Abstract
Background
The enzymatic degradation of quorums sensing (QS) molecules (called quorum quenching, QQ) has been considered as a promising anti-virulence therapy to treat biofilm-related infections and combat antibiotic resistance. The recently-discovered QQ enzyme MomL has been reported to efficiently degrade different N-acyl homoserine lactones (AHLs) of various Gram-negative pathogens. Here we investigated the effect of MomL on biofilms formed by two important nosocomial pathogens, Pseudomonas aeruginosa and Acinetobacter baumannii.
Methods
MomL was expressed in E.coli BL21 and purified. The activity of MomL on AHLs with hydroxyl substituent was tested. Biofilms of P. aeruginosa PAO1 and Acinetobacter strains were formed in 96-well microtiter plates. Biofilm formation was evaluated by crystal violet staining, plating and fluorescence microscopy. The effect of MomL on biofilm susceptibility to antibiotics was also tested. We further evaluated MomL in dual-species biofilms formed by P. aeruginosa and A. baumannii, and in biofilms formed in a wound model. The effect of MomL on virulence of A. baumannii was also tested in the Caenorhabditis elegans model.
Results
MomL reduced biofilm formation and increased biofilm susceptibility to different antibiotics in biofilms of P. aeruginosa PAO1 and A. baumannii LMG 10531 formed in microtiter plates in vitro. However, no significant differences were detected in the dual-species biofilm and in wound model biofilms. In addition, MomL did not affect virulence of A. baumannii in the C. elegans model. Finally, the effect of MomL on biofilm of Acinetobacter strains seems to be strain-dependent.
Discussion
Our results indicate that although MomL showed a promising anti-biofilm effect against P. aeruginosa and A. baumanii biofilms formed in microtiter plates, the effect on biofilm formation under conditions more likely to mimic the real-life situation was much less pronounced or even absent. Our data indicate that in order to obtain a better picture of potential applicability of QQ enzymes for the treatment of biofilm-related infections, more elaborate model systems need to be used.
Keywords: Quorum quenching, Biofilm, MomL, Pseudomonas aeruginosa, Acinetobacter baumannii, Quorum sensing inhibition, Quorum sensing
Introduction
Quorum sensing (QS) is a widespread communication process that allows bacteria to coordinate their group behavior based on the production, detection and response to extracellular signal molecules (Bassler & Losick, 2006; Williams et al., 2007). QS regulates gene expression related to biofilm formation, motility and production of virulence factors in many Gram-negative and Gram-positive pathogens, and interfering with QS has been intensively studied as a promising anti-virulence therapy to combat bacterial infections and antibiotic resistance (Hentzer & Givskov, 2003; LaSarre & Federle, 2013; Rutherford & Bassler, 2012). Many natural and synthetic compounds have been found to inhibit QS, and several quorum quenching (QQ) enzymes mainly targeting N-acyl homoserine lactone (AHL) based QS in Gram-negative bacteria have been described as well (Brackman & Coenye, 2015; Fetzner, 2015; Rasmussen & Givskov, 2006; Tang & Zhang, 2014). Some of these QS inhibitors (QSIs) and QQ enzymes have shown promising anti-virulence effects both in vitro and in vivo. For instance, furanones which resemble AHLs and are able to bind to QS receptors have been reported to reduce biofilm formation and enhance bacterial clearance in Pseudomonas aeruginosa lung infection in mice (Hentzer et al., 2002; Wu et al., 2004). Baicalin hydrate and cinnamaldehyde (QSIs targeting AHL-based QS in P. aeruginosa and Burkholderia cepacia complex) as well as hamamelitannin (a QSI targeting the peptide-based QS system present in Staphylococcus aureus) increase biofilm susceptibility to antibiotics and survival of infected Galleria mellonella larvae and Caenorhabditis elegans, as well as decrease the microbial load in a mouse pulmonary infection model (Brackman et al., 2011). As for QQ enzymes, an AiiM-producing P. aeruginosa mutant showed reduced lung injury and increased survival in an in vivo study on mice with pneumonia (Migiyama et al., 2013), and an inhaled lactonase SsoPox-I was also reported to reduce virulence of P. aeruginosa and mortality in rat pneumonia (Hraiech et al., 2014).
Previously MomL, a novel AHL lactonase belonging to the metallo-β-lactamase superfamily was identified and characterized (Tang et al., 2015). It has high degrading activities towards short- and long-chain AHLs with or without an oxo-group at the C-3 position (Tang et al., 2015). MomL can reduce pyocyanin and protease production by P. aeruginosa and attenuated the virulence of P. aeruginosa in a C. elegans infection model (Tang et al., 2015), but its effect on biofilm formation of P. aeruginosa and other Gram-negative pathogens was not tested yet.
