Abstract
Mouse embryonic fibroblast (MEF) cells are an important in vitro model for developmental biology, disease, and reprogramming studies. However, as with other primary cells, they are challenging to transfect. Although viral gene-delivery methods achieve high gene-delivery efficiency, challenges with cell mutagenesis and safety among others have led to the use and improvement of non-viral gene-delivery methods in MEF cells. Despite the importance of gene delivery in MEF cells, there is limited comparison of method/reagent efficacy. In this study, we compared the effectiveness of different gene-delivery methods and several reagents currently available in MEF cells by introducing a plasmid containing enhanced green fluorescent protein (EGFP). We analyze transfection efficiency by EGFP fluorescence. Our results suggest that two gene-delivery methods—electroporation and magnetofection in combination with a lipid reagent, are the most efficient transfection methods in MEF cells. This study provides a foundation for the selection of transfection methods or reagents when using MEF cells.
Keywords: dendrimer, electroporation, lipid-based transfection, magnetofection, MEF
INTRODUCTION
MEF cells are a useful model to study the cellular, molecular, and/or biochemical mechanisms underlying the developmental phenotypes of genetically modified mice.1 MEF cells are also helpful when studying the molecular basis of diseases, such as cancer.2 In addition, MEF cells are used to study the factors that induce reprogramming toward pluripotency and transdifferentiation into different cell types.3–5 For all of these studies, it is necessary to have efficient gene-delivery methods.6 This is particularly important when the studies involve transient expression of genes.
Viral methods using integrating viruses achieve high gene-delivery efficiency; however, virus integration into the cell’s genome can cause insertional mutagenesis.6, 7 On the other hand, nonintegrating viruses, such as adenovirus, show low gene-delivery efficiencies and lead to inflammatory responses.6, 7 Non-viral gene-delivery methods are significantly less efficient than viral methods; however, they have a number of advantages, such as being nonmutagenic, noninfectious, less immunogenic, and easier to handle compared with viral methods.6, 7 In addition, they lead to transient gene expression, which is advantageous when prolonged expression of the gene is toxic. For these reasons, a number of recent studies focus on the use and improvement of non-viral gene-delivery methods, particularly in MEF cells.6
Despite the importance of transfection in MEF cells, there is limited comparison of transfection reagent efficacy. Only a few studies in MEF cells report transfection efficiencies, with a majority of them being studies that compare novel, non-viral transfection methods with 1 or 2 established methods. In this study, we compared the transfection efficiency of several non-viral transfection reagents, including lipid based [Lipofectamine LTX (Thermo Fisher Scientific, Waltham, MA, USA), TransFectin (Bio-Rad Laboratories, Hercules, CA, USA), GenJet In Vitro DNA Transfection Reagent (Ver II; SignaGen Laboratories, Rockville, MD, USA), DreamFect Gold (OZ Biosciences, San Diego, CA, USA)], dendrimer based [NanoJuice (EMD Millipore, Billerica, MA, USA)], polyamine based [GeneJuice (EMD Millipore)], and electroporation. We also tested the effect of magnetofection [CombiMag (OZ Biosciences)] on the transfection efficiency of the lipid reagents.
MATERIALS AND METHODS
MEF Cell Isolation
C57BL/6 wild-type embryos were isolated at embryonic d 13.5 (E13.5) and placed on ice-cold PBS, 1× Ca2+, Mg2+ (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4, 1 mM CaCl2, 0.5 mM MgCl2). Mice were used according to a Boston University School of Medicine Institutional Animal Care and Use Committee (IACUC)-approved protocol and in compliance with the Guide for the Care and Use of Laboratory Animals. Embryo bodies were minced after removing their liver, spleen, and intestine and then digested with 0.25% Trypsin-EDTA (#MT25053Cl; Corning, Manassas, VA, USA). Trypsin-EDTA incubation was stopped with complete media [high-glucose DMEM (#15017CM; Corning)]; supplemented with 10% heat-inactivated FBS (#FB5001; Denville Scientific, Holliston, MA, USA)], 50 U/ml penicillin/streptomycin (#30003Cl; Corning), and 4 mM L-glutamine (#25005Cl; Corning); washed with PBS; plated in complete media; and incubated at 37°C in 5% CO2. Medium was replaced every 2 d. MEFs were frozen at Passage 1 in complete media, supplemented with 40% FBS and 10% DMSO. After thawing, at Passage 3, MEF cells were dissociated from plasticware using 0.05% Trypsin-EDTA and seeded into 24-well plates for transfection. In this study, MEF cells were transfected at Passage 4.
Plasmid Preparation and Transfection
EGFP-encoding plasmid (pEGFP-N1; #6085-1; Clontech Laboratories, Mountain View, CA, USA) was prepared in INVαF′ Escherichia coli (#C2020-03; Thermo Fisher Scientific) using a maxiprep plasmid kit (#12143; Qiagen GmbH, Hilden, Germany). DNA concentration was adjusted to 1 mg/ml in 0.05% Tris-EDTA, and plasmids were stored at −20°C.
