Abstract
Goals
Computational Analysis of Swallowing Mechanics is a method that utilizes multivariate shape change analysis to uncover covariant elements of pharyngeal swallowing mechanics associated with impairment using videofluoroscopic swallowing studies. The goals of this preliminary study were to (1) characterize swallowing mechanics underlying stroke related dysphagia, (2) decipher the impact of left and right hemispheric stroke on pharyngeal swallowing mechanics, and (3) determine pharyngeal swallowing mechanics associated with penetration-aspiration status.
Materials and Methods
Videofluoroscopic swallowing studies of 18 dysphagic patients with hemispheric infarcts and age and gender matched controls were selected from well-controlled data sets. Patient data including laterality, and penetration-aspiration status was collected. Coordinates mapping muscle group action during swallowing were collected from videos. Multivariate morphometric analyses of coordinates associated with stroke, affected hemisphere, and penetration-aspiration status were performed.
Findings
Pharyngeal swallowing mechanics differed significantly in the following comparisons: stroke vs. controls (D=2.19, p<.0001); right hemispheric stroke vs. controls (D=3.64, p<.0001); left hemispheric stroke vs. controls (D=2.06, p<.0001); right hemispheric stroke vs. left hemispheric stroke (D=2.89, p<.0001); and penetration-aspiration vs. within normal limits (D=2.25, p<.0001). Differences in pharyngeal swallowing mechanics associated with each comparison were visualized using eigenvectors.
Conclusion
While current literature focuses on timing changes in stroke-related dysphagia, this data suggests that mechanical changes are also functionally important. Pharyngeal swallowing mechanics differed by affected hemisphere and penetration-aspiration status. Computational Analysis of Swallowing Mechanics can be used to identify patient specific swallowing impairment associated with stroke injury that could help guide rehabilitation strategies to improve swallowing outcomes.
Keywords: ischemic hemispheric stroke, dysphagia, swallowing mechanics, aspiration
Introduction
Dysphagia commonly follows stroke and is a predictor of poor outcomes including aspiration, lengthened hospital stay, need for institutional care, pneumonia, and even mortality [1–5]. During pharyngeal swallowing, the pharynx is transformed from a respiratory channel into an alimentary conduit. In the current study, pharyngeal swallowing mechanics of dysphagic ischemic stroke patients are compared to healthy controls in order to better appreciate the impact of stroke on swallowing. We compared left and right hemispheric stroke groups to understand the impact of each on swallowing mechanics. Furthermore, we investigated the sample’s pharyngeal mechanics associated with penetration and aspiration.
Current literature of the functional changes in stroke related dysphagia emphasizes delays in timing [6–8], aspiration risk associated with timing [9–11], and related sensorineural deficits [12–14] with mechanistic deficits receiving less attention. Reports suggest differences in hyoid and laryngeal displacement but are mixed (Table 1) [11, 15–18]. A more comprehensive understanding of covariant swallowing mechanics including hyoid and laryngeal movement, tongue base retraction, pharyngeal shortening, and head and neck extension could not only highlight the dysfunction underlying stroke-associated penetration and aspiration, but also suggest which treatment goals would be more salient [19, 20].
Table 1.
Previous literature reporting differences in swallowing mechanics associated with stroke.
| Landmark | Authors | Relevant Finding |
|---|---|---|
| Hyoid | Bingjie et al [11] |
reduced
vertical movement in aspirating versus non-aspirating stroke patients horizontal showed no significant difference between any group—stroke or healthy |
| Paik et al [15] |
no significant difference for vertical movement decreased anterior movement in dysphagic stroke patients as compared to normal |
|
| Kim et al [16] | slightly decreased but non-significant difference in aspirating versus non-aspirating stroke patients in vertical and horizontal directions | |
| Seo et al [17] | non-significant decreases in movement for non-recovered stroke aspirators compared to recovered | |
| Larynx | Bingjie et al [11] | reduced vertical displacement in aspirating versus non-aspirating stroke patients |
| Hyolaryngeal complex | Han et al | penetration-aspiration status predicted reduced hyolaryngeal elevation in stroke survivors |
Another important factor that could be contributing to dysfunction of swallowing mechanics is the impact of left versus right hemispheric lesions. While the consensus in the literature supports the idea that swallowing is bilaterally innervated [21–24], some suggest that one hemisphere is more dominant, varying by person [25–29]. Increasingly, recent studies indicate that right hemisphere lesion is associated with deficits in the pharyngeal stage of swallowing [30–32]. Teismann et al. (2011) and Mihai et al. (2014) showed a shift of neural activity to the right hemisphere during the pharyngeal stage of swallowing in healthy subjects [33, 34]. Other authors support the notion that the two hemispheres do, in fact, control different aspects of swallowing [35]. Support for this theory dates back to 1993 when Robbins et al. found right hemispheric strokes to be associated with longer pharyngeal transit and response times as well as increased risk of penetration and aspiration when compared to left hemispheric strokes [36]. However, consensus on whether there is a dominant hemisphere, varying by person or whether there are instead distinct roles assigned to each hemisphere remains unclear. Furthermore, the specific impact of hemispheric stroke on the multiple muscle group actions underlying pharyngeal swallowing mechanics is unreported.
Conventional univariate displacement measurements from videofluoroscopic swallowing studies are helpful to quantify stroke impact, for example, on hyoid movement. The impact of multiple stroke variables, however, on multiple interacting elements of swallowing mechanics requires a different approach. Pharyngeal swallowing is complex. Computational Analysis of Swallowing Mechanics (CASM) utilizes geometric morphometrics to quantify overall shape differences associated with variables of interest. Once these overall differences are determined, eigenvectors are used to visualize the impact of variables on covariant pharyngeal swallowing mechanics. As such, the traditional outcome of distance measurements is replaced by eigenvectors that provide information characterizing the direction and magnitude of variations in shape.
CASM maps anatomical landmarks of the swallowing apparatus. These landmarks can be reliably obtained from imaging data including hyoid movement, laryngeal elevation, tongue base retraction, pharyngeal shortening, and head and neck posture (Figure 1) [37, 38]. Configurations of coordinates represent the interaction of multiple muscle groups underlying pharyngeal swallowing mechanics as these landmarks are displaced. Hence, shape analysis of landmarks throughout the swallow permits statistical evaluation and statistical visualization of elements of pharyngeal swallowing. Sets of coordinates are compared mathematically to determine differences in shape by variables of interest (group, lesion site, bolus type, penetration-aspiration status, etc.) [39]. Eigenvectors show the magnitude and direction of variation for each coordinate. The impact of a named variable on the action of multiple muscle groups is therefore inferred allowing for an approach to quantify and visualize the gestalt mechanics of pharyngeal swallowing using imaging data.
