Abstract
Secretion systems are key virulence factors, modulating interactions between pathogens and the host's immune response. Six potential secretion systems (types 1–6; T1SS–T6SS) have been discussed in classical bordetellae, respiratory commensals/pathogens of mammals. The prototypical Bordetella bronchiseptica strain RB50 genome seems to contain all six systems, whilst two human-restricted subspecies, Bordetella parapertussis and Bordetella pertussis, have lost different subsets of these. This implicates secretion systems in the divergent evolutionary histories that have led to their success in different niches. Based on our previous work demonstrating that changes in secretion systems are associated with virulence characteristics, we hypothesized there would be substantial divergence of the loci encoding each amongst sequenced strains. Here, we describe extensive differences in secretion system loci; 10 of the 11 sequenced strains had lost subsets of genes or one entire secretion system locus. These loci contained genes homologous to those present in the respective loci in distantly related organisms, as well as genes unique to bordetellae, suggesting novel and/or auxiliary functions. The high degree of conservation of the T3SS locus, a complex machine with interdependent parts that must be conserved, stands in dramatic contrast to repeated loss of T5aSS ‘autotransporters’, which function as an autonomous unit. This comparative analysis provided insights into critical aspects of each pathogen's adaptation to its different niche, and the relative contributions of recombination, mutation and horizontal gene transfer. In addition, the relative conservation of various secretion systems is an important consideration in the ongoing search for more highly conserved protective antigens for the next generation of pertussis vaccines.
Introduction
One of the important factors of pathogenicity in many Gram-negative bacteria is the possession of secretion systems that can transport molecules from bacterial cells into the environment or into host cells (Shrivastava & Mande, 2008). Six secretion systems (types 1–6; T1SS–T6SS) and other single-membrane-spanning secretion systems have specialized components that transport effector proteins across the Gram-negative bacterial cell wall (Costa et al., 2015; Filloux et al., 2008). The T1SS is widespread in pathogenic Gram-negative bacteria such as Vibrio cholerae or Escherichia coli, and known to secrete adhesins, glycanases and rhizobial proteins that are involved in biofilm formation in Agrobacterium tumefaciens and Pseudomonas syringae pv. tomato (Delepelaire, 2004; Fauvart & Michiels, 2008; Reddy et al., 2007; Thomas et al., 2014). The T2SS is Sec-dependent, highly similar to the type 4 pilus assembly system (T4PS), and required for virulence of V. cholerae, enterotoxigenic E. coli and Pseudomonas aeruginosa (Cianciotto, 2005; Filloux, 2004; Nivaskumar & Francetic, 2014). The T3SS, which is closely related to a flagella subunit export system, is an injectisome that delivers effector proteins to host cells through a needle-like apparatus (Cornelis, 2006; Diepold & Wagner, 2014; Grant et al., 2006; Mota et al., 2005; Saier, 2004). The T4SS has been shown to transport nucleic acids in addition to complex proteins (Christie et al., 2005, 2014; Christie & Cascales, 2005). T5SSs can be divided into five subclasses (a–e): the T5aSS is an autotransporter (AT), the T5bSS is a two-partner secretion (TPS) system, the T5cSS is the trimeric AT, the T5dSS is a fusion between AT and TPS, and finally the T5eSS is the inverted AT (Gawarzewski et al., 2013; Leo et al., 2012). They have been shown to be required for virulence in Haemophilus influenzae, Yersinia enterocolitica, Neisseria gonorrhoeae, Helicobacter pylori and Bordetella pertussis (Bernstein, 2007; Dautin & Bernstein, 2007; Henderson et al., 2004; Jacob-Dubuisson et al., 2004; Marr et al., 2008; Noofeli et al., 2011). Lastly, the T6SS appears to constitute a phage-tail-spike-like injectisome and is required for virulence in several pathogens, including V. cholerae, P. aeruginosa, Francisella tularensis and Bordetella bronchiseptica (Bingle et al., 2008; Cascales, 2008; Filloux et al., 2008; Pukatzki et al., 2007; Shrivastava & Mande, 2008; Wu et al., 2008). Additionally, the T6SS is involved in intraspecies and interspecies bacterial interactions (Cambronne & Roy, 2006; Hood et al., 2010; Russell et al., 2011; Schwarz et al., 2010; Zhang et al., 2013). All six possible secretion systems have been described in the classical bordetellae and some have been shown to be important for bacterial pathogenesis (Buboltz et al., 2009; Mattoo & Cherry, 2005; Park et al., 2012; Weyrich et al., 2012). However, further studies are essential to classify these possible secretion systems and understand their role in pathogenesis. In fact, little is understood about their evolutionary history or their respective contributions to the different biology of these closely related subspecies that differ in some of the most interesting characteristics of bacterial pathogens, including host range, persistence and virulence/pathogenesis.
B. bronchiseptica is a Gram-negative bacterium that colonizes the respiratory tract of a broad range of mammalian hosts (Sebaihia et al., 2006). B. pertussis and Bordetella parapertussis, the causative agents of whooping cough in humans, are believed to have evolved independently from a B. bronchiseptica-like progenitor (Diavatopoulos et al., 2005). Over 90 % of the 16 million pertussis-related cases, and 195 000 deaths each year occur in developing countries; however, there have even been epidemics in developed countries, such as Australia, the UK, Canada and the USA, despite high vaccine coverage (CDC, 2015a, b). These three subspecies of bacteria offer a valuable opportunity to study the evolution of virulence factors as they share >95 % sequence identity across entire genomes, but differ more substantially in gene content, mostly due to gene loss that took place independently in the B. pertussis and B. parapertussis lineages as each adapted to become highly virulent, human-specific pathogens. We have shown previously that the differential presence/absence of virulence factors contributes to the different virulence characteristics of these lineages (Buboltz et al., 2008, 2009; Heininger et al., 2002).
Although six potential types of secretion systems have been described in the classical bordetellae, only a subset has been shown to play roles in pathogenesis and some of these appear to be specific to a subset of lineages. The T1SS has been shown to transport adenylate cyclase toxin (ACT) (Laoide & Ullmann, 1990), which contributes to pathogenesis of B. pertussis and B. bronchiseptica strain RB50, but the locus encoding the toxin and its secretion system are missing in some B. bronchiseptica strains (Buboltz et al., 2008). The fimbrial subunits, which are important protective antigens and virulence factors, appear to be secreted by the T2SS (Shrivastava & Miller, 2009). The T3SS is required to secrete effectors, such as Bordetella T3SS effector A (BteA), but its expression patterns and roles in infection appear to differ substantially amongst lineages (Buboltz et al., 2009). The T4SS secretes the eponymous toxin, pertussis toxin (PTX), which at one time was believed to be sufficient to induce most of the symptoms of B. pertussis disease (Pittman, 1984, 1986), but PTX is not produced by B. parapertussis, which causes substantially the same disease (Weiss et al., 1993). Increased secretion of effectors through these secretion systems has been associated with hypervirulence (Buboltz et al., 2009; Weiss et al., 1993). The T5SS transports adherence factors, including filamentous haemagglutinin, pertactin and tracheal colonization factor (Shrivastava & Miller, 2009). Lastly, the newly discovered T6SS is required for colonization and cytotoxicity toward macrophages and dendritic cells, even though specific effectors have not yet been identified (Weyrich et al., 2012). As many secreted proteins are virulence factors that contribute to pathogenesis in different ways, understanding their different evolutionary histories will help us determine how distinct lineages of bordetellae have evolved different complex interactions with their hosts to allow for their remarkable success in a variety of host populations.