For transfection, MEF cells were seeded in 24-well plates at a density of 1 × 105 cells/well for Lipofectamine, TransFectin, DreamFect, NanoJuice, GeneJuice, and CombiMag. For GenJet, MEF cells were seeded in 24-well plates at a density of 1.5 × 105 cells/well. According to the manufacturer’s instructions, cells were grown overnight to reach a confluence of 80–95% before transfection. Variables indicated in the text (amounts of plasmid DNA and reagent and incubation time) were tested. Transfection complexes were formed at room temperature in serum-free medium before adding them dropwise, as is described below. For electroporation, 2× P100 at 80% confluence were trypsinized in 0.05% Tris-EDTA, washed, counted, and resuspended at 5 × 105 cells/0.5 ml. Cell suspension plus DNA (0.5 ml; 5 μg) was electroporated in a Gene Pulser cuvette (0.4 cm; Bio-Rad Laboratories) at 0.350 μF and at the voltage described in the text and figure legends. Electroporated cells were transferred to 1 well of a 6-well plate. Commercially available transfection reagents were tested using the DNA: reagent ratios described in the text and figure legends according to the manufacturers’ instructions. All protocols are per well of a 24-well plate.
Lipofectamine LTX reagent with Plus reagent (Cat. #15338100)
DNA was diluted in 25 μl DMEM without serum and 8 μl Plus reagent and gently mixed. Two microliters of Lipofectamine was diluted in 25 μl DMEM. Mixtures were incubated for 5 min, combined, and incubated for 15 min at room temperature. Complexes were added to cells in media minus FBS and antibiotics.
Lipofectamine is made of cationic lipids with a positively charged head group and 1 or 2 hydrocarbon chains. The positive charge of the surface mediates the interaction between the nucleic acid and the cell membrane. This allows for a fusion of the liposome-nucleic acid transfection complex with a negatively charged cell membrane. This transfection complex enters the cell through endocytosis.
TransFectin lipid reagent (Cat. #1703351)
DNA was diluted in 50 μl DMEM. TransFectin reagent (2 μl) was also diluted in 50 μl DMEM for each sample. DNA and TransFectin solutions were mixed by pipetting and incubated for 20 min at room temperature. The 100 μl DNA-TransFectin complexes were added directly to the cells in complete media.
TransFectin has a similar mechanism to Lipofectamine.
GenJet In Vitro DNA Transfection Reagent (Cat. #SL100489)
DNA was diluted into 50 μl DMEM and vortexed gently. GenJet reagent (4 μl) was added to another 50 μl DMEM and mixed by pipetting. The diluted GenJet reagent was then immediately added to the diluted DNA and mixed by pipetting. After incubating the DNA-GenJet mixture for 15 min, this mixture was then added dropwise to the cells in complete media. The DNA/GenJet complex was then aspirated out at 5, 12, or 18 h post-transfection and replaced with complete culture medium.
GenJet is a liposome-based reagent containing hydrophobic and hydrophilic regions similar to the phospholipids in the membrane bilayer, making it easier for the reagent to interact with the cell membrane. GenJet captures nucleic acids, and the complex enters the cell through endocytosis.
DreamFect Gold (Cat. #DG81000)
DNA was diluted in 50 μl DMEM and mixed by pipetting. DreamFect Gold reagent (4 μl) was also diluted in 50 μl DMEM-free medium for each sample and mixed by pipetting. The diluted DNA solution was added to the diluted DreamFect Gold solution within 5 min, mixed by pipetting, and incubated for 20 min at room temperature. The DNA-reagent complexes were added dropwise directly into the wells in complete media.
DreamFect Gold is a lipid-based transfection reagent. It associates nucleic acids with cationic lipids, resulting in molecular complexes known as lipoplexes. The cells take up this complex through endocytosis.
Lipofectamine with CombiMag (Cat. #CM21000)
DNA (1 μg) was diluted in 25 μl DMEM and was then added to 8 μl Lipofectamine, diluted in another 100 μl DMEM by pipetting. Four tubes were prepared containing none and 0.5, 1, and 2 μl CombiMag, respectively, and the DNA-Lipofectamine solution was added to them, mixed by pipetting, and incubated for 15 min. The DNA-Lipofectamine-CombiMag solution was then added dropwise into the wells grown in media minus FBS and antibiotics, and plates were placed for 12 min on a magnetic plate.
CombiMag is a reagent based on magnetic nanoparticles coated with cationic polymers. The complexes of CombiMag plus lipid-reagents/DNA are then transported into the cells supported by a magnetic field.