Figure 1.
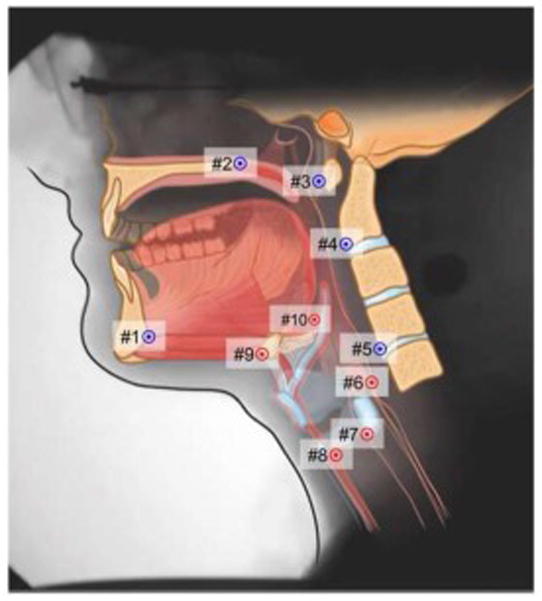
Coordinates mapping anatomical landmarks are used here to characterize the actions of muscles underlying pharyngeal swallowing mechanics. Coordinates 1 to 5 map the interactions of the skeletal levers (mandible, cranial base, and vertebrae) that suspend swallowing muscles. Coordinates 6 to 10 map distal attachments of muscles that displace swallowing structures. Coordinate 6 maps palatopharyngeus shortening the pharynx (nucleus ambiguus), Coordinate 7 maps stylopharyngeus (nucleus ambiguus) elevating the larynx posteriorly, Coordinates 8 and 9 map mylohyoid (trigeminal motor nucleus), geniohyoid and thyrohyoid (C1 gray matter) displacing the hyoid and larynx anterosuperiorly, and coordinate 10 maps the styloglossus and hyoglossus retracting the base of tongue (hypoglossal nucleus).
Herein we consider the independent variables of test group, hemisphere affected by stroke, and penetration-aspiration status. The goal of the present study was to analyze and visualize pharyngeal swallowing mechanics associated with these variables. The authors hypothesized that: (H1) multiple elements of pharyngeal swallowing mechanics of dysphagic stroke patients are reduced when compared with age and gender matched controls, (H2) right-sided lesions more negatively impact pharyngeal stage swallowing mechanics than left, and (H3) within the stroke cohort, impaired pharyngeal swallowing mechanics are associated with penetration-aspiration status.
Methods
Subjects and Imaging
The study included 18 supratentorial ischemic stroke subjects (averaged 70.1 ± 15.4 years of age, ranged from 40 to 90 years and included 11 females and 7 males) and 18 age and gender matched healthy control subjects. All stroke subjects were right handed; 6 had right hemispheric infarcts and 12 had left hemispheric infarcts. Local Institutional Review Boards approved the study and all participants gave written informed consent.
Inclusion criteria for the stroke patients were a unilateral, cortical or subcortical ischemic stroke. To qualify, each stroke patient had to have some degree of dysphagia, defined penetration of the bolus to the true vocal folds or lower in at least one swallow as judged by members of a swallowing lab [40]. The most important exclusion criteria for this study were: previous history of swallowing difficulty, previous history of stroke unless the lesion hemisphere was the same, history of a progressive neurological disorder, severe deficits in language comprehension, and other potential confounders to swallowing such as Chronic Obstructive Pulmonary Disease (COPD) or cancer.
All subjects underwent a Videofluoroscopic Swallowing Study (VFSS) within 1–6 days after their stroke. All VFSSs were recorded at 30 frames per second under a swallowing protocol carried out by trained speech-language pathologists. Swallow images were framed in a lateral view to include the hard palate superiorly, cricoid inferiorly, mental protuberance of mandible anteriorly and vertebral column posteriorly. The VFSS protocol included various bolus trials; 5mL thin and 5mL pudding swallows were selected and evaluated for the present study.
A speech-language pathologist blinded to patient data scored every swallow for every bolus trial using the Penetration-Aspiration Scale (PAS) [40]. Swallows were then classified as within normal limits (PAS of 1 or 2) or as penetration-aspiration (PAS 3–8). If a bolus had multiple swallows, the swallow with the worst PAS score was used to represent that trial. Of the 36 video swallows from the stroke cohort, 19 were within normal limits, 8 showed a penetrating bolus (7 during the swallow) and 9 showed aspiration (8 during the swallow). Concerning sidedness 5 of 18 swallows showed penetration and 1 of 18 showed aspiration for right sided infarcts, and 2 of 18 showed penetration and 7 of 18 showed aspiration for left sided infarcts.
Lesion Localization
All patients underwent MRI in a 1.5 Tesla General Electric scanner with an 8 channel head receive-only coil. The acquired images included T1, T2, FLAIR as well as DTI sequences. For the purpose of this study we only used the DTI T2 trace images (TR = 8000 ms; TE = 96 ms; 128x128 matrix; FOV = 240 voxel size = 1.88x1.88x5mm). We visualized the images for each individual patient using FSL software (4.1.4 http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/) in order to determine the side of the stroke lesion and lesion size.
Important to attributing differences in swallowing mechanics to sidedness in the present study is controlling for stroke severity. Stroke severity measurements including NIH Stroke Scale (NIHSS) and lesion size are here compared. No statistically significant difference was found in NIHSS scores (Left: 12.2±5.6, Right:12.3±6.0) or lesion size (Left: 49.9±34.7 cm3, Right: 30.4±26.3 cm3). A morphometric regression analysis of left and right sided mechanics on lesion size did not reveal a statistically significant relationship (r=.09). While the sample of left-sided stroke subjects is twice the number of right-sided stroke subjects, these data suggest functional equivalency between groups in stroke severity.
