Abstract
Background
Genetic-epidemiological studies that estimate the contributions of genetic factors to variation in tic symptoms are scarce. We estimated the extent to which genetic and environmental influences contribute to tics, employing various phenotypic definitions ranging between mild and severe symptomatology, in a large population-based adult twin-family sample.
Methods
In an extended twin-family design, we analyzed lifetime tic data reported by adult mono- and dizygotic twins (n= 8,323) and their family members (n=7,164; parents and siblings) from 7,311 families in the Netherlands Twin Register (NTR). We measured tics by the abbreviated version of the Schedule for Tourette and Other Behavioral Syndromes (STOBS) (TSAICG, 2007). Heritability was estimated by genetic Structural Equation Modeling (SEM) for four tic disorder definitions: three dichotomous and one trichotomous phenotype, characterized by increasingly strictly defined criteria.
Results
Prevalence rates of the different tic disorders in our sample varied between 0.3 and 4.5% depending on tic disorder definition. Tic frequencies decreased with increasing age. Heritability estimates varied between .25 and .37, depending on phenotypic definitions. None of the phenotypes showed evidence of assortative mating, effects of shared environment, or non-additive genetic effects.
Conclusions
Heritabilities of mild and severe tic phenotypes were estimated to be moderate. Overlapping confidence intervals of the heritability estimates suggest overlapping genetic liabilities between the various tic phenotypes. The most lenient phenotype (defined only by tic characteristics, excluding criteria B, C and D of DSMIV) rendered sufficiently reliable heritability estimates. These findings have implications in phenotypic definitions for future genetic studies.
Keywords: tic symptoms, Tourette Syndrome, heritability, twin, DSM, SEM
Introduction
Tics are defined as involuntary sudden, recurrent, non-rhythmic, stereotypical motor movements or vocalizations (DSM-IV-TR) varying from almost indiscernible eye-blinking to complex motor movements involving multiple muscle systems. The Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) (American Psychiatric Association 2000) distinguishes four categories of tic disorders: Tourette’s Disorder, also called Tourette Syndrome (TS), Chronic Motor or Vocal Tic Disorder (CMT/CVT), transient tic disorder, and tic disorder not otherwise specified (NOS). Tic diagnosis depends on age of onset, duration, and type (motor, vocal or both). Tics typically first manifest between age 4 to 6 years, and peak in severity between 10 and 12 years (Erenberg et al. 1987). Over 70% of patients experience significant reduction in tic frequency and intensity by adulthood (Bloch & Leckman 2009; Cath et al. 2011).
Community-based studies have produced disparate TS prevalence estimates in children and adolescents – ranging from 0.5 to 38 cases per 1000 (Apter et al. 1993; Hirtz et al. 2007; Knight et al. 2012; Robertson et al. 2009; Scahill et al. 2005; Mathews et al. 2014; Miller et al. 2014). In a review study Scahill et al. (2013) concluded that the prevalence of TS in children between age 6 and 18 lies between 0.5% and 0.7%. Estimates for chronic motor tics ranged from 0.3% to 0.8% (Kurlan et al. 2001; Khalifa 2006; Scahill et al. 2006; Kraft et al. 2012; Cubo et al. 2011) in several studies in children. Male-to-female ratios varied between 3- 4 to 1, with higher prevalence rates in boys (1.06 to 4.5% in boys, and 0.25% to 1.7% in girls (Lichtenstein et al. 2010; Knight et al. 2012)).
In adults, tic prevalence rates are considerably lower, with estimates of 0.05% to 0.1% for TS, and of 0.08% to 6.7% for any tic disorders (Knight et al. 2012; Apter et al. 1993; Bar-Dayan et al. 2010; Eapen et al. 2001; Robertson et al. 1994; Schlander et al. 2011; Wenning et al. 2005; Zohar et al. 1992). A Swedish population-based twin study (n=21,911) found prevalence rates of 6.7% for having any tic, and 1.4% for having TS (not taking the DSM-IV-TR criterion of presence of at least one vocal tic into account) (Pinto et al. 2016).
The causes of individual differences in tic disorder characteristics and severity are poorly understood. Genetic and environmental factors contribute to phenotypic variation. There is some suggestion of assortative mating (i.e., spousal resemblance) for tics (Hasstedt et al. 1995; Kurlan et al. 1994) but studies have been scarce. The presence of parental data allows us to take into account assortative mating. This is important as assortative mating in the parental generation may in their offspring increase the genetic correlations between siblings including DZ twins (these are on average 0.5 under random mating). This may bias the results obtained from the classical twin design, with underestimation of heritability and overestimation of shared environmental effects. In family studies, heritability estimates of TS and tic symptoms range from .18 to .77 (Mathews & Grados 2011; Pauls et al. 1991; de Haan et al. 2015; Mataix-Cols et al. 2015; Hirschtritt et al. 2015). Tic risk in first degree relatives of tic sufferers is high (Mataix-Cols et al. 2015). In one small clinical twin study (Price et al. 1985) of 30 MZ and 13 DZ twin pairs concordance rates were .53 for MZ pairs and .08 for DZ pairs. When criteria were broadened to include any tic, concordance rates were 0.77 for MZ pairs and 0.23 for DZ pairs.
Three population-based twin heritability studies have been performed in children or adolescents, and one in adults. (Bolton et al. 2007; Anckarsäter et al. 2011; Lichtenstein et al. 2010; Pinto 2016). The longitudinal Child and Adolescent Twin Study in Sweden (CATSS) assessed tic disorders in 17,000 twins aged 9 to 12 years. The assessment consisted of three questions on tic occurrence which the parents’ twins answered during a telephone interview (Anckarsäter et al. 2011). Tics were further assessed using the ‘Autism — Tics, ADHD and other Comorbidities inventory’ (A-TAC) (Hansson & Svanstro 1994; Larson et al. 2010). Correlations for tic disorder were .38 in MZ and .11 in DZ twins, and heritability estimates were .26 in girls and .39 in boys. The heritability estimate of a binary TS diagnosis based on these data (3.1% diagnosed as affected) equalled .56 (Lichtenstein et al. 2010). Furthermore, the Genetic and Environmental Effects on Emotion study (GEMS) estimated the heritability of tic disorders based on a binary diagnosis in 4662 4–6-year old twin pairs (Bolton et al. 2007). Mothers were interviewed in a two stage telephone screen with questions on tic occurrence in their 4-year-old twins. The high scoring sample from stage 1 was selected for stage 2 (n=854 pairs) and re-interviewed. Using a liability threshold model, the heritability estimate was .5. A Japanese twin study employed a liability threshold model to assess the heritability of mother-rated tics in a sample of 1896 twin pairs between 3 and 15 years (Ooki 2005). The mothers rated their twins with respect to the frequency of tic behaviors. Tic heritabilities estimates were .28 (boys) and .29 (girls), with shared environmental effects explaining 41% of the variance in boys and 32% in girls. Finally, Pinto et al (2016) studied the co-variation of tics, OC symptoms and ADHD in adult twins (n=20.821). The tic heritability estimate based on liability threshold modeling was 0.33 (Pinto et al. 2016). In sum, heritability estimates from epidemiological studies vary between ~.28 and ~.56, with different tic definitions and rating methods used and most studies estimating tic heritability of a single phenotypic operationalization.
