Abstract
Breast implant-associated anaplastic large cell lymphoma (BI-ALCL) is a distinct type of T-cell lymphoma arising around breast implants. The United States FDA recently updated the 2011 safety communication, warning that women with breast implants may have a very low risk of developing ALCL adjacent to a breast implant. According to the World Health Organization, BI-LCL is not a breast cancer or cancer of the breast tissue; it is a lymphoma, a cancer of immune cells. BI-ALCL is highly curable in the majority of patients. Informed consent should include the risk of BI-ALCL with breast implant patients. Women with breast implants are encouraged to contact their plastic surgeon if they notice swelling, fluid collections, or unexpected changes in breast shape. Physicians are encouraged to send suspicious peri-prosthetic fluid for CD30 immunohistochemistry, cell block cytology, and culture in symptomatic patients. An observation from reported cases indicates a predominance of textured device involvement. More information is needed to fully understand risk factors and etiology. The association of bacteria and biofilm with ALCL is currently being investigated and one theory is that biofilm may play a role in this disease process stressing the importance of best practice techniques intraoperatively. Recent studies have reported clinical presentation, prognosis, and treatment outcomes with long term followup demonstrating the critical role for surgical management.
Keywords: Breast implant-associated anaplastic large cell lymphoma (BI-LCL), anaplastic large cell lymphoma (ALCL), non-Hodgkin lymphoma, CD30, double capsule, late seroma
Breast implant-associated anaplastic large cell lymphoma (BI-ALCL) background
Over 1.5 million women worldwide receive breast implants annually (1). According to the Unites States Food and Drug Administration, breast implants have a “reasonable assurance of safety and efficacy”, but are not without possible short and long-term complications such as infection, device failure, and capsular contracture (2). Breast implant-associated anaplastic large cell lymphoma (BI-LCL) is a very rare T-cell lymphoma that can arise around breast implants placed either for reconstructive or cosmetic indications. While theories have been postulated on the potential etiology of BI-ALCL, pathogenesis has not yet been clearly defined. Other rare breast implant associations include benign delayed seromas and double capsules, which have been the focus of implant marketing campaigns, but remain poorly understood or described outside of case reports. The purpose of this article is to perform a literature review on the etiology and sequelae of BI-ALCL, benign delayed seromas, and double capsules surrounding breast prostheses.
In 2016, the US FDA updated its 2011 safety communication cautioning about BI-ALCL and included clinical presentation, prognosis, and treatment options (3). The FDA is now aware of 258 adverse event reports of BI-ALCL, however due to the rarity of the disease, the FDA recommended no specific changes to clinical practice for asymptomatic patients with breast implants. Since the FDA safety communication, a number of major worldwide government authorities have developed BI-ALCL patient and physician recommendations. The National Comprehensive Cancer Network (NCCN) released statements on BI-ALCL in 2012, and is expected to issue treatment guidelines in 2017 (4). In response to public concern and published cases, the World Health Organization (WHO) in March 2016 provisionally classified BI-ALCL as a distinct lymphoma with disease-specific surgical treatment recommendations (5). The revision refines diagnostic criteria and emphasizes the importance of surgical management for disease confined to the capsule. The International Agency for Research on Cancer (IARC) in 2014 designated BI-ALCL a priority of further research to determine malignancy etiology and mechanism of pathogenesis (6). In 2015, the US National Cancer Institute (NCI) posted specific surgical recommendations for the treatment of BI-ALCL (7). In 2015, the French National Cancer Institute (Agence Nationale de Sécurité du Médicament, ANSM) released diagnosis and treatment recommendations for BI-ALCL and mandated that all breast implants carry a warning that a clearly established link exists between breast implants and ALCL (8). In July 2016, the ANSM released an update on BI-ALCL based upon their 29 cases and because of a predominance of textured cases, ANSM is calling for all implant manufacturers selling in France to submit clear safety data for textured implants within the year or their respective devices will be restricted from sale (9).
Family of ALCL diseases
Lymphoma is a cancer of the immune system developing from lymphocytes and is the most common malignancy of the blood (10). Lymphoma broadly includes Hodgkin’s lymphoma, Non-Hodgkin’s lymphoma (NHL), and a variety of lymphoproliferative disorders. In the United States, approximately 71,850 cases of NHL were diagnosed in 2014 (11). Estimated incidence of T-cell NHL diagnoses in the United States in 2014 was 17,302 (11), which included 1982 cases of ALCL of any type (12). Clinicopathologic subtypes of ALCL range from the more aggressive systemic ALCL down to the indolent CD30 positive lymphoproliferative disorders of the skin that includes a benign lymphomatoid papulosis and the relatively indolent primary cutaneous ALCL (5-yr OS >90% to 95%, lymph node metastases 5%) (13). Multiple sites of disease, frequent lymphadenopathy, and wide dissemination characterize systemic ALCL. Systemic ALCL is further classified by either the expression or absence of the anaplastic lymphoma kinase (ALK) tyrosine kinase receptor gene translocation. ALK+ ALCL accounts for approximately 50 to 80 percent of all ALCLs, and occurs most commonly in males (male/female ratio: 6.5:1) under the age of thirty, and has a 5-yr overall survival (OS) of 70% (5). In contrast, systemic ALK-ALCL is an immunophenotypically and cytogenetically heterogeneous group, and has a 5-year OS 31%. Standard first line chemotherapy is cyclophosphamide, hydroxydaunorubicin, vincristine, and prednisone (CHOP) (14). When treated with chemotherapy, ALK+ ALCL has a higher overall 5-year survival rate than systemic ALK-ALCL (58% versus 34%, respectively) (15,16). As a percentage of all T-cell lymphomas, ALK+ ALCL is more common in North America than Europe or Asia (16.0% vs. 6.4% vs. 3.2%, respectively). Systemic ALK-ALCL is more common in Europe than North America or Asia (9.4% vs. 7.8% vs. 2.6%, respectively) (17).
Overview of BI-ALCL
BI-ALCL is distinct from primary breast lymphoma (PBL), which is a disease of the breast parenchyma, and is predominantly a B-cell lymphoma (65–90%) (18,19), while BI-ALCL is purely a T-cell lymphoma arising either in an effusion surrounding the implant or in the scar capsule surrounding a breast implant (20,21). All confirmed reported cases of BI-ALCL are ALK—and express the CD30 cell surface protein (Table 1,Figure 1). Most cases are diagnosed during implant revision surgery performed for a late onset (>1 year), persistent seroma and may be associated with symptoms of pain, breast lumps, swelling, or breast asymmetry. The number of BI-ALCL cases reported in primary augmentation and reconstruction for breast cancer or prophylaxis are nearly equivalent. BI-ALCL most commonly follows an indolent course provided adequate surgical ablation of the implant and surrounding capsule without systemic therapy, but aggressive exceptions, disease progression, and death have been reported (22).
Table 1. Criteria for diagnosis of breast implant-associated anaplastic large cell lymphoma.
| A tumor with adequate pathological specimen for analysis, involving an effusion either surrounding a breast implant lining a breast implant capsule |
| Neoplasm with large lymphoid cells with abundant cytoplasm and pleomorphic nuclei |
| Tumor demonstrates T-cell markers with uniform expression of CD30 by immunohistochemistry or flow cytometry |
| Negative for anaplastic lymphoma kinase (ALK) protein or translocations involving the ALK gene at chromosome 2q23 |
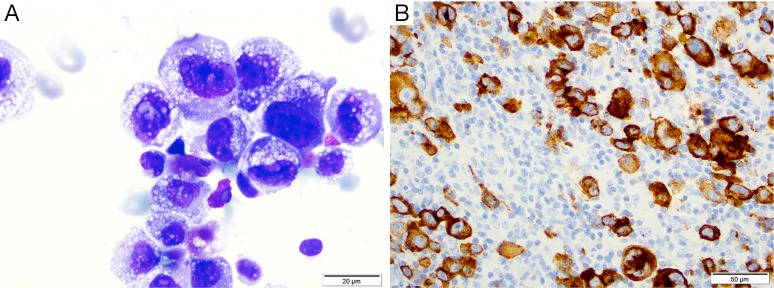
Figure 1.