Comparing sequenced classical bordetellae genomes, we have observed many similarities and notable differences in each secretion system. For each secretion system, we predicted a network that identifies conserved components, consistent with functional orthologues of known activities, as well as accessory components with potentially new functions. In addition, computational analysis identified variation in secretion systems amongst the bordetellae and reflected the different evolutionary histories for each secretion system. Notably, the data suggest that point mutations are the dominant force that shape most secretion systems, but recombination plays an important role in evolutionary changes of the T6SS. We also present evidence that some secretion systems may have undergone horizontal gene transfer, which is relatively rare in the bordetellae. The diversity of secretion systems in the bordetellae and their evolution likely reflects the relative roles of each in the distinct natural history of bordetellae lineages that have adapted to cause different diseases in diverse hosts. This analysis exposes novel aspects of the recent divergent evolution of the classical bordetellae and suggests different roles for each secretion system in their different ecological niches.
Methods
Secretion system sequence, domain and functional network comparison
Genes and the loci that encode the previously discussed secretion systems (T1SS–T6SS) from the annotated B. bronchiseptica strain RB50 genome were compared amongst the 11 Bordetella genomes via the Artemis Comparison Tool (Carver et al., 2005). Percentage sequence similarity was calculated based on the RB50 sequence via blast using default settings (Altschul et al., 1990) and genes that contained either a frameshift mutation or an in-frame stop codon or that are absent were highlighted with different colours in the heatmap generated by r (R Development Core Team, 2008). The phylogenetic tree (gene content tree) based on the presence or absence of each gene in the secretion system loci was superimposed on the heatmap. Conserved domains in each gene of the secretion system loci in RB50 were searched against the National Center for Biotechnology Information Conserved Domain Database (http://www.ncbi.nlm.nih.gov/Structure/cdd/cdd.shtml). The functional protein network for each secretion system was predicted based on protein sequences of RB50 using string (Search Tool for Recurring Instances of Neighbouring Genes) with the default settings (Snel et al., 2000). string iteratively searches for the conserved genomic organization for each of the secretion system components in multiple species by calculating confidence scores based on co-occurrence in other bacteria species, experimental interactions, databases, co-expression analysis and text-mining of research journal articles.
Horizontal gene transfer detection
Putative horizontally acquired regions in Bordetella strains were predicted by Alien_hunter, which is a program capable of detecting genomic regions that may originate from foreign sources using interpolated variable order motifs (Vernikos & Parkhill, 2006). For the phylogenetic tree comparison, multiple alignments of each secretion system locus were generated by mega5 software (Tamura et al., 2011), and maximum-likelihood trees were constructed with a Tamura–Nei model and 1000 bootstrap replicates.
Mutation and recombination rate calculation
The recombination/mutation (ρ/θ) ratio for each secretion system, except the T5aSS, amongst the classical bordetellae strains was calculated with the rdp4 suite with the default settings (Martin et al., 2010).
Results
Comparison of secretion systems in classical bordetellae
Secretion systems can involve complex molecular structures that require several genes to encode various components with necessary and cooperative functions, and other genes with regulatory or accessory functions that may not always be required. As such, there are sets of genes that are highly conserved, often encoding the structural and other required components. Other sets of genes are more frequently lost because their role is less central, contributing to only a subset of functions. Here, we assessed the presence/absence of all genes associated with possible secretion systems in B. bronchiseptica reference strain RB50 (Fig. 1, Table S1, available in the online Supplementary Material).
Fig. 1.
Schematic presentation of secretion pathways in B. bronchiseptica strain RB50: individual secretion systems are presented with the RB50 gene name. Components that are absent in RB50 are indicated by dotted lines. OM, Outer membrane; IM, inner membrane.
RB50 contains genes encoding T1SS components required for the secretion of the important virulence factor ACT. These include the genes encoding the ATP-binding cassette transporter (cyaB), the membrane fusion protein (cyaD) and the outer membrane factor (cyaE) (Holland et al., 2005), as well as genes encoding ACT (cyaA) and the enzyme that acylates it (cyaC).
In contrast to the high conservation of the T1SS, RB50 contains only a partial and annotated as T2SS locus that is missing seven out of 13 core components (Gsp C–O) of the T2SS of Nitrosomonas europaea, Leptospira interrogans and Rhodopirellula baltica (Cianciotto, 2005; Korotkov et al., 2012; Nivaskumar & Francetic, 2014; Sandkvist, 2001). There are several putative membrane proteins that may contribute as alternative components of the T2SS in RB50, replacing the missing components (Gsp CHIKLMN), which are inner-membrane proteins (Thanassi & Hultgren, 2000). An incomplete set of T2SS homologues (i.e. Gsp DEFGHIJKO) is associated with the presence of type 4 piliation in other bacteria (Cianciotto, 2005). In addition to the present T2SS components (Gsp DEFGJO), the RB50 tbl2SS locus encodes a putative ATPase protein, PilT, which is unique to the T4PS (Mahmoud & Koval, 2010; Nivaskumar & Francetic, 2014). Based on these two observations, the T2SS in RB50 may more closely resemble the T4PS, which is known to be evolutionarily related to the T2SS (Peabody et al., 2003). Further experimental studies may shed more light on the origin and identity of this secretion system locus, but for now we will refer to it as the T2SS-like/T4PS locus.
RB50 has an intact T3SS-encoding locus with all of the >20 known core components (Abby & Rocha, 2012; Cornelis, 2006) as well as additional regulatory proteins, reflecting the importance of this secretion system. RB50 also has a T4SS locus containing all the known core components (ptlABCDEFGHI) encoding homologues of VirB2, VirB3, VirB4, VirB6, VirB7, VirB8, VirB9, VirB10 and VirB11, respectively, but missing genes for VirB1, VirB5 and VirD4 (Fronzes et al., 2009), which are core components in other bacteria. However, these components are not required parts of the apparatus of the secretion systems in A. tumefaciens (Fronzes et al., 2009) and may not be required for some or all functions in the classical bordetellae (O'Callaghan et al., 1999). PTX secretion occurs by a two-step process, as in the A. tumefaciens complex (Christie et al., 2014; Locht et al., 2011; Pantoja et al., 2002).
In addition to filamentous haemagglutinin, which is secreted by the TPS system (T5bSS), most T5SSs are classical ATs (T5aSS) that function independently, with their own signal sequence, passenger domain and translocation unit (Henderson et al., 2004; Julio & Cotter, 2005). The signal sequence allows targeting of the protein to the inner membrane for export, whilst the passenger domain has diverse effector functions. The translocation unit consists of a linker region with a β-domain, which facilitates translocation of the passenger domain through the outer membrane (Henderson et al., 2004). Our phylogenetic analysis confirms that the ATs of B. bronchiseptica strain RB50 fall into three different groups (Henderson et al., 2004) (Fig. S1). Group 1 includes about half of the genes, including those encoding the known virulence factors pertactin, Vag8, BrkA and tracheal colonization factor. Group 2 includes adhesins related to E. coli ATs. Finally, known proteases are clustered into group 3, including SphB1, 2 and 3. Interestingly, BB2324 is not a recognizable member of any of these groups and may form its own functional group.
