Abstract
The soluble epoxide hydrolase (sEH) is a regulatory enzyme responsible for the metabolism of bioactive lipid epoxides of both omega-6 and omega-3 long chain polyunsaturated fatty acids. These natural epoxides mediate cell signaling in several physiological functions including blocking inflammation, high blood pressure and both inflammatory and neuropathic pain. Inhibition of the sEH maintains the level of endogenous bioactive epoxy-fatty acids (EpFA) and allows them to exert their generally beneficial effects. The Akita (Ins2Akita or Ins2C96Y) mice represent a maturity-onset of diabetes of the young (MODY) model in lean, functionally unimpaired animals, with a sexually dimorphic disease phenotype. This allowed for a test of male and female mice in a battery of functional and nociceptive assays to probe the role of sEH in this system. The results demonstrate that inhibiting the sEH is analgesic in diabetic neuropathy and this occurs in a sexually dimorphic manner. Interestingly, sEH activity is also sexually dimorphic in the Akita model, and moreover correlates with disease status particularly in the hearts of male mice. In addition, in vivo levels of oxidized lipid metabolites also correlate with increased sEH expression and the pathogenesis of disease in this model. Thus, sEH is a target to effectively block diabetic neuropathic pain but also demonstrates a potential role in mitigating the progression of this disease.
Keywords: soluble epoxide hydrolase (sEH), epoxy-fatty acid (EpFA), anti-nociception, chronic pain, diabetic neuropathy
1. Introduction
Diabetes is a growing worldwide epidemic and results in many comorbidities including hypertension, nephropathy, retinopathy and neuropathy[1]. Diabetic neuropathy has a profound negative impact on patients and available therapies have severe side effects and are often counter indicated for other comorbid conditions [2]. There is a great need for new therapeutics as well as relevant models to test them that have a potential for translation to man.
The Akita mouse model was discovered in the late 1990s and has been developed as a non-obese, maturity-onset of diabetes of the young (MODY) mouse model for Type I diabetes (Yoshioka 1997). It has important advantages compared to other models of diabetes used to study neuropathy, among other disorders. First, it is an autosomal dominant murine Ins2C96Y mutation with a progressive disease into adulthood which has a syntenic chromosomal conversion to human, and thus, is similar to the development of disease in humans. This obviates much of the argumentation around using streptozocin to chemically induce diabetes since streptozocin has been at times suggested to be neurotoxic [3]. Second, it is a non-obese model, and therefore is not subject to the complications of impaired motor function for behavioral tasks as observed with ob/ob or db/db mouse models. Third, it allows for an investigation of a dimorphic phenotype. The heterozygous Akita mice, particularly the males, progress to pernicious hyperglycemia and resulting secondary pathologies of diabetes providing a unique opportunity to study the physiopathologies of the disease and therapeutic interventions to alleviate them. Homozygous Ins2 Akita males are subject to high mortality at a young age [4] while the homozygous wildtype littermates are used as asymptomatic controls. Here we employ this mouse model to study the effects of enzyme inhibition on the developed diabetic neuropathy and probe differences in the sexually dimorphic expression of the phenotype to answer basic questions about the pathology in these animals.
The experiments here use the naturally occurring Akita mouse model to test the hypothesis that inhibiting the soluble epoxide hydrolase enzyme (sEH) mediates analgesia against the chronic pain of diabetic neuropathy. The sEH is a master regulatory enzyme in the arachidonic acid (ARA) cascade downstream of the cytochrome P450 enzymes that act on long chain polyunsaturated fatty acids (PUFA) as substrates. The sEH transforms epoxy-fatty acids (EpFA) into the vicinal diols of several classes of lipids including both omega-3 and 6 derived metabolites. The EpFA are stabilized in vivo by the inhibition of the sEH and have demonstrated anti-inflammatory, antihypertensive, and analgesic properties [5–7]. The EpFA have also demonstrated efficacy against chemically induced diabetic neuropathy [8]. Here we use the Akita mouse model to inquire if sEH inhibition is analgesic in this naturally progressing disease, and importantly, if there are sexual dimorphisms in the pain responses. We used the conditioned place preference assay to investigate if sEHI block neuropathic pain. We then tested both male and female Akita mice for sEH enzyme activity and examined the oxylipin profile in the same groups.
2. Materials and Methods
2.1 Animals
All procedures and animal care adhered the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications 8th Edition 2011) and were performed in accordance with the protocols approved by the Animal Use and Care Committee (IACUC) of the University of California, Davis. Great care was taken to minimize suffering of the animals and to reduce the number of animals used. Experiments on heterozygous Akita and wildtype littermate controls used mice bred from wild type C57BL/6J females crossed with heterozygous Akita males purchased from Jackson Laboratories (Sacramento, CA) and maintained at the facilities of the University of California, Davis. The Akita mice were housed under standard conditions (25°C) in a fixed 12-h light/dark cycle with ad libitum food and water. Male and female Akita offspring and their wildtype littermate controls were used in the studies. For all the behavioral tests, the Akita mice were cohoused with their littermate controls for the duration of the experiments without identification of their disease state. They were randomly assigned to treatment or vehicle groups and tested intermingled for the studies. Only at sacrifice were the individual mice identified as Akita or littermate control and added to the appropriate groups. After completion of the nociceptive assays the mice were assessed for hyperglycemia (blood glucose measured in mg/dl) with a commercial blood glucose meter (Bayer, Contour). Previously when this colony was assessed by genotyping with DNA purified from tail snips taken after sacrifice [9] the results corresponded overwhelmingly with the phenotypic characterization and therefore the phenotypic identification was used for the current study.
For brevity we refer to the Akita (Ins2Akita or Ins2C96Y) heterozygous diabetic mice as the Akita mice and the homozygous wildtype asymptomatic mice as the littermate controls for both sexes. Akita male mice develop robust hyperglycemia starting at 5 weeks of age and subsequent neuropathy while the Akita females develop hyperglycemia later and less robustly. At 8 and 12 weeks the mice were assessed with a small battery of functional tests and for a decrease in hind paw mechanical withdrawal thresholds (MWTs) indicating allodynia.
