Abstract
Cartilage is an avascular, aneural, and alymphatic connective tissue with a limited capacity caused by low mitotic activity of its resident cells, chondrocytes. Natural repair of full thickness cartilage defects usually leads to the formation of fibrocartilage with lower function and mechanical force compared with the original hyaline cartilage and further deterioration can occur. Tissue engineering and regenerative medicine is a promising strategy to repair bone and articular cartilage defects and rehabilitate joint functions by focusing on the optimal combination of cells, material scaffolds, and signaling molecules. The unique physical and topographical properties of nanofibrous structures allow them to mimic the extracellular matrix of native cartilage, making an appropriate resemblance to induce cartilage tissue regeneration and reconstruction. To improve simulation of native cartilage, the incorporation of nanofibrous scaffolds with suitable corresponsive cells could be effective. In this review article, an attempt was made to present the current state of cartilage tissue engineering using nanofibrous scaffolds and stem cells as high proliferative immune privilege cells with chondrogenic differentiation ability. The comprehensive information was retrieved by search of relevant subject headings in Medline/Pubmed and Elsevier databases.
Keywords: Cartilage, Nanofibers, Scaffolds, Stem cells, Tissue engineering
Introduction
Because of limited capacity for spontaneous repair, cartilage tissue cannot be restored to its normal function and structure after damages caused by trauma, osteoarthritis disease, accidents and so forth. Surgical strategies to repair cartilage chondral or osteochondral defects have been used to restore joint function and eliminate associated pain, including stimulation of the marrow by microfracture, mosaicplasty and cell-based therapies. Although surgical strategies reduce patient pain and increase joint mobility, the regenerated tissue is morphologically, biochemically and biomechanically inferior to the native cartilage. Additional surgery is often required to regain complete function, resulting in the progression to partial or total knee replacement. Therefore, there is a tremendous need for new regenerative medicine approaches to augment the repair process and to facilitate adequate tissue regeneration and longevity.
The novel strategy for regeneration of cartilage defects involves cells seeded biomaterials with appropriate growth factors 1. Biomaterial as a proper microenvironment for the cells provides mechanical support for engineered tissues. Recently, commercially available synthetic and natural matrix has been tested in animal models or clinical trials for repair of cartilage and the overall short-term clinical outcome is favorable 2. Therefore, tissue engineering is a promising option for the treatment of cartilage defects. Using different materials and production methods, many forms of biomaterial scaffolds with different properties have been developed for cartilage tissue engineering.
In the past decade, nanofibrous structures have attracted much interest as tissue engineered scaffolds because of their unique physical and topographical properties. The nanosized structure of a scaffold plays an important role to mimic the Extracellular Matrix (ECM) Structure 3.
Nanofiber scaffolds composed of ultra-fine biodegradable polymeric fibers morphologically similar to natural ECM have been widely emerged as potential scaffolds for cartilage tissue engineering.
It is worth mentioning that while nanofibrous structures could mimic similar fiber diameters, composition, and alignment of the ECM of articular cartilage, the synchronization of these scaffolds with suitable corresponsive cells could help us to achieve the best tissue engineering results for articular cartilage 4. Due to some characteristics of stem cells such as self-renewal, high proliferation and trans-differentiation capacity that reduce the challenges propounded about chondrocytes 5, these non-specialized cells are the focus of interest in tissue engineering and regenerative medicine field. This article reviewed and presented actual status of in vitro and in vivo studies on the application of nanofibrous structures and stem cells for cartilage tissue reconstruction. For extraction of related publications, keywords of cartilage tissue engineering, nanofibers and stem cells as MeSH terms in PubMed were used. All data belong to the publications and efforts in the field of cartilage tissue engineering and nanofibers that was achieved to date.
Different methods for fabrication of nanofiber scaffolds
Different synthetic nanomaterials have been fabricated to create the microenvironment that seeded cells can be encouraged to expand and differentiate into desired lineages, including chondrocytes 6,7. The biomemetic properties and good physiochemical features of nano-materials play a key role in stimulation of chondrocyte growth and cartilage tissue regeneration 8,9. Their physical characteristics promote advantageous biological responses of seeded cells in vitro, including increased cell proliferation and attachment while maintaining chondrocytic phenotype 9,10. In addition, application of nanofibrous scaffolds enables incorporation of nanospheres containing different growth factors. Exogenous transforming growth factor (TGF-β) family has been proved to stimulate cell proliferation and chondrogenesis both in vivo and in vitro. The factor TGF-β1 is naturally found in human platelets, bone, and other tissues and has been shown as an inducer of chondrogenesis 11. The controlled release of Bone Morphogenetic Protein (BMP-7) from nanospheres-containing scaffolds has induced significant ectopic bone formation in vivo 12. Based on these findings, nanofibrous scaffold and nanospheres, combined with chondrogenic and osteogenic factors, have been introduced as potential candidates to reconstruct the osteochondral defect for the regeneration of bone, cartilage, and their interface simultaneously 13. To provide ECM-like nanofibrous scaffolds, a variety of techniques have been developed, including electrospinning, self-assembly, phase separation and drawing 14.
Electrospinning
The most conventional method for processing of polymeric biomaterials into nanofibrous scaffolds is electrospinning with promising results for tissue engineering applications. This process is a simple economical technique to produce nanofibers from a wide range of synthetic and natural polymers in randomly-oriented or aligned manner 10. Electrospun nanofibers have a high specific surface area and can be functionalized with bioactive macromolecules 15,16. Electrospinning outcome is influenced by several parameters, including molecular weight of polymer, polymer solution properties, electric potential, distance between capillary and metal collector, etc.
In spite of the benefits electrospinning has to offer, it suffers from limitations including jet instability, toxic solvent, packaging, handling 17, and the production of two-dimensional (2D) matrices with small pores, which inhibits cell penetration and vascular ingrowth 18. In order to elicit the maximum benefit from this method, there are some advancements or modifications to the processing conditions 19. Coaxial electrospinning technique 20 enables the controlled release of active bio-molecules by producing core-shell nanofibers trapping drugs or bioactive molecules. Several attempts have been made to fabricate three-dimensional macroporous nanofibrous electrospun scaffolds by modifying the electrospinning conditions or using post-treatments. Process modifications include low-temperature electrospinning 21, needleless electrospinning using disc as spinneret 22, application of different collector plates, such as parallel plate 23 and screws 24, and introducing micrometer-sized fibers 25–27 or inert particle spacers, such as salts 28,29, poly(ethylene oxide) (PEO) 30, gas 31, etc. Using solutions with polyelectrolyte nature (a high charge density material) leads to the extension of fibers outwards from the collector under conditions which induce repulsion between neighboring fibers 32. In brief, post-treatments include photo-masking 33 or stacking layered mats 34.
Self-assembly
Novel nanofibrous scaffolds have been fabricated by self-assembling peptides through molecular self-assembly by mimicking regulatory mechanisms of natural ECM. Self-assembly is a manufacturing process in which small molecules-as basic building blocks- will be added-up to form nanofibres. These structures have gained much progress in repairing different injured tissues such as cartilage, bone, nerve, heart and blood vessel 35. Two significant approaches have been proposed to proximate peptide nanofiber scaffolds to ECM: (1) modification with functional motifs (e.g. RGD, IKVAV and YIGSR) and (2) controlled release of molecular signals such as Fibroblast Growth Factor (FGF-2) and Vascular Endothelial Growth Factor (VEGF). In self-assembly, intermolecular forces determine the properties and shape of nanofibers. Nanofibers can be assembled with various polymeric configurations such as diblock copolymers, triblock copolymers, triblock polymers (of peptide amphiphile and dendrimers), and bolaform (of glucosamide and its deacetylated derivatives) 36. In vitro assessment of many peptide nanofiber scaffolds have revealed the ability to induce cell proliferation, differentiation, migration and ECM production 37–39. Poor mechanical property of peptide nanofiber scaffolds might limit its application to non-load-bearing sites 40.
Phase-separation
Phase-separation is a method for fabrication of 3D nanofibrous structures with nanofibers that closely mimic dimension of collagen fibrils of ECM (50–500 nm) 41. This technique is based on the physical incompatibility of polymers and their tendency to separate into two phases for nanofiber production 17. Phase-separation provides the possibilities of scaffold fabrication for a desired anatomical shape and presenting the nano and macro architecture simultaneously 42. Although the fabrication process is convenient and requires simple instrumentation, it is limited to only certain specific polymer-solvent combinations. Also, fiber dimensions cannot be controlled and the mechanical properties of the fiber are not suitable for load-bearing applications due to the highly porous structure. The controlling parameters include polymer type, polymer concentration, solvent type and thermal treatment 41.
Drawing
In the drawing process, a micropipette, a few micrometers in diameter, is dipped into a polymer liquid and withdrawn at a fixed speed resulting in production of nanofibers. This process is simple and is suitable for viscoelastic materials bearing strong deformations while being united enough to support the stresses developed under pulling. However, it is limited to laboratory scale as nanofibers are formed one by one. Another limitation is that, there is no control on fiber dimensions and only fibers with diameters in the micrometer size can be produced. Also, an additional step such as weaving is needed to make scaffolds for tissue engineering applications 17,36.
Some advantages and disadvantages of the above mentioned techniques in terms of their fabrication, reproducibility and controllability have been summarized in table 1.
Table 1.
Advantages and disadvantages of different methods for fabrication of nanofibers
| Manufacturing process | Control on fiber dimension | Advantages | Disadvantages |
|---|---|---|---|
| Electrospinning | Yes (from few nanometers to several microns) |
|
|
| Drawing | No |
|
|
| Phase-separation | No |
|
|
| Self-assembly | No |
|
|
The advantages of stem cells for cartilage tissue engineering purposes
In native tissues, cells are constantly interacting with the surrounding ECM that leads to transferring information between the extracellular and intracellular space, directing their behavior. Chondrocytes are the sole cell type in articular cartilage that mostly has been served as the cell source for articular cartilage repair in clinic. However, their utilization in clinic is accompanied with some limitations. For example, autologous chondrocyte availability is limited and cannot provide the high cellular demand of articular cartilage repair. Although some in vitro cell expansion methods have been developed to increase cell numbers for transplantation, the risk of chondrocytes dedifferentiation during in vitro culture is a big challenge 43,44.
Although there exists a wide range of studies on transplantation of more available chondrocyte sources such as allogeneic or xenogeneic chondrocytes instead of autologous chondrocytes, these chondrocytes can potentially induce immune responses or transmit diseases. Thus, the application of allogeneic and xenogeneic chondrocytes requires further investigations to remove such concerns. Since chondrocytes from each of the four zones exhibit different properties, another strategy is the use of separately seeded zonal chondrocytes toward regenerating biomimetic functional cartilage tissue 45,46. Due to the aforementioned limitations of chondrocyte sources, there is much effort to find out alternative cell sources. In these years, fascinating characteristics of stem cells especially adult stem cells such as accessibility, availability and chondrogenic capacity have introduced these cells as promising cell sources for articular cartilage tissue engineering 5.