Lastly, all 16 previously predicted core components of the T6SS are present in B. bronchiseptica strain RB50. Although regulators present in other organisms have not yet been identified in this locus (Weyrich et al., 2012), there are five proteins encoded within the locus [TssA (BB0794), TssN (BB0808), TssP (BB0811), TssQ (BB0812) and TssV (BB0818)] that have neither known T6SS functional domains nor known clusters of orthologous group domains, suggesting they may have B. bronchiseptica-specific functions (Table S1).
Overall, this comparative analysis indicates that B. bronchiseptica strain RB50 contains most core components of the six possible secretion systems. Several secretion system loci, including T2SS-like/T4PS, T4SS and T6SS, in RB50 are missing some components and/or contain genes that are not in the respective loci in other bacteria, suggesting that RB50 secretion systems might have different regulation and/or functions compared with other bacteria.
Functional networks of each secretion system
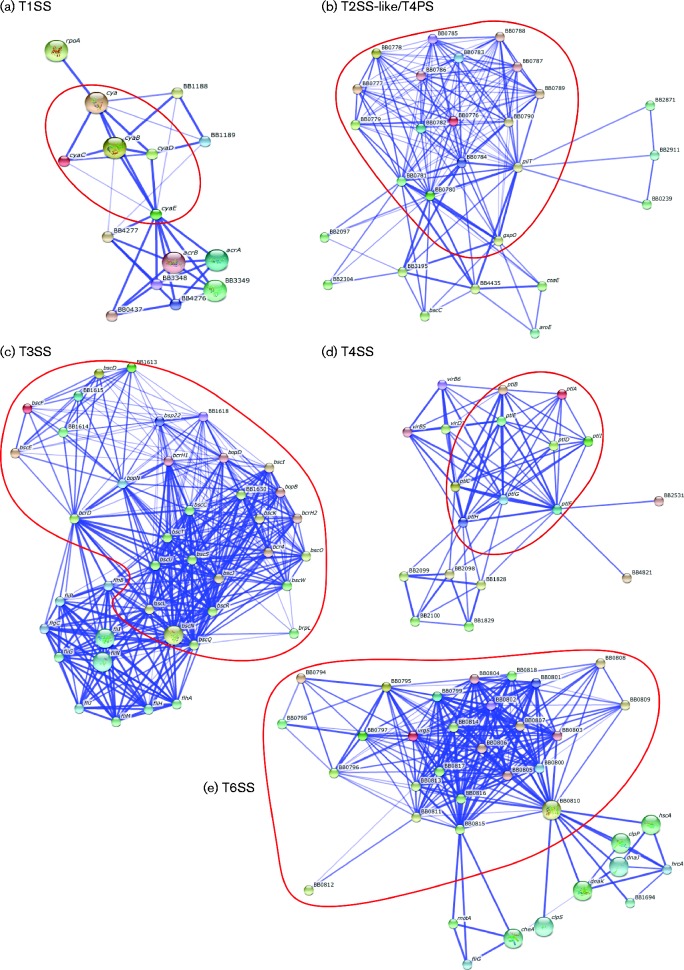
Genes encoding functionally associated proteins tend to occur within the same operon even in distantly related genomes. We assessed the functional association between components in the B. bronchiseptica RB50 secretion systems using string to narrow down candidate genes for further study (Snel et al., 2000) (Fig. 2). string iteratively searches for the conserved genomic organization for a given gene in multiple, phylogenetically distant species with confidence scores, based on co-occurrence in other bacterial species, experimental interactions, databases, co-expression analysis and text-mining of research journal articles. Interestingly, T1SS components are associated with ABC transporter (BB1189), haemolysin D (BB0437, BB1188 and BB4277), and DNA-directed RNA polymerase subunit (RpoA) (Table 1), which may affect transcription in B. bronchiseptica strain RB50. In addition, T1SS components are associated with AcrAB, which is well-known for drug transport (Seeger et al., 2006), and other efflux system proteins (BB3348 and BB3349) (Thomas et al., 2014).
Fig. 2.
Protein network for the (a) T1SS, (b) T2SS-like/T4PS, (c) T3SS, (d) T4SS and (e) T6SS predicted by string (Snel et al., 2000) based on protein sequences in B. bronchiseptica strain RB50. Confidence scores were calculated based on co-occurrence in other bacterial species, experimental interactions, databases, co-expression analysis and text-mining of research journal articles. Genes within red circles encode secretion system components. Intensity of colour is based on confidence scores and only 10 additional candidates are shown in the network.
Table 1. Proteins (B. bronchiseptica strain RB50/B. pertussis strain Tohama I) and their predicted functions associated with each secretion system.
| Secretion system | Protein | Predicted function |
|---|---|---|
| T1SS | BB1188/– | Haemolysin D |
| BB1189/– | ABC transporter | |
| AcrA/AcrA | Acriflavine resistance protein A precursor | |
| AcrB/AcrB | Acriflavine resistance protein B | |
| BB3348/BP2076 | Efflux system transmembrane protein | |
| BB3349/BP2075 | Efflux system inner membrane protein | |
| BB4276/BP0407 | Acriflavine resistance protein | |
| BB4277/– | Haemolysin D | |
| BB0437/– | Haemolysin D | |
| RpoA/RpoA | DNA-directed RNA polymerase subunit α | |
| T2SS-like/T4PS | BB2871/BP1320 | YggS family pyridoxal phosphate enzyme |
| BB2911/BP1280 | Pyrroline 5-carboxylate reductase | |
| BB0239/BP0560 | YggS family pyridoxal phosphate enzyme | |
| BscC/BscC | Type III secretion system | |
| BB2304/BP1113 | Competence protein | |
| AroE/AroE | Shikimate 5-dehydrogenase | |
| CoaE/CoaE | Dephospho-CoA kinase | |
| BB4435/BP3818 | Type 4 prepilin-like protein leader peptide processing enzyme | |
| BB3195/BP1832 | PulE/GspE-like ATPase of the Tfp pilus assembly | |
| BB2097/BP2526 | Flp pilus assembly protein CpaC/GspD family | |
| T3SS | FliP/FliP | Flagellar biosynthesis protein |
| FlgC/FlgC | Flagellar basal body rod protein | |
| FliG/FliG | Flagellar motor switch protein | |
| FliH/FliH | Flagellar assembly protein H | |
| FliI/FliI | Flagellum-specific ATP synthase | |
| FliJ/FliJ | Flagellar protein | |
| FliM/FliM | Flagellar motor switch protein | |
| FliN/FliN | Flagellar motor switch protein | |
| FlhB/FlhB | Flagellar biosynthesis protein | |
| FlhA/FlhA | Flagellar biosynthesis protein | |
| T4SS | BB1828/BP1998 | Type II secretion system |
| BB1829/BP1999 | TadB-like Flp pilus assembly protein | |
| BB2098/BP2525A | Secretory protein | |
| BB2099/BP2525 | Putative membrane protein | |
| BB2100/BP2524A | Pilus assembly protein TadC | |
| BB2531/BP0988 | Putative exported protein | |
| BB4821/BP3734 | SCO1/SenC family protein | |
| VirB5/– | Plasmid-related exported protein | |
| VirD2/– | Plasmid-related protein | |
| VirB6/– | Plasmid-related membrane protein | |
| T6SS | DnaJ/DnaJ | Chaperone protein |
| DnaK/DnaK | Molecular chaperone | |
| HscA/HscA | Chaperone protein | |
| ClpP/ClpP | ATP-dependent Clp protease proteolytic subunit | |
| ClpS/BP2756 | ATP-dependent Clp protease adaptor protein | |
| CheA/CheA | Chemotaxis protein | |
| MotA/MotA | Flagellar motor protein | |
| FliG/FliG | Flagellar motor switch protein | |
| HrcA/BP2504 | Heat-inducible transcription repressor | |
| BB1694/– | Phage-related Clp protease |
The T2SS-like/T4PS components are also associated with other proteins whose genes are located elsewhere on the chromosome (Fig. 2). Interestingly, some of those proteins are encoded in two of the three additional T4PS loci on the genome (BB1826–BB1834, BB2093–BB2100, BB3192–BB3199). BB2097 is a member of the Flp pilus assembly protein CpaC/GspD family whilst BB3195 is predicted to be a PulE/GspE-like ATPase of the Tfp pilus assembly (Table 1). In addition, phosphate enzymes (BB2871 and BB0239) are associated with this T2SS-like/T4PS, as it was shown that phosphate starvation in Caulobacter crescentus induces T2SS-dependent release of the lipoprotein ElpS (Le Blastier et al., 2010).
