Abstract
Background
A previous meta-analysis of 3 zinc acetate lozenge trials estimated that colds were on average 40% shorter for the zinc groups. However, the duration of colds is a time outcome, and survival analysis may be a more informative approach. The objective of this individual patient data (IPD) meta-analysis was to estimate the effect of zinc acetate lozenges on the rate of recovery from colds.
Methods
We analyzed IPD for 3 randomized placebo-controlled trials in which 80–92 mg/day of elemental zinc were administered as zinc acetate lozenges to 199 common cold patients. We used mixed-effects Cox regression to estimate the effect of zinc.
Results
Patients administered zinc lozenges recovered faster by rate ratio 3.1 (95% confidence interval, 2.1–4.7). The effect was not modified by age, sex, race, allergy, smoking, or baseline common cold severity. On the 5th day, 70% of the zinc patients had recovered compared with 27% of the placebo patients. Accordingly, 2.6 times more patients were cured in the zinc group. The difference also corresponds to the number needed to treat of 2.3 on the 5th day. None of the studies observed serious adverse effects of zinc.
Conclusions
The 3-fold increase in the rate of recovery from the common cold is a clinically important effect. The optimal formulation of zinc lozenges and an ideal frequency of their administration should be examined. Given the evidence of efficacy, common cold patients may be instructed to try zinc acetate lozenges within 24 hours of onset of symptoms.
Keywords: common cold, meta-analysis, randomized controlled trials, respiratory tract infections, zinc acetate
Five previous meta-analyses concluded that there is strong evidence that zinc lozenges can shorten the duration of colds [1–5], although there were shortcomings in the Cochrane review [5–7]. The benefit of zinc lozenges in the controlled trials has not been uniform; however, most of the negative trials appear to have been the result of either too low a daily zinc dose or inappropriate excipients that bound the zinc ions [1, 2, 8–10].
The 5 previous meta-analyses that supported zinc lozenges’ impact on colds investigated the effect on the duration of the common cold [1–5]. Two of them focused on the same set of 3 trials as the current study: one used a t test [3] and another used a linear model of individual patient data (IPD) [4] to analyze common cold duration. However, duration is a time outcome, and survival analysis may be a more appropriate approach than a t test to analyze such data.
Survival analysis allows visual inspection of survival curves, which is more informative than comparing mean and standard deviation values of a t test. Furthermore, survival analysis is not hampered by censored data, ie, patients who were followed but dropped out before recovery; however, in a t test analysis, such observations need recovery day imputation. In addition, outlier patients with extended colds will increase the standard deviation estimates and decrease the statistical power of a t test. In contrast, a few patients who have extended colds minimally influence survival analysis.
Two zinc salts have been used in the zinc lozenge trials: zinc gluconate and zinc acetate. Because acetate binds zinc ions less strongly than gluconate, zinc acetate has been proposed as the optimal salt for lozenges [1, 10]. Three controlled trials examined the effect of >75 mg/day zinc as zinc acetate lozenges on the duration of the common cold [11–13]. For this IPD meta-analysis, we used the data sets of the 3 trials.
The goal of this IPD analysis was (1) to analyze the effect of zinc acetate lozenges on the rate of recovery from the common cold and (2) to determine whether the effect of zinc is modified by age, sex, race, allergy, smoking, or by baseline common cold severity.
METHODS
Selection of the Trials
This IPD meta-analysis was restricted to placebo-controlled trials on zinc acetate lozenges for patients with naturally acquired common cold infections in which the dosage of elemental zinc was >75 mg/day. We restricted our analysis to high-dose trials, because previous studies indicated lack of effect of low doses, <75 mg/day of zinc [1, 2, 5, 10]. Previous searches of the literature [1, 2, 5] identified 3 trials that meet these selection criteria [11–13]. No additional zinc acetate lozenge trials were found by searching PubMed and Scopus using the free search terms “zinc” and “lozenge*” (February 13, 2017). The 3 sets of IPD were available with the collaboration of the authors of the studies with the first author. Details of the 3 included trials are shown in Supplementary File 1.
Outcomes and Extraction of the Data
The outcome in this meta-analysis was the day of recovery from the common cold. In the original trial report, Petrus et al [11] published both the mean duration of various cold symptoms and the duration of the longest cold symptom. We used the latter as the outcome in this study because it is consistent with the outcome definition in the Prasad et al [12, 13] studies. There were no censored observations in the 3 included trials.
