Abstract
Building research infrastructure capacity to address clinical and translational gaps has been a focus of funding agencies and foundations. Clinical and Translational Sciences Awards, Research Centers in Minority Institutions Infrastructure for Clinical and Translational Research (RCTR) and the Institutional Development Award Infrastructure for Clinical and Translational Research funded by United States (US) government to fund clinical translational research programs have existed for over a decade to address racial and ethnic health disparities across the US. While the impact on the nation’s health can’t be made in a short period, assessment of a program’s impact could be a litmus test to gauge its effectiveness at the institution and communities. We report the success of a Pilot Project Program in the University of Hawaii RCTR Award in advancing careers of emerging investigators and community collaborators. Our findings demonstrated that the investment has a far-reaching impact on engagement with community-based research collaborators, career advancement of health disparities investigators, and favorable impacts on health policy.
Keywords: health disparity, clinical research, health inequity, translational research
INTRODUCTION
Health inequities continue to persist in communities across the disease spectrum throughout the United States (US) and globally1–3. Contributing to the culture of health disparities has partly been a function of the imbalance of biomedical research funding from government agencies, private foundations, industry and other sources4–7. Innovative concepts and bold initiatives to increase collaborations and partnerships were established through government, industry and foundations to increase translation of research into practice to accelerate medical research6–10. Concepts and ideas to increase capacity to address clinical and translational gaps in the health of the population included new approaches to discovery, developing partnerships between government and private sectors, identifying research teams, and redesigning the clinical research paradigm6, 10. A number of research initiatives were instituted by various funding agencies to address clinical and translational gaps to bring entities together to accelerate the pace of discovery, from the earliest discovery stages through clinical research11–17. What followed in the USA, amongst many other infrastructure programs, were the Clinical and Translational Sciences Award program (CTSA) funded by the National Center for Advancing Translational Sciences (NCATS)18, 19, Research Centers in Minority Institutions (RCMI) Infrastructure for Clinical and Translational Research (RCTR) funded by National Institute of Minority Health and Health Disparities (NIMHD)20, and the Institutional Development Award Program Infrastructure for Clinical and Translational Research (IDeA-CTR) funded by National Institute of General Medical Sciences (NIGMS), Table 1.
Table 1.
Clinical Translational Research Infrastructure Programs, Funding Agencies & Institutions
| Programs/Agencies/Institutions | Abbreviations |
|---|---|
| Air Force Research Laboratory | AFRL |
| Clinical and Translational Science Award | CTSA |
| Department of Defense | DOD |
| Health Resources and Services Administration | HRSA |
| Institutional Development Award | IDeA |
| National Center for Research Resources | NCRR |
| National Heart, Lung, and Blood Institute | NHLBI |
| National Institute of Diabetes and Digestive and Kidney Diseases | NIDDK |
| National Institute of General Medical Sciences | NIGMS |
| National Institute of Minority Health and Health Disparities | NIMHD |
| National Institute of Neurological Diseases & Stroke | NINDS |
| National Institute on Alcohol Abuse and Alcoholism | NIAAA |
| National Science Foundation | NSF |
| National Institute on Drug Abuse | NIDA |
| Office of Naval Research | ONR |
| Pacific Command | PACOM |
| Research Centers in Minority Institutions | RCMI |
| Research Centers in Minority Institutions Infrastructure for Clinical and Translational Research | RCTR |
| United States Special Operations Command | USSOCOM |
Recognizing that building capacity to engage in clinical translational research at institutions across the USA requires collaborations and partnerships, the CTSA mechanism was launched to support programs and infrastructures for translational science7, 19. The success of the CTSA programs impacted geographical regions based at the institutions involved with their respective CTSA programs21–25. On a smaller scale and owing to the limitations placed by the NIH IDeA Program, institutions from IDeA-eligible states developed clinical and translational research capacity at their institutions beginning in 201426. In a similar fashion, RCMI institutions were provided opportunities to develop infrastructures to conduct clinical and translational research11–17. The University of Hawaii (UH), as an RCMI institution, established the RCMI Multidisciplinary And Translational Research Infrastructure Expansion (RMATRIX) Program as an RCTR which has grown to be a successful clinical and translational research foundation for faculty members at UH.20 Nationally, millions of federal and foundation dollars have been invested into these and other clinical and translational research infrastructure programs. We report the success of a Pilot Project Program of RMATRIX at UH which not only built research capacity locally but also impacted clinical and translational research far beyond Hawaii through the support of academic investigators with Pilot Project Awards. One of unique characteristics of Hawaii was that as the 50th state of the USA, its geographical isolation in the Pacific Ocean distinguished it as having the only accredited USA medical school within a 2400 mile radius. With its diverse ethnic population including Native Hawaiians, Pacific Islanders, Asians and Filipinos, the RMATRIX program at UH was uniquely situated with its infrastructure to potentially contribute to the clinical translational landscape in Hawaii to benefit its population. One component of the RMATRIX program was the Pilot Project program which provided resources and funds for emerging investigators to support research projects focusing on health inequities in the communities. We report that the investment not only built the clinical and translational research infrastructure at UH but the dollars had a far-reaching impact that connected communities and collaborators both locally and nationally where health inequities also persisted.