BI-ALCL histology. (A) Wright Giemsa staining from a malignant effusion of BI-ALCL demonstrating pleomorphic cells with horseshoe shaped nuclei, nuclear folding and abundant vacuolated cytoplasm (1,000× magnification); (B) immunohistochemistry in tissue section demonstrates sheets of large cells positive for CD30 in a case of BI-ALCL. BI-ALCL, breast implant-associated anaplastic large-cell lymphoma. (Reprinted with permission from Clemens MW, Miranda RN. Coming of Age: Breast Implant-Associated Anaplastic Large Cell Lymphoma After 18 Years of Investigation. Clin Plast Surg 2015;42:605-13.)
Etiology of BI-ALCL
No risk factors have been clearly identified for ALCL though many have been theorized including the presence of a subclinical biofilm, response to particulate from textured implants, a consequence of capsular contracture or repeated capsular trauma, and/or genetic predisposition but these observations have not been confirmed in formal epidemiological studies (23). Recent studies have demonstrated a possible pathogenic mechanism of chronic T-cell stimulation with local antigenic drive, ultimately leading to the development of lymphoma (24). The immune system’s response to chronic inflammation surrounding the breast implant in a genetically susceptible patient, may lead to genetic degeneration and dysplasia (25). In a recent study by Hu et al., the authors compared the implant capsules of patients with BI-ALCL to those with normal capsular contracture, finding a high bacterial load and significantly different microbiome in the BI-ALCL specimens with a high predominance of the bacteria Ralstonia pickettii, a gram-negative rod and common contaminant of drinking water (26). Previous work comparing the capsules of textured and smooth implants in pigs showed that there are increased lymphocytes on textured breast implants, with a T-cell predominance, further supporting texturing as a link to chronic inflammation and T-cell response. Kadin and colleagues demonstrated that patterns of cytokine and transcription factor expression such as SOCS3, JunB, SATB1 are suggestive of a Th1 phenotype which further supports the theory that BI-ALCL may arise from chronic bacterial antigen stimulation of T-cells (27). Further research is required to identify modifiable risk factors and susceptible populations, however based upon these preliminary findings, standard of care sterility technique such as antibiotic prophylaxis and no-touch practice remains prudent when placing a breast implant (28).
Epidemiology of BI-ALCL
Since 1997, approximately 136 patients have been reported in case reports of BI-ALCL (29-53). A number of epidemiologic studies have failed to show an association between breast augmentation and risk of lymphoma, however these studies were limited by the number of patients enrolled or by the insufficient time period of follow up (54-56). These studies underscore the difficulty of determining the incidence and prevalence of a very rare and recently recognized clinical entity. Large prospective outcomes studies of textured implants are rare. McGuire and colleagues reported four patients with BI-ALCL occurring in a prospective cohort of 17,656 patients with textured implants at a mean follow up of 3.4 years (57). de Jong and colleagues reported an individually matched case control study from the Netherlands nationwide pathology database (58). The authors found a positive association for the development of ALCL in women with breast implants compared to those without an implant with an odds ratio of 18.2 (95% confidence interval, 2.1–156.8). Based upon these data, the authors estimated an incidence of 0.1 to 0.3 per 100,000 BI-ALCL cases for women with prostheses per year. Another way of representing these numbers is that 1 in 500,000 women are at risk of developing the disease after 1 year. However, the disease on average occurs at 10 years and therefore at that time point, 1 in 50,000 women with breast implants are at risk of developing the disease.
Presentation and diagnosis of BI-ALCL
Two-thirds of BI-ALCL patients will present as a malignant effusion associated with the fibrous capsule surrounding an implant occurring on average 8 to 10 years after implantation. Any seroma occurring greater than 1 year after implantation not readily explainable by infection or trauma should be considered suspicious for disease (Figure 2). One third of patients present with a mass which may indicate a more aggressive clinical course. Adrada and colleagues reviewed 44 BI-ALCL patients with imaging studies and reported on the sensitivity/specificity for detecting an effusion using ultrasound (84%/75%), computerized tomography (55%/83%), magnetic resonance imaging (82%/33%), and positron emission tomography (38%/83%) (59). Additionally, the sensitivity/specificity to detect a BI-ALCL mass was reported for ultrasound (46%/100%), computerized tomography (50%/100%), magnetic resonance imaging (82%/33%), and positron emission tomography (64%/88%). The sensitivity of mammography was found to be inferior for BI-ALCL effusion and mass. Ultrasound is utilized at our institution as a screening tool for suspected cases and in combination with PET for confirmed cases to determine extension and for surveillance of disease (Figure 2).
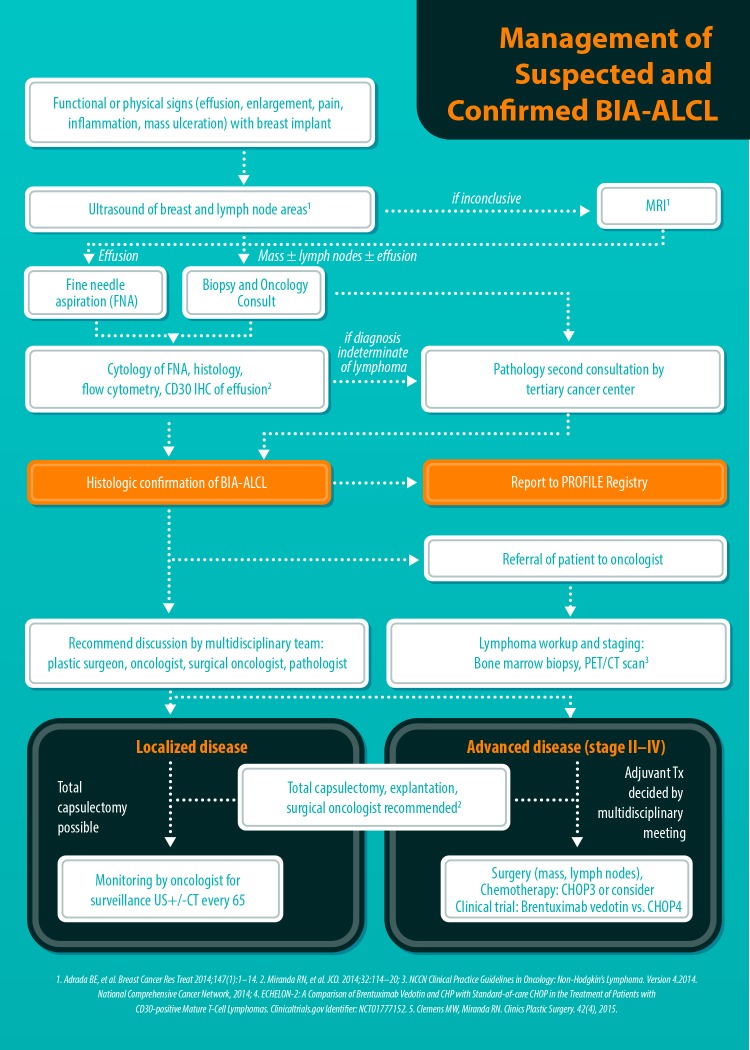
Figure 2.
Algorithmic approach to a patient with a delayed seroma and suspected BI-ALCL. This algorithm was developed at MD Anderson and subsequently adopted by the American Society of Plastic Surgeons (ASPS) and the American Society of Aesthetic Plastic Surgery (ASAPS). BI-ALCL, breast implant-associated anaplastic large-cell lymphoma. [Reprinted with permission from Clemens MW, Butler CE. ASPS/PSF Efforts on BIA-ALCL. Plastic Surgery News 2015;26(7).]
For suspected patients, any aspiration of periprosthetic fluid should be sent to pathology for cytologic evaluation and include a clinical history with the stated intent to “rule out BI-ALCL”. Pathologic evaluation may demonstrate BI-ALCL as individual cells, cell clusters in aggregates, or as cohesive sheets. Diagnosis using Wright-Giemsa or hematoxylin and eosin staining alone is usually insufficient, however BI-ALCL will demonstrate strong and uniform membranous expression of CD30 immunohistochemistry. Ultrasound may help define the extent of an effusion, and can be helpful in identifying any associated capsule masses. Clinical examination should include evaluation of regional lymph nodes. Volumes of an effusion can range from 50 to 1,000 mL and is typically more viscous than a benign seroma owing to the high protein content and cellularity. The surrounding capsule may be thickened and fibrous or may be deceptively unremarkable on gross examination consistent with the under appreciation of this lymphoma. If a mass is present, it can protrude into the implant creating a mass effect distortion on imaging, or the mass may protrude outward into the soft tissue.
