Abstract
The monoamine serotonin (5-HT), a well known neurotransmitter, is also important in peripheral tissues. Several studies have suggested that 5-HT is involved in bone metabolism. Starting from our original observation of increased 5-HT2B receptor (5-HT2BR) expression during in-vitro osteoblast differentiation, we investigated a putative bone phenotype in vivo in 5-HT2BR knockout mice. Interestingly, 5-HT2BR mutant female mice displayed reduced bone density that was significant from age 4 months, and had intensified by 12 and 18 months. This histomorphometrically-confirmed osteopenia seems to be due to reduced bone formation since (1) the CFU-FALP+ capacity of bone marrow precursors was markedly reduced in the 5-HT2B receptor mutant mice from 4 to 12 months of age, (2) ex-vivo primary osteoblasts from mutant mice exhibited reduced proliferation and delayed differentiation, and (3) calcium incorporation was markedly reduced in osteoblasts after 5-HT2BR depletion (produced genetically or by pharmacological inactivation). These findings support the hypothesis that the 5-HT2BR receptor facilitates osteoblast recruitment and proliferation, and that its absence leads to osteopenia that worsens with age. We show here, for the first time, that the 5-HT2B receptor is a physiological mediator of 5-HT in bone formation and, potentially, in the onset of osteoporosis in aging women.
Keywords: Animals; Mice, Knockout; Aging; Bone Density; Bone Diseases, Metabolic; Cell Differentiation; Cell Proliferation; Cells, Cultured; Female; Gene Expression Regulation; Mice; Osteoblasts; Receptors, Serotonin
Introduction
Osteoporosis is a disorder responsible for fragility fractures and is caused by an imbalance between bone formation and resorption, both of which are controlled by both systemic and local factors (1). There is considerable current interest in neurohormonal regulators of bone metabolism, which have been shown to influence both the formation and resorption of bone. Bone cells can express receptors for VIP (2), CGRP (3), catecholamines (4) and glutamate (5) and leptin has been reported recently to control bone formation via a central mechanism (6), implying the involvement of the sympathetic nervous system and CART, which have been shown to decrease bone formation dramatically (7).
The monoamine serotonin (5-hydroxytryptamine, or 5-HT) has mainly been investigated as a neurotransmitter principally involved in mood control. However, it also mediates a wide range of peripheral functions. 5-HT is synthesized by a two-step pathway in which tryptophan hydroxylase is the rate-limiting enzyme. Circulating 5-HT is principally stored in platelet dense granules. Extra-cellular levels of 5-HT are determined by the 5-HT transporter (5-HTT) (8). The diversity of actions of 5-HT results from the existence of multiple 5-HT receptors (5-HTRs), which have been divided into seven classes (5-HT1R to 5-HT7R) on the basis of their signaling pathway (9). The main targets of 5-HT in peripheral tissues are the cardiovascular system, the gastrointestinal tract, and platelets (8).
Several studies suggest that 5-HT is also involved in bone metabolism (10). For instance, 5-HTT has been detected in osteoblasts, and its inactivation leads to reduced bone accrual (11, 12). Expression of various combinations of 5-HT1 (5-HT1A and/or 5-HT1D) and 5-HT2 (5-HT2A and/or 5-HT2B and/or 5-HT2C) receptors have been reported in vitro in chicken, rat and mouse osteoblastic cell lines and primary osteoblast cultures, but their functional relationships to bone physiopathology have not been clearly established (11, 13–15).
During mouse embryogenesis, 5-HT appears to regulate epithelial/mesenchymal interactions during craniofacial development (16, 17). 5-HT2BR seems to be particularly important in mediating the effects of 5-HT on embryonic morphogenesis (18), and is involved during osteogenesis in an inducible mesoblastic murine cell line (19).
We report here that during osteoblast differentiation, only 5-HT1A, 5-HT2A and 5-HT2BRs are expressed, and that only 5-HT2BRs expression is increased. We also show that targeted inactivation of the 5-HT2BR gene in mice (5-HT2BR −/−) leads to osteopenia and reduced bone formation in aging mice, and that osteoblast proliferation and recruitment is reduced in 5-HT2BR depleted primary cultures. The present study shows that 5-HT2BR plays a major role in bone formation.
Materiel and methods
Chemicals
Alpha-Minimal essential medium (α-MEM) supplemented with L-glutamine (Invitrogen, Cergy-Pontoise, France), penicillin-streptomycin suspension (Invitrogen) and 10% fetal calf serum (FCS) (Sigma-Aldrich, St Quentin Fallavier, France) depleted of 5-HT (20) was used. Acid ascorbic, β-glycerophosphate, ritanserin, 5-HT binoxalate and BW 723C86 (1-[5(2-thienylmethoxy)-1H-3-indolyl]propan-2-amine hydrochloride) were purchased from Sigma-Aldrich. [45Ca]2+ (specific activity, 1.85 GBq/mg) was from GE Healthcare (Orsay, France). The radioligands (Perkin Elmer Life and Analytical Sciences (Boston, MA) or GE Healthcare) [3H]-8-OH-DPAT ((±)-8-hydroxy-2-(di-n-propylamino)tetralin, specific activity, 4.74 TBq/mmol), [125I]-DOI ((±)-2,5-dimethoxy-4-iodoamphetamine hydrochloride, specific activity, 81.4 TBq/mmol), [125I]-GTI (5-hydroxytryptamine-5-O-carboxymethylglycyltyrosinamide, specific activity, 72.9 TBq/mmol), [3H]-5-HT binoxalate (specific activity, 845 GBq/mmol), [3H]-LY 278584 (1-methyl-N-(8-methyl-8-azabicyclo[3.2.1]oct-3-yl)1H-indazole-3-carboxamide, specific activity, 2.31 TBq/mmol), [125I]-SB 207710 (1-butyl-4-piperidinylmethyl-8-amino-7-iodo-1,4-benzodioxan-5-carboxylate, specific activity, 21.2 TBq/mmol), [3H]-LSD (lysergic acid diethylamide, specific activity, 2.23 TBq/mmol), [3H]-MDL 100907 ((±)2,3-dimethoxyphenyl-1-[2-(4-piperidine)-methanol], specific activity, 2.63 TBq/mmol), [3H]-LY 266097 (1-(2-chloro-3,4-dimethoxybenzyl)-6-methyl-1,2,3,4-tetrahydro-9Hpyrido[3,4-b]indole hydrochloride, specific activity, 925 GBq/mmol), [3H]-mesulergine (specific activity, 2.83TBq/mmol), [3H]-SB 258585 (4-iodo-N-[4-methoxy-3-(4-methyl-piperazin-1-yl)-phenyl]-benzenesulfonamide, specific activity, 2.32 TBq/mmol) and [3H]-SB 269970: (R)-3-(2-(2-(4-methylpiperidin-1-yl)ethyl)pyrrolidine-1-sulfonyl)phenol, specific activity, 2.33 TBq/mmol) were used to assess the presence of 5-HTRs. RS-127445 (2-amino-4-(4-fluoronaphtyl-1-yl)-6-isopropyl-pyrimidine) and [3H]-LY 266097 were generous gifts of Drs Mc Namara (Syntex, Palo-Alto, USA) and Würch (Roche, Basel, Switzerland). All other chemicals were of the purest grade available and were from usual commercial sources.