METHODS
RMATRIX Program
The Institutional Review Board (IRB) of the UH Human Studies Program reviewed the principle of the project. The RMATRIX Program was initially funded as an RCTR in 2010 for 3 years with a 1 year no-cost extension in response to an NIH Program Announcement, PAR-09-261, "Limited Competition for Research Centers in Minority Institutions Infrastructure for Clinical and Translational Research". Following a competitive renewal application, RMATRIX was refunded for 5 years in 2014. Through the RCTR award, RMATRIX was established as the integrated "home" for clinical and translational science in Hawaii through the UH.
In the first 4 years, we reorganized and expanded existing (and previously disconnected) RCMI research infrastructure programs at UH working with community and hospital partners. The RMATRIX Key Functions were carefully designed to provide the clinical and translational research infrastructure to expand proposed HEALTH (Health, Equity and Lifestyle Transformation in Hawai’i) Initiatives to address health disparities throughout Hawaii. The RMATRIX Key Functions which served as resources to support investigators (during study development and implementation and career development) included: Program Administration, Professional Development, Collaborations and Partnerships, Biomedical Informatics, Clinical Research Resources and Facilities, Community-Based Research, Research Design and Biostatistics, Regulatory Knowledge, and Evaluation. These 8 Key Functions focused initially on projects and investigators interested in health disparity issues related to six HEALTH Initiatives: cardiovascular; respiratory; nutrition & metabolic; cancer; perinatal, growth, & development; and aging & neurocognition. In the successful renewal application, lessons learned allowed the program to focus on three HEALTH Initiatives which resonated with the communities and investigators through needs assessments: nutrition & metabolic health; reproductive growth & developmental health; and aging & chronic disease prevention/management.
While RMATRIX supported investigators interested in clinical and translational research across the spectrum of the HEALTH Initiatives, this report will highlight the continuing activities of investigators from the first three years of the RMATRIX Pilot Project Program. Data from 3–5 years after the pilot projects were award provided facts and figures on the ongoing impact of the RMATRIX program which informed this report.
RMATRIX Pilot Project Program
The Pilot Project Program promoted inter-professional translational research in health disparities by supporting research endeavors and collaborations among emerging investigators. The Pilot Project Program released an annual institution-wide request for proposals which encouraged multiple principal investigators and/or collaborators across disciplines. Presentations at department and school faculty meetings were also held to disseminate information about the funding opportunities. Direct costs up to $30,000 per year for 1-year projects were allowed, with priority given to those studies which focused on one of the HEALTH Initiatives. From 2010–2012, 18 Pilot Project Investigators were awarded from 55 Pilot Project applications which were submitted in response to the RFP. At the time of the RFP release, webinars were held to guide and provide potential applicants information on the Pilot Project application process. Each cycle of applications underwent external review with at least 2 experienced external reviewers assigned to each application and scored similarly to the current NIH extramural grant review guidelines.27 The level of funding available in 2010–2012 determined the number of Pilot Project Awards which were provided in each year: 3 awards to 3 investigators in 2010; 8 awards to 8 investigators in 2011; and 6 awards to 7 investigators in 2012. The Pilot Project investigators’ disciplines and Pilot Project titles are summarized in Table 2.
Table 2.
