Abstract
Background
Preoperative chemotherapy is a strategy for conversion to resection and/or assessing disease biology prior to operation. The utility of such an approach in gallbladder carcinoma (GBCA) is unknown. This study evaluates outcomes of GBCA patients treated with chemotherapy for locally advanced or lymph node involved tumors.
Study Design
Patients that received systemic chemotherapy for locally advanced or lymph node positive GBCA were identified from a departmental database. Patients were excluded if there was any evidence of distant metastases or if records were inadequate to determine initial chemotherapy and response. Response (RECIST), operative results, and overall survival (OS) were assessed.
Results
Seventy-four patients were included from 1992–2015. Eighty-nine percent of patients (n=64) were treated with gemcitabine and 57% with gemcitabine/platinum (n=42). At initial response assessment, 17 patients (23%) had progression. The remaining patients had stable disease (n=38, 51%) or partial response (n=19, 26%). Twenty-two patients (30%) underwent attempt at resection which was definitive for 10 patients (14%). Median OS for the entire cohort was 14 months (95% CI:11.3–17.9). Among patients with surgery, definitive resection was associated with a median OS of 51 months (95% CI:11.7–55.3) compared to 11 months (95% CI:4.1–23.6) for those that were unresectable (p=0.003).
Conclusions
Even without distant metastases, locally advanced or lymph node positive GBCA is associated with poor outcomes. Definitive resection was possible in a subset of patients selected for surgery after a favorable response to chemotherapy and was associated with long-term survival. We recommend surgical re-evaluation following chemotherapy to select potential operative candidates.
Introduction
Gallbladder carcinoma (GBCA) is an uncommon malignancy with a poor prognosis. In the United States, 10,000 new diagnoses are made each year (1). George Pack first advocated radical resection of gallbladder cancer in 1955, but clinical management has remained challenging with historical series reporting 5 year survival rates between 5% and 13% (2–4). Definitive surgery includes resection of hepatic segments 4 and 5 but may also require major hepatectomy, bile duct excision, or additional-organ resection to obtain tumor clearance (5). However, definitive resection is only achievable in approximately 25% of patients with GBCA (6). In such an aggressive malignancy, preoperative chemotherapy is an appealing treatment strategy to increase rates of resection and assess disease biology prior to operation, particularly in patients with advanced disease associated with a high risk of recurrence. Although this strategy has been employed successfully for other malignancies, its utility in GBCA is unknown (7, 8).
Prognosis is particularly poor for patients with locally advanced GBCA. Both pre-operative jaundice and lymph node metastases are associated with worse outcomes when present in GBCA (9, 10). While positive portal nodes are evidence of a more advanced tumor and stage (11), biopsy proven retroperitoneal lymph nodes have traditionally been justification to abandon exploration due to disseminated disease beyond the extent of resection and overwhelmingly poor outcomes. Similarly, preoperative jaundice indicates involvement of the tumor in the porta hepatis either by enlarged lymph nodes or direct tumor extension causing biliary obstruction. Locally advanced or lymph node involved GBCA represents an ideal high-risk subset of patients in which to explore the treatment strategy of preoperative chemotherapy.
With publication of the ABC-02 Trial in 2010, gemcitabine and cisplatin became the standard chemotherapy for locally advanced GBCA based on the results of the drug combination in metastatic and unresectable biliary tract cancer (12). Prior to that study, various treatment regimens were employed for locally advanced GBCA with the goal of subsequent resection. The percentage of patients with locally advanced GBCA that respond to the initial chemotherapy regimen and then go on to definitive surgery is unclear, and likewise, the long-term survival benefit of resection in this setting is not well described.
This study evaluates surgical outcomes and overall survival in GBCA patients treated with chemotherapy for locally advanced or lymph node involved tumors in the absence of distant metastases.
Methods
Patient Selection
This project received approval from the Institutional Review Board for waiver of informed consent. All patients with GBCA evaluated at Memorial Sloan Kettering Cancer Center by a hepatopancreatobiliary surgeon were recorded in a prospectively-maintained database. This database includes demographics, pathology results, operative details, complications, and outcomes. Patients included in this study were identified from the database between 1992 and 2015. Additional data for analysis was retrospectively collected from the electronic medical record. Patient demographics, clinical history, pathology, laboratory results, and survival were examined.
Patients were considered locally advanced if they presented with jaundice or with vascular and biliary involvement precluding resection. Node-positive GBCA was defined as enlarged lymph nodes along the cystic duct, common bile duct, portal vein, or hepatic artery (N1) or nodal involvement or the aortocaval, retropancreatic, celiac or superior mesentery artery lymph nodes (N2) on imaging or biopsy-proven disease in the same location according to current AJCC guidelines (13). Patients with distant metastases (including discontinuous disease within the liver) were excluded. Elevated CA 19-9 was any value greater than 37 U/mL (our institutional higher limit of normal) at the start of treatment. Incidental GBCA was defined as malignancy diagnosed following laparoscopic cholecystectomy for presumed benign biliary disease, and all other patients were classified as having primary GBCA.
Patients were pooled from those that presented with 1) imaging or biopsy with evidence of locally advanced or node positive gallbladder cancer or 2) previous surgical exploration without resection due to locally advanced disease or lymph node involvement in the absence of distant metastases. Patients were excluded if no data was available in the electronic medical record regarding the type of chemotherapy or response to chemotherapy administered for unresected locally advanced or lymph node involved tumors. Previous exploration was defined as a prior attempt at definitive resection, and does not include the index cholecystectomy for patients with incidental GBCA. Patients with gallbladder cancer were cared for with a multi-disciplinary approach at our institution. Care was planned at a hepatobiliary disease management conference combining surgery, medical oncology, pathology, and radiology. The decision for operation reflected the choice of the surgeon and disease management team regarding the length and duration of chemotherapy.
