Abstract
Background
Standardized dental diagnostic terminologies (SDDxTs) were introduced decades ago. Their use has been on the rise, accompanying the adoption of electronic health records (EHRs). One of the most broadly used terminologies is the Dental Diagnostic System (DDS). Our aim was to assess the adoption of SDDxTs by US dental schools by using the Rogers diffusion of innovations framework, focusing on the DDS.
Methods
The authors electronically surveyed clinic deans in all US dental schools (n = 61) to determine use of an EHR and SDDxT, perceived barriers to adoption of an SDDxT, and the effect of implementing an SDDxT on clinical productivity.
Results
The response rate was 57%. Of the 35 responses, 91% reported using an EHR to document patient care, with 84% using axiUm, and 69% reported using an SDDxT to document patient diagnoses; 41% used the DDS. Fifty-four percent of those who did not use an SDDxT had considered adopting the DDS, but 39% had not, citing barriers such as complexity and compatibility.
Conclusions
Adoption of an SDDxT, particularly the DDS, is on the rise. Nevertheless, a large number of institutions are in the Rogers late majority and laggards categories with respect to adoption. Several factors may discourage adoption, including the inability to try out the terminology on a small scale, poor usability within the EHR, the fact that it would be a cultural shift in practice, and a perception of unclear benefits. However, the consolidation of the DDS and American Dental Association terminology efforts stands to encourage adoption.
Keywords: Diffusion of innovation, terminology, dental schools, electronic health records
Some new things catch on, and others do not. As Atul Gawande1 pointed out, anesthesia and antisepsis, 2 bedrock elements of the practice of dentistry, had different diffusion trajectories. Once it was demonstrated publicly, ether anesthesia spread across the world in a matter of months, whereas antisepsis took a generation to become common practice. Here, we are going to consider the adoption of a standardized dental diagnostic terminology (SDDxT) through the lens of innovation diffusion.
Without innovation, there would be no progress, and dentistry certainly has enjoyed a lot of progress over the millennia.2 We are grateful for that: If not, modern-day dentists might be using the bow drill tipped with a flint head used in the Neolithic age instead of the high-speed handpiece.3 In 1957, the Borden Airotor, a high-speed air turbine contra-angle handpiece, was introduced.4 By 1962, more than 90% of dentists were using a turbine contra-angle handpiece.5-7 Like Gawande's anesthesia example, this innovation was greeted with rapid, full adoption.
The implant is another innovation in dentistry that has been adopted widely, though its trajectory was more like that of antisepsis.1 The first titanium dental implant was placed in a person in 1965, at Brånemark's clinic in Gothenburg, Sweden. Four years later, the American Dental Association (ADA) position was that these devices were highly experimental.8 The turning point came in 1982 at the Toronto Conference on Osseointegration in Clinical Dentistry,9 where Brånemark's team presented the scientific evidence and clinical success of osseointegration to educational leaders in the fields of oral surgery and prosthodontics from North American schools. That same year, the US Food and Drug Administration approved the use of titanium dental implants. By 2006, US dentists had placed 5,505,720 implants.10
Not all innovations ultimately are embraced. Consider the rubber dam: Centuries after its invention, it is not broadly used.5 In their studies, the Dental Practice-Based Research Network practitioners found that only 44% always used a rubber dam when performing endodontic therapy and 63% never used a rubber dam for restorative treatment.11 This partial adoption is vexing because patient harm can be prevented through the use of the rubber dam, by decreasing the chance of aspiration and contamination. In addition, there is some evidence that rubber dams may reduce restoration failure rates.12
How can we describe adoption of an innovation? We have covered examples of full adoption, late full adoption, and partial adoption. In addition to these, there is rejection. Together, as Figure 1 shows, these are the 4 archetypal diffusion trajectories.
Figure 1.
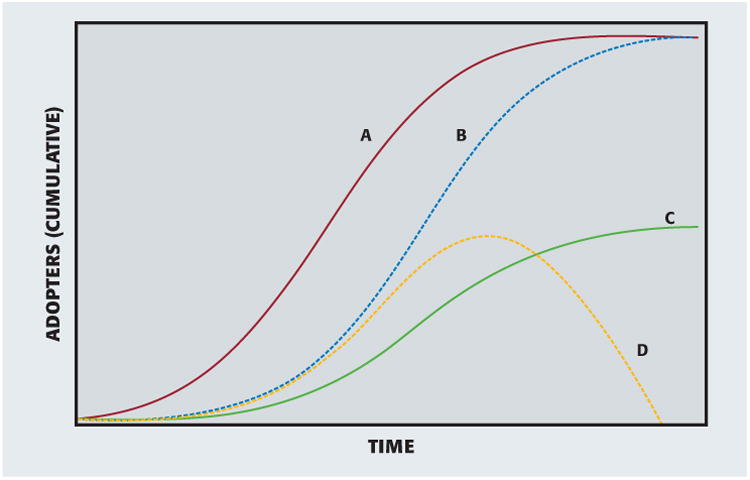
Adoption outcomes. A: Full adoption. B: Late full adoption. C: Partial adoption. D: Rejection.
