Abstract
Breast augmentation is the most commonly performed surgical procedure in aesthetic plastic surgery. Accurate pre-operative planning is crucial to obtain the best outcomes. We present our planning method deriving from a more than 30-year experience in aesthetic breast surgery, matching together patients tissues’ characteristics and patients’ wishes. We schematized our planning method in an easy-to-use flow diagram to help the decisional process in breast augmentation.
Keywords: Breast augmentation, breast implants, pre-operative planning
Introduction
Breast augmentation is the most commonly performed surgical procedure in aesthetic plastic surgery (1,2).
Accurate pre-operative planning is crucial to obtain the best outcomes and to reduce re-intervention rates.
The entire decisional process in breast augmentation was initially determined exclusively by patient’s wishes and surgeons’ preference, being the choice of implant size, type of implant, implant position and type of incision an arbitrary decision.
This led to high re-intervention rates for patient’s dissatisfaction with implant size and other post-operative complications (3-5).
Tebbetts described four main areas of post-operative issues after primary breast augmentation and dissatisfaction with implant size was one of them (6), the rate of requests for change of implants purely for size issues ranging from 2% to 20.6% (7).
Many techniques aiming to refine the pre-operative decisional process in breast augmentation have been developed in the last 10 years, leading to a significant reduction of re-operation rates (8-10).
Lower re-intervention rates are associated with the application of tissue-based planning methods, decisional systems matching implants to patient’s tissues and breast dimensions (11-14).
A national survey conducted among consultant plastic surgeons in United Kingdom showed how two schools of thought have emerged among the recent attempts to rationalize the choice of breast implants: those relying on standardized measurement systems and those that are guided by volume. The survey showed that over one third of surgeons take an intermediate approach using different forms of breast measurement (most commonly breast base) in combination with volumetric external sizing (15).
We present our planning method deriving from a more than 30-year experience in aesthetic breast surgery, matching together patients tissues’ characteristics and patients’ wishes.
We schematized our planning method in an easy-to-use flow diagram to help the decisional process in breast augmentation.
How to guide decisional processes in breast augmentation
We firmly believe a scientific and rigorous approach towards breast augmentation to be mandatory in order to obtain good outcomes, long-lasting results, low complication and re-intervention rates and high women’s satisfaction levels.
A rigorous approach starts with an accurate first consultation, listening to patient’s wishes, analyzing skin and soft tissues characteristics, the size of the chest wall and the breast and breast shape, always remembering that if you fail to plan, you plan to fail.
After accurate planning and shared decision making, a properly performed surgery with a complete knowledge of the devices we are using, with a correct and standardized follow-up will be next drivers towards the best and long-lasting results in breast augmentation.
We always have to balance the wishes of the patient with her tissue characteristics, identifying potential desired result/soft tissue mismatch. When the patient’s wish is recognized to be not achievable, further consultation and patient education is mandatory. Very useful tools to enhance patient understanding of the achievable results during the consultation are represented by the external sizers.
We developed a planning method to guide the decisional process in breast augmentation based on skin and soft tissue characteristics, breast and chest wall size, breast shape and patient’s wishes.
When planning a breast augmentation, the surgeon will assess implant size, implant type, implant pocket position and incision location and each decision will strongly impact on final outcomes.
The entire decisional process could be based on objective and quantifiable data deriving from patient’s tissue characteristics or arbitrary choices deriving from surgeon’s preference or patient’s specific requests.
We must pursue evidence-based surgery and to achieve predictable outcomes with low re-operation rates, we have to build our results on objective data.
Assessing implant size and type
Several dimensional systems have been developed to pre-operatively assess implant size in breast augmentation, one of the most commonly used being the BioDimensional System licensed by Inamed Corporation in 1994 (16), later evolved in the TEPID system, a planning method prioritizing long-term outcomes and minimizing re-intervention rates (17).
We firmly believe methods to assess implant size should put together patient’s wishes with tissue characteristics, making women understand the real possibilities of their tissues and the limits of the achievable outcomes, basing on objective, quantifiable measurements.