Animal model
This study was carried out using the 5-HT2BR knockout mouse model in which the exon 2 of the 5-HT2BR locus had been substituted for the selective bacterial neo cassette (21). Genotyping has been described previously (21). In the mutant mice population, one third of embryonic mice die mid-gestation due to trabecular defects in heart, and one third die at birth from cardiac failure. The mice that survive have a cardiac phenotype, but a normal life span (21). The 5-HT2BR knockout mice are a pure 129sv/PAS background, and wild-type (WT) 129sv/PAS background mice used as controls were purchased from the Charles River Laboratory (L’arbresle, France). The mice were examined when aged 5 weeks, 10 weeks, 4 months, 12 months and 18 months. Urine samples were collected individually 3 days before sacrifice. Mice were weighed and anesthetized by intraperitoneal injection of ketamine (45 mg/kg) and xylazine (5 mg/kg) (Sigma-Aldrich, St Quentin Fallavier, France). Blood plasma (sodium heparinate) samples were collected by eye puncture. The sacrifice was realized under anesthesia by cervical dislocation, and the femurs were harvested for histomorphometry and the tibia for 3D micro-computed tomography (CT) analysis. The animals were allowed free access to food and water in full compliance with French Government and European community animal welfare policy.
Radiography, Dual-energy X-ray absorptiometry (DEXA) and Micro-CT analysis
Femurs were analyzed by contact radiography using an X-ray cabinet (Faxitron Xray Corp., Wheeling, IL, USA). 5-HT2BR−/− and WT mice at different ages were weighed (g), anesthetized and their BMD (g/cm2) determined by DEXA, using a PIXImus II Densitometer, (Lunar, GE Healthcare, Lambesc, France). 3D micro-CT analysis was performed at the tibial metaphysis using a 3D micro-CT scanner (Scanco Medical, Bassersdorf, Switzerland) as previously described (22).
Bone histomorphometry
To evaluate the dynamic bone formation parameters by histomorphometry, skeletons were doubly labeled by tetracycline and calcein as previously described. Before sacrifice, first a tetracycline injection was administered (20 mg/kg, Pfizer, Amboise, France), followed by a second injection of calcein (20 mg/kg, Sigma-Aldrich) 5 days later for the 4 month-old mice or 6 days later for the 18 month-old mice. Female mice were killed 24 h after the second injection. For cortical and trabecular histomorphometry, the left femurs from 4-and 18 month-old mice were dissected and stored in 70 % ethanol. They were dehydrated in ascending alcohol concentrations, defatted in xylene, and then embedded in methyl methacrylate. All histomorphometric analyses were performed according to the guidelines of American Society of Bone and Mineral Research histomorphometry nomenclature committee (23), as previously described (24).
Biochemical analysis
To quantify osteoclastic bone resorption, urinary samples were collected from 4-month and 18-month old female mice in the morning to measure the level of deoxypyridinoline cross-linkage using a chemiluminescent assay on an Immulite 2000 automated analyzer (DPC Siemens Medical Solutions Diagnostics, La Garenne-Colombes, France). Animals were fasted overnight and urine specimens were collected during a 2–3 hour-period. Values are reported relative to creatinine concentrations as determined by a standardized colorimetric assay using alkaline picrate with Advia 2400 automat (Siemens Medical Solutions Diagnostics, Puteaux, France). To quantify osteoblast formation, osteocalcin (mouse osteocalcin IRMA Kit, Immunotopics, San Clemente, California, USA) and alkaline phosphatase (ALP) activity (Advia 2400 automated analyzer, Siemens Medical Solutions Diagnostics) were determined colorimetrically in the plasma (sodium heparinate) of 4-and 18 month-old female mice (at least ten mice for each group).
Calvarial osteoblast primary culture
Primary osteoblasts were enzymatically isolated from calvariae of neonatal (2–3 day old) WT and transgenic mice as previously described. Briefly, calvariae were dissected aseptically, and sequentially digested for 70 minutes in a phosphate-buffered saline (PBS) collagenase solution containing 0.2% collagenase IV (Sigma-Aldrich) and 0.01% deoxyribonuclease (Sigma-Aldrich) at 37 °C. Calvarial cells were collected by centrifuging, and then washed and plated at 106 cells per 75 cm2 flasks. Cells were expanded for 5 days in α-MEM supplemented with 10% FCS depleted of 5-HT, 2 mM glutamine, 100 U/ml penicillin and 100μg/ml streptomycin. Cells were harvested with trypsin/EDTA (Invitrogen), and plated at a density of 105 cells/cm2 in the differentiation medium (50 μg/ml ascorbic acid with 10 mM β-glycerophosphate [β-GP]). To determine the ALP activity, cells were washed twice in ice-cold phosphate buffer saline (PBS), and then stored in purified water at −80°C till analysis. ALP activity in the lysate was measured as previously described for plasma. Total intracellular protein contents were measured using the BCA protein assay reagent kit (Pierce Perbio Science France, Brebières, France), and ALP activity was normalized in terms of protein content. The capacity of the osteoblasts to mineralize was assessed by alizarin staining on days 13 and 21. The number of mineralized nodules was measured using an image analyzer (Microvision, Les Ullis, France).
Radioligand Binding assays
To prepare crude membranes for radioligand binding assays essentially as described (25), calvarial osteoblast cells were washed twice in ice-cold phosphate buffer saline (PBS), then scraped off the plate into 1.5 ml PBS containing 4 mM EDTA, 1 mM EGTA, 10 mM imidazole, pH 7.30 supplemented with a set of protease inhibitors completeTM (Roche Molecular Biochemicals, Meylan, France) (buffer 1), and then treated with 1 mg/ml collagenase and 4 mM EDTA. Samples were centrifuged and pellets frozen at -70°C. Frozen cell pellets were thawed at 4°C, homogenized and re-suspended in 10 ml of buffer 1. Extracts were centrifuged for 10 min at 5,000 × g. Supernatants were collected, poured onto a 20% sucrose cushion, and then centrifuged for 90min at 100,000 g. The resulting membrane-containing pellets were resuspended in 75 mM KCl, 5 mM MgCl2, 1 mM EGTA, 10 mM imidazole buffer, pH 7.30, for use in binding assays. Protein concentrations were determined by BCA protein assay reagent kit (cf. above).
RT-PCR, semi-quantitative and quantitative PCR (qPCR)
Total RNA from calvarial osteoblasts was isolated using the Mini kit QIAGEN RNeasy (Qiagen, Courtaboeuf, France) according to the Manufacturer’s procedures using DNase. One μg of each RNA was converted into cDNA using the High Capacity cDNA Archive Kit (Applied Biosystems, Courtaboeuf, France), and amplifications were performed using standard protocols in a GeneAmp PCR 7500 (Applied Biosystems).
Quantitative real-time PCR expression analysis was performed using a light cycler Roche, ABsolute SYBR™Green Capillary Mix (Abgene, Courtaboeuf, France). The primers with the FAM-labeled TaqMan probe of 5-HT1AR, 5-HT2AR, 5-HT2BR gene and GAPDH for normalization (Assays-on-Demand TM, Applied Biosystems) were used. The sets of primer used for the osteoblast differentiation markers (Runx-2, ALP, type I collagen and osteocalcin) were: Collagen type 1 (forward: 5′-CTTGGTGGTTTTGTATTCGATGAC-3′; reverse: 5′-GCGAAGGAACAGTCGCT-3′); ALP (forward: 5′-AAGGCTTCTTCTTGCTGGTG-3′; reverse: 5′-GCCTTACCCTCATGATGTCC-3′); osteocalcin (forward: 5′-CTCACAGATGCCAAGCCCA-3′; reverse: 5′-CCAAGGTAGCGCCGGAGTCT-3′); Endogenous Runx2 (forward: 5′-TTGACCTTTGTCCCAATGC-3′; reverse: 5′-AGGTTGGAGGCACACATAGG-3′). Aldolase A (forward: 5′-TGAAGCGCTGCCAGTATGTTA-3′; reverse: 5′-GGTCGCTCAGAGCCAGTATGGTTA-3′); 18S (forward: 5′-CGGCTACCAATCCAAGGAA-3′; reverse: 5′-GCTGGAATTACCGCGGCT-3′); Aldolase and 18S were used for normalization.