Statistical Methods
We used the coxph procedure of the survival package of R-project, version 3.2.5 [14] to separately analyze the 3 individual studies to yield the rate ratio (RR) for the recovery from the common cold and its 95% confidence interval (CI). The cox.zph procedure was used to confirm that the proportional hazard assumption was satisfied for the 3 trials (see calculations in Supplementary File 2).
We pooled the data of the 3 trials using Cox mixed-effects modeling; the modeling was completed using the coxme procedure of the coxme package of R [14]. We used the study as a random variable for the zinc lozenge effect and also as an independent explanatory variable [15, 16]. We also calculated the stratified Cox model that allows for a distinct baseline hazard in each study by using the strata statement in the coxph procedure. Finally, we calculated the pooled Cox model by consolidating the data from the 3 different studies with the coxph procedure, ignoring study as a clustering variable.
The interaction between the zinc lozenge effect and a subgroup was calculated by first adding zinc and the subgroup to the Cox model; then the interaction term between zinc and the subgroup was added. The P value for the zinc and subgroup interaction was calculated from the Analysis of Deviance Table by the analysis of variance. The ratio of RRs for 2 subgroups was also calculated to compare the effectiveness of zinc in the complementary subgroups. The Kaplan-Meier estimates were calculated using the survfit procedure of R. Two-tailed P values were used. Our calculations are described in detail in Supplementary File 2.
RESULTS
The 3 included trials were randomized, double-blind, placebo-controlled trials with few dropouts. Subgroup distributions of baseline variables are shown in Table 1. The 3 trials had a total of 199 common cold patients. The majority were white and women; 80% were in the age range 20 to 50 years old. One third of the patients had allergies. One quarter of patients were African Americans, and 10% reported “other” as their ethnic background. Although Petrus et al [11] did not collect information about smoking, one quarter of the participants in the 2 studies by Prasad et al [12, 13] were smokers. The daily dose of elemental zinc from the lozenges was 80 to 92 mg/day in the 3 studies. Prasad et al [12, 13] included patients only if they had had colds for 24 hours or less; the delay between the onset of symptoms and treatment initiation is not available for the Petrus et al [11] trial. See further methodological and other details of the 3 trials in Supplementary File 1.
Table 1.
Demographic Characteristics of Subjects Included in the 3 Trials
| Characteristic | All Participants | Petrus et al [11] | Prasad et al [12] | Prasad et al [13] |
|---|---|---|---|---|
| All participants | 199 | 101 | 48 | 50 |
| Age (yr) | ||||
| Median | 27.0 | 22.0 | 37.0 | 34.5 |
| Range | 17–61 | 18–54 | 18–61 | 17–60 |
| Males (%) | 41% | 47% | 38% | 34% |
| Allergies (%) | 31% | 46% | 13% | 20% |
| Smokers (%)a | 29% | — | 27% | 30% |
| Ethnic group | ||||
| White (%) | 66% | 72% | 61% | 60% |
| Black (%) | 24% | 15% | 33% | 32% |
| Other (%) | 10% | 13% | 6% | 8% |
aThe Petrus study (1998) did not collect data on smoking, the 29% indicates proportion of the 98 participants in the Prasad trials.
Petrus et al [11] instructed patients to dissolve 1 lozenge in the mouth every 1.5 hours while awake on the first day and then 1 lozenge every 2 hours on the following days; lozenges dissolved in approximately 15 minutes [11]. Prasad et al instructed patients to dissolve in their mouth 1 lozenge every 2–3 hours while awake; lozenges dissolved in approximately 30 minutes [12, 13]. The effect of zinc acetate lozenges in the studies and their pooled effects are shown in Table 2. When the study was used as the random effects variable for the zinc lozenge effect, the Cox mixed-effects model indicated that the rate of recovery from the common cold was improved by RR of 3.1 in the zinc group. The stratified Cox regression model was calculated as a sensitivity approach, and it provided a similar estimate of zinc lozenge effect (Table 2). Finally, if the results of the trials were combined ignoring the study as a clustering variable, then the rate of recovery was 2.6 times higher among the zinc participants. The latter estimate of effect is lowest, which is explained by the substantially greater zinc effect in the 2 small studies by Prasad et al [12, 13] (N = 48 and N = 50, respectively), compared with the smaller effect in the larger study by Petrus et al [11] (N = 101) (see Table 2).