Chemotherapy Administration and Response
Chemotherapy choice and administration was at the discretion of the primary medical oncologist. As a tertiary referral center, patients often chose to receive therapy locally and returned for repeat imaging. As such, number of cycles and toxicity was not reliably available for all patients in this retrospective review. According to practice, the initial assessment of response occurred at approximately 2 months after chemotherapy initiation with computed tomography (CT) scan of the abdomen and pelvis. The radiographic and clinical reports were used to assign treatment response. Definitions of response were assigned based on RECIST guidelines (version 1.1) using the first post-chemotherapy CT scan (14). Patients that died prior to response assessment were included with those that demonstrated disease progression. Response was classified based on the first imaging assessment of response. Some patients had subsequent CT scans to assess further response. Patients with curative-attempt exploration at any interval after chemotherapy administration were included in the operative group.
Surgical Approach
The authors’ surgical approach for GBCA has been described previously (5, 11, 15). Laparoscopy was used selectively before laparotomy in cases with concern for metastatic disease. When the patients appeared to have localized disease without evidence of distant metastases, patients underwent a laparotomy. At exploration, surgeons mobilized and palpated the liver, duodenum, pancreatic head, and retroperitoneum. They performed ultrasonography of the liver to assess for discontinuous metastatic disease and assess involvement of major vasculature. Frozen section biopsies were taken of any suspicious hepatic or extrahepatic lesions. Patients who had peritoneal metastases, discontinuous liver metastases, or involved lymph nodes beyond the scope of resection were considered to have non-curative, incomplete resections. Disease invading the adjacent liver in continuity with the gallbladder was not a contraindication to resection. Definitive, complete resection was performed in instances where it was possible to attain negative margins. Among selected patients with a previous curative-intent exploration, the second exploration after systemic chemotherapy followed a similar operative approach. In this group, the first operation must have been abandoned due to locally unresectable disease or positive N1/N2 lymph nodes without evidence of peritoneal or liver metastases.
Statistical Analysis
Patient characteristics were described using counts and percentages for categorical variables and medians and ranges for continuous variables. Differences in patient characteristics were assessed using the Fisher’s exact test and the Wilcoxon Rank Sum test where appropriate. For all survival analyses, Kaplan Meier (KM) methods were used to calculate the 1 year, 3 year and median survival along with 95% confidence intervals. Differences in survival between groups were assessed using the log rank test. Overall survival (OS) was calculated from the start of chemotherapy until death. Patients alive at last followup were censored. Survival time was landmarked at date of response to assess the difference in survival based on response and all patients were included. Survival time landmarked at date of surgery was used to assess the difference of surgical resection status and only those who had surgery after chemotherapy were included. P values less than 0.05 were considered statistically significant. All analyses were done using SAS 9.4 (The SAS Institute, Cary, NC).
Results
Demographics
Overall, 148 patients from 1992–2015 met the inclusion criteria of locally advanced or lymph node positive GBCA in the absence of known distant metastases. However, 74 patients (50%) had adequate records for retrospective review including chemotherapy regimen, date of initiation, response assessment, operative details, and clinical outcomes and were included in the study. The median age for our sample was 65 years (range 43–86). There were 38 female patients (51%). The median BMI was 26.8 kg/m2 (range 17–44.5). Additional patient characteristics are detailed in Table 1.
Table 1.
Patient Characteristics (n=74)
| Characteristic | |
|---|---|
| Demographics | |
| Sex, female, n (%) | 38 (51.4) |
| Age at diagnosis, y, median (range) | 65.1 (43.2–85.6) |
| Major comorbidity, n (%) | 28 (37.8) |
| BMI, kg/m2, median (range) (n=69) | 26.8 (17–44.5) |
| Race, n (%) | |
| Caucasian | 55 (74.3) |
| South/East Asian | 7 (9.5) |
| Black | 6 (8.1) |
| South/Central American | 6 (8.1) |
| Clinical characteristics, n (%) | |
| Primary Finding | 49 (66.2) |
| Gallstones | 49 (66.2) |
| Prior exploration | 33 (44.6) |
| CA 19-9 > 37, (n=47) | 35 (74.5) |
| Jaundice at presentation | 32 (43.2) |
| Reasons for chemotherapy, n (%) | |
| Locally advanced disease | 14 (18.9) |
| Nodal disease | 35 (47.3) |
| Locally advanced & nodal disease | 22 (29.7) |
| Spillage, residual disease | 3 (4.1) |
| Gemcitabine-based chemotherapy, (n=72), n (%) | 64 (88.9) |
| Platinum chemotherapy, (n=72), n (%) | 42 (58.3) |
| Histology, n (%) | |
| Adenocarcinoma | 71 (95.9) |
| Adenosquamous carcinoma | 3 (4.1) |
| Grade, n (%) | |
| Unknown | 14 (18.9) |
| Well differentiated | 1 (1.4) |
| Moderately differentiated | 29 (39.2) |
| Poorly differentiated | 30 (40.5) |
| Pre-chemotherapy stage, n (%) | |
| IIIA | 2 (3.3) |
| IIIB | 10 (16.4) |
| IVA | 6 (9.8) |
| IVB | 43 (70.5) |
| Days from chemotherapy initiation to response assessment, d (range) | 64 (22–215) |
| Days from response assessment to resection (n=22), d, median (range) | 50.5 (12–223) |
| Operation after chemotherapy, n (%) | 22 (29.7) |
CA 19-9:, carbohydrate antigen 19–9.