2.2 Chemicals
For these experiments the sEH inhibitor t-TUCB: trans-4-[4-(3-trifluoromethoxyphenyl-l-ureido)-cyclohexyloxy]-benzoic acid (also referred to as UC1728) was synthesized in house and characterized previously [10, 11] . Doses of t-TUCB were formulated immediately before use in PEG400 for the experiments and injected subcutaneously in a 50 μl volume.
2.3 Nociceptive and Motor Skill Bioassays
The conditioned place preference (CPP) assay used a previously described apparatus [12]. Briefly, the CPP apparatus is a 30×16×20 cm acrylic box with equal sides of distinct stimuli using wall patterns (visual) and floors (tactile). Mice are habituated to the box on 2 consecutive days. After this, a pre-conditioning measurement is assessed in the apparatus with free access to both chambers (30 min). This is followed by 3 days of conditioning where the mice receive vehicle and are immediately isolated to one chamber in the morning. At least four hours after the vehicle, the same mouse is given an sEH inhibitor and immediately isolated to the counterbalanced chamber. On the next day, mice are tested without injection for preference in the apparatus with free access to both chambers for 30 min. Measurements are reported as the test time minus preconditioning time, expressed in seconds, in the drug paired chamber. Increased time spent in the drug-paired chamber (non-preferred chamber) indicates preference for that chamber. The vehicle control group receives PEG400 in the non-preferred chamber at least 4 hrs after sham saline injection in the preferred chamber. In preliminary tests to refine the experimental conditions PEG400 vehicle or saline injections showed no differences compared to naïve mice.
For observation of spontaneous locomotion, mice were assessed in an open-field arena (40×40×30 cm) of a 16-square grid clean floor as previously described [12]. The open field assay was tested for 2 minutes and then mice were returned to their home cage. The open field assay was scored manually with the score a combination of vertical rears and lines crossed completely with both hind paws. Scores are reported as the average per group ± S.E.M.
All groups of mice were also tested in the von Frey assay. For this an electronic von Frey aesthesiometer (IITC, Woodland Hills, CA) was used to quantify allodynia in the mice. Mice were acclimated to the apparatus made of clear acrylic chambers on a steel mesh floor. The mouse hind paw was probed through the mesh with a rigid tip probe to measure the grams of force required to elicit a hind paw withdrawal. The mechanical withdrawal thresholds (MWTs) were measured 3 times per mouse at 1 minute intervals for each point. The report is an average and S.E.M. for each group of mice tested under similar conditions. The mean MWT for the Akita 12WK male mice averaged 6.0± 03 grams of force to elicit a hind paw withdrawal which was significantly lower than the littermate controls indicating allodynia (identification or groups was determined at the end of study and testing was blinded). MWTs for female Akita 12WK mice were 6.5± 0.3 grams and were not significantly different from female or male controls at the same time period.
The same gram scale meter was used to with a T-bar attachment to assess the grip strength of the mice. For this test mice are allowed to grasp the T-bar with their front paws and then are pulled away from the bar which records their grip strength in grams. As with previous tests the grip strength was measured 3 times per mouse at 1 minute intervals for each point. The report is the average and S.E.M. for each group of mice tested under similar conditions.
A hot plate (IITC, Woodland Hills, CA) set at 55ºC was used to determine the thermal sensitivity of the mice. For the assay the mice were placed on the pre-heated hotplate and observed for the time until they displayed a hind paw lick or jump and then were immediately removed. The hotplate response was measured 3 times per mouse with at least a one-minute interval between each score. The report is the average latency in seconds and S.E.M. for each group of mice tested under similar conditions.
2.4 sEH activity assay
Tissues samples were taken at necropsy and were flash frozen at after sampling and stored at −80ºC until further processing. Prior to the assay, tissues were weighed and homogenized in ice cold PBS buffer with 0.1% EDTA and protease inhibitor (PMSF 1mM and DTT 1mM). These homogenates were then aliquoted and flash frozen for sEH activity assays and oxylipin analysis. The sEH activity for these homogenates was then determined by previously reported methods using 3H trans-diphenyl-propene oxide (t-DPPO) as substrate [13].
2.5 Oxylipin analysis
Plasma samples for eicosanoid analysis were obtained from cardiac puncture of deeply anesthetized mice that after exsanguination were necropsied for tissues. Plasma was separated, flash frozen and stored at −80C until analysis. Tissues were flash frozen at necropsy and stored at −80C until further processing. Tissues were weighed and homogenized in ice cold PBS buffer with 0.1% EDTA and protease inhibitor. These homogenates were then aliquoted and flash frozen for sEH activity assays and oxylipin analysis. The oxylipin analysis followed the method of Yang et al. [14]. Briefly internal standards were added to the homogenates, the samples extracted with a solid phase extraction column (Oasis-HLB, Waters), the samples were evaporated until dry and reconstituted with a second internal standard in 50 μL methanol. The solution was then analyzed by LC-MS/MS per published methods.
2.6 Statistics
Data were analyzed using SigmaPlot 11.0 for windows (Systat Software Inc., San Jose, CA). The applied statistical methods are reported in the results section with p values ≤ 0.05 considered significant.
3. Results
3.1 Inhibiting sEH induces a CPP response in Akita mice with developed neuropathy
For all the behavioral tests the Akita mice were cohoused with their littermate controls for the duration of the experiments and randomly assigned to treatment or vehicle groups and tested intermingled for the studies. Only at sacrifice were the individual mice identified as Akita or littermate control and added to the appropriate groups. The Akita mouse model was used for these experiments because the Akita diabetic mice are lean, motor competent mice that can be assessed in these assays as a good functional comparison to controls. Male mice were tested at 8 weeks (8WK) old. At this time the mice have been hyperglycemic for several weeks. As expected, a 10 mg/kg dose of t-TUCB administered each day of the 3-day conditioning had no CPP effect in control mice compared to the combined vehicle group (combined: control and Akita vehicle treated mice). This dose also lacked statistical significance in the heterozygous mice although there was a trend toward an induced CPP response (Fig. 1, p = 0.280, One Way ANOVA, n= 8–15). The 8WK Akita mice are hyperglycemic by this age and demonstrated allodynia with significantly lower von Frey scores compared to littermate controls (Table 1).