The string network assembled for the T3SS of RB50 (Fig. 2) shows that these genes are very tightly associated with each other, as well as with flagellar operons (Table 1), as T3SSs are known to be closely related to flagella subunit export systems (Cornelis, 2006; Diepold & Wagner, 2014; Saier, 2004; Stone et al., 2010). In addition, the gene encoding BrpL, which has been shown to regulate the Bordetella T3SS (Nicholson, 2007), still appears to be associated with the T3SS in this network.
T4SS genes are tightly associated with each other, and genes encoding VirB5, VirD2 and VirB6 are functionally associated with the T4SS. In addition, proteins from the T4PS loci (BB1828–BB1829 and BB2098–BB2100) are also interconnected with T4SS components like T2SS-like/T4PS components (Table 1). It has been hypothesized that T2SS-like/T4PS and T4SS have evolved similar extrusion mechanisms for secretion of these large, multisubunit A/B toxins (Christie et al., 2014).
T5aSS-encoding genes are not clustered within the same operon, but are scattered throughout the genome. The lack of association suggests that their regulation and function are independent, as would be expected for ‘ATs’ that are not dependent on each other for their functions.
In contrast to the independent ATs, several aspects of the T6SS-encoding genes are consistent with their function as a complex molecular machine with many interdependent components. Interestingly, BB0810 appears to be an ATPase functionally related to DnaK, which is a molecular chaperone shown to be important for Brucella melitensis growth in macrophage infection, suggesting that the Bordetella T6SS could be involved in surviving interactions with immune cells (Wang et al., 2009). T6SS components are also associated with other chaperone proteins and flagellar-related proteins (Table 1).
Diversity of secretion system loci amongst the classical bordetellae
To examine differences in the secretion systems of the various strains/lineages, we compared the presence/absence of all the secretion system-encoding genes and their sequence similarity amongst the 11 classical bordetellae strains. The T3SS genes are highly conserved (>93 % identity) in all strains. This is an important observation in light of prior evidence that the T3SS of B. pertussis is not expressed and more recent evidence that its expression is rapidly lost during culture in vitro (Rambow-Larsen & Weiss, 2004; Fennelly et al., 2008). Together, these results indicate that T3SS genes have been under strong purifying selection during the diverse evolutionary history of these strains in nature, although there may be positive selection for mutation or loss of expression when grown under laboratory conditions. The T5aSS genes are the most divergent (as low as 85 % identity), with many pseudogenes (Fig. 3), which is expected as they are ATs that function independently (Henderson et al., 2004) and they are distributed across the entire genomes, indicating lack of local constraint for gene context, so one can be lost or mutated without affecting the function of others. The T1SS locus is conserved in all strains except in B. bronchiseptica strain 253 and other strains of this MLST type (Buboltz et al., 2009), which are missing the entire locus. B. bronchiseptica strains 1289, D445, Bbr77, MO149 and 18323 all contain a frameshift mutation in cyaB, encoding the putative ABC transporter, but retain the rest of the locus. The T2SS-like/T4PS locus is well conserved amongst B. bronchiseptica strains, although sequencing gaps in strains D445 and Bbr77 prevent a conclusive claim. In contrast, human B. parapertussis (B. parapertussishu) and B. pertussis strains are missing the entire locus, and there are four pseudogenes in this locus of ovine B. parapertussis (B. parapertussisov) strain Bpp5, indicating that the locus is in a state of decay. The T4SS genes are conserved in all the strains except B. bronchiseptica strains Bbr77 and MO149. Lastly, the T6SS locus is conserved in four out of six B. bronchiseptica strains. D445 is missing the entire T6SS locus, whilst Bbr77 has one pseudogene in the locus, suggesting a recent inactivation of the T6SS or identifying a non-essential gene. Similarly, there are multiple missing genes and/or pseudogenes in the T6SS loci of the B. parapertussis host-restricted strains (both human and ovine), and all three B. pertussis strains lack the entire T6SS locus, suggesting its functions are not required for the closed and human host-specific ecological niche of these lineages.
Fig. 3.
Diversity in secretion system loci amongst the classical bordetellae compared with B. bronchiseptica strain RB50. The heatmap was generated based on nucleotide percentage identity compared with RB50 for each gene/locus. Absence of a certain gene and presence of a pseudogene are highlighted with white and sky blue colour with a hash symbol. An asterisk indicates the genes missing due to the draft status of the genome, which have not been confirmed. The gene content tree, the dendrogram of hierarchical clustering of the gene content in the secretion system loci matrix, was superimposed on top of the heatmap. Manhattan distances, linkage method and 1000 bootstrap replicates were used for the clustering.
The bordetellae are believed to have evolved and specialized via genome decay and loss of up to 25 % of their genes (Parkhill et al., 2003). The loss of a single gene required for a complex function like a secretion system should have affected the selective pressure posed on the remaining genes of that secretion system. These secretion systems would then be expected to undergo rapid degradation and/or loss. To examine evolutionary history as it applies to secretion systems and relates to the phylogeny, we reconstructed a tree based on the presence and absence of individual genes within each secretion system locus (Fig. 3). This tree appears to be similar to genome-wide SNP trees (Park et al., 2012) in its clustering of B. pertussis strains (18323, Tohama I and CS) together as well as its association of the two lineages (human and ovine) of B. parapertussis. Similar to a previously published gene content tree (Park et al., 2012), B. bronchiseptica strains compose two separate clusters, referred to as complex I (RB50, 1289 and 253) and complex IV (Bbr77 and MO149) (Fig. 3). In addition, B. parapertussis human (12822) and ovine (Bpp5) strains are both clustered together, consistent with the genome-wide SNP-based tree. However, unlike the genome-wide trees (Park et al., 2012), the secretion system gene content tree grouped B. bronchiseptica complex IV strain D445 closer to B. pertussis strains than to the other complex IV strains, most likely due to the loss of the entire T6SS locus. These data reveal an intriguing tendency of secretion system loci to be lost or divergent in the human isolates (B. parapertussishu strain 12822, B. pertussis strains CS, Tohama I and 18323, and some B. bronchiseptica complex IV strains D445, Bbr77 and MO149), potentially suggesting a link between secretion systems and their apparent recent adaptation to a closed life cycle and/or to human host.