Clinical Data
Primary GBCA accounted for 66% of patients (49/74) with the remaining 34% of patients (25/74) presenting with incidental GBCA following cholecystectomy (Table 1). Pain (35/74, 47%) and jaundice (32/74, 43%) were the two most common causes of presentation. The infrequent causes of presentation were cholangitis, incidental imaging, fatigue, and weight loss (7/74, 10%).
Patients were classified into 4 groups with regard to the reason for chemotherapy. Fourteen patients (19%) were locally advanced, 35 patients (47%) had biopsy-proven or imaging evidence of nodal disease, 22 patients (30%) were classified as having both criteria, and 3 patients (4%) received preoperative chemotherapy because of incidental gallbladder cancer and perforation. Full pre-treatment stage was available for 61 patients and the majority were stage IVB (43/74, 58%) due to evidence of N2 nodal disease by imaging, biopsy, or exploration. Thirty-three patients (45%) had previous exploration without definitive resection.
Of the 41 patients who were included without previous exploration, 32 (78%) were given preoperative chemotherapy because of characteristic imaging or biopsy indicating a minimum of lymph node disease. The remaining patients had imaging suspicious for locally advanced disease only (6/41, 15%) or had high-risk T3 incidental GBCA with concern for spillage, positive margin, and residual disease in the gallbladder fossa (3/41, 7%).
Chemotherapy Regimens and Response Assessment
Chemotherapy records were available for 72 of the 74 patients (97%), and all patients were assigned a treatment response. Sixty four (87%) patients received gemcitabine and 42 patients (57%) received combination platinum-based chemotherapy. Gemcitabine was utilized in all 32 patients (100%) during or after 2010 and included platinum-based chemotherapy in 30 patients (94%). Prior to 2010, gemcitabine was utilized in 32 of the 42 chemotherapeutic regimens (76%) and included platinum-chemotherapy in only 12 instances (29%). Other chemotherapy choices included 5-FU/leucovorin (6/74), sorafenib (1/74), IL-2/Xeloda (1/74), and unknown (2/74). Of note, patients with adenosquamous histology (3/74) all received gemcitabine and platinum chemotherapy.
Chemotherapy response was routinely assessed at approximately 8 weeks from initiation using contrast-enhanced CT scan. The median time between the start of chemotherapy and initial response assessment was 64 days (range 22–215). Seven patients (9%) died prior to re-staging CT scan and were included in the 17 patients (23%) demonstrating disease progression. Partial response was observed in 19 patients (26%) with the remaining 38 patients (51%) having stable disease on repeat imaging (Figure 1). In the cohort of 22 patients selected for attempt at curative resection, the median time from response assessment to the operation was 51 days (range 12–223).
Figure 1.
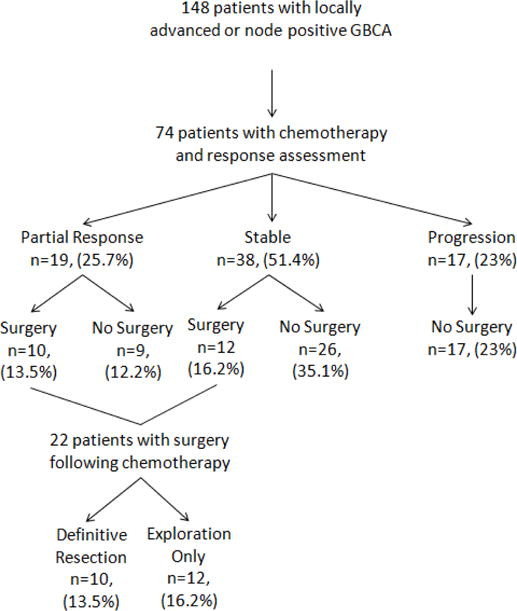
Flowchart for the study. GBCA, gallbladder carcinoma.
Surgery
Twenty two of the 57 (39%) patients with stable disease or partial response were taken for attempt at curative resection. The remaining 35 patients (61%) with stable or partial response did not proceed to surgery for various reasons. The most common reasons were progression on a second CT scan while receiving continued treatment (15/35, 43%) or clinical deterioration (13/35, 37%). Types of clinical deterioration and comorbidities observed were biliary obstruction, bleeding gastric ulcers, cardiac ischemia, ascites, pneumonia, deep venous thrombosis, and overall functional status. The remaining 7 patients remained unresectable with continued evidence of biliary or vascular involvement or enlarged N2 nodes and were not selected for attempt at resection. Figure 1.
Patient characteristics stratified by attempt at surgical resection following chemotherapy are detailed in Table 2. A lower proportion of patients who went to surgery (5/22, 23%) had prior exploration compared to those who did not (28/52, 54%) (p=0.021). Additionally, patients who went to surgery after chemotherapy were stage IVB (8/22, 53%) or stage IIIB (7/22, 47%) at the beginning of treatment. By contrast, patients who did not undergo attempted resection were stage IVB (35/52, 76%), stage IVA (6/52, 13%) IIIB (3/52, 6.5%) or IIIA (2/52, 4.3%) (p=0.003). No significant differences were seen between those who had surgery versus those who didn’t for the other covariates including age (median 63 years vs. 66 years, p=0.34), race (Caucasian 82% vs. 71%, p=0.33), gender (55% vs. 50% p=0.80), jaundice on presentation (32% vs 48%, p=0.21) or grade (poorly differentiated 43% vs. 54%, p=0.32) (Table 2).