RMATRIX Pilot Project Investigators with Associated Grants
| Pilot Project Investigator |
Pilot Project Investigator’s Discipline |
Pilot Project Title | Grants After RMATRIX Pilot Project Award | ||
|---|---|---|---|---|---|
| Agency/Grant# | Role* | Years | |||
| 1 | Native Hawaiian Health |
PILI ’Aina Project: Partnerships to Overcome Obesity Disparities in Hawaii |
NIMHD/R24MD001660 NIMHD/RL5MD009591 NIMHD/T37MD006909 NHLBI/R01HL126577 |
PI Sub-PI Sub-PI PI |
2013–16 2014–19 2014–18 2015–20 |
| 2 | Neurology | Neuroimaging Correlates of Monocyte/Macrophage Infiltration in HIV- infected Individuals: A Cross-sectional Pilot Study Using IV Furomoxytol |
NINDS/R21NS087951 | PI | 2015–17 |
| 3 | Cell & Molecular Biology |
Protection from Cardiac Hypertrophy via Pharmacological Inhibition of the Ion Channel TRPV1 |
DOD AFRL | Co-PI | 2013–18 |
| 4 | Anatomy, Biochemistry & Physiology |
Correlation of Genetic Risk Factors with Gestational Diabetes and Preeclampsia in Women from Hawaii |
NIGMS/P20GM103457 Project 4 |
PI | 2014–19 |
| 5 | Cancer | Discrimination and Substance Abuse Among Adolescents in Hawaii |
NIDA/NINDS/NIAAA/U 01DA041117 |
Co-PI | 2015–20 |
| 6 | Tropical Medicine, Med Micro, Pharm |
Identification and Characterization of Cryptic Fleming Bay Marine Stingers |
USSOCOM/H92222-14- P-0058 USSOCOM/H92222-15- P-0067 ONR/BAA 10-001 PACOM/0000 |
PI PI PI PI |
2014–15 2015–16 2015–17 2015–16 |
| 7 | Engineering | Effect of Corticosteroids on Pulmonary Surfactant of Premature Newborns |
NSF/CBET-1236596 NSF/CBET-1254795 |
PI PI |
2012–16 2013–18 |
| 8 | Medicine | Cerebrovascular Risk Factors and Altered Brain Structure in Healthy Individuals |
NINDS/R21NS080656 | PI | 2013–17 |
| 9, 10 | Engineering, Medicine |
Integration and Assessment of Physiologic Radar Technology in Sleep Monitoring Systems |
NSF STTR | Co-PI | 2014–15 |
| 11 | Pediatrics | Project PONO - Prevention of Negative Outcomes |
NHLBI/HL130780 | PI | 2015–16 |
| 12 | Cardiology | Metabolite Profile as a Postpartum Predictor of Endothelial Dysfunction Following Preeclampsia |
NHLBI/OD/R21HL11539 8 |
PI | 2013–17 |
| 13 | Native Hawaiian Health |
Hula & Hypertension: Ola Hou Pilot Study |
NIDDK/R01DK102728 (U. of Washington) |
PI | 2015–20 |
| 14 | Nursing | Perceptions of Native Hawaiian Community Partners about Community-Based Participatory Research |
HRSA/D09HP28681 | Sub-PI | 2015–18 |
AFRL: Air Force Research Laboratory; DOD: Department of Defense; HRSA: Health Resources and Services Administration; NCRR: National Center for Research Resources; NHLBI: National Heart, Lung, and Blood Institute; NIAAA: National Institute on Alcohol Abuse and Alcoholism; NIDA: National Institute on Drug Abuse; NIDDK: National Institute of Diabetes and Digestive and Kidney Diseases; NIGMS: National Institute of General Medical Sciences; NIMHD: National Institute of Minority Health and Health Disparities; NINDS: National Institute Neurological Diseases & Stroke; NSF: National Science Foundation; ONR: ONR: Office of Naval Research; OD: Office of the Director; USSOCOM: United States Special Operations Command; PACOM: Pacific Command
PI: Principal Investigator; Co-PI: Co-Principal Investigator; Sub-PI: Subcontract PI
RMATRIX Pilot Project Program Evaluation
The RMATRIX evaluators (from the second funding cycle) consisted of one MD and one MA/MPH with over 30 years each in health research including federal funding on multi-year grants and contracts as well as infrastructure development grants in minority health involving academia, medical centers, and community organizations throughout Hawaii. The Evaluators were from an independent, nonprofit organization with research backgrounds who were each funded by grants and contracts and each understanding challenges that researchers faced. They employed qualitative methodology using semi-structured interviews to collect information on Pilot Project Investigators’ perspectives and experiences. The semi-structured interview process permitted maximum use of face-to-face time with each Pilot Project Investigator. Interviews were conducted at a location selected by the Pilot Project Investigator; usually a conference room at their department or in their office at the University or Medical Center.