Proliferation and apoptosis assays
For proliferation, we used both 5-Bromo-2′-deoxy-uridine (BrdU) labeling of osteoblast cells with the Cell Proliferation kit (GE Healthcare, Orsay, France), and [3H]-thymidine (GE Healthcare) incorporation. Briefly, cells were plated in 96-well plates at a density of 5.103/well, and cultured in medium containing 10% 5-HT-depleted FCS until 80–90% confluence (48 h). Cells were cultured for an additional 18 h in a medium devoid of FCS. Then BrdU (final concentration: 10 mM) or [3H]thymidine (1 μCi/well) was added to the cultures for 24 h. Subsequently, BrdU labeling was measured as described by the manufacturer. The free radioactive thymidine was washed off using 5% trichloroacetic acid, and the incorporated radioactive thymidine was quantified by scintillation counting.
For apoptosis, caspase-3 activity was measured after 72 h of culture using the Caspase-3 Fluorometric Assay Kit (R&D. Systems, Lille, France) according to the manufacturer’s protocol. Protein concentrations were measured by BCA protein assay reagent kit (cf. above).
Calcium Incorporation
Cellular [45Ca]2+ uptake (19) by confluent calvarial osteoblasts from WT or mutated mice was measured in 24-well dishes after various culture times (day 6, 9, 12) in the differentiation medium supplemented with 10% 5-HT-depleted FCS. Twenty-four hours before cellular [45Ca]2+ uptake, the initial medium was replaced by a “fresh” medium containing 1% (v/v) 5-HT-depleted FCS. The cultures were then rinsed with PBS, and incubated in calcium- and serum-free medium containing ascorbic acid, βGP, and 5 μCi/ml [45Ca]2+ for 4 h. The medium was removed, and the cultures incubated for 1h in a medium containing serum and calcium. The monolayer was collected by scraping in PBS containing 0.01% SDS, and then extracted for 3 h in 0.5 N HCl before the radioactivity of the whole sample was determined. Quadruplicate determinations were done for each experimental point. Data were pooled from two independent experiments using [45Ca]2+ of similar specific activities.
Alkaline-phosphatase positive Colony forming unit (CFU-FALP+) assays
Alkaline-phosphatase positive colony forming units (CFUs) were assayed as described previously (26). Briefly, marrow stromal cells were collected from the femurs and tibias of 4-and 12-month-old female mice. Bone marrow was flushed out from each bone with α-MEM supplemented with FCS (15%) using a syringe and a 26-gauge needle. After a short spin, the cell pellets were re-suspended in the same culture medium. They were further filtered through a 0.45 m-pore-diameter nylon filter (Millipore, Molsheim, France). Cell suspensions were treated with a lysis solution (155 mM NH4Cl, 10 mM KHCO3, 0.1 mM EDTA, pH 7.4) to remove the red blood cells. Cells were seeded at 3.106 cells/well in a 6-well plate. After culturing for four days, 100μg/ml of ascorbic acid was added to the culture medium until the end of the experiment. After 11 days, cell colonies were fixed and stained for ALP by adding the BCIP/NBT (bromochloroindoyl-phosphate/nitroblue tetrazolium chloride) Sigma fast substrate buffer (Sigma-Aldrich). The number of CFU-FALP+ cells per dish was counted.
Statistical analysis
Statistical analysis was performed using the StatView 4.5 software (Abacus Concepts Inc., Berkeley CA, USA). Statistical differences between experimental groups were assessed using analysis of variance (ANOVA). The significance threshold was set at p <0.05. All values are shown as mean ± SEM.
Results
The 5-HT2BR was the only 5-HTR to display increased expression during osteoblast differentiation
We used various radioligands to identify 5-HT binding sites on WT calvarial osteoblasts. Before differentiation, the cells bound significant amounts of [3H]-5-HT, indicating the presence of serotonergic receptors, and of [125I]-DOI, suggesting the presence of 5-HT2-like binding sites. In contrast, [3H]-LY 278584 and [125I]-SB 207710 were not bound, demonstrating the absence of 5-HT3 and 5-HT4 binding sites respectively. Moreover, the cells bound neither [3H]-mesulergine nor [3H]-LSD in the presence of 100 nM MDL 100907, indicating the absence of 5-HT2C, 5-HT5, 5-HT6 and 5-HT7 binding sites. The absence of 5-HT6 and 5-HT7 binding sites was re-assessed by the absence of any specific binding of [3H]-SB 258585 or [3H]-SB 269970. Then specific labeling with [3H] 8-OH-DPAT, [3H]-MDL 100907 and [3H]-LY 266097, indicated the presence of 5-HT1A, 5-HT2A and 5-HT2B binding sites respectively. Finally, the absence of labeling with [125I]-GTI revealed the absence of 5-HT1B/1D binding sites.
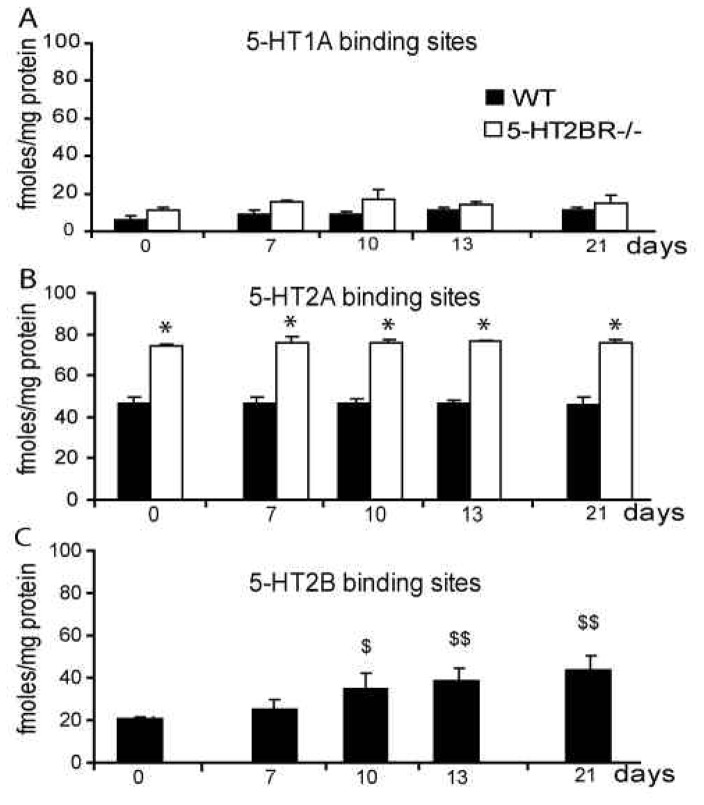
To investigate the potential role of serotonergic receptors in osteogenesis, binding assays were performed during the differentiation of primary calvarial osteoblast cultures. The binding-site profile (5-HT1A, 5-HT2A and 5-HT2B binding sites) remained unchanged throughout differentiation, as did the numbers of 5-HT1A (Fig. 1A) and 5-HT2A (Fig. 1B) binding sites. In contrast, 5-HT2B binding sites increased for 10 days, and then reached a plateau (Fig. 1C). The level of 5-HT2B mRNA remained constant during osteoblast differentiation (data not shown), indicating that its regulation must occur post-transcriptionaly. Taken together, these findings suggest that 5-HT2BRs may be important during osteoblast differentiation.