Figure 1. Inhibition of sEH does not induce a statistically significant CPP response in Akita 8WK male mice.
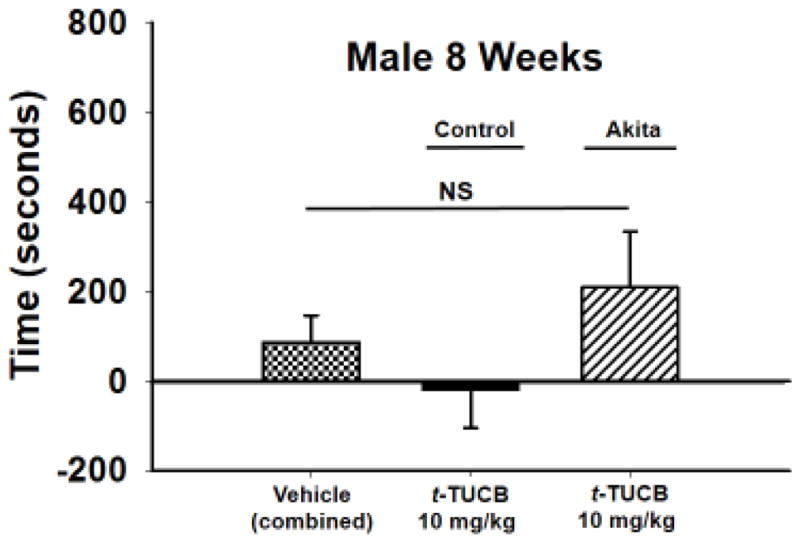
The conditioned place preference assay uses negative reinforcement to assess pain relief in neuropathic animals. When tested in the CPP assay the 8WK Akita male mice demonstrated a trend toward a conditioned response but it lacked statistical significance (p = 0.280). There was no response to the same treatment of 10 mg/kg t-TUCB in the male littermate controls. The vehicle (combined) group consists of vehicle tested in Akita and littermate controls. Measurements are reported as the test time minus preconditioning time (seconds), in the drug paired chamber.
Table 1.
Functional and Nociceptive Assays of 8 Week Diabetic Males.
| Assay | Akita Males | +/− | Control Males | Akita Males | +/− | Control Males | Akita Females | +/− | Control Females |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| 8 weeks | 12 weeks | ||||||||
|
|
|||||||||
| Open Field (cross + rear) | 88 ± 24 | 89 ± 18 | *72 ± 4.8 | 85 ± 4.2 | 82 ± 4.3 | 90 ± 6.2 | |||
| Von Frey (gram) | *5.9 ± 0.2 | 6.8 ± 0.3 | *6.0 ± 0.3 | 7.3 ± 0.5 | 6.5 ± 0.3 | 5.7 ± 0.4 | |||
| Grip Strength (gram) | *81 ± 16 | 94 ± 17 | *84 ± 3.6 | 95 ± 3.8 | 91 ± 4.4 | 84 ± 2.5 | |||
| Hot Plate 55°C (seconds) | 11 ± 2.1 | 9.7 ± 2.7 | 11 ± 0.6 | 12 ± 0.6 | 11 ± 1.0 | 10 ± 0.8 | |||
|
| |||||||||
| Weight (gram) | *20 ± 1.3 | 23 ± 1.7 | *22 ± 0.6 | 24 ± 0.7 | 21 ± 0.4 | 20 ± 0.5 | |||
|
| |||||||||
| Blood Glucose (mg/dl) | 531 ± 19 | 148 ± 29 | 553 ± 31 | 153 ± 6.6 | 363 ± 28 | 123 ± 9.3 | |||
8 Week males, control versus Akita by T-tests: von Frey t = −2.542, 36 degrees of freedom, p = 0.015), open field t = −0.111, 36 degrees of freedom, p = 0.912, grip strength t = −2.352, 36 degrees of freedom p= 0.024, hot plate t = 1.019, 36 degrees of freedom p = 0.315, body weight (grams) t = −5.351, 36 degrees of freedom p≤0.001.
12 Week males, control versus Akita by T-tests: von Frey, Mann-Whitney Rank Sum Test, T = 117.000 n(small)= 12 n(big)= 13, p = 0.036, open field, t-Test, t = −2.094, 23 degrees of freedom p = 0.048, grip strength t = −2.082, 23 degrees of freedom p = 0.049, hot plate, t = −0.721, 23 degrees of freedom, p = 0.478, body weight, Mann-Whitney Rank Sum Test, T = 108.500 n(small)= 12 n(big)= 13, p = 0.010.
12 Week females, control versus Akita by T-tests: von Frey t-Test, t = −1.499, 24 degrees of freedom, p = 0.147, open field t = 1.871 with 24 degrees of freedom, p = 0.074, grip strength t = −1.471 with 17 degrees of freedom, p= 0.160, hot plate t = −0.892 with 25 degrees of freedom, p = 0.381, body weight t = −1.471 with 25 degrees of freedom, p= 0.154.
