Abstract
Natural scrapie in sheep occurs in classical and atypical forms, which may be distinguished on the basis of the associated neuropathology and properties of the disease-associated prion protein on Western blots. First detected in 1998, atypical scrapie is known to have occurred in UK sheep since the 1980s. However, its aetiology remains unclear and it is often considered as a sporadic, non-contagious disease unlike classical scrapie which is naturally transmissible. Although atypical scrapie tends to occur in sheep of prion protein (PRNP) genotypes that are different from those found predominantly in classical scrapie, there is some overlap so that there are genotypes in which both scrapie forms can occur. In this search for early atypical scrapie cases, we made use of an archive of fixed and frozen sheep samples, from both scrapie-affected and healthy animals (∼1850 individuals), dating back to the 1960s. Using a selection process based primarily on PRNP genotyping, but also on contemporaneous records of unusual clinical signs or pathology, candidate sheep samples were screened by Western blot, immunohistochemistry and strain-typing methods using tg338 mice. We identified, from early time points in the archive, three atypical scrapie cases, including one sheep which died in 1972 and two which showed evidence of mixed infection with classical scrapie. Cases with both forms of scrapie in the same animal as recognizable entities suggest that mixed infections have been around for a long time and may potentially contribute to the variety of scrapie strains.
Introduction
Natural scrapie in sheep is one of a group of diseases, affecting several mammalian species, known as transmissible spongiform encephalopathies (TSEs) or prion diseases. A hallmark of TSEs is the detection in brain (and sometimes also lymphoreticular tissues) of an abnormal form of the prion protein, known by various short forms including PrPSc and PrPd to distinguish it from the normal cellular protein PrP or PrPC (Bolton et al., 1982; Hope et al., 1986). PrPSc is relatively proteinase K-resistant and on Western blots usually has a distinct three-banded pattern (the result of differential glycosylation), and particular patterns and sizes of the bands can be used as part of strain typing of TSEs (Gavier-Widén et al., 2005). Incidence of scrapie is highly dependent on PRNP genotype at codons 136, 154 and 171 with, for example, V136R154Q171/VRQ animals at very high risk of disease, and ARR/ARR and heterozygotes at low risk (for review, see Goldmann, 2008).
A different form of ovine TSE, termed Nor98 or atypical scrapie, was discovered in Norway in 1998 (Benestad et al., 2003). It is biologically, neuropathologically and biochemically distinct from classical natural scrapie (Table S1, available in the online Supplementary Material). For example, atypical scrapie PrPSc is less proteinase K-resistant than classical scrapie PrPSc and has a more variable pattern on Western blots, including characteristic low-molecular-mass band(s) variously estimated at ∼7–12 kDa, not found in classical scrapie (Le Dur et al., 2005). Since 1998, atypical scrapie cases have been identified throughout Europe, including the UK, mainly through active surveillance of asymptomatic sheep (Buschmann et al., 2004; De Bosschere et al., 2004; Nentwig et al., 2007; Orge et al., 2004; Polak et al., 2010). Atypical scrapie tends to occur in older sheep and in animals with PRNP genotypes considered to be resistant to classical scrapie (Benestad et al., 2008; Saunders et al., 2006), such as those with AHQ and ARR alleles. It is also associated with codon 141, which varies only on the ARQ allele, such that genotypes including the AF141RQ allele are more susceptible than those with AL141RQ. There is some overlap of susceptibility, however, as some genotypes are found in both atypical and classical scrapie cases, e.g. VRQ/AL141RQ and AL141RQ/AL141RQ (Fediaevsky et al., 2008). Indeed, there is evidence for both scrapie forms occurring in a single AL141RQ/AF141RQ animal (Mazza et al., 2010).
Incidence of atypical scrapie in the UK is low, but consistent. In 2012 and 2013, it was found in ∼0.1 % of the >18 000 sheep which were tested in abattoir and fallen-stock surveys each year (Ortiz-Pelaez & Arnold, 2013). Atypical scrapie is not thought to be naturally transmissible, although successful experimental transmissions have been achieved in sheep and transgenic mice; in the latter with similar pathology to that seen in the original sheep (Andréoletti et al., 2011; Le Dur et al., 2005; Simmons et al., 2007, 2010).
Originally it was not certain whether atypical scrapie was a newly emerging TSE or whether a pre-existing disease had been identified by increased surveillance. Archive searches found cases in the UK from 1989 (Bruce et al., 2007; Foster et al., 2008) and 1987 (Webb et al., 2009) indicating that it is not a new disease. We took advantage of the Neuropathogenesis Unit (NPU) sheep tissue archive (now stored at the Roslin Institute), which has samples dating back to the 1960s, to search for additional early examples of atypical scrapie in order to establish its history as far as possible. It was of particular interest to look for examples of TSE infections with features of both atypical and classical scrapie as the origin of atypical scrapie is unknown and one possibility is that it developed from a type of classical scrapie. Here, we report evidence for early unusual scrapie cases with individual sheep apparently showing signs of multiple-strain infections.
Results
Selection of candidate atypical scrapie cases
We examined two sheep tissue archives: (1) sheep (n ≈ 350) from throughout the UK and (2) sheep (n ≈ 1500) from our own flock (NPU Cheviots). Samples, which varied considerably in quality due to long-term storage, were put through a non-rigorous selection process designed to maximize the chances of finding atypical scrapie at early dates, but not expected to find every case present. Further details are given in Methods and Fig. S1.
Two UK archive sheep were considered to be candidate atypical scrapie cases. The first, L4824 (AHQ/ARR), was one of a pair of suspect scrapie cases from 1988 from a flock in Scotland, both female Cheviots of unrecorded age. Whilst L4824 had been judged in 1988 to be negative for brain vacuolation in the medulla, the companion case [L4823 (VRQ/AL141RQ)] was diagnosed as classical scrapie based on positive brain vacuolar pathology. Fixed tissue was available for us to carry out immunohistochemistry (IHC) with BG4 antibody and L4824 displayed the disease-related PrP (PrPd) deposition characteristic of atypical scrapie, with marked labelling in the molecular layer in the cerebellum but very little in obex and basal ganglia (Fig. S2). There was also evidence of microvacuolation in the cerebellum, clearly not spotted in 1988. In contrast, L4823 had PrPd labelling in obex and basal ganglia rather than the cerebellum (Fig. S2), which suggested it was classical scrapie.
The second UK archive candidate atypical scrapie case was H800, a female Poll Dorset sheep (VRQ/AF141RQ PRNP genotype) which died in 1977. There is no further recorded information about this animal and no fixed tissue for IHC. It was discovered by Western blot analysis, detailed below.
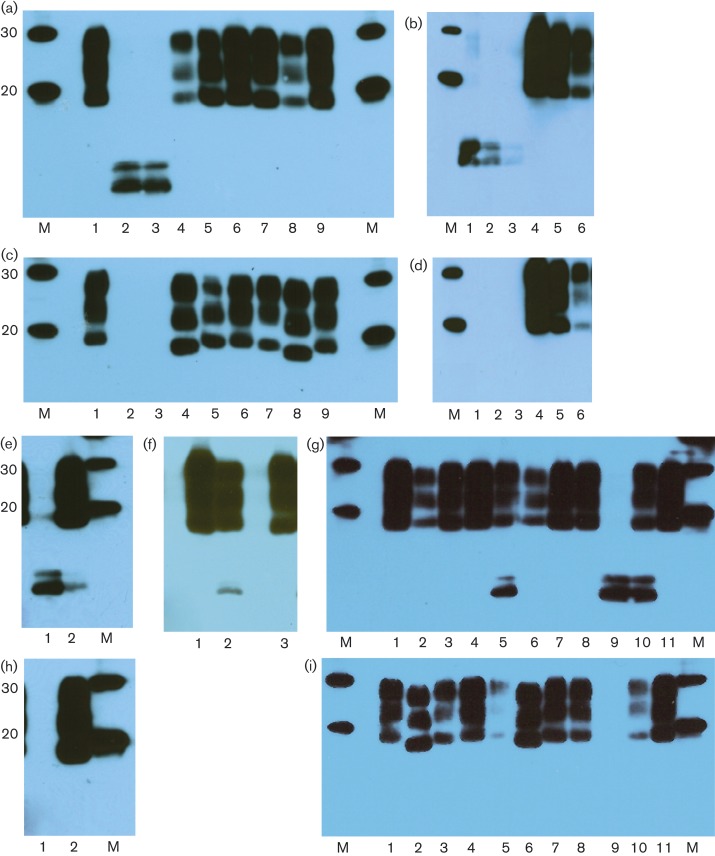
All three sheep, i.e. L4824, L4823 and H800, were positive by Western blot for PrPSc. L4824 displayed a low-molecular-mass PrPSc band (Fig. 1a), estimated on this Western blot at 7–9 kDa, indicative of atypical scrapie and recognized by P4 but not 6H4 antibodies (Fig. 1a, c), similar to cases described previously by ourselves and others (Foster et al., 2008). L4823 had the pattern of PrPSc expected from classical scrapie (Fig. 1a, c). Western blots of H800 with P4 revealed a very faint low-molecular-mass PrPSc band (Fig. 1b), estimated as ∼8 kDa, reminiscent of atypical scrapie and absent with 6H4 (Fig. 1d). Suspecting degradation of protein in storage, we attempted to reproduce the pattern using different sheep brain samples which had been similarly stored, but found only classical scrapie patterns (not shown). H800 was therefore classed as unusual.
