Abstract
The ‘waterfall effect’ is a descriptive term to indicate a sliding ptosis of parenchymal breast tissue over a fixed or encapsulated implant. It occurs more frequently than surgeons anticipate and especially over the longer term after augmentation. Certain breast implants are more prone to contribute to this problem as are implants placed in submuscular pockets that ride high, especially in women with anatomical musculoskeletal variance or asymmetry. This article describes the aetiology of sliding ptosis in more detail, the relevant anatomy and the surgical correction. Understanding the problem enables the surgeon to plan the appropriate procedure and obtain proper informed consent. It is possible that a two stage procedure is necessary should the upper pole of breast require a debulk, either early (3 to 12 months) or later as the breast may slide with ageing of the tissues. The waterfall effect of breast parenchyma over implants is only apparent when the upper torso of the woman is undressed and she is in an erect posture. A significant number of women are happy with this situation and therefore no further action is required. Those that want an improved appearance in these circumstances can try autologous fat transfer to rebulk the surrounding tissues but generally the most likely solution involves a mastopexy with or without implant exchange. The results are highly rewarding but the scars are the legacy. Mastopexy augmentation is a difficult procedure and should only be performed by experienced surgeons. Many surgeons prefer a two stage approach with either an implant based augmentation first to limit scars and see if the patient is happy with the outcome or a first stage mastopexy to decide whether implants or fat graft are actually required as a secondary procedure.
Keywords: Breast mastopexy, breast implants, silicone, polyurethane breast implants, breast ptosis, pectoralis major muscle, breast parenchyma
Introduction
By definition the Waterfall effect describes a sliding of breast tissue (ptosis) over a fixed structure or object or a combination of both. The fixed structure is the skeleton and ptosis is related to the longitudinal chest wall vector as described by Frame et al. in 2015 (1) in association with the ability of breast parenchyma to gravitationally glide. The fixed object is of course the breast implant and how it retains its shape and form within its own capsule and in relation to the adjacent skeleton posteriorly and surrounding breast tissue or fascia anteriorly, determines the potential for sliding ptosis of overlying breast parenchyma.
Breast Augmentation is the most common Aesthetic Surgery procedure performed by Plastic Surgeons in the UK and although there is no effective implant registry at the present time, it is estimated that between 8,000 to 20,000 women have implants per year in the UK (2) but in the USA there are over 300,000 breast augments performed per year which over many decades correlates to about 4% of women in the USA having implants at the present time (3).
With an estimated 300,000 implants sold per year in Brazil and South America the total global numbers are therefore huge, with an estimated between 5 and 10 million women globally having breast implants. Concomitant to these numbers it has also been shown that up to 30% of women have implants removed or exchanged within 10 years after augmentation (4).
The most common complication after primary augmentation is capsular contracture and although the published incidence rates have decreased as both surgical technique and product design has improved, it is still the cases that up to 20% of implanted women have Baker 3 or 4 capsules at 10 years. Capsular contracture causes a progressive tightening around an implant thus changing the shape, thinning the overlying breast thus giving a mobile overlying breast parenchyma the opportunity to slide over the capsule allowing the classical post augmentation waterfall effect (Figure 1). This is more commonly seen in women that yo-yo diet, are multiparous but also may have high incorrectly positioned submuscular implants (Figure 2), a positive longitudinal vector skeleton and a short areola to submammary fold distance yet good breast volume (Figure 3). Ptotic breasts prior to implant augmentation often waterfall later in life (Figure 4). However even subglandular and subfascial implants can adhere to deeper structures and this gives the opportunity for any overlying loose and possibly excessive breast parenchyma to glide over the fixed capsule This is especially important to consider where polyurethane implants are being used (Figure 5) because they bio-integrate via five recognised layers into the surrounding tissues (5).
Figure 1.
Breast augmentation with round McGhan implants within submammary pockets showing sliding ptosis of breast parenchyma 10 years later. The breast volume filled a C cup bra pre-augmentation.
Figure 2.
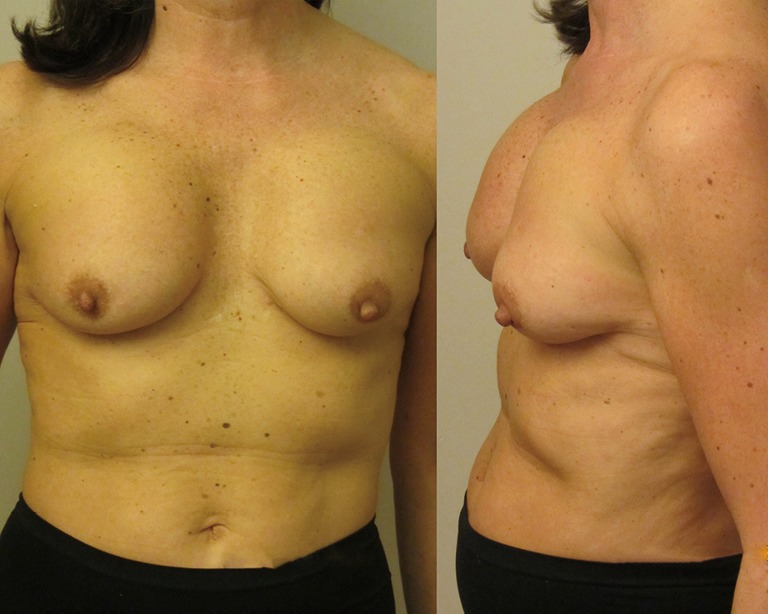
Subpectoral anatomical shaped McGhan implants have rotated and are now positioned too high on the chest wall causing a pseudoptosis effect of the overlying mobile breast tissue. The breast parenchyma is in fact in the correct position.
