Abstract
Background
Proliferator activator receptor (PPAR)-γ coactivator (PGC-1α) gene and Sirtuin-1 (SIRT1) respond to physiological stimuli and regulate insulin resistance. Inflammatory markers TNF-α, interleukin-6, C-reactive protein (CRP) and the soluble forms of intracellular adhesion molecule (sICAM-1) and vascular CAM-1 (sVCAM-1) are associated with increased risk of diabetes and coronary heart disease. Resistive training reduces hyperinsulinemia and improves insulin action in chronic stroke. Yet, the molecular mechanisms for this are unknown. This study will determine the effects of RT on skeletal muscle PGC-1α and Sirtuin-1 (SIRT1) mRNA expression and inflammatory and vascular markers.
Methods
Stroke survivors (50–76 years) underwent a fasting blood draw for measurement of TNF-α, IL-6, CRP, serum amyloid A, sICAM-1, sVCAM-1 and bilateral vastus lateralis biopsies before and after RT. Participants were also assessed using bilateral multi-slice thigh CT scans from the knee to the hip, a total body scan by DXA, and one-repetition (1-RM) strength testing. Subjects performed two sets of three lower extremity RT exercises 3x/week for 12-weeks.
Results
Bilateral leg press and leg extension strength increased ~30–50% with RT (P<0.001). Body weight, total body fat mass, and fat-free mass did not change. Thigh muscle area and volume increased in both legs (P<0.05). Non-paretic muscle PGC-1α mRNA expression increased 14% (P<0.05) after RT and SIRT1 mRNA decreased 24% (P<0.05) and 31% (P<0.01) in paretic and non-paretic muscle. There were no significant changes in plasma inflammation with training.
Discussion
RT in chronic stroke induces changes in key skeletal muscle regulators of metabolism, without effecting circulating inflammation.
Keywords: Exercise, Skeletal Muscle, Stroke, Inflammation, Strength, Vascular
Introduction
Inflammation is a risk factor for stroke and contributes to the progression of cardiovascular disease1. Moreover, low-grade inflammation is a pathophysiological mechanism underlying sarcopenia2. The paretic thigh of stroke survivors has 20% lower muscle area and 25% higher intramuscular fat than the non-paretic thigh3 demonstrating substantial atrophy and muscle composition change4. We have previously reported that resistive training (RT) results in muscle hypertrophy and loss of intramuscular fat in chronic stroke, while reducing skeletal muscle expression of myostatin5, a member of the transforming growth factor beta family of secreted growth factors, and a significant regulator of skeletal muscle development and size6. To our knowledge, no studies have examined RT-induced changes to key inflammatory and metabolic biomarkers in the circulation after stroke or their role in paretic muscle wasting.
C-reactive protein (CRP) and the soluble forms of intracellular adhesion molecule (sICAM-1) and vascular CAM-1 (sVCAM-1) are vascular inflammatory markers associated with increased risk of diabetes and coronary heart disease7, 8. Elevated circulating concentrations of TNF-α and interleukin-6 also occur in type 2 diabetes and predict its development in middle-aged and elderly adults9, 10. In addition, PPAR-γ coactivator (PGC-1α) and Sirtuin-1 (SIRT1) respond to physiological stimuli and regulate insulin resistance though distinct mechanisms11. Regular aerobic exercise modulates intracellular pathways to improve glucose uptake, in part by attenuating epigenetic modifications on PGC-1α and its downstream regulators12.
We showed that resistive training can reduce hyperinsulinemia and improve insulin action in chronic stroke13, a population with a high prevalence of insulin resistance and diabetes14. Herein, we test the hypothesis that resistive training reduces systemic inflammation and alters the gene expression of PGC-1α and SIRT1 in paretic and non-paretic skeletal muscle. Thus, the purpose of this study was to determine the effects of resistive training (RT) on systemic inflammatory and vascular markers, and paretic (P) and non-paretic (NP) skeletal muscle PGC-1α and SIRT1 mRNA expression in chronic stroke.
Methods
Subjects
Of the 24 ischemic stroke subjects enrolled (>six months latency), six individuals did not complete the study due to time constraints or medical issues unrelated to study participation. The 18 individuals (12 men, 6 women) who completed the study were between 55–76 years with BMIs between 21–39 kg/m2. All 18 underwent either a blood drawn for assessment of circulating inflammatory markers, or bilateral-skeletal muscle tissue biopsies for gene expression analysis. Fifteen came from our previously published study5 but the blood and tissue biomarkers covered in this paper were not part of the prior work. All stroke survivors had mild to moderate hemiparetic gait deficits and had completed conventional rehabilitation therapy. Evaluations included medical history, physical examination, fasting blood profile, and screening for dementia15 and depression16 to ensure adequate informed consent. Subjects were excluded for unstable angina, congestive heart failure (NYHA II), severe peripheral arterial disease, major post-stroke depression, dementia, severe receptive aphasia, and orthopedic or chronic pain conditions.