TransFectin, GenJet, and DreamFect Gold with CombiMag
DNA was diluted in 50 μl DMEM and then mixed with 2 μl Lipofectamine diluted in another 50 μl DMEM by pipetting, as describe above for each reagent. Four tubes were prepared containing none and 0.5, 1, and 2 μg CombiMag, respectively, and to each tube, the DNA-reagent solution was added and mixed by pipetting. After 15 min of incubation, the DNA-TransFectin-CombiMag solution was added dropwise to the wells grown in complete media, and plates were placed for 12 min on a magnetic plate.
NanoJuice (Cat. #71902-3)
Various combinations of NanoJuice Core Transfection (0.8 and 1.6 μl) and NanoJuice Transfection Booster (0.8, 1.6, 2.4, and 3.2 μl) were added in this order to 20 μl DMEM, gently mixed by pipetting, and subsequently incubated for 5 min at room temperature. Then, 0.25 μg DNA was added to each tube by gentle pipetting. This transfection mixture was incubated at room temperature for 15 min and added dropwise to the cells grown in media without antibiotics.
NanoJuice combines Priostar dendrimers with a polycationic liposomal formulation to bind DNA.
GeneJuice transfection reagent (Cat. #70967-3)
Varying amounts of GeneJuice reagent (0.5, 0.75, 1, 1.25, and 1.5 μl) were added to 20 μl DMEM and mixed by vortexing. Then, 0.25 μg plasmid DNA was added into the GeneJuice mixture and incubated at room temperature for 15 min. The mixture was added dropwise to the cells grown in complete medium.
GeneJuice is composed of a nontoxic cellular protein and a small amount of a novel polyamine. The active ingredient is the polyamine that contains a partially positive charge so it interacts with the DNA plasmid. Its hydrophobic region brings the DNA inside the cell.
Gene Pulser Electroporation Buffer (Cat. #1652676)
MEF cells were resuspended in electroporation buffer at 5 × 105 cells/0.5 ml. Cell suspension containing DNA (0.5 ml; 5 μg) was electroporated in a 4 mm Gene Pulser cuvette (#1652088). Electroporation was performed at 4 different voltages (250, 300, 325, and 350 V) at a capacitance of 350 μF using the Gene Pulser II Electroporation System (Bio-Rad Laboratories). The pulse duration was 20 ms. Electroporated cells were transferred to 1 well of a 6-well plate in complete medium.
With an electric field, electroporation creates pores on the lipid bilayers of the cell, allowing the cells to be more permeable. The vectors are able to move into the bilayers, which lead to a delivery of nucleic acids into the nucleus of the targeted cells.
Quantification of Transfection Efficiency
Bright-field and fluorescent photographs of fields of MEF cells were taken at the times described in the figure legends in each culture dish with a Nikon Deconvolution Wide-Field Epifluorescence microscope. Photographs were taken at 10× magnification. Four to 6 bright-field/fluorescent photographs were analyzed, where controls contained at least 100 cells; if the transfection combination decreased the number of cells surviving, then it is indicated in the text. Cells were counted by 2 independent observers. The transfection efficiency was calculated by dividing the number of EGFP-positive cells by the total number of surviving cells in the photographs. The total number of surviving cells in the photographs was used to report cell numbers. Student’s t test was used to determine significance (P < 0.05 was considered significant). Brightness and contrast were adjusted in the MEF cell photographs.
RESULTS
MEF cells obtained from E13.5 mouse embryos were transfected with a plasmid containing the reporter gene, EGFP. Transfection efficiencies varied by over 24-fold (range: 2–48%) among the transfection reagents tested.
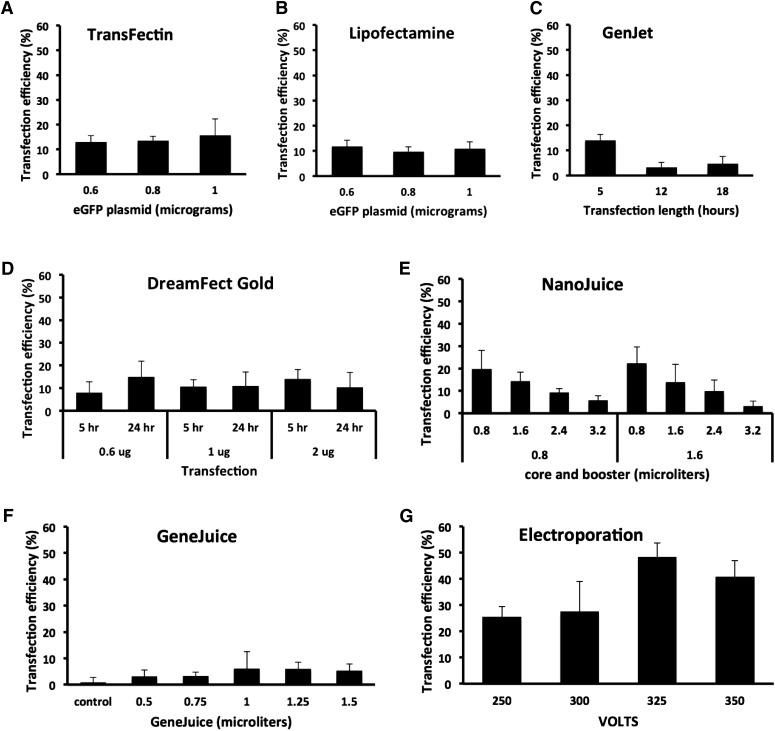
For cationic lipid reagents TransFectin and Lipofectamine LTX, different amounts of DNA plasmid were added to 2 μl reagent (Figs. 1 and 2). Maximum transfection efficiency was 15.7% (±6.5%; 1 μg) for TransFectin and 11.8% (±2.4%; 0.6 μg) for Lipofectamine (n = 6; Fig. 1A and B). Figure 3A and B shows images of the transfected cells. Figure 3H shows an example of a control transfection with plasmid vector alone. For both reagents, there was no statistical difference in the transfection efficiency for the different plasmid amounts (Fig. 1A and B). Increasing plasmid concentration did not result in a decrease in cell numbers after transfection (P > 0.05). EGFP fluorescence could be detected as early as 5 h after transfection, and it was still detected at 44 h (not shown).