Besides P. aeruginosa, Acinetobacter baumannii has also been recognized as an increasingly prevalent Gram-negative opportunistic pathogen responsible for severe nosocomial infections (Gonzalez-Villoria & Valverde-Garduno, 2016; Peleg, Seifert & Paterson, 2008). Resistance of P. aeruginosa and A. baumannii strains to multiple antibiotic classes complicates the treatment for these infections and poses considerable therapeutic challenges worldwide (Potron, Poirel & Nordmann, 2015). One of the main factors contributing to their reduced antibiotic susceptibility and to treatment failure is biofilm formation both on tissues and abiotic surfaces (Donlan & Costerton, 2002; Hall-Stoodley, Costerton & Stoodley, 2004; Longo, Vuotto & Donelli, 2014). Biofilms of both P. aeruginosa and A. baumannii are known to be regulated by AHL-based QS. In P. aeruginosa, N-(3-oxododecanoyl)-L-homoserine lactone (3-oxo-C12-HSL) and N-butyryl-L-homoserine lactone (C4-HSL) are used by the Las and Rhl QS system, respectively (Pesci et al., 1997), and these AHLs can both be degraded by MomL (Tang et al., 2015). One AHL synthase belonging to LuxI family, AbaI, has been reported to catalyze the synthesis of N-(3-hydroxydodecanoyl)-L-HSL (3-OH-C12-HSL) in Acinetobacter nosocomialis M2 (Niu et al., 2008), but other AHLs with varied chain lengths and substituents are also found in Acinetobacter strains (Bhargava, Sharma & Capalash, 2010; González et al., 2009). The biofilm-forming ability of an abaI mutant was reduced by around 40% compared to the corresponding wildtype strain (Niu et al., 2008). Compared to the extensive literature on inhibiting QS pathways and virulence in P. aeruginosa (Aybey & Demirkan, 2016; Furiga et al., 2016; Hentzer et al., 2003; O’Loughlin et al., 2013; Yin et al., 2015), there are fewer reports on inhibiting QS and biofilm formation in A. baumannii (Bhargava et al., 2015; Chow et al., 2014; Saroj & Rather, 2013).
In the present study, we tested the anti-biofilm activity of MomL against P. aeruginosa and A. baumannii, and further evaluated the effect of MomL under more complex conditions such as in dual-species biofilm and in a wound model system with the aim to obtain a better knowledge base regarding the possible development of QQ enzymes as anti-virulence therapy.
Material & Methods
Bacterial strains, culture conditions and chemicals
P. aeruginosa PAO1, A. calcoaceticus LMG 10517, A. nosocomialis M2 and A. baumannii LMG 10520, LMG 10531 and AB5075 were cultured on tryptic soy agar (TSA) or in Mueller-Hinton broth (MH) at 37 °C. Escherichia coli BL21(DE3) harboring MomL expression plasmid pET24a(+)-momL-(−SP) (Tang et al., 2015) was cultured on Luria-Bertani (LB) agar supplemented with kanamycin (50 μg/mL) at 37 °C. The AHL biosensor Agrobacterium tumefaciens A136 (pCF218) (pCF372) (Zhu et al., 1998) was maintained on LB agar supplemented with spectinomycin (50 μg/mL) and tetracycline (4.5 μg/mL), and grown in AT minimal medium (Tempé et al., 1977) containing 0.5% (wt/vol) glucose for detecting AHLs in the liquid X-Gal (5-bromo-4-chloro-3-indolyl- β-D-galactopyranoside) assay. 3-OH-C12-HSL was purchased from Sigma-Aldrich and dissolved in dimethyl sulfoxide (DMSO) as stock solution (100 mM). Caenorhabditis elegans N2 (glp-4; sek-1) was propagated under standard conditions, synchronized by hypochlorite bleaching, and cultured on nematode growth medium using E. coli OP50 as a food source (Stiernagle, 1999).
MomL expression and purification
MomL was expressed and purified according to Tang et al. (2015). In brief, protein expression was induced by 0.5 mM IPTG (isopropyl- β-D-thiogalactopyranoside) when E. coli cells in LB reaching an optical density at 600 nm (OD600) of 0.5–0.7. The induction was carried out at 16 °C with moderate shaking (150 rpm) for 12 h. Cells were harvested and sonicated, and the obtained supernatant was loaded on NTA-Ni (Qiagen, Hilden, Germany) columns for purification according to the manufacturer’s instruction. Desalting of the protein solution was accomplished by Amicon Ultra-15 centrifugal filter devices, and the purified MomL was stored at −20 °C in Tris–HCl buffer (50 mM, pH 6.5) with 25% glycerol.