Why do some things catch on quickly, but others do not? To be sure, the diffusion of an innovation depends on its characteristics. Rogers identified 5 attributes that can affect diffusion: relative advantage, compatibility, complexity, trialability, and observability (Table 1).5 In brief, an innovation that is perceived to have an advantage over the current technology or idea, is easy to use, is consistent with existing values and experiences, can be experimented with on a small scale, and has easily observed results will be more likely to be adopted.6
Table 1. Five attributes that can affect diffusion*.
| Attribute | Description |
|---|---|
| Relative Advantage | The degree to which an innovation is perceived as being better than the idea it supersedes. |
| Compatibility | The degree to which an innovation is perceived as consistent with the existing values, past experiences, and needs of the potential adopter. |
| Complexity | The degree to which an innovation is perceived as relatively difficult to understand and use. |
| Trialability | The ability for the innovation to be tried or used in a test mode on a small scale. |
| Observability | The ability for the outcome of an innovation to be observed and measured easily by others. |
Source: Rogers.5
With that said, the so-called diffusion of innovations does not depend only on the innovation itself but also on its communication channels, time, and the people who make up the system interacting with the innovation. In the case of antisepsis, there were prominent people who simply could not believe that a physician's hand could transmit disease. In the words of Dr. Charles Meigs, a leading obstetrician in the eighteenth century, “Doctors are gentlemen, and a gentleman's hands are always clean.”13 Members of a social system tend to adopt an innovation at different rates, which is correlated to their attitudes and personality traits. Although some are open to change and may even be risk takers, others will need a little more persuasion from peers and colleagues, and some others will not adopt until they directly observe advantages or superiority of the innovation.
There are other characteristics that correspond to adoption rates: Study results have shown differences in the rate of adoption of dental technologies between specialists and nonspecialists and between dentists in larger and smaller practices.9 Rogers5 classified adoptersinto 5 categories, ranging from innovators to laggards (Table 2). Beyond the characteristics of people, how the people are connected into a social network also affects the diffusion of an innovation.14 Coleman and colleagues15 conducted the classic study of this phenomenon in 1966: They showed that physicians' willingness to prescribe the then-new antibiotic tetracycline diffused through professional contacts. Receiving a seal of approval from a professional organization also can influence the adoption of innovations. Examples of the value of such endorsements are evident in the increased rate of adoption of the dental operating microscope after recommendations from the American Association of Endodontists to the Commission on Dental Accreditation of the ADA16 and in the use of sealants in the early1980s after recommendations of sealant use by the ADA and the National Institutes of Health. Surveyed dentists in the state of Washington reported an increase in sealant use by 67% after they had read the recommendations.17,18
Table 2. Title*.
| Adopter CATEGORY | Characteristics |
|---|---|
| Innovators (2.5%) | Crave change and innovation Seen as slightly radical Cope well with uncertainty |
| Early Adopters (13.5%) | Gatekeepers of new ideas into a system, the opinion leaders |
| Early Majority (34%) | Will adopt new ideas after deliberation |
| Late Majority (34%) | Might adopt as a result of increased pressure from peers Skeptical of change |
| Laggards (16%) | Isolated from social network Will be the last to change Suspicious of change agents |
Source: Rogers.5
Terminologies and Dentistry
Standardized diagnostic terminologies are integral to medical care and catalyze research. For example, health services researchers use the terms to look for variations in access, quality, cost, and effectiveness of care, including the tracking of disparities.19,20 Without these standards, it would be difficult to compare outcomes of different treatments for the same diagnosis, to articulate the prognosis of a diagnosis, and to assess the appropriateness of a treatment for a diagnosis. Being consistent about what we call things also helps us to communicate more clearly with patients and colleagues.
Standardized medical diagnostic terms have deep roots, extending back to seventeenth century efforts to classify the causes of death, which culminated in the creation of the first version of the International Classification of Diseases (ICD) in the late nineteenth and early twentieth centuries. In addition to the ICD, the Systematized Nomenclature of Medicine Clinical Terms (SNOMED CT) is another, much larger, terminology in use in health care. To put this in perspective, the ICD, Tenth Revision, Clinical Modification22 includes 71,924 procedure codes and 69,823 diagnosis codes, but SNOMED CT already included 255,538 concepts in the January 2008 release but more importantly 418,320 hierarchies (that is, abscess, tumor, cyst) based on defining relationships between concepts.23 SNOMED CT spans not only procedures and diagnoses but also body structures, devices, pharmaceuticals, events, specimens, and so on.24 The International Health Terminology Standards Development Organization owns SNOMED CT.25
Dentistry has lagged behind medicine in developing and adopting an SDDxT. Although a handful of isolated dental organizations developed dental diagnostic terms to meet their local needs, most dental diagnoses have been indicated through charting of carious lesions or a free-text description rather than with standardized diagnostic terms.