FIGURE 1.
Transfection efficiencies by different reagents in MEF cells. Transfection efficiency was calculated as described in Materials and Methods and represented as histograms. (A and B) For TransFectin and Lipofectamine, different amounts of DNA plasmid (0.6, 0.8, and 1 μg) were combined with 2 μl reagent. Bars represent the means ± sd of 6 cell field photographs at 44 h. Student's t test showed not significant differences. For TransFectin, 0.6 vs. 0.8 μg (P = 0.6), 0.6 vs. 1 μg (P = 0.4), and 0.8 vs. 1 μg (P = 0.5). For Lipofectamine, 0.6 vs. 0.8 μg (P = 0.1), 0.6 vs. 1 μg (P = 0.5), and 0.8 vs. 1 μg (P = 0.5). Average number of cells counted per histogram bar is 674 for TransFectin and 461 for Lipofectamine. (C) For GenJet, different times of incubation of the transfection reagent were tested (5, 12, and 18 h). Bars represent the means ± sd of 6 cell field photographs at 44 h. Student’s t test: 5 vs. 12 h (P = 0.0007), 5 vs. 18 h (P = 0.001), and 12 vs. 18 h (P = 0.4). Average number of cells counted per histogram bar is 671. (D) For DreamFect Gold, different amounts of DNA plasmid (0.6, 0.8, and 1 μg) and different times of transfection incubation time (5 and 24 h) were tested. Bars represent the means ± sd of 6 cell field photographs at 44 h. Student’s t test: at 5 h, 0.6 vs. 2 μg (P = 0.04). No other statistically significant differences were found. Average number of cells counted per histogram bar is 432. (E) For NanoJuice, we combined various amounts of NanoJuice Core Transfection (0.8 and 1.6 μl) and NanoJuice Transfection Booster (0.8, 1.6, 2.4, and 3.2 μl). Bars represent the means ± sd of 4 cell field photographs at 21 h. Student’s t test: 0.8/0.8 vs. 0.8/3.2 (P = 0.02), 0.8/1.6 vs. 0.8/3.2 (P = 0.01), 1.6/0.8 vs. 0.8/2.4 (P = 0.03), and 1.6/0.8 vs. 0.8/3.2 (P = 0.003). Average number of cells counted per histogram bar is 180. (F) For GeneJuice, we tested DNA plasmid amount (0.5, 0.75, 1, 1.25, and 1.5 μg) in combination with 0.75 μl transfection reagent. Bars represent the means ± sd of 4 cell field photographs at 21 h. Student’s t test: control vs. 1.25 (P = 0.02) and control vs. 1.5 (P = 0.04). Average number of cells counted per histogram bar is 161. (G) For electroporation, 4 different pulse magnitudes (250, 300, 325, and 350 V) were tested. Bars represent the means ± sd of 4 cell field photographs at 21 h. Student’s t test: 250 vs. 325 V (P = 0.0006), 250 vs. 350 V (P = 0.006), and 300 vs. 325 V (P = 0.02). Average number of cells counted per histogram bar is 72.