Embryonic Stem Cells (ESCs) and induced Pluripotent Stem Cells (iPSCs) are cell sources with high chondrogenic potentials; however, there are concerns on their immunogenicity, potential for malignancy, ethical issues (for ESCs), and heterogeneous differentiation. Therefore, these cell sources cannot be the best candidate for cartilage tissue engineering 47.
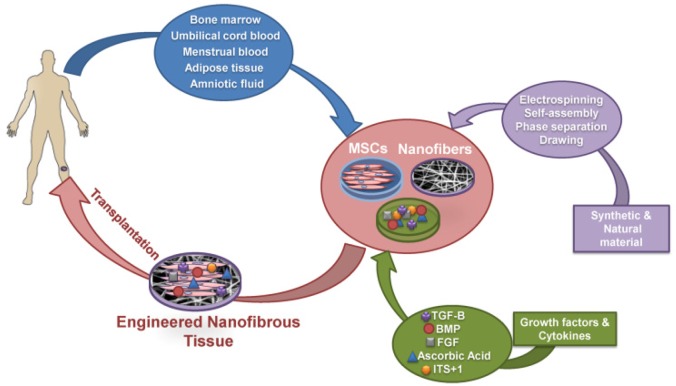
As shown in figure 1, adult stem cells being derived from different tissues such as bone marrow, cord blood, placenta, adipose tissue, amniotic fluid and menstrual blood combined with nanofibrous scaffolds have been widely used for cartilage tissue engineering 13,48–50. Compared with adult chondrocytes, they can easily be obtained and manipulated as they are able to undergo several passages before losing their differentiation potential.
Figure 1.
Schematic diagram of cartilage tissue engineering process using nanofibers and stem cells. Mesenchymal stem cells derived from different sources are expanded ex vivo and subsequently cultured in nanofiber scaffolds to initiate differentiation in presence of growth factors and cytokines. Finally, the engineered nanofibrous tissues were implanted in vivo for cartilage tissue regeneration. MSCs: Mesenchymal Stem Cell, BMP: Bone Morphogenetic Protein, TGF-B: Transforming Growth Factor-Beta, FGF: Fibroblast Growth Factor, ITS+1: Insulin-Transferrin-Selenium+ Bovine Serum Albumin and Linoleic Acid.
Bone Marrow Mesenchymal Stem Cells (BMMSCs), for example, are multi-potential stem cells with the capacity to differentiate into a variety of tissue types including bone, cartilage, fat, muscle, tendon and other tissues when induced by the appropriate cues both in vitro or in vivo 51,52. They can provide a suitable cell source for osteochondral tissue reconstruction 13 and have also been previously explored for the engineering of fibro-cartilaginous tissues such as the annulus fibrous of the inter-vertebral disc and the knee meniscus 53,54. For example, Li et al stated that adult BMMSCs seeded on electrospun polycaprolactone (PCL) combined with TGF-β1 differentiated into a chondrocytic phenotype at levels comparable to traditional pellet cultures. The designed constructs showed a zonal morphology with a layer of cartilaginous matrix composed of collagen type II, cartilage proteoglycan link protein, and aggrecan 55.
Shafiee et al have studied the in vitro characteristics and chondrogenic capacity of four available human adult stem/progenitor cell sources using aligned electrospun polycaprolactone/poly (L-lactic acid) (PCL/PLLA) nanofibers. The studied cells include BMMSCs, adipose tissue-derived MSC (AD-MSC), Articular Chondrocyte Progenitors (ACP), and nasal septum-derived progenitors (NSPs). Accordingly, NSPs exhibited the highest proliferation potential and chondrogenic capacity 47.
More recently, menstrual blood has been identified as an easily accessible and renewable stem cell source with the higher proliferative rate compared with umbilical cord and bone marrow derived mesenchymal stem cells 56,57. Our group presented the evidence introducing menstrual blood stem cells (MenSCs) as a suitable stem cell population candidate for cartilage tissue engineering. Indeed, the chondrogenic capacity of MenSCs is a major issue which may support future application of MenSCs as a reliable source for cell therapy of cartilage defects.
In vitro findings on recapitulation of ECM environment for cartilage tissue engineering using nanofibrous scaffolds
Single polymer-based nanofibrous matrices
Electro-spun nanofibers with different compositions have been widely studied for osteochondral differentiation (Table 2). Chondrogenic differentiation of BMMSCs has been extensively studied on 2D electrospun nanofibrous matrices using single polymer, such as PCL 58,59 and poly (D,L-lactide-co-glycolide) (PLGA) 60,61. Wise et al found that cell orientation is minimally influenced by soluble factors and is mainly controlled by physical cues (oriented micro- and nano-fibers in this study); however, cell shape was affected by chondrogenic factors 58. Cells cultured in chondrogenic media on nanofibers showed a significant increase in the sGAG content and expression of collagen type II in comparison with culturing in normal growth media and on microfiber scaffolds 58. Alves da Silva et al cultured BMMSCs on electrospun PCL nanofiber mesh in a multi-chamber flow perfusion bioreactor to produce cartilagineous extracellular matrix 59. Statically cultured cells had a fibroblast-like morphology, while dynamic condition induced round-shaped morphology with increased amount of sGAG and collagen type I and II. However, there was no significant difference between gene expression of chondrogenic markers in two culture conditions. Another study has shown that PLGA electrospun nanofibers assisted the growth and differentiation of human BMMSCs as well as their osteogenic and chondrogenic potential 60.
Table 2.
In vitro studies on cartilage tissue engineering using stem cells and nanofibers
| Species | Cells Source | Cells Type | Biomaterials | Stimulating Factors | Results | Ref. |
|---|---|---|---|---|---|---|
| Rabbit | Bone Marrow | Mesenchymal Stem Cells (MSCs) | Poly (Vinyl Alcohol)/Poly (E-Caprolactone): PVA/PCL | TGF-B1, FGF-2, Dexamethasone, Ascorbate 2-Phosphate, ITS+1 premix, | MSCs seeded on PVA/PCL scaffolds showed the mRNA expression of collagen type II and Aggrecan after 21 days of chondrogenic differentiation | (3) |
| Goat | Bone Marrow | MSCs | Poly (Vinyl Alcohol) - methacrylate (PVA-MA) PVA-hondroitin Sulfate- methacrylate (PVA-CS-MA) | TGF-B1, Ascorbate 2-Phosphate, Dexamethasone, L-Proline, Sodium Pyruvate, ITS-Plus Premix | A higher collagen type II/type I gene expression ratio in PVA-CS-MA compared with PVA-MA fibers alone | (7) |
| Fetal Bovine | Epiphyseal Cartilage | Chondrocytes | PCL | Ascorbate 2-Phosphate, Dexamethasone, Sodium Pyruvate, Proline, ITS-Plus Premix | Chondrocytes seeded on the PCL scaffold maintained their chondrocytic phenotype by gene expressing of collagen types IIB and IX, aggrecan, and cartilage oligomeric matrix protein | (9) |
| Human | Bone Marrow | MSCs | PCL and sodium hyaluronate (HA) | TGF- B1, Bovine Serum Albumin (BSA) | Initial release of HA is sufficient in terms of directing the implanted MSCs toward a chondrogenic end, whereas a late release of TGF-B1 is preferred to foster type II and avoid type I collagen expression | (11) |
| Human | Bone Marrow | MSCs | Poly (L-lactic) acid (PLLA) | TGF- B1 | In the presence of TGF- B1, cartilage tissue developed on PLLA scaffolds had high level of Sulfated glycosaminoglycans (sGAG), Sox-9 and collagen type II | (13) |
| Human | Umbilical Cords | MSCs | Poly L-lactide-co-glycolic acid (PLGA) and PCL | TGF-B3, TGF-B1, IGF, BMP6, Ascorbate 2-Phosphate, ITS-Plus Premix, Dexamethasone, L-Proline | Level of sGAG and sulfated proteoglycans and also the ratio of collagen type II to collagen type I expression was up-regulated in differentiated MSCs on PLGA. Cells differentiated on the scaffold | (48) |
| Human | Menstrual blood | Menstrual blood-derived stem cells (MenSCs) | PCL | TGF-B3, IGF-1, Sodium Pyruvate, Ascorbate 2-Phosphate Dexamethasone, ITS+1 premix | had high level of collagen type II and also proteoglycan production compared to 2D system | (56) |
| Human | Bone Marrow | MSCs | PCL | TGF-B1, Ascorbate 2-Phosphate, Sodium Pyruvate, L-Proline, ITS-Plus Premix | Gene expression of collagen types II and IX and also the level of sGAG was up-regulated in nanofibrous system compared with control culture | (55) |
| Human | Bone Marrow | MSCs | PLGA | TGF-B3 | MSCs seeded in PLGA nanofiber scaffold in chondrogenic induced medium began to produce high level of sGAG compared to MSCs seeded in PLGA nanofibers without chondrogenic differentiations | (60) |
| Bovine | Carpometacarpal joints of the forelimbs | Chondrocytes | PLLA | TGF-B1, IGF-1, Ascorbate 2-Phosphate, Dexamethasone, Sodium Pyruvate, Proline ITS-Plus Premix | The dynamic culture condition and IGF-1/TGF-b1 treatments upregulated collagen and sGAG production in packed cell nanofiber composite cultures | (88) |
| Human | Placentas | MSCs | nano-sized calcium-deficient hydroxyapatite (nCDHA) and/or a recombinant protein containing arginine–glycine–aspartate (RGD) into the alginate gel and PLGA | TGF-B3, Ascorbate 2-Phosphate, Dexamethasone, l-proline | The amount of sGAG and collagen type II accumulated was found to be the greatest for human Placenta-derived MSCs embedded in the alginate/nCDHA/RGD gel and injected and cultivated in the PLGA scaffold | (50) |
| Bone marrow | ||||||
| Rat | Subcutaneous Fat | |||||
| Human | Bone Marrow | MSCs | PCL | TGF- B1, Ascorbate 2-Phosphate, Sodium Pyruvate, Dexamethasone, l-proline | The expression of collagen type II and aggrecan was upregulated significantly in MSCs seeded on the nanofibrous PCL scaffold | (58) |