Data were collected beginning 1 year after each Pilot Project Investigator was awarded the RMATRIX Pilot Award through December 2015. RMATRIX Pilot Project Investigators received their awards in 2010, 2011, and 2012, providing 3 to 5 years of follow-up data for analysis. A standardized list of outcomes was used to guide the interview process while not restricting the Pilot Project Investigator from sharing additional information he/she felt important. This included: publications; presentations; patents; further research after pilot award; continuation of pilot or new research; research role; awards/recognitions; promotions; job/career change; research findings that brought change to clinical or social service practice, resulted in change in organizational, local, state, or federal policies; findings used in advocacy; collaborators; disciplines; community involvement; and federal contacts, Table 3. A copy of the outcomes to be discussed as well as evaluation questions were sent to all Pilot Project Investigators before their interview. Each Pilot Project Investigator was requested to send an updated resume to the Evaluators prior to the in-person meeting. This allowed the Evaluators to review the resume, note outcomes, and identify additional questions for the meeting. This helped to maximize the interview process and keep the total interview time to one hour. The academic professional outcomes and measurements were reported based on the interview information of the pilot project investigators, Table 3.
Table 3.
Outcome Measurements Used to Assess Impact of Pilot Projects
| Assessment Outcome | Measurements |
|---|---|
| Scholarly Work | Publications, presentations at national/international meetings, funded grants/awards |
| Professional Academic Promotion | Academic promotion, new academic position |
| Entrepreneurship | New startup company, patents, intellectual property agreements |
| Training the Next Generation of Faculty Researchers | Providing mentorship to students (medical students, Master Candidates, PhD Candidates, undergraduate students), physicians, postdocs, faculty |
| Awards/Honors | Awards and recognitions from regional, national and international institutions and organizations |
| Scientific Impact | Scientific impact on how the pilot project impacted access, delivery and outcomes of care, or contributed to health among Native Hawaiians and Other Pacific Islanders |
| Advocacy | Impact of the pilot project on the population in Hawaii through new policy and advocacy. |
| Synergy and Collaborations | As a result of the pilot projects, new collaborations which were formed amongst academia, communities, and industry from local, national, and international regions. |
RESULTS
Eighteen Pilot Project Investigators received seventeen pilot awards from 2010–2012 in RMATRIX. Seventeen of eighteen investigators in RMATRIX were contacted; however, the lack of contact information for the last investigator prevented follow-up. Fifteen investigators provided updated resumes and met with the Evaluators. One Pilot Project Investigator provided a resume but elected to not participate in the interview while a second who moved to the mainland United States furnished an updated resume and answered the evaluation questions via email. The data presented summarizes information from 17 investigators based on self-report and public documentation. The academic demographics of the investigators represented diverse disciplines across the spectrum and academic ranks, Table 4.
Table 4.
Professional & Academic Demographics of Pilot Project Investigators
| Professional Discipline | Highest Academic Degree Obtained |
Academic Rank | Academic Promotions |
Metric Outcomes |
|---|---|---|---|---|
| Public Health, Psychology, Nursing, Engineering, Tropical Medicine, Cell and Molecular Biology, Medicine |
PhD (n=12), MD/PhD (n=2), MD (n=3) |
Assistant Professor (n=10), Associate Professor (n=3), Professor (n=4) |
Promoted to Associated Professor (n=2); to Professor (n=2); New academic appointments (n=2) |
224 publications and 311 presentations (141 local, 93 national, and 77 international) |
Scholarly Assessment
Scholarly assessment was measured by the publications, presentations at scientific meetings and by funded grants. The successful achievements of the Pilot Project Investigators were highlighted by publications in peer-reviewed journals and books since receiving their pilot awards as well as their contributions at scientific meetings with presentations, Table 4. Since the completion of their Pilot Project, the 17 investigators had 47 grants which were currently active at the time of this report. An additional 34 grants were funded and completed since their Pilot Projects. In addition to the funding agencies noted in Table 2, private foundations and pharmaceutical companies also contributed as sources. The 81 grants contributed over $79.5 million in research with each of the 17 investigators being Principal Investigators (PI) on 60 grants which totaled $22.4 million in direct award dollars. The litmus test for RMATRIX investigators’ research successes was based on being awarded federally-funded grants4.