Treatment and staging of BI-ALCL
Patients with biopsy-proven BI-ALCL must be referred to a lymphoma oncologist ideally prior to any surgical intervention to allow for proper oncologic evaluation. Surgical treatment of BI-ALCL requires complete tumor ablation, which includes removal of the implant, complete removal of any disease mass with negative margins, and total capsulectomy. Because an implant capsule may drain to multiple regional lymph node basins, there does not appear to be a role for sentinel lymph node biopsy in the treatment of BI-ALCL. Fine needle aspiration of enlarged lymph nodes can yield a false negative and therefore excisional biopsies should be performed of any suspicious lymph nodes. The involvement of a surgical oncologist in the management of this lymphoma is recommended. An incomplete resection or inadequate local surgical control may subject the patient recurrence, or to the need for adjunctive treatments such as chemotherapy and radiation therapy whereas complete resection may be the definitive treatment in the majority of cases. Surgery should be performed with strict oncologic technique including use of specimen orientation sutures placement of surgical clips within the tumor bed, and use of new instruments if performing a contralateral explantation (Figure 3). At this time, the FDA does not recommend screening or prophylactic implant removal for asymptomatic patients or patients with familial susceptibility cancer. All implant patients can have a minute amount of periprosthetic fluid which is normal and does not require screening (Figure 4). Although reimplantation with a textured implant is not recommended, several BI-ALCL patients have received implant replacement with a smooth implant following definitive treatment, and these patients are being closely monitored for any disease recurrence.
Figure 3.
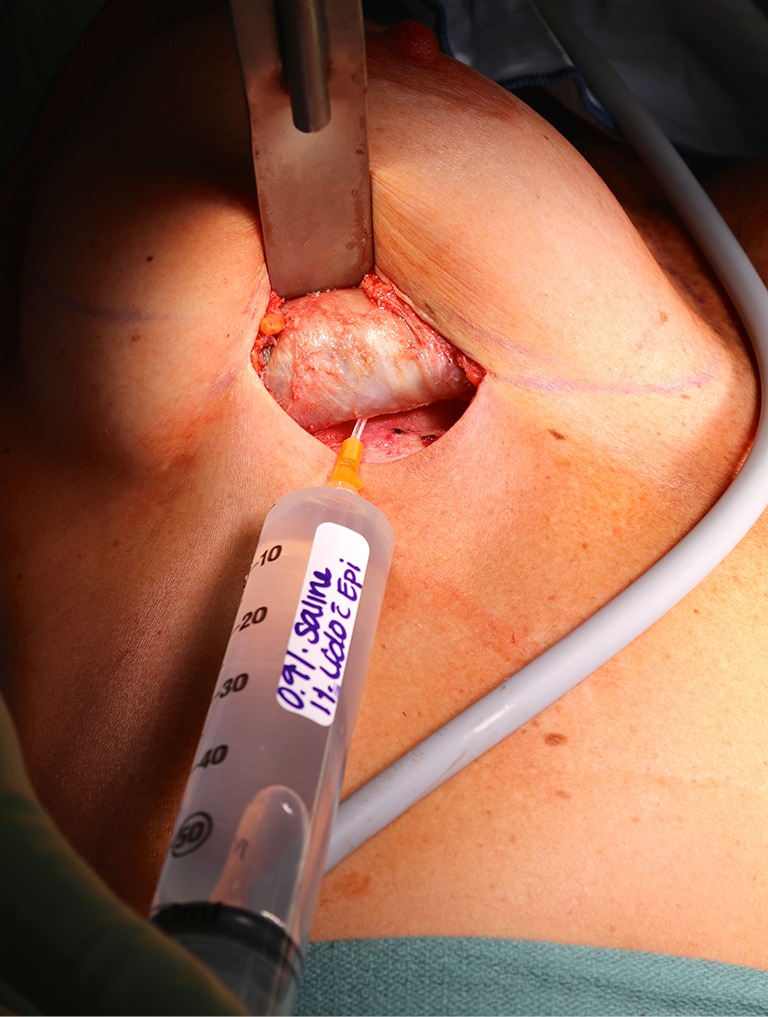
Complete capsulectomy. Treatment of BI-ALCL includes total capsulectomy with excision of any associated masses as residual disease left on the chest wall may continue to progress and require further treatment such as chemotherapy. With subpectoral implants, elevation of the posterior capsular wall off of the rib cage may be difficult but is still essential. Pictured is standard tumescence being infiltrated by angiocath into the posterior capsule of a BI-ALCL patient to facilitate complete removal of the capsules. BI-ALCL, breast implant-associated anaplastic large-cell lymphoma.
Figure 4.
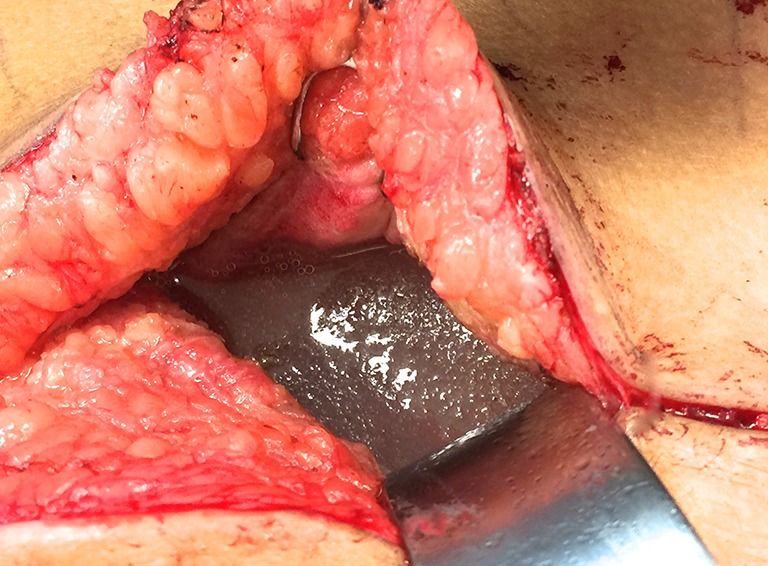
Normal periprosthetic fluid. All breast implant patients can have a minute amount of periprosthetic fluid which is normal. Small collections of fluid incidentally found on imaging or at an implant exchange in an asymptomatic patient do not require screening with CD30 immunohistochemistry.
Patients with BI-ALCL or any type of lymphoma are traditionally staged by the 1971 Ann Arbor Classification which stages disease based upon progression as a “liquid tumor” (60). Under this classification, nearly all BI-ALCL patients fall under one of two stages, either stage 1E (84%) or stage IIE (16%). Although most patients with BI-ALCL have a relatively indolent clinical course, reports of deaths attributable to disease emphasize the importance of a timely diagnosis and adequate treatment with appropriate surveillance or else disease progression can occur with detrimental effect. Patients with BI-ALCL who have died were described with local or regional extension of their disease, and very rare distant organ metastasis. This pattern of progression suggests that BI-ALCL is a distinct entity, and displays progression more similar to solid tumors than to other non-Hodgkin lymphomas. This pattern of disease spread is also better suited to a clinical and pathologic staging system modeled after the American Joint Committee on Cancer (AJCC) TNM (Tumor, Lymph node, Metastasis) system for staging solid tumors (61). A recent MD Anderson BI-ALCL TNM staging system seems to be more applicable than the Ann Arbor system for predicting prognosis and for evaluating treatment regimens in patients with BI-ALCL (62) (Figure 5,Table 2).
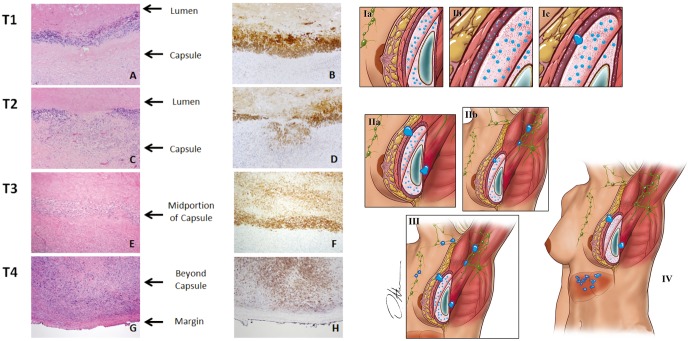
Figure 5.