| Human | Cartilage | Chondrocytes | Polylactic acid (PLA) microfibers and PCL nanofibers | TGF-B1, Ascorbate 2-Phosphate, ITS+1 premix, Dexamethasone | The pore sizes in the scaffolds were tailored and increased from nanometer scale in purely nanofibrous scaffolds to hundreds of micrometers in scaffolds of nanofiber-coated microfibers. Also, SEM analysis indicated that the chondrocytes adhered and spread on composite scaffolds and produced high level of extracellular matrix. | (89) |
| Porcine | Articular Cartilage | Chondrocytes | PLGA nanofiber and membrane scaffold | Ascorbate 2-Phosphate | The DNA content and normalized sGAG content of the nanofiber based scaffolds were significantly higher than those of the membrane-type scaffolds. | (90) |
| Rabbit | Bone Marrow | MSCs | Natural Nanofibrous Articular Cartilage extracellular matrix (ACECM) and PLGA composite oriented scaffold | - | Cell proliferation test showed that the number of MSCs in ACECM and composite scaffolds was noticeably higher than that in PLGA scaffold, which was coincident with results of SEM observation and cell viability staining | (91) |
| Human | Bone Marrow | MSCs | PCL Microfibers and Nanofibers | TGF-B3, Ascorbate 2-Phosphate, L-proline, Dexamethasone, ITS+1 premix | Cellular proliferation and sGAG and collagen production were enhanced on microfiber in comparison to nanofiber scaffolds, with high initial seeding densities being required for significantchondrogenic differentiation and extracellular matrix (ECM) deposition. Moreover, the collagen type II/I ratio, as a indicator of hyaline cartilage phenotype, was significantly greater for the higher seeding densities on microfibers than nanofibers and in comparison to the lower seeding densities | (92) |
| Human | Adipose Tissue | Adipose-Derived Stem Cells (ASCs) | PCL and cartilage-derived matrix (CDM) | TGF-B1, BMP-6, Dexamethasone,, Ascorbate 2-Phosphate, L-proline | Incorporation of CDM into seeded scaffolds with hASCs stimulated sGAG synthesis and collagen type 10A1 gene expression. Also, compared with single-layer scaffolds, multilayer scaffolds enhanced cell infiltration and ACAN gene expression | (93) |
| Human | UmbilicalCord | Umbilical Cord Wharton’s Jelly Stem Cells (WJSCs) | PCL/Collagen | TGF-B3, FGF-2,L-proline, ITS+1 Premix, Dexamethasone,Ascorbate 2-Phosphate, Sodium Pyruvate | Seeded scaffolds with WJSCs and MSCs showed positive staining in 21 days for the chondrogen related proteins collagen type II and SOX9 and also sGAG values compared to controls | (94) |
| Bone Marrow | MSCs | |||||
| Rat | Bone Marrow | MSCs | PCL nanofibers encapsulated with Hyaluronic acid (HYA) and CS | - | Collagen type II was expressed more in the scaffolds with nanofibers inclusive of CS and HYA than in the scaffolds with vertically oriented nanofibers | (95) |
| Human | Bone Marrow | MSCs | PLLA Microfibers and Nanofibers | TGF-B3, ITS +1premix Dexamethasone, Ascorbic acid-2-phosphate, Sodium Pyruvate, L-proline | Chondrogenic markers of aggrecan, chondroadherin, sox9, and collagen type II were the highest for cells on micron-sized fibers in comparison to cells on nano-sized fibers | (96) |
| - | - | C3H10T1/2 murine embryonic mesenchymal progenitor cells | core-shell poly(ether sulfone)- PCL (PES-PCL) | rhBMP-2 | Results from chondrogenic differentiation of cells on scaffolds indicated that the lower modulus PCL fibers provided more appropriate microenvironments for chondrogenesis, by upregulation of Sox9, collagen type II and aggrecan gene expression and sGAG production compared to core-shell PES-PCL fibers | (97) |
| Human | Bone Marrow | MSCs | PLLA | TGF-B1, IGF-1, Dexamethasone, Ascorbic acid-2-phosphate, Sodium Pyruvate, L-proline, ITS+1 premix | The mRNA levels of aggrecan and collagen type II in TGF-B1/IGF-I treated cultures were notably higher than those treated only with TGF-B1, although these differences were not statistically significant. However, collagen type II/collagen type I ratio was high in TGFB1/IGF-I treated cultures. Also, in tow conditions, both sGAG and hydroxyproline accumulation showed significant changes over culture time | (98) |
| Human | Bone Marrow | MSCs | PCL nanofibers | TGF-B1, Ascorbic acid-2-phosphate, Dexamethasone, Sodium Pyruvate, ITS+1 premix | Constructs cultured in the presence of chondrogenic medium supplemented with TGF-B1 revealed significantly upregulated expression of aggrecan and Collagen type II and also abundant proteoglycan-rich ECM compared to constructs cultured in the presence of chondrogenic medium alone | (99) |
| Human | Articular Cartilage | Chondrocytes | Micro and Nanofibers PLLA | TGFB1, ITS+1 premix, Dexamethasone, Ascorbic acid-2-phosphate | In both types, scaffolds indicated an increase in sGAG production and Collagen type II expression over time | (100) |
| Canine | Articular Cartilage | Chondrocytes | Electrospun poly(D,L-lactide)/poly(L lactide) (PDLA/PLLA) or poly(D,L lactide)/polycaprolacton e (PDLA/PCL) with chitosan-based hydrogel | Ascorbic acid-2-phosphate | Primary canine chondrocytes produced collagen type II and proteoglycans while being cultured on scaffolds composed of electrospun PDLA/PCL and chitosan hydrogel | (101) |
| Rabbit | Articular Cartilage | Chondrocytes | PLLA nanofibers modified with cationized gelatin (CG) (CG-PLLA) | - | In vitro studies indicated that CG-PLLA could enhance viability, proliferation and differentiation of rabbit articular Chondrocytes compared with pristine PLLA nanofibers. In addition, these cell–scaffold constructs were able to maintain the expression of characteristic markers (collagen II, aggregan and SOX 9) of chondrocytes | (102) |
| Human | Bone Marrow | MSCs | PLLA nanofibers | TGF-B1, Ascorbic acid-2-phosphate, L-proline, Dexamethasone, Sodium Pyruvate, ITS+1 premix | PLLA-scaffold seeded with MSCs transfected with SOX-9 showed an increase in aggrecan mRNA expression over controls | (103) |
| Human | Bone Marrow | MSCs | PLGA nanofibers | Chondrogenic induction medium (CM, hMSC Differentiation BulletKit-chondrogenic, Lonza), TGF-B3 | Production of proteoglycan and type-II collagen and also the high expression levels of SOX9 and COL10A1 were observed in differentiated BMMSCs on nanofibers in comparison to two-dimensionally cultured cells | (61) |
| - | - | ATDC5 chondrogenic cell line | Collagen-PLA, Collagen-PLGA | - | The addition of collagen has a dual influence of making the scaffolds more hydrophilic and reinforcing the mechanical properties. Furthermore, the soft scaffolds composed of the highly biodegradable PLGA50:50 and collagen, in two ratios (40:60 and 60:40), were optimal for chondrogenesis with ECM production and enhanced cartilage specific gene expression | (62) |
| Human | Articular Cartilage | Chondrocytes | poly(3hydroxybutyrate)/poly (3hydroxyoctanoate) P(3HB)/P(3HO) | - | The finding revealed that two ratios of P(3HB)/P(3HO) enhanced the aggregation of hyaline-like cartilage matrix and type II collagen after three weeks of culture with chondrocytes | (63) |
| Rabbit | Articular Cartilage | Chondrocytes | PLLA/ silk fibroin (PLLA/SF) | - | The PLLA/SF composite scaffold supports adhesion, proliferation, and growth of chondrocyte higher than PLLA scaffold without SF | (64) |
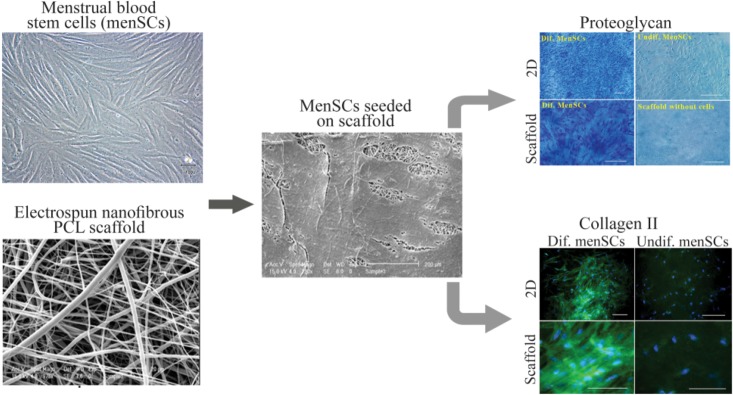
Our group has demonstrated that MenSCs, with higher proliferation capacity than BMMSCs, have the potential to undergo chondrogenic differentiation on PCL nanofibers 56,57. In addition, culturing on PCL nanofibers improved level of sGAG and proteoglycan production compared to PCL film (Figure 2).
Figure 2.
Culture and chondrogenic differentiation of MenSCs on nanofibrous scaffold. The image analyses of the scanning electron microscopy show that cells penetrated and adhered well on the surface of the mesh. Development of cartilage-like tissue in cultured constructs has been examined histologically with respect to the presence of proteoglycan and collagen type II (Scale bar: 100 μm). PCL: Polycaprolactone, Dif: Differentiated, 2D: Two Dimensional. (Adopted from Kazemnejad et al 2014 40, with minor modification).
Dahl et al investigated the potential of human Umbilical Cord Mesenchymal Stem Cells (UCMSCs) for chondrogenic differentiation on PLGA and PCL electrospun nanofibers 48. Cell culturing on nanofibers resulted in the production of higher levels of sGAG and sulfated proteoglycans. The ratio of collagen type II to type I expression was considered as the differentiation index (DI) in cartilage tissue engineering. There was a significant increase in the DI between PLGA and pellet control while no differences between PCL and PLGA cultures or between the PCL and pellet cultures were detected. While the expression level of elastin was not different between pellet controls and the two nanofiber conditions, significant increase in collagen type X on PLGA nanofiber scaffolds was found when compared to pellet controls.
Hybrid nanofibrous matrices
Randomly-oriented nanofibers: Shafieeet al have proved the potential of hybrid PVA/PCL nanofiber mesh seeded with rabbit BMMSCs in terms of cartilage tissue engineering in vitro and in vivo 3. Electrospinning PVA concurrently with PCL improved the capacity of nanofibrous scaffold for cell attachment and interactions and consequently improved cell proliferation rate.