Different evolutionary histories of secretion system loci
The presence/absence of individual secretion systems or their components amongst different sets of strains seems to suggest a different evolutionary history for each system. To explore each system, additional phylogenetic trees were reconstructed based on individual secretion system sequences (Fig. 4b–g). Consistent with the phylogenetic relatedness of all the secretion system loci together (Fig. 4a), individual T1SS and T3SS phylogenetic trees showed that B. pertussis strains and B. bronchiseptica complex IV strains form two separate clusters, and B. parapertussis strains and B. bronchiseptica complex I isolates cluster together (Park et al., 2012). This suggests that T1SS and T3SS in B. pertussis strains and B. bronchiseptica complex IV strains have a different evolutionary relationship, whilst B. parapertussis strains and B. bronchiseptica complex I isolates, from non-human sources, are very similar to each other. Interestingly, the T2SS-like/T4PS, T4SS, T5aSS and T6SS phylogenetic trees have a structure different from T1SS and T3SS phylogenetic trees. The T2SS-like/T4PS phylogenetic tree is consistent with the genome-wide SNP tree on the branch containing B. parapertussisov strain Bpp5 and B. bronchiseptica complex IV strains. The marked separation of B. bronchiseptica complex I strains from this clade potentially suggests different evolutionary pressures in this lineage. The T4SS loci of B. parapertussis strains and B. pertussis strains are closely related to each other, consistent with their common host adaptation, but B. bronchiseptica strain D445 has a very long branch, suggesting a very different evolutionary history. Intriguingly, the T5aSS tree is drastically different from other trees in that Bbr77, unlike other B. bronchiseptica complex IV strains, appears much closer to B. bronchiseptica RB50 than to B. pertussis strains. In addition, the diversity of T5aSS is more drastic than other secretion systems based on the tree branch lengths, suggesting different evolutionary histories or different selective pressures on these ATs. In the T6SS tree, the B. bronchiseptica strains, including cluster IV isolates (Bbr77 and MO149), are separated from B. parapertussis strains. The different phylogenetic trees for the secretion systems indicate more complex phylogenetic relationships, and highlight the potential contribution of various diversity-generating mechanisms and/or selective pressures acting on each secretion system.
Fig. 4.
Different phylogenetic relationships of 11 classical bordetellae strains based on secretion system loci. The phylogenetic trees of (a) all secretion systems, (b) T1SS, (c) T2SS-like/T4PS, (s) T3SS, (e) T4SS, (f) T5aSS and (g) T6SS were reconstructed by maximum-likelihood methods with 1000 bootstrap replicates based on individual secretion system loci sequences. The strains that contain horizontally transferred secretion system loci are represented by red bars.
To distinguish and compare the relative contributions of recombination and mutation on the Bordetella secretion systems, we assessed the ρ/θ ratio of the secretion systems that occur in clusters of secretion system genes (all but T5aSS, which are not clustered) (Table 2). The T1SS, T2SS-like/T4PS, T3SS and T4SS ρ/θ ratios showed low values, between 0.027 and 0.197, reflecting relatively little recombination in these loci. In contrast, the T6SS ρ/θ ratio was >1 (2.216), suggesting that recombination appears to be the predominant mechanism generating diversity in this locus. This is further supported by the fact that the T6SS locus in the analysed strains is split into two clusters of genes in Bordetella petrii, which is the environmental species of the genus Bordetella, as well as the fact that a cluster of T6SS genes is missing in B. parapertussis strain Bpp5 (Fig. 3). We also analysed each secretion system with Alien_hunter (Vernikos & Parkhill, 2006) to detect horizontally transferred genome segments. Segments of T1SS, T3SS and T4SS loci in B. pertussis, B. parapertussishu and/or B. bronchiseptica complex IV (D445) strains were predicted to be horizontally transferred (Fig. 4, red lines).
Table 2. Recombination/mutation (ρ/θ) ratio of individual secretion systems, except the T5aSS, in the classical bordetellae strains as calculated by rdp4.
| Secretion system | ρ/θ |
|---|---|
| T1SS | 0.065 |
| T2SS-like/T4PS | 0.197 |
| T3SS | 0.062 |
| T4SS | 0.027 |
| T5aSS | – |
| T6SS | 2.216 |
Discussion
In theory, the simple function of secreting proteins could be performed by a single complete secretion system. However, the different important roles in bacterial pathogenesis that each of several secretion systems plays indicate that they do much more than simply transport molecules out of bacteria. Individual dedicated secretion systems have been shown to mediate complex interactions with the host immune system and intense competition with other bacteria (Tseng et al., 2009), and it is likely that we are only beginning to understand their roles and functions. The six possible secretion systems described in the bordetellae are poorly understood, but several are already known to be important during infection. As these systems are encoded by many genes and can be very energetically costly, they are likely to be rapidly lost as lineages diverge and adapt to lifestyles that do not require them. Therefore, the differential conservation, divergence or loss of secretion system genes is likely to be related to the recent divergence of the bordetellae, which differ in some of the most interesting characteristics of pathogens, including host range, virulence and persistence.
The classical bordetellae contain three possible Sec-dependent secretion systems, i.e. the T2SS-like/T4PS, T4SS and T5aSS, and three Sec-independent secretion systems, i.e. the T1SS, T3SS and T6SS (Costa et al., 2015; Locht et al., 2011; Pantoja et al., 2002). These secretion systems are very similar to other bacterial secretion systems in that many core components are highly conserved amongst all species and strains. For example, most secretion systems contain all the known essential components in the systems (Figs 1 and 3). Interestingly, the T2SS-like/T4PS of B. bronchiseptica strain RB50 has an incomplete set of T2SS core components and appears to be closely related to the T4PS. In contrast, the loci encoding the T3SS and the T6SS contain more genes than the corresponding loci in other bacteria, suggesting that they encode proteins that have species-specific activities that could add accessory functions to the systems. In particular, there are five genes in the T6SS locus that do not have any previously known bacterial functional domains, suggesting novel functions (Table S1). We also identified putative exported proteins, chaperones and flagellar proteins that are associated with secretion systems and may mediate new secretion system functions (Fig. 2, Table 1). Thus, confirming and understanding the role of these novel putative proteins are likely to shed light on Bordetella-specific secretion system functions.
In addition to the various novel potential components of these secretion systems, we also noted substantial variations in these loci (Fig. 3). The fact that the T3SS is the most conserved secretion system amongst the classical bordetellae confirms the significant role of this system in the many shared characteristics of these common and successful mammalian respiratory pathogens (Marshall & Finlay, 2014). Some of the variations observed in other secretion systems are likely to contribute to the known phenotypic differences amongst these species. For example, B. bronchiseptica strain 253 and closely related strains that are missing the T1SS required for ACT secretion are non-haemolytic and hypovirulent in mice (Buboltz et al., 2008). B. pertussis strains and B. parapertussishu strain 12822 are missing the entire T2SS-like/T4PS locus, which appears to have been lost by each lineage during their independent adaptation to an apparently closed life cycle in exclusively human hosts (Parkhill et al., 2003). Intriguingly, B. bronchiseptica complex IV strains are missing either the T4SS (Bbr77 and MO149) or the T6SS (D445), but are hypervirulent in the mouse model of infection (Ahuja et al., 2012; Buboltz et al., 2008). This phenotype is associated with increased expression of T3SS effectors, perhaps implicating these secretion systems in some complex immunomodulation (Ahuja et al., 2012; Buboltz et al., 2008).