The aim of the present study is to examine the genetic and environmental contributions to tic symptoms using different DSM-IV-TR derived tic phenotypic definitions in a population-based adult twin-family sample. As different studies use different measures of tics, it is highly useful to explore the influence of these varying measurement methods on variation in tic heritability. In addition, for future GWAS or other studies using genetic variants, it seems paramount to use those phenotypic tic definitions that capture the most optimal heritability estimates; “most optimal” meaning a combination of significant nonzero heritability and narrower CIs, reflecting the largest information content.
In addition, an adult twin-family sample has the advantage that lifetime tics are taken into account, allowing tics to be included that develop in adolescence. An extended twin design was used, including twins, siblings and their parents. The presence of parental data allowed us to further study the influence of assortative mating, a topic that has been scarcely addressed in TS (Hanna, Janjua, Contant, & Jankovic, 1999; Kurlan, Eapen, Stern, McDermott, & Robertson, 1994; McMahon et al., 1996). This is important as assortative mating may increase the additive genetic correlation among DZ twins (i.e., .5 given random mating), which may bias the results obtained analyzing only data from twins. Specifically assortative mating results in overestimation of shared environmental effects, and underestimation of genetic effects. In addition, an extended twin design confers greater power than the classical twin design (Posthuma & Boomsma 2000). Our aims were to 1) quantify the genetic contributions to the various tic phenotypes, using both lenient and strict phenotypic definitions of tics and tic severity; 2) explore the role of assortative mating and dominance effects; and 3) determine how much the heritability estimates vary with phenotypic definition.
Method
Participants
This study is part of an ongoing longitudinal study of twins and families registered in the Netherlands Twin Register (NTR), in which participants complete a series of questionnaires on health and behavior every two to four years. A tic questionnaire was included in the 2008 survey (see Willemsen et al. 2013 for a more detailed description of the data collection). Data from 8,323 adult twins and 7,164 family members (clustered in 7,311 families) were available. Family members included twins, and parents and siblings of twins. From each family, data from two twins, two additional siblings, and their parents, if available, were selected. Non-biological parents and non-full siblings were excluded. In cases of triplets or higher-order multiples, the first- and second-born twins were included. In cases of more twin pairs per family, one twin pair was included. Online Supplementary table 1 gives the number of family members. Data from both twins were present in 2748 families (38%), and data from twins as well as parents were present in 804 (11%) of the families. Zygosity of same-sex twins was determined by blood type, DNA markers, or questionnaire (Rietveld et al. 2000). There were 2,714 complete twin pairs with known zygosity (98.8% of all complete twin pairs): 388 MZ and 200 DZ male pairs, 1129 MZ and 507 DZ female pairs, and 490 DZ pairs of opposite sex (DOS). The age of twins ranged from 17 to 97 years (mean=33.1, SD=14.5), and the age of siblings from 11 to 88 years (mean=37.1 and SD=13.8) and of the 5,441 parents from 37 to 94 (mean=54.9 and SD=8.6). Ethical approval for the study was obtained from the Medical Ethical Committee of the VU University Medical Centre.
Measures
Data on tics from NTR Survey 8 were collected using the abbreviated Schedule for Tourette and Other Behavioral Syndromes (STOBS-ABBR) that provides a semi-structured assessment on tics, OC, and ADHD symptoms (Pauls & Hurst 1996). This scale has been used widely by the Tourette Syndrome Association International Consortium for Genetics (TSAICG), both as interview and as self-report measure. For the NTR 2008 survey, the STOBS was abbreviated to include 9 items on the most frequent tics occurring in clinical samples (Cath et al. 2011; Freeman et al. 2000); see online supplementary table 2 for the STOBS-ABBReviated. Participants indicated for each tic type whether they ever/never experienced it. When given the response ‘ever’, they indicated whether the tic had occurred 0–1 year ago, 1–5 years ago, or more than 5 years ago. Subsequently, given a positive response on tic presence, items were filled in on age at onset, duration of tics (<1 year versus >1 year), and tic frequency/severity in three additional self-report items. A paper version of the questionnaire was completed by 7,028 participants (45%), and an online version was completed by 8,459 participants.
Using the STOBS-ABBR, all participants were classified according to DSM-IV-TR criteria (American Psychiatric Association 2000) into the following mutually exclusive categories: probable TS, probable chronic (motor or vocal) tic disorder, probable transient tic disorder, or probable tic disorder NOS (see table 1 for a summary of tic definitions). We added the term “probable”, since subjects were classified based on self-report, whereas a tic diagnosis is usually established through interview and observation by experienced clinical experts, a requirement that we were unable to fulfill in this large population-based study. The DSM-IV-TR requires an age at onset before 18 years to fulfill criteria for a tic disorder diagnosis. However, in view of the age at onset distributions of our data (figure 1) and as used by the Tourette Syndrome Study Group (Anon 1993), we adopted an age of onset≤21 years as a requirement for the definitions of “probable TS”, “probable chronic (motor or vocal) tic disorder”, and “probable transient tic disorder”. “Probable tic disorder NOS” did not require the age-of-onset criterion.
Table 1.
DSM-IV-TR criteria for the different tic disorders
| Motor tic(s) | Vocal tic(s) | > 4 weeks | > 1 year | Age of onset before adulthood | Many tics a day | Other requirements | ||
|---|---|---|---|---|---|---|---|---|
| Probable TS | yes >1 | AND | yes | yes | yes | yes | yes | – |
| Probable Chronic td | yes | OR | yes | yes | yes | yes | yes | – |
| Probable Transient td | yes | AND/OR | yes | yes | no | yes | yes | – |
| Probable Td-NOS | yes | AND/OR | yes | – | – | – | – | no other tic disorder |
TS = Tourette Syndrome; td = tic disorder; Td-NOS= tic disorder ‘not-otherwise specified’.
Figure 1.
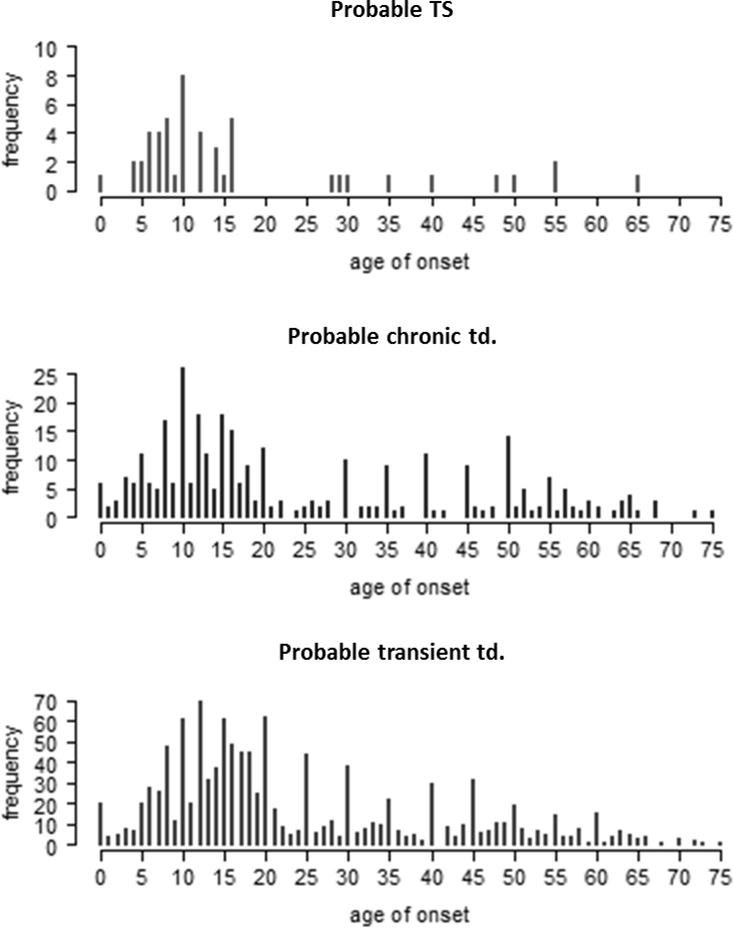
Reported age of onset of tics for participants that fulfill criteria of Tourette syndrome, chronic tic disorder and transient tic disorder (including the age of duration criterium but without the age of onset-criterium).