Figure 3.
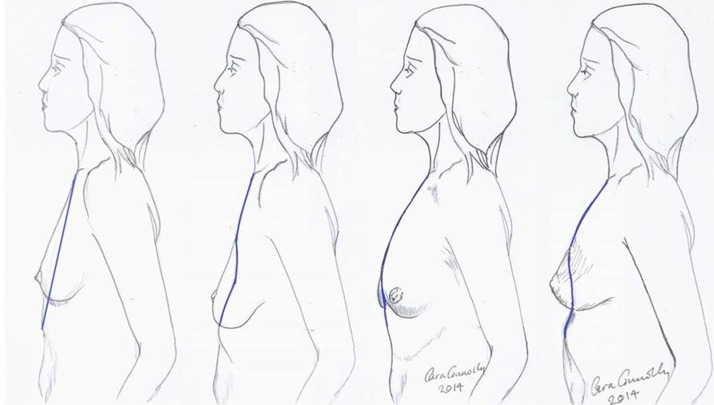
The longitudinal chest wall vectors, as originally drawn by Dr. Cara Connelly FRCS (Plast).
Figure 4.
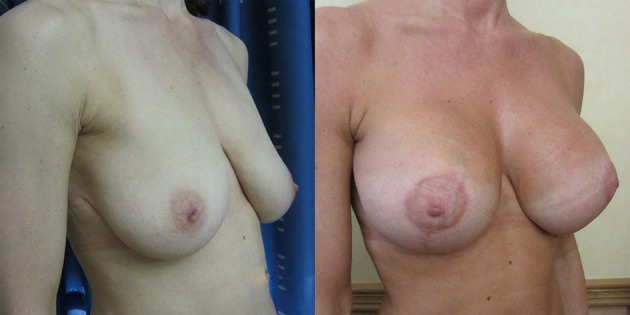
With good volume breast parenchyma there is always the possibility of a degree of sliding ptosis as the breast fat diminishes with pressure from the weight of implant. In this case the effect leaves a slightly fuller medial cleavage with mild waterfall of overlying parenchyma.
Figure 5.
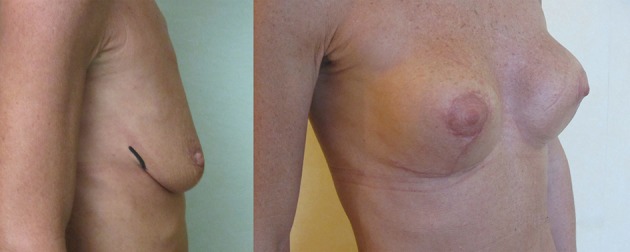
Superior pole mastopexy over adherent polyurethane implants. The upper pole waterfall effect is caused by adherence of breast tissue to the lower implant together with a reduced volume to the lower breast parenchyma after reduction.
Of course implants that do not adhere and remain mobile within enlarged pockets will have less chance of hinging the parenchymal tissues over the breast and so reduce the chance of the waterfall effect, however alternative issues are more likely to occur including lateral displacement of implant, rotation and excessive visible rippling (Figure 6).
Figure 6.
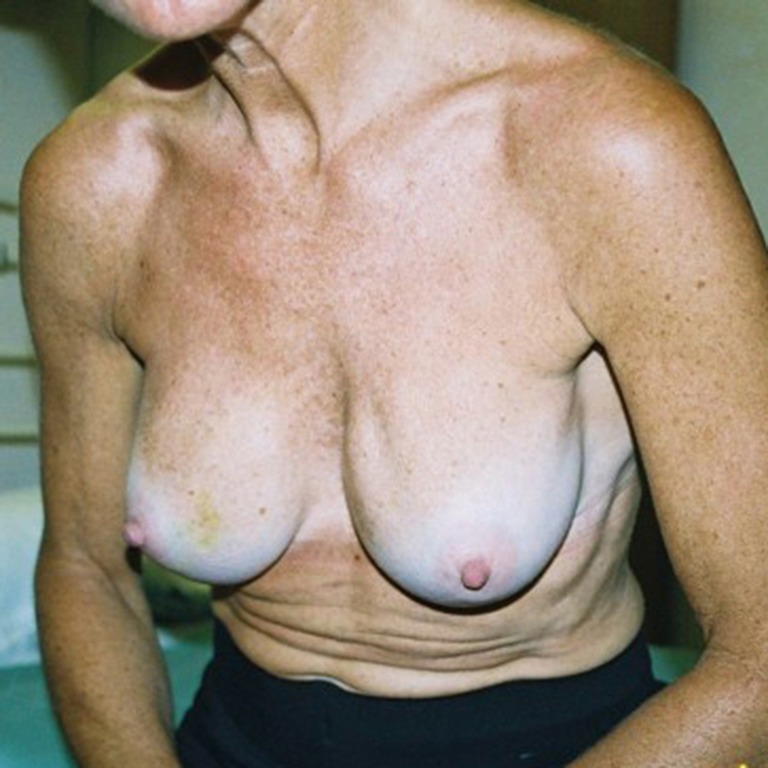
Late rippling seen during a leaning forwards posture with mentor augmented breasts. The implants are in subfascial pockets and are mobile.