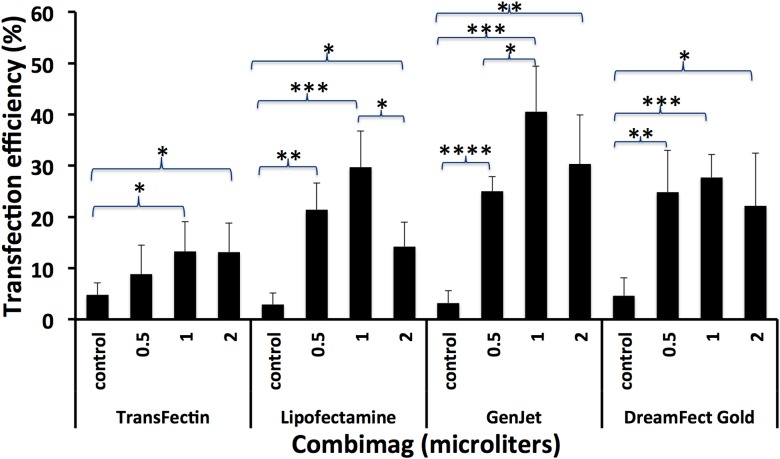
FIGURE 2.
CombiMag increases the transfection efficiency of lipid reagents in MEF cells. Different amounts of CombiMag [0 (control), 0.5, 1, and 2 μl] were added to the different transfection reagents. Histograms represent the effect of CombiMag on the transfection efficiencies of lipid reagents in MEF cells at 22 h. Bars represent the means ± sd of 4 cell fields. Student’s t test: *P < 0.05, **P < 0.005, ***P < 0.0005, ****P < 0.00005. Average number of cells counted was 200.
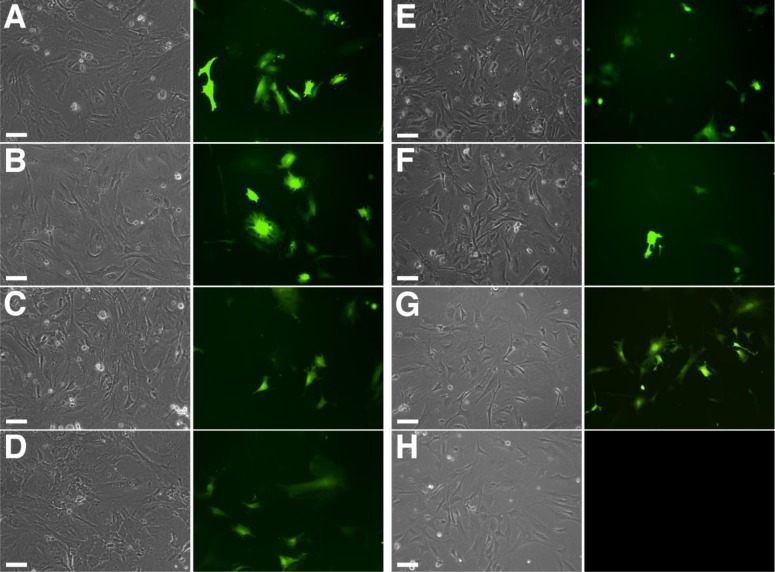
FIGURE 3.
EGFP fluorescence in transfected MEF cells, which were examined 22 h after transfection of an EGFP-expressing plasmid construct by fluorescence microscopy. Panels show bright-field (grayscale) and corresponding UV images (color) for each reagent. (A) TransFectin, (B) Lipofectamine, (C) GenJet, (D) DreamFect Gold, (E) NanoJuice, (F) GeneJuice, (G) Electroporation, and (H) control transfection with plasmid vector alone. Photographs were taken at 10× original magnification. Original scale bars, 100 μm.
For GenJet (1:4 DNA to reagent), the time of incubation with the transfection reagent was tested (5, 12, or 18 h). The transfection efficiency for 5 h of incubation was the highest (14 ± 4.5%), and it was significantly higher than the other time points (n = 6; Fig. 1C). Figure 3C shows images of the transfected cells. The increasing length of GenJet incubation did not result in a decrease in cell numbers after transfection.
For DreamFect Gold, the amount of plasmid (0.6, 1, or 2 μg/4 μl reagent) and time (5 or 24 h) of incubation of the reagent were tested (n = 6; Fig. 1D). For 0.6 μg plasmid, the transfection efficiency increased significantly in proportion to the time of reagent incubation (7.9 ± 4.9% at 6 h to 14.5 ± 6.9% at 24 h). Transfection efficiency did not increase significantly in proportion to the plasmid amount. Figure 3D shows images of the transfected cells. Incubation of DreamFect for 24 h resulted in an ∼50% lower number of cells surviving than the corresponding 5 h of incubation at all plasmid amounts tested (t test; n = 6; 0.6 μg, P = 0.02; 1 μg, P = 0.003; 1 μg, P = 0.04).