To classify as probable TS, the following was required: 1) positive responding (‘ever’) to at least two motor and one vocal tics; 2) age of onset≤21 years; and 3) a tic duration of ≥ one year. The same criteria were used to classify as a probable chronic tic disorder, except that either one vocal or one motor tic was required. These subjects were further subdivided based on the nature of their tics (motor/vocal). “Probable transient tic disorder” required: 1) one or more motor and/or vocal tics, 2) age at onset≤21, and 3) tic duration of <1 year. Participants who reported at least one tic, but without an age at onset≤21, and/or with a tic duration of <1 year were categorized as a probable tic disorder NOS.
For genetic modeling we classified subjects as affected or non-affected according to different inclusion criteria: 1) all subjects who scored any tic at any age of onset for any period of time included as affected (‘any probable tic’ – the most lenient phenotype); 2) subjects with “probable TS”, “probable chronic tic disorder–motor”, or “probable tic disorder–vocal” were classified as affected; 3) all subjects with probable TS and probable chronic tic–motor tics as were classified affected. One additional definition was considered with 3 categories: “no tic disorder” (unaffected), “probable tic disorder NOS” combined with “probable transient tic disorder”(affected), and “probable chronic tic disorder—motor”, “probable chronic tic disorder—vocal” and “probable TS” (affected).
Statistical analyses
Population prevalences of the different tic disorder definitions were estimated in the entire sample of 15,487 individuals. Fitting the genetic models and calculating correlations between family members was done by assuming that a normally distributed liability underlies the discrete phenotypes (Falconer 1965; Falconer 1967). In the case of a dichotomous phenotype, the threshold separates the two classes of subjects, namely the “affected” and “unaffected”. For the trichotomous phenotype, two thresholds were estimated, separating three classes following the definition described above.
To assess the significance of covariates prior to the genetic modeling, we performed logistic regression, examining the covariates sex, age at filling in the questionnaire, method of reporting (paper versus internet survey) and their interactions. To correct for family clustering, we used generalized estimation equation (GEE; Dobson & Barnett 2008) with the R-package ‘GEE’ and the logistic link function. For all analyses and model fitting procedures the threshold for significance was set at alpha=.05. We obtained initial estimates of familial resemblance by estimating tetrachoric and polychoric correlations between the liabilities of the family members using the R-package ‘polychor’ (https://cran.r-project.org/web/packages/polycor/index.html).
Genetic model fitting was conducted in R-package OpenMx version 2.2.4 (Boker et al. 2011). Parameters were estimated by raw data maximum-information likelihood. We first tested whether parent-offspring correlations were equal to DZ and sibling correlations (as all share on average 50% of their segregating genes). For the two strictest variable definitions, we encountered computational problems due to the low prevalence, giving rise to empty cells in the tables. We therefore excluded data from siblings and parents. Next, we fitted genetic variance decomposition models. These decompose variance in the liability to have tics into additive genetic (A), unique environmental (E), common environmental (C), and/or dominant genetic factors (D). Since C and D cannot be estimated together, we included C if the MZ correlation was less than twice the DZ twin correlation. If the MZ correlation was larger than twice the DZ correlation, we included D. The influence of common environmental factors and of genetic dominance was tested by comparing a nested AE model with either the ACE or the ADE model using likelihood-ratio tests. The AE models are depicted in online supplementary Figure 1 and 2.
Results
Descriptive statistics
Prevalence rates of STOBS-ABBR tic items are summarized in online supplementary table 3. Given these symptoms we derived four probable tic disorder diagnoses in accordance with the DSM-IV-TR (i.e.: TS, Chronic motor/vocal tic disorder, transient tic disorder and tic disorder NOS). Prevalence rates of these disorder diagnoses varied from 0.3% (probable TS) to 4.5% (probable transient tic disorder; Table 2).
Table 2.
Descriptive statistics: number of participants and prevalence rates of DSM-IV probable tic disorders to the STOBS
| Probable tic disorder | N (%) | Male/Female | |
|---|---|---|---|
| Participants classified with a tic disorder | Probable TS | 44 (0.3%) | 23/21 |
| Probable Chronic tic disorder (motor) | 150 (1.0%) | 71/79 | |
| Probable Chronic tic disorder (vocal) | 42 (0.3%) | 22/20 | |
| Probable Transient tic disorder | 658 (4.5%) | 264/394 | |
| Probable Tic disorder NOS | 637 (4.4%) | 316/321 | |
|
| |||
| Total | 1531 (10.5%) | 696/835 | |
Genetic analyses were performed on the four tic phenotypes grouped together in different ways for the various genetic analyses as described above in the methods section. Figure 2 shows the prevalence rates for each of these four (three dichotomous and one trichotomous). Depending on the strictness of the phenotype definition, prevalence rates varied from 1.3% (‘probable TS or probable chronic motor tic disorder’) to 12.5% (‘any probable tic’, the most lenient phenotype).
Figure 2.
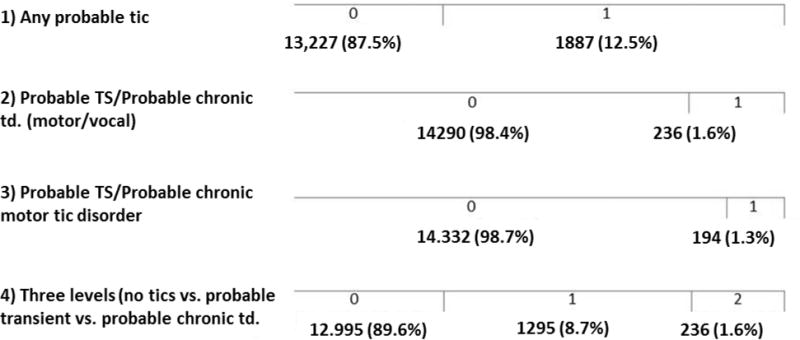
Descriptive statistics: Number of unaffected (‘0’) and affected (‘1’ and ‘2’) participants, according to each of the four phenotypes. The fourth phenotype has two thresholds and is a combination of the first and second dichotomous phenotype.