BI-ALCL staging system: this TNM system was modeled after the American Joint Committee on Cancer (AJCC) TNM (tumor, lymph node, metastasis) staging system for solid tumors. T1: lymphoma cells confined to the effusion or a layer on the luminal side of the capsule (IA); T2: lymphoma cells superficially infiltrate the luminal side of the capsule (IB); T3: clusters or sheets of lymphoma cells infiltrate into the thickness of the capsule (IC); and T4: lymphoma cells infiltrating beyond the capsule (IIA), into the adjacent soft tissue or breast parenchyma (III, IV). (×100). (Reprinted with permission from Clemens MW, Medeiros LJ, Butler CE, et al. Complete Surgical Excision Is Essential for the Management of Patients With Breast Implant-Associated Anaplastic Large-Cell Lymphoma. J Clin Oncol 2016;34:160-8.)
Table 2. Clinicopathologic features of patients with breast implant-associated anaplastic large-cell lymphoma.
| Clinical feature | Patients with BI-ALCL |
|---|---|
| Age, years | |
| Median | 54 |
| Range | 28–87 |
| Laterality | |
| Right | 46 (52.9) |
| Left | 37 (42.5) |
| Bilateral | 4 (4.6) |
| Reason for initial implantation | |
| Cosmetic | 51 (58.6) |
| Breast cancer reconstruction | 36 (41.4) |
| Type of implant (n=81) | |
| Silicone | 40 (49.4) |
| Saline | 41 (50.6) |
| Texture of implant (n=48) | |
| Purely textured | 45 (93.7) |
| Purely smooth | 0 (0) |
| Both smooth/textured | 3 (2.3) |
| Interval to lymphoma diagnosis, years | |
| Median | 8 |
| Mean | 9.1 |
| Range | 2–25 |
| Clinical presentation | |
| Effusion only | 52 (59.8) |
| Mass only | 15 (17.2) |
| Effusion and mass | 17 (19.5) |
| No mass, no effusion | 3 (3.4) |
| T stage | |
| T1 | 31 (35.6) |
| T2 | 11 (12.6) |
| T3 | 14 (16.1) |
| T4 | 30 (34.5) |
| N stage | |
| 0 | 74 (85.1) |
| 1 | 13 (14.9) |
| Clinical feature | Patients with BI-ALCL |
| Ann Arbor stage at presentation | |
| IE | 74 (86.2) |
| IIE | 13 (13.8) |
| TNM stage at presentation | |
| IA | 31 (35.6) |
| IB | 10 (11.5) |
| IC | 12 (13.8) |
| IIA | 22 (25.3) |
| IIB | 4 (4.6) |
| III | 8 (9.2) |
| IV | 0 (0) |
| Chemotherapy (n=51) | |
| CHOP | 44 (86.3) |
| 3 cycles | 11 |
| 4 cycles | 2 |
| 6 cycles | 28 |
| NA | 3 |
| CHOEP | 11 (21.6) |
| 6 cycles | 11 |
| NS | 2 (3.9) |
| ABVD | 2 (3.9) |
| Hyper-CVAD | 1 (1.9) |
| Follow-up, months | |
| Median | 30 |
| Mean | 45 |
| Range | 3–217 |
Data are given as No. (%) unless otherwise noted. Abbreviations: ABVD, adriamycin, bleomycin, vinblastine, and dacarbazine; BI-ALCL, breast implant-associated anaplastic large-cell lymphoma; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; CHOEP, CHOP plus etoposide; hyper-CVAD course A, hyperfractionated; cyclophosphamide, vincristine, doxorubicin, and dexamethasone with course B: methotrexate and cytarabine; NA, not available; NS, chemotherapy not specified. (Reprinted with permission from Clemens MW, Medeiros LJ, Butler CE, et al. Complete Surgical Excision Is Essential for the Management of Patients With Breast Implant-Associated Anaplastic Large-Cell Lymphoma. J Clin Oncol 2016;34:160-8.)
Clinical characteristics and BI-ALCL outcomes
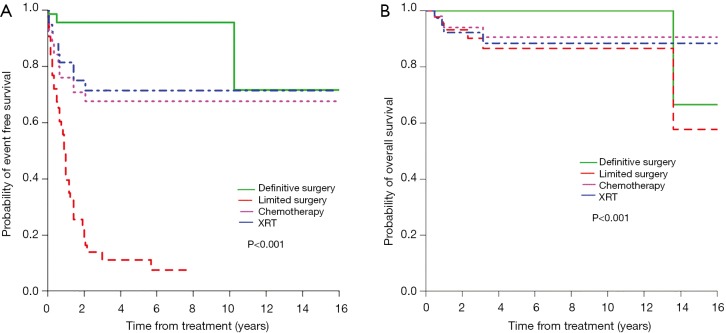
The clinico-pathologic features of BI-ALCL have been reported in several literature reviews. In 2014, Miranda et al. reviewed the long-term follow up of 60 BI-ALCL patients (52). The mean age was 52 years old (range, 28 to 87 years) with a median of 9 years (range, 1 to 32 years) between implantation and lymphoma diagnosis. Patients presented with either a malignant effusion improperly called seroma (70%) or a distinct mass (30%). The median overall survival (OS) was 12 years (median follow-up, 2 years; range, 0–14 years). A total capsulectomy with implant removal was performed in 93% of patients. OS and progression free survival (PFS) were similar between patients who received and did not receive chemotherapy (P=0.44 and P=0.28, respectively), suggesting that some patients may achieve optimal outcomes with an adequate surgical approach (Figure 6). Radiation therapy has also been used for local control of disease, and further research is required to determine specific indications for adjunctive treatments. Patients with a breast mass had a worse OS and PFS (P=0.052 and P=0.03, respectively). At this time, it is unclear whether the association of mass and worse prognosis indicates a more aggressive variant, more progressed disease, or perhaps a consequence of inadequate surgical ablation of tumor infiltration. Event free survival and overall survival by different treatment modalities are summarized in Figure 7.
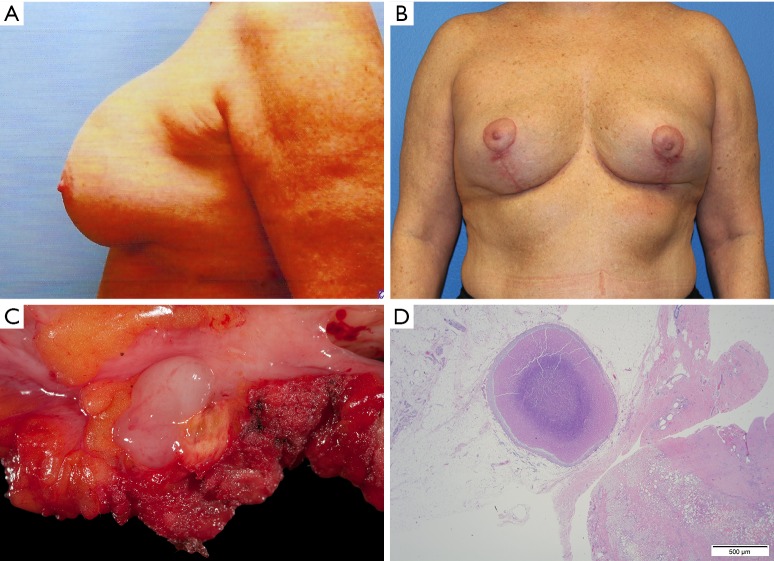
Figure 6.
Clinical image of a 52-year-old woman who received a cosmetic breast augmentation with textured silicone implants. Twenty years after implantation, she developed swelling of the left breast of recent onset which was aspirated multiple times. (A) Patient then received a partial capsulectomy, implant removal and mastopexy; (B) adequate tumor resection was questionable and despite being asymptomatic, the patient underwent a completion capsulectomy with excision of the posterior capsular wall; postoperative pathology again demonstrated persistent BI-ALCL disease of her chest wall and now with free margins; (C) tissue section demonstrates a nodule with compact tumor cells. Immunohistochemistry demonstrates that most tumor cells (D) which express CD30. Patient received no further adjunctive treatments and is currently 2 years disease free. BI-ALCL, breast implant-associated anaplastic large-cell lymphoma. (Reprinted with permission from Clemens MW, Miranda RN. Coming of Age: Breast Implant-Associated Anaplastic Large Cell Lymphoma After 18 Years of Investigation. Clin Plast Surg 2015;42:605-13.)