Table 2.
Comparison of Patients by Surgical and Resection Status
| Surgery after chemotherapy | Resection status | |||||
|---|---|---|---|---|---|---|
| No surgery, (n=52) | Surgery after chemotherapy, (n=22) | p Value | Definitive resection, (n=10) | Incomplete resection, (n=12) | p Value | |
| Primary vs incidental, n (%) | ||||||
| Primary | 35 (67.3) | 14 (63.6) | 0.79 | 9 (90) | 5 (41.7) | 0.031* |
| Incidental | 17 (32.7) | 8 (36.4) | 1 (10) | 7 (58.3) | ||
| Sex, n (%) | ||||||
| Male | 26 (50) | 10 (45.5) | 0.80 | 2 (20) | 8 (66.7) | 0.043* |
| Female | 26 (50) | 12 (54.5) | 8 (80) | 4 (33.3) | ||
| Race, n (%) | ||||||
| Caucasian | 37 (71.2) | 18 (81.8) | 0.33 | 9 (90) | 9 (75) | 0.22 |
| South/East Asian | 4 (7.7) | 3 (13.6) | 0 (0) | 3 (25) | ||
| Black | 5 (9.6) | 1 (4.5) | 1 (10) | 0 (0) | ||
| South/Central American | 6 (11.5) | 0 (0) | 0 (0) | 0 (0) | ||
| Age at diagnosis, y, median (range) | 66.32 (43.22– 85.64) | 63.4 (46.03–85.34) | 0.34 | 67.99 (46.03–85.34) | 61.17 (55.66–77.95) | 0.77 |
| Major comorbidity, n (%) | ||||||
| No | 32 (61.5) | 14 (63.6) | >0.95 | 7 (70) | 7 (58.3) | 0.67 |
| Yes | 20 (38.5) | 8 (36.4) | 3 (30) | 5 (41.7) | ||
| BMI kg/m2, median (range) | 27 (18.7–42.7) | 26.05 (17–44.5) | 0.53 | 29 (20.9–44.5) | 25.6 (17–37.7) | 0.62 |
| Gallstones, n (%) | ||||||
| No | 17 (32.7) | 8 (36.4) | 0.79 | 3 (30) | 5 (41.7) | 0.67 |
| Yes | 35 (67.3) | 14 (63.6) | 7 (70) | 7 (58.3) | ||
| Prior exploration, n (%) | ||||||
| No | 24 (46.2) | 17 (77.3) | 0.021* | 6 (60) | 11 (91.7) | 0.14 |
| Yes | 28 (53.8) | 5 (22.7) | 4 (40) | 1 (8.3) | ||
| CA 19-9 > 37, n (%) | ||||||
| No | 10 (31.3) | 2 (13.3) | 0.29 | 1 (11.1) | 1 (16.7) | >0.95 |
| Yes | 22 (68.8) | 13 (86.7) | 8 (88.9) | 5 (83.3) | ||
| Jaundice on presentation, n (%) | ||||||
| No | 27 (51.9) | 15 (68.2) | 0.21 | 7 (70) | 8 (66.7) | >0.95 |
| Yes | 25 (48.1) | 7 (31.8) | 3 (30) | 4 (33.3) | ||
| Gemcitabine-based chemotherapy, n (%) | ||||||
| No | 6 (12) | 2 (9.1) | >0.95 | 0 (0) | 2 (16.7) | 0.48 |
| Yes | 44 (88) | 20 (90.9) | 10 (100) | 10 (83.3) | ||
| Platinum chemotherapy, n (%) | ||||||
| No | 24 (48) | 6 (27.3) | 0.12 | 2 (20) | 4 (33.3) | 0.65 |
| Yes | 26 (52) | 16 (72.7) | 8 (80) | 8 (66.7) | ||
| Grade, n (%) | ||||||
| Well differentiated | 0 (0) | 1 (4.8) | 0.32 | 1 (10) | 0 (0) | 0.39 |
| Moderately differentiated | 18 (46.2) | 11 (52.4) | 6 (60) | 5 (45.5) | ||
| Poorly differentiated | 21 (53.8) | 9 (42.9) | 3 (30) | 6 (54.5) | ||
| Overall stage, n (%) | ||||||
| IIIA | 2 (4.3) | 0 (0) | 0.003* | 0 (0) | 0 (0) | >0.95 |
| IIIB | 3 (6.5) | 7 (46.7) | 3 (50) | 4 (44.4) | ||
| IVA | 6 (13) | 0 (0) | 0 (0) | 0 (0) | ||
| IVB | 35 (76.1) | 8 (53.3) | 3 (50) | 5 (55.6) | ||
Significant.
CA 19-9, carbohydrate antigen 19-9
At surgery, 10 (45%) of 22 patients underwent definitive resection. A higher proportion of those with a complete resection were female (8/10, 80% vs 4/12, 33%, p=0.043) and a lower proportion had incidental gallbladder cancer (1/10, 10% vs 7/12, 58%, p=0.031). No other clinical factors were significantly different between the groups (p=0.14–0.95) (Table 2).