In another study, Ahmed et al suggested that soft scaffolds composed of the highly biodegradable PLGA and collagen, in two ratios (40:60 and 60:40) were optimal for chondrogenesis 62. Most recently, Ching et al suggested that P(3HB)/P(3HO) nanofiber scaffolds fabricated by electrospinning reduce the risk of developing secondary osteoarthritis and may be suitable for clinical use 63. Moreover, it has been newly indicated that the PLLA/silk fibroin (PLLA/SF) composite scaffold supports adhesion, proliferation, and growth of chondrocyte more than PLLA scaffold without SF, introducing this scaffold a suitable material with potential application in cartilage tissue engineering 64.
Aligned nanofibers: A study has been conducted on seeding BMMSCs and fibrochondrocytes on PCL-PEO aligned nanofibrous meshes and demonstrated that aligned nanofibrous topography could influence human BMMSCs fibrochondrogenesis by mimicking the naturally-occurring ECM more closely than micro-patterned features such as ridges or grooves 65.
In this way, Shafiee et al evaluated cell proliferation and chondrogenesis on aligned (A) and randomly (R) oriented electrospun PLLA/PCL hybrid scaffolds. They demonstrated that NSPs exhibit different behavior in two scaffolds. NSPs seeded on R fibers were expanded in all directions and exhibited a polygonal morphology and displayed multipolar shape. Conversely, NSPs were oriented only in the longitudinal direction of A fibers and showed bipolar extension along the fiber course alignment. Also, NSPs cultured on A fibers showed significantly higher expression of markers related to chondrogenesis process compared to cells cultivated on R fibers. The authors emphasized the role of the physical and topographical characteristics of scaffolds in the development of efficient stem cell-scaffold complexes and concluded that the aligned nanofibrous scaffolds can significantly enhance chondrogenic differentiation of nasal septum derived progenitors 66.
Micro-nano fibrous scaffolds
While nanoscale features are desired due to mimicking the ECM components such as collagen fibers, it is believed that high concentrations of nanoscale fibers could increase cell spreading and limit cellular infiltration 67,68. Therefore, fabrication of multi-scale scaffolds combining microfibers with nanofibers has been considered with the aim of providing larger pore sizes and improving cell differentiation and ECM production 69. For this purpose, Levorson et al fabricated electrospun scaffolds consisting of two differently scaled fibers interspersed evenly throughout an entire construct as well as scaffolds containing fibers of fibrin and PCL. The prepared samples were scaffolds containing PCL microfibers (Pμ), PCL microfibers with PCL nanofibers (PμPn), and PCL microfibers and fibrin nanofibers (PμFn) being electrospun by a dual extrusion process. Both PμFn and PμPn scaffolds displayed similar porosities higher than microfibers alone. However, the Pμ scaffolds bore significantly larger pore sizes than the scaffolds containing nanofibers. Additionally, the PμPn scaffolds had the highest density of nanofibers and the smallest average pore size of all samples. The seeded human UCMSCs on PμPn scaffolds appeared to exhibit a flattened, broad polygonal morphology and spread along microfibers while cells on the Pμ and PμFn scaffolds showed more elongated and spindle-like morphologies and extended between the microfibers. Furthermore, analysis of cellular infiltration by Fast Green staining showed more scattered distribution of cells within the PμPn scaffolds while cells were primarily located on the surface of the other two scaffold types. Histological examination also exhibited more deposition of sGAG in PμPn and PμFn in contrast to the scaffolds composed of microfibers alone suggesting that the inclusion of nanofibers within a microfiber is useful towards the production and distribution of sGAG and may be beneficial for cartilage regeneration. The authors emphasized on tuning the density of nanofibers with respect to microfibers in an effort to control the positive influence of nanofibers on cell attachment and ECM production while minimizing any negative effects such as limited infiltration 70.
Three dimensional nanofibrous scaffolds
To achieve 3D highly porous nanofibrous structure for cartilage tissue engineering, Hu et al used a phase separation method to fabricate a desirable scaffold made of PLLA. They showed that fabricated nanofiber scaffolds could efficiently support chondrogenesis of human BMMSCs in the presence of TGF-β1. The expression of chondrogenic markers in human BMMSCs grown on nanofiber matrix was significantly higher compared with cells raised on smooth film culture 13.
Li et al examined the differentiation of adult BMMSCs to chondrocytic phenotype on a nanofibrous PCL scaffold. They found that in Nanofibrous Scaffold (NFS) chondrocyte-like cells produced higher level of cartilaginous ECM compared with high-density Cell Pellet (CP) culture. In addition, specifically, collagen type IX was expressed to an upper level in nanofibrous system compared to CP culture. Furthermore, the level of sulfated Glycosaminoglycan (sGAG) synthesis in NFS culture was over two-fold higher than CP culture over a 21-day culture period. Their experimental results suggested that, while a 3D environment and TGF-β1 were both necessary to induce chondrogenesis, the PCL-based NFS significantly enhanced the chondrogenic differentiation of BMMSCs compared to the CP culture and could be considered as a candidate scaffold for cell-based tissue engineering approaches to cartilage repair compared to the CP culture 55.
Biomolecules-loaded nanofibrous structures
The potential of electrospun nanofibrous and micro-fibrous PCL scaffolds to release TGF-β1 and stimulate chondrogenic differentiation of BMMSCs has also been investigated by Schagemann et al. They found that the augmentation of nanofibrous texture with or without TGF-β1 and/or hyaluronan was helpful in terms of directing the implanted BMMSCs toward a chondrogenic end. In addition, their results demonstrated that nanofibrous scaffold groups have different trends with microfibrous scaffolds via release level of TGF-β1 and chondrogenic development. The microfibrous scaffolds release TGF-β1 more than nanofibrous scaffolds; however, expression of cartilage marker in nanofibrous scaffold groups was higher than that in microfibrous scaffolds 48.
Recently, injectable microspheres were suggested as an attractive stem cell and growth factors carriers for tissue regeneration. In a study by Zhang et al, Transforming Growth Factor-β1 mimicking peptide cytomodulin (CM), was conjugated onto the functional nanofibrous hollow microspheres (FNF-HMS) to induce distinct differentiation pathways of rabbit BMMSCs. Their finding indicated that novel FNF-HMS effectively presents CM to BMMSCs and successfully induces their chondrogenesis for cartilage formation in both in vitro and in vivo studies 71.
In vivo studies on repair of cartilage defects using constructs composed of nanofibers and stem cells
Single polymer-based nanofibrous matrices
Implantation of nanofibers-based tissue engineered cartilage eliminates the need for an extra covering material to secure and protect the implant, such as periosteum which is used in the current autologous chondrocyte transplantation procedure. Harvesting periosteum comes with morbidity and complications, thus it is clinically preferable to avoid the use of periosteum 72.
In the recent decade, effectiveness of implanted electrospun PCL nanofibrous scaffold with/without cells has been evaluated for repair of cartilage defects in animal models (Table 3). Li et al demonstrated the potential of BMMSCs-seeded PCL-based nanofibrous scaffolds to repair full-thickness cartilage defects in a swine model. This cell-scaffold construct renewed hyaline cartilage-like tissue and restored a smooth cartilage surface as compared with other groups, including acellular constructs and untreated group. Furthermore, the studied group, which was chondrocyte-seeded scaffold, produced fibrocartilage-like tissue with an irregular superficial cartilage contour 72.
Table 3.
In vivo studies for repair of cartilage defects using constructs composed of nanofibers and stem cells
| Host | Cells source | Cells type | Biomaterials | Stimulating factors | Results | Ref. |
|---|---|---|---|---|---|---|
| Pig | Bone Marrow | Mesenchymal Stem Cells (MSCs) | Hyaluronate/type I collagen/fibrin composite Scaffold containing polyvinyl alcohol (PVA) nanofibers and | FGF-2 and Insulin | The cell-free composite scaffold improved migration of the bone marrow stem cells into the defect, and their differentiation into chondrocytes and also enhanced the regeneration of osteochondral defects towards hyaline cartilage and/or fibrocartilage in contrast to control cases that were left untreated and were filled with fibrous tissue | (1) |
| Rabbit | Bone Marrow | MSCs | Collagen and Polyl-Lactic Acid (PLA) | - | Compared with collagen scaffold, implantation of collagen-nanofiber scaffold seeded with cells induced more rapid subchondral bone appearance, and better cartilage development, which led to better functional repair of deep osteochondral defects in rabbits | (2) |
| Rabbit | Bone Marrow | MSCs | PVA/ poly (ε-caprolactone) (PCL) nanofiber (PVA/PCL) | - | A high similarity in ECM patterns between regenerated tissue in the group which received cell-seeded scaffold and normal tissues was observed. Also, the production of collagen type II in these groups was high compared to other groups | (3) |
| Rat | - | - | Poly (Vinyl Alcohol) - methacrylate (PVA-MA) and Chondroitin Sulfate (CS) | - | CS fibers combined with PVA fibers induced statistically higher type II collagen production compared with the PVA fibers alone and empty defects | (7) |
| Swine | Articular Cartilage | Allogeneic Chondrocytes | PCL | - | In contrast to acellular constructs and the no-implant control groups, MSC-seeded scaffolds renewed hyaline cartilage-like tissue and restored a smooth cartilage surface. In addition, the chondrocyte-seeded scaffolds produced fibrocartilage-like tissue with an irregular superficial cartilage contour | (72) |
| Human | Bone marrow | Xenogeneic MSC | ||||
| Rabbit | - | - | PCL with Chitosan | TGF-B1, Ascorbate-2-phosphate | Cartilage formation and production of sGAG in the uncoated scaffolds increased at the end of implantation time compared to chitosan-coated scaffolds. Also, significantly more mineral dep osition was detected inTGF-β1-injected and uncoated scaffolds compared to vehicle-injected and coated scaffolds | (73) |
| Rabbit | Bone Marrow | MSCs | oriented poly (L-lactic acid)-copoly (e-caprolactone) P(LLA-CL) yarn collagenI/hyaluronate hybrid scaffold (Yarn-CH) as a chondral phase and Porous beta-TCP as a osseous phase | TGF-B1, Dexamethasone, Ascorbate-2-phosphate,L-proline, Sodium pyruvate, ITS+1 Premix | In differentiated MSCs/YarnCH/TCP and MSCs/CH/TCP biphasic scaffold groups, the regenerated defects almost completely full with hyaline-like repaired tissue appeared to be integrated with the surrounding tissues. In undifferentiated MSCs/YarnCH/TCP and MSCs/CH/TCP biphasic scaffold groups, defects were covered by rough tissue with irregular surfaces which were clearly distinguishable from the normal cartilage. Furthermore, immunohistochemical staining showed high level of collagen type II in the BMSCs/YarnCH/TCP biphasic scaffold groups than in the other groups | (76) |
| Rabbit | - | - | porous hydroxyapatite/collagen (HAp/Col) scaffold | FGF-2 | Abundant bone formation was observed in the HAp/Col implanted groups as compared to the control group. Furthermore, HAp/Col impregnated with FGF-2 displayed not only abundant bone regeneration but also the most satisfactory cartilage regeneration, with cartilage presenting a hyaline-like appearance | (78) |
Hybrid nanofibrous matrices
An autologous cell-based cartilage repair approach has been developed to eliminate harvesting of healthy cartilage and in vitro culture. In this study, PCL nanofiber scaffolds 73 (with/without chitosan coating) were implanted under periosteum in six months old rabbits with injection of GF-β1 into the implant site. Cell infiltration was observed in all groups while sGAG production and cartilage formation was more typical in the uncoated scaffolds compared to chitosan-coated scaffolds. In addition, TGF-β1-injection and application of uncoated scaffolds resulted in significantly more mineral deposition.