Figure 1. Osteoblasts express several 5-HTRs; 5-HT2BRs increase during osteoblast differentiation.
Binding assays in osteoblast primary cultures were carried out at plating (day 0) and after 7, 10, 13 and 21 days. 5-HT1A (A), 5-HT2A (B), and 5-HT2B (C) binding sites were determined in WT (black bars) and in 5HT2BR−/− osteoblastic cells (open bars). *, significant difference from WT at p<0.001. $, significant difference from day 0 at p<0.01. $$, significant difference from day 0 at p<0.001.
Female 5-HT2BR−/− mice display decreased BMD that worsens as they age
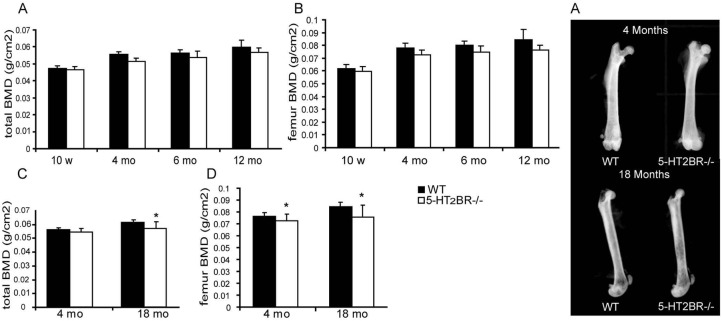
To determine the role of 5-HT2BRs on bone in vivo, the bone mineral density (BMD) of the 5-HT2BR−/− and WT mice was assessed at different times from 5 weeks to 18 months. During this period, the increases in body weight and size were not statistically different in WT and 5-HT2BR−/− mice (data not shown). No difference was observed between 5-HT2BR−/− and WT mice with regard to basal locomotion {L.Maroteaux, unpublished}. There was no difference in BMD in 5-HT2BR−/− and WT male mice at any time (data not shown). In contrast, aged 5-HT2BR−/− female mice (over 4-months of age) had lower BMD than equivalent WT mice. All subsequent in-vivo analyses were therefore performed in females. The time-dependent increase in BMD observed in WT mice was blunted in 5-HT2BR−/− mice, both for the whole body (Fig. 2A) and for the femur (Fig. 2B). At 4 months of age, 5-HT2BR−/− mice had lower BMD than WT mice, but only at the femur (−5.2%, Fig. 2C). At 18 months of age, the difference between WT and 5-HT2BR−/− BMD was more pronounced, and detected both for the whole body (−6.5%, Fig. 2C), and for femur (−10.6%, Fig. 2D). Radiography of the femur did not reveal any obvious difference between WT and 5-HT2BR−/− 4-month-old females, whereas the radiopacity of the femur in 18-month-old 5-HT2BR−/− females was lower than that in WT (Fig. 2E). These data show that 5-HT2BR knockout induces an age-dependent decrease in bone density in female mice.
Figure 2. 5-HT2BR−/− mice display decreased BMD that worsens with aging.
BMD was determined for the whole body (A) and femurs (B) of 5HT2BR−/− and wild-type (WT) female mice at the times indicated (n=5 per group). ANOVA for repeated measures revealed a statistically significant change related to genotype over time for both whole body and femoral BMDs (p < 0.05). BMD was also evaluated in 4- and 18-month-old 5-HT2BR−/− and WT mice (n = 15 to 20 per group) for the whole body (C) and the femur (D). Radiographies were performed on the femurs (E). *: significant (p < 0.05) difference between BMDs of 5-HT2BR−/− and WT mice. Mean values ± SEM (bars) are shown.
Female 5-HT2BR−/− mice display trabecular and cortical osteopenia attributable to decreased bone formation
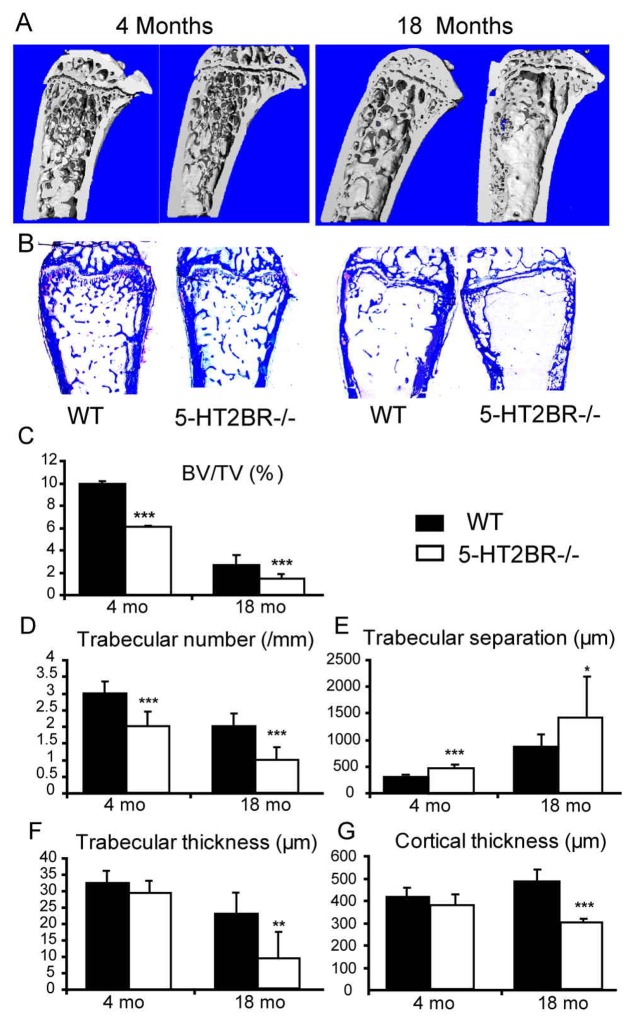
Histomorphometry and 3D-micro-CT reconstruction were performed in 4- and 18-month-old WT and 5-HT2BR−/− mice in order to characterize changes in microarchitecture, (Figs. 3 and 4). Trabecular bone volume to total bone volume (BV/TV, Fig. 3C), trabecular number (Fig. 3D), trabecular and cortical thickness (Figs. 3F and 3G) were all significantly lower in 5-HT2BR−/− mice. Accordingly, the trabecular separation (Fig. 3E) was significantly greater in 5-HT2BR−/− mice. Moreover, both trabecular and cortical bone decreased with age. The mineral apposition rate (MAR, Fig. 4C) was not affected, indicating that osteoblast function was normal in 5-HT2BR−/− female mice. In contrast, the mineralizing surface (MS/BS, Fig. 4B) and bone formation rate (BFR/BS, Fig. 4D) were both lower in 5-HT2BR−/− mice than in WT mice at both 4 and 18 months of age, suggesting that the number of osteoblasts might be reduced in 5-HT2BR−/− mice. This decrease in bone formation was confirmed by the low plasma levels of ALP activity (Fig. 4F) and osteocalcin (Fig. 4G). Finally, urinary deoxypyridinoline (Dpd), a marker of osteoclast activity, was not significantly different in 5-HT2BR−/− and WT mice at 4- or 18-months of age (Fig. 4E). Taken together, these findings indicate that low bone mass in 5-HT2BR−/− mice is associated with a decreased number of bone forming cells that may account for the marked decrease in bone formation.