All tests were performed before and after the three-month training intervention. All methods and procedures were approved by the Institutional Review Board of the University of Maryland as well as the VA R&D committee. Each participant provided written informed consent.
VO2 peak and Body Composition
Exercise testing with open circuit spirometry was conducted to measure VO2peak using a graded submaximal treadmill test17. Height and weight were measured. Fat mass, lean tissue mass and %body fat were determined by DXA (Prodigy LUNAR GE version 7.53.002). Thigh CT scans were performed every 4 cm starting at the patella and ending at the femoral head (Siemens Somatom Sensation 64 Scanner) and a single mid-thigh slice was used to quantify skeletal muscle area, total fat area, low density lean tissue area3, and muscle attenuation in both the paretic and non-paretic thighs. Scans were analyzed using MIPAV (Medical Image Processing, Analysis and Visualization, v.7.0, NIH).
Strength Testing
Bilateral 1 repetition maximum (1-RM) strength tests were conducted on pneumatic leg press and leg extension RT equipment built for single leg movement (Keiser, Fresno, CA), to account for strength discrepancies between the paretic and non-paretic limbs. Two familiarization sessions were included prior to baseline 1-RM testing to avoid the confounding effects of learning on baseline strength measures.
Blood Draw and Analysis
Subjects underwent an overnight 12-hour fast and the following morning had a blood draw (n=15) and two-hour oral glucose tolerance test. Blood samples were collected in heparinized syringes, placed in pre-chilled test tubes containing 1.5 mg of ethylenediaminetetraacetic acid per mL of blood, centrifuged at 2,000 x g for 10 minutes at 4°C, and aliquoted for storage at −80°C until analysis. Plasma glucose concentrations were measured in duplicate using the glucose oxidase method (2300-STAT Plus, YSI, Yellow Springs, OH). Plasma insulin was determined in duplicate using radioimmunoassay (Millipore, Billerica, MA). Fasting plasma for CRP, SAA, sICAM-1, and sVCAM-1 was measured in duplicate, with CV less than 10% according to electrochemiluminescence using a multi-spot microplate (SECTOR Imager-2400, Meso Scale Discovery, Gaithersburg, MD). Fasting plasma for TNF-α, IL-6, IL-1β, and IL-8 was measured in triplicate also using a multi-spot microplate (SECTOR Imager-2400, Meso Scale Discovery, Gaithersburg, MD).
Skeletal Muscle Biopsies, RNA extraction and Reverse transcription for Real-time RT-PCR
Percutaneous needle biopsies were obtained from the vastus lateralis muscle. These were done ~12–13 cm above the patella on the anterolateral aspect of each thigh using a Bergstrom needle (Stille, Solna, Sweden) with a suction-enhancement technique. The biopsies were performed before and after training under local anesthesia from 10 subjects after a 12-hour fast for the measurement of gene expression for PGC-1α and SIRT-1. Post-training biopsies occurred 24–36 hours after the last bout of RT. The acquired tissue was used to measure PGC-1α and SIRT-1 gene expression (paretic and non-paretic). Muscle was immediately freeze-clamped and stored at −80°C. Approximately 50–80 mg of muscle was used for RNA isolation.
Total RNA was extracted from skeletal muscle by the guanidinium isothiocyanate/phenol/chloroform method developed by Chomcynski and Sacchi18. The RNA pellet was resuspended in RNAsecure Resuspension solution (Cat. #7010, Ambion Inc.) and RNA concentrations were measured in a spectrophotometer. One μg of total RNA for each sample was reverse transcripted (RT) into first strand cDNA using Transcriptor First Strand cDNA Synthesis Kit (Cat# 04 896 866 001, Roche Applied Science) according to the detailed manufacturer’s protocol and as previously described5. Quantitative Real-time PCR (qPCR) and data analysis for PGC-1α and SIRT-1 were performed in a LightCycler 480 Real-Time PCR System with LightCycler® 480 software (Roche Applied Science). LightCycler 480 Multiwell plate 384 (Cat# 04 729 748 001), LightCycler 480 Probes Master kit (Cat# 04 887 301 001) and Taqman gene expression primer/probe set (Thermo Fisher Scientific Inc. Applied Biosystems, Foster City, CA.) were used. Each qPCR reaction was carried out in a final volume of 10μl, consisting of 2μl 1:4 diluted template cDNA, 5μl LightCycler 480 Probes Master. 0.5μl Taqman gene expression primer and probe mix, and 2.5μl nuclease-free water. Water instead of cDNA served as the no template control. According to the manufacturer’s instruction, the qPCR protocol was adopted for all samples: after incubation at 95°C for 10 min to activate the DNA polymerase, 45 cycles of 95°C for 10s and 60°C for 30s each were performed to facilitate the PCR reaction. 36B4 served as an internal control for normalization. Data acquisition occurred at real time during the annealing/elongation incubation at 60°C. All samples were amplified in triplicate from the same RNA preparation. Gene expression data were analyzed by Roche LightCycle 480 system Software version 1.5 advanced relative quantification program. The average of three determinations for each sample and the normalized ratio of Target/Reference was used in statistical analyses.