The iPSCs can be produced by reprogramming of terminally differentiated cells to primary stem cells with pluripotency. To benefit from the breakthrough of iPSCs, the effect of electrospun PCL/gelatin nanofibrous scaffolds on the chondrogenesis of iPSCs and articular cartilage defect restoration was investigated. It was indicated that iPSCs expressed higher levels of chondrogenic markers on the scaffolds than the culture plate. Additionally, in an animal model, cartilage defects implanted with the scaffold-iPSCs composite exhibited an enhanced gross appearance and histological improvements, higher cartilage-specific gene expression and protein levels, as well as subchondral bone regeneration. Therefore, it was shown that scaffolds enhanced the chondrogenesis of iPSCs and that iPSCs-containing scaffolds improved the re-establishment of cartilage defects to a greater degree than did scaffolds alone in vivo 74.
In another study, efficiency of the fabricated hybrid PVA/PCL nanofibers seeded with autologous BMMSCs was evaluated in the knees defect of rabbits. The authors indicated improved regeneration of cartilage in full-thickness defects that treated with BMMSCs-loaded PVA/PCL electrospun scaffolds compared to scaffold alone or untreated defects. After 12 weeks of implantation, almost all defects that were treated with cell-scaffold constructs were completely enclosed with smooth tissue and edges of the grafted areas were hardly detectable. In addition, unlike the group who received only PVA/PCL scaffolds, a high similarity in ECM patterns between regenerated and normal tissues was observed and collagen type II staining was positive 3.
In another study by He et al, the cell seeded electrospun nanofibers containing collagen-poly (L-lactic acid-co-ε-caprolactone) (collagen-PLCL) and chondrocytes were implanted subcutaneously into nude mice followed by evaluation of the quality of neocartilage. Their results revealed that collagen-PLCL membranes facilitate the formation of cartilage-like tissue in animals and thus could mimic the natural ECM with good cell affinity 75.
Recently, bi-layer scaffolds have gained considerable attention for the restoration of osteochondral defects affecting both the articular cartilage and the underlying subchondral bone 74. Combination of collagen and electrospun nanofibers as bi-layer scaffold has been demonstrated to help cartilage and bone regeneration. In 2013, Zhang et al reported efficiency of a fabricated bi-layer microporous scaffold with collagen and electrospun PLLA nanofibers (collagen-PLLA) for repair of osteochondral defects. Compared with collagen scaffold, implantation of collagen-PLLA scaffold seeded with BMMSCs induced more rapid subchondral bone appearance and better cartilage development, which led to better functional repair of deep osteochondral interfacial tissue structure in rabbits 2.
Liu et al have also studied the efficiency of a biphasic complex to repair the osteochondral defects in a rabbit model, which was composed of oriented electrospun poly (L-lactic acid)-co poly(e-caprolactone) P(LLA-CL) Yarn-collagen type I/hyaluronate hybrid scaffold (Yarn-CH) as a chondral phase and porous beta tricalcium phosphate (TCP) as a osseous phase. The regenerated defects treated by differentiated BMMSCs/Yarn-CH/TCP and BMMSCs/CH/TCP (control) biphasic scaffold groups were completely repaired by hyaline-like tissue that appeared to be integrated with the surrounding tissues. In undifferentiated BMMSCs/Yarn-CH/TCP and BMMSCs/CH/TCP biphasic scaffold groups, defects were covered by rough tissue with irregular surfaces which were clearly distinguishable from the normal cartilage. In addition, the cell distribution and cell morphology in the regenerated cartilage were almost identical to the native host cartilage including the superficial zone in differentiated groups compared to undifferentiated groups. Furthermore, immuno-histochemical staining showed higher level of collagen type II in the BMMSCs/Yarn-CH/TCP biphasic scaffold groups than in the other groups. Greater improvement of the compressive modulus was also shown in differentiated-BMMSCs/Yarn-CH/TCP biphasic scaffold group compared to other groups 76.
Three dimensional nanofibrous scaffolds
In 2010, a three-dimensional PLGA/nano-hydroxyapatite (PLGA/NHA) scaffold was fabricated by a thermally induced phase separation method and its efficacy to repair articular osteochondral defects in murine model was investigated. The defects in the PLGA/NHA-MSCs treated group were filled with smooth and hyaline-like cartilage with profuse glycosaminoglycan and collagen type II deposition12 weeks after operation 77.
For interacting cells with the surrounding ECM, which gives rise to a dynamic transfer of information between the extracellular and intracellular space, researchers have introduced several biological signals, including chondroitin sulfate, hyaluronic acid, and collagen into tissue-engineered scaffolds to encourage tissue specificity 43. In 2012, 3D electrospun nanofiber network composed of PVA-methacrylate (PVA-MA) and a composite of PVA-MA/chondroitin sulfate-methacrylate (CS-MA) (PVA-MA/CS-MA) were used for articular cartilage repair. After evaluation of the scaffolds for cartilage-like tissue formation in vitro, the constructs were implanted into rat osteochondral defects. Their results showed that nanoscaffolds with chondroitin sulfate (PVA-MA/CS-MA) supported chondrogenesis more than PVA-MA alone, judged by collagen type II and proteoglycan production in defects 43.
Biomolecules-loaded nanofibrous structures
Some investigators evaluated impregnation of nanofibrous scaffolds with various growth factors to promote repair of articular cartilage defects. For example, Maehara et al assessed efficacy of a bi-layer porous hydroxyapatite/collagen (HAp/collagen) nano-composite prepared by coprecipitation method and impregnated with FGF-2 for repairing the osteochondral defects in a rabbit model. Their results showed that HAp/collagen scaffolds impregnated with FGF-2 not only regenerate bone tissue but also resulted in development of satisfactory cartilage regeneration with a hyaline-like appearance. Their finding suggested that porous HAp/collagen with FGF-2 augmented the cartilage repair 78 .
Filova et al developed a novel drug delivery system using nano-composite for repair of osteochondral defects in miniature pigs. The fabricated cell-free composite scaffolds that contained PVA nanofibers enriched with liposomes, FGF-2, and insulin were subsequently embedded in a fibrin gel including hyaluronate/collagen type I. Interestingly, the cell-free composite scaffold improved migration of the BMMSCs into the defect and their differentiation into chondrocytes as compared with untreated group. As a result, composite scaffold containing nanofibers with liposomes functionalized with growth factors was able to enhance the regeneration of osteochondral defects towards hyaline cartilage and/or fibrocartilage compared with untreated defects that were filled predominantly with fibrous tissue 1 .
Conclusion
One key challenge in tissue engineering especially cartilage reconstruction is mimicking the architecture of ECM. At present, nanofibrous scaffolds irrespective of their method of synthesis are the most promising matrix to generate artificial ECM. These scaffolds are characterized by high surface area and enhanced porosity, which are highly desired for tissue engineering and drug delivery applications. There are four dominant methods to fabricate nanofibers for cartilage tissue engineering: electrospinning, molecular self-assembly, phase separation, and drawing 8,14.
Of these methodologies, electrospinning is the most common approach for cartilage tissue engineering since this technique offers great flexibility in terms of the choice of scaffold material and fiber diameter from the micrometer down to nanometer range. Electrospun polymeric fibrous meshes also present a higher surface area for cell attachment. Indeed, fabrication of electrospun nanofibers is easy, inexpensive and relatively reproducible 79,80. Due to difficulties in controlling porosity and pore size and architecture, three other techniques have been utilized less than electrospinning for cartilage tissue engineering purposes.
The availability of a wide range of natural and synthetic biomaterials has broadened the scope for development of nanofibrous scaffolds. Synthetic polymer-based systems offer additional advantages with their adjustable mechanical properties, as well as ease of surface modification via protein coatings, or conjugation of specific signaling molecules 81. The most common electrospun nanofibers designed for cartilage tissue engineering are made of poly (α-hydroxyesters) 82. Although the synthetic nanofibers prepared form these materials are capable to support chondrocyte proliferation and differentiation, some strategies have been applied to improve cell tendency of these materials that help us to achieve better results in future repair of cartilage defects. One applied strategy is hybridization of these synthetic materials by natural polymers like collagen 62. The combination of synthetic materials with natural polymers in nanofibers has resulted in better cell attachment, proliferation and chondrogenic development compared to synthetic polymers alone 64. Moreover, some in vivo studies implied better repair of cartilage defects by hybrid nanofibers compared to simple nanofiber composed of only synthetic polymers. Another strategy is modifying the surface of scaffolds through physical and chemical methods to improve the bioactivity of materials for cell adhesion and distribution.
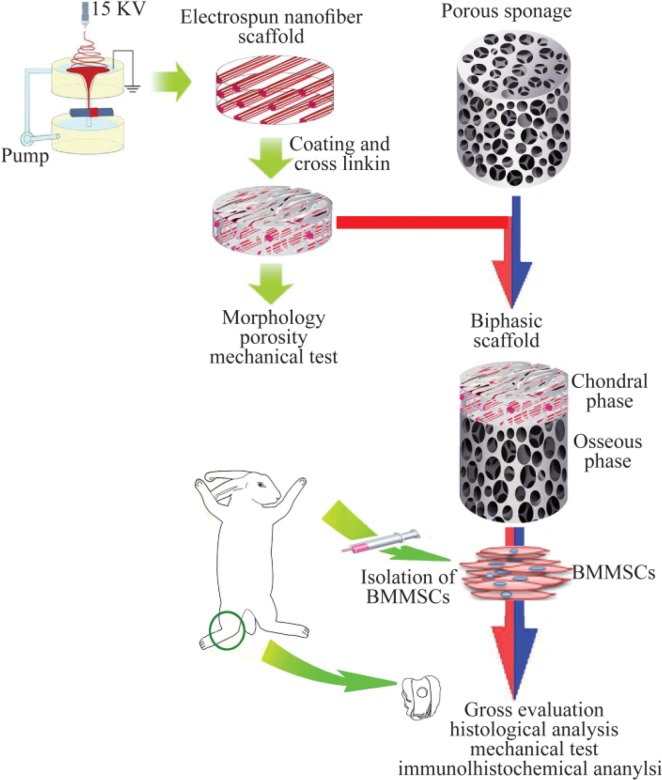
One approach to improve cell affinity is surface modification of the nanofibers by plasma treatment 83. Some others have improved the cell affinity of nanofibers by attaching Arg-Gly-Asp (RGD) peptides to the nanofibers surfaces. Indeed, these strategies play an important role in governing cellular responses and helping the scaffold to play a more efficient role as bioactive systems rather than just passive cell carriers. To prove this assumption, different in vitro and in vivo studies narrated that integration of fabrication techniques with surface modification methods has resulted in closer properties of nanofibrous scaffolds to native ECM, encouraged cell attachment and development into chondrocyte lineage. To repair the osteochondral defects that have two different structures, incorporation of stem cells with biphasic scaffolds containing hybrid nanofibers for chondral phase and porous sponge scaffolds for osseous phase seems to be a good strategy (Figure 3).