Coordinate Data Collection
Observers segmented and de-identified control video files (.avi converted to .mov) and stroke video files (.mp4) into 72 (18 subjects x 2 groups [stroke vs. control] x 2 different bolus swallows [thin vs. pudding]) short 1–4 second clips capturing the oral propulsive stage and pharyngeal stage of swallowing [41]. A medical student (NM) who met reliability criterion for coordinate mapping (inter-rater r >.95 for all coordinates) annotated swallows employing a semiautomated MATLAB tracking tool designed specifically for this purpose by Natarajan et al. [42] (Figure 2). The rater tracked all 10 anatomical landmarks in every frame to capture the swallowing apparatus as a whole during the oral propulsive stage and pharyngeal stage of swallowing. The beginning of the oral propulsive stage was marked by the initiation of posterior propulsion of the bolus from the anterior oral cavity that resulted in successfully moving the bolus into the pharynx. The pharyngeal stage was defined as commencing at the first anterior jump of the hyoid that led to a swallow and concluding at the first closure of the UES. The MATLAB tracking tool produced an .mp4 video displaying the annotation for review and a .csv file with coordinates of each point and respective frame. A head and neck anatomist reviewed the .mp4 files containing point placement from each evaluated swallow for error and re-annotated all observed inaccuracies.
Figure 2.
A MATLAB Tracker tool is used to map 10 coordinates in each frame during oral propulsive stage and the pharyngeal stage of swallowing as follows: 1) Genial tubercle of the mandible, 2) hard palate, 3) C1 tubercle, 4) C2, 5) C4, 6) upper esophageal sphincter, 7) posterior cricoid, 8) anterior cricoid, 9) hyoid, and 10) pit of valleculae.
Computational Analysis of Swallowing Mechanics
Files of coordinates representing oral propulsive stage and pharyngeal stage of swallowing were concatenated with each set of 10 coordinates per frame assigned a unique identifier (n=1,749). Categorical variables were also assigned to each unique identifier including group (stroke vs. control), swallowing stage (oral propulsive vs. pharyngeal), affected hemisphere (left vs. right), bolus type (5mL thin vs. 5mL pudding), sex (male vs. female), and penetration-aspiration status (PAS 1–2 vs. PAS 3–8). Coordinates and classifier variables were uploaded into MorphoJ integrated software for multivariate morphometric analysis [43]. Following a Procrustes fit, a canonical variate analysis with post hoc discriminant function (pair-wise) analysis (DFA) was performed for hypothesis testing. DFA eigenvectors were used to visualize differences in swallowing mechanics associated with classifier variables of interest. To account for multiple comparisons of 10 coordinates, a Bonferroni correction was applied and statistical significance was set at a p-value of 0.005 or less for all analyses.
Hypothesis testing
A canonical variate analysis by test group, swallowing stage, and affected hemisphere with a post-hoc DFA comparing dysphagic stroke patients with age and gender matched controls was used to test H1. To test H2, we used pharyngeal stage data only (n=1120 frames) performing a canonical variate analysis by test group and affected hemisphere to determine if pharyngeal swallowing mechanics from right-sided lesions differed more from controls than left-sided lesions. H3 was tested by a DFA comparing pharyngeal mechanics of stroke patients (n=488 frames) with penetration-aspiration vs. stroke patients within normal limits. Post-hoc DFA eigenvector graphs show the magnitude and direction of coordinate variation allowing for visualization of gestalt pharyngeal swallowing mechanics associated with each comparison. A description of each eigenvector graph is included in the results.
Results
H1: Stroke vs. Controls
Canonical variate analysis of test group, swallowing stage, and hemisphere affected showed highly significant differences (p<.0001) across all comparisons. DFA pairwise comparisons of stroke vs. controls groups showed significant differences in pharyngeal swallowing mechanics between the cohorts (D=2.17, p<.0001). DFA eigenvectors (Figure 3) indicate reduced hyoid elevation, reduced tongue base retraction, and reduced pharyngeal shortening, as well as hyperextension of the head and neck throughout the oral propulsive and pharyngeal stages of swallowing.
Figure 3.
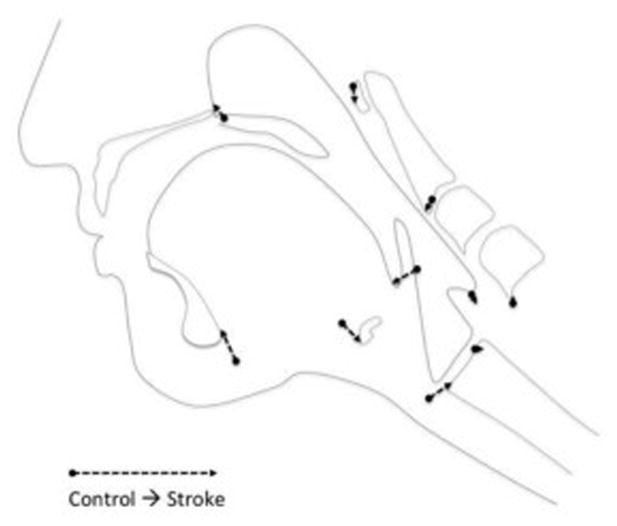
Eigenvectors of controls versus stroke indicate reduced hyoid elevation, reduced tongue base retraction, and reduced pharyngeal shortening, as well as hyperextension of the head and neck throughout the oral propulsive and pharyngeal stages of swallowing in stroke subjects.
H2: Mechanics associated with hemispheric stroke
Canonical variate analysis of group and hemisphere affected showed highly significant differences (p<.0001) across all comparisons (Figure 4a). Swallowing mechanics associated with right hemispheric lesions showed greater differences than left hemispheric lesions when compared to controls as demonstrated by a greater D value (right vs. controls: D=3.64, p<.0001; left vs. controls: D=2.06, p<.0001). Differences were also noted between right and left hemispheric lesions (right vs. left: D=2.89, p<.0001). DFA eigenvectors (Figure 4b) indicated reduced hyoid movement, reduced laryngeal elevation, reduced tongue base retraction and reduced pharyngeal shortening in pharyngeal swallowing mechanics in left-sided stroke subjects compared to controls. Contrastingly, right-sided strokes showed increased pharyngeal shortening, decreased tongue base retraction, reduced hyoid movement, and a posteriorly repositioned larynx compared to controls.
Figure 4.