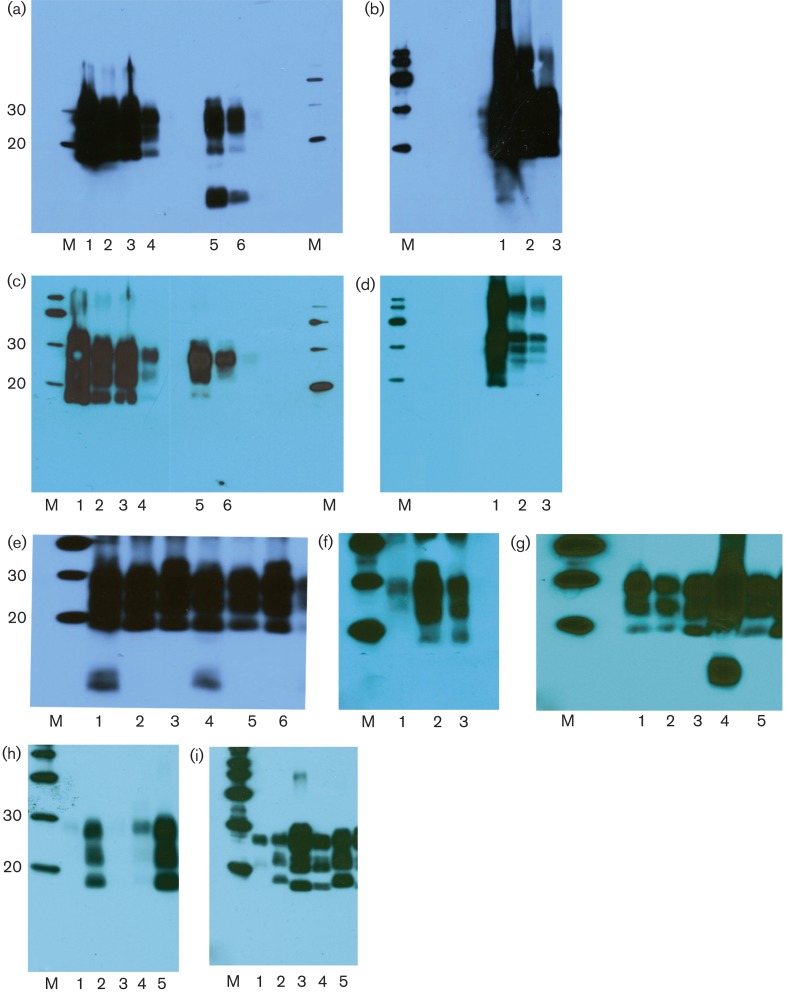
Fig. 1.
(a–i) Western blots of PrPSc from sheep brain of unusual scrapie cases and controls using antibodies P4 (a, b, e, g, h) and 6H4 (c, d, f, i). M, molecular mass markers (kDa). (a, c) Lanes 1–4, L4823, serial 1 : 3 dilutions; lanes 5 and 6, L4824, serial 1 : 3 dilutions. (b, d) Lanes 1–3, H800, serial 1 : 3 dilutions. (e) Lane 1, 13 × 85; lanes 2 and 3, two concurrent natural scrapie sheep; lane 4, 13 × 85, 1 : 3 dilution; lanes 5 and 6, concurrent natural scrapie sheep, 1 : 3 dilutions. (f) Lane 1, unrelated sample; lane 2, SSBP/1; lane 3, 13 × 85. (g) Lanes 1, 3 and 5, natural scrapie controls; lane 2, 13 × 69; lane 4, 13 × 85. (h, i) Lanes 1 and 4, CH1641; lanes 2 and 5, SSBP/1; lane 3, unrelated sample.
In the second archive (NPU Cheviots), of the sheep that died before 1980, a single animal (13 × 85) with P4 showed PrPSc with a clear additional low-molecular-mass band estimated as ∼7–9 kDa (Fig. 1e, g) which was absent with 6H4 (Fig. 1f). This was a female Cheviot (AF141RQ/AF141RQ), which was born in 1966 and died at 6 years of age in 1972. It had been challenged with experimental scrapie (details below), and was recorded in 1972 as having positive clinical signs of scrapie with a ‘very unusual clinical syndrome’ and with ‘widespread moderate lesions’ in the brain. We have no further written records and have no fixed tissue available to re-examine the pathology in this animal.
Sheep 13 × 85 was one of a group of 11 animals which were used in a study of scrapie strain interference and challenged subcutaneously, first with SSBP/1 scrapie and secondly, after 2 years without clinical signs, with CH1641. Three (all AF141RQ/AF141RQ), including 13 × 85, of the 11 sheep became clinically affected with scrapie signs at 1224–1524 days after SSBP/1 challenge and 500–800 days after CH1641 challenge. Only one (13 × 69) of the other two scrapie-affected sheep had stored frozen tissue allowing a comparison by Western blot; however, this sheep showed a classical scrapie-like PrPSc pattern without the ∼7–9 kDa molecular mass band seen with 13 × 85 (Fig. 1g). For comparison, Western blots of both CH1641 and SSBP/1 are also shown in Fig. 1(h, i). Whilst PrPSc from SSBP/1 was recognized by both 6H4 and P4, CH1641 showed much reduced staining with P4 compared with 6H4, which also revealed a lower (∼19 kDa) unglycosylated band than is seen with natural scrapie or SSBP/1.
Strain typing of candidate atypical scrapie cases by mouse bioassay
Non-transgenic inbred mice
Owing to the unusual clinical signs (unfortunately not recorded) in sheep 13 × 85, transmissions were set up in 1972 to a wide range of inbred mouse lines but never previously published. The results from RIII, C57/Bl (Prnpa mice) and VM (Prnpb) (Table 1) showed 100 % attack rate and mean incubation periods of 267 ± 26, 297 ± 25 and 468 ± 73 days, respectively. This was noted at the time as surprising as concurrent transmissions from SSBP/1, CH1641 and natural classical scrapie from an NPU flock VRQ/VRQ sheep (47 × 79) gave much lower attack rates and longer incubation periods (>480 days) (Table 1). Results from more recent atypical scrapie-negative transmissions, including that from sheep 51 × 45, to the same mouse lines are also shown in Table 1.
Table 1. Attack rates and incubation periods of transmissions of sheep brain in three different inbred mouse lines, carried out between 1971 and 2002.
Attack rate is given as number of clinically positive, vacuolation-positive mice (Ns)/number injected (Ni). Incubation periods are given as means when there were more than five positive cases; otherwise individual mouse incubation periods are given. na, Not applicable.
| Inoculum | Transmission date | Mouse line | |||||
|---|---|---|---|---|---|---|---|
| RIII | C57 | VM | |||||
| Attack rate (Ns/Ni) | Incubation period [days (sd)] | Attack rate (Ns/Ni) | Incubation period [days (sd)] | Attack rate (Ns/Ni) | Incubation period [days (sd)] | ||
| 13 × 85, unusual scrapie | 1972 | 6/6 | 267 (26) | 6/6 | 297 (25) | 5/5 | 468 (73) |
| SSBP/1 | 1971 | 2/4 | 486, 505 | 5/5 | 517 (3) | 5/5 | 506 (47) |
| CH1641 | 1989 | 7/18 | 481 (66) | 3/18 | 682, 689, 764 | 3/18 | 717, 717, 683 |
| 47 × 79, classical scrapie, natural | 1994 | 0/23 | na | 9/23 | 592 (83) | 3/15 | 544, 596, 604 |
| Scr2, atypical scrapie* | 1990 | 0/23 | na | 0/13 | na | 0/18 | na |
| 51 × 45, atypical scrapie | 2002 | 0/23 | na | 0/19 | na | 0/24 | na |
Data adapted from Bruce et al. (2002).