Thresholds and Covariate effects
The locations of the thresholds and effects of covariates are shown in online supplementary figure 3. In all baseline models, separate thresholds were estimated for offspring and parents (e.g., for the ‘any probable tic’ phenotype when only twins and parents were included, one threshold estimate instead of four resulted in a significantly worse fit: χ2(3)=15.25, p=.002; two threshold estimates, one for parents and one for offspring, did not significantly reduce the fit: χ2(2)=3.07, p=.22). Thus, for the ‘any probable tic’ phenotype, threshold estimates for parents were higher than for offspring, indicating that parents reported less tics. This was not seen in the more strict dichotomous phenotypes, indicating that the frequency of more severe tic disorders, based on self-report, and after correction for age, did not differ between parents and offspring.
Covariate effects were similar for the dichotomous variables (with the ‘any probable tic’ phenotype corresponding to the lowest threshold and the ‘probable TS or probable chronic tic disorder’ and ‘probable TS or probable chronic motor tic disorder’ phenotypes corresponding to the second threshold). Males were affected more often than females (e.g., for the ‘any probable tic’ dichotomous variable: b=−0.38, se=0.07, p<.001). We observed a decrease in the reporting of tics with increasing age (‘any probable tic’ phenotype, with standardized age: b=−0.17, se=0.05, p<.001). Participants who answered the paper-version of the questionnaire (instead of the online-version) reported more tics (using the ‘any probable tic’ phenotype: b=−0.16, se=0.08, p=.045; for the stricter tic disorder phenotypes this was not significant; online supplementary table 4). The interaction between age and method of reporting for the second dichotomous phenotype (probable chronic tic disorder and TS versus mild or no tic disorder) was found to be significant (b=−.33, se=0.15, p=.03).
Familial correlations and Assortative mating
Familial correlations are shown in Table 3. MZ twin correlations were higher than DZ twin correlations and correlations in other first-degree family members. Online Supplementary Table 5 summarizes the correlations between other family members. Since the correlation structure among relatives did not provide consistent evidence for either dominant genetic or common environmental effects, models with both dominance (ADE) and common environmental effects (ACE) were considered.
Table 3.
MZ and DZ twin polychoric correlations (and standard errors) for each phenotype
| phenotype | MZ twin corr. (se) | DZ twin corr.(se) |
|---|---|---|
| 1) Any probable tic | .37 (0.05) | .18 (0.07) |
| 2) Probable TS/probable chronic tic disorder | .24 (0.21) | .15 (0.21) |
| 3) Probable TS/probable chronic motor tic disorder. | .32 (0.21) | .19 (0.21) |
| 4) Three levels (no tics vs. probable transient/NOS tic disorder. vs. TS/probable chronic tic disorder | .37 (0.06) | .17 (0.07) |
With respect to exploration of the influence of assortative mating: our data do not support evidence for assortative mating using any of the phenotypic definitions.
Genetic Model fitting
Table 4 shows the results of genetic model fitting, where ACE and ADE differ in that the first model attributes familial resemblance to additive genetic and common environmental factors, and the second model attributes resemblance to additive and non-additive (dominance) genetic factors. In all models the C and D parameters were not significant: comparison with the more parsimonious AE model did not show a significant drop in the fit (e.g. for the first dichotomous phenotype, when twins, parents and siblings were included; AE vs. ACE: χ2(1)<.001, p>.99, and AE vs. ADE: χ2(1)=2.59, p=.11). Heritability point estimates ranged from .25 to .37. Thus, familial resemblance can be explained solely by additive genetic factors. The 95% confidence intervals were wide and all overlapping. The ‘any probable tic’ phenotype showed the narrowest confidence interval (0.31, 95% CI [0.23, 0.38]).
Table 4.
Estimated parameters, and fit indices of genetic analyses for each of the four phenotypes
| family members included | baseline model | a2 (95%CI) * | fit (AE compared to baseline model)
|
thr 1 offspr/parents | thr 2 offspr/parents | |||
|---|---|---|---|---|---|---|---|---|
| diff -2LL | Diff df | p | ||||||
| 1) any probable tic | tw+par | sat. | .31 (.23–.40) | 11.98 | 11 | .63 | .92/1.06 | – |
| tw+par+sibs | constr.sat | .30 (.23–.38) | 8.23 | 7 | .69 | .93/1.06 | – | |
|
| ||||||||
| 2) Probable TS/chr. TD | tw+par | – | .37 (.08–.61) | 1.99/1.95 | – | |||
| tw+par+sibs | – | .31 (.04–.55) | 2.04/1.95 | – | ||||
| tw | sat. | .25 (.02–.60) | .008 | 1 | .93 | 2.03 | – | |
|
| ||||||||
| 3) Probable TS/chr. motor TD. | tw+par | – | .32 (.02–.61) | 2.10/2.07 | – | |||
| tw+par+sibs | – | .28 (.02–.56) | 2.16/2.06 | – | ||||
| tw | sat. | .34 (.02–.68) | .018 | 1 | .89 | 2.12 | – | |
|
| ||||||||
| 4) three levels** | tw+par | sat. | .34 (.24–.44) | 6.45 | 11 | .84 | .98/1.09 | 1.93/2.04 |
| tw+par+sibs | constr.sat | .33 (.24–.42) | 3.36 | 7 | .85 | 1.00/1.08 | 1.97/2.06 | |
Note.
c2 and d2 were tested but never significant; the remainder of the variance comes from unique environmental effects (e2);
no tics vs. probable transient tic disorder/probable tic disorder NOS vs. probable TS/probable chronic tic disorder; ‘TS/chr. TD’ = Tourette Syndrome/Chronic tic disorder; ‘thr’ = threshold; ‘tw’ = twins; ‘par’ = parents; ‘sibs’ = siblings; ‘sat.’ = saturated model; ‘constr.sat.’ = constrained saturated model (i.e. parent-offspring correlations are set equal and full sibling correlations are set equal
Discussion
The aim of this study was to estimate the heritability of increasingly strict phenotypic definitions of lifetime tic disorders that were mostly in line with current DSMIV and DSM 5 criteria for tic disorders, in a large adult population-based sample Further, using an extended twin design, we estimated the relative contribution of additive and non-additive genetic effects, effects of common and unique rearing environment, and the role of assortative mating. In line with Walkup et al (2010), we were specifically interested in obtaining a clear understanding of the core phenomenological features of tics, taking one step further, i.e. by investigating whether and to what extent the various phenotypic definitions influence estimates of genetic and environmental contributions to tics (Walkup, Ferrao, Leckman, Stein, & Singer, 2010). The abbreviated STOBS that we used is in line with both DSMIVTR and DSM5 criteria of the various tic disorders with respect to their core criteria of tic characteristics, duration, and age at onset, except for criterion D (i.e.: the disturbance is not attributable to a medical condition). In sum, the first 9 items of the abbreviated STOBS asked about tic characteristics (pertaining to criterion A), one additional item asked about age at onset (before versus after age 18) and one item asked about duration of tics (<1 year or > 1 year).