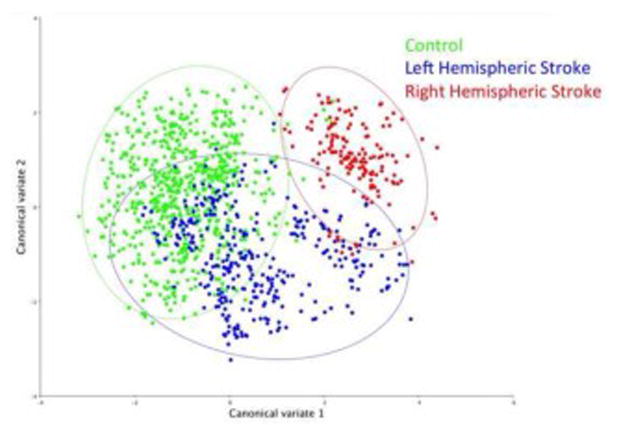
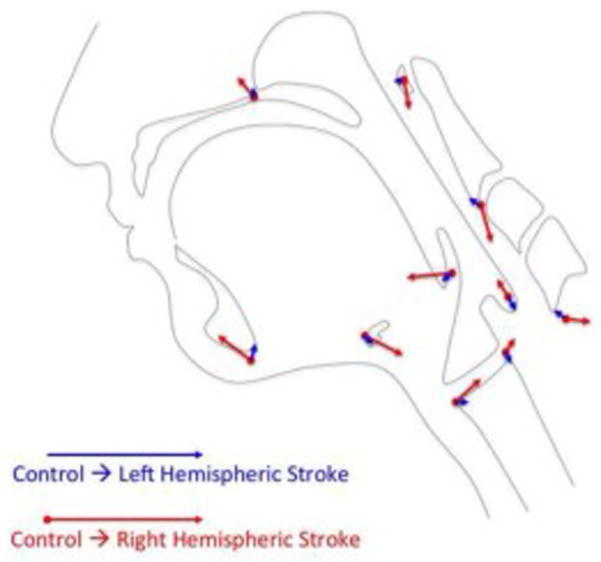
Figure 4a. Canonical variate analysis showing pharyngeal mechanics differed by group and lesion sidedness (right-sided lesion vs. controls: D=3.64, p<.0001; left-sided lesion vs. controls: D=2.06, p<.0001; right-sided lesion vs. left-sided lesion: D=2.89, p<.0001).
Figure 4b. Eigenvectors of left hemispheric stroke vs. control pharyngeal (in blue) swallowing mechanics indicate mild reductions in hyoid elevation (9), laryngeal elevation (7,8), tongue base retraction (10), and pharyngeal shortening (6). Overlaid are eigenvectors of right hemispheric stroke vs. control pharyngeal swallowing mechanics (in red) which indicate increased pharyngeal shortening, reduced tongue base retraction, reduced hyoid elevation, extension of the head and neck, and a posteriorly repositioned larynx.
H3: Mechanics associated with penetration-aspiration status in stroke subjects
DFA comparing swallows of stroke subjects associated with the penetration-aspiration vs. within normal limits showed significant differences in pharyngeal swallowing mechanics (D=2.25, p<.0001). DFA eigenvectors (Figure 5) indicate that impaired hyoid excursion, tongue base retraction, and pharyngeal shortening underlie penetration-aspiration within the stroke cohort.
Figure 5.
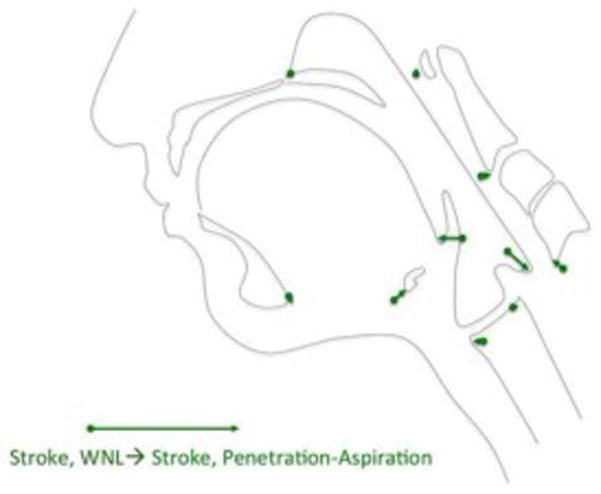
Eigenvectors of pharyngeal swallowing mechanics associated with penetration-aspiration (PEN-ASP) in stroke subjects compared within normal limits (WNL) in stroke patients. Reductions in hyoid movement, tongue base retraction, and pharyngeal shortening are associated with penetration-aspiration mechanics within in this sample of stroke subjects.
Discussion
In this study, differences in pharyngeal swallowing mechanics were evaluated and visualized using CASM to assess associations with stroke, lesion site (left vs. right hemisphere), and penetration-aspiration status. These findings suggest that mechanical changes resulting from stroke are an important consideration in dysphagia management and knowing the specific mechanical impairment will allow for more specific therapy goals, possibly hastening and increasing the chances of recovery.
Stroke vs. Controls
Swallowing mechanics in the stroke cohort include reduced superior hyoid movement, decreased tongue-base retraction, hyperextension of the head and neck and a curious repositioning of the larynx when compared to age and gender matched controls. The hyoid finding agrees with Bingje et al., but differs from other prior reports [11, 15–18](Table 1). Impaired tongue base retraction has been previously proposed but is here verified with data [44, 45]. Hyperextension of the head and neck is a new finding and is likely a compensatory mechanism for decreased hyoid movement.
The repositioning of the larynx is a finding that is new to the literature. There are no muscle groups that would directly relocate the larynx posteriorly within the visceral compartment of the neck during swallowing. Extension of the head and neck may underlie this apparent repositioning of the larynx. However, this same pattern has been observed in a patient following Respiratory-Swallow Phase Training [46, 47]. Tran and colleagues (2016) used a patient specific CASM comparing swallowing at full lung volume compared to mid to low expiratory volume. In this paper the authors hypothesize that negative pressure in the mediastinum at full lung volume creates traction on the visceral compartment of the neck that is opposed by pharyngeal dilators which results in a posterior displacement of the larynx against the prevertebral compartment. If this hypothesis holds, then respiratory-swallow phase training may be a viable rehabilitation strategy where indicated by respiratory swallow analysis for stroke patients [46]. Novel to the CASM method is the use of vectors to visualize the impact of stroke on multiple interacting elements of swallowing mechanics. These data infer patterns of neuromuscular deficits associated with variables of interest such as sidedness and lesion site. Coupled with a robust stroke data set, CASM could lead to more fully understanding the neurobiology of swallowing.