In 2010, with support from a grant (1R01DE021051) funded by the National Institutes of Health, a group of dental faculty members, including some of us (R.B.R., J.M.W., M.F.W., E.K.), created a work group and developed an SDDxT—the Dental Diagnostic System (DDS).26-28 We designed DDS as an interface terminology. This means that it was meant to be sufficiently concise to be navigable within the electronic health record (EHR) while still being rich enough to capture diagnoses seen in the dental clinic. It interfaces to more extensive reference terminologies, such as SNOMED CT. We designed the DDS to adhere to guiding principles of terminology development—for example, having terms arranged in a hierarchical order; structuring concepts into categories and subcategories; evaluating and refining the terminology on a regular basis; and, importantly, linking diagnoses to procedure codes.29,30
Since its development in 2009, the DDS terminology has undergone 6 major revisions—the addition of new major headings (such as orthodontics) and subheadings (such as the International Caries Detection and Assessment System, headache, healthy); modification of diagnostic categories on the basis of the current thinking in the specialty (for example, periodontics and temporomandibular joint diseases); and the removal of outmoded diagnoses such as microcavitation, pre-eruptive caries, and so on. We also substantially enhanced the terminology by developing assertional knowledge (definitions, rich synonymy, differential diagnoses, prevalence information, colloquial designations, and treatment suggestions based on best practices). Beyond these, the structure has been built out to accommodate all types of users—those who prefer highly granular terms, and those who prefer less specific diagnoses. In 2015, it was adopted as a standard (norm) in the Netherlands.31
The ADA had been working on a complementary set of standardized dental diagnostic terms, the Systematized Nomenclature of Dentistry (SNODENT), since the early 1990s. Until 2012, there was no implementation of SNODENT, perhaps because it was available only by paid license. In 2012, SNODENT Version II was incorporated into SNOMED CT. Two of us (J.M.W. and E.K.) and key ADA staff members are members of the dental special interest group responsible for the further refinement of dental terms in SNOMED CT. Through this collaboration, we have been able to integrate the DDS terms into SNOMED CT, thereby harmonizing our efforts with those of the ADA. All DDS terms are now available in SNODENT. To mark this step, in 2016, SNODENT and DDS were renamed as SNODENT, a dental diagnostic terminology and SNO-DDS, respectively. This announcement was met with great enthusiasm at our March 2016 dental diagnostic terminology conference in Los Angeles. In addition, we have developed SNO-DDS General Dentistry, a reference set (smaller subsets of terms out of a larger terminology) to SNO-DDS, for the smaller general dental office and are developing additional reference sets for the specialties (for example, oral surgery, periodontics).
The ADA has submitted SNODENT and SNO-DDS to become an American National Standards Institute standard, and they have been accepted; the SNO-DDS will function as SNODENT's reference set by the end of 2016. This move is important because dental organizations have been waiting for this seal of approval before being comfortable adopting a dental diagnostic terminology. A reference set is a subset of the reference terminology (here, SNODENT) that contains concepts that are relevant to a specific domain and that may have alternative navigation hierarchies. These reference sets can serve as interface terminologies, which we described earlier. Figure 2 shows the relationships among the various terminologies ().
Figure 2.
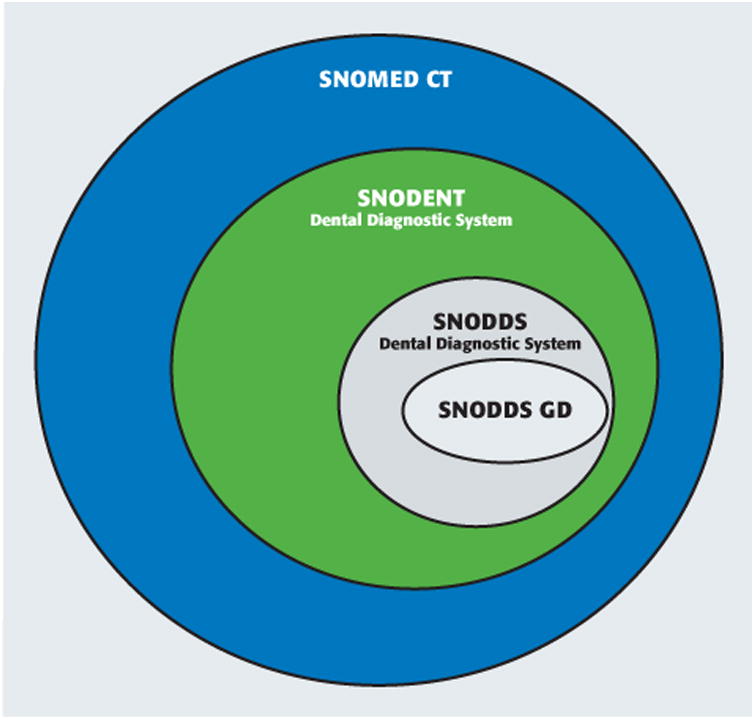
Relationships among the Systematized Nomenclature of Medicine Clinical Terms (SNOMED CT), Systematized Nomenclature of Dentistry (SNODENT) (comprehensive clinical terminology for dentistry that includes diagnoses, findings, and anatomy), and SNO-DDS (comprehensive dental diagnostic terminology for use in electronic health record user inferfaces) terminologies, including a smaller SNODDS General Dentistry (GD), which is a subset (reference set) that includes only terms related to general dentistry.