Resistive Training Protocol
The training protocol was designed to provide a high volume, high intensity training stimulus for maximal adaptation in skeletal muscle mass across a 3 month period. Subjects trained 3x/week for 12 weeks, performing two sets of 20 unilateral repetitions on the leg press, leg extension and leg curl machines (Keiser, pneumatic resistance, Fresno, CA) at every session. Generally, resistance was set at a level that would cause muscle failure somewhere between the 10th and 15th repetition. Resistance would then be gradually reduced to allow completion of the full 20 repetition set. Participants trained each leg separately to account for differences in strength and progression requirements between limbs. Resistance was gradually increased every two to three weeks to account for strength gains and to maximize the intensity of the training.
Statistical Analyses
Baseline gene expression levels were compared between paretic and non-paretic legs using paired Student t-tests. Training-induced changes for all circulating biomarkers as well as within leg gene expression levels were assessed across time using repeated measures ANOVA. All data were analyzed using SPSS 12.0. Data are presented as means ± SD. P values <0.05 are statistically significant.
Results
Physical Characteristics (Table 1)
Table 1.
Characteristics of stroke survivors before and after RT.
| Variable | Pre | Post |
|---|---|---|
| Age (years) | 64 ± 6 | ------- |
| Latency since stroke (years) | 8 ± 6 | ------- |
| BMI (kg/m2) | 27.5 ± 4.4 | ------- |
| Weight (kg) | 82.0 ± 16.8 | 81.7 ± 16.4 |
| VO2peak (ml.kg.min−1) | 21.2 ± 7.0 | 21.4 ± 6.6 |
| Paretic 1RM Leg Extension (lbs) | 57 ± 35 | 84 ± 39† |
| Non-paretic 1RM Leg Extension (lbs) | 110 ± 36 | 140 ± 31† |
| Paretic 1RM Leg Press (lbs) | 299 ± 145 | 378 ± 151** |
| Non-paretic 1RM Leg Press (lbs) | 439 ± 136 | 556 ± 119† |
| Percent body fat | 35.2 ± 7.7 | 34.9 ± 8.1 |
| Fat mass (kg) | 30.2 ± 9.2 | 29.7 ± 9.6 |
| Fat-free mass (kg) | 55.0 ± 10.5 | 55.6 ± 10.6 |
| Paretic muscle area (cm2) | 63.2 ± 10.6a | 73.7 ± 13.1**a |
| Non-paretic muscle area (cm2) | 83.9 ± 16.1 | 91.8 ± 21.2** |
| Paretic subcutaneous fat area (cm2) | 85.7 ± 41.2 | 83.3 ± 42.2c |
| Non-paretic subcutaneous fat area (cm2) | 81.1 ± 41.5 | 77.0 ± 38.3 |
| Paretic low density lean tissue area (cm2) | 25.5 ± 11.1b | 25.7 ± 12.2 |
| Non-paretic low density lean tissue area (cm2) | 23.4 ± 10.3 | 23.9 ± 11.1 |
| Paretic muscle attenuation (HU) | 25.2 ± 4.3d | 29.1 ± 2.4c |
| Non-paretic muscle attenuation (HU) | 31.9 ± 3.9 | 35.7 ± 2.4* |
Note lbs for strength levels are derived from pneumatic resistance equipment. BMI = body mass index; VO2peak = peak oxygen consumption. Values are means ± SD.
pre vs. post,
P<0.05,
P<0.01 and
P<0.0001
paretic vs. non-paretic,
P<0.005;
P<0.01;
P<0.05;
P=0.06
Stroke survivors (n=18) were predominately male (67%) and split along racial lines (56% Caucasian and 44% African-American). Six had impaired glucose tolerance upon study entry but none had type 2 diabetes. VO2peak did not significantly change with RT. As expected, muscle leg press strength improved by 27% in the non-paretic leg (P<0.0001) and 26% in the paretic leg (P<0.005). Leg extension increased 27% and 47% in non-paretic and paretic legs, respectively (P<0.001). A between leg effect reflected a greater increase in paretic leg extension strength across the RT intervention (P<0.001). There were no significant changes in body weight, total body fat mass, fat-free mass, and %fat by DXA with RT.