Mechanics associated with hemispheric stroke
Confirming our hypothesis (H2), the DFA indicate that pharyngeal swallowing mechanics in right-sided lesions was more impaired than left-sided lesions when compared to controls, supporting the notion that the right hemisphere is more involved in the pharyngeal stage than the left hemisphere as observed by others [33–36]. DFA eigenvectors also indicate different patterns of impairment by hemisphere. Left-sided lesions resulted in an equivalent reduction in all elements of swallowing mechanics–that is, there was only slight reduction in hyolaryngeal elevation, pharyngeal shortening and tongue base retraction with little change in head and neck position. Right-sided lesions resulted in severely altered swallowing mechanics including drastic reduction in hyoid elevation, reduced tongue base retraction, increased head and neck extension and the previously mentioned curious repositioning of the larynx. Of note, previous studies have found that right-sided lesions correlate more strongly with pneumonia than do left. While our sample size was insufficient to investigate this, our findings of greater change in mechanics in right-sided lesions align with this data [48,49]. More study is needed to uncover the underlying mechanism of this laryngeal posture. As noted above, it may result from a compensatory extension of the head and neck or may be associated with an interruption to respiratory-swallow patterns to right hemispheric stroke [47]. Interestingly, pharyngeal shortening is improved, perhaps to compensate for these other impairments.
Right-sided stroke has been shown to delay initiation and increase the duration of pharyngeal swallowing [36]. If respiratory cessation during pharyngeal swallowing is a brainstem function and impaired right cortical drive is associated with delayed pharyngeal swallowing, then perhaps this suggests a mechanism for the uncoupling of swallowing drive and respiratory cessation in right-sided cortical stroke patients. Additionally, swallow function has been shown to improve following expiratory muscle strength training [50, 51]. While this training addresses a different problem, perhaps improved respiratory function somehow retrains respiratory-swallow coordination. It would be important to integrate structural, neurologic, respiratory, and swallowing data to address these questions.
The impact of hemispheric stroke on swallowing is an open question [52]. Some studies have shown the right side to be more active than the left later in the swallow while the left hemisphere is active throughout [30, 33, 34]. Our findings support the idea that both hemispheres are important in pharyngeal swallowing, and that the left and right hemispheres may have distinct, non-random roles. In our sample, NIHSS scores were equivalent which could mask left sided severity as left sided functionality is more heavily weighted in the scale [53]; however, severity of stroke by lesion volume showed non-significant greater insult on the left side compared to right. Overall, the data in the present study suggest a mechanical correlate to sidedness that needs more investigation.
Mechanics associated with penetration-aspiration status
Swallowing mechanics associated with penetration-aspiration (n=13 video swallows) compared to within normal limits (n=23 video swallows) showed that reduced hyoid excursion, reduced tongue base retraction and reduced pharyngeal shortening were associated with penetration-aspiration but not hyperextension of the head and neck or repositioning of the larynx. These results implicate clinical treatment to address the specific mechanical dysfunction, as in the Shaker exercise or chin tuck against resistance to increase hyoid excursion [54, 55], the Masako maneuver to increase tongue base retraction [56], and effortful pitch glide to engage the long pharyngeal muscles underlying pharyngeal shortening [57].
Interestingly, a post hoc morphometric regression analysis showed that pharyngeal swallowing mechanics associated with right vs. left hemispheric stroke were not predictive of mechanics underlying penetration-aspiration (r2=.02). Further work is needed to investigate the implications of this finding. It is important to note that other factors such as sensory or timing deficits can contribute to penetration-aspiration; however, a post hoc analysis showed no significant differences in timing of oropharyngeal transport between left or right-sided lesions in this small sample. In addition, of the 36 stroke swallows evaluated only 2 incidents of a penetration-aspiration occurred before swallowing. This may suggest greater importance for mechanical changes over timing changes, although the sample size is small. It is possible that other stroke variables such as lesion site confound this outcome. The limited and very small boluses used for analyses may have also precluded the true penetration-aspiration status of the patients. It could also be posited that while the reduced mechanics did not lead to penetration or aspiration, they could lead to other outcomes of dysphagia such as residue. Inclusion of such variables in future studies will allow for greater insight into the other insidious effects of post-stroke deficits. Consistent with previous findings in the literature, an additional post-hoc morphometric regression analysis did show that low bolus viscosity is predictive of mechanics associated with penetration-aspiration (r2=.59) [58]. Whether or not higher viscosity bolus types are merely compensatory or neurorehabilitative requires more study.
Limitations and Further Work
These finding should be tempered with understanding that not all elements of pharyngeal swallowing mechanics are presently characterized in CASM such as soft palate closure and pharyngeal stripping wave. However, CASM represents the only current approach to quantify and visualize the gestalt mechanics of pharyngeal swallowing using imaging data. Future work includes establishing means of expanding this approach including recent work establishing the reliability of mapping the pharyngeal constrictor muscles [59]. This dataset included an every frame analysis (1,749 sets of coordinates) from 72 well-controlled 30fps video swallows including 12 swallows from six right hemispheric stroke patients, 24 swallows from 12 left hemispheric stroke patients, and 36 age and gender matched controls. However, even with strong statistical power of CASM, it is questionable that these findings are generalizable given the low number of subjects (n=18) and limited bolus trials. CASM allows for multidimensional statistical analysis and visualization of covariant swallowing mechanics associated with etiology of dysphagia, site of lesion, penetration-aspiration status, or any other categorical variable of interest. CASM can be applied to big data, cohort, or patient specific analyses. Future directions for stroke include a big data CASM repository to determine impaired swallow mechanics associated with specific lesion sites and loads and testing patient specific results of stroke intervention such as transcranial direct current stimulation and targeted rehabilitative exercise [60].
Conclusions
Our results from this limited dataset show a global impairment of swallowing mechanics after stroke. Moreover, these data suggest that sidedness of stroke lesion meaningfully affects the resulting mechanical changes with right-sided hemispheric strokes having a greater negative impact on pharyngeal swallowing mechanics than left-sided hemispheric strokes when compared to controls. CASM is a method that provides new insight into impaired swallowing mechanics associated with stroke including mechanics that underlie penetration-aspiration status.