The use of a standardized diagnostic terminology is a substantial change for the dental profession. Four years after the development of the DDS, we sought to understand its diffusion, implementation, and adoption among US dental academic clinics.
Methods
Instrument development
We developed a dental school survey to assess the diffusion, adoption, and perception of implementation of the DDS terminology (Appendix, available online at the end of this article). There were 21 questions that covered the size of the doctor of dental surgery and doctor of dental medicine classes and type of dental clinics, use of an EHR and EHR type, diagnostic terminology used, familiarity with the DDS terminology, channel through which the school became aware of the DDS terminology, implementation status of the DDS terminology in the school's EHR (and if the school regularly upgraded to newer versions of the terminology), ease in gaining approval to implement the DDS terminology, and effect of DDS terminology implementation on clinical productivity.
The research team members developed the pilot survey instrument and tested and modified it iteratively to ensure implementation fidelity (for example, correct skip logic). The full-length survey took 10 minutes, on average, to complete. The research team sent out the final survey for reliability testing. We selected 6 faculty members from 3 academic dental institutions that already had implemented the DDS to ensure that they would be eligible to respond to all questions. To determine intrarater reliability and consistency, we asked each respondent to complete the survey on 2 separate occasions with an interval of 2 weeks. On the initial dissemination, the 6 testers responded to the survey, but only 3 testers (50%) completed the survey at retest. We assumed the dropouts to be noninformative missing data.32,33 The intrarater reliabilities for each of these testers were 0.90, 0.78, and 0.89, respectively (κ of 0.81-1 represents almost perfect agreement).34 No changes to the survey were required as a result of testing.
Participant recruitment and consent
The Harvard Longwood Medical Area Office of Human Research Administration Institutional Review Board granted approval for this study. We identified the names and e-mail addresses of deans of clinics (or deans performing analogous roles) from the 61 dental schools listed in the American Dental Education Association's 2014-15 Directory of Institutional Members and Association Officers. We sent an invitational e-mail to the prospective respondents in which we explained the aims of the study and included a link to a secure online survey hosted by Qualtrics. By clicking on the link, participants consented to participate in the survey. We sent out the survey via e-mail, and we sent nonrespondents reminders once a week for 5 consecutive weeks at which time we achieved a satisfactory response proportion (>50%).35
Data cleaning and analyses
We carefully reviewed survey responses to ensure exclusion of duplicate responses. We summarized quantitative data responses as frequencies (and percentages) and as means; we coded qualitative data in themes and reported them as such.
Results
We received 35 responses from the 61 deans who received the survey for a response rate of 57%, which is considerably above the average response proportion (34%) usually reported for Web-based surveys.35 All schools reported having a predoctoral clinic for their doctor of dental surgery and doctor of dental medicine classes. Class size ranged from 23 to 194 students, with a median of 88 and mean of 89. Twenty-nine schools (85%) reported having postdoctoral, resident, and specialty clinics for students, and 29 schools (85%) reported having a faculty group or private practice. One school reported having a dental hygiene class, and another reported being associated with a federally qualified health center.
EHR use
Since the 2009 Health Information Technology for Economic and Clinical Health Act,36 numerous eligible health organizations began aligning their organizations with the meaningful use criteria necessary to receive incentives for implementing certified EHRs. Dental schools are no exception. Of the 35 schools, 32 reported using an EHR to document patient care, as well as for administrative purposes, such as billing (Table 3). The remainder indicated that they did not use an EHR for the clinical documentation of patient care. Of these, 84% used axiUm (Exan). Table 3 documents the distribution of types of EHRs used.
Table 3. EHR* use and type in US dental academic institutions.
| EHR Use | No. of respondents | Response | No. (%)† |
|---|---|---|---|
| Are you using an EHR at your school for documenting patient care (not just for billing and administrative functions)? | 35 | Yes | 32 (91) |
| No | 3 (9) | ||
| Which EHR system are you using at your school for documenting patient care? | 32 | axiUm (Exan) | 27 (84) |
| GSD (General Systems Design) | 2 (6) | ||
| Dentrix (Henry Schein) | 1 (3) | ||
| NextGen (QSIDental) | 1 (3) | ||
| Eaglesoft (Patterson Dental) | 1 (3) |
EHR: Electronic health record.
Percentages do not total 100 because of rounding.
Twenty-nine of the 35 respondents (83%) were familiar with the DDS. The survey asked the schools using an EHR which terminology they used when documenting diagnoses in the EHR. The plurality (41%) reported that their institution used the DDS: Table 3 shows the breakdown of responses.
For the purpose of this article, we considered adoption of the DDS as the decision to acquire the DDS and load it in the EHR. This means that the DDS could be in use either in the development (testing) mode or in the production (live) mode in the EHR. Of the 32 EHR users, 16 (50%) had the DDS loaded in their EHR, all of whom were axiUm users. Among these 16 EHR users, 10 reported that the DDS terminology was in clinical use (that is, in production mode); 5 respondents had the DDS in development (testing) mode. One institution did not respond to this item. In the survey, we further queried the institutions identified as clinical users about the ease of implementation of the DDS through questions that assessed the following: the level of difficulty obtaining approval for implementing the DDS, the level of difficulty obtaining the DDS from the vendor or developer, and level of disruption implementing the DDS caused in the daily workflow and production of the users. Most reported that it was not difficult to obtain approval to implement the DDS or to obtain the DDS. Most also did not feel that there was a loss of clinical productivity during the implementation and associated training. Table 4 reports the results.