Transgenic mice (tg338)
We did not attempt additional strain typing in WT mice as atypical scrapie, and frequently also classical scrapie, does not transmit well to non-transgenic mice (see Table 1). As a consequence, the scrapie cases selected for further study (L4824, H800 and 13 × 85) were transmitted to tg338 mice, which express the ovine VRQ allotype (Le Dur et al., 2005), and compared with classical and atypical scrapie controls, also in tg338 mice, including L4823 as a concurrent flock-mate control for L4824. We were not able to perform such transmissions with the similar control for 13 × 85 (13 × 69) because no sterile material suitable for bioassay was available.
Incubation period results are shown in Table 2 and a summary is given in Table 3. The classical scrapie control (68 × 81) had a very long incubation period of 584 ± 57 days. In contrast, SSBP/1, which is a ‘rapid strain’ in tg338 mice, gave the expected short incubation period of 76 ± 7 days. CH1641 and the two atypical isolates, Scr2 and 51 × 45, all gave clinically positive mice with medium incubation periods of 157 ± 3, 191 ± 46 and 175 ± 23 days, respectively. The unusual cases L4824 and 13 × 85 also gave medium incubation periods of 173 ± 13 and 135 ± 14 days, respectively, although 13 × 85 gave an attack rate of < 50 %. H800 also had a low attack rate ( < 50 %) and a wide range of medium incubation periods (276 ± 113 days), and L4823 produced very low numbers (two out of 11) of affected animals, with long and very long incubation periods of 364 and 572 days.
Table 2. Origins and characteristics of the sheep inocula and tg338 mouse transmission features (attack rate and incubation period).
Attack rate is given as number of clinically positive, vacuolation-positive mice (Ns)/number injected (Ni). Incubation periods are given as means when there were more than five positive cases; otherwise individual mouse incubation periods are given. na, Not applicable.
| Inoculum and/or sheep identity | Scrapie type | Origin (year of death) | Genotype | Attack rate in tg338 mice (Ns/Ni) | Mean incubation period [days (sd)] | Range, or actual incubation period if fewer than five mice (days) |
|---|---|---|---|---|---|---|
| 68 × 81 | Classical 21 kDa, natural | NPU flock (2000) | VRQ/VRQ | 12/12 | 584 (57) | 482–677 |
| SSBP/1 | Classical 21 kDa, rapid | NPU (pre-1960) | Pooled clinical sheep brain (all VRQ encoding) | 6/6 | 76 (7) | 64–83 |
| CH1641 (J2916) | Classical, 19 kDa | NPU (2000) | ARQ/AHQ | 12/12 | 157 (3) | 155–163 |
| Scr2 | Atypical | UK (1989) | AHQ/AHQ | 10/11 | 191 (46) | 165–310 |
| 51 × 45 | Atypical | NPU flock (2001) | AHQ/AHQ | 11/12 | 175 (23) | 150–217 |
| L4824 | Unusual case | UK (1988) | AHQ/ARR | 10/11 | 173 (13) | 151–196 |
| L4823 | L4824 flock-mate | UK (1988) | VRQ/AL141RQ | 2/11 | na | 364, 572 |
| H800 | Unusual case | UK (1977) | VRQ/AF141RQ | 5/11 | 276 (113) | 163–462 |
| 13 × 85 | Unusual case | NPU (1972) | AF141RQ/AF141RQ | 5/11 | 135 (14) | 125–160 |
Table 3. Summary of all data from sheep brain samples and transmissions to WT and transgenic mice (tg338), and conclusions on strain typing.
Summary of data from three WT mouse lines abstracted from Table 1. Incubation period (VL, very long; L, long; M, medium; S, short) and IHC designations are as defined in text. Western blot data represent lowest molecular mass (kDa) of PrPSc labelled by either of the antibodies 6H4 or P4. Attack rate is as defined in Tables 1 and 2. nd, Not done; na, not applicable; X, nothing labelled on the Western blot.
| Inoculum | Sheep, source of inoculum | WT mice, three lines | tg338 mice | Conclusion on strain type | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IHC | Western blots: lowest-molecular-mass band (kDa) | Attack rate (%) | Incubation period | Attack rate (%) | Incubation period | Vacuolation profile type | IHC type | Western blots: lowest-molecular-mass band (kDa) | ||||
| 6H4 | P4 | 6H4 | P4 | |||||||||
| Classical scrapie: 68 × 81(WT mice) and 47 × 79 (tg338) | Classical scrapie | 21 | 21 | 0–39 | VL | 100 | VL | B1 | B1 | 21 | 21 | Classical (1) |
| SSBP/1 | Classical scrapie | 21 | 21 | 50–100 | VL | 100 | S | B2 | I | 21 | 21 | Classical (2) |
| CH1641 (J2916) | Classical scrapie | 19 | X | 17–39 | VL | 100 | M | B1 | I | 19 | 21 | Classical (3) |
| Scr2 | Atypical scrapie | X | 7–9 | 0 | na | 91 | M | A | A | X | 7–9 | Atypical |
| 51 × 45 | Atypical scrapie | X | 7–9 | 0 | na | 92 | M | A | A | X | 7–9 | Atypical |
| L4824 | Atypical scrapie | 21 | 7–9 | nd | na | 91 | M | A | A | X | 7–9 | Atypical |
| L4823 | Classical scrapie | 21 | 21 | nd | na | 18 | L | A+B1 | A+B1 | 21 | 7–9 | Mixed atypical and classical (1) |
| H800 | nd | 21 | ∼8 | nd | na | 45 | L | B1 | B1 | 21 | 21 | Classical (1) |
| 13 × 85 | nd | 21 | 7–9 | 100 | L | 45 | M | Mixed, A+another strain: B1 and/or B2 | A+I | 21 | 7–9 | Mixed atypical and classical (2/3) |
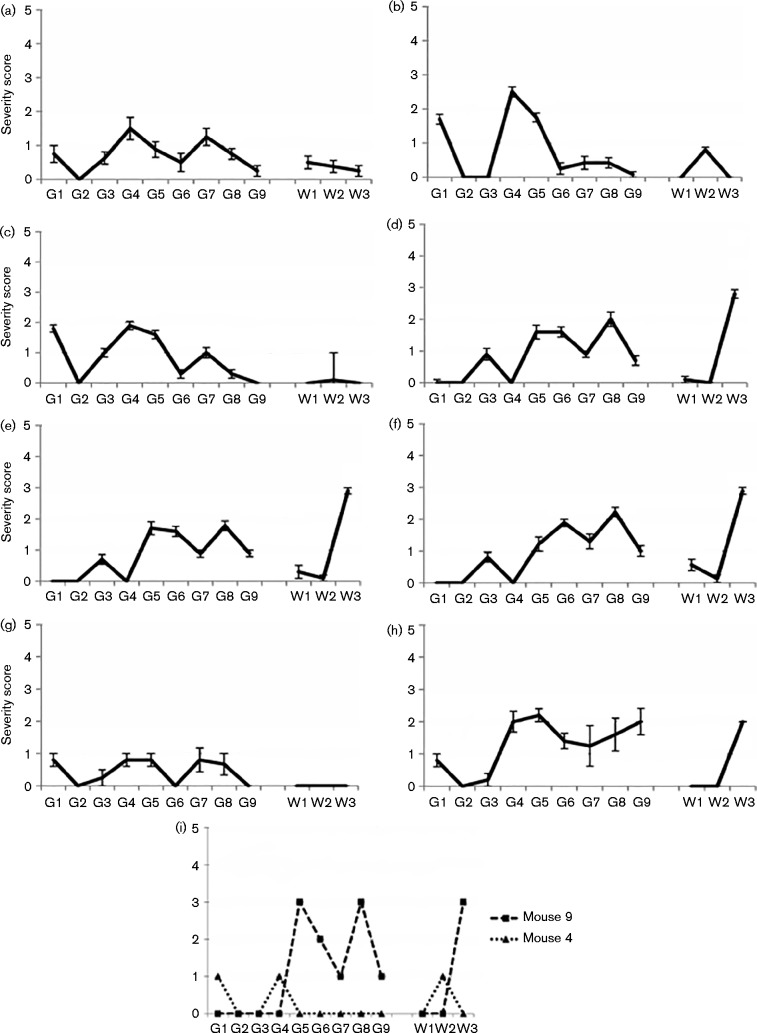
Vacuolation profiles in the clinically affected mice are illustrated in Fig. 2. The natural classical scrapie control (68 × 81; Fig. 2a) showed a fairly flat lesion profile similar to CH1641 (Fig. 2c) and is arbitrarily designated type B1 in summary Table 3. SSBP/1 (Fig. 2b) showed more vacuolation in regions G4 and G5 (type B2 in Table 3). The two atypical scrapie controls, Scr2 and 51 × 45, gave very similar and characteristic lesion profiles (Fig. 2d, e, respectively; type A in Table 3), particularly striking in the damage caused in the white matter area W3 (basal cerebral peduncle), as expected (Griffiths et al., 2010). Of the unusual scrapie transmissions, the vacuolar profile of L4824 (Fig. 2f) was very similar to that of atypical scrapie (type A), H800 (Fig. 2g) resembled classical scrapie (type B1) and 13 × 85 (Fig. 2h) had similarities to atypical scrapie in white matter damage, but in grey matter looked different from any of the other tested strains (designated mixed type in Table 3). L4823 produced only two clinical cases so no lesion profile could be produced for direct comparison; however, the lesion damage patterns from each individual clinical mouse are shown in Fig. 2(i). L4823 mouse 4 (L4823/4), incubation period 572 days, showed pathology resembling natural scrapie (type B1), whereas mouse 9 (L4823/9), incubation period 364 days, had a distinct pattern very like atypical scrapie (type A).