Final breast shape will depend on coverage tissue (breast skin, glandular parenchyma and fat) characteristics and implants.
After an objective definition of specific patient’s parameters [chest wall width, base width of the existing breast, nipple-to-inframammary fold (IMF) distance under maximal stretch, medial, lateral, superior and central pinch thickness of the existing tissues, clivage, sternal notch to nipple distance], the surgeon will be able to choose the best width, height and projection of the implant (Figure 1).
Figure 1.

Key measurements in the pre-operative planning of breast augmentation.
Dimensions will determine volume and not vice versa.
The surgeon will be able to assess breast volume, classified in very small/small/medium.
In case of medium-sized breasts, ptosis will be also assessed according to Regnault classification (18). Minimal ptosis (Regnault I) can be solved with the correct use of extra projected high cohesivity anatomical implant (Allergan Style 510, Irvine, CA, USA); or corresponding CPG model 333 Anatomical implants (Mentor Inc., Texas, USA). In case of moderate ptosis (Regnault II), an adjunctive round-block mastopexy will help obtaining the best outcome. If facing a severe ptotic breast (Regnault III), a Wise (inverted T) pattern mastopexy could be considered together with the augmentation. Simultaneous breast augmentation and mastopexy could represent one of the most difficult procedures in aesthetic breast surgery if not accurately pre-operatively planned and meticulously performed. In case of augmentation mastopexy, we suggest the surgeon to consider round implants use if not completely confident with anatomical implants. When needing adjunctive procedures to lift the breast we strongly advice trying to minimize implant contamination during the surgery.
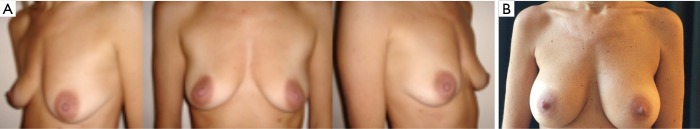
The surgeon will also consider patient’s wishes. Women asking for a full-filled upper pole will be offered an Extra-Projected Style 410 Cohesive Gel Implant (Allergan, Irvine, CA, USA). Women wishing a sweeter upper pole will be offered an Extra-Projected Style 510 Dual Gel implant (Allergan, Irvine, CA, USA) (Figure 2); or corresponding CPG model 333 anatomical implants (Mentor Inc., Texas, USA). If desiring a soft breast, the surgeon will consider a Low, Medium or Full Projected Style 410 Soft Touch Gel implant (Allergan, Irvine, CA, USA) (Figures 3,4); or corresponding CPG model 322-332 Anatomical implants (Mentor Inc., Texas, USA).
Figure 2.
Dual plane breast augmentation with Allergan Style 510 Dual Gel implants (width 12 cm; height 11.1 cm; volume 290 cc). Pre-operative view (A); 1 year (B); 3 years (C) and 6 years (D) follow-up.
Figure 3.
Dual plane breast augmentation using Allergan Style 410 MM Soft Touch gel implants (volume 215 cc). Pre-operative view (A); post-operative follow-up at 8 years (B).
Figure 4.
Dual plane breast augmentation using Allergan Style 410 FF Soft Touch implant (Width 11.5 cm; height 12 cm; volume 290 cc). Pre-operative view (A); post-operative follow-up at 6 years (B).
Even though we could obtain good outcomes with both round and anatomical implants in women with a good breast tissue coverage, we prefer anatomical implants. They help enhancing cosmetic results, allowing long-lasting results and remain mandatory in challenging situations, when correcting congenital malformations, when considering breast augmentation in very thin patients or patients with low/moderate breast ptosis (19,20).
Assessing implant pocket location and IMF positioning
The surgeon will then assess skin and soft tissue characteristics, through the soft-tissue medial, lateral, superior and central pinch thickness.
If upper pole pinch thickness less than 2 cm (medium/poor soft tissues), he will consider a dual-plane technique to ensure good tissue coverage (21).