The anatomy
Established anatomy textbooks do not position the breast correctly on the chest wall. The breast is situated within the deep fatty layer immediately behind the superficial (scarpa’s) fascia, but on the deep surface the breast is held by ligaments of Cooper to the deep fascia on the chest wall. These ‘ligaments’ are essentially condensations of pectoralis fascia. Strictly speaking they are not ligaments of course because they have no bony attachments. The lower breast is contained and held over the submammary fold, which is another condensation of deep fascia, but this extends to the more superficial tissues closer to skin. The degree of droop over this fold in the erect position defines the degree of ptosis (Figure 7) and this is influenced by volume and consistency of breast, relative compliance of skin, genetics and the variable shapes and vectors of underlying skeletal frame. The lower lateral breast is the tissue that can migrate laterally over the thorax but suspended by the axillary tail of breast including the lateral vascular pedicle hidden within Würinger ligament (6). The key to understanding the degree of mobility of the breast on the chest wall understands the loose areola connective tissue in Levicks triangle and the significant differences of musculoskeletal anatomy within and between women. Levicks triangle is the area between the lower free border of the pectoralis major muscle and the chest wall (7). There is no deep fascial adherence of breast within Levicks triangle. This is the area that surgeons utilize to easily access subpectoral pockets for implant augmentation. It is the area over which the supine patient finds a loose floppy breast dislocating into the axilla and lateral chest wall.
Figure 7.
The levels of breast ptosis as defined by positioning of the nipple in relationship with the sub-mammary fold (SMF). The nipple areola complex (NAC) to SMF distance is crucial when deciding the need for mastopexy and indeed the projecting capability when using higher profile implants.
The breast does not therefore always lie over the 2nd to 6th ribs and costal cartilages and the neurovascular arrangements are therefore variable in position, even if they have their expected origin.
The breast itself is contained within a superficial fascial envelope. If, as often described, Cooper’s ligaments do pass through from deep fascia to dermis then there is considerable centripetal force upon them and a heavy breast would be expected to always become ptotic. Intramammary the 15 to 20 breast ducts themselves have variable depths within the parenchyma and inversion is not uncommon as a presentation later in life with benign breast tissue as the breast becomes more ptotic. Breast is composed of a variable conglomerate of duct, milk gland hormone dependent cysts, fibrous collagen veneers and fat. The infrastructure compliance is dependent upon how the fat bulks out the veneers of collagen. In solid firm consistency breasts the main component in slim women is usually fibrous tissue. During lactation and breast feeding the normal glandular tissue expands 3 to 5 times in a yo-yo fashion and this causes fatty atrophy of the breast. When lactation ceases and the woman physiology returns to normal the glandular component regresses but the fat may not return. The consequence is a loss of compliance, looseness and droop which we call ptosis. To many this is normal and acceptable but some women are unhappy with the changes and resort to surgery.
From the surgical anatomy point of view it is important to know about variance in breast parenchyma, skin elasticity, neurovascular anatomy, the biomechanics of pectoralis muscle activity, variability of the thoracic skeleton and muscles and to devise surgical options to address each of the issues. Most importantly the consequences of breast implant associated surgery must be considered and fully explained to the patient. The surgeon has to take into account the patients’ skin type, healing, potential to form poor scars and possible need for corrective surgery which should occur in less than 3% of cases. It is clear that if more children are planned surgery should be deferred.
Surgical correction
Understanding the causation allows us to consider the options:
❖ No surgery. This can be acceptable to the patient if the positioning of breast within bra and clothes gives symmetry and with reasonable cleavage. Clearly the result may not be acceptable without bra support but the risks of surgery have to be counterbalanced proportionately;
❖ Exchange and resite implants. Possibly change to a more projecting implant or select a different implant style or shape from another reputable manufacturer and or resite in front of the pectoralis major muscle. This will only work if the lower half of the breast parenchyma is not too bulky or sliding. In this event a lower pole reduction has to happen;
❖ Remove implants. This will probably still leave a disappointed patient because of the loss of upper pole breast fullness. The waterfall effect disappears though;
❖ Incorporate a mastopexy over suitably resisted or replaced implants. Generally speaking this is the preferred option for patients that have decided to proceed to consultation after discussing within the family unit. Occasionally autologous fat grafting has something to offer particularly if there is asymmetry. A second consultation after photographic imaging (preferably using the IC 360 system) (Figure 8), is always advisable so that the patient can understand her own anatomy in more detail and compare the pre- to post-operative result from corrective surgery.