Figure 3.
A schematic model for in vivo study on repair of osteochondral defects using constructs composed of nanofibers and stem cells. The nanofiber is considered as the chondral phase. Porous sponage is used as the osseous phase. After combining with BMMSCs, biphasic complex was utilized to repair osteochondral defects in the animal model (Adopted from Liu et al 201456, with modification).
In spite of the great achievements behind the design of nanofibrous scaffolds, there is still plenty of room for improvement. Integration of nanofibers into micro-fabricated 3D scaffolds has resulted in obtaining more desirable scaffolds with providing larger pore sizes and improving cell differentiation and ECM production.
The future research on nanofibrous architecture may be focused on the new nanofabrication techniques. In combination with new nanofabrication technologies, nanofibrous scaffold could be decorated with nano-topographic patterns, such as ridges and grooves to better match the nanostructure of ECM achieving a better control of ECM-mimicry.
Based on this review, the efficiency of cell-seeded nanofibers in repair of cartilage defects is significantly more than the scaffold alone. It sounds that the seeded cells via secretion of growth factors and cytokines help sGAG production and mediate better situation to mimic ECM environment 8.
Two primarily considered criteria to determine the optimal source of cells for cartilage repair are the performance of the cells and their accessibility. Regarding performance, primary or low passage articular chondrocytes provide several advantages due to their high level of matrix synthesis and lack of hypertrophy. However, for larger defects, which require a larger number of cells, it is generally accepted that the dedifferentiation which occurs during monolayer expansion is a significant hurdle 84.
On the other hand, due to requirements of two-step intra-articular procedures for clinical use of autologous chondrocytes, one to harvest the cartilage and one to re-implant, many groups are attempting to develop allogenic sources of cells to be used in articular cartilage repair. Although cartilage is considered an immune privileged site, newer data indicate that chondrocytes have immunological properties that limit host immune reaction 85.
Following the search for immune privilege cell source that can readily provide large numbers of undifferentiated progenitors with chondrogenic potential, adult stem cells were introduced as interesting cells for tissue engineering and regenerative medicine purposes.
The most commonly used stem cells for cartilage tissue engineering especially in nanofibrous structures are the stem cells derived from bone marrow. It is due to the high chondrogenic differentiation ability and the availability of great knowledge about immunological characteristics and nature of this source of stem cells compared to other adult mesenchymal stem cells 5,86. However, due to some problems such as invasive techniques for sample collection and low availability, BMMSCs are introduced as not an ideal source and still some challenges for tissue engineering application exist. With introducing more available and accessible stem cell sources with similar immunological properties and great proliferation and trans-differentiation ability such as menstrual blood and adipose tissue stem cells, it is expected that these newer stem cells would be synchronized with nanofibers for future studies on cartilage tissue engineering.
Notably, besides improvement of nanofiber fabrication technique, utilization of other stem cell sources instead of BMMSCs and incorporation of nanofibers with differentiation promoting growth factors such as BMP-6 87 are future research priorities of cartilage reconstruction. In this manner, designing and applying suitable bioreactors that ultimately help in more ECM production and achievement of artificial constructs simulating native cartilage tissues, should not be ignored. In conclusion, although many experiments have been carried out to simulate native cartilage using nanofibers and stem cells with some promising reports about efficiency of these constructs for repair of cartilage defects in animal models, much joint effort by scientists from multiple disciplines is still required for transition of the data from in vitro to in vivo phase. To facilitate the future applicability of constructs composed of stem cells and nanofibers, a time frame is required for development of bench to bedside strategies.
References
- 1.Filová E, Rampichová M, Litvinec A, Držík M, Míčková A, Buzgo M, et al. A cell-free nanofiber composite scaffold regenerated osteochondral defects in miniature pigs. Inte J Pharm 2013;447(1–2):139–149. [DOI] [PubMed] [Google Scholar]
- 2.Zhang S, Chen L, Jiang Y, Cai Y, Xu G, Tong T, et al. Bi-layer collagen/microporous electrospun nanofiber scaffold improves the osteochondral regeneration. Acta Biomater 2013;9(7):7236–7247. [DOI] [PubMed] [Google Scholar]
- 3.Shafiee A, Soleimani M, Chamheidari GA, Seyedjafari E, Dodel M, Atashi A, et al. Electrospun nanofiber-based regeneration of cartilage enhanced by mesenchymal stem cells. J Biomed Mater Res A 2011;99(3):467–478. [DOI] [PubMed] [Google Scholar]
- 4.Chiang H, Jiang CC. Repair of articular cartilage defects: review and perspectives. J Formos Med Assoc 2009;108 (2):87–101. [DOI] [PubMed] [Google Scholar]
- 5.Koga H, Engebretsen L, Brinchmann JE, Muneta T, Sekiya I. Mesenchymal stem cell-based therapy for cartilage repair: a review. Knee Surg Sports Traumatol Arthrosc 2009;17(11):1289–1297. [DOI] [PubMed] [Google Scholar]
- 6.Chen H, Truckenmuller RK, Blitterswijk CA van, Moroni L. Fabrication of nanofibrous scaffolds for tissue engineering applications. In: Gaharwar AK, Sant S, Hancock MJ, Hacking AA. (Eds.). Nanomaterials in tissue engineering: Fabrication and applications. Series in Biomaterials (56). USA: Woodhead Publishing; 2013. P.158–182. [Google Scholar]
- 7.Coburn JM, Gibson M, Monagle S, Patterson Z, Elisseeff JH. Bioinspired nanofibers support chondrogenesis for articular cartilage repair. Proc Natl Acad Sci 2012;109 (25):10012–10017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang L, Hu J, Athanasiou KA. The role of tissue engineering in articular cartilage repair and regeneration. Crit Rev Biomed Eng 2009;37(1–2):1–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li WJ, Danielson KG, Alexander PG, Tuan RS. Biological response of chondrocytes cultured in three-dimensional nanofibrous poly(epsilon-caprolactone) scaffolds. J Biomed Mater Res A 2003;67(4):1105–1114. [DOI] [PubMed] [Google Scholar]
- 10.Li WJ, Laurencin CT, Caterson EJ, Tuan RS, Ko FK. Electrospun nanofibrous structure: a novel scaffold for tissue engineering. J Biomed Mater Res 2002;60(4):613–621. [DOI] [PubMed] [Google Scholar]
- 11.Schagemann J, Paul S, Casper M, Rohwedel J, Kramer J, Kaps C, et al. Chondrogenic differentiation of bone marrow-derived mesenchymal stromal cells via biomimetic and bioactive poly-ε-caprolactone scaffolds. J Biomed Mater Res A 2013;101(6):1620–1628. [DOI] [PubMed] [Google Scholar]
- 12.Wei G, Jin Q, Giannobile WV, Ma PX. The enhancement of osteogenesis by nano-fibrous scaffolds incorporating rhBMP-7 nanospheres. Biomaterials 2007;28(12):2087–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu J, Feng K, Liu X, Ma PX. Chondrogenic and osteogenic differentiations of human bone marrow-derived mesenchymal stem cells on a nanofibrous scaffold with designed pore network. Biomaterials 2009;30(28):5061–5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang L, Webster TJ. Nanotechnology and nano-materials: promises for improved tissue regeneration. Nano Today 2009;4(1):66–80. [Google Scholar]
- 15.Jannesari M, Varshosaz J, Morshed M, Zamani M. Composite poly (vinyl alcohol)/poly (vinyl acetate) electrospun nanofibrous mats as a novel wound dressing matrix for controlled release of drugs. Int J Nano-medicine 2011;6:993–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luong-Van E, Grøndahl L, Chua KN, Leong KW, Nurcombe V, Cool SM. Controlled release of heparin from poly(epsilon-caprolactone) electrospun fibers. Biomaterials 2006;27(9):2042–2050. [DOI] [PubMed] [Google Scholar]
- 17.James R, Toti US, Laurencin CT, Kumbar SG. Electrospun nanofibrous scaffolds for engineering soft connective tissues. Methods Mol Biol 2011;726:243–258. [DOI] [PubMed] [Google Scholar]
- 18.Kai D, Jin G, Prabhakaran MP, Ramakrishna S. Electrospun synthetic and natural nanofibers for regenerative medicine and stem cells. Biotechnol J 2013;8(1):59–72. [DOI] [PubMed] [Google Scholar]
- 19.Shuakat MN, Lin T. Recent developments in electrospinning of nanofiber yarns. J Nanosci Nanotechnol 2014;14 (2):1389–1408. [DOI] [PubMed] [Google Scholar]
- 20.Moghe A, Gupta B. Co-axial Electrospinning for Nanofiber Structures: Preparation and Applications. Polym Rev (Phila Pa) 2008;48(2):353–377. [Google Scholar]
- 21.Simonet M, Schneider OD, Neuenschwander P, Stark WJ. Ultraporous 3D polymer meshes by low-temperature electrospinning: use of ice crystals as a removable void template. Polym Eng Sci 2007;47(12):2020–2026. [Google Scholar]
- 22.Huang C, Niu H, Wu C, Ke Q, Mo X, Lin T. Disc-electrospun cellulose acetate butyrate nanofibers show enhanced cellular growth performances. J Biomed Mater Res A 2013;101(1):115–122. [DOI] [PubMed] [Google Scholar]