Figure 3. 5-HT2BR−/− mice display trabecular and cortical osteopenia.
3D reconstruction by μCT of metaphyseal regions of tibia (A) and the metaphyseal region of distal femur (B) stained with toluidine blue in 4- and 12-month-old female mice illustrate the trabecular and cortical differences between 5-HT2BR−/− and WT mice. Histomorphometric structural parameters (C–G) in 4- and 18-month-old female mice were measured in the femoral distal metaphysis. Mean values ± SEM (bars) are reported (n = 10–12 sections per group). Asterisks indicate a significant difference between genotypes (* = P < 0.05, ** = P < 0.01, *** = P < 0.001).
Figure 4. Bone formation decreases in the absence of 5-HT2BR.
Static and dynamic bone parameters and biochemical markers were measured in 4- and 18-month-old 5-HT2BR−/− (open bars) and WT female mice (black bars). Representative tetracycline/calcein double-labeling in 4-month-old 5-HT2BR−/− and WT bone sections are shown (A). Dynamic formation parameters (mineralizing surface/bone surface = MS/BS) (B), mineralizing apposition rate = MAR) (C) and bone formation rate = BFR) (D), as well, as biochemical markers of bone resorption (urinary deoxypyridinoline cross-links = Dpd) (E), biochemical markers of bone formation, plasma alkaline phosphatase activity = ALP activity) (F) and plasma osteocalcin level (G) were determined. Data are shown as means ± SEM (n=10–12 sections per sample). Asterisks indicate significant difference between genotypes (* = P < 0.05, ** = P < 0.01, *** = P < 0.001).
5-HT2BR knockout impacts on osteoblast proliferation and recruitment
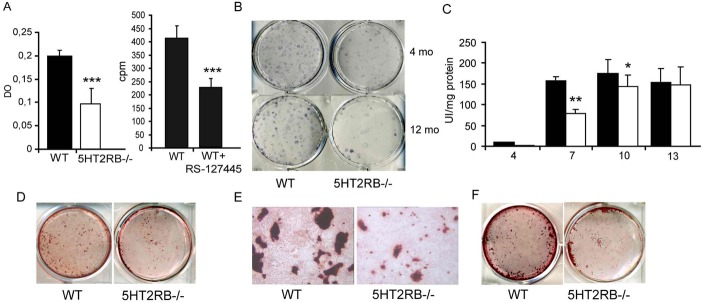
To investigate the cell defects that lead to low bone formation, we assessed the recruitment, proliferation, and survival of osteoblasts in WT and 5-HT2BR−/− mice. Caspase-3 activation appeared to be the same in WT and 5-HT2BR−/− osteoblasts (data not shown), indicating that there was no major change in apoptosis. In contrast, osteoblast proliferation was markedly lower in cultures of primary osteoblasts from 5-HT2BR−/− mice (−55%, Fig. 5A). Furthermore, RS-127445, a specific 5-HT2BR antagonist, decreased proliferation in WT osteoblasts (−45%, Fig. 5A) alone. Bone marrow adiposity was not affected by 5-HT2BR knockout (data not shown), but the number of alkaline-phosphatase cell forming units (CFU-FALP+) was dramatically lower in 5-HT2BR−/− mice than in WT mice at both 4-months (60 ± 11 vs. 182 ± 25/dish) and 12-months (26 ± 6 vs. 114.0 ± 16) of age (Fig. 5B).
Figure 5. 5-HT2BR impacts on osteoblast recruitment and proliferation.
(A) Proliferation was assessed after a 2 day-culture period by BrdU incorporation in WT and 5-HT2BR−/− calvarial osteoblasts, and by [3H] thymidine incorporation in the absence or presence of RS-127445 (5 nM). Each value is the mean ± SEM of 12 wells. Data from two independent experiments were pooled. Significant difference from WT cells (*** = p < 0.001). (B) CFU analysis was performed using bone marrow progenitors from the femurs of 4- and 12-month-old female mice. The numbers of CFU-FALP+ colonies/dish were significantly lower in 5-HT2BR−/− mice as compared to WT mice. This graph is representative of two independent experiments with quadruplicate determinations. (C) ALP activities were measured in WT and 5-HT2BR-deficient primary osteoblast cultures. Each value is the mean ± SEM of 12 wells. Data were pooled from two independent experiments. Difference from WT at day 7 (** = p < 0.001) and 10 (* = p <0.03). (D–F) At day 13 (D) and day 21 (F), the capacity of WT and HT2BR−/− primary osteoblast to produced mineralized nodules was determined by alizarin staining; the mean area of the WT nodules was higher than that of the HT2BR−/− nodules (p<0.001). The nodules formed in culture appeared to be smaller in WT than in 5-HT2BR-deficient osteoblasts (E).
To find out whether the 5-HT2BR-deficient osteoblasts had retained their ability to differentiate, the activity of ALP, a marker of osteoblast differentiation, was determined in primary osteoblast cultures on days 4, 7, 10 and 13. ALP activity was lower in 5-HT2BR−/− osteoblast cultures on days 7 (−50%) and 10 (−22%), but was no longer lower than WT on day 13 (Fig. 5C). There were significantly fewer mineralized nodules in 5-HT2BR−/− than WT osteoblast cultures only at day 13 (Fig. 5D, 95±15 vs. 134±10), but not at day 21 (Fig. 5F, 105±15 vs. 126±12), and they were also smaller in size (Fig. 5E).
Taken together, these findings indicate that 5-HT2BR knockout induces a marked decrease in osteoblast proliferation and recruitment, without markedly impairing terminal differentiation.
5-HT2BR is the main 5-HTR impacting on osteoblasts
As expected, we found that primary osteoblasts obtained from 5-HT2BR−/− mice expressed only 5-HT1AR and 5-HT2AR. Before differentiation, the number of 5-HT1AR binding sites in 5-HT2BR−/− mice was not significantly different from that in WT (Fig. 1A), whereas the knockout mice had twice as many 5-HT2AR binding sites (Fig. 1B). No change in this situation occurred during differentiation.