Our prevalence rates of a tic disorder are in the expected range (i.e. between 0.3–4.5%). In epidemiological studies in children, prevalence rates are between 3–8 cases per 1000 between ages 6 and 18 (Scahill et al. 2013). Our rates are higher than reported in most epidemiological studies in adults (0.001–0.05%), but in line with the other tic twin study in adults using self-reports by Pinto et al. (2016), who reported prevalence rates of TS between 0.4–1.4% depending on strictness of phenotypic definition. Also, the prevalence rates of the most lenient definition of “any probable tic” of 12.5% in our sample is by and large in accordance (although somewhat higher) with the rates reported by Pinto et al. of 7.2% for any tic in men and 6.5% in women (Pinto et al. 2016). An explanation for the somewhat higher rates in our twin study and previous epidemiological studies (Pinto et al., 2016) might be that, using DSM-III and IV criteria (American Psychiatric Association 1994), earlier studies included an impairment/disability criterion for TS which has been subsequently removed from the DSM-IV-TR and DSM-V (American Psychiatric Association 2000). This may have caused a relative underestimation of tic prevalence.
In our cross-sectional sample self-reported tic frequencies tended to decrease during adolescence and throughout adulthood, which is fully in line with interview-based epidemiological and clinical studies across the lifespan indicating that self-reported tic measures can be reliably used in large scaled studies. The decrease in tic frequencies with age might be the result of maturation of the frontal lobes, and -as a result- increased inhibitory efficiency of the cortico-striato-thalamo-cortical (CSTC) circuitry (Felling & Singer 2011). However, recall bias, resulting in under-reporting of milder tics with age, should also be taken into account as a contributor to decrease of tic severity and frequency with age.
In this study, (narrow-sense) heritability estimates ranged from 0.25–0.37, with large confidence intervals that overlapped across the phenotypic definitions and that were in line with the other tic twin study in adults (Pinto et al. 2016) and somewhat lower than some family studies and twin-family studies in children (Bolton et al. 2007; Anckarsäter et al. 2011; Lichtenstein et al. 2010; Mathews & Grados 2011).
Possibly, with time, unique environmental mediators become increasingly important in the expression of these complex disorders. To conclude, in the present study heritability estimates for both mild and severe tic phenotypes were consistent, ranging from 0.25 to 0.37. However, the prevalences of the severe tic phenotypes were low, resulting in relatively low power to estimate the most strict thresholds models. As a consequence, confidence intervals of the heritabilities for the severe tic disorders are wide, and the narrow-sense heritability for severe tic disorders might be as large as 56% (the upper border of the confidence interval when siblings are included). Family-based studies specifically ascertaining probands with TS corroborate the data provided by this study, and suggest that heritability estimates might actually be on the high end of this estimate (58%–77%) (Hirschtritt et al. 2015).
Interestingly, we found that heritability estimates of the “any tic” definition showed the narrowest Confidence Intervals, yielding moderate heritability estimates. Thus, the “core” tic phenotype that included only DSMIVTR and 5 criterion A of the various tic disorders definitions (i.e. presence of a tic), seems to render the most reliable heritability estimates. In our opinion, in line with Walkup et al., 2010, this pleas for a relatively clear and simple phenotypic definition of tics in future data collection efforts for genetic studies, provided that the core phenotypic characteristics of tic disorders have been met, i.e. presence of tics, defined as “sudden, rapid, recurrent, non-rhythmic, stereotyped motor movements or vocalisations”.
We found no evidence for assortative mating with respect to any of the tic phenotypes. In addition, we found no evidence of a contribution of common environment (C) or non-additive genetic effects (D), implying that all phenotypic definitions of probable tic disorders (mild/severe) are influenced by additive genetic factors and unique environmental factors. The absence of C is consistent with the Swedish twin study in children (Lichtenstein et al. 2010; Anckarsäter et al. 2011; Pinto et al. 2016). The discrepant findings by a Japanese twin study (Ooki 2005) who found a large contribution of shared environmental effects on tics, might be due to cultural differences; i.e. cultural adaptations reflect differences in shared environmental contributions to heritability estimates in cross-groups comparisons.
The heritability estimates mentioned so far were estimated using the twin method. Davies et al. (2013) used SNP data from a GWAS of clinical TS cases to estimate the heritability attributable to the contribution of SNPs (e.g., GCTA; (Yang, Lee, Goddard, & Visscher, 2011)). In contrast to our findings, Davis et al. found a high chip-based heritability estimate of .58, which is remarkably high compared to most SNP-based studies of complex disease (Wray & Maier 2014). We do not have a clear explanation for these divergent findings, although power issues and sample selection (clinical versus epidemiological) might play a role.
We did not attempt to model all phenotypic operationalizations simultaneously, as this is impossible due to the many empty cells in the cross tables. However, we assume that the variation in scoring of the various phenotypic definitions has a direct bearing on the diagnostic threshold, but not on the underlying liability. This implies that the estimates of the genetic and environmental contributions to individual differences in the liability should be equal. Our results are consistent with this, as the confidence intervals largely overlap across phenotype definitions, suggesting a continuous normally distributed liability for having a mild or severe tic phenotype. However, as the different phenotypic definitions did yield small but significantly different heritability estimates, this suggests that small (but significant) quantitative differences exist in genetic liability to tics.
The relatively modest heritabilities as found in this study, coupled with relatively large contribution of unique environmental influences, are consistent with the conceptualization of TS as a complex disorder like other complex psychiatric disorders, such as OCD and Anxiety Disorders (Zilhao et al. 2014; Pauls 2010; van Grootheest et al. 2005; Shimada-Sugimoto et al. 2015; Hettema et al. 2001; Van Grootheest et al. 2007). In line with this, various environmental factors (such as stress, fatigue and life events) have been found to be relevant to the expression of tics (Findley et al. 2003; Swain & Leckman 2005). Importantly, this study has relevance for molecular genetics and GWASs. GWASs in complex traits have not been very successful to date, partly as a consequence of difficulties in defining and standardizing phenotypes (Sabb et al. 2009; Smith et al. 2013; Wray et al. 2012; Wray & Maier 2014). Our work indicates that the heritability estimates from multiple tic phenotypic definitions largely overlap, strongly suggesting that future studies may use lower thresholds for tic classification, hence taking advantage of the increased power due to the higher number of cases that can be included in GWASs.
Results from this study should be interpreted in light of some limitations. The data collected are based on self-report measures (as this is a population-based study) and not on clinician-administered structural interviews, which might have led to misclassification. Additionally, since lifetime tics have been reported retrospectively, recall bias might have caused inaccuracy in recollecting past occurrences of tics.
In conclusion, our results indicate that genetic and unshared environmental factors contribute to the phenotypic variability across the full range of tic disorders. No shared environmental or genetic dominance effects were found to contribute. Finally there was no/little evidence for assortative mating. Our findings replicate and extend previous work in adults (Pinto et al., 2016), suggesting a relatively large contribution of environmental factors to the phenotype. However, these environmental influences might also include epigenetic or even genetic effects (private mutations). The heritability estimates of the different phenotypic definitions estimates are comparable (considering the confidence intervals), which is consistent with the liability threshold model, in which alternative scoring has a bearing on the threshold(s), but much less on the contributions of genetic and environmental factors to individual differences in the liability.
Supplementary Material
Acknowledgments
We are grateful to the twin families for their participation. This project has been financed by FP7-People-2012-ITN, project: TS-Eurotrain, grant number 316978; BBR Foundation (NARSAD) 21668; ZonMW(Addiction) 31160008; and European Research Council (ERC-230374).