We also tested whether magnetofection (CombiMag) increased the transfection efficiency of lipid reagents in MEF cells. Different amounts of CombiMag (0, 0.5, 1, and 2 μl) and 1 μg DNA were used with each lipid transfection reagent. The transfection efficiencies of control samples containing no magnetic nanoparticles were between 3 and 5% (n = 4; Fig. 2). There was a statistically significant increase in transfection efficiencies in samples with CombiMag when compared with control samples containing no magnetic nanoparticles (Fig. 2). This was true for all transfection reagents tested, although at different levels: 13.3% (±5.8%) for TransFectin (3 times higher), 29.7% (±7%) for Lipofectamine (10 times higher), 40.5% (±8.9%) for GenJet (13 times higher), and 27.7% (±4.5%) for DreamFect Gold (6 times higher). For TransFectin (n = 4), incubation with 1 or 2 μg CombiMag resulted in an ∼50% (P = 0.01) and 80% (P = 0.00005) lower number of cells, respectively. For GenJet (n = 4), incubation with 0.5, 1, and 2 μg CombiMag resulted in an ∼40% (P = 0.00006), 65% (P = 0.002), and 75% (P = 0.0005) lower number of cells, respectively. For DreamFect (n = 4), incubation with 2 μg CombiMag resulted in an ∼40% (P = 0.048) lower number of cells. For Lipofectamine, CombiMag amounts did not significantly affect cell numbers after transfection.
NanoJuice, a dendrimer and polycation liposome-based reagent, was tested with 0.25 μg plasmid DNA. We combined various amounts of NanoJuice Core Transfection (0.8 and 1.6 μl) and NanoJuice Transfection Booster (0.8, 1.6, 2.4, and 3.2 μl), per the suggestion of the manufacturer. Maximum transfection efficiency was found with both 0.8 and 1.6 μl core, plus 0.8 μl booster (22.2 ± 7.4%; n = 4; Fig. 1E). Addition of increasing amounts of booster reduced the transfection efficiency. Increasing amounts of plasmid, core, or booster did not result in a decrease in cell numbers. Figure 3E shows images of the transfected cells.
GeneJuice, a polyamine and cell protein-based reagent, was tested with 0.25 μg plasmid DNA. We tested various amounts of GeneJuice (0.5, 0.75, 1, 1.25, and 1.5 μl) in combination with 0.75 μl transfection reagent. The highest transfection efficiency (6.1 ± 6.5%) was found at 1 μg DNA (n = 4; Fig. 1F). Increasing amounts of plasmid did not result in a decrease in cell numbers. Figure 3F shows images of the transfected cells.
Electroporation was performed at 4 different pulse magnitudes (250, 300, 325, and 350 V). There was a statistically significant increase in transfection efficiency correlating with increased pulse magnitude from 25.3% (±4.1%) at 250 V to 48.1% (±5.6%) at 325 V (n = 4; Fig. 1G). Figure 3G shows images of the transfected cells. The number of cells at pulse magnitudes of 300 V and higher was ∼50% lower than at 250 V. Therefore, electroporation was toxic for MEF cells at high voltages (t test; n = 4; 250 vs. 300, P = 0.02; 250–325, P = 0.005; 250 vs. 350, P = 0.004).
DISCUSSION
Our study extensively compared the efficacy of different non-viral gene-delivery methods in MEF cells and can be used as a foundation for the selection of reagents when using MEF cells. Electroporation or magnetofection plus a lipid reagent achieved the best transfection efficiency in MEF cells among the non-viral transfection methods that we tested here. Differences in transfection efficiency depended mostly on the transfection method/reagent used, although other variables, such as the amount of plasmid used and the length of transfection, also played a role.
The lipid transfection reagents resulted in transfection efficiencies of 12–22% in MEF cells. Our results are slightly higher than reported in the literature for other lipid transfection reagents in MEF cells isolated in the laboratory. For example, Lipofectamine and FuGENE are reported to result in 3–8% transfection efficiency.8, 9 However, it is possible to achieve higher transfection efficiencies in laboratory-isolated MEF cells by consecutive transfections (e.g., 50% for FuGENE after 4 consecutive transfections).10 The literature also shows that consecutive transfections are particularly useful when generating induced pluripotent stem (iPS) cells by reprogramming MEF cells, as this strategy helps maintain the percentage of cells expressing the gene of interest.10 Furthermore, even immortalized MEF cells (NIH3T3) show relatively low transfection efficiencies. Published data show transfection efficiencies in NIH3T3, ∼22% using Lipofectamine11 and ∼27% using Attractene.12 Transfection efficiencies were not found in the literature for TransFectin and GenJet.
The differences in transfection efficiencies between our data and reported data using lipid transfection reagents could be a result of a number of factors, such as time of incubation of the reagent, amount of plasmid DNA, time of analysis, and MEF cell passage number. It was challenging to compare our data with published data, as these variables differ or are not described, but some basic guidelines can be inferred. First, as described above, some of these reagents can be toxic at long incubation times or high amounts of plasmid DNA. Second, the time to analyze may account for some of the differences between published data. For example, it is reported that without retransfection, the percentage of cells expressing the gene of interest (i.e., GFP) falls by 15–28% in 2 d.13 Lastly, transfection efficiency in MEF cells depends on the passage number. Maximum transfection efficiency is found in early passages (<5) in both lipid transfection and electroporation.10, 14 Indeed, for iPS generated from MEF cells, it is recommended to use MEF cells on or before Passage 3.10 This was also our experience, as by Passage 6, the transfection efficiency was almost 0%. Therefore, even though MEF cells are typically difficult to transfect with lipid methods, relatively high transfection efficiencies can be achieved when variables are adjusted carefully.