Detection for degradation of 3-OH-C12-HSL
The amount of 3-OH-C12-HSL was quantified using A. tumefaciens A136 liquid X-gal assay and expressed as the normalized β-galactosidase activity as previously described (Tang et al., 2013). The correction factor a and b were obtained and calculated for our experimental conditions, and the final formula to calculate the normalized β-galactosidase activity is . To test the degradation of 3-OH-C12-HSL by MomL, 3-OH-C12-HSL (10 μM) was mixed with MomL in different concentrations (0.05–5 μg/mL) and incubated at 37 °C for 1 h. No MomL was added to the control. Afterwards the residual 3-OH-C12-HSL was quantified by adding 10 μL solution to the A136 biosensor, as described previously (Tang et al., 2013).
Biofilm formation assays
Overnight cultures of P. aeruginosa and Acinetobacter strains in MH broth were diluted to contain approximately 5 × 107 CFU/mL. 90 μL of this diluted bacterial suspension was transferred to the wells of a round-bottomed 96-well microtiter plate. Uninoculated MH broth served as blank control. To test the effect of MomL on biofilms, 10 μL purified enzyme (in different concentrations) was added to the wells, while 10 μL Tris–HCl buffer (50 mM, pH 6.5) with 25% glycerol was added to the control. The plate was incubated at 37 °C for 4 h before the supernatant was removed. The wells were rinsed once with sterile physiological saline (PS) and re-filled with fresh medium (90 μL) and MomL (10 μL). The plate was incubated at 37 °C for an additional 20 h. The biofilm biomass was quantified by crystal violet (CV) staining as described previously (Peeters, Nelis & Coenye, 2008). After rinsing the wells with sterile PS, the biofilm was fixed with 100 μL 99% methanol for 15 min and stained with 100 μL 0.1% CV for 20 min. The excess CV was removed by washing the plates under running tap water and bound CV was released by adding 150 μl of 33% acetic acid. The absorbance was measured at 590 nm.
Biofilm susceptibility assays
After a 24 h-biofilm of P. aeruginosa or A. baumannii strains was formed as described above either in presence of MomL or not, the plate was emptied and biofilm cells were rinsed with sterile PS. Antibiotics were dissolved in PS and 90 μL of these solutions were added to treat the biofilm for another 24 h, either with or without 10 μl MomL. Tobramycin (TOB; 4 μg/mL as final concentration), ciprofloxacin (CIP; 0.5 μg/mL), meropenem (MEM; 16 μg/mL) and colistin (CST; 16 μg/mL) were used to treat the biofilm of P. aeruginosa PAO1; TOB (6 μg/mL), CIP (4 μg/mL), MEM (8 μg/mL) and CST (16 μg/mL) were used to treat the biofilm of the A. baumannii strains. The supernatant was removed and the wells were washed once with sterile PS. To release bacterial cells from biofilm, two cycles of vortex (5 min) and sonication (5 min) were performed, and the number of CFU/biofilm was determined by plating the resulting suspensions on TSA.
Fluorescence microscopy
Biofilms of P. aeruginosa PAO1 or A. baumannii strains were formed in the absence or presence of MomL and treated with antibiotics as described above using a flat-bottomed 96-well microtiter plates. A total of 3 μL SYTO9 and 3 μL propidium iodide were diluted to 1mL in sterile PS, and 100 μL of this staining solution was transferred to each well. The plate was incubated for 15 min at room temperature and fluorescence microscopy was performed with EVOS FL Auto Imaging System (Life Technologies, Carlsbad, CA, USA). The red fluorescent signal was detected with 531/40 nm excitation filter cube and 593/40 nm emission filter cube and the green fluorescent signal was detected with 470/22 nm excitation filter cube and 510/42 nm emission filter cube.
Dual-species biofilm formation
Overnight cultures of P. aeruginosa and A. baumannii strains in MH broth were diluted to contain approximately 5 × 105 CFU/mL and 5 × 107 CFU/mL, respectively, and equal volume of suspensions of P. aeruginosa and A. baumannii were mixed. MomL (200 μg/mL) and tobramycin (6 μg/mL) were added as described above. To quantify CFU in the dual-species biofilm, Pseudomonas Isolation Agar (Difco) and TSA supplemented with 5 μg/mL cefsulodin were used as selective media for P. aeruginosa and A. baumannii respectively.