Table 2.
Stroke characteristics collected from MRI including hemisphere affected, vascular territories indicated, and specific lesion sites for each subject.
| Side | Vascular territory | Frontal Cortex | Parietal Cortex | Insular Cortex | Temporal Cortex | Occipital Cortex | Subcortical white matter | Internal capsule | Basal Ganglia | Thalamus |
|---|---|---|---|---|---|---|---|---|---|---|
| L | ACA MCA PCA |
x | x | x | x | x | x | |||
| L | MCA | x | x | |||||||
| L | MCA | x | x | x | x | x | x | |||
| L | MCA | x | x | x | x | x | ||||
| L | MCA | x | x | x | ||||||
| L | MCA | x | x | x | ||||||
| L | MCA | x | x | x | x | x | x | x | ||
| L | MCA | x | x | x | ||||||
| L | MCA | x | x | x | x | x | x | |||
| L | MCA | x | x | x | ||||||
| L | MCA PCA |
x | x | x | x | x | ||||
| L | MCA PCA |
x | x | x | x | x | ||||
| R | ACA, MCA | x | x | x | x | x | x | |||
| R | MCA | x | x | x | ||||||
| R | MCA | x | x | |||||||
| R | MCA | x | x | x | ||||||
| R | MCA PCA |
x | x | x | x | x | ||||
| R | MCA PCA |
x | x | x |
Acknowledgments
Grant Support:
Grant support for stroke data from National Institute On Deafness And Other Communication Disorders of the National Institutes of Health under Award Number R01DC012584 (Kumar). The Grant Support for Control Data from Veterans Affairs, Rehabilitation Research and Development under award number CDA-1 1IK1RX001628-01A1 (PI: (Focht) Garand), the National Institute on Deafness and Other Communication Disorders of the National Institutes of Health under award number K24DC12801 (PI: Martin-Harris), and the South Carolina Clinical & Translational Research (SCTR) Institute, with an academic home at the Medical University of South Carolina, NIH/NCATS Grant number TL1 TR000061 (PI: Brady; Project PI: Garand).
Research reported in this publication was supported by the National Institute on Deafness and Other Communication Disorders of the National Institutes of Health under Award Number R01DC012584 (Kumar). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors would like to acknowledge Dr. Kendrea Garand and Dr. Bonnie Martin-Harris for the contribution of control data partially supported by the Veterans Affairs, Rehabilitation Research and Development under award number CDA-1 1IK1RX001628-01A1 (PI: (Focht) Garand), the National Institute on Deafness and Other Communication Disorders of the National Institutes of Health under award number K24DC12801 (PI: Martin-Harris), and the South Carolina Clinical & Translational Research (SCTR) Institute, with an academic home at the Medical University of South Carolina, NIH/NCATS Grant number TL1 TR000061 (PI: Brady; Project PI: Garand).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Singh S, Hamdy S. Dysphagia in stroke patients. Postgrad Med J. 2006;82:383–91. doi: 10.1136/pgmj.2005.043281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R. Dysphagia after stroke incidence, diagnosis, and pulmonary complications. Stroke. 2005;36:2756–63. doi: 10.1161/01.STR.0000190056.76543.eb. [DOI] [PubMed] [Google Scholar]
- 3.Smithard D, O’neill P, Park C, Morris J, Wyatt R, England R, et al. Complications and outcome after acute stroke does dysphagia matter? Stroke. 1996;27:1200–4. doi: 10.1161/01.str.27.7.1200. [DOI] [PubMed] [Google Scholar]
- 4.Silver FL, Norris JW, Lewis AJ, Hachinski V. Early mortality following stroke: a prospective review. Stroke. 1984;15:492–6. doi: 10.1161/01.str.15.3.492. [DOI] [PubMed] [Google Scholar]
- 5.Bounds JV, Wiebers DO, Whisnant JP, Okazaki H. Mechanisms and timing of deaths from cerebral infarction. Stroke. 1981;12:474–7. doi: 10.1161/01.str.12.4.474. [DOI] [PubMed] [Google Scholar]
- 6.Veis S, Logemann J. Swallowing disorders in persons with cerebrovascular accident. Arch Phys Med Rehabil. 1985;66:372–5. [PubMed] [Google Scholar]
- 7.Johnson ER, McKenzie SW, Rosenquist CJ, Lieberman JS, Sievers AE. Dysphagia following stroke: quantitative evaluation of pharyngeal transit times. Arch Phys Med Rehabil. 1992;73:419–23. [PubMed] [Google Scholar]
- 8.Robbins J, Levine RL. Swallowing after unilateral stroke of the cerebral cortex: preliminary experience. Dysphagia. 1988;3:11–7. doi: 10.1007/BF02406275. [DOI] [PubMed] [Google Scholar]
- 9.Kim Y, McCullough GH. Stage transition duration in patients poststroke. Dysphagia. 2007;22:299–305. doi: 10.1007/s00455-007-9085-4. [DOI] [PubMed] [Google Scholar]
- 10.Power ML, Hamdy S, Goulermas JY, Tyrrell PJ, Turnbull I, Thompson DG. Predicting aspiration after hemispheric stroke from timing measures of oropharyngeal bolus flow and laryngeal closure. Dysphagia. 2009;24:257–64. doi: 10.1007/s00455-008-9198-4. [DOI] [PubMed] [Google Scholar]
- 11.Bingjie L, Tong Z, Xinting S, Jianmin X, Guijun J. Quantitative videofluoroscopic analysis of penetration-aspiration in post-stroke patients. Neurology India. 2010;58:42–7. doi: 10.4103/0028-3886.60395. [DOI] [PubMed] [Google Scholar]
- 12.Warabi T, Ito T, Kato M, Takei H, Kobayashi N, Chiba S. Effects of stroke-induced damage to swallow-related areas in the brain on swallowing mechanics of elderly patients. Geriatr Gerontol Int. 2008;8:234–42. doi: 10.1111/j.1447-0594.2008.00473.x. [DOI] [PubMed] [Google Scholar]
- 13.Power ML, Fraser CH, Hobson A, Singh S, Tyrrell P, Nicholson DA, et al. Evaluating oral stimulation as a treatment for dysphagia after stroke. Dysphagia. 2006;21:49–55. doi: 10.1007/s00455-005-9009-0. [DOI] [PubMed] [Google Scholar]