Fig. 2.
Lesion profiles of unusual scrapie cases and controls in brain of clinically affected tg338 mice. Patterns of severity of vacuolation (with se bars) in nine grey matter areas (G1–G9: medulla, cerebellum, superior colliculus, hypothalamus, thalamus, hippocampus, septum, retrosplenial cortex, and cingulate and motor cortex) and three white matter areas (W1–W3: cerebellum, superior cerebral peduncle and basal cerebral peduncle). (a) Classical scrapie 68 × 81, (b) SSBP/1, (c) CH1641, (d) atypical scrapie Scr2, (e) atypical scrapie 51 × 45, (f) L4824, (g) H800, (h) 13 × 85 and (i) vacuolation damage in two individual clinically positive mice inoculated with L4823 [mouse 4 (L4823/4) and mouse 9 (L4823/9)].
IHC on tg338 mouse transmissions
With small variations in magnitude, mice within each inoculation group showed indistinguishable patterns and distribution of PrPd accumulation (identical with 2G11 and SAF84), except for the two positive L4823 mice, as detailed below (Table S2, Figs 3 and 4). Mice infected with either of the two atypical scrapie controls Scr2 (Fig. 3a) and 51 × 45 (not shown) and L4824 (Fig. 3c) had prominent, multifocal and bilateral coalescing PrPd aggregates in the thalamus which in some mice had the appearance of non-vascular plaque-like deposits. In the most severe cases, those coalescing accumulations were present also in neighbouring areas of the parietal cerebral cortex. Fine particulate PrPd deposits were also found in the same locations, whilst other brain areas appeared devoid of PrPd. Intracellular deposits were inconspicuous. Vacuolation was very severe (Table S2), particularly in white matter tracts of the thalamus and midbrain, but was also present in the cerebellum (grey and white matter) and other brain areas. This IHC pattern is designated type A in summary Table 3.
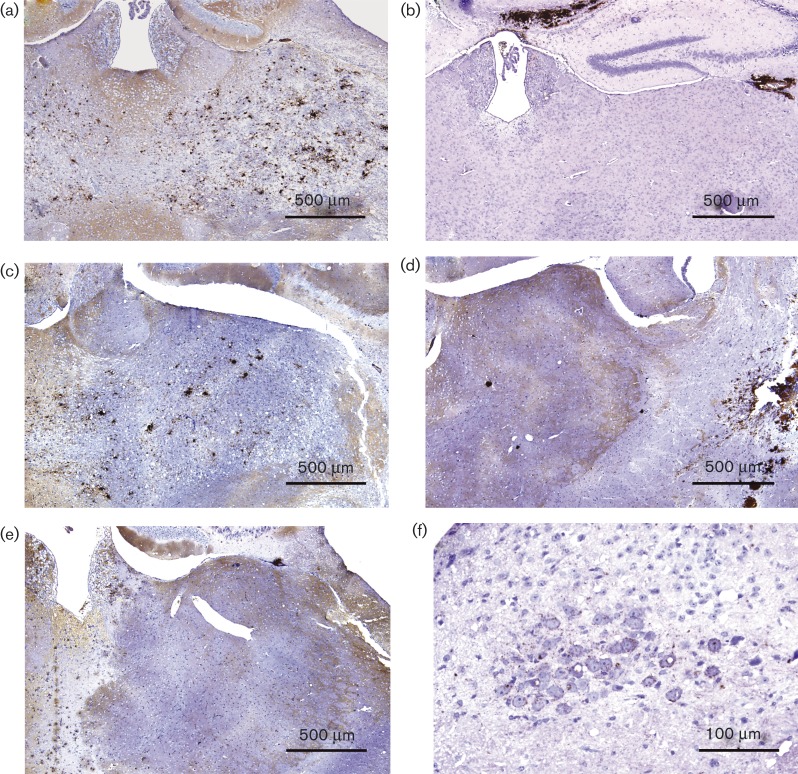
Fig. 3.
Immunohistochemical PrPd features of tg338 mice. Thalamus from mice inoculated with (a) atypical scrapie (Scr2), (b) classical scrapie (68 × 81), (c) L4824, (d) H800, (e) L4823/9 and (f) dorsal motor nucleus of the vagus from mouse inoculated with SSBP/1. IHC with 2G11 or (f only) SAF84 with haematoxylin counterstaining.
The IHC patterns in the classical scrapie control 61 × 81 (Fig. 3b), H800 (Fig. 3d) and mouse L4823/4 (Fig. 3e) were, in contrast, dominated by vascular and non-vascular PrPd plaques located at the injection site, and in subependymal and subpial areas throughout the brain, without involvement of the thalamus. Moderate fine particulate PrPd deposits and mild to moderate intracellular PrPd aggregates were observed in vestibular nuclei, habenula, hypothalamus, midbrain and cerebral cortex. In some mice, intracellular aggregates were also seen in obex and deep cerebellar nuclei. Vacuolation was mild to moderate (Table S2). This IHC pattern is designated type B1 in Table 3.
SSBP/1 and CH1641 were characterized by absence of plaques or coalescing PrPd deposits, and mild to moderate particulate and intracellular aggregates (Fig. 3f) with very little vacuolation (Table S2), designated IHC type I in Table 3.
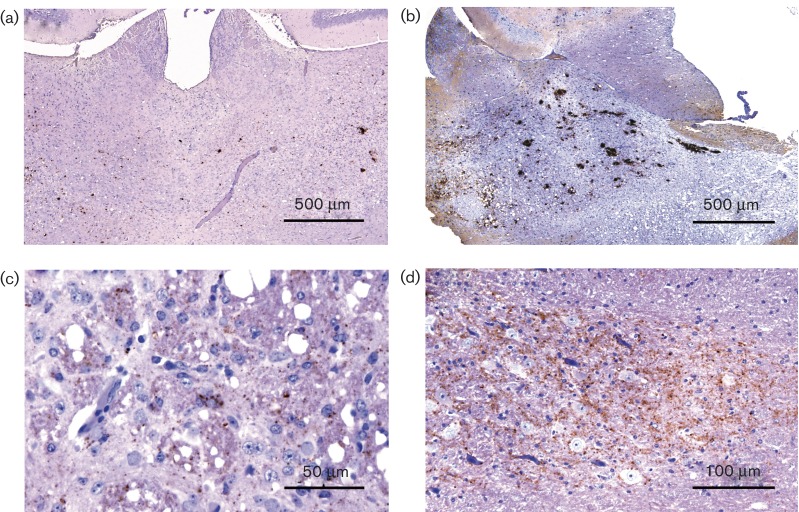
Mice from the 13 × 85 transmission and mouse L4823/9 interestingly showed mixed features. With 13 × 85, PrPd accumulation (Fig. 4a, b) was very similar to atypical scrapie (bilateral coalescing PrPd deposits in the thalamus and severe vacuolation; Table S2). However, like SSBP/1 and CH1641, particulate deposits were more prominent and widespread, also having intracellular (intraneuronal and intramicroglial) PrPd accumulation in the midbrain, habenula, hypothalamus, striatum and cerebral cortex (IHC type A+I in Table 3). L4823/9 showed a mixture of features of atypical scrapie (coalescing PrPd deposits in the thalamus and prominent vacuolation; Fig. 4c) and of classical scrapie (PrPd plaques and particulate deposits; Fig. 4d; IHC type A+B1 in Table 3).