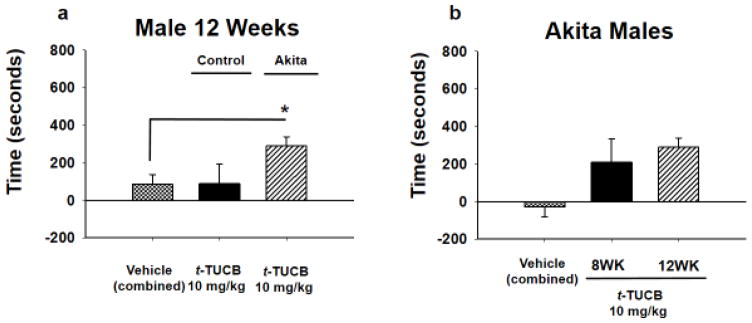
When Akita male mice were tested at 12 weeks (12WK) with the same 10 mg/kg dose, t-TUCB elicited a significant CPP response indicating pain relief (Fig 2A, p = 0.024, One Way ANOVA, Holm-Sidak post hoc, n=4–15). The Akita 12WK mice demonstrated lower MWTs (allodynia) in the von Frey assay and these mice also scored lower in the open field and grip strength compared to littermate controls (Table 1, includes statistics). When the responses were compared, the Akita 12WK mice demonstrated a significant response compared to the combined vehicle controls (combined: 8WK and 12WK Akita vehicle treated mice) and the Akita 8WK male mice did not reach significance. (Fig 2B, p = 0.019, Kruskal-Wallis One Way ANOVA on Ranks, n=7–11).
Figure 2. sEH inhibition at 12 weeks in AK males induces a CPP response.
(A) A 10 mg/kg dose of t-TUCB was tested in a group of 12WK Akita males it elicited a CPP response indicating pain relief (*p = 0.024, combined vehicle is all control and Akita vehicle treated mice). The same dose in the littermate controls had no effect. (B) The 12WK Akita males had a significantly elevated CPP response compared to 8WK Akita males and combined controls indicating pain relief (*p = 0.019, combined vehicle is all 8Wk and 12WK Akita vehicle treated mice). Thus, this observable trend in 8WK Akita males may have correlated with the progression of the disease state in the male Akita mice.
The mechanical withdrawal thresholds (MWTs) of 8WK Akita males revealed a significant decrease compared to the littermate controls indicating allodynia (Table 1, includes statistics). They also were robustly hyperglycemic at 8WK correlating with the decline in MWTs. The 8WK Akita males additionally scored lower in grip strength compared to matched controls as anticipated, but, they had no change in the hot plate response. There is precedent for the diabetic neuropathic mice to become insensate to thermal stimuli while becoming mechanically more sensitive [15]. The open field showed no significant difference in activity at 8 weeks. The 12WK Akita males displayed a significant decline in MWT compared to littermate controls as well as grip strength and open field activity. The 12WK Akita females, although hyperglycemic, displayed no different functional behaviors than the control males. In sum the hyperglycemic females showed no statistical change compared to the littermate controls in any of the assays. The females are less hyperglycemic than the males at the same age which may affect their behaviors in the nociceptive and functional assays.
3.2 The dimorphic phenotype of the Akita model is reflected in the CPP results
Akita and littermate control females were also tested at 12 weeks for their response to sEH inhibition in the CPP assay. Only 12WK females were tested given the females do not develop as severe a hyperglycemia as the males particularly at earlier stages. The Akita females were hyperglycemic at 12 weeks but not to the severity of the Akita males including the 8WK male scores (Table 1). Despite their hyperglycemia, when the identification and grouping of the mice was completed the Akita females demonstrated similar scores in the von Frey assay, open field and grip strength assays compared to their littermate controls (Table 1). When the Akita females were tested in the CPP assay compared to the treated female littermate controls or vehicle there was no significant response to the 10 mg/kg dose of t-TUCB (Fig. 3, p = 0.608, One Way ANOVA, n= 3–6).
Figure 3. sEH inhibition does not induce a CPP in Akita female mice.
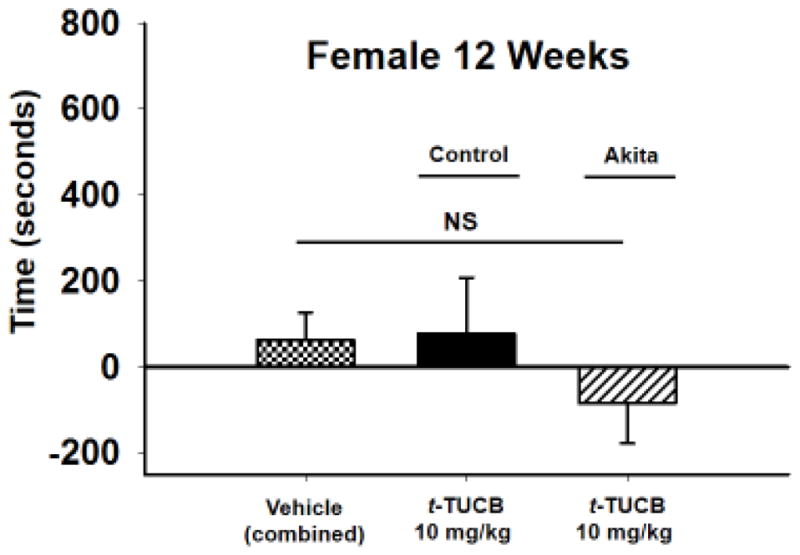
Female 12WK hyperglycemic mice were administered the same 10 mg/kg dose of t-TCUB but it did not elicit a response in the CPP assay. There was also no response in the female littermate controls (p = 0.608).
3.3 sEH enzyme activity reflects the sexual dimorphism of diabetes in Akita mice
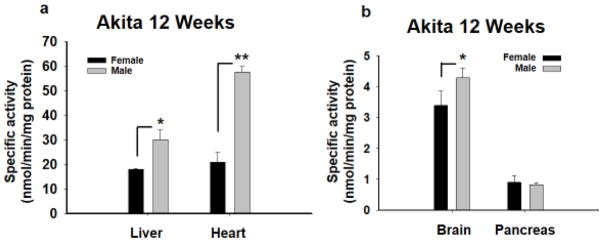
After behavioral testing was complete a sample of all the groups of 12WK mice (male and female) were assessed for sEH activity (Fig. 4). sEH activity is higher in liver (p= 0.001), brain (p = 0.009) but also most notably in the heart of Akita males versus females Akita mice (p≤0.001, One Way ANOVA per tissue, Holm-Sidak post hoc, n=4). (B) However, activity was not increased in the pancreas when Akita males and females were compared (p =0.488, One Way ANOVA per tissue, Holm-Sidak post hoc, n=4).