Table 2.
The Effect of Zinc Acetate Lozenges on the Rate of Recovery From the Common Cold in the 3 Included Trials
| Trials | No. of Participants | Mean Duration of Colds in the Placebo Group (Days) | Effect of Zinc Lozenges on the Rate of Recovery | |
|---|---|---|---|---|
| RR | 95% CI | |||
| Petrus et al [11] | 101 | 7.1 | 1.77 | 1.16–2.7 |
| Prasad et al [12] | 48 | 8.1 | 7.5 | 3.5–16 |
| Prasad et al [13] | 50 | 7.1 | 22 | 7.7–64 |
| The 3 Trials Pooled | ||||
| Cox model: study as a random effect | 199 | 3.1 | 2.1–4.7 | |
| Cox model: stratified by study | 199 | 3.6 | 2.5–5.1 | |
| Cox model: study ignored as a clustering variable | 199 | 2.6 | 1.9–3.5 | |
Abbreviations: CI, confidence interval; RR, rate ratio.
Table 3 shows the subgroup analyses of the zinc lozenge effects between the 2 complementary subgroups. The effect of zinc acetate lozenges was not modified by age, sex, race, allergy, smoking, or baseline severity of the common cold. Age was also analyzed as a continuous variable, and it did not interact with the zinc lozenge effect. In addition, because the age ranges of the 3 trials were substantially different, the interaction between the zinc effect and age was also calculated within the 3 trials, and no substantial modification was seen (Supplementary File 2). Finally, we also calculated the ratios of the RRs in the complementary subgroups and their 95% CIs (Supplementary File 2).
Table 3.
Difference in Zinc Acetate Lozenge Efficacy Between Subgroups
| Subgroup | No. Common Cold Patients | Effects in Subgroups | ||
|---|---|---|---|---|
| RR | 95% CI | Test of Interaction (P) | ||
| All participants | 199 | 3.1 | 2.1–4.7 | |
| Age (yr) | ||||
| 17–27 | 100 | 2.4 | 1.5–3.6 | 0.15 |
| 28–61 | 99 | 3.8 | 2.5–5.9 | |
| Sex | ||||
| Male | 82 | 3.6 | 2.1–6.3 | 0.5 |
| Female | 117 | 2.9 | 1.8–4.8 | |
| Ethnic groupa | ||||
| White | 132 | 2.8 | 1.9–4.1 | 0.4 |
| Black | 47 | 3.7 | 2.0–6.9 | |
| Allergy | ||||
| No | 137 | 3.0 | 1.8–4.9 | 0.5 |
| Yes | 62 | 3.7 | 1.9–7.1 | |
| Smokerb | ||||
| No | 70 | 8.7 | 4.0–19 | 0.8 |
| Yes | 28 | 9.9 | 3.7–27 | |
| Severity of the Common Cold at the Baselinec | ||||
| Below median | 102 | 4.0 | 2.3–6.9 | 0.2 |
| Above median | 97 | 2.8 | 1.6–4.7 | |
Abbreviations: CI, confidence interval; RR, rate ratio.
aEthnic groups other than white or black were excluded from this comparison.
bThe Petrus et al [11] study did not collect data on smoking. The RR estimates are based only on the 2 studies by Prasad et al [12, 13].
cThe severity scales of the 3 trials were different, and, therefore, the severity of each study was dichotomized by its own median severity score. The common cold severity above median is ≥8 points in the Petrus et al [11] study, ≥11 points in the Prasad et al [12] study, and ≥8 points in the Prasad et al [13] study (see Supplementary File 1 for details).