Fig. 4.
Immunohistochemical PrPd features of tg338 mice inoculated with sheep brain. (a, b) Thalamus from mice inoculated with 13 × 85, (c) corpus striatum and (d) deep cerebellar nuclei from mouse L4823/9. IHC with 2G11 and haematoxylin counterstaining.
Western blotting of mouse transmissions
Mouse brain samples from clinical and pathology-positive animals were subjected to Western blots to examine the patterns of PrPSc (Fig. 5). L4824 and 51 × 45 PrPSc both showed low-molecular-mass doublet bands (∼7–9 kDa) not detected by 6H4 (Fig. 5a, c, lanes 2 and 3) as expected from atypical scrapie. PrPSc from H800 (Fig. 5a, c, lane 7) had similar staining properties to classical scrapie (Fig. 5a, c, lanes 1 and 9) and SSBP/1 (Fig. 5b, d, lanes 4–6) showing a three-banded pattern with ∼21 kDa lower-molecular-mass band and stronger staining with P4 than with 6H4. Even with over-exposure (not shown) there was no sign of the faint ∼8 kDa band seen in the original sheep sample. PrPSc from 13 × 85 showed features of both atypical and classical scrapie on the Western blots. Initially (Fig. 5a, c, lane 5), the pattern resembled classical scrapie, but by increasing the loading (Fig. 5e, h, lane 1 and f, lane 2), a band at ∼7 kDa was clearly visible with P4 but not with 6H4. CH1641 also had different staining with the two antibodies (Fig. 5g, i, lanes 4 and 8). With 6H4, the lower-molecular-mass band (∼19 kDa) typical of CH1641 in sheep was reproduced in the tg338 mice; however, with P4 this band was not stained and instead an ∼21 kDa band was visible, unlike PrPSc in CH1641-affected sheep which is largely unlabelled by P4 (Jeffrey et al., 2006a).
Fig. 5.
(a–i) Western blots of PrPSc from brain of tg338 mice inoculated with unusual or control scrapie sheep brain using antibodies P4 (a, b, e, f, g) and 6H4 (c, d, h, i). M, molecular mass markers (kDa). Inocula: (a, c) lanes 1, 6 and 9, natural scrapie control (68 × 81); lane 2, L4824; lane 3, atypical scrapie control (51 × 45); lanes 4 and 8, CH1641(J2916); lane 5, 13 × 85; lane 7, H800; (b, d) lanes 1–3, 1 : 3 dilutions of atypical scrapie Scr2; lanes 4–6, 1 : 3 dilutions of SSBP/1; (e) lane 1, atypical scrapie (51 × 45); lane 2, 13 × 85 loaded × 6; (f) lanes 1 and 3, natural scrapie controls; lane 2, 13 × 85 loaded × 6; (g, h) lanes 1, 4 and 11, natural scrapie control (68 × 81); lanes 2 and 6, CH1641 (J2916); lanes 3, 7 and 8, single mouse L4823/4; lanes 5 and 10, single mouse L4823/9; lane 9, atypical scrapie control (51 × 45).
The two clinically positive mice from the L4823 transmissions are shown in Fig. 5(g, i) with each mouse (L4823/4 and L4823/9) showing different patterns. L4823/4 was similar to classical scrapie, with the typical three-banded pattern showing more intense staining with P4 than 6H4 (Fig. 5g, i, lane 3). L4823/9 also showed this pattern but in addition, with P4, had the low-molecular-mass doublet at ∼7–9 kDa characteristic of atypical scrapie (Fig. 5g, i, lanes 5 and 10). Negative control mice, tg338 with no inoculation, showed no staining whatsoever with 6H4 or P4 (not shown).
A summary of the findings for each inoculum and our conclusions on strain identity are given in Table 3.
Discussion
In this study of sheep samples dating back to the 1960s, ∼1850 animals from our combined UK and NPU Cheviot archives were searched for signs of atypical scrapie using a screening process designed to establish the earliest date at which it had occurred, but not expected to find every case. The characteristics of atypical scrapie are well established (Tranulis et al., 2011), but it is not clear whether there is a single atypical strain (e.g. Nor98) or several different similar strains. Western blot patterns and pathology can vary from isolate to isolate; however, atypical scrapie transmissions suggest that this results from unknown host factors rather than strain variability (Arsac et al., 2009; Götte et al., 2011). Here, we have used the term atypical scrapie to encompass both possibilities – either a single entity or a group of similar strains.
We used IHC, Western blotting and PRNP genotyping to select sheep samples for TSE strain typing in tg338 mice in which classical natural scrapie, SSBP/1, CH1641 (designated in Table 3 as classical types 1, 2 and 3, respectively) and atypical scrapie can be distinguished using details of pathology and differential antibody staining. tg338 mice, expressing the sheep VRQ allele, have been used successfully to analyse sheep scrapie cases, both classical and atypical, in a way which was not previously very easy due to the difficulties of transmission to WT mice (Andréoletti et al., 2011; Le Dur et al., 2005; Thackray et al., 2012). Results were, however, compared with WT mouse transmissions, where available.
In Table 3, we presented our conclusions on strain identity. From our UK archive, one sheep, H800, initially thought to be atypical scrapie because of a faint ∼8 kDa band on Western blot, resembled classical scrapie by lesion profiling, IHC and Western blot on transmission to mice. Moreover, its genotype (VRQ/AF141RQ) is rarely affected by atypical scrapie and it is therefore more likely that the low-molecular-mass band in Western blots from the original sheep was the result of tissue degradation in storage. Clearly, the presence of ∼7–9 kDa protein bands in PrPSc preparations needs to be interpreted with caution.
L4824, which died in 1988, had characteristic histopathology and was of a PRNP genotype (AF141RQ/AL141RQ) common for atypical scrapie. On transmission to tg338 mice, the lesion and IHC profiles were very similar to the two atypical scrapie controls. Both sheep and mouse Western blot patterns were similar in having a doublet band at 7–9 kDa recognized by P4 and not 6H4 – a characteristic of atypical scrapie. L4824 had a companion case from the same flock (L4823) which had several features consistent with classical scrapie, including IHC and Western blot patterns, and was selected for transmission as a convenient concurrent control. Surprisingly, on transmission to tg338 mice, however, one of the two clinically affected mice had pathology and Western blot patterns consistent with classical scrapie (L4823/4), whilst the other (L4823/9) had a mixture of classical and atypical scrapie features. One possible explanation is that the sheep had both classical and atypical scrapie with the features of the latter masked by those of classical scrapie (IHC and vacuolation) and/or lost due to degradation on storage (Western blots). Once transmitted to tg338 mice, which are highly sensitive to very low titres of atypical scrapie (Andréoletti et al., 2012), a single mouse (L4823/9) may have detected the atypical scrapie agent against a background of equally low effective titres of classical scrapie.