In case of very good soft tissues (upper pole pinch thickness more than 2 cm), the surgeon will choose a sub-fascial breast augmentation.
Our preference for incision location is at the IMF, in order to minimize implant contamination. However incision location will be defined in relation with patient’s wishes, surgical skills trying to reduce tissue trauma and trade-offs.
When considering an incision at the IMF, the estimation of the level of the new IMF appears mandatory. Several methods have been described in order to define the level of the new IMF, as the ICE principle (22) or the method reported by Tebbetts with the TEPID system (17). Other authors prefer to calculate the position of the new IMF adding the half parenchymal thickness to the implant’s lower ventral curvature. This new IMF calculation method has been validated with Allergan implants (Irvine, CA, USA) and we actually do not know if it could be extended to other types of implants.
Our preference for new IMF positioning derives from an extension of Tebbetts’ method: the new IMF position will be calculated adding the half of the width of the implant to a measure deriving from the patient’s tissue stretching: if low tissue amount we will add 1 cm, if moderate tissue amount less than 1 cm, if good tissue amount no further addings will be considered.
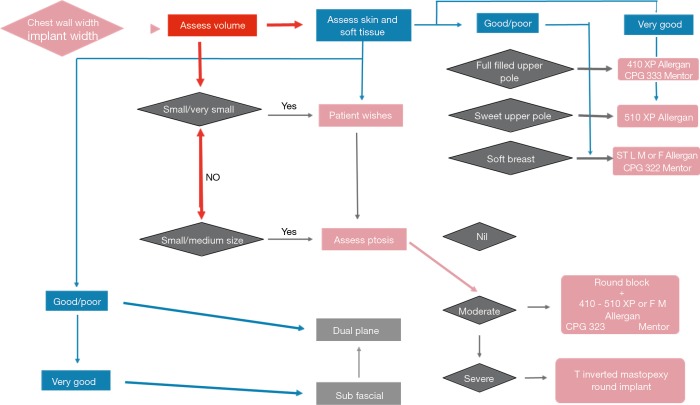
Our decisional process in breast augmentation is summarized in the breast augmentation flow-diagram (Figure 5).
Figure 5.
The breast augmentation flow diagram.
Discussion
We firmly believe that the best outcomes in breast augmentation could be achieved only through a standardized pre-operative planning of the surgery, a complete knowledge of the available devices, the application of an impeccable surgical technique and a scheduled follow-up.
The pre-operative planning should derive from a balance of patient’s tissue characteristics and patient’s wishes. Quantifiable, objective parameters should drive decisions for implant choice and implant pocket position, but the patient’s desire could further define the final outcome if the surgeon has clear in his mind the whole available “armamentarium” for a “scientific” breast augmentation.
Pinch thickness to guide decisions about implant coverage and pocket location, chest wall width, breast base width, nipple-to-IMF distance, skin stretch to drive implant volume assessment and still arithmetics to define new IMF position.
Objective measurements will help obtaining long-lasting results and fulfilling women’s desires, significantly reducing re-intervention rates.
When considering a specific volume, implant width will be the most important parameter, but the surgeon has to take into full account the height of the implant as well, depending on the characteristics of the overlying tissues, implant filler characteristics and implant shell-filler interactions. When using non-form stable implants, the height of the device is difficult to measure accurately so implant width and projection remain the most significant parameters.
Accurate measurements, but also impeccable surgical technique and standardized follow-up. Our recommended follow-up starts at 1 week, changing drapes and then maintaining paper tape on the surgical scars for 2 months, avoiding strong muscular exercise for three months, wearing post-surgical bras day and night for 2 months and then only at night for 1 more month. Clinical evaluation will be performed at first, second, sixth month after surgery and then yearly together with breast imaging.
The decisional algorithm we developed, graphically summarize the complex process behind a breast augmentation and aims to help standardizing decisions, basing on quantifiable parameters and abandoning arbitrary and subjective assessments methods.
Evidence-based surgery aiming to evidence-based outcomes mandatory requires scientific analysis of the decisional pathways.