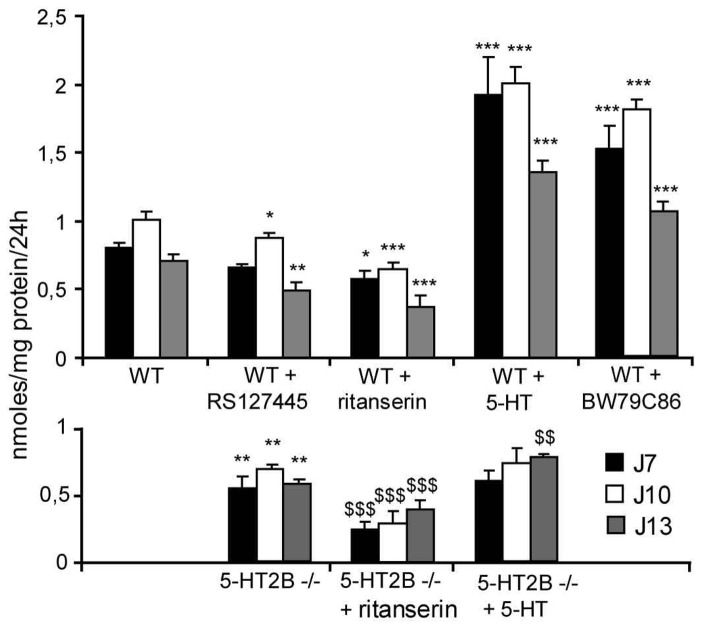
In order to investigate the role of the different 5-HT2Rs in osteoblasts, we measured [45Ca]2+ uptake (Fig. 6). In the absence of exogenous ligand, [45Ca]2+ uptake was lower for 5-HT2BR−/− primary cells than for WT cells (−23%), suggesting an intrinsic activity of the osteoblast 5-HT2BR. This hypothesis was supported by a higher [45Ca]2+ incorporation by WT osteoblasts treated with the selective 5-HT2BR antagonist RS-127445 (−10%) than in 5-HT2BR−/− osteoblasts. However, the osteoblast 5-HT2BR activity was principally agonist-dependent, and a similar increase in [45Ca]2+ incorporation was found when WT cells were treated with either 5-HT (+ 50%) or BW79C86 (+ 44%), a preferential agonist of 5-HT2BR. Furthermore, exposing WT osteoblasts to RS 127445, led to a decrease (−20%) in [45Ca]2+ incorporation. However, treatment with ritanserin, an inverse 5-HT2R agonist, led to a further decrease (−37%) in [45Ca]2+ incorporation, suggesting that the 5-HT2ARs (as well as the 5-HT2BRs) also impact in [45Ca]2+ incorporation. This point was confirmed by the finding that treating 5-HT2BR−/− osteoblasts with ritanserin produced a further decrease (−34%) in [45Ca]2+ incorporation and that treatment with 5-HT induced an increase in [45Ca]2+ incorporation only at day 13. Interestingly, at day 13, 5-HT doubled [45Ca]2+ incorporation in WT osteoblasts, whereas in 5-HT2BR-deficient cells the increase induced was only 25% (Fig. 6), showing that the 5-HT2BR is responsible for most of the effect of 5-HT on osteoblasts.
Figure 6. 5-HT2BR is the main 5-HTR impacting on osteoblasts.
[45Ca]2+ uptake Incorporation of [45Ca]2+ after incubating for 24 hours was measured after different times of primary osteoblast culture. Data were obtained at day 7, 10 and 13 for WT and 5-HT2BR−/− calvarial cells. WT calvarial cells were treated with the 5-HT2BR partial agonist BW 723C86 (10 nM), 5-HT (50 nM), the 5-HT2BR specific antagonist RS-127445 (20 nM) and the 5-HT2R inverse agonist ritanserin (100 nM). Results from 2 experiments performed in quadruplicate are shown as means ± SEM.* indicates p < 0.05, ** p < 0.01, *** p < 0.001 significantly different from WT calvarial cells. $$ indicates p < 0.01, $$$ p < 0.001 significantly different from 5-HT2BR−/− calvarial cells.
Discussion
So far, no direct physiological role of 5-HT on bone had been identified (12, 14, 15, 27). We show here for the first time, that 5-HT2BR has a key role among the various 5-HTRs expressed on bone cells. Female 5-HT2BR−/−mice exhibit an osteopenic phenotype (reduced BMD and trabecular and cortical bone volumes) that appears at 4 months of age, and progresses with senescence. We provide evidence that 5-HT2BRs impact on recruitment and proliferation of osteoblasts in aging mice. The phenotype of low-turnover osteopenia is indicative of reduced bone formation, one of the hallmarks of human and murine models of osteoporosis (1, 28, 29).
In vitro, we observed decreased osteoblast recruitment from mesenchymal stem cells from 5-HT2BR−/− mice. The 5-HT2BR has been reported to regulate embryonic mouse hind limb mesenchymal cell proliferation (17, 30, 31), and to increase the osteogenic differentiation of a mesoblastic cell line (19). In our study, the recruitment of osteoblastic precursors (CFU-FALP+ colonies) from mesenchymal stem cells was reduced in bone marrow from 4- and 18-month-old 5-HT2BR−/− mice. Interestingly, a significant decrease in bone density is first observed at the age of 4 months in these mice. Decreased bone formation in aging mice is associated with a decrease in the number of osteoblast colonies from the bone marrow (26, 32). We can, therefore, postulate that the reduced ability of 5-HT2BR −/− cells to produce alkaline-positive colonies might amplify the low osteoblast progenitor recruitment of aging mice. Aged-dependant osteoporosis in humans has been associated with decrease proliferative capacity of osteoprognitor cells (33) and also with an increase in the number of adipocytes (34). The same holds true in SAMP6 mice, a model of accelerated senescence (29, 35). In our 5-HT2BR−/− mice, decreased osteogenesis was not associated with a shift toward the adipocyte lineage in either bone sections or cell cultures. Our findings show that 5-HT2BRs impact on the recruitment of osteoblasts without producing a shift toward another pathway.
The 5-HT2BR appears to activate cell proliferation in several systems (18, 36): it activates the Ras pathway (20, 37) and transactivates tyrosine kinase receptors, such as the PDGF-R (36). Although mutant osteoblastic cells appear to have the same mineralizing rate as WT in vivo, in vitro we observed a decreased proliferation of 5-HT2BR−/− primary osteoblasts. In addition, the mineralized nodules generated by 5-HT2BR−/− primary cells presented a mineralization delay and were smaller in size than those generated by WT cells. [45Ca]2+ incorporation and alkaline phosphatase levels were lower in 5-HT2BR−/− than in WT cells between 7 and 13 days of culture. This transient delay in differentiation was probably due to decreased proliferation, as a reduction in the number of osteoblasts would reduce the intercellular contacts through cadherins that are important for acquisition of the osteoblast phenotype (38, 39). The decreased proliferation of osteoblasts is associated with reduced recruitment of progenitor cells from the bone marrow that may result in fewer bone forming surfaces in vivo. Moreover, 5-HT may increase interleukin 6 (IL6) production by stimulating 5-HT2Rs (40). This cytokine is an important target of 5-HT2BR (41) and play a role in osteoblast formation (42). In our study, we observed a decrease of IL6 in 5-HT2BR−/− osteoblast cultures as compared to WT osteoblasts (preliminary data). This downregulation in 5-HT2BR−/− mice could impact on osteoblast recruitment and proliferation.
The study of [45Ca]2+ incorporation in 5-HT2BR−/− osteoblast cultures demonstrates that 5-HT2BRs have a clear impact on osteoblast functions. The decrease in [45Ca]2+ uptake in the absence of 5-HT2BRs or the presence of a selective antagonist demonstrates the role of this receptor in calcium uptake. However, a further decrease in [45Ca]2+ incorporation was observed when the 5-HT2AR was inhibited by ritanserin, and 5-HT increased [45Ca]2+ incorporation in osteoblasts with the 5-HT2BR mutant. The mitogenic activity of 5-HT in other organs, such as the liver (43) and renal artery (44) has been linked to both 5-HT2B and 5-HT2ARs. It is therefore likely that these two 5-HT2Rs have partial overlapping functions.
Our findings support the notion that 5-HT does indeed influence bone metabolism. So far, no data exclude the possibility that neuronal 5-HT could influence bone cell behavior. Also, platelets, which store serotonin, could release 5-HT in contact or near osteoblasts (10). Finaly, osteoblasts may also produce 5-HT that could act in an autocrine/paracrine fashion. Tryptophan hydroxylase-1 (TPH1) mRNA, the rate limiting enzyme in 5-HT synthesis, has been detected in the osteoblast and osteocyte cell lines (14), and we detected significant levels of 5-HT in the supernatants of WT and 5-HT2BR −/− osteoblast cultures (preliminary data). In our study, the activity of 5-HT2BRs was mainly agonist-dependent, but a constitutive activity of 5-HT2BRs in osteoblast cannot be ruled out.