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (4th ed) 4th. American Psychiatric Association; 1994. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. 4th. American Psychiatric Association; 2000. [Google Scholar]
- Anckarsäter H, Lundström S, Kollberg L, Kerekes N, Palm C, Carlström E, Långström N, Magnusson Patrik KE, Halldner L, Bölte S, Gillberg C, Gumpert C, Råstam M, Lichtenstein P. The Child and Adolescent Twin Study in Sweden (CATSS) Twin research and human genetics: the official journal of the International Society for Twin Studies. 2011;14:495–508. doi: 10.1375/twin.14.6.495. [DOI] [PubMed] [Google Scholar]
- Definitions and classification of tic disorders. The Tourette Syndrome Classification Study Group. Archives of neurology. 1993;50:1013–6. doi: 10.1001/archneur.1993.00540100012008. Anon. [DOI] [PubMed] [Google Scholar]
- Apter A, Pauls DL, Bleich A, Zohar AH, Kron S, Ratzoni G, Dycian A, Kotler M, Weizman A, Gadot N. An epidemiologic study of Gilles de la Tourette’s syndrome in Israel. Archives of General Psychiatry. 50:734–738. doi: 10.1001/archpsyc.1993.01820210068008. [DOI] [PubMed] [Google Scholar]
- Bar-Dayan Y, Arnson Y, Elishkevits K. Screening for Common Neurologic Diseases Among Israeli Adolescents. Journal of Child Neurology. 2010;25:348–351. doi: 10.1177/0883073809339878. [DOI] [PubMed] [Google Scholar]
- Bloch MH, Leckman JF. Clinical course of Tourette syndrome. Journal of psychosomatic research. 2009;67:497–501. doi: 10.1016/j.jpsychores.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boker S, Neale M, Maes H, Wilde M, Spiegel M, Brick T, Spies J, Estabrook R, Kenny S, Bates T, Mehta P, Fox J. OpenMx: An Open Source Extended Structural Equation Modeling Framework. Psychometrika. 2011;76:306–317. doi: 10.1007/s11336-010-9200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton D, Rijsdijk F, O’Connor TG, Perrin S, Eley TC. Obsessive-compulsive disorder, tics and anxiety in 6-year-old twins. Psychological medicine. 2007;37:39–48. doi: 10.1017/S0033291706008816. [DOI] [PubMed] [Google Scholar]
- Cath DC, Hedderly T, Ludolph AG, Stern JS, Murphy T, Hartmann A, Czernecki V, Robertson MM, Martino D, Munchau A, Rizzo R, Androutsos C, Aschauer H, Baird G, Bos-Veneman N, Brambilla A, Cardona F, Cavanna A, Dehning S, Eapter A, Farkas L, Gadaros J, Hauser E, Heyman I, Hoekstra PJ, Korsgaard A, Jackson GM, Larsson L, Menghetti C, Debes NM, Muller N, Muller-Vahl K, Munchau A, Musil R, Nagy P, Nurnberger J, Oostra B, Paschou P, Pasquini M, Plessen KJ, Porta M, Rickards H, Rizzo R, Roessner V, Rothenberger A, Servello D, Skov L, Strand G, Tarnok Z, Termine C, Van Der Griendt J, Verdellen C, Visser-Vandewalle V, Wannag E, Wolanczyck T. European clinical guidelines for Tourette Syndrome and other tic disorders. Part I: Assessment. European Child and Adolescent Psychiatry. 2011;20:155–171. doi: 10.1007/s00787-011-0164-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubo E, Gabriel y Galán JM, Villaverde VA, Velasco SS, Benito VD, Macarrón JV, Guevara JC, Louis ED, Benito-León J. Prevalence of tics in schoolchildren in central Spain: a population-based study. Pediatric Neurology. 2011;45(2):100–108. doi: 10.1016/j.pediatrneurol.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Davis LK, Yu D, Keenan CL, Gamazon ER, Konkashbaev AI, Derks EM, Neale BM, Yang J, Lee SH, Evans P, Barr CL, Bellodi L, Benarroch F, Berrio GB, Bienvenu OJ, Bloch MH, Blom RM, Bruun RD, Budman CL, Camarena B, Campbell D, Cappi C, Cardona S, Julio C, Cath DC, Cavallini MC, Chavira D, Chouinard S, Conti DV, Cook EH, Coric V, Cullen BA, Deforce D, Delorme R, Dion Y, Edlund CK, Egberts K, Falkai P, Fernandez TV, Gallagher PJ, Garrido H, Geller D, Girard SL, Grabe HJ, Grados MA, Greenberg BD, Gross-Tsur V, Haddad S, Heiman GA, Hemmings SMJ, Hounie AG, Illmann C, Jankovic J, Jenike MA, Kennedy JL, King RA, Kremeyer B, Kurlan R, Lanzagorta N, Leboyer M, Leckman JF, Lennertz L, Liu C, Lochner C, Lowe TL, Macciardi F, McCracken JT, McGrath LM, Mesa Restrepo SC, Moessner R, Morgan J, Muller H, Murphy DL, Naarden AL, Ochoa WC, Ophoff RA, Osiecki L, Pakstis AJ, Pato MT, Pato CN, Piacentini J, Pittenger C, Pollak Y, Rauch SL, Renner TJ, Reus VI, Richter MA, Riddle MA, Robertson MM, Romero R, Rosàrio MC, Rosenberg D, Rouleau GA, Ruhrmann S, Ruiz-Linares A, Sampaio AS, Samuels J, Sandor P, Sheppard B, Singer HS, Smit JH, Stein DJ, Strengman E, Tischfield JA, Duarte AV, Vallada H, Van Nieuwerburgh F, Veenstra-Vanderweele J, Walitza S, Wang Y, Wendland JR, Westenberg HGM, Shugart YY, Miguel EC, McMahon W, Wagner M, Nicolini H, Posthuma D, Hanna GL, Heutink P, Denys D, Arnold PD, Oostra BA, Nestadt G, Freimer NB, Pauls DL, Wray NR, Stewart SE, Mathews CA, Knowles JA, Cox NJ, Scharf JM. Partitioning the heritability of Tourette syndrome and obsessive compulsive disorder reveals differences in genetic architecture. PLoS genetics. 2013;9(10) doi: 10.1371/journal.pgen.1003864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson AJ, Barnett A. An introduction to generalized linear models. 3rd. Chapman and Hall/CRC; 2008. [Google Scholar]
- Eapen V, Laker M, Anfield A, Dobbs J, Robertson MM. Prevalence of tics and Tourette syndrome in an inpatient adult psychiatry setting. Journal of psychiatry & neuroscience: JPN. 2001;26:417–20. [PMC free article] [PubMed] [Google Scholar]
- Erenberg G, Cruse R, Rothner A. The natural history of Tourette syndrome: a follow-up study. Annals of neurology. 1987;22(3):383–385. doi: 10.1002/ana.410220317. [DOI] [PubMed] [Google Scholar]
- Falconer DS. The inheritance of liability to certain diseases, estimated from the incidence among relatives. Annals of Human Genetics. 1965;29:51–76. [Google Scholar]
- Falconer DS. The inheritance of liability to diseases with variable age of onset, with particular reference to diabetes mellitus. Annals of Human Genetics. 1967;31:1–20. doi: 10.1111/j.1469-1809.1967.tb01249.x. [DOI] [PubMed] [Google Scholar]
- Felling RJ, Singer HS. Neurobiology of Tourette Syndrome: Current Status and Need for Further Investigation. 2011;31(35):12387–12395. doi: 10.1523/JNEUROSCI.0150-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findley DB, Leckman JF, Katsovich L, Lin H, Zhang H, Grantz H, Otka J, Lombroso PJ, King RA. Development of the Yale Children’s Global Stress Index (YCGSI) and its application in children and adolescents ith Tourette’s syndrome and obsessive-compulsive disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2003;42(4):450–457. doi: 10.1097/01.CHI.0000046816.95464.EF. [DOI] [PubMed] [Google Scholar]