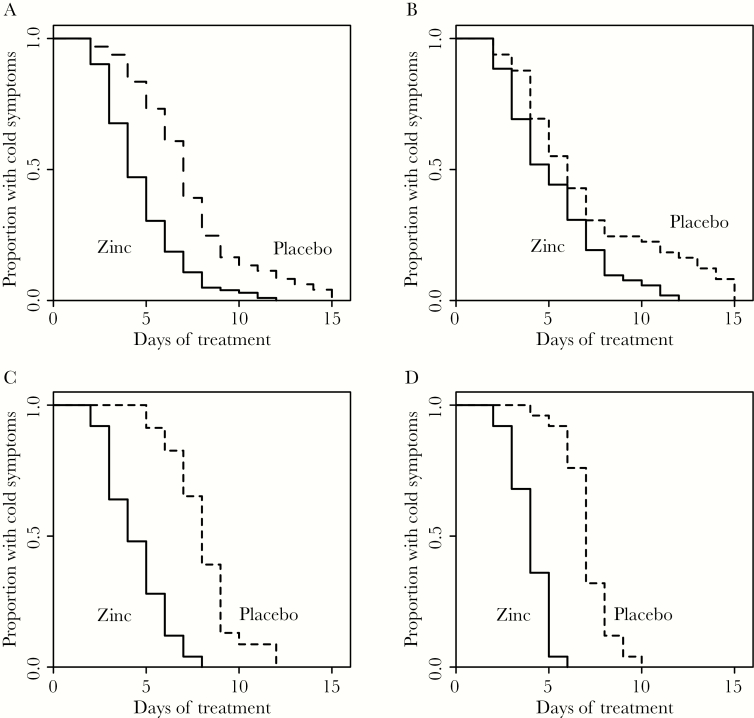
Kaplan-Meier curves of the 3 studies and of the pooled observations are shown in Figure 1. Figure 1A shows the pooled observations for the zinc and placebo groups from the 3 trials, ie, the combined data from the 3 separate trials. Ignoring study as a clustering variable led to a conservative estimation of the zinc effect (see above). Figure 1A is thus biased towards an underestimation of the zinc lozenge effect. Difference between the zinc and placebo groups in the Kaplan-Meier estimates gives the proportion of common cold patients who benefited from zinc. The greatest difference was on the 5th day: 70% of the zinc acetate lozenge patients had recovered from the common cold compared with 27% of the placebo patients (Figure 1A). The ratio of these proportions is 2.6, which is consistent with the Cox regression estimates (Table 2). The estimated difference in proportions equates to 43 percentage points more patients being cured by the zinc lozenges by day 5 compared with placebo. This corresponds to the number needed to treat to benefit 1 patient (NNT) of 2.3. Thus, 1 of every 2.3 patients on average did not suffer from the cold on the 5th day because of the zinc acetate lozenge treatment. Table 4 shows the NNT values for days 2 to 8 of the common cold. On the 3rd to 7th days, the NNT values are less than 4, indicating that in this time range more than 1 of 4 patients benefited from zinc acetate lozenges. Figure 1B−D show the individual Kaplan-Meier curves for the 3 trials.
Figure 1.
Kaplan-Meier curves showing the recovery from the common cold in the 3 trials pooled and in the individual studies by the zinc and placebo treatments. (A) All 3 trials pooled that had at the start 102 zinc and 97 placebo participants in all [11–13]. (B) Petrus et al [11] study, (C) Prasad et al [12] study, and (D) Prasad et al [13] study.
Table 4.
Estimates for NNT From the Included Trialsa
| Day | Proportion of Patients Still Sick on the Given Day | NNT | ||
|---|---|---|---|---|
| Zinc | Placebo | Difference | ||
| 2 | 0.902 | 0.969 | 0.067 | 15 |
| 3 | 0.677 | 0.938 | 0.262 | 3.8 |
| 4 | 0.471 | 0.835 | 0.365 | 2.7 |
| 5 | 0.304 | 0.732 | 0.428 | 2.3 |
| 6 | 0.186 | 0.608 | 0.422 | 2.4 |
| 7 | 0.108 | 0.392 | 0.284 | 3.5 |
| 8 | 0.049 | 0.247 | 0.198 | 5.0 |
Abbreviations: NNT, the average number of common cold patients needed to be treated for 1 patient to become cured by the given day.
aThese calculations are based on the Kaplan-Meier estimates shown in Figure 1A.
DISCUSSION
When we combined the results of 3 zinc acetate lozenge trials, we found that zinc lozenges increased the rate of recovery from the common cold by 3-fold. There was no indication that the effect was modified by age, sex, race, allergy, smoking, or baseline common cold severity. Therefore, the overall estimate of zinc acetate lozenges, the 3-fold increase in the recovery rate, seems to be a useful estimate irrespective of variations by these factors.