It is not surprising to see much greater diversity amongst the T5aSS genes, as they encode ATs that act independently and are scattered across the genomes. Consistent with their independent functions, many of these T5aSS genes are either lost or inactivated in various strains, including B. pertussis and B. parapertussis strains (Fig. 3) (Park et al., 2012; Parkhill et al., 2003). Interestingly, components of the T6SS either are lost or are pseudogenes in B. pertussis and B. parapertussis strains as well as B. bronchiseptica strain D445, which was also isolated from a human, suggesting the T6SS may be expendable or counter-selected during human infection. Although B. bronchisepticahu strains Bbr77 and MO149 do not seem to be much different, they are a little bit more divergent (between 95.48 and 99.7 % identity) than other B. bronchiseptica strains, 253 and 1289 (between 97.5 and 100 % identity). T6SSs are known to play an important role in the complex competition between bacteria and the observation that the T6SS common to other Burkholderia species was lost during the adaptation of Burkholderia mallei to a human host has been attributed to the loss of competition with other micro-organisms. Similarly, loss of the T6SS by several human-adapted Bordetella lineages suggests this specialization has eliminated some source of bacterial competition, possibly in some extra-host environmental niche.
It appears that mutation, recombination and horizontal gene transfer contribute to the different evolutionary histories of individual secretion systems in the classical bordetellae (Figs 3 and 4, Table 2). Mutation appears to be the dominant force contributing to the diversification of T1SS–T5SS. In contrast, the T6SS has accumulated more changes due to recombination, consistent with prior descriptions of recombination of the more distantly related species B. petrii (data not shown). In fact, B. parapertussisov strain Bpp5 is missing some genes in the middle of the T6SS locus (Fig. 3), although B. parapertussishu retains these components. The loss of these genes correlates with the specialization of B. parapertussisov to cause pneumonia in sheep. It is still to be seen whether the lost components contribute some accessory functions not necessary for other core functions that remain important for the sheep pathogen or whether the remaining genes have no function and represent a locus in decay.
The presence/absence and relative conservation of each of the possible six secretion systems are likely to relate to the differential need for each in the natural history of the classical bordetellae, which have recently adapted different strategies and virulence characteristics to become some of the most successful respiratory commensals and/or pathogens of a wide range of hosts, including humans. This comparative analysis should guide the study of the roles of each secretion system in some of the most important aspects of bacterial pathogens.
Acknowledgements
We acknowledge members of the Harvill Lab for helpful discussion of the project, and especially Drs Sara E. Hester, Sarah J. Muse, Laura L. Goodfield and Bodo Linz for their critical review of the manuscript. This work was supported by the National Institutes of Health (5R01GM083113) and by the Agriculture and Food Research Initiative Competitive Grants Program (2010-65110-20488) of the US Department of Agriculture, National Institute of Food and Agriculture.
Supplementary Data
Supplementary Data
Abbreviations:
- ACT
adenylate cyclase toxin
- AT
autotransporter
- hu
human
- ov
ovine
- PTX
pertussis toxin
- T4PS
type 4 pilus assembly system
- TPS
two-partner secretion
- TxSS
type x secretion system
References
- Abby S. S., Rocha E. P. C. (2012). The non-flagellar type III secretion system evolved from the bacterial flagellum and diversified into host-cell adapted systems PLoS Genet 8e1002983. 10.1371/journal.pgen.1002983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahuja U., Liu M., Tomida S., Park J., Souda P., Whitelegge J., Li H., Harvill E. T., Parkhill J., Miller J. F. (2012). Phenotypic and genomic analysis of hypervirulent human-associated Bordetella bronchiseptica BMC Microbiol 12167. 10.1186/1471-2180-12-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. (1990). Basic local alignment search tool J Mol Biol 215403–410 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Bernstein H. D. (2007). Are bacterial ‘autotransporters’ really transporters? Trends Microbiol 15441–447 10.1016/j.tim.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Bingle L. E., Bailey C. M., Pallen M. J. (2008). Type VI secretion: a beginner's guide Curr Opin Microbiol 113–8 10.1016/j.mib.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Buboltz A. M., Nicholson T. L., Parette M. R., Hester S. E., Parkhill J., Harvill E. T. (2008). Replacement of adenylate cyclase toxin in a lineage of Bordetella bronchiseptica J Bacteriol 1905502–5511 10.1128/JB.00226-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buboltz A. M., Nicholson T. L., Weyrich L. S., Harvill E. T. (2009). Role of the type III secretion system in a hypervirulent lineage of Bordetella bronchiseptica Infect Immun 773969–3977 10.1128/IAI.01362-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambronne E. D., Roy C. R. (2006). Recognition and delivery of effector proteins into eukaryotic cells by bacterial secretion systems Traffic 7929–939 10.1111/j.1600-0854.2006.00446.x. [DOI] [PubMed] [Google Scholar]
- Carver T. J., Rutherford K. M., Berriman M., Rajandream M.-A., Barrell B. G., Parkhill J. (2005). ACT: the Artemis Comparison Tool Bioinformatics 213422–3423 10.1093/bioinformatics/bti553. [DOI] [PubMed] [Google Scholar]
- Cascales E. (2008). The type VI secretion toolkit EMBO Rep 9735–741 10.1038/embor.2008.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (2015a). Pertussis: outbreaks.http://www.cdc.gov/pertussis/outbreaks/trends.html..
- CDC (2015b). Pertussis in Other Countries.http://www.cdc.gov/pertussis/countries.html..