Figure 4. sEH enzyme activity reveals a sexual dimorphic increase in Akita mice.
The 12WK old male and female Akita mice were compared for their sEH enzyme activity in several tissues. (A) The 12WK Akita males demonstrated significant increases in sEH activity compared to Akita females in several tissues including the liver and heart with the highest increase in the heart (**p≤0.001). (B) The sEH activity was also significantly higher in the Akita male brain (*p = 0.009) versus Akita females but not pancreas (p =0.488). The sEH activity is expressed as nanomoles of hydrolyzed substrate per minutes per milligram protein.
3.4 Oxylipin ratios are increased in in a sexually dimorphic manner
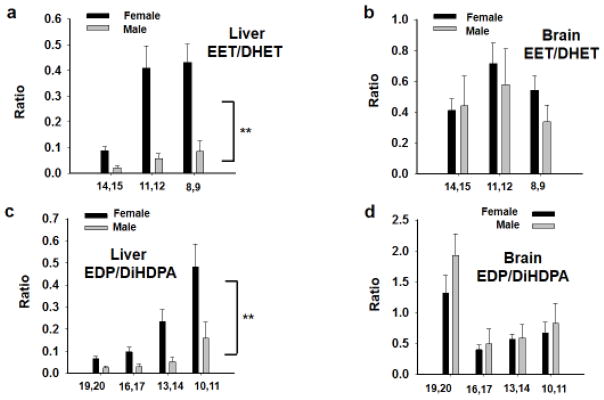
The 12WK mice were then investigated for quantification of selected oxidized lipid metabolites to correlate with enzyme activity. This analysis revealed, perhaps not surprisingly, that ratios in epoxy to dihydroxyl metabolites increased in correlation with lower sEH activity in the same tissues. This is true for ratios of both omega-6 and omega-3 derived EpFA. In the Akita 12WK mice, the omega-6 derived EET/DHET ratios in liver were robustly higher in female liver compared to males (Fig 5A, p ≤0.001, Kruskal-Wallis One Way ANOVA on Ranks, n=4). However, in the brain, although there was a trend of increases in the females, there was no statistical difference between the females and males (Fig 5B, p =0.073, Kruskal-Wallis One Way ANOVA on Ranks, n=4). The omega-3 derived EDPs demonstrated similar effects to the omega-6 class in liver with the metabolite ratios in females being higher than the males (Fig 5C, p≤0.001 Kruskal-Wallis One Way ANOVA on Ranks, n=4). The omega-3 metabolite ratios followed the same scheme as omega-6 in the brain showing no statistical change between females and males (Fig 5D, p=0.213, Kruskal-Wallis One Way ANOVA on Ranks, n=4). A more detailed description of the individual regioisomer epoxide and diol concentrations including plasma analysis appears in the supplementary information (Supplementary Tables 1–4).
Figure 5. Epoxy-fatty acid to diol ratios are elevated in Akita female versus male tissues.
(A) The omega-6 derived EET/DHET ratios in liver were elevated in Akita female liver compared to males (**p≤0.001). (B) In the brain where there was no statistical difference between the females and males (p =0.073). (C) The omega-3 derived EDPs, similar to the omega-6 lipid metabolites, were increased in the liver of Akita females compared to males (**p=0.001). The EDP ratios in the brain had no statistical change between Akita females and males (p=0.213).
4. Discussion
The sEH inhibitors (sEHI) are analgesic in several models of pain including inflammatory lipopolysaccharide and carrageenan induced pain which were some of the first models tested with sEHI [16–18]. Later the investigation broadened into testing neuropathic pain such as streptozocin induced diabetic neuropathy [7, 19]. However, there is debate whether this chemically induced model compares to the human disease [3]. Thus, we report here the naturally progressing Akita MODY model which is more similar to the development of the human disease.
The behavioral assays include the CPP, an operant assay that can assess the tonic nature of neuropathic pain. The use of t-TUCB has previously demonstrated dose dependent action in reducing in pain in diabetic animals [12]. Here Male 8WK mice were compared with male 12WK mice because male Akita heterozygotes are known to exhibit robust hyperglycemia and neuropathy prior to this age [20]. Little electrophysiological data exists for the females although the Akita females here exhibit hyperglycemia well above 200 mg/dl at 12 weeks (Table 1). In our study the Akita females had slightly lower MWTs in the von Frey assay compared to littermate controls but did not display a statistically significant increase in allodynia (Table 1). Overall the results from this naturally developing diabetes model demonstrate that sEH inhibition attenuates neuropathic pain. These data also underscore important prior findings of sEHI administration, that they are effective against pain and have no activity in the absence of pain.
4.1 sEH displays sexual dimorphic activity
The behavioral assays revealed that the Akita females do not have an induced CPP and thus a sexual dimorphism exists which correlates with the enzyme activity and oxylipin analysis. The Akita model is uniquely suited to investigate sexual dimorphism because the females differ in disease status being less susceptible to the diabetic phenotype.
The sexual dimorphic nature of sEH expression has been known since the initial description of the enzyme [21]. This difference is robustly evident in mice but occurs in multiple species [22, 23]. Most of the studies that have investigated sexual dimorphic expression of sEH found the expression or activity is higher in males versus females [21–24]. Additional studies revealed that sEH expression can be regulated by both androgen (induction) and estrogen (suppression) to varying degrees [25, 26]. For example, the addition of testosterone to intact females and castrated males greatly increased sEH activity in kidney compared to shams and ovariectomized females had increased sEH activity compared to controls [25]. More recently, dimorphic sEH expression was demonstrated in spontaneously hypertensive rat (SHR) with a similar paradigm of male and female gonadectomized versus sham surgeries. This study identified endogenous female sex steroids as the more potent driver of change in sEH protein levels [26].