The 3 included studies were randomized, double-blind, and placebo-controlled trials with few dropouts. We had available IPD for the 3 trials that allowed effective analysis of possible subgroup differences in the zinc lozenge effect. The common cold infections in the 3 trials were natural common cold infections, and not infections generated by inoculation of a specific virus. Therefore, the findings may be generalized to the community with diverse respiratory viruses causing the common cold. The 3 studies excluded common cold patients with serious or chronic illnesses, and thus the findings should not be extrapolated to such people. It is also worth noting that the Prasad et al [12, 13] trials included patients only if the cold had lasted 24 hour or less, but the delay in the Petrus et al [11] trial is not available. Thus, it is not evident that zinc lozenges have effects if the cold has lasted for a longer time.
An important benefit of survival analysis is that it allows visual inspection and formal analysis of treatment effects over time. In addition, survival analysis is not confounded by censored data or outlier patients who have particularly long colds. The 3 included trials had no censored observations; however, 2 zinc gluconate lozenge trials had a number of censored observations and thus the benefit of survival analysis materializes in their analysis [17, 18] (see below).
In our current analysis, the mixed-effects Cox regression indicated a 3.1-fold increase in the recovery rate by zinc acetate lozenges (Table 2). This estimate appears substantially greater than the estimated 36% to 40% reduction in the duration of colds in the same 3 trials [4]. In addition, the Kaplan-Meier curves allowed us to calculate NNT = 2.3 on the 5th day, indicating that, on average, 1 in 2.3 participants was cured by the 5th day because of zinc lozenges. Thus, the survival analysis gives useful additional information on the effects of treatment compared with analyzing only the effects on common cold duration.
Our meta-analysis was restricted to 3 trials with zinc acetate lozenges with >75 mg of elemental zinc per day. Zinc gluconate has also been used as a constituent for zinc lozenges and has been examined in controlled trials. The acetate anion binds zinc cations less strongly than gluconate, ie, zinc acetate has a lower stability constant than zinc gluconate. Therefore, zinc acetate has been proposed as a more suitable salt for lozenges than zinc gluconate [1, 10]. Two zinc gluconate lozenge trials [17, 18] published survival curves, and the effect of zinc gluconate on the rate of recovery can be calculated for those studies (see Supplementary File 2).
Eby et al [17] found that zinc gluconate increased the rate of recovery from the common cold with RR of 3.5 (95% CI, 1.8–6.7; 207 mg/day zinc); Mossad et al [18] found that zinc gluconate increased the recovery rate with RR of 2.8 (95% CI, 1.8–4.5; 80 mg/day zinc). Thus, the CIs of those 2 zinc gluconate trials are consistent with the pooled estimate calculated for the 3 zinc acetate trials in the current IPD meta-analysis. On the 5th day of the treatment, NNT was 2.4 in the Eby et al [17] trial and 3.7 in the Mossad et al [18] trial, and these estimates are also quite consistent with the findings from the zinc acetate lozenge trials. A significant effect of zinc gluconate lozenges on common cold duration was also reported by Godfrey et al [19], but survival curves were not published. However, the number of patients cured by the 7th day was published yielding RR of 1.55 (95% CI, 1.13–2.1; NNT = 3.3; 192 mg/day zinc). Thus, even though acetate binds zinc ions less strongly than gluconate, the chemical difference between the 2 salts may not have much importance at the clinical level.
Subgroup analysis of the 2 above-mentioned zinc gluconate trials cannot be carried out because data on individual baseline variables are no longer available [17, 18]. Nevertheless, given that the mechanism of zinc lozenge effect seems to be based on the release of free zinc ions [1, 8–10], it seems unlikely that the anion of the salt might influence the effect of the released zinc ions. Thus, it seems probable that the lack of subgroup differences in the effects of zinc acetate lozenges (Table 3) also applies to the zinc gluconate lozenges.
A few zinc lozenge studies did not find effects on the common cold symptoms, but most of the negative studies used low doses of zinc, <75 mg/day, and/or the lozenges contained citric acid or other substances that bind zinc ions [1, 2, 8–10]. Thus, low availability of free zinc ions explains many negative findings of zinc lozenge trials.
Farr and Gwaltney [20] speculated that bad taste of zinc lozenges might explain their apparent benefit. However, bad taste was not a problem in any of the 3 zinc acetate lozenge trials included in this IPD meta-analysis. There was no substantial difference in the occurrence of adverse effects between the zinc and placebo groups, and only a few participants dropped out [11–13]. A few patients identified the lozenge in the most recent trial, but the efficacy of the zinc lozenges remained the same when the analysis was restricted to those who remained blinded at the end of the trial [13].