Biofilm formation in wound model
Artificial dermis composed of hyaluronic acid and collagen was used in our wound model, as described before (Brackman et al., 2016). Each disk of artificial dermis was placed in 24-well microtiter plate. One mL medium containing Bolton Broth, heparinized bovine plasma and freeze-thaw laked horse blood cells was added on and around the dermis. Suspensions (10 μL) of P. aeruginosa or A. baumannii containing 104 bacterial cells were added on the top of dermis. Final concentrations of MomL added were 200 μg/mL and 10 μg/mL for P. aeruginosa and A. baumannii, respectively. Tobramycin (10 μg/mL) was added after 8 h incubation at 37 °C. After 24 h, the infected dermis was washed with 1 mL PS and was transferred into 10 ml PS. Biofilm cells on the dermis were loosen and collected by three cycles of vortex (30 s) and sonication (30 s). The number of CFU/dermis was quantified by standard plating techniques.
C. elegans survival assay
The C. elegans survival assay was carried out as described before with minor modification (Brackman et al., 2011). Synchronized worms (L4 stage) were suspended in medium containing 95% M9 buffer (3 g of KH2PO4, 6 g of Na2HPO4, 5 g of NaCl, and 1 ml of 1 M MgSO4⋅7H2O in 1 l of water) and 5% brain heart infusion broth (Oxoid), and 25 μL of this nematode suspension was transferred to the wells of a 96-well microtiter plate. Overnight culture of A. baumannii was suspended in the assay medium and added in a final concentration of 2.5 × 107 CFU/ml. MomL was added in a final concentration of 10 μg/mL. The plates were incubated at 25 °C for 24 h. The fraction of dead worms was determined by counting the number of dead worms and the total number of worms in each well.
Statistics
The normal distribution of the data was checked by the D’Agostino–Pearson normality test. Normally distributed data were analyzed by one-way ANOVA, and non-normally distributed data were analyzed by the Kruskal–Wallis test or the Mann–Whitney test. All statistical analyses were carried out using GraphPad Prism 6.0.
Results
Degradation of 3-OH-C12-HSL by purified MomL
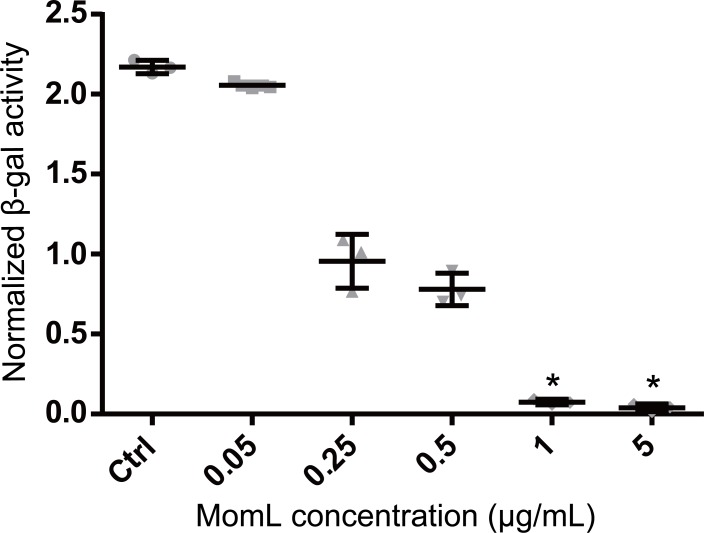
MomL was produced in E. coli and subsequently successfully purified (Fig. 1). Although MomL had been shown to degrade various AHLs (Tang et al., 2015), its activity on AHLs with hydroxyl substituent at the C3 position was not tested yet. We could demonstrate that MomL, in a concentration of 1 μg/mL or higher, can degrade almost all 3-OH-C12-HSLs (10 μM) under the experimental conditions used in the present study (Fig. 2).
Figure 1. SDS-PAGE of purified MomL.