The 1972 case from the NPU Cheviot flock (13 × 85) is also problematic. The sheep 13 × 85 was born in 1966 and formed part of a group in which mixed infection was attempted with two strains of experimental scrapie in separated injections: SSBP/1 and then CH1641. Clinical disease occurred with an incubation period that we now know could have resulted from either strain in AF141RQ/AF141RQ sheep (F. Houston, W. Goldmann and N. Hunter, in preparation). The superinfection with CH1641 could have been blocked by an already replicating SSBP/1 or could have resulted in a mixed experimental infection, layered on top of, or combined with, atypical scrapie which could have arisen at any point in the animal's life. Sheep 13 × 85 developed clinical signs described in 1972 as unusual, and subsequent transmission to WT mice was very efficient and more aggressive than CH1641, SSBP/1, NPU classical scrapie or atypical scrapie alone. No lesion profiles are available for comparison from that original mouse transmission; however, our findings from tg338 mouse transmissions (IHC and Western blots) suggest that 13 × 85 was an atypical scrapie case, superinfected with either or both of CH1641 and SSBP/1. The question is: did this sheep develop atypical scrapie naturally or did it emerge as a result of infection with two experimental sources of classical scrapie? We believe that the latter is unlikely as another sheep subjected to the same combined infection (13 × 69) did not develop features of atypical scrapie.
Natural mixed atypical/classical infection of single sheep has been reported elsewhere (Mazza et al., 2010). Intriguingly, recent PrPSc molecular studies have found evidence of natural mixed infections including CH1641-like strains in sheep (Langeveld et al., 2014) and experimental inoculation studies have suggested that the phenotype of atypical scrapie can in some instances change into that of CH1641 during passage in sheep (Simmons et al., 2015). It is therefore more likely that natural TSE infections of ruminants involve mixtures of strains, or have a more fluid identity, rather than the single strains normally used in laboratory studies. Combinations of infections have been implicated in the development of novel strains with altered host range in lentivirus infections of ruminants (Minardi da Cruz et al., 2013), and such combinations of strains and types of virus/bacteria/prions, particularly in persistent infections, are more likely to represent the real-life situation in the field and could favour the emergence of more highly virulent disease-causing agents. Whereas viruses can recombine their genetic material to form new strains, the concept of the prion being free from any nucleic acid makes it challenging, but also important, to understand how mixed prion strains interact.
Sheep 13 × 85 provides the earliest evidence to date for atypical scrapie in sheep. The finding of cases occurring up to 26 years prior to the original Nor98 animals lends weight to the hypothesis that atypical scrapie is not a newly emerging TSE, but was discovered simply as a result of increased surveillance. Its risk for human health following consumption of sheep products remains unknown, but the length of time atypical scrapie has existed in sheep supports the idea that it represents no additional risk.
Methods
Sheep tissue archives
Our archive of scrapie-affected and healthy sheep tissues dates back to the 1960s and originated at the NPU, but is now held at the Roslin Institute. For this study we divided the archive into two: those from around the UK (UK archive) and those from our own Cheviot flock (NPU archive). The UK archive samples (n ≈ 350) were from sheep, scrapie-affected and healthy, from various locations and with death dates from 1964 to 2004. One UK animal, a Dorset Horn (377J) that died in 1989, was already reported by us to have had atypical scrapie (Bruce et al., 2007). In the NPU archive, at the time this study was carried out, there were ∼1500 sheep represented, with death dates from 1966 to 2005, but the selection process (see below) reduced this number to ∼200 samples for further study. Extensive records cover the foundation of the flock in 1960 to date and studies of its endemic classical scrapie have been reported (Hunter et al., 1996; Matthews et al., 1999, 2001; Redman et al., 2002; Woolhouse et al., 1999) along with details of a case of atypical scrapie from 2001 (Foster et al., 2008).
All information, however limited, that was available in the original records was collated, e.g. clinical signs, PRNP genotype, age at death, flock location, and haematoxylin/eosin-stained vacuolation pathology results. The condition of the samples was highly variable due to long-term storage. Tissues suitable for IHC (formal saline fixed or wax blocks) and/or for Western blotting (frozen) and mouse transmission (sterile and frozen) were available, but not all three for every animal.
Search for atypical scrapie in sheep samples: selection criteria
The selection process, searching for the characteristics of atypical scrapie (Table S1), used three lines of processing (Fig. S1) after removing samples which were too degraded for DNA analysis or too dried out for IHC. First, frozen samples were PRNP genotyped, mostly excluding genotypes associated with classical scrapie, and PrPSc protein analysis was carried out on the relatively few samples which remained. Secondly, fixed tissues suitable for IHC were examined for signs of atypical scrapie pathology. At the final stage of the process, samples were considered for strain typing in mice if atypical scrapie signs had been found by IHC and/or Western blot. A third line of selection involved animals, regardless of genotype, which had records from the time of death suggesting unusual clinical/pathology signs. Only if the latter also had positive atypical signs from Western blots and/or IHC were these sheep considered for mouse strain typing.
Genotyping
Genomic DNA was extracted from blood or tissue samples using a Qiagen DNeasy Blood & Tissue kit, following the manufacturer's instructions. PRNP genotyping was performed on PCR-amplified DNA fragments as described previously (Hunter et al., 2012). Genotypes are presented here in either the three-codon (136, 154, 171) format (e.g. VRQ/ARQ) or, for the ARQ allele on which codon 141 can vary, a four-codon format is used if known (e.g. AL141RQ/AF141RQ). All other alleles in this report were L141.
IHC
Our IHC methods did not result in detection of PrPC, but as no proteinase K is used in the process, the PrP protein detected by IHC is referred to here as disease-associated PrP (PrPd) (González et al., 2005). The term PrPSc is here reserved for the protein detected in Western blots of proteinase K-treated samples.
In certain sheep cases, paraffin-embedded brain tissues were available from previous routine haematoxylin/eosin investigations. These were re-examined with current IHC techniques for the detection of PrPd (Foster et al., 2001; Jeffrey et al., 2006b) using the BG4 anti-PrP mAb at 1 μg ml− 1 (TSE Resource Centre, The Roslin Institute). Following transmission in tg338 mice, formalin-fixed, wax-embedded murine brain tissues were examined as follows. For antigen retrieval, 4 μm tissue sections were immersed in formic acid for 5 min at 20 °C followed by autoclaving in 0.2 % citrate retrieval solution (pH 6.8) at 121 °C for 30 min. After washing in tap water and quenching in hydrogen peroxide (3 % in methanol) for 20 min, tissue sections were blocked with the MOM kit liquid protein concentrate solution (Vector Laboratories) at a dilution of 1 : 20 for 60 min and incubated overnight at 23 °C with 1 μg 2G11 ml− 1 (Novus Biologicals) or 0.2 μg SAF84 ml− 1 (SPI Bio), which are both mouse mAbs recognizing aa 153–158 (Thuring et al., 2004) and aa 166–172 (Jacobs et al., 2011) of ovine PrP, respectively. The subsequent steps of the IHC procedure were performed using an immunoperoxidase Elite ABC kit (Vector Laboratories). Sections were then counterstained with Mayer's haematoxylin.
Biochemical detection of PrPSc
Where sufficient tissue was available, selected samples were subjected to PrPSc analysis by Western blot as described previously (Jeffrey et al., 2006a; Stack et al., 2002) with a non-stringent proteinase K concentration of 50 μg ml− 1. Samples were run on 16 % Tris/glycine gels (Invitrogen) and Western blotted onto PVDF membranes. For detection of PrPSc, two mAbs were used: 6H4 (Prionics) at 2 mg ml− 1, diluted 1 : 5000, and P4 (R-Biopharm) at 1 mg ml− 1, diluted 1 : 2500, followed by ‘visualization’ with a chemiluminescence substrate (Roche) and Lumi-film (Roche). P4 labelling revealed the ∼7–9 kDa protein band(s) characteristic of atypical scrapie and if these low-molecular-mass bands were present, the membrane was stripped of antibody using Restore (Thermo Fisher Scientific) and reprobed with 6H4, which binds to, and reveals, the 18–30 kDa bands, but not the ∼7–9 kDa band(s).
Strain typing in mice
Study of the Roslin Institute data archive revealed that one of the unusual cases, 13 × 85, had been transmitted to WT inbred mice (RIII, C57 and VM) in 1972. These historical results, which have never been published, are presented here along with transmissions of SSBP/1, CH1641, atypical scrapie (Scr2 and 51 × 45) and a VRQ/VRQ NPU classical scrapie case (47 × 79), which were carried out between 1989 and 2002. NPU scrapie has been transmitted to mice many times from different sheep and the older results are very similar to those from more recent transmissions. All of this historical data are now part of the Roslin Institute data archive, and all of the older studies were approved by the relevant ethics committees and conducted under the licensing appropriate at the time. The infection procedures, daily observation of animals and clinical assessments we use now were first established by A. G. Dickinson in the 1960s, and as a result the experiments are directly comparable. Lesion profiles for the 13 × 85 WT mouse transmissions have been lost, however.