- 14.Power ML, Hamdy S, Singh S, Tyrrell PJ, Turnbull I, Thompson DG. Deglutitive laryngeal closure in stroke patients. J Neurol Neurosurg Psychiatry. 2007;78:141–6. doi: 10.1136/jnnp.2006.101857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paik N-J, Kim SJ, Lee HJ, Jeon JY, Lim J-Y, Han TR. Movement of the hyoid bone and the epiglottis during swallowing in patients with dysphagia from different etiologies. J Electromyogr Kinesiol. 2008;18:329–35. doi: 10.1016/j.jelekin.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 16.Kim Y, McCullough GH. Maximal Hyoid Excursion in Poststroke Patients. Dysphagia. 2010;25:20–5. doi: 10.1007/s00455-009-9224-1. [DOI] [PubMed] [Google Scholar]
- 17.Seo HG, Oh B-M, Han TR. Longitudinal changes of the swallowing process in subacute stroke patients with aspiration. Dysphagia. 2011;26:41–8. doi: 10.1007/s00455-009-9265-5. [DOI] [PubMed] [Google Scholar]
- 18.Han H, Shin G, Jun A, Park T, Ko D, Choi E, et al. The Relation Between the Presence of Aspiration or Penetration and the Clinical Indicators of Dysphagia in Poststroke Survivors. Ann Rehabil Med. 2016;40:88–94. doi: 10.5535/arm.2016.40.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vose A, Nonnenmacher J, Singer ML, González-Fernández M. Dysphagia management in acute and sub-acute stroke. Curr Phys Med Rehabil Rep. 2014;2:197–206. doi: 10.1007/s40141-014-0061-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson DN, Herring HJ, Daniels SK. Dysphagia management in stroke rehabilitation. Curr Phys Med Rehabil Rep. 2014;2:207–18. [Google Scholar]
- 21.Hamdy S, Rothwell JC, Brooks DJ, Bailey D, Aziz Q, Thompson DG. Identification of the cerebral loci processing human swallowing with H2 15O PET activation. J Neurophysiol. 1999;81:1917–26. doi: 10.1152/jn.1999.81.4.1917. [DOI] [PubMed] [Google Scholar]
- 22.Mistry S, Hamdy S. Neural control of feeding and swallowing. Phys Med Rehabil Clin N Am. 2008;19:709–28. doi: 10.1016/j.pmr.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 23.Hamdy S. Role of cerebral cortex in the control of swallowing. GI Motility online. 2006 [Google Scholar]
- 24.Daniels SK, Foundas AL. Lesion localization in acute stroke patients with risk of aspiration. J Neuroimaging. 1999;9:91–8. doi: 10.1111/jon19999291. [DOI] [PubMed] [Google Scholar]
- 25.Malandraki GA, Sutton BP, Perlman AL, Karampinos DC, Conway C. Neural activation of swallowing and swallowing-related tasks in healthy young adults: an attempt to separate the components of deglutition. Hum Brain Mapp. 2009;30:3209–26. doi: 10.1002/hbm.20743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michou E, Hamdy S. Cortical input in control of swallowing. Curr Opin Otolaryngol Head Neck Surg. 2009;17:166–71. doi: 10.1097/MOO.0b013e32832b255e. [DOI] [PubMed] [Google Scholar]
- 27.Verin E, Michou E, Leroi A-M, Hamdy S, Marie J-P. “Virtual” Lesioning of the Human Oropharyngeal Motor Cortex: A Videofluoroscopic Study. Arch Phys Med Rehabil. 2012;93:1987–90. doi: 10.1016/j.apmr.2011.10.020. [DOI] [PubMed] [Google Scholar]
- 28.Mosier KM, Liu W-C, Maldjian JA, Shah R, Modi B. Lateralization of cortical function in swallowing: a functional MR imaging study. Am J Neuroradiol. 1999;20:1520–6. [PMC free article] [PubMed] [Google Scholar]
- 29.Hamdy S, Rothwell JC, Aziz Q, Thompson DG. Organization and reorganization of human swallowing motor cortex: implications for recovery after stroke. Clin Sci. 2000;99:151–7. [PubMed] [Google Scholar]
- 30.Teismann IK, Suntrup S, Warnecke T, Steinsträter O, Fischer M, Flöel A, et al. Cortical swallowing processing in early subacute stroke. BMC neurol. 2011;11:34. doi: 10.1186/1471-2377-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dziewas R, Teismann IK, Suntrup S, Schiffbauer H, Steinstraeter O, Warnecke T, et al. Cortical compensation associated with dysphagia caused by selective degeneration of bulbar motor neurons. Hum Brain Mapp. 2009;30:1352–60. doi: 10.1002/hbm.20603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin RE, Sessle BJ. The role of the cerebral cortex in swallowing. Dysphagia. 1993;8:195–202. doi: 10.1007/BF01354538. [DOI] [PubMed] [Google Scholar]
- 33.Teismann IK, Dziewas R, Steinstraeter O, Pantev C. Time-dependent hemispheric shift of the cortical control of volitional swallowing. Hum Brain Mapp. 2009;30:92–100. doi: 10.1002/hbm.20488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mihai PG, Otto M, Platz T, Eickhoff SB. Sequential evolution of cortical activity and effective connectivity of swallowing using fMRI. Hum Brain Mapp. 2014;35:5962–73. doi: 10.1002/hbm.22597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daniels SK, Corey DM, Fraychinaud A, DePolo A, Foundas AL. Swallowing lateralization: the effects of modified dual-task interference. Dysphagia. 2006;21:21–7. doi: 10.1007/s00455-005-9007-2. [DOI] [PubMed] [Google Scholar]
- 36.Robbins J, Levine RL, Maser A, Rosenbek JC, Kempster GB. Swallowing after unilateral stroke of the cerebral cortex. Arch Phys Med Rehabil. 1993;74:1295–300. doi: 10.1016/0003-9993(93)90082-l. [DOI] [PubMed] [Google Scholar]
- 37.Pearson WG, Zumwalt AC. Visualising Hyolaryngeal Mechanics in Swallowing Using Dynamic MRI. Comput Methods Biomech Biomed Eng Imaging Vis. 2014;2:208–16. doi: 10.1080/21681163.2013.846231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pearson WG, Taylor BK, Blair J, Martin-Harris B. Computational analysis of swallowing mechanics underlying impaired epiglottic inversion. Laryngoscope. 2016;126:1854–8. doi: 10.1002/lary.25788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Webster M, Sheets HD, Alroy J, Hunt G. A practical introduction to landmark-based geometric morphometrics. Quantitative Methods in Paleobiology Paleontological Society Papers. 2010;16:163–88. [Google Scholar]