Figure 8.

IC 360 camera imaging of a patient pre- and post-operatively after 3 months (8). Available online: http://www.asvide.com/articles/1479
Selection of implant
The choice of implants is pretty straightforward. Leaving aside the published FDA data on the performance of the triopoly of implants permitted within the USA (Allergan/McGhan/Pfizer, Mentor/Ethicon, Sientra/Silimed), it is vital to understand that the Silicone cohesive gel contained within all implants has essentially the same gel origin, even though the consistency may vary slightly during manufacture of the cohesive gel. The main difference is in the external elastomer shell and the fill capacity. Since Cronin and Gerow used the first silicone gel implants in 1964 it has been obvious that smooth implants have a higher risk of stimulating capsular contracture, even allowing for the higher gel bleed associated with the earlier generations of implant shell technologies. Polyurethane implants were introduced after Ashley in 1970 (9) convincingly demonstrated that capsular contracture rate was lowered. Incidentally the first polyurethane implant was anatomically shaped. Although polyurethane implants are not FDA approved in the USA and Canada, they are being increasingly used all over the world because of their safety record and the lower re-operation rates associated with capsular contracture (10). In the USA and Canada where only silicone or saline implants are available, the importance of texturing has been debated extensively, but all of the evidence indicates that textured implants have less risk of developing capsular contracture than smooth elastomer shell implants (11).
In an attempt to create a textured silicone elastomer implant with comparable low capsular contracture rates with their polyurethane equivalent, Mentor/Ethicon uses polyurethane to impregnate a negative imprint on the outer layer of elastomer. Sientra’s Silimed elastomer implant also has a texture made from the negative imprint of polyurethane. Allergan however, uses a salt to form a coarser pattern on the outer layer of silicone prior to the implant being ‘cured’. This coarse texture pattern is achieved by washing off the crystals (salt-extraction technique). Some believe that this coarse texture is more irritant to the tissues and perhaps is a reason why Anaplastic Large Cell Lymphoma (ALCL) is more prevalent in women with McGhan implants (1 in 1 million), although ALCL can occur in women with any implant (12). Mentor textured implants have no adherence capabilities and therefore are free to move within the size of pocket that the surgeon creates. Many surgeons deliberately enlarge the pocket to avoid a snug fit with mentor implants. Allergan implants are supposed to adhere and may do so in nulliparous firm tissues, but partial adherence is more common and double capsule and fibrous bands are more prevalent than appears in the literature. In the USA and Sweden the teaching to date has been to put implants behind the Pectoralis Major muscle (or in a dual plane) in most cases (13,14) and this is supposed to disguise any prominent edge in the upper pole, especially in thin women. At the same time repeated muscle contracting was thought to help reduce capsular contracture forces developing. Unfortunately up to 25% women dislike the abnormal appearance whilst the Pectoralis Major is contracting and there is also a higher risk of pain where any lateral displacement of the implant will be forced against the lateral perforating branches of the intercostal nerves. Revisional total capsulectomy and implant replacement is often very difficult, particularly when submuscular implants rupture which may be as high as 50% at 20 years when inclusive of ‘silent’ ruptures.
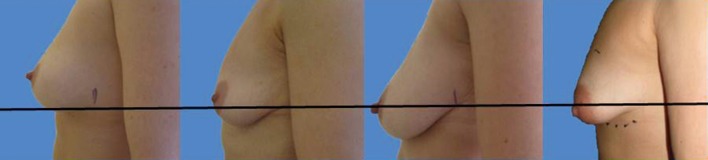
A surgeons preferred implant selection may carry more or less risk of developing the waterfall effect but if the Surgeon is aware of this possibility then certain precautionary steps can be taken. Mentor implants rarely have such problems if they are mobile within capacious soft capsules that are larger than implant diameter. Here the implant is able to naturally displace within the space and remain soft. The overlying parenchyma can alter with posture over the implants. Allergan implants that adhere in high submuscular positions will always show some degree of sliding ptosis if the patient puts on weight or the Surgeon puts them too high on the chest wall (Figure 9). Sliding ptosis will also appear following subglandular implant placement and mild capsular contracture but with comparatively loose and sliding good volume breast tissue (Figures 10,11). Most polyurethane implants are placed in subfascial or dual fascial pockets because with the lower risk of capsular contracture, implant palpation is less obvious in the upper medial quadrant of breast. Polyurethane biointegrates into adjacent tissues and it is vital to place the implants low on the chest wall initially (5). The problem with polyurethane is its efficiency in adherence because overlying breast tissue that is gliding on deep fascia anyway, is more prone to sliding ptosis and the waterfall effect.
Figure 9.