Previously it has been observed that changes in mRNA of proteins did not necessarily reflect the changes in protein levels in the Akita mouse heart [27], but also that sEH protein levels were increased in Akita versus control heart [9]. We therefore focused on assessing sEH activity in heart and additional tissues. Interestingly, the results of sEH activity in Akita mice are in keeping with literature results and suggest possible endocrine involvement. Akita 12WK males had elevated sEH activity compared to females, particularly in the heart. There is a clear correlation between the severity of the disease and sEH activity presenting more in males than less symptomatic females (Fig 4). The lower enzyme activity in Akita females compared to males also correlated to the oxylipin metabolite levels which demonstrated higher epoxide to diol ratios in females versus males in several tissues (Fig 5, Supplemental Tables). Thus, there is the potential that the EpFA and lower sEH activity is protective because the hyperglycemic females are less symptomatic than males.
4.2 sEH activity correlates with disease severity
The Akita females though hyperglycemic were found to lack significant change in sEH activity compared to their littermate controls in any of the tissues investigated (Fig S1). Interestingly, the sEH activity in heart was elevated in Akita males compared to male controls as well as compared to both groups of females. Historical data in Swiss Webster mice demonstrated sEH expression increased with age after 5 weeks and more significantly in males [21]. This expression pattern of sEH increasing rapidly after puberty was recapitulated in rat as well. Dewey et al. examined sEH expression in male Akita mice over the course of disease progression. sEH protein levels showed no change at 3 and 4 weeks but increased 30% at 5 weeks and up to 140% by 12 weeks compared to controls [9]. Thus, increased sEH between sex (Akita male and female), but also age related increases within one sex (Akita males) may contribute to disease pathology.
In the CPP assay sEHI had a significant effect on diabetic neuropathy between littermate control, 8WK Akita, and 12WK Akita males (Fig 2) further demonstrating the relationship between sEH activity and disease state extends beyond only a sexual dimorphism. There is also more sEH activity in the in heart of Akita versus control 12WK males (Fig S3A). It was previously reported that sEH activity was elevated in liver of streptozocin induced diabetic versus control rats [19]. Additionally, sEH measured by qPCR and western blot [9] is increased due to the disease status between age matched Akita and control males. Thus, there is evidence that the pathology of type I diabetes correlates with increased sEH activity independent of age or sex despite differences of induced or natural disease status.
4.3 Cellular stress mechanisms and sEH inhibition
The Akita model was previously used to elucidate mechanisms of endoplasmic reticulum stress (ER stress) because the spontaneous Ins2C96Y mutation leads to a misfolded insulin protein [28]. These studies demonstrated the contribution of C/EBP homologous protein (CHOP) to beta islet cell death using a double transgenic CHOP/Akita mouse model. These mice displayed less beta cell apoptosis, longer onset to hyperglycemia and greater pancreatic insulin content. The Akita model was also previously investigated for influencing diabetes progression and sEH was found to be upregulated in a manner correlating to the developing disease [9]. Recently, the role of epoxy-fatty acids and sEH was examined in the context of ER stress and diabetic peripheral neuropathy [29]. In this study sEHI blocked neuropathic pain, synergized with ER stress inhibitors, and it was concluded that ER stress may contribute to the pathogenesis of neuropathy itself. The caution, however, is that in the double transgenic mouse study CHOP−/−/Akita mice were not improved and the effects of CHOP are nonspecific. Additionally, it has been observed that PERK and eILFα mutant mice do not activate CHOP but fail to resist ER stress [30–32]. Thus, there is a substantial role of ER stress in the pathology of diabetes but it may not be the sole mechanism [33]. There are other mechanisms initiating cellular apoptosis and therefore protecting cells from oxidative stress and inflammation [34] may contribute to improved outcomes.
There are also alternative mechanisms of action possible for sEH inhibition in the diabetic model. sEH inhibition or ablation has demonstrated an effect on blood glucose regulation by increasing insulin secretion [35]. This regulation was relative to amounts of beta cell apoptosis in wild type versus sEH KO mice with streptozocin induced diabetes [35]. It remains possible however, that reducing sEH activity attenuated ER stress and prevented the apoptosis of beta islet cells. Cardiac hypertrophy investigated in Akita mice has also indicated inflammation associated with this pathology in heart [36]. It is possible that the anti-inflammatory action of sEHI contributed here to the improvement in treated mice and contributed to a CPP response. However, cardiac inflammation would not account for the differences in allodynia between Akita and control mice (Table 1). Alternatively, there is evidence of autonomic neuropathy in Akita mice [37]. It is possible the sEHI are neuro-supportive and therefore may act on diabetic autonomic neuropathy as well as peripheral neuropathy. The CPP assay is a powerful tool to assess the non-evoked, tonic pain of diabetic neuropathy. The assay, however, may not distinguish between improved autonomic versus peripheral symptoms of neuropathy. It is possible that improved cardiovascular functioning or cellular stress attenuation contributed to the positive association in the sEHI-paired compartment. Despite this, the CPP response is most likely analgesia given the design of the assay (negative reinforcement), changes in allodynia and previous results in induced diabetic neuropathy that corroborate this outcome [12, 19]. However, future studies are warranted to probe the effects of sEH inhibition in autonomic neuropathy. Additionally, it may be an added beneficial effect that sEHI can potentially reduce the aforementioned cardiac inflammation in addition to blocking neuropathic pain. In either case, these results support the evidence that sEHI block neuropathic pain, are inactive in the absence of pain, and show no responses that are associated with addiction. In summary, there is a strong correlation between the sEH activity assays, the higher epoxide to diol ratios in females, the sexual dimorphic disease states of the animal groups, and the response to sEHI in treating their diabetic neuropathy in the Akita model.
Highlights.
Inhibiting the soluble epoxide hydrolase effectively blocks diabetic neuropathic pain
sEH activity correlates with the disease pathogenesis in the Akita model
Inhibiting sEH has a potential role in mitigating the progression of this pathology
Acknowledgments
This work was supported by the National Institute of Environmental Health Sciences (NIEHS) Grant R01 ES002710, NIEHS Superfund Research Program P42 ES004699, National Institute of Neurological Disorders and Stroke (NINDS) U54 NS079202-01and Grants NIEHS T32ES007059, NIH 5T32DC008072-05 and 5T32HL086350-08 (to K.W.). This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. A.V.G supported by a Hellman Fellowship. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. B.D.H. is a George and Judy Marcus Senior Fellow of the American Asthma Foundation.