Paretic mid-thigh muscle area was lower than non-paretic muscle area before (P<0.005) and after (P<0.0001) RT. Paretic subcutaneous fat area was higher than non-paretic fat area at post-testing but not before training. Low density lean tissue was greater in the paretic than non-paretic leg before training (P<0.01) but was not different between legs after training. Mid-thigh muscle area of the paretic and non-paretic thigh increased 17% (P<0.005) and 10% (P<0.01), respectively after RT. There were no significant changes in subcutaneous fat area and low density lean tissue area with RT. Muscle attenuation of the mid-thigh cross-section tended to increase after RT in both the paretic (15%, P=0.08) and non-paretic thigh by (12%, P<0.05), representing a decrease in intra-muscular fat.
Glucose Metabolism, Cytokines and Vascular Markers (Table 2)
Table 2.
Inflammatory and Vascular Markers before and after RT in stroke survivors.
| Variable | Pre | Post |
|---|---|---|
| Fasting glucose (mg/dl) | 97 ± 8 | 96 ± 8 |
| Fasting insulin (pmol/L) | 118 ± 54 | 122 ± 88 |
| Glucose120 (mg/dl) | 138 ± 46 | 137 ± 49 |
| Insulin120 (pmol/L) | 690 ± 250 | 667 ± 330 |
| IL-6 (pg/ml) | 10.9 ± 11.1 | 12.0 ± 12.6 |
| IL-8 (pg/ml) | 13.6 ± 15.3 | 16.0 ± 13.3 |
| TNF-α (pg/ml) | 16.3 ± 5.9 | 16.5 ± 8.2 |
| IL-1β | 2.6 ± 3.2 | 3.5 ± 4.9 |
| CRP (mg/L) | 6.5 ± 8.5 | 5.7 ± 7.3 |
| SAA (mg/L) | 8.3 ± 11.9 | 4.7 ± 4.5 |
| sICAM-1 (ng/mL) | 580 ± 215 | 536 ± 140 |
| sVCAM-1 (ng/mL) | 1064 ± 607 | 1002 ± 351 |
Values are means ± SD.
There were no significant changes in fasting or two-hour glucose and insulin levels. In addition, two-hour glucose AUC (978 ± 169 vs. 918 ± 197 mmol/L per 120 min) and insulin levels AUC (76,190 ± 24,587 vs. 72,909 ± 40,762 pmol/L per 120 min) did not significantly change. Concentrations of IL-6, IL-8, Il-1β, and TNF-α did not change with RT. Although CRP levels decreased 12%, the change was not significant. In addition, there were no significant changes in SAA, sICAM-1 and sVCAM-1 levels after RT.
Skeletal Muscle PGC-1 and SIRT1 Levels
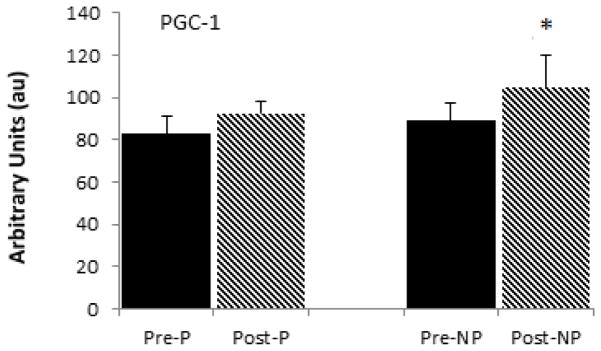
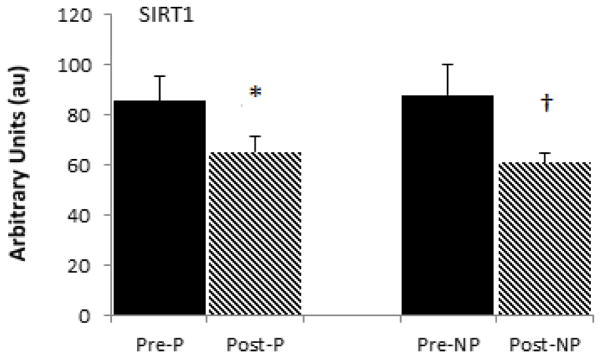
The mRNA levels of PGC-1α were not different between the paretic and non-paretic muscle prior to training (88.8 ± 43.8 vs. 90.0 ± 28.1 AU). There was a 17% increase in PGC-1α mRNA levels in the non-paretic muscle (P<0.05, Figure 1). PGC-1α gene expression increases did not reach statistical significance in paretic muscle (+4%). Similarly, SIRT1 mRNA was not different between paretic and non-paretic muscle prior to RT (80.9 ± 33.8 vs. 75.2 ± 18.4 AU). SIRT1 levels decreased 24% in paretic (P<0.05) and 31% in non-paretic (P<0.01) muscle after RT (Figure 2).