A well-positioned small polyurethane implant in subfascial pocket that has contracted slightly and presents too high with a very mild waterfall effect of the overlying soft tissues. Simply increasing the volume of implant after inferior capsulectomy corrects this problem.
Figure 10.
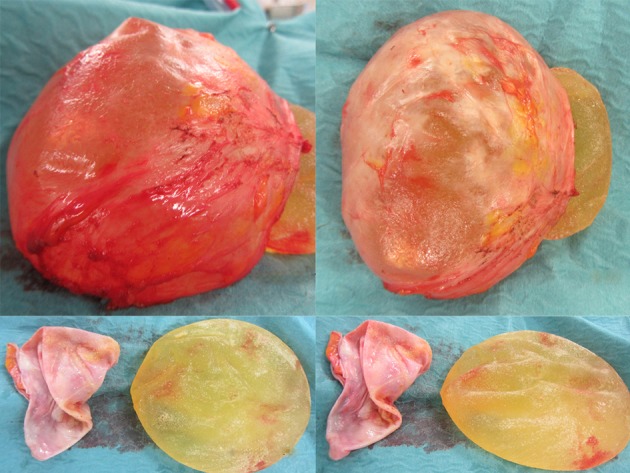
Removal of McGhan and overlying Baker four capsules. The capsule is only 2 mm thick.
Figure 11.

A comparative waterfall effect of breast parenchyma in a breast augment patient part contributed by the short non-expanding NA to sub-mammary fold (SMF) distance.
Addressing the breast parenchyma
Loss of supporting fat within the framework of the breast parenchyma is the fundamental reason to develop the waterfall effect (Figures 12,13,14,15). The nipple ptosis will be more obvious in women where the sub-mammary fold (SMF) to nipple areola distance is far shorter than the implant enhanced breast has been projected, especially if the implant is sited too high, the pectoralis major muscle is bulging superiorly or there is a positive vector curving chest skeleton. The chest wall vectors cannot be changed, so the bulky breast parenchyma has to be addressed because merely re-siting an implant will not necessarily cure the waterfall problem. Correction is achieved by revolumising the breast especially in the upper pole or relocating the breast parenchyma either by autologous fat grafting, a mastopexy or a mastopexy reduction.
Figure 12.
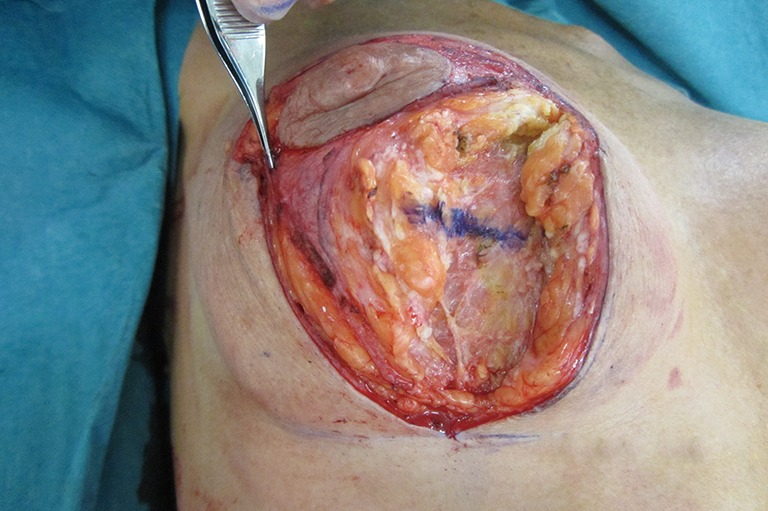
Vertical scar right mastopexy over subfascial polyurethane breast implants. Forceps are distracting the breast parenchyma laterally and a horizontal blue line drawn on the parenchyma.
Figure 13.
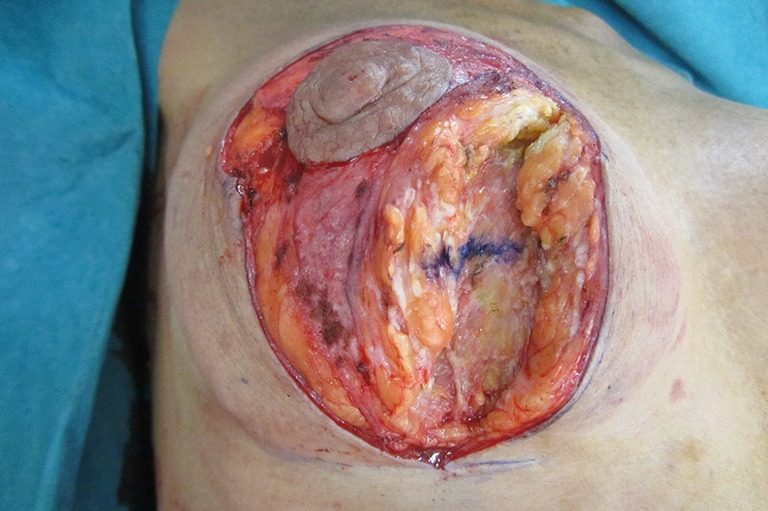
Same patient in a neutral position showing vertical displacement of the blue marker line, even in supine position.