All 10 patients with definitive resection following chemotherapy received gemcitabine-based therapy and were diagnosed after 2008. Disease characteristics and clinicopathologic and operative details regarding these patients are compiled in Table 3. The type of procedure was chosen in order to obtain negative surgical margins. Segment 4/5 resection was performed in 6 patients (60%), hemihepatectomy in 2 patients (20%), and extended hepatectomy in 2 patients (20%). All 10 patients (100%) had lymphadenectomy and the operation included a concurrent bile duct resection in 6 instances (60%). Extra-organ resection was performed in 2 cases (20%) with one pancreaticoduodenectomy and a partial duodenal resection.
Table 3.
Clinicopathologic and Operative Details of Patients with Definitive Resection
| Type | Nodal | Locally advanced | Chemotherapy regimen | Response | Procedure | Bile duct resection | Lymphad enectomy | Extra-organ resection | EBL | Operati ve time, min | Histology | Final stage | Vital status | OS, mo |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Primary | Yes | Yes | Gemcitabine | Partial | Hepatectomy | Yes | Yes | No | 2,00 0 | 365 | Adenoca | T3N1 | Dead | 50.9 |
| Primary | Yes | No | Gemcitabine + Erlotinib | Partial | Segment 4–5 Resection | No | Yes | No | 300 | 248 | Adenoca | T3N2 | Dead | 55.3 |
| Primary | No | Yes | Gemcitabine + Cisplatin + MEK162 | Stable | Hepatectomy | Yes | Yes | No | 500 | 268 | Adenoca | T2N0 | Alive | 3.8 |
| Primary | Yes | No | Gemcitabine + Cisplatin | Stable | Segment 4–5 resection | Yes | Yes | Yes | 750 | 337 | Adenoca | T3N2 | Alive | 8.4 |
| Primary | Yes | Yes | Gemcitabine + Cisplatin | Stable | Extended hepatectomy | Yes | Yes | Yes | 800 | 386 | Adenoca | T3N1 | Alive | 14.7 |
| Primary | No | Yes | Gemcitabine + Cisplatin | Stable | Extended hepatectomy | Yes | Yes | No | 750 | 356 | Adenoca | T3N1 | Dead | 30.3 |
| Primary | Yes | No | Gemcitabine + Cisplatin | Partial | Segment 4–5 resection | No | Yes | No | 50 | 141 | Adenoca | T3N1 | Dead | 19.6 |
| Primary | Yes | No | Gemcitabine + Cisplatin | Stable | Segment 4–5 resection | Yes | Yes | No | 1000 | 160 | Adenoca | T3N0 | Dead | 11.7 |
| Incidental | Yes | No | Gemcitabine + Cisplatin | Stable | Segment 4–5 resection | No | Yes | No | 150 | 177 | Adenoca | T2N1 | Alive | 38.7 |
| Primary | Yes | No | Gemcitabine + Cisplatin | Partial | Segment 4–5 resection | No | Yes | No | 200 | 178 | Adenoca | T0N0 | Alive | 7.3 |
EBL, estimated blood loss, OS, overall survival.
The operative findings that precluded definitive resection were peritoneal involvement (4/12, 33%), discontinuous liver metastases (1/12, 8%), locally unresectable tumors due to vascular, biliary, or multi-organ involvement (5/12, 42%), or disseminated nodal disease beyond scope of resection (2/12, 17%). Vascular reconstruction was a contraindication to resection. These 12 patients were considered to have incomplete resections. The types of procedures performed are as listed: laparoscopy and biopsy (3/12, 25%), laparotomy and biopsy (5/12, 42%), Roux-en-Y biliary enteric bypass (1/12, 8%), palliative cholecystectomy (1/12, 8%), palliative gastrojejunostomy (1/12, 8%), and segment 4/5 resection with unresectable nodes invading renal vein (1/12, 8%). Of these patients, only one (8%) had previously been explored.
Pathology
All 10 patients with definitive resection had adenocarcinoma of the gallbladder- 1 was well differentiated (10%), 6 were moderately differentiated (60%), and 3 were poorly differentiated (30%). Final pathology revealed N1 (5/10) or N2 (2/10) nodal disease in 7 (70%) of the resection specimens. In the others, 2 patients had remarkable responses to chemotherapy with 90% and 100% treatment response respectively on pathologic review. One patient had imaging highly suspicious for nodal disease at presentation, but on final pathology was T3N0 (Table 3).
Overall Survival
At the end of followup, 61 patients had died, and median OS for the entire group of patients was 14.2 months (95% CI: 11.3–17.9). The estimated 1 and 3 year survival were 0.58 (95% CI: 0.46–0.69) and 0.15 (95% CI: 0.07–0.25) (Figure 2a). Median OS differed based on treatment response (p<0.001). Median OS was: 4.2 months for patients with progression (95% CI: 0.2–10.9), 13.5 months for patients with stable disease (95% CI: 9.0–26.1), and 12.1 months for patients with partial response (95% CI: 6.1–19.7) (Figure 2b). Additionally, OS differed based on definitive resection status (p=0.003). Those who had a definitive resection had a median survival of 50.1 months (95% CI: 11.7–55.3 months) compared 10.8 months (95% CI: 4.1–23.6 months) for those who went to operation but did not achieve a curative resection (Figure 2c).
Figure 2.