Figure 1.
Skeletal muscle PGC1 mRNA levels in the paretic and non-paretic legs before and after resistive training (n=10, X± SD) * P< 0.05
Figure 2.
Skeletal muscle SIRT1 mRNA levels in the paretic and non-paretic legs before and after resistive training (n=10, X± SD) * P< 0.05. † P < 0.01
Relationships
Prior to training, those individuals with the highest TNF-α had the highest IL-8 levels (r=0.62, P<0.05) and CRP concentrations (r=0.91, P<0.0001). Baseline SAA was related to baseline IL-1 (r=0.72, P<0.01) and IL-6 (r=0.85, P<0.0001). sICAM-1 was related to IL-1β (r=0.56, P<0.05) and IL-6 (r=0.76, P<0.005). Likewise, sVCAM-1 correlated with IL-1β and IL-6 (r=0.78 and r=0.88, both P<0.005). Cytokines were not related to any measures of glucose metabolism. Baseline muscle area did not correlate with muscle attenuation or baseline levels of circulating inflammatory cytokines. Likewise, muscle area change did not relate to changes in attenuation or cytokines. However, prior to training, fasting glucose and insulin correlated with non-paretic intramuscular fat (r=0.70, P=0.05 and r=0.86, P<0.05, respectively). Similar associations were observed between fasting glucose and insulin and paretic intramuscular fat (r=0.76 and r=0.90, P<0.05). Fasting glucose and insulin were not related to paretic PGC-1α and SIRT-1 mRNA expression. However, non-paretic PGC-1α expression was negatively associated with glucose AUC (r=−0.88, P<0.01) but not insulin AUC.
Discussion
The present study is the first to show increases in PGC-1α mRNA expression with RT after stroke. Further, we provide initial evidence that SIRT1 mRNA expression decreases with RT with no differences in gene expression between the paretic and non-paretic skeletal muscle. We also demonstrate that circulating inflammatory markers do not change with RT in stroke.
We and others report that resistance training increases muscle strength in stroke survivors5, 19–22. Our data indicating that paretic and non-paretic muscle volume is associated with peak eccentric muscle torque4 suggests that the muscle wasting accompanying stroke has functional consequences. Although mechanisms for stroke-related muscle atrophy are unclear, muscle loss and sarcopenia, in general, are linked to several proteolytic systems, including the ubuiquitin-proteasome, lysosome-autophagy, and the TNFα/nuclear factor-kappaB systems23. TNF-α may result in muscle weakness by directly compromising contractile function of limb muscles in a murine model24. Muscle atrophy in type 2 diabetes is reportedly due to a defect in the insulin signaling pathways secondary to inflammation, including NF-kB activation and elevated TNF-α, IL-1 and Il-6 levels25. We proposed a role for inflammatory processes in stroke-induced atrophy and insulin resistance, but were unable to support this as there was lack of an association between cytokines and muscle area or muscle strength (data not shown) or with glucose metabolism. It has been reported that plasma TNF-α and CRP are negatively associated with leg and arm strength, respectively in COPD patients26. Yet, the same group of COPD patients exhibited no relationship between circulating inflammation and muscle mass26, thus similar to our current findings in a stroke population.
We did not find changes in the vascular inflammatory markers for endothelial dysfunction (sICAM-1, sVCAM-1 and CRP), which are associated with coronary heart disease and risk for stroke in large population studies1, 7. Moreover, SAA which stimulates the production of inflammatory cytokines in coronary artery endothelial cells27 did not change with RT. We have previously shown in overweight and obese postmenopausal women that aerobic exercise training combined with weight loss results in a decrease in vascular markers of inflammation28, 29. It is possible that an intervention of RT plus caloric restriction and subsequent loss of body weight may be more effective in reducing inflammation than RT alone as conducted in this study.
Our RT intervention resulted in a significant increase in PGC-1α mRNA levels in the non-paretic but not the paretic limb. These results corroborate investigations showing PGC-1α mRNA expression increases after aerobic exercise in animal models30–32 and in humans33–35. Although acute cycling exercise increased skeletal muscle PGC-1α mRNA expression in young healthy men, the effect of the acute exercise bout was attenuated after 10-days of cycling36 suggesting an adaptation to training. To our knowledge, no studies have examined the effects of resistive training on PGC-1α expression in healthy subjects. Furthermore, it remains to be determined whether PGC-1α expression in stroke skeletal muscle is modified by aerobic exercise training.
We observed a negative relationship between non-paretic PGC-1α expression and glucose area under the curve, indicating lower mitochondrial function among those with more impaired glucose metabolism. Our results confirm findings that PGC-1α levels are associated with insulin resistance as shown in subjects with type 2 diabetes and nondiabetic subjects with a family history of diabetes37. Our small sample size likely precluded observing relationships between changes in skeletal muscle PGC-1α and changes in glucose tolerance.