In contrast with laboratory-obtained MEF cells, some commercially available primary MEF (PMEF) cells show high transfection efficiencies, for example, PMEF-HL cells transfected with Lipofectamine (∼50% transfection efficiency)13 and PMEF-CFL cells with DreamFect Gold (∼60% transfection efficiency).15 Other commercially available PMEF cell lines (PMEF-CF, PMEF-CFL, and PMEF-NL) show low transfection efficiencies (<10%) with Lipofectamine 2000 and X-tremeGENE 9.13, 15, 16 PMEF cells are sold in their third passage; it is possible that their high transfection efficiencies, compared with laboratory-obtained MEF cells, are a result of the fact that they are used at earlier passages (e.g., Passage 4,15). As in laboratory-made MEFs, repeated transfections help maintain the percentage of transfected cells.13
In our analysis, magnetofection (CombiMag), together with lipid reagents, resulted in a 3- to 13-fold increase in transfection efficiency compared with the lipid reagent alone. Published data show transfection efficiencies in MEF cells up to 60% and 50% when DreamFect Gold and X-tremeGENE HP are combined with PEI-Mag2 magnetic nanoparticles, respectively.15 This represents a 2- to 4-fold increase in transfection efficiency with respect to the lipid reagent alone.15 For NIH3T3, nanomagnetic transfection (magnefect-nano) resulted in 22% transfected cells.11
GeneJuice showed low transfection efficiencies (6%). However, other groups have found high transfection efficiency (50%) but toxicity in MEF cells with GeneJuice.10 Other groups also used amide-based reagents. Arginine-terminated polyamidoamine nanoparticles (G4Arg) show 2–3 times higher transfection efficiency (14.25%) than FuGENE HD and Lipofectamine 2000, respectively, in laboratory-made MEF cells.9 Bioreducible linear poly(amido amine) [poly(N,N′-cystaminebisacrylamide-4-amino-1-butanol)] can achieve almost 80% transfection efficiency in PMEF-HL PMEF cells with low toxicity.13
Electroporation had the highest transfection efficiency of the methods tested here (48%). Published results show that electroporation with Gene Pulser MXcell and Amaxa electroporation systems resulted in an 80% and 16% transfection efficiency in MEF cells, respectively.12, 14 The differences between the reported transfection efficiencies could be a result of the system used. However, it could also be a result of the MEF cell passage number, as demonstrated in McCoy et al.14 Here and in other reports, electroporation resulted in decreased cell survival at high voltage.14, 17 Novel electroporation methods, such as capillary electrode and microporation, show better cell survival at high voltages in other primary cells and perhaps could also be used in MEF cells.17, 18 Our results are in accordance with the literature, showing that electroporation may be the most effective transfection method in MEF cells. However, when an electroporation system is not available, magnetofection can achieve relatively high transfection efficiency.
As a result of the high interest in achieving high transfection efficiencies in MEF cells, new delivery reagents are being generated. For example, peptide-based transfection reagents show 17% transfection efficiency in CF-1 PMEF cells. Even though the transfection efficiency is relatively low, this reagent appears to be better than lipid methods for the transfection of viral layered vectors, as they do not affect normal replication-infection cycles of viruses.16
One strength of this report is the use of the same cell passage to test all of the transfection reagents and methods so that they can be compared appropriately. A limitation to our study is that we used MEF cells derived from 1 embryo. We did so, as we believed that this allows for an accurate comparison of the transfection efficiencies. This is similar to other cell culture studies, where researchers use a particular cell line from a single source to avoid adding additional variables. However, future studies could be directed to test different embryos in a litter and embryos from different mating pairs.
In summary, electroporation or magnetofection plus a lipid reagent achieved the best transfection efficiency in MEF cells. Nonetheless, as we discussed above, in some instances, a particular transfection reagent/method is required, even though the transfection efficiency may be lower. In addition, even though reagent cost and time of transfection were not a consideration when selecting the transfection reagents to test in our study, these could be considerations for other laboratories. Although we achieved high transfection efficiencies with magnetofection plus lipid-based methods, it is conceivable that higher transfection efficiencies could be achieved using these reagents in consecutive transfections. Future studies could also be directed to test different transfection reagents in MEF cells obtained from embryos at different gestational ages, as the transfection efficiency could change with age, and the more efficient transfection reagent could also change.