Footnotes
Conflict of interest: The University of California holds patents on the sEH inhibitors used in this study as well as their use to treat inflammation, inflammatory pain, and neuropathic pain. BD Hammock is a co-founder and K Wagner is an employee of EicOsis L.L.C., a startup company advancing sEH inhibitors into the clinic.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, Lin JK, Farzadfar F, Khang Y-H, Stevens GA, Rao M, Ali MK, Riley LM, Robinson CA, Ezzati M. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. The Lancet. 9785;378:31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 2.Rahman MH, Jha MK, Suk K. Evolving Insights into the Pathophysiology of Diabetic Neuropathy: Implications of Malfunctioning Glia and Discovery of Novel Therapeutic Targets. Curr Pharm Des. 2016;22(6):738–57. doi: 10.2174/1381612822666151204001234. [DOI] [PubMed] [Google Scholar]
- 3.Sullivan KA, Lentz SI, Roberts JL, Jr, Feldman EL. Criteria for creating and assessing mouse models of diabetic neuropathy. Curr Drug Targets. 2008;9(1):3–13. doi: 10.2174/138945008783431763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leiter EH, Schile A. Genetic and Pharmacologic Models for Type 1 Diabetes. Current protocols in mouse biology. 2013;3(1):9–19. doi: 10.1002/9780470942390.mo120154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmelzer KR, Kubala L, Newman JW, Kim IH, Eiserich JP, Hammock BD. Soluble epoxide hydrolase is a therapeutic target for acute inflammation. Proc Natl Acad Sci U S A. 2005;102(28):9772–7. doi: 10.1073/pnas.0503279102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imig JD, Zhao X, Zaharis CZ, Olearczyk JJ, Pollock DM, Newman JW, Kim IH, Watanabe T, Hammock BD. An orally active epoxide hydrolase inhibitor lowers blood pressure and provides renal protection in salt-sensitive hypertension. Hypertension. 2005;46(4):975–81. doi: 10.1161/01.HYP.0000176237.74820.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inceoglu B, Jinks SL, Ulu A, Hegedus CM, Georgi K, Schmelzer KR, Wagner K, Jones PD, Morisseau C, Hammock BD. Soluble epoxide hydrolase and epoxyeicosatrienoic acids modulate two distinct analgesic pathways. Proc Natl Acad Sci U S A. 2008;105(48):18901–6. doi: 10.1073/pnas.0809765105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wagner K, Lee KS, Yang J, Hammock BD. Epoxy fatty acids mediate analgesia in murine diabetic neuropathy. Eur J Pain. 2016 doi: 10.1002/ejp.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dewey S, Lai X, Witzmann FA, Sohal M, Gomes AV. Proteomic analysis of hearts from Akita mice suggests that increases in soluble epoxide hydrolase and antioxidative programming are key changes in early stages of diabetic cardiomyopathy. Journal of proteome research. 2013;12(9):3920–33. doi: 10.1021/pr4004739. [DOI] [PubMed] [Google Scholar]
- 10.Hwang SH, Tsai HJ, Liu JY, Morisseau C, Hammock BD. Orally bioavailable potent soluble epoxide hydrolase inhibitors. J Med Chem. 2007;50(16):3825–40. doi: 10.1021/jm070270t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rose TE, Morisseau C, Liu JY, Inceoglu B, Jones PD, Sanborn JR, Hammock BD. 1-Aryl-3-(1-acylpiperidin-4-yl)urea inhibitors of human and murine soluble epoxide hydrolase: structure-activity relationships, pharmacokinetics, and reduction of inflammatory pain. J Med Chem. 2010;53(19):7067–75. doi: 10.1021/jm100691c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wagner K, Yang J, Inceoglu B, Hammock BD. Soluble epoxide hydrolase inhibition is antinociceptive in a mouse model of diabetic neuropathy. The journal of pain : official journal of the American Pain Society. 2014;15(9):907–14. doi: 10.1016/j.jpain.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morisseau C, Hammock BD. Measurement of soluble epoxide hydrolase (sEH) activity. Curr Protoc Toxicol. 2007;4(4) doi: 10.1002/0471140856.tx0423s33. [DOI] [PubMed] [Google Scholar]
- 14.Yang J, Schmelzer K, Georgi K, Hammock BD. Quantitative profiling method for oxylipin metabolome by liquid chromatography electrospray ionization tandem mass spectrometry. Anal Chem. 2009;81(19):8085–93. doi: 10.1021/ac901282n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Obrosova IG. Diabetic painful and insensate neuropathy: pathogenesis and potential treatments. Neurotherapeutics. 2009;6(4):638–47. doi: 10.1016/j.nurt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inceoglu B, Jinks SL, Schmelzer KR, Waite T, Kim IH, Hammock BD. Inhibition of soluble epoxide hydrolase reduces LPS-induced thermal hyperalgesia and mechanical allodynia in a rat model of inflammatory pain. Life Sci. 2006;79(24):2311–9. doi: 10.1016/j.lfs.2006.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inceoglu B, Schmelzer KR, Morisseau C, Jinks SL, Hammock BD. Soluble epoxide hydrolase inhibition reveals novel biological functions of epoxyeicosatrienoic acids (EETs) Prostaglandins Other Lipid Mediat. 2007;82(1–4):42–9. doi: 10.1016/j.prostaglandins.2006.