5-HT2BRs can control uptake activity of the serotonin transporter (5-HTT) in different systems (45, 46). 5-HTT is expressed in osteoclast cell lines and decreases osteoclast differentiation. 5-HTT is also present and functional in primary osteoblasts, osteoblast and osteocyte cell lines (11, 14, 27). Inhibition of 5-HTT by fluoxetine or gene invalidation results in low bone formation in mice (12). The exact biological effect of 5-HTT on bone formation remains unclear, the regulatory mechanism seems to be complex and further studies are required to clarify the relationship with the 5-HT2BR. Nevertheless, we have demonstrated that 5-HT via 5-HT2BRs is a peripheral modulator of osteoblast recruitment in bone formation in aging mice. These findings could provide new opportunities for treatments intended to increase bone formation in aging women.
Acknowledgments
This study was supported by grants from the “Rhumatisme et Travail” association, the Institut National de la Santé et de la Recherche Médicale (INSERM), Université René Diderot and the European Community 6 PCRD (ANABONOS consortium). LM’s work has been supported by funds from the Centre National de la Recherche Scientifique, the Institut National de la Santé et de la Recherche Médicale, the Université Pierre et Marie Curie, and by grants from the Fondation de France, the Fondation pour la Recherche Médicale, the Association pour la Recherche contre le Cancer, the French Ministry of Research (Agence Nationale pour la Recherche) and the European Community. LM’s team is an “Equipe Fondation pour la Recherche Médicale”.
Bibliography
- 1.Raisz LG. Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest. 2005;115:3318–3325. doi: 10.1172/JCI27071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lundberg P, Lundgren I, Mukohyama H, Lehenkari PP, Horton MA, Lerner UH. Vasoactive intestinal peptide (VIP)/pituitary adenylate cyclase-activating peptide receptor subtypes in mouse calvarial osteoblasts: presence of VIP-2 receptors and differentiation-induced expression of VIP-1 receptors. Endocrinology. 2001;142:339–347. doi: 10.1210/endo.142.1.7912. [DOI] [PubMed] [Google Scholar]
- 3.Lerner UH. Deletions of genes encoding calcitonin/alpha-CGRP, amylin and calcitonin receptor have given new and unexpected insights into the function of calcitonin receptors and calcitonin receptor-like receptors in bone. J Musculoskelet Neuronal Interact. 2006;6:87–95. [PubMed] [Google Scholar]
- 4.Suzuki A, Palmer G, Bonjour JP, Caverzasio J. Catecholamines stimulate the proliferation and alkaline phosphatase activity of MC3T3-E1 osteoblast-like cells. Bone. 1998;23:197–203. doi: 10.1016/s8756-3282(98)00099-4. [DOI] [PubMed] [Google Scholar]
- 5.Taylor AF. Osteoblastic glutamate receptor function regulates bone formation and resorption. J Musculoskelet Neuronal Interact. 2002;2:285–290. [PubMed] [Google Scholar]
- 6.Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, Shen J, Vinson C, Rueger JM, Karsenty G. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100:197–207. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- 7.Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, Kondo H, Richards WG, Bannon TW, Noda M, Clement K, Vaisse C, Karsenty G. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434:514–520. doi: 10.1038/nature03398. [DOI] [PubMed] [Google Scholar]
- 8.Sanders-Bush E, Mayer SE. 5-Hydroxytryptamine (serotonin): receptor agonists and antagonists. In: Brunton LL, Lazo JS, Keith LP, editors. Goodman and Gilman’s the pharmacological basis of therapeutics. McGraw-Hill compagnies; Columbus, OH, USA: 2005. pp. 297–316. [Google Scholar]
- 9.Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- 10.Warden SJ, Bliziotes MM, Wiren KM, Eshleman AJ, Turner CH. Neural regulation of bone and the skeletal effects of serotonin (5-hydroxytryptamine) Mol Cell Endocrinol. 2005;242:1–9. doi: 10.1016/j.mce.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Bliziotes MM, Eshleman AJ, Zhang XW, Wiren KM. Neurotransmitter action in osteoblasts: expression of a functional system for serotonin receptor activation and reuptake. Bone. 2001;29:477–486. doi: 10.1016/s8756-3282(01)00593-2. [DOI] [PubMed] [Google Scholar]
- 12.Warden SJ, Robling AG, Sanders MS, Bliziotes MM, Turner CH. Inhibition of the serotonin (5-hydroxytryptamine) transporter reduces bone accrual during growth. Endocrinology. 2005;146:685–693. doi: 10.1210/en.2004-1259. [DOI] [PubMed] [Google Scholar]
- 13.Westbroek I, van der Plas A, de Rooij KE, Klein-Nulend J, Nijweide PJ. Expression of serotonin receptors in bone. J Biol Chem. 2001;276:28961–28968. doi: 10.1074/jbc.M101824200. [DOI] [PubMed] [Google Scholar]
- 14.Bliziotes M, Eshleman A, Burt-Pichat B, Zhang XW, Hashimoto J, Wiren K, Chenu C. Serotonin transporter and receptor expression in osteocytic MLO-Y4 cells. Bone. 2006;39:1313–21. doi: 10.1016/j.bone.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gustafsson BI, Thommesen L, Stunes AK, Tommeras K, Westbroek I, Waldum HL, Slordahl K, Tamburstuen MV, Reseland JE, Syversen U. Serotonin and fluoxetine modulate bone cell function in vitro. J Cell Biochem. 2006;98:139–151. doi: 10.1002/jcb.20734. [DOI] [PubMed] [Google Scholar]
- 16.Shuey DL, Sadler TW, Lauder JM. Serotonin as a regulator of craniofacial morphogenesis: site specific malformations following exposure to serotonin uptake inhibitors. Teratology. 1992;46:367–378. doi: 10.1002/tera.1420460407. [DOI] [PubMed] [Google Scholar]
- 17.Moiseiwitsch JR. The role of serotonin and neurotransmitters during craniofacial development. Crit Rev Oral Biol Med. 2000;11:230–239. doi: 10.1177/10454411000110020601. [DOI] [PubMed] [Google Scholar]
- 18.Choi DS, Ward SJ, Messaddeq N, Launay JM, Maroteaux L. 5-HT2B receptor-mediated serotonin morphogenetic functions in mouse cranial neural crest and myocardiac cells. Development. 1997;124:1745–1755. doi: 10.1242/dev.124.9.1745. [DOI] [PubMed] [Google Scholar]
- 19.Locker M, Bitard J, Collet C, Poliard A, Mutel V, Launay JM, Kellermann O. Stepwise control of osteogenic differentiation by 5-HT(2B) receptor signaling: nitric oxide production and phospholipase A2 activation. Cell Signal. 2006;18:628–639. doi: 10.1016/j.cellsig.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Launay JM, Birraux G, Bondoux D, Callebert J, Choi DS, Loric S, Maroteaux L. Ras involvement in signal transduction by the serotonin 5-HT2B receptor. J Biol Chem. 1996;271:3141–3147. doi: 10.1074/jbc.271.6.3141. [DOI] [PubMed] [Google Scholar]