- Christie P. J., Cascales E. (2005). Structural and dynamic properties of bacterial type IV secretion systems Mol Membr Biol 2251–61 10.1080/09687860500063316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie P. J., Atmakuri K., Krishnamoorthy V., Jakubowski S., Cascales E. (2005). Biogenesis, architecture, and function of bacterial type IV secretion systems Annu Rev Microbiol 59451–485 10.1146/annurev.micro.58.030603.123630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie P. J., Whitaker N., González-Rivera C. (2014). Mechanism and structure of the bacterial type IV secretion systems Biochim Biophys Acta 18431578–1591 10.1016/j.bbamcr.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianciotto N. P. (2005). Type II secretion: a protein secretion system for all seasons Trends Microbiol 13581–588 10.1016/j.tim.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Cornelis G. R. (2006). The type III secretion injectisome Nat Rev Microbiol 4811–825 10.1038/nrmicro1526. [DOI] [PubMed] [Google Scholar]
- Costa T. R. D., Felisberto-Rodrigues C., Meir A., Prevost M. S., Redzej A., Trokter M., Waksman G. (2015). Secretion systems in Gram-negative bacteria: structural and mechanistic insights Nat Rev Microbiol 13343–359 10.1038/nrmicro3456. [DOI] [PubMed] [Google Scholar]
- Dautin N., Bernstein H. D. (2007). Protein secretion in gram-negative bacteria via the autotransporter pathway Annu Rev Microbiol 6189–112 10.1146/annurev.micro.61.080706.093233. [DOI] [PubMed] [Google Scholar]
- Delepelaire P. (2004). Type I secretion in gram-negative bacteria Biochim Biophys Acta 1694149–161 10.1016/j.bbamcr.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Diavatopoulos D. A., Cummings C. A., Schouls L. M., Brinig M. M., Relman D. A., Mooi F. R. (2005). Bordetella pertussis, the causative agent of whooping cough, evolved from a distinct, human-associated lineage of B. bronchiseptica PLoS Pathog 1e45. 10.1371/journal.ppat.0010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diepold A., Wagner S. (2014). Assembly of the bacterial type III secretion machinery FEMS Microbiol Rev 38802–822 10.1111/1574-6976.12061. [DOI] [PubMed] [Google Scholar]
- Fauvart M., Michiels J. (2008). Rhizobial secreted proteins as determinants of host specificity in the rhizobium–legume symbiosis FEMS Microbiol Lett 2851–9 10.1111/j.1574-6968.2008.01254.x. [DOI] [PubMed] [Google Scholar]
- Fennelly N. K., Sisti F., Higgins S. C., Ross P. J., van der Heide H., Mooi F. R., Boyd A., Mills K. H. (2008). Bordetella pertussis expresses a functional type III secretion system that subverts protective innate and adaptive immune responses Infection and Immunity 761257–1266 10.1016/j.bbamcr.2004.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filloux A. (2004). The underlying mechanisms of type II protein secretion Biochim Biophys Acta 1694163–179 10.1016/j.bbamcr.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Filloux A., Hachani A., Bleves S. (2008). The bacterial type VI secretion machine: yet another player for protein transport across membranes Microbiology 1541570–1583 10.1099/mic.0.2008/016840-0. [DOI] [PubMed] [Google Scholar]
- Fronzes R., Schäfer E., Wang L., Saibil H. R., Orlova E. V., Waksman G. (2009). Structure of a type IV secretion system core complex Science 323266–268 10.1126/science.1166101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawarzewski I., Smits S. H. J., Schmitt L., Jose J. (2013). Structural comparison of the transport units of type V secretion systems Biol Chem 3941385–1398 10.1515/hsz-2013-0162. [DOI] [PubMed] [Google Scholar]
- Grant S. R., Fisher E. J., Chang J. H., Mole B. M., Dangl J. L. (2006). Subterfuge and manipulation: type III effector proteins of phytopathogenic bacteria Annu Rev Microbiol 60425–449 10.1146/annurev.micro.60.080805.142251. [DOI] [PubMed] [Google Scholar]
- Heininger U., Cotter P. A., Fescemyer H. W., Martinez de Tejada G., Yuk M. H., Miller J. F., Harvill E. T. (2002). Comparative phenotypic analysis of the Bordetella parapertussis isolate chosen for genomic sequencing Infect Immun 703777–3784 10.1128/IAI.70.7.3777-3784.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson I. R., Navarro-Garcia F., Desvaux M., Fernandez R. C., Ala'Aldeen D. (2004). Type V protein secretion pathway: the autotransporter story Microbiol Mol Biol Rev 68692–744 10.1128/MMBR.68.4.692-744.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland I. B., Schmitt L., Young J. (2005). Type 1 protein secretion in bacteria, the ABC-transporter dependent pathway Mol Membr Biol 2229–39 10.1080/09687860500042013. [DOI] [PubMed] [Google Scholar]
- Hood R. D., Singh P., Hsu F., Güvener T., Carl M. A., Trinidad R. R. S., Silverman J. M., Ohlson B. B., Hicks K. G., other authors (2010). A type VI secretion system of Pseudomonas aeruginosa targets a toxin to bacteria Cell Host Microbe 725–37 10.1016/j.chom.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob-Dubuisson F., Fernandez R., Coutte L. (2004). Protein secretion through autotransporter and two-partner pathways Biochim Biophys Acta 1694235–257 10.1016/j.bbamcr.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Julio S. M., Cotter P. A. (2005). Characterization of the filamentous hemagglutinin-like protein FhaS in Bordetella bronchiseptica Infect Immun 734960–4971 10.1128/IAI.73.8.4960-4971.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korotkov K. V., Sandkvist M., Hol W. G. J. (2012). The type II secretion system: biogenesis, molecular architecture and mechanism Nat Rev Microbiol 10336–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laoide B. M., Ullmann A. (1990). Virulence dependent and independent regulation of the Bordetella pertussis cya operon EMBO J 9999–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Blastier S., Hamels A., Cabeen M., Schille L., Tilquin F., Dieu M., Raes M., Matroule J.-Y. (2010). Phosphate starvation triggers production and secretion of an extracellular lipoprotein in Caulobacter crescentus PLoS One 5e14198. 10.1371/journal.pone.0014198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leo J. C., Grin I., Linke D. (2012). Type V secretion: mechanism(s) of autotransport through the bacterial outer membrane Philos Trans R Soc Lond B Biol Sci 3671088–1101 10.1098/rstb.2011.0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locht C., Coutte L., Mielcarek N. (2011). The ins and outs of pertussis toxin FEBS J 2784668–4682 10.1111/j.1742-4658.2011.08237.x. [DOI] [PubMed] [Google Scholar]
- Mahmoud K. K., Koval S. F. (2010). Characterization of type IV pili in the life cycle of the predator bacterium Bdellovibrio Microbiology 1561040–1051 10.1099/mic.0.036137-0. [DOI] [PubMed] [Google Scholar]
- Marr N., Oliver D. C., Laurent V., Poolman J., Denoël P., Fernandez R. C. (2008). Protective activity of the Bordetella pertussis BrkA autotransporter in the murine lung colonization model Vaccine 264306–4311 10.1016/j.vaccine.2008.06.017. [DOI] [PubMed] [Google Scholar]
- Marshall N. C., Finlay B. B. (2014). Targeting the type III secretion system to treat bacterial infections Expert Opin Ther Targets 18137–152. [DOI] [PubMed] [Google Scholar]
- Martin D. P., Lemey P., Lott M., Moulton V., Posada D., Lefeuvre P. (2010). rdp3: a flexible and fast computer program for analyzing recombination Bioinformatics 262462–2463 10.1093/bioinformatics/btq467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattoo S., Cherry J. D. (2005). Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies Clin Microbiol Rev 18326–382 10.1128/CMR.18.2.326-382.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mota L. J., Journet L., Sorg I., Agrain C., Cornelis G. R. (2005). Bacterial injectisomes: needle length does matter Science 3071278. 10.1126/science.1107679. [DOI] [PubMed] [Google Scholar]
- Nicholson T. L. (2007). Construction and validation of a first-generation Bordetella bronchiseptica long-oligonucleotide microarray by transcriptional profiling the Bvg regulon BMC Genomics 8220. 10.1186/1471-2164-8-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nivaskumar M., Francetic O. (2014). Type II secretion system: a magic beanstalk or a protein escalator Biochim Biophys Acta 18431568–1577 10.1016/j.bbamcr.2013.12.020. [DOI] [PubMed] [Google Scholar]