Table 4. Ease of implementation of the Dental Diagnostic System.
| Ease of implementation | No. of responses (N = 9) |
|---|---|
| Was it difficult obtaining approval from the institution? | |
| No | 7 |
| Yes | 1 |
| Do not know | 1 |
| Was it a challenge to prevent loss of clinical productivity during training and implementation? | |
| Strongly disagree | 1 |
| Disagree | 4 |
| Neither agree nor disagree | 3 |
| Agree | 0 |
| Strongly agree | 1 |
Among the 13 respondents who were aware of the DDS but had not adopted it, 7 (54%) reported that they had not yet implemented DDS terminology but were considering doing so. Five (38%) of the remaining respondents cited complexity, EHR incompatibility, and lack of understanding of the ADA's position as reasons why they were not considering implementing the DDS.
In the survey, we sought to identify the channels of communication through which knowledge of the DDS had been diffused. For respondents who indicated familiarity with the DDS, the survey asked, “How did you hear about the Dental Diagnostic System (DDS) (formerly known as EZCodes)?” The survey allowed respondents to select more than 1 of the following communication channels: conference, publication, nonconference presentation, software vendor, and other. Conference presentation was the diffusion channel most commonly reported (65%), and the least common was via publication or nonconference presentation (7% each) (Table 5).
Table 5. Communication channels (diffusion) of the Dental Diagnostic System.
| Communication Channel | No. of respondents | Total (%) |
|---|---|---|
| Conference | 19 (65) | |
| Exan Summit | 11 | |
| Consortium for Oral Health Research and Informatics | 3 | |
| American Dental Education Association | 1 | |
| Unknown | 4 | |
| Publication | 2 | 2 (7) |
| Nonconference Presentation | 2 | 2 (7) |
| Software Vendor | 3 | 3 (10) |
| Other | 4 (14) | |
| Discussions with colleagues | 3 | |
| Discussions with Dental Diagnostic System development team | 1 |
Discussion
Dental care team members spend a good part of their clinical time entering data into their patients' EHRs: They update medical histories, make note of treatments, and document bleeding on probing. What most do not enter are standardized diagnostic terms. As a case in point, dental schools have been in the vanguard of SDDxT adoption, yet only 10 of the 35 schools surveyed had the DDS loaded into their EHR for clinical use. To our knowledge, only 1 large private group practice, Willamette Dental Group (https://www.willamettedental.com/), has implemented the DDS. In addition, 2 schools were using a self-selected subset terms out of the SNODENT terminology, and 5 schools were using the dental terms in the ICD, Ninth Revision. This lack of and variable adoption is a frustrating truth given the multitude of benefits that would follow from the broad use of a standardized terminology.37-41
One of the early wins among the dental diagnostic term innovators has been the acceleration of discovery and quality improvement activities. Consider that in 2012 members of this research team (R.B.R., J.M.W., E.K.) were successful in creating BigMouth Dental Data Repository, a centralized data repository that contains dental EHR data from 4 dental schools, which established the largest scale multi-institutional dental clinical data repository. The pooled data showed that patients had a high prevalence of caries and periodontal disease on the basis of diagnostic information, not treatment data, an important distinction because treatment data do not reflect the subset with untreated disease.42 Through BigMouth and the use of the DDS terminology, we also have been able to quantify adherence to practice guidelines for managing chronic moderate periodontitis.41 We readily were able to determine that a full 15% of patients with a diagnosis of chronic moderate periodontitis received only prophylaxis, which is below the standard of care set by the American Academy of Periodontology. This is just the tip of the iceberg: Vast knowledge can be extracted efficiently from clinical data if the health care system is instrumented properly.43
These accomplishments stand in contrast to the status quo research enterprise, which generates bespoke data for each project. For instance, to assess adherence to treatment-generalized moderate periodontitis, researchers go through records by hand to assign a diagnosis. This method is highly resource intensive and creates a needless barrier to discovery. Making matters worse, these costly data sets most often are used for only a single study, which must be recreated Sisyphus like for the next study.44
Why do we do this to ourselves, to our profession? Five of us (R.B.R., O.T., J.M.W., M.F.W., E.K.) sought to shed some light on this question by bringing together stakeholders at 2 conferences, 1 at 3 years after the introduction of the DDS (November 2012) and another in March 2016. The objective of both conferences was to identify barriers and discuss progress made in breaking down the obstacles standing in the way of the broad adoption of standardized dental diagnostic terms. Participants included a wide range of stakeholders, including representatives from EHR vendors, insurance companies, government, dental professional organizations, dental academic centers, and large dental group practices. Returning to the Rogers5 framework for the diffusion of innovation indicates that common concerns about implementing standardized diagnostic terms center on compatibility, complexity, relative advantage, and trial-ability, in particular:
Compatibility
Cultural inertia
Documenting diagnoses in a standardized way would be a substantial change in a profession that tends to focus on treatment and procedures rather than diagnoses.