As atypical scrapie does not transmit to WT mice, brains from the three cases of interest plus four control cases were strain typed by intracerebral injection of 20 μl 10 % brain homogenate into tg338 mice after pre-screening for bacterial contamination. The experiments were approved by the Roslin Institute Ethics Committee, and were carried out under UK Home Office personal and project licences.
Control inoculations included (1) three classical scrapie types, two showing 21 kDa unglycosylated PrPSc bands on Western blots (SSBP/1, which produces a very short incubation period in tg338 mice, and an NPU natural scrapie case of VRQ/VRQ genotype, 68 × 81) and a sheep (J2916) affected by CH1641, a classical scrapie with a 19 kDa unglycosylated PrPSc band, and (2) two confirmed atypical scrapie cases: Scr2 (Bruce et al., 2007) and 51 × 45 (Foster et al., 2008). We also included a flock-mate of one of the candidate atypical cases as a concurrent control (L4823).
Mice were observed daily and culled when clear clinical signs of scrapie or other intercurrent disease were noted. Each mouse brain was examined (blinded to the inoculum) for vacuolation in haematoxylin/eosin-stained sections and a lesion profile generated as described previously (Fraser & Dickinson, 1968). Incubation periods were calculated as mean of time between inoculation and death for all clinically positive and vacuolation-positive mice. For some purposes, incubation period mean durations are presented in the following format: short (mean < 100 days), medium, (mean 100–200 days), long (mean 200–400 days) or very long (mean >400 days). Survivors were those mice that lived longer than the earliest positive case.
In addition, for each of the ovine inocula, groups of four clinically affected mice were examined by IHC, with the exception of those inoculated with L4823 (see Table 1), for which only two mice developed neurological signs. The magnitude of PrPd accumulation was scored (blinded to the inoculum) as follows: 0 (absent) to 3 (severe) in brain areas: (1) frontal cortex, corpus striatum, basal ganglia; (2) temporo-parietal cortex, hippocampus, thalamus, hypothalamus; (3) midbrain; (4) cerebellar cortex, deep cerebellar nuclei, pons; and (5) medulla oblongata. The PrPd types observed were intracellular (intraneuronal and intraglial combined), fine particulate, coalescing and plaques (vascular and non-vascular combined). The final score was the mean of the different PrPd types for each group. As differences in the severity of vacuolation were observed in the brains, mice were given an overall score from 0 to 3, which gives a parallel and more general measure of vacuolation than the lesion profiles (see above).
Acknowledgements
The authors would like to acknowledge S. Martin, D. Parnham, D. Drummond and A. Boyle for histology processing, IHC and vacuolation scoring; animal house staff in the Institute for Animal Health for care of animals; and A. G. Dickinson, M. Bruce and J. D. Foster for early data on sheep and mouse transmissions. This study was funded by the Department for Environment, Food and Rural Affairs (contract SE0251) and Biotechnology and Biological Sciences Research Council Institute Strategic Grant BB/J004332/1.
Supplementary Data
Supplementary Data
References
- Andréoletti O., Orge L., Benestad S.L., Beringue V., Litaise C., Simon S., Le Dur A., Laude H., Simmons H., other authors (2011). Atypical/Nor98 scrapie infectivity in sheep peripheral tissues PLoS Pathog 7e1001285. 10.1371/journal.ppat.1001285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andréoletti O., Litaise C., Simmons H., Corbière F., Lugan S., Costes P., Schelcher F., Vilette D., Grassi J., Lacroux C. (2012). Highly efficient prion transmission by blood transfusion PLoS Pathog 8e1002782. 10.1371/journal.ppat.1002782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsac J.-N., Bétemps D., Morignat E., Féraudet C., Bencsik A., Aubert D., Grassi J., Baron T. (2009). Transmissibility of atypical scrapie in ovine transgenic mice: major effects of host prion protein expression and donor prion genotype PLoS One 4e7300. 10.1371/journal.pone.0007300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benestad S.L., Sarradin P., Thu B., Schönheit J., Tranulis M.A., Bratberg B. (2003). Cases of scrapie with unusual features in Norway and designation of a new type, Nor98 Vet Rec 153202–208 10.1136/vr.153.7.202. [DOI] [PubMed] [Google Scholar]
- Benestad S.L., Arsac J.-N., Goldmann W., Nöremark M. (2008). Atypical/Nor98 scrapie: properties of the agent, genetics, and epidemiology Vet Res 3919. 10.1051/vetres:2007056. [DOI] [PubMed] [Google Scholar]
- Bolton D.C., McKinley M.P., Prusiner S.B. (1982). Identification of a protein that purifies with the scrapie prion Science 2181309–1311 10.1126/science.6815801. [DOI] [PubMed] [Google Scholar]
- Bruce M.E., Boyle A., Cousens S., McConnell I., Foster J., Goldmann W., Fraser I. (2002). Strain characterization of natural sheep scrapie and comparison with BSE J Gen Virol 83695–704 10.1126/science.6815801. [DOI] [PubMed] [Google Scholar]
- Bruce M.E., Nonno R., Foster J., Goldmann W.Esposito E., Benestad S.L., Hunter N., Agrimi U. (2007). Nor98-like sheep scrapie in the United Kingdom in 1989 Vet Rec 160665–666 10.1136/vr.160.19.665. [DOI] [PubMed] [Google Scholar]
- Buschmann A., Biacabe A.-G., Ziegler U., Bencsik A., Madec J.-Y., Erhardt G., Lühken G., Baron T., Groschup M.H. (2004). Atypical scrapie cases in Germany and France are identified by discrepant reaction patterns in BSE rapid tests J Virol Methods 11727–36 10.1016/j.jviromet.2003.11.017. [DOI] [PubMed] [Google Scholar]
- De Bosschere H., Roels S., Benestad S.L., Vanopdenbosch E. (2004). Scrapie case similar to Nor98 diagnosed in Belgium via active surveillance Vet Rec 155707–708 10.1136/vr.155.22.707. [DOI] [PubMed] [Google Scholar]
- Fediaevsky A., Tongue S.C., Nöremark M., Calavas D., Ru G., Hopp P. (2008). A descriptive study of the prevalence of atypical and classical scrapie in sheep in 20 European countries BMC Vet Res 419. 10.1186/1746-6148-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J., Goldmann W., Parnham D., Chong A., Hunter N. (2001). Partial dissociation of PrPSc deposition and vacuolation in the brains of scrapie and BSE experimentally affected goats J Gen Virol 82267–273. [DOI] [PubMed] [Google Scholar]
- Foster J., Toovey L., McKenzie C., Chong A., Parnham D., Drummond D., Hunter N. (2008). Atypical scrapie in a sheep in a closed UK flock with endemic classical natural scrapie Vet Rec 162723–724 10.1136/vr.162.22.723. [DOI] [PubMed] [Google Scholar]
- Fraser H., Dickinson A.G. (1968). The sequential development of the brain lesion of scrapie in three strains of mice J Comp Pathol 78301–311 10.1016/0021-9975(68)90006-6. [DOI] [PubMed] [Google Scholar]
- Gavier-Widén D., Stack M.J., Baron T., Balachandran A., Simmons M. (2005). Diagnosis of transmissible spongiform encephalopathies in animals: a review J Vet Diagn Invest 17509–527 10.1177/104063870501700601. [DOI] [PubMed] [Google Scholar]
- Goldmann W. (2008). PrP genetics in ruminant transmissible spongiform encephalopathies Vet Res 3930. 10.1051/vetres:2008010. [DOI] [PubMed] [Google Scholar]
- González L., Terry L., Jeffrey M. (2005). Expression of prion protein in the gut of mice infected orally with the 301V murine strain of the bovine spongiform encephalopathy agent J Comp Pathol 132273–282 10.1016/j.jcpa.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Götte D.R., Benestad S.L., Laude H., Zurbriggen A., Oevermann A., Seuberlich T. (2011). Atypical scrapie isolates involve a uniform prion species with a complex molecular signature PLoS One 6e27510. 10.1371/journal.pone.0027510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths P.C., Spiropoulos J., Lockey R., Tout A.C., Jayasena D., Plater J.M., Chave A., Green R.B., Simonini S., other authors (2010). Characterization of atypical scrapie cases from Great Britain in transgenic ovine PrP mice J Gen Virol 912132–2138 10.1099/vir.0.018986-0. [DOI] [PubMed] [Google Scholar]