- 40.Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996;11:93–8. doi: 10.1007/BF00417897. [DOI] [PubMed] [Google Scholar]
- 41.Matsuo K, Palmer JB. Anatomy and physiology of feeding and swallowing: normal and abnormal. Phys Med Rehabil Clin N Am. 2008;19:691–707. doi: 10.1016/j.pmr.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Natarajan R, Stavness I, Pearson W., Jr Semi-automatic tracking of hyolaryngeal coordinates in videofluoroscopic swallowing studies. Comput Methods Biomech Biomed Eng Imaging Vis. 2015:1–11. [Google Scholar]
- 43.Klingenberg CP. MorphoJ: an integrated software package for geometric morphometrics. Mol Ecol Resour. 2011;11:353–7. doi: 10.1111/j.1755-0998.2010.02924.x. [DOI] [PubMed] [Google Scholar]
- 44.González-Fernández M, Ottenstein L, Atanelov L, Christian AB. Dysphagia after stroke: an overview. Current physical medicine and rehabilitation reports. 2013;1:187–96. doi: 10.1007/s40141-013-0017-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cola MG, Daniels SK, Corey DM, Lemen LC, Romero M, Foundas AL. Relevance of subcortical stroke in dysphagia. Stroke. 2010;41:482–6. doi: 10.1161/STROKEAHA.109.566133. [DOI] [PubMed] [Google Scholar]
- 46.Martin-Harris B, McFarland D, Hill EG, Strange CB, Focht KL, Wan Z, et al. Respiratory-Swallow Training in Patients with Head and Neck Cancer. Arch Phys Med Rehabil. 2015;96:885–93. doi: 10.1016/j.apmr.2014.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tran TTA, Martin Harris B, Pearson WG. Improvements resulting from respiratory-swallow phase training visualized in patient-specific computational analysis of swallowing mechanics. Comput Methods Biomech Biomed Eng Imaging Vis. 2016:1–7. doi: 10.1080/21681163.2016.1152567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kemmling A, Lev MH, Payabvash S, Betensky RA, Qian J, Masrur S, Schwamm LH. Hospital acquired pneumonia is linked to right hemispheric peri-insular stroke. PLoS ONE. 2013;8:8, e71141. doi: 10.1371/journal.pone.0071141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Teasell RW, McRae M, Marchuk Y, Finestone HM. Pneumonia associated with aspiration following stroke. Arch Phys Med Rehabil. 1996;77:707–709. doi: 10.1016/s0003-9993(96)90012-x. [DOI] [PubMed] [Google Scholar]
- 50.Troche MS, Okun MS, Rosenbek JC, Musson N, Fernandez HH, Rodriguez R, et al. Aspiration and swallowing in Parkinson disease and rehabilitation with EMST: a randomized trial. Neurology. 2010;75:1912–9. doi: 10.1212/WNL.0b013e3181fef115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pitts T, Bolser D, Rosenbek J, Troche M, Okun MS, Sapienza C. Impact of expiratory muscle strength training on voluntary cough and swallow function in Parkinson disease. Chest. 2009;135:1301–8. doi: 10.1378/chest.08-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miller AJ. The neurobiology of swallowing and dysphagia. Dev Dis Res Rev. 2008;14:77–86. doi: 10.1002/ddrr.12. [DOI] [PubMed] [Google Scholar]
- 53.Fink JN, Selim M, Kumar S, Silver B, Caplan LR, Linfante I, Schlaug G. Is the association of National Institutes of Health Stroke Scale Scores and acute magnetic resonance stroke volumes equal for patients with right and left-hemispheric ischemic stroke? Stroke. 2002;33:954–958. doi: 10.1161/01.str.0000013069.24300.1d. [DOI] [PubMed] [Google Scholar]
- 54.Shaker R, Kern M, Bardan E, Taylor A, Stewart E, Hoffmann R, et al. Augmentation of deglutitive upper esophageal sphincter opening in the elderly by exercise. Am J Physiol Gastrointest Liver. 1997;272:G1518–G22. doi: 10.1152/ajpgi.1997.272.6.G1518. [DOI] [PubMed] [Google Scholar]
- 55.Yoon WL, Khoo JKP, Liow SJR. Chin tuck against resistance (CTAR): new method for enhancing suprahyoid muscle activity using a Shaker-type exercise. Dysphagia. 2014;29:243–8. doi: 10.1007/s00455-013-9502-9. [DOI] [PubMed] [Google Scholar]
- 56.Fujiu-Kurachi M, Fujiwara S, Tamine K-i, Kondo J, Minagi Y, Maeda Y, et al. Tongue pressure generation during tongue-hold swallows in young healthy adults measured with different tongue positions. Dysphagia. 2014;29:17–24. doi: 10.1007/s00455-013-9471-z. [DOI] [PubMed] [Google Scholar]
- 57.Vasquez-Miloro K, Pearson W, Langmore S. Effortful Pitch Glide: A Potential New Exercise Evaluated by Dynamic MRI. J Speech Lang Hear Res. 2014;57:1243–50. doi: 10.1044/2014_JSLHR-S-13-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Clavé P, De Kraa M, Arreola V, Girvent M, Farre R, Palomera E, et al. The effect of bolus viscosity on swallowing function in neurogenic dysphagia. Aliment Pharmacol Ther. 2006;24:1385–94. doi: 10.1111/j.1365-2036.2006.03118.x. [DOI] [PubMed] [Google Scholar]
- 59.Schwertner, Garand KL, Pearson WG. A novel imaging analysis method for capturing pharyngeal constriction during swallowing. J Imaging Sci. 2016;1:1–6. [PMC free article] [PubMed] [Google Scholar]
- 60.Marchina S, Schlaug G, Kumar S. Study design for the FEASt trial: a randomized controlled intervention for improving dysphagia after acute ischemic stroke. J Stroke Cerebrovasc Dis. 2015;24:511. doi: 10.1016/j.jstrokecerebrovasdis.2014.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]