Complexity
Poor EHR user interface
The computer interface for entering a diagnosis in the EHR may not be user friendly. Vendors do not seem eager to invest in technology to develop adequate interfaces.
Relative advantage
Unclear usefulness
From the individual practitioner's perspective, it may not be clear how using standardized diagnostic terms will improve his or her practice, productivity, or income. Unlike in medicine, where a diagnosis is required to receive a payment for a procedure, the lack of financial incentives clearly plays a role in the slow adoption of diagnostic terms in dentistry, which stands in contrast to the positive effect the meaningful use incentives have had on the implementation of EHRs in dental schools.45
Not required for insurance reimbursement
Because standardized diagnostic terms are not required for billing purposes, dentists do not see the need to adopt them. Insurance companies remain hesitant to require standardized diagnostic terms as long as there is no governmental mandate from Medicaid or the Children's Health Insurance Program.
Fear of a loss of autonomy
Some dentists are concerned that the implementation of dental diagnostic terms will lead to more rigorous and inflexible oversight of the appropriateness of care, which may lead to the denial of insurance claims.
Fear of adopting a terminology that would become outmoded
At the time of our meetings, dentistry had not agreed widely on any of the SDDxTs; there was a hesitance to adopt a terminology that may not be adopted broadly in the long run.
Trialability
EHR-related barriers to adoption
EHR systems do not come preloaded with standardized diagnostic terms or well-designed interfaces.
Because of the large benefits of dental diagnostic terms to patients, research, and the profession, we hope that they ultimately will achieve late full adoption rather than level off at partial adoption.
Because of what we learned from the first meeting, we redoubled our efforts to collaborate with the ADA, which resulted in the SNO-DDS reference set, described previously. We also worked with axiUm to improve the usability of the interface.46 As a result of our modifications, the entire treatment planning module was revamped from an interface that required navigating multiple tabs to a user-friendly, 1-screen module that ties problems, diagnoses, and treatments together. In addition to our laboratory-based findings, the positive effect of our changes has been corroborated by the fact that most of the schools we surveyed reported that they did not experience a loss in clinical productivity during training for implementation of the DDS. Although these are likely to encourage adoption, from a systems perspective, there is more to be done: Schools can emphasize the primacy of diagnosis to proper clinical care,40 to payers, and to group practices, and professional bodies can encourage the documentation of diagnostic terms. EHR vendors can ensure that these terms are available via a usable interface.
Adoption of diagnostic terms will be an important step toward creating a dental learning health system. The goal of any Learning Health System is to
… improve the health of individuals and populations. The LHS [Learning Health System] will accomplish this by generating information and knowledge from data captured and updated over time—as an ongoing and natural by-product of contributions by individuals, care delivery systems, public health programs, and clinical research—and sharing and disseminating what is learned in timely and actionable forms that directly enable individuals, clinicians, and public health entities to separately and collaboratively make informed health decisions.47
Conclusions
At long last, standardized dental diagnostic terms are beginning to be adopted. Will they achieve late full adoption or stall at partial adoption? The answer is in our hands as individual practitioners and as a profession. Each of us can advocate within our social networks, to our institutions, and to our EHR vendors for the implementation and use of these terms so that we—and our patients—can benefit from this most fundamental innovation.
Supplementary Material
Acknowledgments
This research was supported in part by grant 1R01DE023061 from the National Institute of Dental and Craniofacial Research.
Abbreviation Key
- ADA
American Dental Association
- DDS
Dental Diagnostic System
- EHR
Electronic health record
- GD
General dentistry
- ICD
International Classification of Diseases
- SDDxT
Standardized dental diagnostic terminology
- SNODENT
Systematized Nomenclature of Dentistry
- SNOMED CT
Systematized Nomenclature of Medicine Clinical Terms
Footnotes
Supplemental Data: Supplemental data related to this article can be found at http://dx.doi.org/10.1016/j.adaj.2017.01.024.
Disclosure. None of the authors reported any disclosure.
Contributor Information
Dr. Rachel B. Ramoni, Department of Epidemiology & Health Promotion, College of Dentistry, New York University, New York, NY.
Dr. Jini Etolue, Department of Oral Health Policy and Epidemiology, Harvard School of Dental Medicine, Boston, MA.
Dr. Oluwabunmi Tokede, Department of Oral Health Policy and Epidemiology, Harvard School of Dental Medicine, Boston, MA.
Dr. Lyle McClellan, Willamette Dental Group, Skourtes Institute, Hillsboro, OR.
Kristen Simmons, Willamette Dental Group, Skourtes Institute, Hillsboro, OR.
Dr. Alfa Yansane, Department of Preventive and Restorative Dental Sciences, School of Dentistry, University of California, San Francisco, CA.
Dr. Joel M. White, Department of Preventive and Restorative Dental Sciences, School of Dentistry, University of California, San Francisco, CA.