Figure 14.
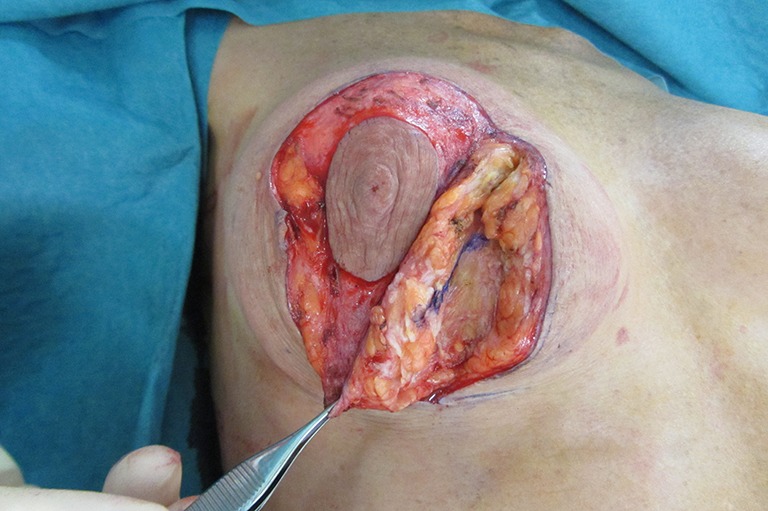
Same patient showing the vertical blue-line glide of tissue over the breast implant capsule with gentle forceps traction.
Figure 15.
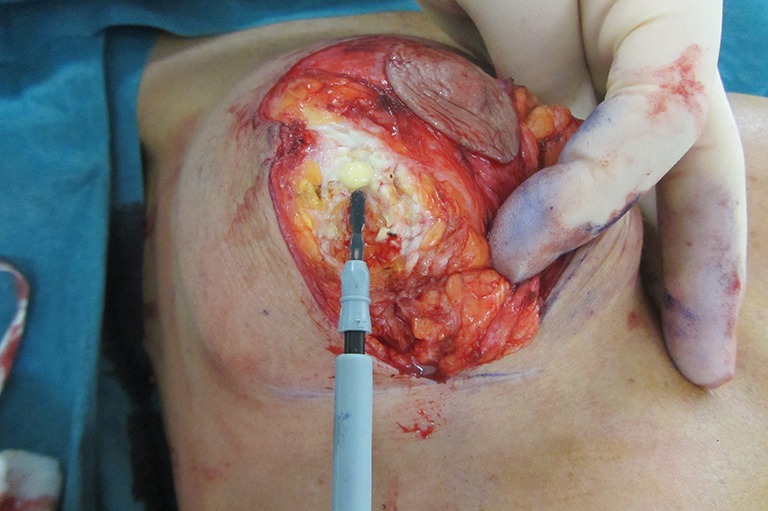
Same patient showing fibrous breast tissue and anatomical glide occurring in the loose areolar tissue layer between the fibrous breast and the implant capsule.
Relocating the breast parenchyma
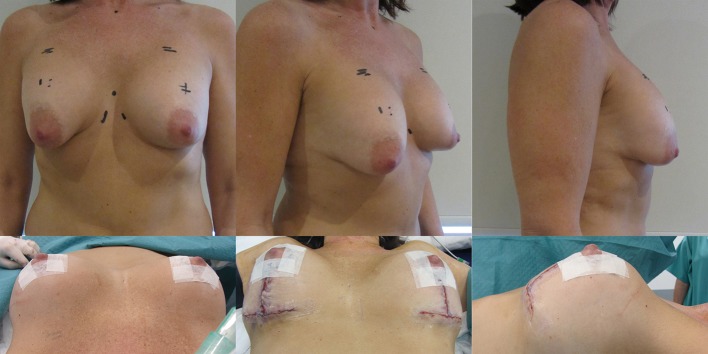
A waterfall of parenchyma over an encapsulated implant has to be addressed by exchanging and relocating a smaller implant into a better position. A mastopexy, perhaps including a controlled parenchymal reduction, can be carried out using a peri-areolar (Figure 16), a short vertical scar or classical inverted “T” approach (Figures 17,18). In my opinion this is best performed over a polyurethane implant because the implant capsule is unlikely to contract or displace over time (5) and therefore the end soft tissue positioning is more predictable. The exchange of implant to polyurethane also allows a potential change of shape to conical, anatomical or round with added benefits (7).
Figure 16.

Pre- and post-operative views after periareolar mastopexy and insertion of low projecting anatomical implants.
Figure 17.
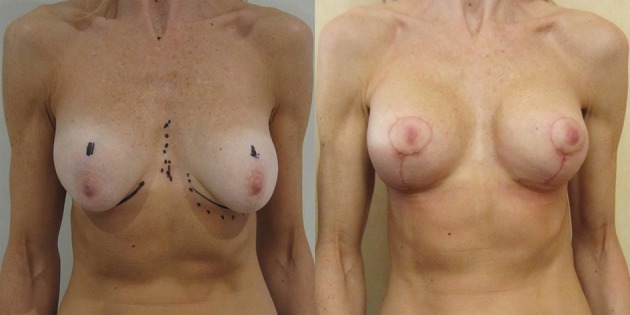
Vertical scar augmentation/mastopexy over conical polyurethane breast implants.