Professional Academic Assessment
Pilot project investigators were assessed on their academic achievements since being awarded the pilot project funds including promotions and new academic appointments, Table 4.
Entrepreneurship Assessment
Entrepreneurship achievements were assessed by successful business ventures or related enterprises, Table 3. Three investigators developed new startup companies based on their research and contributions from their Pilot Project data. These startup companies are employing graduates from UH, providing them with jobs in Hawaii in their discipline. Three patents were received and four intellectual property agreements were filed.
Training the Next Generation of Faculty Researchers Assessment
Career development of faculty includes developing skills and ability to train emerging investigators and students to contribute to the pipeline of new clinical translational research faculty, Table 3. To accomplish this, continued collaborations with other investigators contribute to the foundation. All of the Pilot Project Investigators continued collaborations from their RMATRIX pilot work. Additionally, they have all become mentors themselves for students and other faculty. In 2015 alone, the 17 investigators have mentored 159 mentees including medical students, MDs, Master Candidates, PhD Candidates, PhDs and undergraduate students.
Awards/Honors Assessment
Recognition of the accomplishments as a direct or indirect result of the pilot project awards can contribute to clinical translation research through additional funding opportunities and scientific opportunities, Table 3. Among the numerous awards and recognitions cumulatively bestowed upon the RMATRIX Pilot Project Investigators were: the Robert W. Clopton Award, Distinguished Community Service, Certificate of Recognition and Invited Member National Academy of Inventors, Institute of Electrical and Electronics Engineers Fellow, Hawaii Comprehensive Cancer Coalition Chair, American Cancer Society Hawaii/Pacific Chapter Board, Royal Geographical Society Research Fellow, Migrant Clinicians Network External Advisory Board, Washington State Commission on Asian Pacific American Affairs, Queens’s Health Systems, Queen’s Medical Center and Hawaii Pacific Health Board members, American Academy of Neurology Emerging Leadership Forum Award, NSF Career Award, and American College of Physicians Laureate Award.
Scientific Impact Assessment
The scientific impact of the pilot project and/or contribution by the investigator was assessed by how the knowledge gained or results contributed to the health of the communities, Table 3. Because of Hawaii’s unique geographic location and diverse population, there are rich opportunities to examine how the environment, race and ethnicity, and culture impact the access, delivery and outcomes of care, and contribute to the significant health inequities identified among Native Hawaiians and Other Pacific Islanders. The potential scientific impact that the awarded Pilot Projects proposed were viewed positively by the external reviewers. As such, the success of the Pilot Projects provided scientific contributions across a wide spectrum including: 1) Physiological radar technology for sleep monitoring; 2) Behavioral modification to reduce health disparities and chronic disease in Native Hawaiians and other high risk populations; 3) Neuroimaging technique targeting monocytes for brain inflammation; 4) Properties of pulmonary surfactant in premature infants; 5) Patent award and product development of a compound to effectively treat box jellyfish stings; and 6) Patent on methods for prevention of cardiac hypertrophy.
Advocacy Assessment
The litmus test for effective clinical and translational research that impacts the population in Hawaii is policy and advocacy Table 3. RMATRIX Pilot Project Investigators reported the following actions: 1) Hawaii State Senate Resolution to convene Native Hawaiian Health Task Force addressing health inequities; 2) Establishment of Hawaii State Stroke Registry; and 3) Disparities findings used for advocacy by community leaders and health professionals locally, nationally, and internationally.