Dr. Muhammad F. Walji, Department of Diagnostic and Biomedical Sciences, School of Dentistry, The University of Texas Health Science Center at Houston, Houston, TX.
Dr. Elsbeth Kalenderian, Department of Preventive and Restorative Dental Sciences, School of Dentistry, University of California, 707 Parnassus Ave., Box 0758, Room D3238, San Francisco, CA 94143.
References
- 1.Gawande A. Slow ideas. The New Yorker. 2013 Jul 29; [Google Scholar]
- 2.Wynbrandt J. The Excruciating History of Dentistry: Toothsome Tales & Oral Oddities From Babylon to Braces. New York, NY: St. Martin's Press; 1998. [Google Scholar]
- 3.Coppa A, Bondioli L, Cucina A, et al. Palaeontology: early Neolithic tradition of dentistry. Nature. 2006;440(7085):755–756. doi: 10.1038/440755a. [DOI] [PubMed] [Google Scholar]
- 4.Stephens RR. The dental handpiece: a history of its development. Aust Dent J. 1986;31(3):165–180. doi: 10.1111/j.1834-7819.1986.tb01196.x. [DOI] [PubMed] [Google Scholar]
- 5.Rogers EM. Diffusion of Innovations. 4th. New York, NY: Simon & Schuster; 2010. [Google Scholar]
- 6.Patel RN, Antonarakis GS. Factors influencing the adoption and implementation of teledentistry in the UK, with a focus on orthodontics. Community Dent Oral Epidemiol. 2013;41(5):424–431. doi: 10.1111/cdoe.12029. [DOI] [PubMed] [Google Scholar]
- 7.Herschfeld JJ, Robert J. Nelsen and the development of the high speed handpiece. Bull Hist Dent. 1987;35(1):37–42. [PubMed] [Google Scholar]
- 8.Dental Economics. Dental Economics Division of Petroleum Publishing; 1998. [Google Scholar]
- 9.van der Zande MM, Gorter RC, Aartman IHA, Wismeijer D. Adoption and use of digital technologies among general dental practitioners in the Netherlands. PLoS One. 2015;10(3):e0120725. doi: 10.1371/journal.pone.0120725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Academy of Implant Dentistry. Dental implants facts and figures. [Accessed June 3, 2016]; Available at: http://www.aaid.com/about/press_room/dental_implants_faq.html.
- 11.Anabtawi MF, Gilbert GH, Bauer MR, et al. Rubber dam use during root canal treatment: findings from the Dental Practice-Based Research Network. JADA. 2013;144(2):179–186. doi: 10.14219/jada.archive.2013.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Li C, Yuan H, et al. Rubber dam isolation for restorative treatment in dental patients. Cochrane Database Syst Rev. 2016;9:CD009858. doi: 10.1002/14651858.CD009858.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spiegelman P, Berrett B. Patients Come Second: Leading Change by Changing the Way You Lead. New York, NY: Greenleaf Book Group; 2013. [Google Scholar]
- 14.Valente TW, Palinkas LA, Czaja S, Chu KH, Brown CH. Social network analysis for program implementation. PLoS One. 2015;10(6):e0131712. doi: 10.1371/journal.pone.0131712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coleman JS, Katz E, Menzel H. Medical Innovation: A Diffusion Study. Indianapolis, IN: Bobbs-Merrill; 1966. [Google Scholar]
- 16.Selden HS. The dental-operating microscope and its slow acceptance. J Endod. 2002;28(3):206–207. doi: 10.1097/00004770-200203000-00015. [DOI] [PubMed] [Google Scholar]
- 17.Chapko MK. Diffusion of knowledge in dentistry. Int J Technol Assess Health Care. 2009;4(2):309–318. doi: 10.1017/s0266462300004116. [DOI] [PubMed] [Google Scholar]
- 18.Chapko MK. Time to adoption of an innovation by dentists in private practice: sealant utilization. J Public Health Dent. 1991;51(3):144–151. doi: 10.1111/j.1752-7325.1991.tb02205.x. [DOI] [PubMed] [Google Scholar]
- 19.O'Malley KJ, Cook KF, Price MD, et al. Measuring diagnoses: ICD code accuracy. Health Serv Res. 2005;40(5 pt 2):1620–1639. doi: 10.1111/j.1475-6773.2005.00444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Hadlaq SM, Almadi KH, Alaqla AT, Al-Maflehi NS, Albaker AMA. Adoption of new endodontic technology by dental practitioners in Saudi Arabia. King Saud Univ J Dent Sci. 2011;2(1-2):7–11. [Google Scholar]
- 21.World Health Organization. History of the development of the ICD. [Accessed July 1, 2015]; Available at: http://www.who.int/classifications/icd/en/HistoryOfICD.pdf.
- 22.TechTarget. ICD-10-CM (International Classification of Diseases, Tenth Revision, Clinical Modification) [Accessed November 27, 2016]; Available at: http://searchhealthit.techtarget.com/definition/ICD--10-CM.