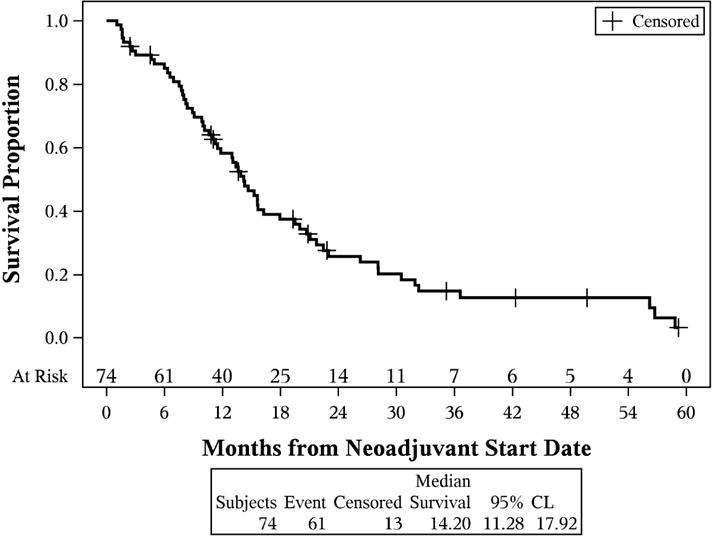
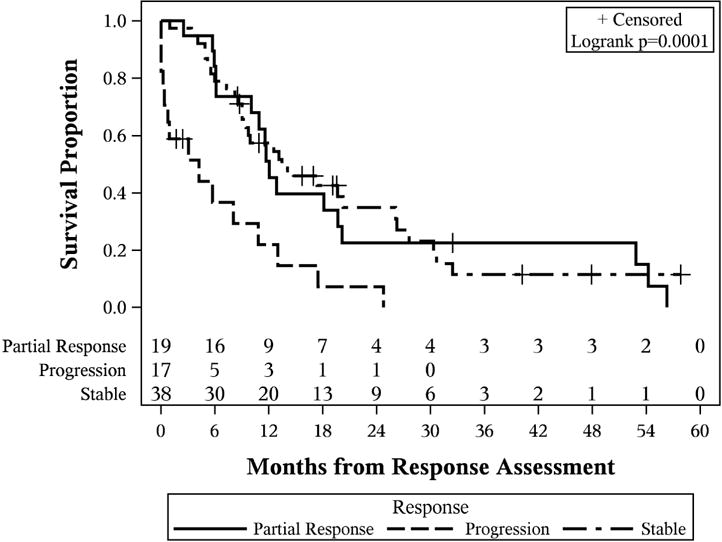
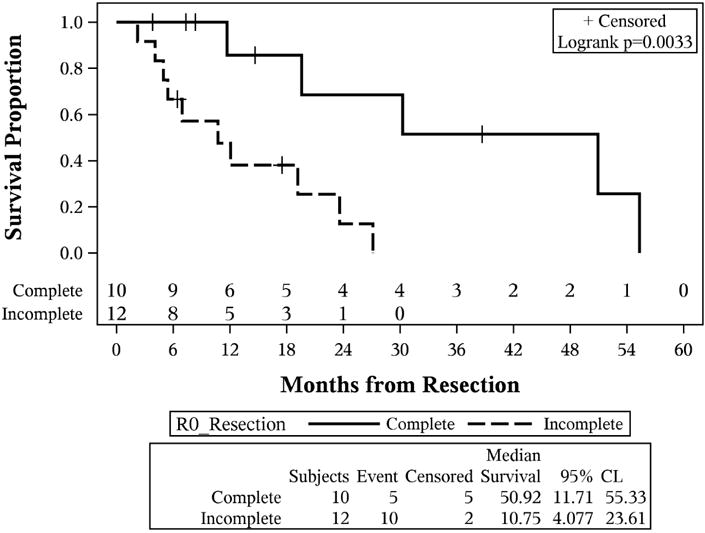
Kaplan-Meier curves. (A) Overall survival of the entire cohort of patients with locally advanced gallbladder carcinoma; (B) overall survival stratified by initial response assessment; (C) overall survival stratified by ability to achieve definitive curative resection.
Discussion
Locally advanced and node positive gallbladder cancer is a rare malignancy that has limited therapeutic options. Patients may have imaging or biopsy-proven evidence of nodal disease or present with jaundice and a locally aggressive portal mass. Regardless of the method of diagnosis, improved survival will be associated with favorable responses to chemotherapy and definitive surgical resection (16). The observed response rates in our retrospective study are consistent with those from the previously published prospective trial (ABC-02) in unresectable and metastatic biliary tract cancer (12). Gemcitabine and cisplatin has become the standard treatment for patients with locally advanced biliary cancer, but in these series there are no patients that achieve a complete response from chemotherapy alone. Therefore, resection continues to be the goal for patients with this disease. Identifying patients that will proceed to surgery and benefit from this procedure remains challenging, and it introduces a discussion regarding the timing of exploration and chemotherapy for all locally advanced tumors. As such, it was first necessary to report the institutional experience with this malignancy and describe the percentage of patients that have received definitive surgery following administration of chemotherapy.
The patients in our study had a median survival of approximately one year (14 months). This reiterates the poor outcomes of locally advanced or lymph node involved GBCA. However, a proportion of patients with stable disease or partial response to chemotherapy proceeded to surgery (30%). Among the whole cohort, advanced stage and previous exploration were different in patients selected for surgery. This association was expected and likely reflects clinical judgment and operative practice regarding this aggressive malignancy. Although selection biases for surgery are present and the number of patients is small, the lack of an association between preoperative factors and resection status may suggest that exploration is sometimes necessary to definitively assess for resection in locally advanced GBCA. As such, approximately one half of patients explored following chemotherapy were able to have complete resections (10/22, 45%). In this group, definitive resection corresponded with an improvement in outcomes just like those patients able to achieve up-front resection without chemotherapy (6).