- Freeman RD, Fast DK, Burd L, Kerbeshian J, Robertson MM, Sandor P. An international perspective on Tourette syndrome: selected findings from 3,500 individuals in 22 countries. Developmental medicine and child neurology. 2000;42:436–447. doi: 10.1017/s0012162200000839. October 1997. [DOI] [PubMed] [Google Scholar]
- van Grootheest D, Bartels M, Cath DC, Beekman AT, Hudziak JJ, Boomsma DI. Genetic and environmental influences on obsessive-compulsive symptoms in adults: a population-based twin-family study. Psychological medicine. 2007;37:1635–1644. doi: 10.1017/S0033291707000980. [DOI] [PubMed] [Google Scholar]
- van Grootheest DS, Cath DC, Beekman AT, Boomsma DI. Twin studies on obsessive-compulsive disorder: a review. Twin research and human genetics: the official journal of the International Society for Twin Studies. 2005;8(5):450–458. doi: 10.1375/183242705774310060. [DOI] [PubMed] [Google Scholar]
- de Haan MJ, Delucchi KL, Mathews CM, Cath DC. Tic symptom dimensions and their heritabilities in Tourette’s syndrome. Psychiatric genetics. 2015;25:112–8. doi: 10.1097/YPG.0000000000000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson SARAL, Svanstro A. Psychiatric telephone interview with parents for screening of childhood autism tics, attention-deficit hyperactivity disorder and other comorbidities (A-TAC) Preliminary reliability and validity. The British Journal of Psychiatry. 1994;187(3):262–267. doi: 10.1192/bjp.187.3.262. [DOI] [PubMed] [Google Scholar]
- Hasstedt SJ, Leppert M, Filloux F, van de Wetering BJ, McMahon WM. Intermediate inheritance of Tourette syndrome, assuming assortative mating. American journal of human genetics. 1995;57:682–9. [PMC free article] [PubMed] [Google Scholar]
- Hettema JM, Neale MC, Kendler KS. A review and meta-analysis of the genetic epidemiology of anxiety disorders. American Journal of Psychiatry. 2001;158:1568–1578. doi: 10.1176/appi.ajp.158.10.1568. [DOI] [PubMed] [Google Scholar]
- Hirschtritt ME, Lee PC, Pauls DL, Dion Y, Grados MA, Illmann C, King RA, Sandor P, McMahon WM, Lyon GJ, Cath DC, Kurlan R, Robertson MM, Osiecki L, Scharf JM, Mathews CA. Lifetime Prevalence, Age of Risk, and Genetic Relationships of Comorbid Psychiatric Disorders in Tourette Syndrome. JAMA Psychiatry. 2015 doi: 10.1001/jamapsychiatry.2014.2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirtz D, Thurman DJ, Gwinn-Hardy K, Mohamed M, Chaudhuri AR, Zalutsky R. How common are the “common” neurologic disorders? Neurology. 2007;68:326–337. doi: 10.1212/01.wnl.0000252807.38124.a3. [DOI] [PubMed] [Google Scholar]
- Khalifa N. Psychopathology in a Swedish Population of School Children With Tic Disorders. Journal of the American Academy of Child & Adolescent Psychiatry. 2006;45(11):1346–1353. doi: 10.1097/01.chi.0000251210.98749.83. [DOI] [PubMed] [Google Scholar]
- Knight T, Steeves T, Day L, Lowerison M, Jette N, Pringsheim T. Prevalence of tic disorders: A systematic review and meta-analysis. Pediatric Neurology. 2012;47:77–90. doi: 10.1016/j.pediatrneurol.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Kraft JT, Dalsgaard S, Obel C, Thomsen PH, Henriksen TB, Scahill L. Prevalence and clinical correlates of tic disorders in a community sample of school-age children. European Child & Adolescent Psychiatry. 2012;21(1):5–13. doi: 10.1007/s00787-011-0223-z. [DOI] [PubMed] [Google Scholar]
- Kurlan R, McDermott MP, Deeley C, Como PG, Brower C, Eapen S, Andresen EM, Miller B. Prevalence of tics in schoolchildren and association with placement in special education. Neurology. 2001;57(8):1383–1388. doi: 10.1212/wnl.57.8.1383. [DOI] [PubMed] [Google Scholar]
- Kurlan R, Eapen V, Stern J, McDermott MP, Robertson MM. Bilineal transmission in Tourette’s syndrome families. Neurology. 1994;44(12):2336–42. doi: 10.1212/wnl.44.12.2336. [DOI] [PubMed] [Google Scholar]
- Larson T, Anckarsäter H, Gillberg C, Ståhlberg O, Carlström E, Kadesjö B, Råstam M, Lichtenstein P, Gillberg C. The Autism – Tics, AD/HD and other Comorbidities inventory (A-TAC): further validation of a telephone interview for epidemiological research. 2010:1–11. doi: 10.1186/1471-244X-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein P, Carlström E, Råstam M, Gillberg C, Anckarsäter H. The genetics of autism spectrum disorders and related neuropsychiatric disorders in childhood. The American journal of psychiatry. 2010;167:1357–1363. doi: 10.1176/appi.ajp.2010.10020223. [DOI] [PubMed] [Google Scholar]
- Mataix-Cols D, Isomura K, Pérez-Vigil A, Chang Z, Rück C, Larsson KJ, Leckman JF, Serlachius E, Larsson H, Lichtenstein P. Familial Risks of Tourette Syndrome and Chronic Tic Disorders: A Population-Based Cohort Study. JAMA psychiatry. 2015 doi: 10.1001/jamapsychiatry.2015.0627. [DOI] [PubMed] [Google Scholar]
- Mathews CA, Scharf JM, Miller LL, Macdonald-Wallis C, Lawlor Da, Ben-Shlomo Y. Association between pre- and perinatal exposures and tourette syndrome or chronic tic disorder in the alspac cohort. British Journal of Psychiatry. 2014;204:40–45. doi: 10.1192/bjp.bp.112.125468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews CA, Grados MA. Familiality of tourette syndrome, obsessive-compulsive disorder, and attention-deficit/hyperactivity disorder: Heritability analysis in a large sib-pair sample. Journal of the American Academy of Child and Adolescent Psychiatry. 2011;50:46–54. doi: 10.1016/j.jaac.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LL, Scharf JM, Mathews CA, Ben-Shlomo Y. Tourette syndrome and chronic tic disorder are associated with lower socio-economic status: Findings from the Avon Longitudinal Study of Parents and Children cohort. Developmental Medicine and Child Neurology. 2014;56:157–163. doi: 10.1111/dmcn.12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooki S. Genetic and environmental influences on stuttering and tics in Japanese twin children. Twin research and human genetics: the official journal of the International Society for Twin Studies. 2005;8:69–75. doi: 10.1375/1832427053435409. [DOI] [PubMed] [Google Scholar]