We developed our decisional algorithm using Allergan implants, or Mentor (Mentor Inc., Texas, USA), but it could be easily adapted to any form-stable breast implant by a skilled user of other types of implants.
Most implant manufacturers actually offer a wide choice of implant device dimensions potentially enabling surgeons to obtain any result a woman could wish.
Long lasting results will derive from the best interactions between the implant and the patient’s tissues: excessive volumes and excessive projections or the wrong implant pocket position derive from wrong interactions between implants and patient’s tissues. Surgeon’s aim should be to tailor the breast augmentation on each single woman.
The optimal breast augmentation remains a team work in which the surgeon should offer the best pre-operative patient education with patient decision support devices and really informed consent processes (6,23).
The proposed algorithm while standardizing decisional pathways, at the same time provides the great opportunity for the surgeon to consider patients’ requests that will definitively determine the final outcome.
We would like to underline how the breast augmentation decisional process remains a complex choice: only pursuing a standardized decisional process, performing an accurate surgery aiming to reduce trade-offs and minimizing contaminations (that does not necessarily mean longer operative times), with a tight-knit and “oiled” surgical team, we could aspire to obtain the best, tailored and long-lasting results.
All aesthetic breast surgeons should analyze their own practice in order to standardize measurements and understanding exactly how measurements determine implant size and how the type of implant impacts on measurement techniques in their experience.
Moreover aesthetic breast surgeons should assess post-operative complications, re-intervention rates and reasons for re-operation and patient-reported outcomes. A national register with compulsory reporting of all breast augmentations with implants and outcomes data would help making a complete picture about different methods of pre-operative planning and their impact on patients’ outcomes (24,25).
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.American Society of Plastic Surgeons. 2015 Plastic Surgery Statistics. Accessed August 2016. Available online: https://www.plasticsurgery.org/news/plastic-surgery-statistics?sub=2015+Plastic+Surgery+Statistics
- 2.ISAPS International Survey on Aesthetic/Cosmetic. Accessed August 2015. Available online: http://www.isaps.org/Media/Default/global-statistics/2016%20ISAPS%20Results.pdf
- 3.Adams WP, Jr, Teitelbaum S, Bengtson BP, et al. Breast augmentation roundtable. Plast Reconstr Surg 2006;118:175S-187S. 10.1097/01.prs.0000247288.70207.24 [DOI] [PubMed] [Google Scholar]
- 4.Bengtson BP. Complications, reoperations, and revisions in breast augmentation. Clin Plast Surg 2009; 36:139-56. 10.1016/j.cps.2008.08.002 [DOI] [PubMed] [Google Scholar]
- 5.Jewell ML, Jewell JL. A comparison of outcomes involving highly cohesive, form-stable breast implants from two manufacturers in patients undergoing primary breast augmentation. Aesthet Surg J 2010;30:51-65. 10.1177/1090820X09360700 [DOI] [PubMed] [Google Scholar]
- 6.Tebbetts JB. An approach that integrates patient education and informed consent in breast augmentation. Plast Reconstr Surg 2002;110:971-8. 10.1097/01.PRS.0000019925.13513.6B [DOI] [PubMed] [Google Scholar]
- 7.Berry MG, Cucchiara V, Davies DM. Breast augmentation: part III--preoperative considerations and planning. J Plast Reconstr Aesthet Surg 2011;64:1401-9. 10.1016/j.bjps.2011.03.028 [DOI] [PubMed] [Google Scholar]