Figure 18.

Same patient oblique view.
The mastopexy should always be performed after implants have been satisfactorily positioned. This allows the vertical subareolar to submammary fold distance to be determined surgically with some accuracy. Pre-operative markings are always useless and probably only serve to allow the surgeon and patient to reach an understanding of the complexity of procedure. They are generally washed off during the skin prep and remarked after the implants have been inserted and the degree of true sliding ptosis id revealed in a semi sitting position. In most cases the vertical scar component should be between 6 and 9 cm depending upon the size of breast and the patients’ constitution. Five to 6 cm periareolar scars should settle well providing that the areola is not closed under tension and the horizontal scars should be kept as short as possible. If the nipple diameter is short, then it must remain at least the same size post-operatively. Trying to enlarge the nipple surgically usually only stretches the scar. Nipple size can always be extended by the judicious use of surgical tattoo. The horizontal scars should neither be visible medially nor laterally in the upright stance with arms by the side, but obviously they will be apparent when the arms are abducted and extended upwards.
Surgical procedure to exchange implants and mastopexy
❖ Pre-operative selection of patient. Camera imaging. Understanding the problem and the relevant anatomical relationships;
❖ Skin marking in a sitting position must take into account skeletal asymmetry, scoliosis, tilted shoulders and posture;
❖ Supine position with a marginal reverse trendelenburg. General anaesthetic plus local anaesthetic if preferred;
❖ Remove implant via a 5- to 6-cm submammary fold incision or the old scar;
❖ Insert a new implant into a re-defined space using either capsulotomy, capsulorrhaphy, capsulectomy and then close the augmentation wound in three layers. The subsequent mastopexy avoids communication between the implant space and the mastopexy wound by using a careful plane of dissection only up to the level of lower areolar margin on the chest wall but in a plane just superficial to the pectoralis fascia;
❖ Assess sliding ptosis over the implant by on table manipulation and then re-mark the proposed new nipple position. If using polyurethane implants, mark up to 2 cm higher than anticipated height especially if using a superior pedicle mastopexy;
❖ Mark any periareolar skin excision to fit a 5- to 6-cm nipple diameter;
❖ Mark a vertical elliptical excision, but aim to excise within the boundary lines to avoid undue tension upon the closure;
❖ De-epithelialise the marked areas;
❖ Plan to reduce the lower pole bulk of breast parenchyma. This is done in a structured way via the superior ‘pedicle’ technique. The superior pedicle though is in fact a superiorly and centrally based pedicle because it is important to only mobilize the parenchyma up to nipple level and to avoid dissecting beyond Levicks triangle laterally. This then leaves a good central, lateral, medial and superiorly based blood supply and lymphatic drainage for the nipple and may preserve reasonable sensation;
❖ A long superior pedicle ptosis correcting mastopexy over adherent implants will bunch up the superior pole and make a different type of ptosis (Figure 19). The bulky upper pole merely pivots over the non-mobile lower segment. Care must be taken to avoid this by not excising too much from the lower pole during the mastopexy and using a debulk technique to the upper pole that involves full thickness skin excision and trimming a superficial wedge of breast from the superior pole. Sometimes liposuction and fat graft may judiciously be required for best results. If nipple blood supply appears tenuous during surgery it is better to have warned the patient prior to surgery that a small secondary debulk under local anaesthetic may be required as a second stage. However, if a broad based superior flap attached to deep fascia is used, then the blood supply to the nipple includes perforating vessels and superior, medial and lateral primary vessels and an the described upper pole debulking procedure can safely be carried out at the primary mastopexy augmentation. It is important to limit the extent of the original implant pocket dissection though and if this was done elsewhere then it would be advisable to plan for a two stage procedure;
❖ All excised tissues should be sent to the laboratory for histological analysis;
❖ Final results should be assessed at 3 months and at least 1 year (Figure 20).
Figure 19.
Pre-operative erect posture views showing sliding ptosis of conical breasts over polyurethane implants. On-table short horizontal scar mastopexy.
Figure 20.