SIRT-1 activity increases after exercise training in rat cardiac muscle38 and electrical stimulation in rat skeletal muscle39. Very few papers have examined changes in human skeletal muscle SIRT-1 with exercise training with inconsistent results. For example, acute cycling exercise increases PGC-1α and SIRT-1 mRNA40, but another study of high intensity interval training showed a significant decrease in SIRT-1 protein levels, contrasting the increases in SIRT-1 activity41. Thus, our data indicating a decline in SIRT-1 gene expression with resistive training in stroke aligns with a prior published decrease in SIRT-1 protein levels, despite the different populations and exercise modes studied. Clearly, more research is needed if the role of SIRT-1 in exercise-induced cardiometabolic adaptation is to be fully understood in those with and without neurologic disability.
Limitations include a small sample size, absence of quantification of PGC-1α and SIRT-1 protein levels, and lack of comparison between circulating inflammatory markers and skeletal muscle inflammatory markers. It is possible that local changes in inflammation could have occurred with the training considering our previous report of increased TNF-α mRNA levels in paretic skeletal muscle compared to non-stroke controls42.
Summary
We provide preliminary evidence that resistive training in chronic stroke induces changes in the gene expression of key skeletal muscle regulators of metabolism, including an increase in PGC-1α and reduction in SIRT-1. Despite the lack of change in systemic inflammatory markers, future investigations should continue to examine the role of local inflammation and insulin resistance in chronic stroke in a larger sample size. It is also important to evaluate muscle inflammation as a potentially modifiable factor in the context of exercise training after stroke.
Acknowledgments
Our appreciation is extended to those stroke survivors who participated in this study. We are grateful to the nurses and laboratory staff in the Geriatrics Services at the Baltimore Veterans Administration (VA) Medical Center, for technical assistance.
Sources of Funding
This study was supported by funds from: VA Senior Research Career Scientist Award (ASR), NIH grants R01-AG030075, Claude D. Pepper Older Americans Independence Center (P30AG028747), the Baltimore VA Geriatric Research, Education, and Clinical Center (GRECC), and VA Rehabilitation Research & Development Maryland Exercise and Robotics Center of Excellence (B3688R; RR&D MERCE).
Footnotes
Declaration of Conflicting Interests
The Author(s) declare (s) that there is no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ridker PM. Inflammatory biomarkers, statins, and the risk of stroke: Cracking a clinical conundrum. Circulation. 2002;105:2583–2585. doi: 10.1161/01.cir.0000017822.82512.62. [DOI] [PubMed] [Google Scholar]
- 2.Michaud M, Balardy L, Moulis G, Gaudin C, Peyrot C, Vellas B, et al. Proinflammatory cytokines, aging, and age-related diseases. J Am Med Dir Assoc. 2013;14:877–882. doi: 10.1016/j.jamda.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 3.Ryan AS, Dobrovolny CL, Smith GV, Silver KH, Macko RF. Hemiparetic muscle atrophy and increased intramuscular fat in stroke patients. Arch Phys Med Rehabil. 2002;83:1703–1707. doi: 10.1053/apmr.2002.36399. [DOI] [PubMed] [Google Scholar]
- 4.Ryan AS, Buscemi A, Forrester L, Hafer-Macko CE, Ivey FM. Atrophy and intramuscular fat in specific muscles of the thigh: Associated weakness and hyperinsulinemia in stroke survivors. Neurorehabil Neural Repair. 2011;25:865–872. doi: 10.1177/1545968311408920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryan AS, Ivey FM, Prior S, Li G, Hafer-Macko C. Skeletal muscle hypertrophy and muscle myostatin reduction after resistive training in stroke survivors. Stroke. 2011;42:416–420. doi: 10.1161/STROKEAHA.110.602441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new tgf-beta superfamily member. Nature. 1997;387:83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- 7.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 8.Festa A, D’Agostino R, Jr, Tracy RP, Haffner SM. Elevated levels of acute-phase proteins and plasminogen activator inhibitor-1 predict the development of type 2 diabetes: The insulin resistance atherosclerosis study. Diabetes. 2002;51:1131–1137. doi: 10.2337/diabetes.51.4.1131. [DOI] [PubMed] [Google Scholar]