- 8.Bengtson BP, Van Natta BW, Murphy DK, et al. Style 410 highly cohesive silicone breast implant core study results at 3 years. Plast Reconstr Surg 2007;120:40S-48S. 10.1097/01.prs.0000286666.29101.11 [DOI] [PubMed] [Google Scholar]
- 9.Maxwell GP, Van Natta BW, Murphy DK, et al. Natrelle style 410 form-stable silicone breast implants: core study results at 6 years. Aesthet Surg J 2012;32:709-17. 10.1177/1090820X12452423 [DOI] [PubMed] [Google Scholar]
- 10.Maxwell GP, Van Natta BW, Bengtson BP, et al. Ten-year results from the Natrelle 410 anatomical form-stable silicone breast implant core study. Aesthet Surg J 2015;35:145-55. 10.1093/asj/sju084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tebbetts JB, Adams WP. Five critical decisions in breast augmentation using five measurements in 5 minutes: the high five decision support process. Plast Reconstr Surg 2005;116:2005-16. [PubMed] [Google Scholar]
- 12.Tebbetts JB. Achieving a zero percent reoperation rate at 3 years in a 50-consecutive-case augmentation mammaplasty premarket approval study. Plast Reconstr Surg 2006;118:1453-7. 10.1097/01.prs.0000239602.99867.07 [DOI] [PubMed] [Google Scholar]
- 13.Adams WP. The High Five Process: tissue-based planning for breast augmentation. Plast Surg Nurs 2007;27:197-201. 10.1097/01.PSN.0000306185.95812.c3 [DOI] [PubMed] [Google Scholar]
- 14.Adams WP., Jr The process of breast augmentation: four sequential steps for optimizing outcomes for patients. Plast Reconstr Surg 2008;122:1892-900. 10.1097/PRS.0b013e31818d20ec [DOI] [PubMed] [Google Scholar]
- 15.Holmes WJ, Timmons MJ, Kauser S. Techniques used by United Kingdom consultant plastic surgeons to select implant size for primary breast augmentation. J Plast Reconstr Aesthet Surg 2015;68:1364-9. 10.1016/j.bjps.2015.06.002 [DOI] [PubMed] [Google Scholar]
- 16.Tebbetts JB. Dimensional Augmentation Mammaplasty Using the BioDimensional System. Santa Barbara, Calif.: McGhan Medical Corporation, 1994:1-90. [Google Scholar]
- 17.Tebbetts JB. A system for breast implant selection based on patient tissue characteristics and implant-soft tissue dynamics. Plast Reconstr Surg 2002;109:1396-409. 10.1097/00006534-200204010-00030 [DOI] [PubMed] [Google Scholar]
- 18.Regnault P. Breast ptosis. Definition and treatment. Clin Plast Surg 1976;3:193-203. [PubMed] [Google Scholar]
- 19.Largent JA, Reisman NR, Kaplan HM, et al. Clinical trial outcomes of high- and extra high-profile breast implants. Aesthet Surg J 2013;33:529-39. 10.1177/1090820X13484035 [DOI] [PubMed] [Google Scholar]
- 20.Tebbetts JB, Teitelbaum S. High- and extra-high-projection breast implants: potential consequences for patients. Plast Reconstr Surg 2010;126:2150-9. 10.1097/PRS.0b013e3181f44564 [DOI] [PubMed] [Google Scholar]
- 21.Tebbetts JB. Dual plane breast augmentation: Optimizing implant-soft tissue relationships in a wide range of breast types. Plast Reconstr Surg 2006;118:81S-98S; discussion 99S-102S. [DOI] [PubMed]
- 22.Mallucci P, Branford OA. Design for Natural Breast Augmentation: The ICE Principle. Plast Reconstr Surg 2016;137:1728-37. 10.1097/PRS.0000000000002230 [DOI] [PubMed] [Google Scholar]
- 23.Adams WP, Jr, Small KH. The Process of Breast Augmentation with Special Focus on Patient Education, Patient Selection and Implant Selection. Clin Plast Surg 2015;42:413-26. 10.1016/j.cps.2015.06.001 [DOI] [PubMed] [Google Scholar]
- 24.Cooter RD, Barker S, Carroll SM, et al. International importance of robust breast device registries. Plast Reconstr Surg 2015;135:330-6. 10.1097/PRS.0000000000000885 [DOI] [PubMed] [Google Scholar]
- 25.Nahabedian MY. Discussion: international importance of robust breast device registries. Plast Reconstr Surg 2015;135:337-8. 10.1097/PRS.0000000000000921 [DOI] [PubMed] [Google Scholar]