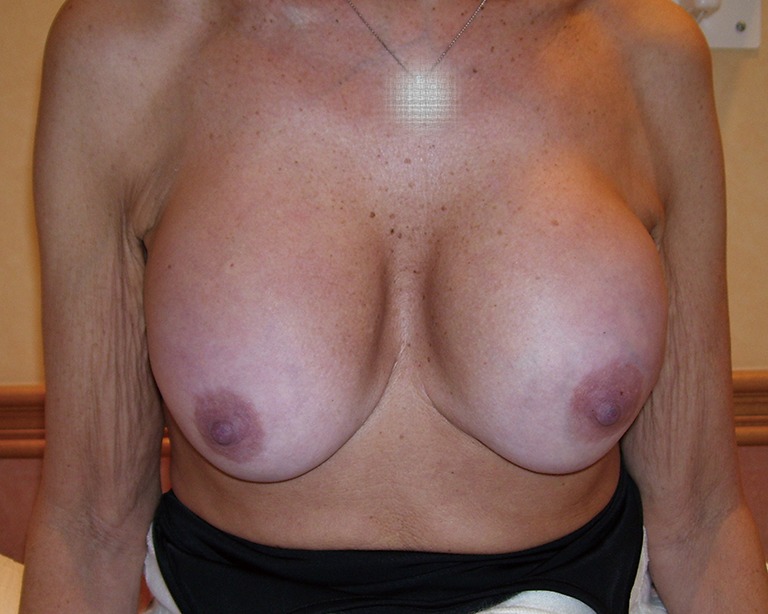
Showing pre-operative views and follow ups at 3 months and 3 years after mastopexy with conical breast implants. There is no evidence of sliding ptosis.
Summary
Mastopexy augmentation is a difficult procedure and should only be performed by experienced surgeons. Many surgeons therefore favor a two stage approach with implant based augmentation first to limit scars and see if the patient is happy with the outcome. A secondary procedure up to years later, may include a lower pole debulk and mastopexy over the settled implant if there remains or develops a significant degree of secondary ptosis.
In established waterfall it is often better to exchange the implants bearing in mind the expected longevity and complications of even the most up to date implants. Correcting ptosis over new implants is a challenging procedure and can create problems necessitating delayed revisional surgery to the upper pole. Clearly informed consent should involve considerable counseling to avoid disappointment and of course litigation.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- 1.Frame JD, Connolly C. Framing the Breast. Aesthet Surg J 2015;35:NP106-12. 10.1093/asj/sju088 [DOI] [PubMed] [Google Scholar]
- 2.McCulley S. How Many Women Have Breast Implants? Available online: http://www.stephenmcculley.co.uk/how-many-women-have-breast-implants/
- 3.UK Progressive. Dear Mona, What Percentage Of Women Have Breast Implants? Available online: http://www.ukprogressive.co.uk/dear-mona-what-percentage-of-women-have-breast-implants/article31743.html
- 4.Center for Devices and Radiological Health U.S. Food and Drug Administration. FDA Update on the Safety of Silicone Gel-Filled Breast. Available online: http://www.fda.gov/downloads/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/BreastImplants/UCM260090.pdf
- 5.Frame J, Kamel D, Olivan M, et al. The In Vivo Pericapsular Tissue Response to Modern Polyurethane Breast Implants. Aesthetic Plast Surg 2015;39:713-23. 10.1007/s00266-015-0550-4 [DOI] [PubMed] [Google Scholar]
- 6.van Deventer PV, Graewe FR, Würinger E. Improving the longevity and results of mastopexy and breast reduction procedures: reconstructing an internal breast support system with biocompatible mesh to replace the supporting function of the ligamentous suspension. Aesthetic Plast Surg 2012;36:578-89. 10.1007/s00266-011-9845-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Georgeu GA, Frame JD, Jr, Frame JD. Conical polyurethane implants: an uplifting augmentation. Aesthet Surg J 2013;33:1116-28. 10.1177/1090820X13512855 [DOI] [PubMed] [Google Scholar]
- 8.Frame J. IC 360 camera imaging of a patient pre- and post-operatively after 3 months. Asvide 2016;3:171. Available online: http://www.asvide.com/articles/1479
- 9.Ashley FL. A new type of breast prosthesis. Preliminary report. Plast Reconstr Surg 1970;45:421-4. 10.1097/00006534-197005000-00001 [DOI] [PubMed] [Google Scholar]
- 10.Castel N, Soon-Sutton T, Deptula P, et al. Polyurethane-coated breast implants revisited: a 30-year follow-up. Arch Plast Surg 2015;42:186-93. 10.5999/aps.2015.42.2.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collis N, Coleman D, Foo IT, et al. Ten-year review of a prospective randomized controlled trial of textured versus smooth subglandular silicone gel breast implants. Plast Reconstr Surg 2000;106:786-91. 10.1097/00006534-200009020-00005 [DOI] [PubMed] [Google Scholar]
- 12.Rupani A, Frame JD, Kamel D. Lymphomas Associated with Breast Implants: A Review of the Literature. Aesthet Surg J 2015;35:533-44. 10.1093/asj/sjv016 [DOI] [PubMed] [Google Scholar]
- 13.Tebbetts JB. Dual plane breast augmentation: optimizing implant-soft-tissue relationships in a wide range of breast types. Plast Reconstr Surg 2001;107:1255-72. 10.1097/00006534-200104150-00027 [DOI] [PubMed] [Google Scholar]
- 14.Hedén P, Jernbeck J, Hober M. Breast augmentation with anatomical cohesive gel implants: the world's largest current experience. Clin Plast Surg 2001;28:531-52. [PubMed] [Google Scholar]