The 80 to 92 mg/day doses used in the zinc acetate lozenge trials are substantially higher than the recommended daily intakes of 11 mg/day for men and 8 mg/day for women in the United States [21]. However, zinc has been administered in doses of 100 to 150 mg/day to certain patient groups for months with few adverse effects [22–27]. Furthermore, starting from adolescence, 150 mg/day of zinc is currently one of the standard treatments for Wilson’s disease, which usually means taking such doses for the rest of the life [28–31]. In the treatment of Wilson’s disease, zinc has had an excellent safety profile, although it has caused gastric irritation in 5%–10% of the patients [29]. Therefore, it seems highly unlikely that the dosage used in the 3 included trials, 80–92 mg/day for 1–2 weeks, might lead to long-term adverse effects. Furthermore, if a patient considers that some zinc lozenges taste bad, he or she can discontinue their usage.
Although there is strong evidence that properly composed zinc lozenges can increase the rate of recovery from the common cold, the majority of zinc lozenges on the market appear to have either too low doses of zinc or they contain substances that bind zinc ions, such as citric acid [1]. Thus, the results of this meta-analysis should not be directly extrapolated to the wide diversity of zinc lozenges on the current market.
CONCLUSIONS
Our IPD meta-analysis found that zinc acetate lozenges (>75 mg of zinc per day) may triple the rate of recovery from the common cold, and the effect is not modified by age, sex, race, allergy, smoking, or baseline common cold severity. The calculated 3-fold increase in the rate of recovery is a clinically important effect. The optimal formulation of zinc lozenges and the most efficacious frequency of their administration should be further examined. Nevertheless, given the strong evidence of efficacy and the low risk of adverse effects, common cold patients may already be encouraged to try zinc acetate lozenges not exceeding 100 mg of elemental zinc per day for treating their colds within 24 hours of onset of symptoms.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Acknowledgments
Authors contributions. A. P., J. T. F., and E. J. P. organized the 3 trials and collected the data that were analyzed in this study. H. H. planned and carried out this meta-analysis and wrote a draft manuscript. A. P., J. T. F., and E. J. P. participated in the revision of the manuscript.
Financial support. This research received no grant from any funding agency in the public, commercial or not-for-profit sectors.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Eby GA. Zinc lozenges as cure for the common cold—a review and hypothesis. Med Hypotheses 2010; 74:482–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hemilä H. Zinc lozenges may shorten the duration of colds: a systematic review. Open Respir Med J 2011; 5:51–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hemilä H, Chalker E. The effectiveness of high dose zinc acetate lozenges on various common cold symptoms: a meta-analysis. BMC Fam Pract 2015; 16:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hemilä H, Petrus EJ, Fitzgerald JT, Prasad A. Zinc acetate lozenges for treating the common cold: an individual patient data meta-analysis. Br J Clin Pharmacol 2016; 82:1393–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Singh M, Das RR. Zinc for the common cold. Cochrane Database Syst Rev 2013; 6:CD001364. [DOI] [PubMed] [Google Scholar]
- 6. Hemilä H. Concerns about unattributed copying of text and data, and about numerous other problems in the Cochrane review “Zinc for the Common Cold” by Singh M, Das RR (2013). 2015. Available at: http://hdl.handle.net/10138/153180. Accessed 20 April 2017. [Google Scholar]
- 7. Singh M, Das RR. WITHDRAWN: Zinc for the common cold. Cochrane Database Syst Rev 2015; 4:CD001364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Godfrey JC. Zinc for the common cold. Antimicrob Agents Chemother 1988; 32:605–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eby GA. Elimination of efficacy by additives in zinc acetate lozenges for common colds. Clin Infect Dis 2001; 32:1520. [DOI] [PubMed] [Google Scholar]
- 10. Eby GA. Zinc lozenges: cold cure or candy? Solution chemistry determinations. Biosci Rep 2004; 24:23–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Petrus EJ, Lawson KA, Bucci LR, Blum K. Randomized, double-masked, placebo-controlled clinical study of the effectiveness of zinc acetate lozenges on common cold symptoms in allergy-tested subjects. Curr Ther Res 1998; 59:595–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Prasad AS, Fitzgerald JT, Bao B, et al. Duration of symptoms and plasma cytokine levels in patients with the common cold treated with zinc acetate: a randomized, double-blind, placebo-controlled trial. Ann Intern Med 2000; 133:245–52. [DOI] [PubMed] [Google Scholar]
- 13. Prasad AS, Beck FW, Bao B, et al. Duration and severity of symptoms and levels of plasma interleukin-1 receptor antagonist, soluble tumor necrosis factor receptor, and adhesion molecules in patients with common cold treated with zinc acetate. J Infect Dis 2008; 197:795–802. [DOI] [PubMed] [Google Scholar]
- 14. R Core Team. R Project for Statistical Computing Available at: https://www.r-project.org. Accessed 20 April 2017.