Figure 7.
Survival curves according to treatment approaches. Event-free survival (A), overall survival (B). (Reprinted with permission from Clemens MW, Medeiros LJ, Butler CE, et al. Complete Surgical Excision Is Essential for the Management of Patients With Breast Implant-Associated Anaplastic Large-Cell Lymphoma. J Clin Oncol 2016;34:160-8.)
Hart et al. performed a meta-analysis and identified 53 BI-ALCL patients (63). For patients with available clinical data, the authors noted rates of 17.3% extracapsular disease and 23.1% with the presence of a mass. 39.6% of patients were treated with surgery alone, 9.4% with surgery and radiation, 18.9% with surgery and chemotherapy, 30.2% with surgery, chemotherapy, and radiation, and 1.9% with chemotherapy alone. At a median follow up of 15 months (range, 3.6–90 months), disease recurrence was 28.3%, of which 73.3% were treated with salvage chemotherapy. BI-ALCL was attributed to four patient deaths. Extracapsular disease extension was associated with increased risk for recurrence (P<0.0001) and patient death (P=0.0008). This study also found a statistically significant difference in three and 5-year survival rates between patients presenting with and without a mass (P=0.0308 and P=0.0308, respectively). While BI-ALCL may follow a relatively indolent course in most patients, reports of disseminated cancer and deaths attributed to the disease emphasizes the importance of timely diagnosis and adequate treatment with appropriate surveillance.
Brody and colleagues recently reported a summary of 79 published patients and 94 previously unreported cases (64). The authors report that for all implants where surface characteristics were known, there was at least one textured device used within the patient’s surgical history. The authors reconfirm that there are no known pure smooth implant cases and importantly note that BI-ALCL has been reported in association with all major forms of implant texturing techniques from current implant manufacturers. Our institutional experience has been similar and is summarized in Table 3. We have noted most patients having slow disease progression and a good prognosis when adequately treated, but conceded occasional lymphadenopathy, metastases, and nine attributable deaths.
Table 3. Distribution BI-ALCL patients by Manufacturer. Note MD Anderson and USC reporting are worldwide whereas FDA MAUDE database is predominantly a United States catchment of cases.
| Institution | Manufacturer | N (%) |
|---|---|---|
| Worldwide cases by MD Anderson Tracking 2016 | Unknown | 87 (47.8) |
| Allergan/Inamed/McGhan | 76 (41.8) | |
| Mentor | 6 (3.3) | |
| Nagor | 3 (1.7) | |
| Eurosilicone | 1 (0.6) | |
| PIP | 5 (2.8) | |
| Sientra/Silimed | 3 (1.7) | |
| Bioplasty | 1 (0.6) | |
| Worldwide Cases Reported by USC 2015 | Unknown | 61 (35.0) |
| Allergan/Inamed/McGhan | 97 (56.0) | |
| Mentor | 3 (1.7) | |
| Nagor | 3 (1.7) | |
| Eurosilicone | 0 | |
| PIP | 5 (2.9) | |
| Sientra/Silimed | 1 (0.5) | |
| US MAUDE Database as of 2015 | Unknown | 22 (9.6) |
| Allergan/Inamed/McGhan | 184 (80.3) | |
| Mentor | 20 (8.7) | |
| CUI | 1 (0.4) | |
| Sientra | 1 (0.4) |
Future research is warranted to determine if certain patients have a predisposition to the development of the disease and if any modifiable risk factors exist either with the patient or type of implant utilized. Advances in the treatment of T cell lymphomas give promise for BI-ALCL refractory to surgical therapy alone. Brentuximab vedotin is a novel anti-CD30 monoclonal antibody that has improved the management of systemic ALCL with a reported objective response rate of 86% and complete remission rate of 59% in relapsed or refractory systemic ALCL in clinical trials (65,66). Prospective trials of BI-ALCL patients at major referral centers may help delineate chemotherapeutic sensitivity and efficacy of novel agents.
Informed consent and reporting
Following recommendations made by the FDA in 2011, implant manufacturers added language warning on the existence of BI-ALCL to breast implant package inserts within the United States and Canada. Breast implant informed consent examples that include the risk of BI-ALCL were subsequently produced by the American Society of Plastic Surgeons (ASPS) and are available for download from the www.plasticsurgery.org website. With respect to BI-ALCL, risk disclosure should have at least three basic objectives (67). The first objective is to make patients aware of the existence of this rare disease. The second objective is for patients to understand common presenting symptoms such as a breast mass or delayed-presentation seroma or effusion. The final objective is to compel patients to take action and follow up with a physician should these symptoms occur. Printed copies of a patient’s signed consent as well as procedure specific informational pamphlets at an initial consultation will enable patients to further digest surgical details at their leisure in their own homes, and engenders more effective recall.
Many resources exist for oncologists and surgeons treating a BI-ALCL patient. The Plastic Surgery Foundation paired the American Society of Plastic Surgeons and the US FDA to form the PROFILE registry (Patient Registry and Outcomes For breast Implants and anaplastic large cell Lymphoma etiology and Epidemiology). The FDA specifically recommends the reporting of confirmed cases to the PROFILE registry. The purpose of PROFILE is to increase the scientific data on BI-ALCL in women with breast implants as well as to support research to characterize BI-ALCL and to elucidate the exact role of breast implants in the etiology of the disease. In addition to providing plastic surgeons, oncologists, and patients with information they need about breast implants and treatment of BI-ALCL, confirmed cases in the registry will be available for analytical epidemiological studies. Treating physicians are encouraged to report confirmed cases to the PROFILE website which can be found at www.thepsf.org.
Benign delayed seromas
Benign delayed seromas are collections of serous fluid spontaneously occurring around a prosthesis more than 1 year after implantation. While delayed fluid collections are the most common presenting symptom for BI-ALCL patients, the mechanism is completely different as BI-ALCL develops a true malignant effusion due to the high protein content and cellularity of the fluid as well as the effect of interleukin production by the anaplastic cells resulting in local vascular leakage. It is important to differentiate these two distinct unrelated processes as there is a common misperception that they are on a continuum with one leading into the other. Benign fluid collections are not precursors to the development of BI-ALCL, and to date there has not been reported a patient with recurrent benign seromas that then converted to a CD30 positive effusion.
Benign late seromas most commonly follow blunt external trauma (i.e., Motor vehicle accident, closed capsulectomy) or low grade infection, however they can also occur in the absence of any discernible precipitating event (68-70) (Figure 8). Spear and colleagues reported a 5-year experience of 28 late seromas encountered in 25 patients (71). The authors found that 96 percent had a Biocell (Allergan) textured device in place at the time of seroma development. The authors did note that within the surgical practices reviewed, 51% of patients received a smooth implant and 49% of patients received a Biocell textured implant, the only type of texturing utlilized by the authors. This finding points to delayed seromas being primarily though not exclusively a textured implant sequelae. The late seromas in the series were managed with 53.6% complete capsulectomy, seroma drainage, and new implant exchange, 10.7% by seroma drainage and implant exchange, 7.1% by complete capsulectomy/seroma drainage with no implant replacement, 17.9% by only seroma aspiration alone, and 10.7% by antibiotic therapy alone. While all cultures were negative, it’s important to note that advanced biofilm detection techniques such as PCR pyrosequencing and scanning electron microscopy were not employed nor was routine CD30 immunohistochemistry testing to rule out BI-ALCL. It is recommended to test all delayed seromas not readily explainable with CD30 immunohistochemistry.
Figure 8.