Synergy and Collaborations Assessment
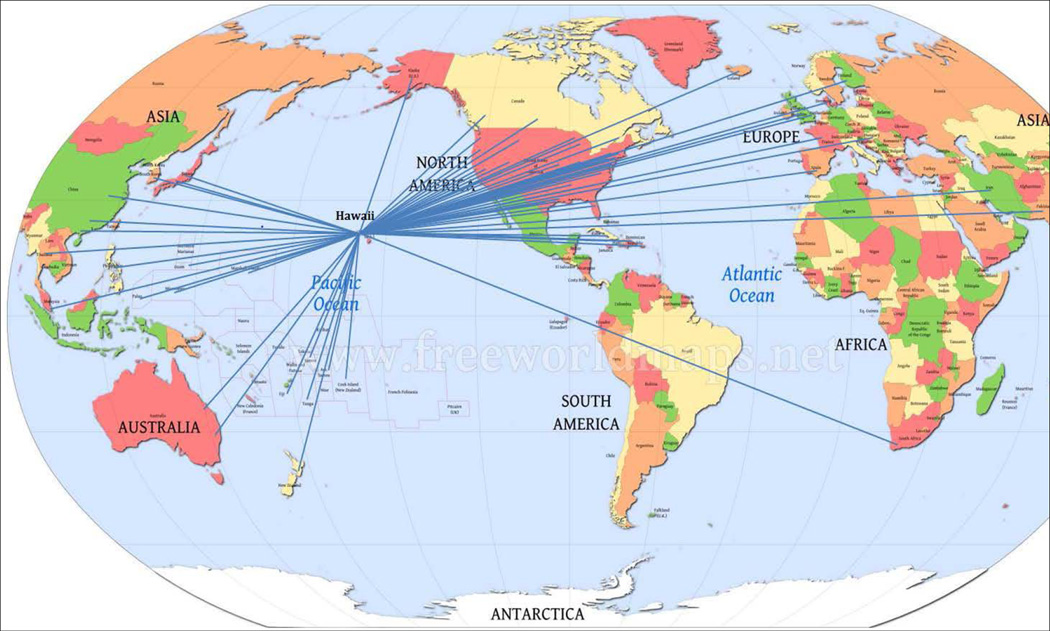
Establishing synergy and collaborations between the pilot project investigator and other leaders locally, nationally, and internationally has the potential to increase the impact that the research has on the population health in Hawaii as well as beyond the state, Table 3. The creation and launching of a new interdisciplinary translational class at UH combining engineering and pediatrics was one of the first of its kind at the institution. A statewide effort involving communities, health professionals, and academia united to support healthy choices to address obesity with launch of a successful program weight control program for children and families. The synergy and collaboration between engineering investigators working alongside health professionals to apply radar for sleep monitoring in the medical center bridged the engineering and medical disciplines in a positive and successful effort. Collaborations amongst academia, communities, and industry from local, national, and international regions are summarized in Figure 1 which also shows the direct connections of the collaborators from other geographical regions of the world.
Figure 1. RMATRIX Pilot Project Investigator Co-Author/Collaborator Network.
Links between the University of Hawaii RMATRIX Pilot Project investigators with investigators/collaborators nationally and internationally.
DISCUSSION
The RMATRIX Pilot Project Program successfully launched and expanded clinical translational research opportunities for the emerging faculty who were instrumental in leveraging the resources and their pilot project data to benefit the institution and state. In addition to enhancing their respective academic careers, their efforts contributed to policies and launched collaborations to start new ventures and projects to potentially benefit the populations in Hawaii and expand beyond the state.
At its inception, RMATRIX was conceived and envisioned to be a catalyst to build a critical mass of investigators who would conduct translational and clinical research in the HEALTH Initiatives that disproportionately affect Native Hawaiian and other Pacific Peoples in Hawaii. For many clinical and translational research infrastructure programs in the US, a similar theme purveys to benefit the populations they serve or with whom they engage in their activities4, 6, 7, 16–18, 20, 23, 26, 28. While the long-term goal of promoting and improving health equity across all stages of life for the most disparate populations (by contributing to improving the health of all people in Hawaii) is an ambitious undertaking and not quickly attainable in a short period of time (3 years), the RMATRIX has put in place a valuable infrastructure for academic and community investigators to work together on approaches for the prevention, diagnosis and treatment of diseases to improve health and reduce health disparities. RMATRIX is committed to further expanding and strengthening the infrastructure to lead by its success. Ultimately, the next steps for RMATRIX will be to demonstrate its success in improving health equities for the population in Hawaii.
RMATRIX has demonstrated the importance of career development and mentorship through the building of a critical mass of investigators committed to eliminating health inequities through clinical and translational health disparities research and collaborations with other institutions and communities as well as various professional disciplines locally, nationally and internationally. RMATRIX further strengthened the synergy between organizations, professional disciplines and individual investigators.