ACKNOWLEDGMENTS
The authors thank Ekaterina Kintsurashvili, Grace Ferri, Alberto Borba, and other members of the Dominguez laboratory for discussions and comments to the manuscript. MEF cells were photographed in the Department of Medicine Imaging Core. The authors thank the technical director of the Imaging Core, Dr. Michael Kirber, for his technical support and enthusiasm. This work was supported with funding from the National Institute of General Medical Sciences (NIGMS; 1R01GM098367) and the U.S. National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) Grant UL1-TR000157. The authors declare not to have any competing interests.
REFERENCES
- 1.Lengner CJ, Lepper C, van Wijnen AJ, Stein JL, Stein GS, Lian JB. Primary mouse embryonic fibroblasts: a model of mesenchymal cartilage formation. J Cell Physiol 2004;200:327–333. [DOI] [PubMed] [Google Scholar]
- 2.Shimabuku T, Tamanaha A, Kitamura B, Tanabe Y, Tawata N, Ikehara F, Arakaki K, Kinjo T. Dual expression of Epstein-Barr virus, latent membrane protein-1 and human papillomavirus-16 E6 transform primary mouse embryonic fibroblasts through NF-κB signaling. Int J Clin Exp Pathol 2014;7:1920–1934. [PMC free article] [PubMed] [Google Scholar]
- 3.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126:663–676. [DOI] [PubMed] [Google Scholar]
- 4.Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Südhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature 2010;463:1035–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Efe JA, Hilcove S, Kim J, Zhou H, Ouyang K, Wang G, Chen J, Ding S. Conversion of mouse fibroblasts into cardiomyocytes using a direct reprogramming strategy. Nat Cell Biol 2011;13:215–222. [DOI] [PubMed] [Google Scholar]
- 6.Kim TK, Eberwine JH. Mammalian cell transfection: the present and the future. Anal Bioanal Chem 2010;397:3173–3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glover DJ, Lipps HJ, Jans DA. Towards safe, non-viral therapeutic gene expression in humans. Nat Rev Genet 2005;6:299–310. [DOI] [PubMed] [Google Scholar]
- 8.Tsuchiya M, Ogawa H, Koujin T, Kobayashi S, Mori C, Hiraoka Y, Haraguchi T. Depletion of autophagy receptor p62/SQSTM1 enhances the efficiency of gene delivery in mammalian cells. FEBS Lett 2016;590:2671–2680. [DOI] [PubMed] [Google Scholar]
- 9.Zhu K, Li J, Lai H, Yang C, Guo C, Wang C. Reprogramming fibroblasts to pluripotency using arginine-terminated polyamidoamine nanoparticles based non-viral gene delivery system. Int J Nanomedicine 2014;9:5837–5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okita K, Hong H, Takahashi K, Yamanaka S. Generation of mouse-induced pluripotent stem cells with plasmid vectors. Nat Protoc 2010;5:418–428. [DOI] [PubMed] [Google Scholar]
- 11.Fouriki A, Dobson J. Nanomagnetic gene transfection for non- viral gene delivery in NIH 3T3 mouse embryonic fibroblasts. Materials 2013;6:255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawasaki H, Kosugi I, Arai Y, Iwashita T, Tsutsui Y. Mouse embryonic stem cells inhibit murine cytomegalovirus infection through a multi-step process. PLoS One 2011;6:e17492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adler AF, Grigsby CL, Kulangara K, Wang H, Yasuda R, Leong KW. Nonviral direct conversion of primary mouse embryonic fibroblasts to neuronal cells. Mol Ther Nucleic Acids 2012;1:e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCoy AM, Collins ML, Ugozzoli LA. Using the gene pulser MXcell electroporation system to transfect primary cells with high efficiency. J Vis Exp 2010;e1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grześkowiak BF, Sánchez-Antequera Y, Hammerschmid E, Döblinger M, Eberbeck D, Woźniak A, Słomski R, Plank C, Mykhaylyk O. Nanomagnetic activation as a way to control the efficacy of nucleic acid delivery. Pharm Res 2015;32:103–121. [DOI] [PubMed] [Google Scholar]
- 16.Pärn K, Viru L, Lehto T, Oskolkov N, Langel Ü, Merits A. Transfection of infectious RNA and DNA/RNA layered vectors of semliki forest virus by the cell-penetrating peptide based reagent PepFect6. PLoS One 2013;8:e69659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim JA, Cho K, Shin MS, Lee WG, Jung N, Chung C, Chang JK. A novel electroporation method using a capillary and wire-type electrode. Biosens Bioelectron 2008;23:1353–1360. [DOI] [PubMed] [Google Scholar]
- 18.Lim JY, Park SH, Jeong CH, Oh JH, Kim SM, Ryu CH, Park SA, Ahn JG, Oh W, Jeun SS, Chang JW. Microporation is a valuable transfection method for efficient gene delivery into human umbilical cord blood-derived mesenchymal stem cells. BMC Biotechnol 2010;10:38. [DOI] [PMC free article] [PubMed] [Google Scholar]