To our knowledge, this is the second retrospective series looking at a single institution’s surgical experience following chemotherapy for locally advanced or node positive gallbladder cancer (17). The rate of definitive resection differed from the previous study by Sirohi et al. In their study, 46% of patients (17/37) had R0 resections after receiving gemcitabine and either cisplatin or oxaliplatin. These results are encouraging, but differ from the rate of 14% observed in this analysis. While both studies demonstrate the possibility of R0 resection and associated improvement in survival, the discrepancy in rate of definitive resections may be related to the extent of disease and selection for up-front surgical exploration. In the study by Sirohi et al, less than half of the patients were selected for neoadjuvant chemotherapy because of suspected nodal involvement (15/37, 41%), and the most common reason for inclusion was invasion of the hepatic parenchyma, which is typically not a contraindication for initial resection. In contrast, the majority of patients in our study had evidence of nodal disease (57/74, 77.0%) as tumor infiltrating the liver was not reason alone to delay immediate resection. Furthermore, patients were excluded in the study by Sirohi et al if there was evidence of locally advanced disease with vascular invasion, and it is not reported what percentage of patients presented with jaundice. By contrast, locally advanced and unresectable lesions based on vascular and biliary involvement were reason for inclusion in the study at our institution. We believe these patients, in which observation of disease biology prior to an attempt at resection would be clinically useful, should be included in an analysis of locally advanced disease. The results of these two studies should therefore be interpreted in light of the patient characteristics and inclusion criteria.
Our findings describe the poor prognosis of locally advanced or lymph node involved GBCA, but also demonstrate that a subset of patients respond to chemotherapy and have improved outcomes with definitive surgery. These results suggest surgical re-evaluation may be beneficial for patients that have a favorable response to chemotherapy to select operative candidates. The treatment strategy of preoperative chemotherapy has potential to increase the number of definitive resections and assess disease biology. Furthermore, GBCA has a tendency to recur early with distant metastases (18), and assessing disease biology prior to an operation would spare the surgical morbidity for a patient with early progression. This technique is being utilized in other malignancies encountered by a hepatobiliary surgeon including borderline resectable colorectal liver metastases and pancreatic cancer (19, 20). This retrospective study reports the surgical outcomes and survival of patients treated with chemotherapy for locally advanced or lymph-node positive GBCA. With improving chemotherapy options and the development of targeted treatments based on mutation profiling, the frequency of this treatment strategy could potentially increase (21, 22). It is also important to note that while this study analyzed patients with chemotherapy administration for locally advanced disease, it is possible that the observation time alone helps improve patient selection. Ausania et al showed that a technique of delayed-restaging (instead of neoadjuvant chemotherapy) in incidental gallbladder cancer prevented 49% of patients from being subjected to an operation with early progression (23). However, a subset of patients in our sample responded to chemotherapy, and this would not have occurred with observation alone. Predicting these patients is not possible, thus the standard care at our institution was to administer chemotherapy to observe disease biology and response with repeat imaging.
This study had several limitations. First, it was retrospective in nature and thus subject to inherent selection biases and missing data. Patients that were included may represent a subset with more favorable biology and performance status since they were referred and assessed by a hepatobiliary surgeon. As such, this selected group may not fully represent the entirety of patients with locally advanced or lymph node involved GBCA as a whole. For this reason, the number of definitive resections achieved in our sample may potentially be an overestimation of all patients with locally advanced GBCA. Second, as a retrospective study, selected patients were not assessed for response at a predefined time point nor did they undergo surgery at the same interval. The time between response assessment and surgery was variable. Some patients had a second CT scan which likely contributed to the selection for surgery, but this was not standardized across all patients. Also, patients were included based on various criteria including imaging, biopsy, or previous exploration. A lower proportion of patients with previous exploration were taken to the operating room for another attempt at resection and therefore the groups (inclusion based on imaging versus previous exploration) introduces a potential bias regarding subsequent surgery. It is possible that routine neoadjuvant chemotherapy with modern regimens may increase the rates of definitive resection, but this study was not able to address that question. Interestingly, all 10 patients with definitive resection following chemotherapy had surgery within the last 7 years. This suggests a potential trend in physician practice towards chemotherapy, reassessment, and surgery for patients with a good response. Lastly, our sample size was small, especially with regard to the number of patients who underwent attempted curative resection. A prospective, appropriately-powered, study with standardized protocol for response assessment and selection for laparoscopy/laparotomy is necessary to truly assess the benefit of neoadjuvant chemotherapy followed by resection for this disease. Nonetheless, this retrospective study provides a necessary basis for subsequent investigations regarding preoperative chemotherapy and surgical outcomes for locally advanced GBCA.
Conclusion
Our institutional experience with locally advanced or lymph node involved gallbladder cancer reinforces that this is a challenging disease with poor outcomes. However, in a subset of well-selected patients with favorable response to chemotherapy, definitive resection is possible and is associated with long-term survival. We recommend surgical re-evaluation following chemotherapy for locally advanced gallbladder cancer to select potential operative candidates. Neoadjuvant chemotherapy for locally advanced or lymph node positive gallbladder cancer should be evaluated in prospective trials.
Précis.