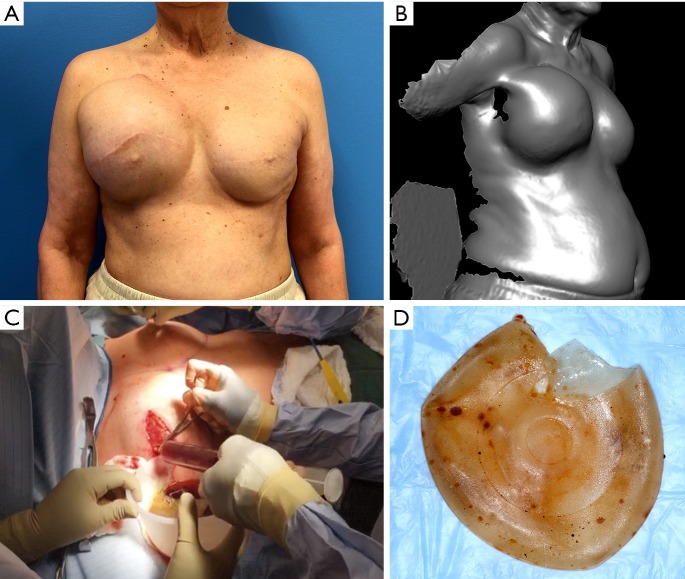
Delayed seroma. A 70-year-old female with history of right breast cancer status post bilateral mastectomy and Mentor textured implant reconstruction in 1992. Twenty-four years post implantation, patient developed a delayed seroma of the right breast. (preoperative A, 3-D imaging topography view B, intraoperative drainage C). Breast implant had no manufacturer markings but was confirmed based on review of original operative notes as well as negative imprint stamp texturing observed on implant. (explant D) Following total capsulectomy and implant exchange, patient had no further fluid collections or complications.
Maxwell and colleagues reported on the 10-year Core data of Allergan’s 410 implant in 941 patients and found a delayed seroma rate of 0.6% (72). The overwhelming majority of late seromas reported in case reports appear to be idiopathic. In a prospective multicenter trial of 17,656 textured implant patients, Mcguire and colleagues reported delayed seromas occurring in 26 of 17,656 subjects (0.15%) and 31 of 31,992 implanted devices (0.10%) (57). These late onset seroma rates are similar to those reported for smooth devices, and therefore texturing has not demonstrated an incremental risk of late seroma relative to other implants.Mazzocchi theorized that possible friction irritation from either a fold in the device or rubbing of a textured implant may be responsible in a minority of delayed seromas (73). A graduated management approach from conservative aspiration progressing to complete capsulectomy will eradicate the vast majority of delayed seromas. Considering many BI-ALCL patients have disease confined to the capsule, it is possible that some patients prior to widespread recognition of the disease were treated with capsulectomy without ever receiving a diagnosis. However, a blind approach is less than optimal when managing a malignancy.
Double capsules
Nearly all synthetic implantable devices including breast implants will form some varying degree of scar encapsulation composed of disorganized collagen and fibroblasts. The observation of double capsules has been described in limited series and case reports predominantly in textured implants (Figure 9). Findlay reported her experience of 14 patients with observed double capsules (74). All occurred in textured implants and specifically with Biocell texturing though Dr. Findlay did not report what type of texturing she prefers for augmentation mammaplasty in general. 50% of the patients were asymptomatic and were incidentally found to have double capsules during revisionary surgery. Five of the patients had capsular contracture though extra capsules do not seem to automatically lead to capsular contraction. A postulated theory of double capsules is that the initial adherence of a capsule to a textured implant surface becomes separated with minor trauma tearing away from the old capsule and developing a new capsule. The resulting rough surfaces may create a seroma in between the capsule planes because of shear forces. Smooth implants may not be as susceptible to reinjury phenomena just as polyurethane implants, because of a more robust tissue ingrowth of the capsule, are also less prone to these sequelae of implants. Anecdotally, we have seen double capsules as well as triple and quadruple capsules around textured breast implants (Figure 10). Collagen fibers exhibit a laminar deposition, sometimes demonstrating the characteristic imprint of the texture surface in between layers of the capsule likely representing an original capsule that either tore or lifted away due to a subclinical biofilm on the surface of the implant. The exact etiology of double capsules is unclear and likely multifactorial. The clinical consequence of a double capsule may be a delayed seroma, capsular contracture, or may be a nonentity as many patients are completely asymptomatic.
Figure 9.
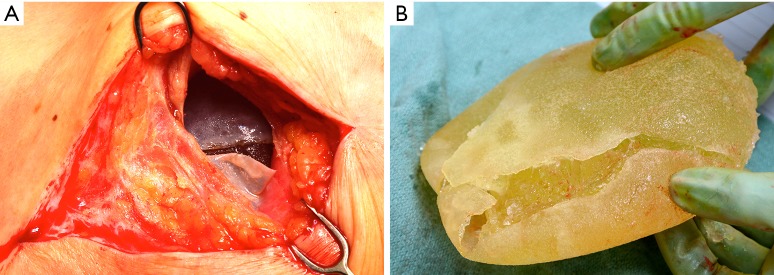
“Double capsule”. A 56-year-old female developed contour deformity of her reconstructed breast following blunt trauma to the chest in a motor vehicle accident. Patient delayed in addressing the deformity for 1 year. At explantation, multiple capsules were encountered (A) over the ruptured implant (B).
Figure 10.
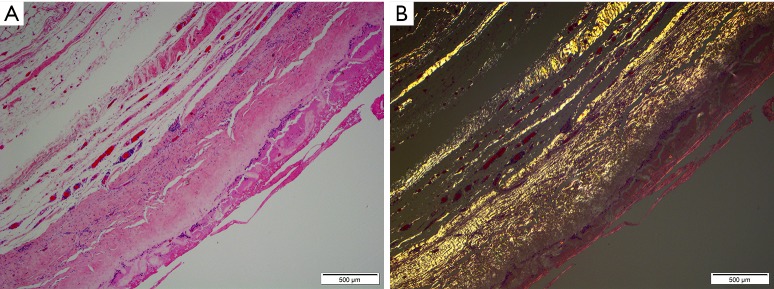
“Double capsule” histology. Haematoxylin and eosin staining of a breast implant capsule (A) and tissue polarization on birefringent lens (B) demonstrates laminar deposition of multiple layers of capsules having developed over two decades.
Conclusions
Unusual sequelae of breast implants such as BI-ALCL, benign delayed seromas, and double capsules remain rare. BI-ALCL is a rare lymphoma associated with breast implants though the exact etiology and pathogenesis remain unclear. Timely diagnosis of BI-ALCL and differentiation from benign seromas is dependent on appropriate testing with CD30 immunohistochemistry. The majority of BI-ALCL patients demonstrate a good prognosis with adequate surgical treatment. Textured implants are most commonly implicated in the development of these rare associations, and further research is critical for identification of risk factors and prevention of future cases.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.ISAPS global statistics 2016. Available online: www.isaps.org/Media/Default/global-statistics/2016%20ISAPS%20Results.pdf, website accessed July 1, 2016.
- 2.U.S. Food and Drug Administration. (2011). Anaplastic Large Cell Lymphoma (ALCL) In Women with Breast Implants: Preliminary FDA Findings and Analyses. Retrieved from www.fda.gov on November 20, 2014.
- 3.U.S. Food and Drug Administration. (2016). Anaplastic Large cell Lymphoma (ALCL) In Women with Breast Implants: Medical Device Reports of Breast Implants in Women with ALCL. Retrieved from www.fda.gov on July 1, 2016.
- 4.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Non-Hodgkin's Lymphomas. January 25, 2012. www.nccn.org, Website accessed August 1, 2015.
- 5.Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016;127:2375-90. 10.1182/blood-2016-01-643569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Report of the Advisory Group to Recommend Priorities for IARC Monographs during 2015–2019, April 18, 2014. Available online: http://monographs.iarc.fr, Website accessed August 1, 2015.
- 7.US National Cancer Institute. Treatment for health professionals. Available online: http://www.cancer.gov/, Website accessed August 1, 2015.
- 8.Institut National du Cancer. Agence Nationale de Sécurité du Médicament. Breast Implant Associated Anaplastic Large Cell Lymphoma: Expert Opinion, February 1, 2015.
- 9.French National Agency for Medicines and Health Product Safety (ANSM) update on BIA-ALCL. Available online: http://ansm.sante.fr/S-informer/Actualite/Lymphome-Anaplasique-a-Grandes-Cellules-associe-aux-implants-mammaires-LAGC-AIM-Point-sur-les-investigations-en-cours-Point-d-Information, accessed July 10, 2016.
- 10.National Cancer Institute. General Information About Adult Non-Hodgkin Lymphoma (NHL). Retrieved November 20, 2014.