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morisseau C, Inceoglu B, Schmelzer K, Tsai HJ, Jinks SL, Hegedus CM, Hammock BD. Naturally occurring monoepoxides of eicosapentaenoic acid and docosahexaenoic acid are bioactive antihyperalgesic lipids. J Lipid Res. 2010;51(12):3481–90. doi: 10.1194/jlr.M006007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inceoglu B, Wagner KM, Yang J, Bettaieb A, Schebb NH, Hwang SH, Morisseau C, Haj FG, Hammock BD. Acute augmentation of epoxygenated fatty acid levels rapidly reduces pain-related behavior in a rat model of type I diabetes. Proc Natl Acad Sci U S A. 2012;109(28):11390–5. doi: 10.1073/pnas.1208708109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Preux Charles AS, Verdier V, Zenker J, Peter B, Medard JJ, Kuntzer T, Beckmann JS, Bergmann S, Chrast R. Global transcriptional programs in peripheral nerve endoneurium and DRG are resistant to the onset of type 1 diabetic neuropathy in Ins2 mice. PLoS ONE. 2010;5(5):e10832. doi: 10.1371/journal.pone.0010832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gill SS, Hammock BD. Distribution and properties of a mammalian soluble epoxide hydrase. Biochem Pharmacol. 1980;29(3):389–95. doi: 10.1016/0006-2952(80)90518-3. [DOI] [PubMed] [Google Scholar]
- 22.Moody DE, Loury DN, Hammock BD. Epoxide metabolism in the liver of mice treated with clofibrate (ethyl-alpha-(p-chlorophenoxyisobutyrate)), a peroxisome proliferator. Toxicol Appl Pharmacol. 1985;78(3):351–62. doi: 10.1016/0041-008x(85)90240-6. [DOI] [PubMed] [Google Scholar]
- 23.Meijer J, Lundqvist G, DePierre JW. Comparison of the sex and subcellular distributions, catalytic and immunochemical reactivities of hepatic epoxide hydrolases in seven mammalian species. Eur J Biochem. 1987;167(2):269–79. doi: 10.1111/j.1432-1033.1987.tb13333.x. [DOI] [PubMed] [Google Scholar]
- 24.Denlinger CL, Vesell ES. Hormonal regulation of the developmental pattern of epoxide hydrolases. Studies in rat liver. Biochem Pharmacol. 1989;38(4):603–10. doi: 10.1016/0006-2952(89)90205-0. [DOI] [PubMed] [Google Scholar]
- 25.Pinot F, Grant DF, Spearow JL, Parker AG, Hammock BD. Differential regulation of soluble epoxide hydrolase by clofibrate and sexual hormones in the liver and kidneys of mice. Biochemical Pharmacology. 1995;50(4):501–508. doi: 10.1016/0006-2952(95)00167-x. [DOI] [PubMed] [Google Scholar]
- 26.Martin DS, Klinkova O, Eyster KM. Regional differences in sexually dimorphic protein expression in the spontaneously hypertensive rat (SHR) Molecular and Cellular Biochemistry. 2012;362(1):103–114. doi: 10.1007/s11010-011-1132-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bugger H, Chen D, Riehle C, Soto J, Theobald HA, Hu XX, Ganesan B, Weimer BC, Abel ED. Tissue-specific remodeling of the mitochondrial proteome in type 1 diabetic akita mice. Diabetes. 2009;58(9):1986–97. doi: 10.2337/db09-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oyadomari S, Koizumi A, Takeda K, Gotoh T, Akira S, Araki E, Mori M. Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J Clin Invest. 2002;109(4):525–32. doi: 10.1172/JCI14550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inceoglu B, Bettaieb A, Trindade da Silva CA, Lee KS, Haj FG, Hammock BD. Endoplasmic reticulum stress in the peripheral nervous system is a significant driver of neuropathic pain. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(29):9082–7. doi: 10.1073/pnas.1510137112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell. 2000;5(5):897–904. doi: 10.1016/s1097-2765(00)80330-5. [DOI] [PubMed] [Google Scholar]
- 31.Scheuner D, Song B, McEwen E, Liu C, Laybutt R, Gillespie P, Saunders T, Bonner-Weir S, Kaufman RJ. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell. 2001;7(6):1165–76. doi: 10.1016/s1097-2765(01)00265-9. [DOI] [PubMed] [Google Scholar]
- 32.Harding HP, Zeng H, Zhang Y, Jungries R, Chung P, Plesken H, Sabatini DD, Ron D. Diabetes mellitus and exocrine pancreatic dysfunction in perk−/− mice reveals a role for translational control in secretory cell survival. Mol Cell. 2001;7(6):1153–63. doi: 10.1016/s1097-2765(01)00264-7. [DOI] [PubMed] [Google Scholar]
- 33.Ron D. Proteotoxicity in the endoplasmic reticulum: lessons from the Akita diabetic mouse. J Clin Invest. 2002;109(4):443–5. doi: 10.1172/JCI15020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meyerovich K, Fukaya M, Terra LF, Ortis F, Eizirik DL, Cardozo AK. The non-canonical NF-κB pathway is induced by cytokines in pancreatic beta cells and contributes to cell death and proinflammatory responses in vitro. Diabetologia. 2016;59(3):512–521. doi: 10.1007/s00125-015-3817-z. [DOI] [PubMed] [Google Scholar]
- 35.Luo P, Chang HH, Zhou Y, Zhang S, Hwang SH, Morisseau C, Wang CY, Inscho EW, Hammock BD, Wang MH. Inhibition or deletion of soluble epoxide hydrolase prevents hyperglycemia, promotes insulin secretion, and reduces islet apoptosis. J Pharmacol Exp Ther. 2010;334(2):430–8. doi: 10.1124/jpet.110.167544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chavali V, Tyagi SC, Mishra PK. Differential expression of dicer, miRNAs, and inflammatory markers in diabetic Ins2+/− Akita hearts. Cell biochemistry and biophysics. 2014;68(1):25–35. doi: 10.1007/s12013-013-9679-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chon KH, Yang B, Posada-Quintero HF, Siu KL, Rolle M, Brink P, Birzgalis A, Moore LC. A novel quantitative method for diabetic cardiac autonomic neuropathy assessment in type 1 diabetic mice. J Diabetes Sci Technol. 2014;8(6):1157–67. doi: 10.1177/1932296814545669. [DOI] [PMC free article] [PubMed] [Google Scholar]