- Noofeli M., Bokhari H., Blackburn P., Roberts M., Coote J. G., Parton R. (2011). BapC autotransporter protein is a virulence determinant of Bordetella pertussis Microb Pathog 51169–177 10.1016/j.micpath.2011.04.004. [DOI] [PubMed] [Google Scholar]
- O'Callaghan D., Cazevieille C., Allardet-Servent A., Boschiroli M. L., Bourg G., Foulongne V., Frutos P., Kulakov Y., Ramuz M. (1999). A homologue of the Agrobacterium tumefaciens VirB and Bordetella pertussis Ptl type IV secretion systems is essential for intracellular survival of Brucella suis Mol Microbiol 331210–1220 10.1046/j.1365-2958.1999.01569.x. [DOI] [PubMed] [Google Scholar]
- Pantoja M., Chen L., Chen Y., Nester E. W. (2002). Agrobacterium type IV secretion is a two-step process in which export substrates associate with the virulence protein VirJ in the periplasm Mol Microbiol 451325–1335 10.1046/j.1365-2958.2002.03098.x. [DOI] [PubMed] [Google Scholar]
- Park J., Zhang Y., Buboltz A. M., Zhang X., Schuster S. C., Ahuja U., Liu M., Miller J. F., Sebaihia M., other authors (2012). Comparative genomics of the classical Bordetella subspecies: the evolution and exchange of virulence-associated diversity amongst closely related pathogens BMC Genomics 13545. 10.1186/1471-2164-13-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhill J., Sebaihia M., Preston A., Murphy L. D., Thomson N., Harris D. E., Holden M. T. G., Churcher C. M., Bentley S. D., other authors (2003). Comparative analysis of the genome sequences of Bordetella pertussis, Bordetella parapertussis and Bordetella bronchiseptica Nat Genet 3532–40 10.1038/ng1227. [DOI] [PubMed] [Google Scholar]
- Peabody C. R., Chung Y. J., Yen M.-R., Vidal-Ingigliardi D., Pugsley A. P., Saier M. H. J., Jr (2003). Type II protein secretion and its relationship to bacterial type IV pili and archaeal flagella Microbiology 1493051–3072 10.1099/mic.0.26364-0. [DOI] [PubMed] [Google Scholar]
- Pittman M. (1984). The concept of pertussis as a toxin-mediated disease Pediatr Infect Dis 3467–486 10.1097/00006454-198409000-00019. [DOI] [PubMed] [Google Scholar]
- Pittman M. (1986). Neurotoxicity of Bordetella pertussis Neurotoxicology 753–67. [PubMed] [Google Scholar]
- Pukatzki S., Ma A. T., Revel A. T., Sturtevant D., Mekalanos J. J. (2007). Type V secretion system translocates a phage tail spike-like protein into target cells where it cross-links actin Proc Natl Acad Sci U S A 10415508–15513 10.1073/pnas.0706532104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambow-Larsen A. A., Weiss A. A. (2004). Temporal expression of pertussis toxin and Ptl secretion proteins by Bordetella persussis J Bacteriol 18643–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team (2008). R: a Language and Environment for Statistical Computing Vienna: R Foundation for Statistical Computing. [Google Scholar]
- Reddy J. D., Reddy S. L., Hopkins D. L., Gabriel D. W. (2007). TolC is required for pathogenicity of Xylella fastidiosa in Vitis vinifera grapevines Mol Plant Microbe Interact 20403–410 10.1094/MPMI-20-4-0403. [DOI] [PubMed] [Google Scholar]
- Russell A. B., Hood R. D., Bui N. K., LeRoux M., Vollmer W., Mougous J. D. (2011). Type VI secretion delivers bacteriolytic effectors to target cells Nature 475343–347 10.1038/nature10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr (2004). Evolution of bacterial type III protein secretion systems Trends Microbiol 12113–115 10.1016/j.tim.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Sandkvist M. (2001). Biology of type II secretion Mol Microbiol 40271–283 10.1046/j.1365-2958.2001.02403.x. [DOI] [PubMed] [Google Scholar]
- Schwarz S., West T. E., Boyer F., Chiang W.-C., Carl M. A., Hood R. D., Rohmer L., Tolker-Nielsen T., Skerrett S. J., Mougous J. D. (2010). Burkholderia type VI secretion systems have distinct roles in eukaryotic and bacterial cell interactions PLoS Pathog 6e1001068. 10.1371/journal.ppat.1001068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebaihia M., Preston A., Maskell D. J., Kuzmiak H., Connell T. D., King N. D., Orndorff P. E., Miyamoto D. M., Thomson N. R., other authors (2006). Comparison of the genome sequence of the poultry pathogen Bordetella avium with those of B. bronchiseptica, B. pertussis and B. parapertussis reveals extensive diversity in surface structures associated with host interaction J Bacteriol 1886002–6015 10.1128/JB.01927-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger M. A., Schiefner A., Eicher T., Verrey F., Diederichs K., Pos K. M. (2006). Structural asymmetry of AcrB trimer suggests a peristaltic pump mechanism Science 3131295–1298 10.1126/science.1131542. [DOI] [PubMed] [Google Scholar]
- Shrivastava S., Mande S. S. (2008). Identification and functional characterization of gene components of type VI secretion system in bacterial genomes PLoS One 3e2955. 10.1371/journal.pone.0002955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrivastava R., Miller J. F. (2009). Virulence factor secretion and translocation by Bordetella species Curr Opin Microbiol 1288–93 10.1016/j.mib.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snel B., Lehmann G., Bork P., Huynen M. A. (2000). string: a web-server to retrieve and display the repeatedly occurring neighbourhood of a gene Nucleic Acids Res 283442–3444 10.1093/nar/28.18.3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone C. B., Bulir D. C., Gilchrist J. D., Toor R. K., Mahony J. B. (2010). Interactions between flagellar and type III secretion proteins in Chlamydia pneumoniae BMC Microbiol 1018. 10.1186/1471-2180-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S. (2011). mega5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods Mol Biol Evol 282731–2739 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanassi D. G., Hultgren S. J. (2000). Multiple pathways allow protein secretion across the bacterial outer membrane Curr Opin Cell Biol 12420–430 10.1016/S0955-0674(00)00111-3. [DOI] [PubMed] [Google Scholar]
- Thomas S., Holland I. B., Schmitt L. (2014). The type 1 secretion pathway — the hemolysin system and beyond Biochim Biophys Acta 18431629–1641 10.1016/j.bbamcr.2013.09.017. [DOI] [PubMed] [Google Scholar]
- Tseng T.-T., Tyler B. M., Setubal J. C. (2009). Protein secretion systems in bacterial–host associations, and their description in the gene ontology BMC Microbiol 9 (Suppl 1., S2. 10.1186/1471-2180-9-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernikos G. S., Parkhill J. (2006). Interpolated variable order motifs for identification of horizontally acquired DNA: revisiting the Salmonella pathogenicity islands Bioinformatics 222196–2203 10.1093/bioinformatics/btl369. [DOI] [PubMed] [Google Scholar]
- Wang Y., Chen Z., Qiao F., Ying T., Yuan J., Zhong Z., Zhou L., Du X., Wang Z., other authors (2009). Comparative proteomics analyses reveal the virB of B. melitensis affects expression of intracellular survival related proteins PLoS One 4e5368. 10.1371/journal.pone.0005368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A. A., Johnson F. D., Burns D. L. (1993). Molecular characterization of an operon required for pertussis toxin secretion Proc Natl Acad Sci U S A 902970–2974 10.1073/pnas.90.7.2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyrich L. S., Rolin O. Y., Muse S. J., Park J., Spidale N., Kennett M. J., Hester S. E., Chen C., Dudley E. G., Harvill E. T. (2012). A type VI secretion system encoding locus is required for Bordetella bronchiseptica immunomodulation and persistence in vivo PLoS One 7e45892. 10.1371/journal.pone.0045892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H.-Y., Chung P.-C., Shih H.-W., Wen S.-R., Lai E.-M. (2008). Secretome analysis uncovers an Hcp-family protein secreted via a type VI secretion system in Agrobacterium tumefaciens J Bacteriol 1902841–2850 10.1128/JB.01775-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Zhang H., Gao Z.-Q., Wang W.-J., Liu G.-F., Xu J.-H., Su X.-D., Dong Y.-H. (2013). Structure of the type VI effector-immunity complex (Tae4-Tai4) provides novel insights into the inhibition mechanism of the effector by its immunity protein J Biol Chem 2885928–5939 10.1074/jbc.M112.434357. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data