- 11.SEER Data Fact Sheets: Non-Hodgkin Lymphoma. Available online: http://seer.cancer.gov/statfacts/html/nhl.html, accessed July 2015
- 12.A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin's lymphoma. The Non-Hodgkin's Lymphoma Classification Project. Blood 1997;89:3909-18. [PubMed] [Google Scholar]
- 13.Jacobsen E. Anaplastic large-cell lymphoma, T-/null-cell type. Oncologist 2006;11:831-40. 10.1634/theoncologist.11-7-831 [DOI] [PubMed] [Google Scholar]
- 14.Fisher RI, Gaynor ER, Dahlberg S, et al. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin's lymphoma. N Engl J Med 1993;328:1002-6. 10.1056/NEJM199304083281404 [DOI] [PubMed] [Google Scholar]
- 15.Savage KJ, Harris NL, Vose JM, et al. ALK- anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: report from the International Peripheral T-Cell Lymphoma Project. Blood 2008;111:5496-504. 10.1182/blood-2008-01-134270 [DOI] [PubMed] [Google Scholar]
- 16.Savage KJ, Chhanabhai M, Gascoyne RD, et al. Characterization of peripheral T-cell lymphomas in a single North American institution by the WHO classification. Ann Oncol 2004;15:1467-75. 10.1093/annonc/mdh392 [DOI] [PubMed] [Google Scholar]
- 17.Vose J, Armitage J, Weisenburger D, et al. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol 2008;26:4124-30. 10.1200/JCO.2008.16.4558 [DOI] [PubMed] [Google Scholar]
- 18.Cao YB, Wang SS, Huang HQ, et al. Primary breast lymphoma--a report of 27 cases with literature review. Ai Zheng 2007;26:84-9. [PubMed] [Google Scholar]
- 19.Gholam D, Bibeau F, El Weshi A, et al. Primary breast lymphoma. Leuk Lymphoma 2003;44:1173-8. 10.1080/1042819031000079195 [DOI] [PubMed] [Google Scholar]
- 20.Kim B, Roth C, Chung KC, et al. Anaplastic large cell lymphoma and breast implants: a systematic review. Plast Reconstr Surg 2011;127:2141-50. 10.1097/PRS.0b013e3182172418 [DOI] [PubMed] [Google Scholar]
- 21.Clemens MW, Miranda RN. Commentary on: Lymphomas Associated With Breast Implants: A Review of the Literature. Aesthet Surg J 2015;35:545-7. 10.1093/asj/sjv056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim B, Roth C, Young VL, et al. Anaplastic large cell lymphoma and breast implants: results from a structured expert consultation process. Plast Reconstr Surg 2011;128:629-39. 10.1097/PRS.0b013e31821f9f23 [DOI] [PubMed] [Google Scholar]
- 23.Yoshida SH, Swan S, Teuber SS, et al. Silicone breast implants: immunotoxic and epidemiologic issues. Life Sci 1995;56:1299-310. 10.1016/0024-3205(95)00081-X [DOI] [PubMed] [Google Scholar]
- 24.Ferreri AJ, Govi S, Pileri SA, et al. Anaplastic large cell lymphoma, ALK-negative. Crit Rev Oncol Hematol 2013;85:206-15. 10.1016/j.critrevonc.2012.06.004 [DOI] [PubMed] [Google Scholar]
- 25.Bizjak M, Selmi C, Praprotnik S, et al. Silicone implants and lymphoma: The role of inflammation. J Autoimmun 2015;65:64-73. 10.1016/j.jaut.2015.08.009 [DOI] [PubMed] [Google Scholar]
- 26.Hu H, Jacombs A, Vickery K, et al. Chronic biofilm infection in breast implants is associated with an increased T-cell lymphocytic infiltrate: implications for breast implant-associated lymphoma. Plast Reconstr Surg 2015;135:319-29. 10.1097/PRS.0000000000000886 [DOI] [PubMed] [Google Scholar]
- 27.Kadin ME, Deva A, Xu H, et al. Biomarkers Provide Clues to Early Events in the Pathogenesis of Breast Implant-Associated Anaplastic Large Cell Lymphoma. Aesthet Surg J 2016;36:773-81. 10.1093/asj/sjw023 [DOI] [PubMed] [Google Scholar]
- 28.Deva AK, Adams WP, Jr, Vickery K. The role of bacterial biofilms in device-associated infection. Plast Reconstr Surg 2013;132:1319-28. 10.1097/PRS.0b013e3182a3c105 [DOI] [PubMed] [Google Scholar]
- 29.Gaudet G, Friedberg JW, Weng A, et al. Breast lymphoma associated with breast implants: two case-reports and a review of the literature. Leuk Lymphoma 2002;43:115-9. 10.1080/10428190210189 [DOI] [PubMed] [Google Scholar]
- 30.Alobeid B, Sevilla DW, El-Tamer MB, et al. Aggressive presentation of breast implant-associated ALK-1 negative anaplastic large cell lymphoma with bilateral axillary lymph node involvement. Leuk Lymphoma 2009;50:831-3. 10.1080/10428190902795527 [DOI] [PubMed] [Google Scholar]
- 31.Miranda RN, Lin L, Talwalkar SS, et al. Anaplastic large cell lymphoma involving the breast: a clinicopathologic study of 6 cases and review of the literature. Arch Pathol Lab Med 2009;133:1383-90. [DOI] [PubMed] [Google Scholar]
- 32.Li S, Lee AK. Silicone implant and primary breast ALK1-negative anaplastic large cell lymphoma, fact or fiction? Int J Clin Exp Pathol 2009;3:117-27. [PMC free article] [PubMed] [Google Scholar]
- 33.Farkash EA, Ferry JA, Harris NL, et al. Rare lymphoid malignancies of the breast: a report of two cases illustrating potential diagnostic pitfalls. J Hematop 2009;2:237-44. 10.1007/s12308-009-0043-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bishara MR, Ross C, Sur M. Primary anaplastic large cell lymphoma of the breast arising in reconstruction mammoplasty capsule of saline filled breast implant after radical mastectomy for breast cancer: an unusual case presentation. Diagn Pathol 2009;4:11. 10.1186/1746-1596-4-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lazzeri D, Agostini T, Giannotti G, et al. Null-type anaplastic lymphoma kinase-negative anaplastic large cell lymphoma arising in a silicone breast implant capsule. Plast Reconstr Surg 2011;127:159e-62e. 10.1097/PRS.0b013e318213a1bd [DOI] [PubMed] [Google Scholar]
- 36.Talwalkar SS, Miranda RN, Valbuena JR, et al. Lymphomas involving the breast: a study of 106 cases comparing localized and disseminated neoplasms. Am J Surg Pathol 2008;32:1299-309. 10.1097/PAS.0b013e318165eb50 [DOI] [PubMed] [Google Scholar]
- 37.Lechner MG, Lade S, Liebertz DJ, et al. Breast implant-associated, ALK-negative, T-cell, anaplastic, large-cell lymphoma: establishment and characterization of a model cell line (TLBR-1) for this newly emerging clinical entity. Cancer 2011;117:1478-89. 10.1002/cncr.25654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carty MJ, Pribaz JJ, Antin JH, et al. A patient death attributable to implant-related primary anaplastic large cell lymphoma of the breast. Plast Reconstr Surg 2011;128:112e-118e. 10.1097/PRS.0b013e318221db96 [DOI] [PubMed] [Google Scholar]
- 39.Aladily TN, Medeiros LJ, Amin MB, et al. Anaplastic large cell lymphoma associated with breast implants: a report of 13 cases. Am J Surg Pathol 2012;36:1000-8. 10.1097/PAS.0b013e31825749b1 [DOI] [PubMed] [Google Scholar]
- 40.Lazzeri D, Zhang YX, Huemer GM, et al. Capsular contracture as a further presenting symptom of implant-related anaplastic large cell lymphoma. Am J Surg Pathol 2012;36:1735-6; author reply 1736-8. [DOI] [PubMed]
- 41.Bautista-Quach MA, Nademanee A, Weisenburger DD, et al. Implant-associated primary anaplastic large-cell lymphoma with simultaneous involvement of bilateral breast capsules. Clin Breast Cancer 2013;13:492-5. 10.1016/j.clbc.2013.08.009 [DOI] [PubMed] [Google Scholar]
- 42.Farace F, Bulla A, Marongiu F, et al. Anaplastic large cell lymphoma of the breast arising around mammary implant capsule: an Italian report. Aesthetic Plast Surg 2013;37:567-71. 10.1007/s00266-013-0120-6 [DOI] [PubMed] [Google Scholar]