- 21.Nebigil CG, Choi DS, Dierich A, Hickel P, Le Meur M, Messaddeq N, Launay JM, Maroteaux L. Serotonin 2B receptor is required for heart development. Proc Natl Acad Sci U S A. 2000;97:9508–9513. doi: 10.1073/pnas.97.17.9508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Merciris D, Marty C, Collet C, de Vernejoul MC, Geoffroy V. Overexpression of the transcriptional factor Runx2 in osteoblasts abolishes the anabolic effect of parathyroid hormone in vivo. Am J Pathol. 2007;170:1676–1685. doi: 10.2353/ajpath.2007.061069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 24.Geoffroy V, Marty-Morieux C, Le Goupil N, Clement-Lacroix P, Terraz C, Frain M, Roux S, Rossert J, de Vernejoul MC. In vivo inhibition of osteoblastic metalloproteinases leads to increased trabecular bone mass. J Bone Miner Res. 2004;19:811–822. doi: 10.1359/JBMR.040119. [DOI] [PubMed] [Google Scholar]
- 25.Kellermann O, Loric S, Maroteaux L, Launay JM. Sequential onset of three 5-HT receptors during the 5-hydroxytryptaminergic differentiation of the murine 1C11 cell line. Br J Pharmacol. 1996;118:1161–1170. doi: 10.1111/j.1476-5381.1996.tb15519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen TL. Inhibition of growth and differentiation of osteoprogenitors in mouse bone marrow stromal cell cultures by increased donor age and glucocorticoid treatment. Bone. 2004;35:83–95. doi: 10.1016/j.bone.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 27.Battaglino R, Fu J, Spate U, Ersoy U, Joe M, Sedaghat L, Stashenko P. Serotonin regulates osteoclast differentiation through its transporter. J Bone Miner Res. 2004;19:1420–1431. doi: 10.1359/JBMR.040606. [DOI] [PubMed] [Google Scholar]
- 28.Parfitt AM, Villanueva AR, Foldes J, Rao DS. Relations between histologic indices of bone formation: implications for the pathogenesis of spinal osteoporosis. J Bone Miner Res. 1995;10:466–473. doi: 10.1002/jbmr.5650100319. [DOI] [PubMed] [Google Scholar]
- 29.Jilka RL, Weinstein RS, Takahashi K, Parfitt AM, Manolagas SC. Linkage of decreased bone mass with impaired osteoblastogenesis in a murine model of accelerated senescence. J Clin Invest. 1996;97:1732–1740. doi: 10.1172/JCI118600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhasin N, Kernick E, Luo X, Seidel HE, Weiss ER, Lauder JM. Differential regulation of chondrogenic differentiation by the serotonin2B receptor and retinoic acid in the embryonic mouse hindlimb. Dev Dyn. 2004;230:201–209. doi: 10.1002/dvdy.20038. [DOI] [PubMed] [Google Scholar]
- 31.Bhasin N, LaMantia AS, Lauder JM. Opposing regulation of cell proliferation by retinoic acid and the serotonin2B receptor in the mouse frontonasal mass. Anat Embryol (Berl) 2004;208:135–143. doi: 10.1007/s00429-004-0380-7. [DOI] [PubMed] [Google Scholar]
- 32.Bonyadi M, Waldman SD, Liu D, Aubin JE, Grynpas MD, Stanford WL. Mesenchymal progenitor self-renewal deficiency leads to age-dependent osteoporosis in Sca-1/Ly-6A null mice. Proc Natl Acad Sci U S A. 2003;100:5840–5845. doi: 10.1073/pnas.1036475100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stenderup K, Justesen J, Clausen C, Kassem M. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone. 2003;33:919–926. doi: 10.1016/j.bone.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 34.Meunier P, Aaron J, Edouard C, Vignon G. Osteoporosis and the replacement of cell populations of the marrow by adipose tissue. A quantitative study of 84 iliac bone biopsies. Clin Orthop Relat Res. 1971;80:147–154. doi: 10.1097/00003086-197110000-00021. [DOI] [PubMed] [Google Scholar]
- 35.Kajkenova O, Lecka-Czernik B, Gubrij I, Hauser SP, Takahashi K, Parfitt AM, Jilka RL, Manolagas SC, Lipschitz DA. Increased adipogenesis and myelopoiesis in the bone marrow of SAMP6, a murine model of defective osteoblastogenesis and low turnover osteopenia. J Bone Miner Res. 1997;12:1772–1779. doi: 10.1359/jbmr.1997.12.11.1772. [DOI] [PubMed] [Google Scholar]
- 36.Lesurtel M, Graf R, Aleil B, Walther DJ, Tian Y, Jochum W, Gachet C, Bader M, Clavien PA. Platelet-derived serotonin mediates liver regeneration. Science. 2006;312:104–107. doi: 10.1126/science.1123842. [DOI] [PubMed] [Google Scholar]
- 37.Nebigil CG, Launay JM, Hickel P, Tournois C, Maroteaux L. 5-hydroxytryptamine 2B receptor regulates cell-cycle progression: cross-talk with tyrosine kinase pathways. Proc Natl Acad Sci U S A. 2000;97:2591–2596. doi: 10.1073/pnas.050282397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng SL, Shin CS, Towler DA, Civitelli R. A dominant negative cadherin inhibits osteoblast differentiation. J Bone Miner Res. 2000;15:2362–2370. doi: 10.1359/jbmr.2000.15.12.2362. [DOI] [PubMed] [Google Scholar]
- 39.Shin CS, Lecanda F, Sheikh S, Weitzmann L, Cheng SL, Civitelli R. Relative abundance of different cadherins defines differentiation of mesenchymal precursors into osteogenic, myogenic, or adipogenic pathways. J Cell Biochem. 2000;78:566–577. [PubMed] [Google Scholar]
- 40.Kubera M, Maes M, Kenis G, Kim YK, Lason W. Effects of serotonin and serotonergic agonists and antagonists on the production of tumor necrosis factor alpha and interleukin-6. Psychiatry Res. 2005;134:251–258. doi: 10.1016/j.psychres.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 41.Jaffre F, Callebert J, Sarre A, Etienne N, Nebigil CG, Launay JM, Maroteaux L, Monassier L. Involvement of the serotonin 5-HT2B receptor in cardiac hypertrophy linked to sympathetic stimulation: control of interleukin-6, interleukin-1beta, and tumor necrosis factor-alpha cytokine production by ventricular fibroblasts. Circulation. 2004;110:969–974. doi: 10.1161/01.CIR.0000139856.20505.57. [DOI] [PubMed] [Google Scholar]
- 42.Franchimont N, Wertz S, Malaise M. Interleukin-6: An osteotropic factor influencing bone formation? Bone. 2005;37:601–606. doi: 10.1016/j.bone.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 43.Ruddell RG, Oakley F, Hussain Z, Yeung I, Bryan-Lluka LJ, Ramm GA, Mann DA. A role for serotonin (5-HT) in hepatic stellate cell function and liver fibrosis. Am J Pathol. 2006;169:861–876. doi: 10.2353/ajpath.2006.050767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Watts SW, Thompson JM. Characterization of the contractile 5-hydroxytryptamine receptor in the renal artery of the normotensive rat. J Pharmacol Exp Ther. 2004;309:165–172. doi: 10.1124/jpet.103.062562. [DOI] [PubMed] [Google Scholar]
- 45.Launay JM, Schneider B, Loric S, Da Prada M, Kellermann O. Serotonin transport and serotonin transporter-mediated antidepressant recognition are controlled by 5-HT2B receptor signaling in serotonergic neuronal cells. Faseb J. 2006;20:1843–1854. doi: 10.1096/fj.06-5724com. [DOI] [PubMed] [Google Scholar]
- 46.Callebert J, Esteve JM, Herve P, Peoc’h K, Tournois C, Drouet L, Launay JM, Maroteaux L. Evidence for a control of plasma serotonin levels by 5-hydroxytryptamine(2B) receptors in mice. J Pharmacol Exp Ther. 2006;317:724–731. doi: 10.1124/jpet.105.098269. [DOI] [PubMed] [Google Scholar]