- Pauls D, Hurst C. Schedule for Tourette and Other Behavioural Syndromes. New Haven: New Haven, CT: Child Study Center, Yale University School of Medicine; 1996. The Yale family/genetic study self-report questionnaire for tics, obsessivecompulsiveness, attentional difficulties, impulsivity and motor hyperactivity. [Google Scholar]
- Pauls DL, Raymond CL, Stevenson JM, Leckman JF. A family study of Gilles de la Tourette syndrome. American journal of human genetics. 1991;48:154–163. [PMC free article] [PubMed] [Google Scholar]
- Pauls DL. The genetics of obsessive-compulsive disorder: a review. Dialogues in clinical neuroscience. 2010;12:149–163. doi: 10.31887/DCNS.2010.12.2/dpauls. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto R, Monzani B, Leckman JF, Rück C, Serlachius E, Lichtenstein P, Mataix-Cols D. Understanding the Covariation of Tics, Attention-Deficit/Hyperactivity, and Obsessive-Compulsive Symptoms: A Population-Based Adult Twin Study. 2016 doi: 10.1002/ajmg.b.32436. [DOI] [PubMed] [Google Scholar]
- Posthuma D, Boomsma DI. A note on the statistical power in extended twin designs. Behavior Genetics. 2000;30:147–158. doi: 10.1023/a:1001959306025. [DOI] [PubMed] [Google Scholar]
- Price RA, Kidd KK, Cohen DJ, Pauls DL, Leckman JF. A twin study of Tourette syndrome. Archives of general psychiatry. 1985;42:815–20. doi: 10.1001/archpsyc.1985.01790310077011. [DOI] [PubMed] [Google Scholar]
- Rietveld MJ, van Der Valk JC, Bongers IL, Stroet TM, Slagboom PE, Boomsma DI. Zygosity diagnosis in young twins by parental report. Twin research: the official journal of the International Society for Twin Studies. 2000;3:134–41. doi: 10.1375/136905200320565409. [DOI] [PubMed] [Google Scholar]
- Robertson MM, Verril M, Mercer M, James B, Pauls DL. Tourette’s syndrome in New Zealand. A postal survey. The British journal of psychiatry: the journal of mental science. 1994;164:263–266. doi: 10.1192/bjp.164.2.263. [DOI] [PubMed] [Google Scholar]
- Robertson MM, Eapen V, Cavanna AE. The international prevalence, epidemiology, and clinical phenomenology of Tourette syndrome: A cross-cultural perspective. Journal of Psychosomatic Research. 2009;67:475–483. doi: 10.1016/j.jpsychores.2009.07.010. [DOI] [PubMed] [Google Scholar]
- Sabb FW, Burggren AC, Higier RG, Fox J, He J, Parker DS, Poldrack RA, Chu W, Cannon TD, Freimer NB, Bilder RM. Challenges in phenotype definition in the whole-genome era: multivariate models of memory and intelligence. Neuroscience. 2009;164:88–107. doi: 10.1016/j.neuroscience.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scahill L, Williams S, Schwab-Stone M, Applegate J, Leckman JF. Disruptive behavior problems in a community sample of children with tic disorders. Advances in Neurology. 2006;99:184–190. [PubMed] [Google Scholar]
- Scahill L, Sukhodolsky DG, Williams SK, Leckman JF. Public health significance of tic disorders in children and adolescents. Adv Neurol. 2005;96:240–248. [PubMed] [Google Scholar]
- Scahill L, Dalsgaard S, Bradbury K. The prevalence of tourette syndrome and its relationship to clinical features. In: Martino D, Leckman JF, editors. Tourette Syndrome. Oxford: Oxford University Press; 2013. pp. 121–133. [Google Scholar]
- Schlander M, Schwarz O, Rothenberger A, Roessner V. Tic disorders: administrative prevalence and co-occurrence with attention-deficit/hyperactivity disorder in a German community sample. European psychiatry: the journal of the Association of European Psychiatrists. 2011;26:370–4. doi: 10.1016/j.eurpsy.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Shimada-Sugimoto M, Otowa T, Hettema JM. Genetics of anxiety disorders: Genetic epidemiological and molecular studies in humans. Psychiatry and Clinical Neurosciences. 2015:388–401. doi: 10.1111/pcn.12291. [DOI] [PubMed] [Google Scholar]
- Smith S, Hay El H, Farhat N, Rekaya R. Genome wide association studies in presence of misclassified binary responses. BMC genetics. 2013;14:124. doi: 10.1186/1471-2156-14-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Leckman JF. Tourette Syndrome and Tic Disorders. Psychiatry (Edgmont) 2005;2(203):26–36. [PMC free article] [PubMed] [Google Scholar]
- Walkup JT, Ferrao Y, Leckman JF, Stein DJ, Singer H. Tic disorders: some key issues for DSM-V. Depression and anxiety. 2010;27:600–610. doi: 10.1002/da.20711. [DOI] [PubMed] [Google Scholar]
- Wenning GK, Kiechl S, Seppi K, Müller J, Högl B, Saletu M, Rungger G, Gasperi A, Willeit J, Poewe W. Prevalence of movement disorders in men and women aged 50–89 years (Bruneck Study cohort): A population-based study. Lancet Neurology. 2005;4:815–820. doi: 10.1016/S1474-4422(05)70226-X. [DOI] [PubMed] [Google Scholar]
- Willemsen G, Vink JM, Abdellaoui A, den Braber A, van Beek JHDA, Draisma HHM, van Dongen J, van ‘t Ent D, Geels LM, van Lien R, Ligthart L, Kattenberg M, Mbarek H, de Moor MHM, Neijts M, Pool R, Stroo N, Kluft C, Suchiman HED, Slagboom PE, de Geus EJC, Boomsma DI. The Adult Netherlands Twin Register: twenty-five years of survey and biological data collection. Twin research and human genetics: the official journal of the International Society for Twin Studies. 2013;16:271–81. doi: 10.1017/thg.2012.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray NR, Lee SH, Kendler KS. Impact of diagnostic misclassification on estimation of genetic correlations using genome-wide genotypes. European Journal of Human Genetics. 2012;20:668–674. doi: 10.1038/ejhg.2011.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray NR, Maier R. Genetic Basis of Complex Genetic Disease: The Contribution of Disease Heterogeneity to Missing Heritability. Current Epidemiology Reports. 2014;1(4):220–227. [Google Scholar]
- Yang J, Lee SH, Goddard ME, Visscher PM. GCTA: a tool for genome-wide complex trait analysis. American journal of human genetics. 2011;88(1):76–82. doi: 10.1016/j.ajhg.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilhao NR, Smit DJA, Den Braber A, Dolan CV, Willemsen G, Boomsma DI, Cath DC. Genetic and Environmental Contributions to Stability in Adult Obsessive Compulsive Behavior. Twin Research and Human Genetics. 2014;18(1):52–60. doi: 10.1017/thg.2014.77. [DOI] [PubMed] [Google Scholar]
- Zohar AH, Ratzoni G, Pauls DL, Apter A, Bleich A, Kron S, Rappaport M, Weizman A, Cohen DJ. An epidemiological study of obsessive-compulsive disorder and related disorders in Israeli adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 1992;31:1057–1061. doi: 10.1097/00004583-199211000-00010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