In locally advanced gallbladder cancer, definitive resection was possible in a subset of patients selected for operation after a favorable response to chemotherapy and was associated with better survival outcomes. We recommend surgical re-evaluation after chemotherapy to select potential operative candidates. Neoadjuvant chemotherapy should be evaluated in a prospective trial.
Acknowledgments
This work was supported in part by the NIH/NCI P30 CA008748 Cancer Center Support Grant. Poster presented at the Western Surgical Association 124th Scientific Session, Coronado, CA, November 2016.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Information: Nothing to disclose.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Cubertafond P, Gainant A, Cucchiaro G. Surgical treatment of 724 carcinomas of the gallbladder. Results of the French Surgical Association Survey. Ann Surg. 1994;219:275–280. doi: 10.1097/00000658-199403000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilkinson DS. Carcinoma of the gall-bladder: an experience and review of the literature. Australian N Zealand J Surg. 1995;65:724–727. doi: 10.1111/j.1445-2197.1995.tb00545.x. [DOI] [PubMed] [Google Scholar]
- 4.Pack GT, Miller TR, Brasfield RD. Total right hepatic lobectomy for cancer of the gallbladder; report of three cases. Ann Surg. 1955;142:6–16. doi: 10.1097/00000658-195507000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D'Angelica M, Dalal KM, DeMatteo RP, et al. Analysis of the extent of resection for adenocarcinoma of the gallbladder. Ann Surg Oncol. 2009;16:806–816. doi: 10.1245/s10434-008-0189-3. [DOI] [PubMed] [Google Scholar]
- 6.Fong Y, Jarnagin W, Blumgart LH. Gallbladder cancer: comparison of patients presenting initially for definitive operation with those presenting after prior noncurative intervention. Ann Surg. 2000;232:557–569. doi: 10.1097/00000658-200010000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 8.Li W, Qin J, Sun YH, Liu TS. Neoadjuvant chemotherapy for advanced gastric cancer: a meta-analysis. WorldJ Gastroenterol. 2010;16:5621–5628. doi: 10.3748/wjg.v16.i44.5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hawkins WG, DeMatteo RP, Jarnagin WR, et al. Jaundice predicts advanced disease and early mortality in patients with gallbladder cancer. Ann Surg Oncol. 2004;11:310–315. doi: 10.1245/aso.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 10.Fong Y, Wagman L, Gonen M, et al. Evidence-based gallbladder cancer staging: changing cancer staging by analysis of data from the National Cancer Database. Ann Surg. 2006;243:767–771. doi: 10.1097/01.sla.0000219737.81943.4e. discussion 771–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ito H, Ito K, D’Angelica M, et al. Accurate staging for gallbladder cancer implications for surgical therapy and pathological assessment. Ann Surg. 2011;254:320–325. doi: 10.1097/SLA.0b013e31822238d8. [DOI] [PubMed] [Google Scholar]
- 12.Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 13.Edge SB, American Joint Committee on Cancer . AJCC cancer staging manual. 7th. xiv. New York: Springer; 2010. p. 648. [DOI] [PubMed] [Google Scholar]
- 14.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 11) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 15.Butte JM, Gonen M, Allen PJ, et al. The role of laparoscopic staging in patients with incidental gallbladder cancer. HPB. 2011;13:463–472. doi: 10.1111/j.1477-2574.2011.00325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dixon E, Vollmer CM, Jr, Sahajpal A, et al. An aggressive surgical approach leads to improved survival in patients with gallbladder cancer: a 12-year study at a North American Center. Ann Surg. 2005;241:385–394. doi: 10.1097/01.sla.0000154118.07704.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sirohi B, Mitra A, Jagannath P, et al. Neoadjuvant chemotherapy in patients with locally advanced gallbladder cancer. Future Oncol (London, England) 2015;11:1501–1509. doi: 10.2217/fon.14.308. [DOI] [PubMed] [Google Scholar]
- 18.Jarnagin WR, Ruo L, Little SA, et al. Patterns of initial disease recurrence after resection of gallbladder carcinoma and hilar cholangiocarcinoma: implications for adjuvant therapeutic strategies. Cancer. 2003;98:1689–1700. doi: 10.1002/cncr.11699. [DOI] [PubMed] [Google Scholar]
- 19.Lehmann K, Rickenbacher A, Weber A, et al. Chemotherapy before liver resection of colorectal metastases: friend or foe? Ann Surg. 2012;255:237–247. doi: 10.1097/SLA.0b013e3182356236. [DOI] [PubMed] [Google Scholar]
- 20.Blazer M, Wu C, Goldberg RM, et al. Neoadjuvant modified (m) FOLFIRINOX for locally advanced unresectable (LAPC) and borderline resectable (BRPC) adenocarcinoma of the pancreas. Ann Surg Oncol. 2015;22:1153–1159. doi: 10.1245/s10434-014-4225-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Javle M, Rashid A, Churi C, et al. Molecular characterization of gallbladder cancer using somatic mutation profiling. Human Pathol. 2014;45:701–708. doi: 10.1016/j.humpath.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Javle M, Churi C, Kang HC, et al. HER2/neu-directed therapy for biliary tract cancer. J Hematol Oncol. 2015;8:58. doi: 10.1186/s13045-015-0155-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ausania F, Tsirlis T, White SA, et al. Incidental pT2–T3 gallbladder cancer after a cholecystectomy: outcome of staging at 3 months prior to a radical resection. HPB. 2013;15:633–637. doi: 10.1111/hpb.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]