- 23.Li D, Wang Y, Xia Y. Electrospinning nanofibers as uniaxially aligned arrays and layer-by-layer stacked films. Adv Mater 2004;16(4):361–366. [Google Scholar]
- 24.Wang Y, Li H, Wang G, Yin T, Wang B, Yu Q. Electrospinning of polymer nanofibers with ordered patterns and architectures. J Nanosci Nanotechnol 2010;10(3): 1699–1706. [DOI] [PubMed] [Google Scholar]
- 25.Pham QP, Sharma U, Mikos AG. Electrospun poly (epsilon-caprolactone) microfiber and multilayer nanofiber/microfiber scaffolds: characterization of scaffolds and measurement of cellular infiltration. Biomacromolecules 2006;7(10):2796–2805. [DOI] [PubMed] [Google Scholar]
- 26.Gentsch R, Boysen B, Lankenau A, Borner HG. Single-step electrospinning of bimodal fiber meshes for ease of cellular infiltration. Macromol Rapid Commun 2010;31 (1):59–64. [DOI] [PubMed] [Google Scholar]
- 27.Tuzlakoglu K, Bolgen N, Salgado AJ, Gomes ME, Piskin E, Reis RL. Nano- and micro-fiber combined scaffolds: a new architecture for bone tissue engineering. J Mater Sci Mater Med 2005;16(12):1099–1104. [DOI] [PubMed] [Google Scholar]
- 28.Nam J, Huang Y, Agarwal S, Lannutti J. Improved cellular infiltration in electrospun fiber via engineered porosity. Tissue Eng 2007;13(9):2249–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wright L, Andric T, Freeman J. Utilizing NaCl to increase the porosity of electrospun materials. Mater Sci Eng: C 2011;31(1):30–36. [Google Scholar]
- 30.Baker BM, Gee AO, Metter RB, Nathan AS, Marklein RA, Burdick JA, et al. The potential to improve cell infiltration in composite fiber-aligned electrospun scaffolds by the selective removal of sacrificial fibers. Biomaterials 2008;29(15):2348–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chung HJ, Kim IK, Kim TG, Park TG. Highly open porous biodegradable microcarriers: in vitro cultivation of chondrocytes for injectable delivery. Tissue Eng Part A 2008;14(5):607–615. [DOI] [PubMed] [Google Scholar]
- 32.Bonino CA, Efimenko K, Jeong SI, Krebs MD, Alsberg E, Khan SA. Three-dimensional electrospun alginate nanofiber mats via tailored charge repulsions. Small 2012;8(12):1928–1936. [DOI] [PubMed] [Google Scholar]
- 33.Sundararaghavan HG, Metter RB, Burdick JA. Electrospun fibrous scaffolds with multiscale and photopatterned porosity. Macromol Biosci 2010;10(3):265–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Srouji S, Kizhner T, Suss-Tobi E, Livne E, Zussman E. 3-D Nanofibrous electrospun multilayered construct is an alternative ECM mimicking scaffold. J Mater Sci Mater Med 2008;19(3):1249–1255. [DOI] [PubMed] [Google Scholar]
- 35.He B, Yuan X, Zhou A, Zhang H, Jiang D. Designer functionalised self-assembling peptide nanofibre scaffolds for cartilage tissue engineering. Expert Rev Mol Med 2014;16:e12. [DOI] [PubMed] [Google Scholar]
- 36.Nayak R, Padhye R, Kyratzis IL, Truong YB, Arnold L. Effect of viscosity and electrical conductivity on the morphology and fiber diameter in melt electrospinning of polypropylene. Textile Research Institute 2013;83(6): 606–617. [Google Scholar]
- 37.Kisiday J, Jin M, Kurz B, Hung H, Semino C, Zhang S, et al. Self-assembling peptide hydrogel fosters chondrocyte extracellular matrix production and cell division: implications for cartilage tissue repair. Proc Natl Acad Sci USA 2002;99(15):9996–10001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu J, Song H, Zhang L, Xu H, Zhao X. Self-assembly-peptide hydrogels as tissue-engineering scaffolds for three-dimensional culture of chondrocytes in vitro. Macromol Biosci 2010;10(10):1164–1170. [DOI] [PubMed] [Google Scholar]
- 39.Jayawarna V, Richardson SM, Hirst AR, Hodson NW, Saiani A, Gough JE, et al. Introducing chemical functionality in Fmoc-peptide gels for cell culture. Acta Biomater 2009;5(3):934–943. [DOI] [PubMed] [Google Scholar]
- 40.Vasita R, Katti DS. Nanofibers and their applications in tissue engineering. Int J Nanomedicine 2006;1(1):15–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Asmatulu R, Yildirim MB, Khan WS, Adeniji A, Wamocha HL. Nanofiber fabrication and characterization for the engineering education. Proceedings of the 2007 ASEE Midwest Regional Conference; 2007 Sept 19–21; Wichita, KS 9 p. [Google Scholar]
- 42.Ma PX, Choi JW. Biodegradable polymer scaffolds with well-defined interconnected spherical pore network. Tissue Eng 2001;7(1):23–33. [DOI] [PubMed] [Google Scholar]
- 43.Athanasiou KA, Darling EM, Hu JC. Articular cartilage tissue engineering. Synthesis Lectures on Tissue Eng 2009;1(1):1–182. [Google Scholar]
- 44.Darling EM, Athanasiou KA. Rapid phenotypic changes in passaged articular chondrocyte subpopulations. J Orthop Res 2005;23(2):425–432. [DOI] [PubMed] [Google Scholar]
- 45.Yu H, Grynpas M, Kandel RA. Composition of cartilagenous tissue with mineralized and non-mineralized zones formed in vitro. Biomaterials 1997;18(21):1425–1431. [DOI] [PubMed] [Google Scholar]
- 46.Aydelotte MB, Kuettner KE. Differences between subpopulations of cultured bovine articular chondrocytes. I. Morphology and cartilage matrix production. Connect Tissue Res 1988;18(3):205–222. [DOI] [PubMed] [Google Scholar]
- 47.Shafiee A, Kabiri M, Langroudi L, Soleimani M, Ai J. Evaluation and comparison of the in vitro characteristics and chondrogenic capacity of four adult stem/progenitor cells for cartilage cell-based repair. J Biomed Mater Res A 2015. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 48.Dahl JP, Caballero M, Pappa AK, Madan G, Shockley WW, van Aalst JA. Analysis of human auricular cartilage to guide tissue-engineered nanofiber-based chondrogenesis implications for microtia reconstruction. Otolaryngology Head Neck Surg 2011;145(6):915–923. [DOI] [PubMed] [Google Scholar]
- 49.Khanmohammadi M, Khanjani S, Bakhtyari MS, Zarnani AH, Edalatkhah H, Akhondi MM, et al. Proliferation and chondrogenic differentiation potential of menstrual blood-and bone marrow-derived stem cells in two-dimensional culture. Int J Hematol 2012;95(5):484–493. [DOI] [PubMed] [Google Scholar]
- 50.Hsu SH, Huang TB, Cheng SJ, Weng SY, Tsai CL, Tseng CS, et al. Chondrogenesis from human placenta-derived mesenchymal stem cells in three-dimensional scaffolds for cartilage tissue engineering. Tissue Eng Part A 2011;17(11–12):1549–1560. [DOI] [PubMed] [Google Scholar]
- 51.Caplan AI. Mesenchymal stem cells. J Orthop Res 1991; 9(5):641–650. [DOI] [PubMed] [Google Scholar]
- 52.Väänänen HK. Mesenchymal stem cells. Ann Med 2005; 37(7):469–479. [DOI] [PubMed] [Google Scholar]
- 53.Nerurkar NL, Baker BM, Sen S, Wible EE, Elliott DM, Mauck RL. Nanofibrous biologic laminates replicate the form and function of the annulus fibrosus. Nat Mater 2009;8(12):986–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baker BM, Mauck RL. The effect of nanofiber alignment on the maturation of engineered meniscus constructs. Biomaterials 2007;28(11):1967–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li WJ, Tuli R, Okafor C, Derfoul A, Danielson KG, Hall DJ, et al. A three-dimensional nanofibrous scaffold for cartilage tissue engineering using human mesenchymal stem cells. Biomaterials 2005;26(6):599–609. [DOI] [PubMed] [Google Scholar]
- 56.Kazemnejad S, Akhondi MM, Soleimani M, Zarnani AH, Khanmohammadi M, Darzi S, et al. Characterization and chondrogenic differentiation of menstrual blood-derived stem cells on a nanofibrous scaffold. Int J Artif Organs 2012;35(1):55–66. [DOI] [PubMed] [Google Scholar]
- 57.Kazemnejad S, Zarnani AH, Khanmohammadi M, Mobini S. Chondrogenic differentiation of menstrual blood-derived stem cells on nanofibrous scaffolds. Methods Mol Biol 2013;1058:149–169. [DOI] [PubMed] [Google Scholar]
- 58.Wise JK, Yarin AL, Megaridis CM, Cho M. Chondrogenic differentiation of human mesenchymal stem cells on oriented nanofibrous scaffolds: engineering the superficial zone of articular cartilage. Tissue Eng Part A 2008; 15(4):913–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alves da Silva ML, Martins A, Costa-Pinto A, Costa P, Faria S, Gomes M, et al. Cartilage tissue engineering using electrospun PCL nanofiber meshes and MSCs. Biomacromolecules 2010;11(12):3228–3236. [DOI] [PubMed] [Google Scholar]
- 60.Xin X, Hussain M, Mao JJ. Continuing differentiation of human mesenchymal stem cells and induced chondrogenic and osteogenic lineages in electrospun PLGA nanofiber scaffold. Biomaterials 2007;28(2):316–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sonomoto K, Yamaoka K, Kaneko H, Yamagata K, Sakata K, Zhang X, et al. Spontaneous differentiation of human mesenchymal stem cells on poly-lactic-co-glycolic acid nano-fiber scaffold. PloS one 2016;11(4): e0153231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ahmed M, Ramos TA, Damanik F, Quang Le B, Wieringa P, Bennink M, et al. A combinatorial approach towards the design of nanofibrous scaffolds for chondrogenesis. Sci Rep 2015;5:14804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ching K, Andriotis OG, Li S, Basnett P, Su B, Roy I, et al. Nanofibrous poly (3-hydroxybutyrate)/poly (3-hydroxyoctanoate) scaffolds provide a functional microenvironment for cartilage repair. J Biomater Appl 2016;31 (1):77–91. [DOI] [PubMed] [Google Scholar]
- 64.Li Z, Liu P, Yang T, Sun Y, You Q, Li J, et al. Composite poly (l-lactic-acid)/silk fibroin scaffold prepared by electrospinning promotes chondrogenesis for cartilage tissue engineering. J Biomater Appl 2016;30(10):1552–1565. [DOI] [PubMed] [Google Scholar]