- 43.Ivaldi C, Perchenet AS, Jallut Y, et al. Two cases of lymphoma in an implant capsule: A difficult diagnosis, an unknown pathology. Ann Chir Plast Esthet 2013;58:688-93. 10.1016/j.anplas.2013.04.003 [DOI] [PubMed] [Google Scholar]
- 44.Parthasarathy M, Orrell J, Mortimer C, et al. Chemotherapy-resistant breast implant-associated anaplastic large cell lymphoma. BMJ Case Rep 2013;2013. pii: bcr2013201950. [DOI] [PMC free article] [PubMed]
- 45.Thompson PA, Prince HM. Breast implant-associated anaplastic large cell lymphoma: a systematic review of the literature and mini-meta analysis. Curr Hematol Malig Rep 2013;8:196-210. 10.1007/s11899-013-0164-3 [DOI] [PubMed] [Google Scholar]
- 46.Taylor KO, Webster HR, Prince HM. Anaplastic large cell lymphoma and breast implants: five Australian cases. Plast Reconstr Surg 2012;129:610e-7e. 10.1097/PRS.0b013e3182450aae [DOI] [PubMed] [Google Scholar]
- 47.George EV, Pharm J, Houston C, et al. Breast implant-associated ALK-negative anaplastic large cell lymphoma: a case report and discussion of possible pathogenesis. Int J Clin Exp Pathol 2013;6:1631-42. [PMC free article] [PubMed] [Google Scholar]
- 48.Sørensen K, Murphy J, Lennard A, et al. Anaplastic large cell lymphoma in a reconstructed breast using a silicone implant: a UK case report. J Plast Reconstr Aesthet Surg 2014;67:561-3. 10.1016/j.bjps.2013.09.015 [DOI] [PubMed] [Google Scholar]
- 49.Smith TJ, Ramsaroop R. Breast implant related anaplastic large cell lymphoma presenting as late onset peri-implant effusion. Breast 2012;21:102-4. 10.1016/j.breast.2011.07.007 [DOI] [PubMed] [Google Scholar]
- 50.Olack B, Gupta R, Brooks GS. Anaplastic large cell lymphoma arising in a saline breast implant capsule after tissue expander breast reconstruction. Ann Plast Surg 2007;59:56-7. 10.1097/SAP.0b013e31804d442e [DOI] [PubMed] [Google Scholar]
- 51.Newman MK, Zemmel NJ, Bandak AZ, et al. Primary breast lymphoma in a patient with silicone breast implants: a case report and review of the literature. J Plast Reconstr Aesthet Surg 2008;61:822-5. 10.1016/j.bjps.2007.03.027 [DOI] [PubMed] [Google Scholar]
- 52.Miranda RN, Aladily TN, Prince HM, et al. Breast implant-associated anaplastic large-cell lymphoma: long-term follow-up of 60 patients. J Clin Oncol 2014;32:114-20. 10.1200/JCO.2013.52.7911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hart AM, Lechowicz MJ, Peters KK, et al. Breast Implant-Associated Anaplastic Large Cell Lymphoma: Report of 2 Cases and Review of the Literature. Aesthet Surg J 2014;34:884-94. 10.1177/1090820X14539503 [DOI] [PubMed] [Google Scholar]
- 54.Largent J, Oefelein M, Kaplan HM, et al. Risk of lymphoma in women with breast implants: analysis of clinical studies. Eur J Cancer Prev 2012;21:274-80. 10.1097/CEJ.0b013e328350b0ae [DOI] [PubMed] [Google Scholar]
- 55.Brinton LA. The relationship of silicone breast implants and cancer at other sites. Plast Reconstr Surg 2007;120:94S-102S. 10.1097/01.prs.0000286573.72187.6e [DOI] [PubMed] [Google Scholar]
- 56.Lipworth L, Tarone RE, McLaughlin JK. Breast implants and lymphoma risk: a review of the epidemiologic evidence through 2008. Plast Reconstr Surg 2009;123:790-3. 10.1097/PRS.0b013e318199edeb [DOI] [PubMed] [Google Scholar]
- 57.McGuire P, Reisman NR, Murphy DK. Risk Factor Analysis for Capsular Contracture, Malposition, and Late Seroma in Subjects Receiving Natrelle 410 Form-Stable Silicone Breast Implants. Plast Reconstr Surg 2017;139:1-9. 10.1097/PRS.0000000000002837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.de Jong D, Vasmel WL, de Boer JP, et al. Anaplastic large-cell lymphoma in women with breast implants. JAMA 2008;300:2030-5. 10.1001/jama.2008.585 [DOI] [PubMed] [Google Scholar]
- 59.Adrada BE, Miranda RN, Rauch GM, et al. Breast implant-associated anaplastic large cell lymphoma: sensitivity, specificity, and findings of imaging studies in 44 patients. Breast Cancer Res Treat 2014;147:1-14. 10.1007/s10549-014-3034-3 [DOI] [PubMed] [Google Scholar]
- 60.Carbone PP, Kaplan HS, Musshoff K, et al. Report of the Committee on Hodgkin's Disease Staging Classification. Cancer Res 1971;31:1860-1. [PubMed] [Google Scholar]
- 61.Sobin LH, Gospodarowicz MK, Wittekind C. editors. The TNM classification of malignant tumours 7th edition. Hoboken: New Jersey, Wiley-Blackwell, 2009. [Google Scholar]
- 62.Clemens MW, Medeiros LJ, Butler CE, et al. Complete Surgical Excision Is Essential for the Management of Patients With Breast Implant-Associated Anaplastic Large-Cell Lymphoma. J Clin Oncol 2016;34:160-8. 10.1200/JCO.2015.63.3412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hart A, Lechowicz MJ. Breast Implant-Associated Anaplastic Large Cell Lymphoma: Treatment Experience In 53 Patients. Blood 2013;122:5089. [Google Scholar]
- 64.Brody GS, Deapen D, Taylor CR, et al. Anaplastic large cell lymphoma occurring in women with breast implants: analysis of 173 cases. Plast Reconstr Surg 2015;135:695-705. 10.1097/PRS.0000000000001033 [DOI] [PubMed] [Google Scholar]
- 65.Younes A, Bartlett NL, Leonard JP, et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med 2010;363:1812-21. 10.1056/NEJMoa1002965 [DOI] [PubMed] [Google Scholar]
- 66.Pro B, Advani R, Brice P, et al. Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: results of a phase II study. J Clin Oncol 2012;30:2190-6. 10.1200/JCO.2011.38.0402 [DOI] [PubMed] [Google Scholar]
- 67.Clemens MW, Miranda RN, Butler CE. Breast Implant Informed Consent Should Include the Risk of Anaplastic Large Cell Lymphoma. Plast Reconstr Surg 2016;137:1117-22. 10.1097/01.prs.0000481103.45976.b1 [DOI] [PubMed] [Google Scholar]
- 68.Pajkos A, Deva AK, Vickery K, et al. Detection of subclinical infection in significant breast implant capsules. Plast Reconstr Surg 2003;111:1605-11. 10.1097/01.PRS.0000054768.14922.44 [DOI] [PubMed] [Google Scholar]
- 69.Wuest WL. Breast implant seroma in pregnancy. Br J Plast Surg 1992;45:328. 10.1016/0007-1226(92)90065-6 [DOI] [PubMed] [Google Scholar]
- 70.Hasham S, Akhtar S, Fourie LR. Persistent seroma following breast prosthesis explantation: a case report and review. Eur J Plast Surg 2006;28:490-3. 10.1007/s00238-005-0011-4 [DOI] [Google Scholar]
- 71.Spear SL, Rottman SJ, Glicksman C, et al. Late seromas after breast implants: theory and practice. Plast Reconstr Surg 2012;130:423-35. 10.1097/PRS.0b013e3182589ea9 [DOI] [PubMed] [Google Scholar]
- 72.Maxwell GP, Van Natta BW, Bengtson BP, et al. Ten-year results from the Natrelle 410 anatomical form-stable silicone breast implant core study. Aesthet Surg J 2015;35:145-55. 10.1093/asj/sju084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mazzocchi M, Dessy LA, Corrias F, et al. A clinical study of late seroma in breast implantation surgery. Aesthetic Plast Surg 2012;36:97-104. 10.1007/s00266-011-9755-3 [DOI] [PubMed] [Google Scholar]
- 74.Hall-Findlay EJ. Breast implant complication review: double capsules and late seromas. Plast Reconstr Surg 2011;127:56-66. 10.1097/PRS.0b013e3181fad34d [DOI] [PubMed] [Google Scholar]