With the large number of clinical and translational research infrastructure awards in existence7, 12, 14, 16, 17, 24, 26, 29, 30, RMATRIX is likely not unique in its impact or importance to the institution, state, or communities. Other successful infrastructure programs have Pilot Project mechanisms to jumpstart research careers of faculty28, 30, however, because of the uniqueness of its size, location, and modest landscape in clinical and translational research at the UH, the magnitude of its impact may be more pronounced. As one of our pilot investigators noted, RMATRIX changed the intellectual atmosphere at UH and JABSOM, raising awareness of ethnic disparities in health, work that is supported by funding agencies, and bringing local indigenous investigators together with other groups nationally and internationally providing an avenue for new research. This successful program demonstrated how support for clinical and translational research could be leveraged and could set the cornerstone for similar programs to collaborate to ultimately benefit the health and well-being of the populations.
It has taken decades to recognize and acknowledge health inequities in our communities. Addressing these issues now using the mechanism of successful clinical and translational research infrastructure programs will help communities move from identification of the causes of health inequities toward the training of investigators with strong community partners who will develop effective interventions with strong scientific support that will lead to commercial products, changes in health professional practice, and well-grounded health policy that can turn the tide of health disparities affecting the population.
Acknowledgments
The authors would like to thank the RMATRIX Key Function Directors (Venkataraman Balaraman, Kathryn Braun, John Chen, Judith Inazu, J. Keawèaimoku Kaholokula, Kari Kim, Marjorie Mau, Neal Palafox, Cecilia Shikuma, Alexander Stokes, JoAnn Tsark) and Grace Matsuura and Lauren Soto. Support was provided by the National Institute on Minority Health and Health Disparities (U54MD007584; U54MD008149), National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
COMPLIANCE WITH ETHICAL STANDARDS
The scope of the work did not involve human participants as reviewed by the University of Hawaii Institutional Review Board.
The authors have no other potential conflicts of interests except for the funding agencies as acknowledged.
REFERENCES
- 1.Chin MH, Alexander-Young M, Burnet DL. Health care quality-improvement approaches to reducing child health disparities. Pediatrics. 2009;124(Suppl 3):S224–S236. doi: 10.1542/peds.2009-1100K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fink AM. Toward a new definition of health disparity: a concept analysis. J Transcult Nurs. 2009;20(4):349–357. doi: 10.1177/1043659609340802. [DOI] [PubMed] [Google Scholar]
- 3.McPheeters ML, et al. Closing the quality gap: revisiting the state of the science (vol. 3: quality improvement interventions to address health disparities) Evid Rep Technol Assess (Full Rep) 2012;(208.3):1–475. [PMC free article] [PubMed] [Google Scholar]
- 4.Kaiser J. Biomedical research funding. NIH uncovers racial disparity in grant awards. Science. 2011;333(6045):925–926. doi: 10.1126/science.333.6045.925. [DOI] [PubMed] [Google Scholar]
- 5.Moses H, 3rd, et al. Financial anatomy of biomedical research. JAMA. 2005;294(11):1333–1342. doi: 10.1001/jama.294.11.1333. [DOI] [PubMed] [Google Scholar]
- 6.Portilla LM, Alving B. Reaping the benefits of biomedical research: partnerships required. Sci Transl Med. 2010;2(35):35cm17. doi: 10.1126/scitranslmed.3001137. [DOI] [PubMed] [Google Scholar]
- 7.Shirey-Rice J, et al. The CTSA Consortium's Catalog of Assets for Translational and Clinical Health Research (CATCHR) Clin Transl Sci. 2014;7(2):100–107. doi: 10.1111/cts.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sajdyk TJ, et al. Project development teams: a novel mechanism for accelerating translational research. Acad Med. 2015;90(1):40–46. doi: 10.1097/ACM.0000000000000528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sampselle CM, et al. Nurse engagement and contributions to the clinical and translational science awards initiative. Clin Transl Sci. 2013;6(3):191–195. doi: 10.1111/cts.12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zerhouni E. Medicine. The NIH Roadmap. Science. 2003;302(5642):63–72. doi: 10.1126/science.1091867. [DOI] [PubMed] [Google Scholar]