- 23.National E-Health Transition Authority. e-Clinical Terminology. SNOMED CT Basics. 2008 [Google Scholar]
- 24.International Health Terminology Standards Development Organisation. SNOMED CT Starter Guide. Copenhagen, Denmark: International Health Terminology Standards Development Organisation; 2014. [Google Scholar]
- 25.International Health Terminology Standards Development Organization. Release of SNOMED CT. [Accessed May 4, 2013]; Available at: http://www.ihtsdo.org/snomed-ct/release-of-snomed-ct/
- 26.Taylor TD, Agar JR. Twenty years of progress in implant prosthodontics. J Prosthet Dent. 2002;88(1):89–95. [PubMed] [Google Scholar]
- 27.Parashos P, Messer HH. The diffusion of innovation in dentistry: a review using rotary nickel-titanium technology as an example. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):395–401. doi: 10.1016/j.tripleo.2005.02.064. [DOI] [PubMed] [Google Scholar]
- 28.Eshleman JR, Sarrett DC. How the development of the high-speed turbine handpiece changed the practice of dentistry. JADA. 2013;144(5):474–477. doi: 10.14219/jada.archive.2013.0148. [DOI] [PubMed] [Google Scholar]
- 29.Cimino JJ. Desiderata for controlled medical vocabularies in the twenty-first century. Methods Inf Med. 1998;37(4-5):394–403. [PMC free article] [PubMed] [Google Scholar]
- 30.Cimino JJ. In defense of the Desiderata. J Biomed Inform. 2006;39(3):299–306. doi: 10.1016/j.jbi.2005.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.NEN. Diagnostic terms and codes for dentistry. Delft, The Netherlands: Nederlands Normalisatie-instituut; 2015. p. 128. [Google Scholar]
- 32.Hamer RM, Simpson PM. Last observation carried forward versus mixed models in the analysis of psychiatric clinical trials. Am J Psychiatry. 2009;166(6):639–641. doi: 10.1176/appi.ajp.2009.09040458. [DOI] [PubMed] [Google Scholar]
- 33.Ducheyne F, De Bourdeaudhuij I, Lenoir M, Cardon G. Test-retest reliability and validity of a child and parental questionnaire on specific determinants of cycling to school. Pediatr Exerc Sci. 2012;24(2):289–311. doi: 10.1123/pes.24.2.289. [DOI] [PubMed] [Google Scholar]
- 34.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. [PubMed] [Google Scholar]
- 35.Shih T, Fan X. Comparing response rates from Web and mail surveys: a meta-analysis. Field Methods. 2008;20(3):249–271. [Google Scholar]
- 36.HealthIT.gov. EHR incentives & certification: how to attain meaningful use. [Accessed April 8, 2016]; Available at: http://www.healthit.gov/providers-professionals/how-attain-meaningful-use.
- 37.Kalenderian E, Ramoni RL, White JM, et al. The development of a dental diagnostic terminology. J Dent Educ. 2011;75(1):68–76. [PMC free article] [PubMed] [Google Scholar]
- 38.Kalenderian E, Ramoni RB, White JM, et al. The importance of using diagnostic codes. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112(1):4–5. doi: 10.1016/j.tripleo.2011.01.047. [DOI] [PubMed] [Google Scholar]
- 39.Ramoni RB, Walji MF, Kim S, et al. Attitudes toward and beliefs about the use of a dental diagnostic terminology: a survey of dental care providers in a dental practice. JADA. 2015;146(6):390–397. doi: 10.1016/j.adaj.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tokede O, Walji M, Ramoni R, et al. Treatment planning in dentistry using an electronic health record: implications for undergraduate education. Eur J Dent Educ. 2013;17(1):e34–e43. doi: 10.1111/j.1600-0579.2012.00759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kalenderian E, Tokede B, Ramoni R, et al. Dental clinical research: an illustration of the value of standardized diagnostic terms. J Public Health Dent. 2016;76(2):152–156. doi: 10.1111/jphd.12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walji MF, Kalenderian E, Stark PC, et al. BigMouth: a multi-institutional dental data repository. J Am Med Inform Assoc. 2014;21(6):1136–1140. doi: 10.1136/amiajnl-2013-002230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mandl KD, Kohane IS. Federalist principles for healthcare data networks. Nat Biotechnol. 2015;33(4):360–363. doi: 10.1038/nbt.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alighieri D. The Inferno: Canto 7. New Brunswick, NJ: Rutgers University Press; p. 1954.p. 288. Ciardi J, Trans.; original work published 1320. [Google Scholar]
- 45.Kalenderian E, Walji M, Ramoni RB. “Meaningful use” of EHR in dental school clinics: how to benefit from the U.S. HITECH Act's financial and quality improvement incentives. J Dent Educ. 2013;77(4):401–415. [PubMed] [Google Scholar]
- 46.Walji MF, Kalenderian E, Tran D, et al. Detection and characterization of usability problems in structured data entry interfaces in dentistry. Int J Med Inform. 2013;82(2):128–138. doi: 10.1016/j.ijmedinf.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Learning Health Community. Community: mission, vision, and values. [Accessed January 21, 2017]; Available at: http://www.learninghealth.org/about-the-community.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