- 65.Baker BM, Nathan AS, Gee AO, Mauck RL. The influence of an aligned nanofibrous topography on human mesenchymal stem cell fibrochondrogenesis. Biomaterials 2010;31(24):6190–6200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shafiee A, Seyedjafari E, Taherzadeh ES, Dinarvand P, Soleimani M, Ai J. Enhanced chondrogenesis of human nasal septum derived progenitors on nanofibrous scaffolds. Mater Sci Eng C Mater Biol Appl 2014;40:445–454. [DOI] [PubMed] [Google Scholar]
- 67.Pham QP, Sharma U, Mikos AG. Electrospun poly(epsilon-caprolactone) microfiber and multilayer nanofiber/microfiber scaffolds: characterization of scaffolds and measurement of cellular infiltration. Biomacromolecules 2006;7(10):2796–2805. [DOI] [PubMed] [Google Scholar]
- 68.Shalumon K, Binulal N, Deepthy M, Jayakumar R, Manzoor K, Nair S. Preparation, characterization and cell attachment studies of electrospun multi-scale poly (caprolactone) fibrous scaffolds for tissue engineering. J Macromolecular Sci Part A 2010;48(1):21–30. [Google Scholar]
- 69.Srinivasan S, Jayakumar R, Chennazhi K, Levorson EJ, Mikos AG, Nair SV. Multiscale fibrous scaffolds in regenerative medicine. Biomed Appl Polym Nanofibers 2012;246:1–20. [Google Scholar]
- 70.Levorson EJ, Raman Sreerekha P, Chennazhi KP, Kasper FK, Nair SV, Mikos AG. Fabrication and characterization of multiscale electrospun scaffolds for cartilage regeneration. Biomed Mater 2013;8(1):014103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang Z, Gupte MJ, Jin X, Ma PX. Injectable peptide decorated functional nanofibrous hollow microspheres to direct stem cell differentiation and tissue regeneration. Adv Funct Mater 2015;25(3):350–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li WJ, Chiang H, Kuo TF, Lee HS, Jiang CC, Tuan RS. Evaluation of articular cartilage repair using biodegradable nanofibrous scaffolds in a swine model: a pilot study. J Tissue Eng Regen Med 2009;3(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Casper ME, Fitzsimmons JS, Stone JJ, Meza AO, Huang Y, Ruesink TJ, et al. Tissue engineering of cartilage using poly-epsilon-caprolactone nanofiber scaffolds seeded in vivo with periosteal cells. Osteoarthritis Cartilage 2010;18(7):981–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu J, Nie H, Xu Z, Niu X, Guo S, Yin J, et al. The effect of 3D nanofibrous scaffolds on the chondrogenesis of induced pluripotent stem cells and their application in restoration of cartilage defects. PLoS One 2014;9(11): e111566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.He X, Fu W, Feng B, Wang H, Liu Z, Yin M, et al. Electrospun collagen/poly (L-lactic acid-co-ε-caprolactone) hybrid nanofibrous membranes combining with sandwich construction model for cartilage tissue engineering. J Nanosci Nanotechnol 2013;13(6):3818–3825. [DOI] [PubMed] [Google Scholar]
- 76.Liu S, Wu J, Liu X, Chen D, Bowlin GL, Cao L, et al. Osteochondral regeneration using an oriented nanofiber yarn-collagen type I/hyaluronate hybrid/TCP biphasic scaffold. J Biomed Mater Res A 2015;103(2):581–592. [DOI] [PubMed] [Google Scholar]
- 77.Xue D, Zheng Q, Zong C, Li Q, Li H, Qian S, et al. Osteochondral repair using porous poly (lactide-co-glycolide)/nano-hydroxyapatite hybrid scaffolds with undifferentiated mesenchymal stem cells in a rat model. J Biomed Mater Res A 2010;94(1):259–270. [DOI] [PubMed] [Google Scholar]
- 78.Maehara H, Sotome S, Yoshii T, Torigoe I, Kawasaki Y, Sugata Y, et al. Repair of large osteochondral defects in rabbits using porous hydroxyapatite/collagen (HAp/Col) and fibroblast growth factor-2 (FGF-2). J Orthop Res 2010;28(5):677–686. [DOI] [PubMed] [Google Scholar]
- 79.Lim SH, Mao HQ. Electrospun scaffolds for stem cell engineering. Adv Drug Deliv Rev 2009;61(12):1084–1096. [DOI] [PubMed] [Google Scholar]
- 80.Sill TJ, von Recum HA. Electrospinning: applications in drug delivery and tissue engineering. Biomaterials 2008; 29(13):1989–2006. [DOI] [PubMed] [Google Scholar]
- 81.Dahlin RL, Kasper FK, Mikos AG. Polymeric nanofibers in tissue engineering. Tissue Eng Part B Rev 2011;17 (5):349–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li WJ, Cooper JA, Mauck RL, Tuan RS. Fabrication and characterization of six electrospun poly (α-hydroxy ester)-based fibrous scaffolds for tissue engineering applications. Acta Biomater 2006;2(4):377–385. [DOI] [PubMed] [Google Scholar]
- 83.Arasteh S, Kazemnejad S, Khanjani S, Heidari-Vala H, Akhondi MM, Mobini S. Fabrication and characterization of nano-fibrous bilayer composite for skin regeneration application. Methods 2016;99:3–12. [DOI] [PubMed] [Google Scholar]
- 84.Johnstone B, Alini M, Cucchiarini M, Dodge GR, Eglin D, Guilak F, et al. Tissue engineering for articular cartilage repair--the state of the art. Eur Cell Mater 2013; 25:248–267. [DOI] [PubMed] [Google Scholar]
- 85.Adkisson HD, Milliman C, Zhang X, Mauch K, Maziarz RT, Streeter PR. Immune evasion by neocartilage-derived chondrocytes: Implications for biologic repair of joint articular cartilage. Stem Cell Res 2010;4(1):57–68. [DOI] [PubMed] [Google Scholar]
- 86.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood 2007;110(10):3499–3506. [DOI] [PubMed] [Google Scholar]
- 87.Estes BT, Diekman BO, Gimble JM, Guilak F. Isolation of adipose-derived stem cells and their induction to a chondrogenic phenotype. Nat Protoc 2010;5(7):1294–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li WJ, Jiang YJ, Tuan RS. Cell-nanofiber-based cartilage tissue engineering using improved cell seeding, growth factor, and bioreactor technologies. Tissue Eng Part A 2008;14(5):639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Thorvaldsson A, Stenhamre H, Gatenholm P, Walkenström P. Electrospinning of highly porous scaffolds for cartilage regeneration. Biomacromolecules 2008;9(3): 1044–1049. [DOI] [PubMed] [Google Scholar]
- 90.Shin HJ, Lee CH, Cho IH, Kim YJ, Lee YJ, Kim IA, et al. Electrospun PLGA nanofiber scaffolds for articular cartilage reconstruction: mechanical stability, degradation and cellular responses under mechanical stimulation in vitro. J Biomater Sci Polym Ed 2006;17(1–2): 103–119. [DOI] [PubMed] [Google Scholar]
- 91.Zheng X, Yang F, Wang S, Lu S, Zhang W, Liu S, et al. Fabrication and cell affinity of biomimetic structured PLGA/articular cartilage ECM composite scaffold. J Mater Sci Mater Med 2011;22(3):693–704. [DOI] [PubMed] [Google Scholar]
- 92.Bean AC, Tuan RS. Fiber diameter and seeding density influence chondrogenic differentiation of mesenchymal stem cells seeded on electrospun poly (ε-caprolactone) scaffolds. Biomed Mater 2015;10(1):015018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Garrigues NW, Little D, Sanchez-Adams J, Ruch DS, Guilak F. Electrospun cartilage-derived matrix scaffolds for cartilage tissue engineering. J Biomed Mater Res A 2014;102(11):3998–4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fong CY, Subramanian A, Gauthaman K, Venugopal J, Biswas A, Ramakrishna S, et al. Human umbilical cord Wharton’s jelly stem cells undergo enhanced chondrogenic differentiation when grown on nanofibrous scaffolds and in a sequential two-stage culture medium environment. Stem Cell Rev 2012;8(1):195–209. [DOI] [PubMed] [Google Scholar]
- 95.Lee P, Tran K, Chang W, Shelke NB, Kumbar SG, Yu X. Influence of chondroitin sulfate and hyaluronic acid presence in nanofibers and its alignment on the bone marrow stromal cells: Cartilage regeneration. J Biomed Nanotechnol 2014;10(8):1469–1479. [DOI] [PubMed] [Google Scholar]
- 96.Shanmugasundaram S, Chaudhry H, Arinzeh TL. Micro-scale versus nanoscale scaffold architecture for mesenchymal stem cell chondrogenesis. Tissue Eng Part A 2011;17(5–6):831–840. [DOI] [PubMed] [Google Scholar]
- 97.Nam J, Johnson J, Lannutti JJ, Agarwal S. Modulation of embryonic mesenchymal progenitor cell differentiation via control over pure mechanical modulus in electrospun nanofibers. Acta Biomater 2011;7(4):1516–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Janjanin S, Li WJ, Morgan MT, Shanti RM, Tuan RS. Mold-shaped, nanofiber scaffold-based cartilage engineering using human mesenchymal stem cells and bioreactor. J Surg Res 2008;149(1):47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li WJ, Tuli R, Huang X, Laquerriere P, Tuan RS. Multilineage differentiation of human mesenchymal stem cells in a three-dimensional nanofibrous scaffold. Biomaterials 2005;26(25):5158–5166. [DOI] [PubMed] [Google Scholar]
- 100.Stenhamre H, Thorvaldsson A, Enochson L, Walkenström P, Lindahl A, Brittberg M, et al. Nanosized fibers’ effect on adult human articular chondrocytes behavior. Mater Sci Eng C Mater Biol Appl 2013;33(3):1539–1545. [DOI] [PubMed] [Google Scholar]
- 101.Wright LD, McKeon-Fischer KD, Cui Z, Nair LS, Freeman JW. PDLA/PLLA and PDLA/PCL nanofibers with a chitosan-based hydrogel in composite scaffolds for tissue engineered cartilage. J Tissue Eng Regen Med 2014;8(12):946–954. [DOI] [PubMed] [Google Scholar]
- 102.Chen JP, Su CH. Surface modification of electrospun PLLA nanofibers by plasma treatment and cationized gelatin immobilization for cartilage tissue engineering. Acta Biomater 2011;7(1):234–243. [DOI] [PubMed] [Google Scholar]
- 103.Richardson SM, Curran JM, Chen R, Vaughan-Thomas A, Hunt JA, Freemont AJ, et al. The differentiation of bone marrow mesenchymal stem cells into chondrocyte-like cells on poly-L-lactic acid (PLLA) scaffolds. Bio-materials 2006;27(22):4069–4078. [DOI] [PubMed] [Google Scholar]