- Hope J., Morton L.J.D., Farquhar C.F., Multhaup G., Beyreuther K., Kimberlin R.H. (1986). The major polypeptide of scrapie-associated fibrils (SAF) has the same size, charge distribution and N-terminal protein sequence as predicted for the normal brain protein (PrP) EMBO J 52591–2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter N., Foster J.D., Goldmann W., Stear M.J., Hope J., Bostock C. (1996). Natural scrapie in a closed flock of Cheviot sheep occurs only in specific PrP genotypes Arch Virol 141809–824 10.1007/BF01718157. [DOI] [PubMed] [Google Scholar]
- Hunter N., Houston F., Foster J., Goldmann W., Drummond D., Parnham D., Kennedy I., Green A., Stewart P., Chong A. (2012). Susceptibility of young sheep to oral infection with bovine spongiform encephalopathy decreases significantly after weaning J Virol 8611856–11862 10.1128/JVI.01573-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J.G., Sauer M., van Keulen L.J.M., Tang Y., Bossers A., Langeveld J.P.M. (2011). Differentiation of ruminant transmissible spongiform encephalopathy isolate types, including bovine spongiform encephalopathy and CH1641 scrapie J Gen Virol 92222–232 10.1099/vir.0.026153-0. [DOI] [PubMed] [Google Scholar]
- Jeffrey M., González L., Chong A., Foster J., Goldmann W., Hunter N., Martin S. (2006a). Ovine infection with the agents of scrapie (CH1641 isolate) and bovine spongiform encephalopathy: immunochemical similarities can be resolved by immunohistochemistry J Comp Pathol 13417–29 10.1016/j.jcpa.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Jeffrey M., Martin S., González L., Foster J., Langeveld J.P.M., van Zijderveld F.G., Grassi J., Hunter N. (2006b). Immunohistochemical features of PrPd accumulation in natural and experimental goat transmissible spongiform encephalopathies J Comp Pathol 134171–181 10.1016/j.jcpa.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Langeveld J.P.M., Jacobs J.G., Erkens J.H.F., Baron T., Andréoletti O., Yokoyama T., van Keulen L.J.M., van Zijderveld F.G., Davidse A., other authors (2014). Sheep prions with molecular properties intermediate between classical scrapie, BSE and CH1641-scrapie Prion 8296–305 10.4161/19336896.2014.983396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Dur A., Béringue V., Andréoletti O., Reine F., Laï T.L., Baron T., Bratberg B., Vilotte J.L., Sarradin P., other authors (2005). A newly identified type of scrapie agent can naturally infect sheep with resistant PrP genotypes Proc Natl Acad Sci U S A 10216031–16036 10.1073/pnas.0502296102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews L., Woolhouse M.E.J., Hunter N. (1999). The basic reproduction number for scrapie Proc Biol Sci 2661085–1090 10.1098/rspb.1999.0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews L., Coen P.G., Foster J.D., Hunter N., Woolhouse M.E.J. (2001). Population dynamics of a scrapie outbreak Arch Virol 1461173–1186 10.1007/s007050170113. [DOI] [PubMed] [Google Scholar]
- Mazza M., Iulini B., Vaccari G., Acutis P.L., Martucci F., Esposito E., Peletto S., Barocci S., Chiappini B., other authors (2010). Co-existence of classical scrapie and Nor98 in a sheep from an Italian outbreak Res Vet Sci 88478–485 10.1016/j.rvsc.2009.11.015. [DOI] [PubMed] [Google Scholar]
- Minardi da Cruz J.C., Singh D.K., Lamara A., Chebloune Y. (2013). Small ruminant lentiviruses (SRLVs) break the species barrier to acquire new host range Viruses 51867–1884 10.3390/v5071867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nentwig A., Oevermann A., Heim D., Botteron C., Zellweger K., Drögemüller C., Zurbriggen A., Seuberlich T. (2007). Diversity in neuroanatomical distribution of abnormal prion protein in atypical scrapie PLoS Pathog 3e82. 10.1371/journal.ppat.0030082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orge L., Galo A., Machado C., Lima C., Ochoa C., Silva J., Ramos M., Simas J.P. (2004). Identification of putative atypical scrapie in sheep in Portugal J Gen Virol 853487–3491 10.1099/vir.0.80246-0. [DOI] [PubMed] [Google Scholar]
- Ortiz-Pelaez A., Arnold M.E. (2013). Sheep and Goat Scrapie Surveillance 2013. Joint Descriptive Report for Great Britain Defra https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/358335/pub-tse-stats-scrapie.pdf.
- Polak M.P., Larska M., Langeveld J.P.M., Buschmann A., Groschup M.H., Zmudzinski J.F. (2010). Diagnosis of the first cases of scrapie in Poland Vet J 18647–52 10.1016/j.tvjl.2009.07.032. [DOI] [PubMed] [Google Scholar]
- Redman C.A., Coen P.G., Matthews L., Lewis R.M., Dingwall W.S., Foster J.D., Chase-Topping M.E., Hunter N., Woolhouse M.E.J. (2002). Comparative epidemiology of scrapie outbreaks in individual sheep flocks Epidemiol Infect 128513–521 10.1017/S0950268802007008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders G.C., Cawthraw S., Mountjoy S.J., Hope J., Windl O. (2006). PrP genotypes of atypical scrapie cases in Great Britain J Gen Virol 873141–3149 10.1099/vir.0.81779-0. [DOI] [PubMed] [Google Scholar]
- Simmons M.M., Konold T., Simmons H.A., Spencer Y.I., Lockey R., Spiropoulos J., Everitt S., Clifford D. (2007). Experimental transmission of atypical scrapie to sheep BMC Vet Res 320. 10.1186/1746-6148-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons M.M., Konold T., Thurston L., Bellworthy S.J., Chaplin M.J., Moore S.J. (2010). The natural atypical scrapie phenotype is preserved on experimental transmission and sub-passage in PRNP homologous sheep BMC Vet Res 614. 10.1186/1746-6148-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons M.M., Moore S.J., Lockey R., Chaplin M.J., Konold T., Vickery C., Spiropoulos J. (2015). Phenotype shift from atypical scrapie to CH1641 following experimental transmission in sheep PLoS One 10e0117063. 10.1371/journal.pone.0117063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack M.J., Chaplin M.J., Clark J. (2002). Differentiation of prion protein glycoforms from naturally occurring sheep scrapie, sheep-passaged scrapie strains (CH1641 and SSBP1), bovine spongiform encephalopathy (BSE) cases and Romney and Cheviot breed sheep experimentally inoculated with BSE using two monoclonal antibodies Acta Neuropathol 104279–286. [DOI] [PubMed] [Google Scholar]
- Thackray A.M., Hopkins L., Lockey R., Spiropoulos J., Bujdoso R. (2012). Propagation of ovine prions from poor transmitter scrapie isolates in ovine PrP transgenic mice Exp Mol Pathol 92167–174 10.1016/j.yexmp.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Thuring C.M.A., Erkens J.H.F., Jacobs J.G., Bossers A., Van Keulen L.J.M., Garssen G.J., Van Zijderveld F.G., Ryder S.J., Groschup M.H., other authors (2004). Discrimination between scrapie and bovine spongiform encephalopathy in sheep by molecular size, immunoreactivity, and glycoprofile of prion protein J Clin Microbiol 42972–980 10.1128/JCM.42.3.972-980.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranulis M.A., Benestad S.L., Baron T., Kretzschmar H. (2011). Atypical prion diseases in humans and animals Top Curr Chem 30523–50 10.1007/128_2011_161. [DOI] [PubMed] [Google Scholar]
- Webb P.R., Powell L., Denyer M., Marsh S., Weaver C., Simmons M.M., Johns E., Sheehan J., Horsfield P., other authors (2009). A retrospective immunohistochemical study reveals atypical scrapie has existed in the United Kingdom since at least 1987 J Vet Diagn Invest 21826–829 10.1177/104063870902100609. [DOI] [PubMed] [Google Scholar]
- Woolhouse M.E.J., Matthews L., Coen P., Stringer S.M., Foster J.D., Hunter N. (1999). Population dynamics of scrapie in a sheep flock Philos Trans R Soc Lond B Biol Sci 354751–756 10.1098/rstb.1999.0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data