- 11.Estape ES, et al. Incorporating translational research with clinical research to increase effectiveness in healthcare for better health. Clin Transl Med. 2014;3:20. doi: 10.1186/2001-1326-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fleming ES, et al. Addressing health disparities through multi-institutional, multidisciplinary collaboratories. Ethn Dis. 2008;18(2 Suppl 2):S2-161–S2-167. [PMC free article] [PubMed] [Google Scholar]
- 13.Hammatt ZH, et al. Partnering to harmonize IRBs for community-engaged research to reduce health disparities. J Health Care Poor Underserved. 2011;22(4 Suppl):8–15. doi: 10.1353/hpu.2011.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JE, et al. Importance of capacity assessment for an early staged-research network designed to eliminate health disparity: lessons from RTRN. Ethn Dis. 2010;20(1 Suppl 1):S1-150–S1-154. [PMC free article] [PubMed] [Google Scholar]
- 15.Lee JE, et al. User-Friendly Data-Sharing Practices for Fostering Collaboration within a Research Network: Roles of a Vanguard Center for a Community-Based Study. Int J Environ Res Public Health. 2016;13(1) doi: 10.3390/ijerph13010034. p. ijerph13010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ofili EO, et al. Models of interinstitutional partnerships between research intensive universities and minority serving institutions (MSI) across the Clinical Translational Science Award (CTSA) consortium. Clin Transl Sci. 2013;6(6):435–443. doi: 10.1111/cts.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quarshie A, et al. Establishing the Morehouse School of Medicine (MSM) R-CENTER clinical and translational research web-portal: the role of focus groups. J Health Care Poor Underserved. 2011;22(4 Suppl):165–173. doi: 10.1353/hpu.2011.0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leshner AI, et al., editors. Washington DC: National Academy of Sciences; 2013. The CTSA Program at NIH: Opportunities for Advancing Clinical and Translational Research. [PubMed] [Google Scholar]
- 19.Manson SM, et al. Vision, Identity, and Career in the Clinical and Translational Sciences: Building upon the Formative Years. Clin Transl Sci. 2015;8(5):568–572. doi: 10.1111/cts.12316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hedges JR, Shiramizu B, Seto T. RMATRIX - Clinical Translational Research Award. Hawaii Med J. 2011;70(1):18–19. [PMC free article] [PubMed] [Google Scholar]
- 21.Byington CL, et al. The CTSA mentored career development program: supporting the careers of child health investigators. Clin Transl Sci. 2014;7(1):44–47. doi: 10.1111/cts.12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chung B, et al. Faculty Participation in and Needs around Community Engagement within a Large Multiinstitutional Clinical and Translational Science Awardee. Clin Transl Sci. 2015;8(5):506–512. doi: 10.1111/cts.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inkelas M, et al. Enhancing Dissemination, Implementation, and Improvement Science in CTSAs through Regional Partnerships. Clin Transl Sci. 2015;8(6):800–806. doi: 10.1111/cts.12348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meyers FJ, et al. Strengthening the career development of clinical translational scientist trainees: a consensus statement of the Clinical Translational Science Award (CTSA) Research Education and Career Development Committees. Clin Transl Sci. 2012;5(2):132–137. doi: 10.1111/j.1752-8062.2011.00392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morrato EH, et al. Dissemination and implementation of comparative effectiveness evidence: key informant interviews with Clinical and Translational Science Award institutions. J Comp Eff Res. 2013;2(2):185–194. doi: 10.2217/cer.13.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor F. Institutional Development Award. 2016 [Google Scholar]
- 27.Sattler DN, et al. Grant Peer Review: Improving Inter-Rater Reliability with Training. PLoS One. 2015;10(6):e0130450. doi: 10.1371/journal.pone.0130450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holzer J, Kass N. Community engagement strategies in the original and renewal applications for CTSA grant funding. Clin Transl Sci. 2014;7(1):38–43. doi: 10.1111/cts.12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kost RG, et al. Accrual and recruitment practices at Clinical and Translational Science Award (CTSA) institutions: a call for expectations, expertise, and evaluation. Acad Med. 2014;89(8):1180–1189. doi: 10.1097/ACM.0000000000000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Main DS, et al. A community translational research pilot grants program to facilitate community--academic partnerships: lessons from Colorado's clinical translational science awards. Prog Community Health Partnersh. 2012;6(3):381–387. doi: 10.1353/cpr.2012.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]