- 9.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 10.Duncan BB, Schmidt MI, Pankow JS, Ballantyne CM, Couper D, Vigo A, et al. Low-grade systemic inflammation and the development of type 2 diabetes: The atherosclerosis risk in communities study. Diabetes. 2003;52:1799–1805. doi: 10.2337/diabetes.52.7.1799. [DOI] [PubMed] [Google Scholar]
- 11.Muoio DM, Koves TR. Skeletal muscle adaptation to fatty acid depends on coordinated actions of the ppars and pgc1 alpha: Implications for metabolic disease. Appl Physiol Nutr Metab. 2007;32:874–883. doi: 10.1139/H07-083. [DOI] [PubMed] [Google Scholar]
- 12.Dos Santos JM, Moreli ML, Tewari S, Benite-Ribeiro SA. The effect of exercise on skeletal muscle glucose uptake in type 2 diabetes: An epigenetic perspective. Metabolism. 2015;64:1619–1628. doi: 10.1016/j.metabol.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 13.Ivey FM, Ryan AS. Resistive training improves insulin sensitivity after stroke. J Stroke Cerebrovasc Dis. 2014;23:225–229. doi: 10.1016/j.jstrokecerebrovasdis.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ivey FM, Ryan AS, Hafer-Macko CE, Garrity BM, Sorkin JD, Goldberg AP, et al. High prevalence of abnormal glucose metabolism and poor sensitivity of fasting plasma glucose in the chronic phase of stroke. Cerebrovasc Dis. 2006;22:368–371. doi: 10.1159/000094853. [DOI] [PubMed] [Google Scholar]
- 15.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 16.Radloff LS, Rae DS. Susceptibility and precipitating factors in depression: Sex differences and similarities. J Abnorm Psychol. 1979;88:174–181. doi: 10.1037//0021-843x.88.2.174. [DOI] [PubMed] [Google Scholar]
- 17.Macko RF, Katzel LI, Yataco A, Tretter LD, DeSouza CA, Dengel DR, et al. Low-velocity graded treadmill stress testing in hemiparetic stroke patients. Stroke. 1997;28:988–992. doi: 10.1161/01.str.28.5.988. [DOI] [PubMed] [Google Scholar]
- 18.Chomczynski P, Sacchi N. Single-step method of rna isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 19.Yang YR, Wang RY, Lin KH, Chu MY, Chan RC. Task-oriented progressive resistance strength training improves muscle strength and functional performance in individuals with stroke. Clin Rehabil. 2006;20:860–870. doi: 10.1177/0269215506070701. [DOI] [PubMed] [Google Scholar]
- 20.Kim CM, Eng JJ, MacIntyre DL, Dawson AS. Effects of isokinetic strength training on walking in persons with stroke: A double-blind controlled pilot study. J Stroke Cerebrovasc Dis. 2001;10:265–273. doi: 10.1053/jscd.2001.123775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee MJ, Kilbreath SL, Singh MF, Zeman B, Davis GM. Effect of progressive resistance training on muscle performance after chronic stroke. Med Sci Sports Exerc. 2010;42:23–34. doi: 10.1249/MSS.0b013e3181b07a31. [DOI] [PubMed] [Google Scholar]
- 22.Ouellette MM, LeBrasseur NK, Bean JF, Phillips E, Stein J, Frontera WR, et al. High-intensity resistance training improves muscle strength, self-reported function, and disability in long-term stroke survivors. Stroke. 2004;35:1404–1409. doi: 10.1161/01.STR.0000127785.73065.34. [DOI] [PubMed] [Google Scholar]
- 23.Sakuma K, Aoi W, Yamaguchi A. Current understanding of sarcopenia: Possible candidates modulating muscle mass. Pflugers Arch. 2015;467:213–229. doi: 10.1007/s00424-014-1527-x. [DOI] [PubMed] [Google Scholar]
- 24.Reid MB, Lannergren J, Westerblad H. Respiratory and limb muscle weakness induced by tumor necrosis factor-alpha: Involvement of muscle myofilaments. Am J Respir Crit Care Med. 2002;166:479–484. doi: 10.1164/rccm.2202005. [DOI] [PubMed] [Google Scholar]
- 25.Workeneh B, Bajaj M. The regulation of muscle protein turnover in diabetes. The international journal of biochemistry & cell biology. 2013;45:2239–2244. doi: 10.1016/j.biocel.2013.06.028. [DOI] [PubMed] [Google Scholar]