- 15. Debray TP, Moons KG, van Valkenhoef G, et al. Get real in individual participant data (IPD) meta-analysis: a review of the methodology. Res Synth Methods 2015; 6:293–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stewart GB, Altman DG, Askie LM, et al. Statistical analysis of individual participant data meta-analyses: a comparison of methods and recommendations for practice. PLoS One 2012; 7:e46042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eby GA, Davis DR, Halcomb WW. Reduction in duration of common cold by zinc gluconate lozenges in a double-blind study. Antimicrob Agents Chemother 1984; 25:20–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mossad SB, Macknin ML, Medendorp SV, Mason P. Zinc gluconate lozenges for treating the common cold: a randomized, double-blind, placebo-controlled study. Ann Intern Med 1996; 125:81–8. [DOI] [PubMed] [Google Scholar]
- 19. Godfrey JC, Conant Sloane B, Smith DS, et al. Zinc gluconate and the common cold: a controlled clinical study. J Int Med Res 1992; 20:234–46. [DOI] [PubMed] [Google Scholar]
- 20. Farr BM, Gwaltney JM., Jr The problems of taste in placebo matching: an evaluation of zinc gluconate for the common cold. J Chronic Dis 1987; 40:875–9. [DOI] [PubMed] [Google Scholar]
- 21. National Research Council. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington, DC: The National Academies Press; 2001. [PubMed] [Google Scholar]
- 22. Serjeant GR, Galloway RE, Gueri MC. Oral zinc sulphate in sickle-cell ulcers. Lancet 1970; 2:891–2. [DOI] [PubMed] [Google Scholar]
- 23. Greaves MW, Skillen AW. Effects of long-continued ingestion of zinc sulphate in patients with venous leg ulceration. Lancet 1970; 2:889–91. [DOI] [PubMed] [Google Scholar]
- 24. Hallböök T, Laner E. Serum-zinc and healing of venous leg ulcers. Lancet 1972; 2: 780–2. [DOI] [PubMed] [Google Scholar]
- 25. Czerwinski AW, Clark ML, Serafetinides EA, et al. Safety and efficacy of zinc sulfate in geriatric patients. Clin Pharmacol Ther 1974; 15:436–41. [DOI] [PubMed] [Google Scholar]
- 26. Bamford JT, Gessert CE, Haller IV, et al. Randomized, double-blind trial of 220 mg zinc sulfate twice daily in the treatment of rosacea. Int J Dermatol 2012; 51:459–62. [DOI] [PubMed] [Google Scholar]
- 27. Hadwan MH, Almashhedy LA, Alsalman AR. Oral zinc supplementation restores high molecular weight seminal zinc binding protein to normal value in Iraqi infertile men. BMC Urol 2012; 12:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hoogenraad TU. Zinc treatment of Wilson’s disease. J Lab Clin Med 1998; 132:240–1. [DOI] [PubMed] [Google Scholar]
- 29. Brewer GJ, Askari FK. Wilson’s disease: clinical management and therapy. J Hepatol 2005; 42 (Suppl 1):S13–21. [DOI] [PubMed] [Google Scholar]
- 30. Marcellini M, Di Ciommo V, Callea F, et al. Treatment of Wilson’s disease with zinc from the time of diagnosis in pediatric patients: a single-hospital, 10-year follow-up study. J Lab Clin Med 2005; 145:139–43. [DOI] [PubMed] [Google Scholar]
- 31. Ala A, Walker AP, Ashkan K, et al. Wilson’s disease. Lancet 2007; 369:397–408. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.