostic utility comparable to cardiopulmonary exercise testing in ambulatory outpatients with systolic heart failure. J Am Coll Cardiol. 2012;60:2653–61. doi: 10.1016/j.jacc.2012.08.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiarantini D, Volpato S, Sioulis F, et al. Lower extremity performance measures predict long-term prognosis in older patients hospitalized for heart failure. J Card Fail. 2010;16:390–5. doi: 10.1016/j.cardfail.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 24.Witham MD, Fulton RL, Greig CA, et al. Efficacy and cost of an exercise program for functionally impaired older patients with heart failure: a randomized controlled trial. Circ Heart Fail. 2012;5:209–16. doi: 10.1161/CIRCHEARTFAILURE.111.963132. [DOI] [PubMed] [Google Scholar]
- 25.Giallauria F, Vigorito C, Tramarin R, et al. Cardiac rehabilitation in very old patients: data from the Italian Survey on Cardiac Rehabilitation-2008 (ISYDE-2008)--official report of the Italian Association for Cardiovascular Prevention, Rehabilitation, and Epidemiology. J Gerontol A Biol Sci Med Sci. 2010;65:1353–61. doi: 10.1093/gerona/glq138. [DOI] [PubMed] [Google Scholar]
- 26.Bodilsen AC, Pedersen MM, Petersen J, et al. Acute hospitalization of the older patient: changes in muscle strength and functional performance during hospitalization and 30 days after discharge. Am J Phys Med Rehabil. 2013;92:789–96. doi: 10.1097/PHM.0b013e31828cd2b6. [DOI] [PubMed] [Google Scholar]