- 26.Ferrari R, Caram LM, Faganello MM, Sanchez FF, Tanni SE, Godoy I. Relation between systemic inflammatory markers, peripheral muscle mass, and strength in limb muscles in stable copd patients. International journal of chronic obstructive pulmonary disease. 2015;10:1553–1558. doi: 10.2147/COPD.S85954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang RZ, Lee MJ, Hu H, Pollin TI, Ryan AS, Nicklas BJ, et al. Acute-phase serum amyloid a: An inflammatory adipokine and potential link between obesity and its metabolic complications. PLoS Med. 2006;3:e287. doi: 10.1371/journal.pmed.0030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ryan ASGS, Blumenthal JB, Serra MC, Prior SJ, Goldberg AP. Aerobic exercise and weight loss reduce vascular markers of inflammation and improve insulin sensitivity in obese women. Journal of American Geriatrics Society. 2014 doi: 10.1111/jgs.12749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang RZ, Blumenthal JB, Glynn NM, Lee MJ, Goldberg AP, Gong DW, et al. Decrease of circulating saa is correlated with reduction of abdominal saa secretion during weight loss. Obesity (Silver Spring) 2014;22:1085–1090. doi: 10.1002/oby.20657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baar K, Wende AR, Jones TE, Marison M, Nolte LA, Chen M, et al. Adaptations of skeletal muscle to exercise: Rapid increase in the transcriptional coactivator pgc-1. FASEB J. 2002;16:1879–1886. doi: 10.1096/fj.02-0367com. [DOI] [PubMed] [Google Scholar]
- 31.Akimoto T, Pohnert SC, Li P, Zhang M, Gumbs C, Rosenberg PB, et al. Exercise stimulates pgc-1alpha transcription in skeletal muscle through activation of the p38 mapk pathway. J Biol Chem. 2005;280:19587–19593. doi: 10.1074/jbc.M408862200. [DOI] [PubMed] [Google Scholar]
- 32.Goto M, Terada S, Kato M, Katoh M, Yokozeki T, Tabata I, et al. Cdna cloning and mrna analysis of pgc-1 in epitrochlearis muscle in swimming-exercised rats. Biochem Biophys Res Commun. 2000;274:350–354. doi: 10.1006/bbrc.2000.3134. [DOI] [PubMed] [Google Scholar]
- 33.Norrbom J, Sundberg CJ, Ameln H, Kraus WE, Jansson E, Gustafsson T. Pgc-1alpha mrna expression is influenced by metabolic perturbation in exercising human skeletal muscle. J Appl Physiol (1985) 2004;96:189–194. doi: 10.1152/japplphysiol.00765.2003. [DOI] [PubMed] [Google Scholar]
- 34.Pilegaard H, Saltin B, Neufer PD. Exercise induces transient transcriptional activation of the pgc-1alpha gene in human skeletal muscle. J Physiol. 2003;546:851–858. doi: 10.1113/jphysiol.2002.034850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Russell AP, Feilchenfeldt J, Schreiber S, Praz M, Crettenand A, Gobelet C, et al. Endurance training in humans leads to fiber type-specific increases in levels of peroxisome proliferator-activated receptor-gamma coactivator-1 and peroxisome proliferator-activated receptor-alpha in skeletal muscle. Diabetes. 2003;52:2874–2881. doi: 10.2337/diabetes.52.12.2874. [DOI] [PubMed] [Google Scholar]
- 36.Stepto NK, Benziane B, Wadley GD, Chibalin AV, Canny BJ, Eynon N, et al. Short-term intensified cycle training alters acute and chronic responses of pgc1alpha and cytochrome c oxidase iv to exercise in human skeletal muscle. PLoS One. 2012;7:e53080. doi: 10.1371/journal.pone.0053080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of pgc1 and nrf1. Proc Natl Acad Sci U S A. 2003;100:8466–8471. doi: 10.1073/pnas.1032913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferrara N, Rinaldi B, Corbi G, Conti V, Stiuso P, Boccuti S, et al. Exercise training promotes sirt1 activity in aged rats. Rejuvenation research. 2008;11:139–150. doi: 10.1089/rej.2007.0576. [DOI] [PubMed] [Google Scholar]
- 39.Gurd BJ, Yoshida Y, Lally J, Holloway GP, Bonen A. The deacetylase enzyme sirt1 is not associated with oxidative capacity in rat heart and skeletal muscle and its overexpression reduces mitochondrial biogenesis. J Physiol. 2009;587:1817–1828. doi: 10.1113/jphysiol.2008.168096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dumke CL, Mark Davis J, Angela Murphy E, Nieman DC, Carmichael MD, Quindry JC, et al. Successive bouts of cycling stimulates genes associated with mitochondrial biogenesis. Eur J Appl Physiol. 2009;107:419–427. doi: 10.1007/s00421-009-1143-1. [DOI] [PubMed] [Google Scholar]
- 41.Gurd BJ, Perry CG, Heigenhauser GJ, Spriet LL, Bonen A. High-intensity interval training increases sirt1 activity in human skeletal muscle. Appl Physiol Nutr Metab. 2010;35:350–357. doi: 10.1139/H10-030. [DOI] [PubMed] [Google Scholar]
- 42.Hafer-Macko CE, Yu S, Ryan AS, Ivey FM, Macko RF. Elevated tumor necrosis factor-alpha in skeletal muscle after stroke. Stroke. 2005;36:2021–2023. doi: 10.1161/01.STR.0000177878.33559.fe. [DOI] [PubMed] [Google Scholar]