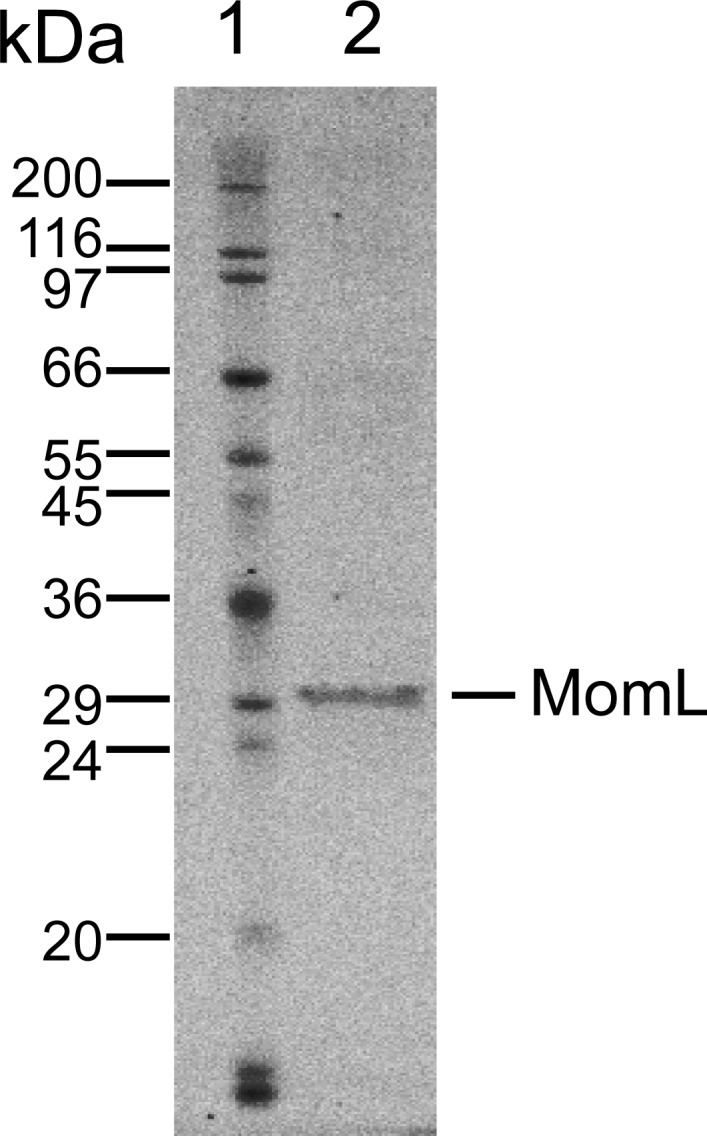
Lane1, molecular mass markers; Lane 2, purified recombinant MomL with molecular mass of nearly 31 kDa.
Figure 2. Degradation of 3OH-C12-HSL by MomL.
The amount of residual 3-OH-C12-HSL was expressed as the normalized β-galactosidase activity. Data shown are average of three technical replicates (n = 3), error bars represent standard deviation. *, P < 0.05 when compared with non-MomL treated control (Kruskal-Wallis test).
Effect of MomL on biofilm formation by P. aeruginosa and A. baumannii
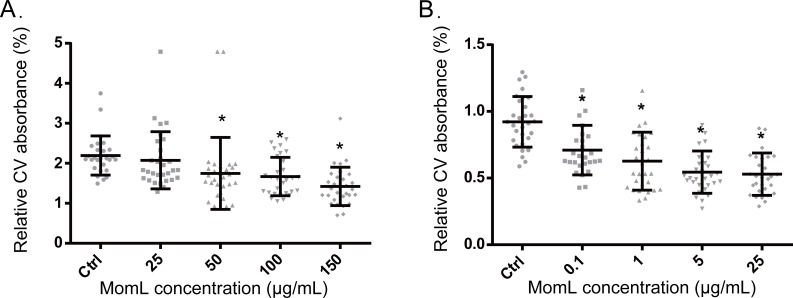
Following biofilm formation in 96-well microtiter plates and quantification by crystal violet staining, a significant difference was observed between P. aeruginosa PAO1 control biofilms and biofilms grown in the presence of MomL (concentration > 50 μg/mL) (Fig. 3A). When grown with 150 μg/mL MomL, an average decrease of approximately 35% was observed. MomL inhibited A. baumannii LMG 10531 biofilm formation at concentrations as low as 0.1 μg/mL, and the biofilm biomass was reduced by approximately 42% when exposed to 5 μg/mL MomL (Fig. 3B). No further decrease was observed when A. baumannii LMG 10531 biofilms were grown in the presence of higher concentration of MomL.
Figure 3. Effect of MomL on biofilm formation by P. aeruginosa PAO1 (A) and A. baumannii LMG 10531 (B).
Biofilms were quantified by CV staining and amount of biofilm left is expressed as CV absorbance (OD 590). Data shown are average of three biological replicates with variable numbers of technical replicates each (n ≥ 27), error bars represent standard deviation. *, P < 0.05 when compared with non-MomL treated control in Kruskal-Wallis test (A) or one-way ANOVA(B).
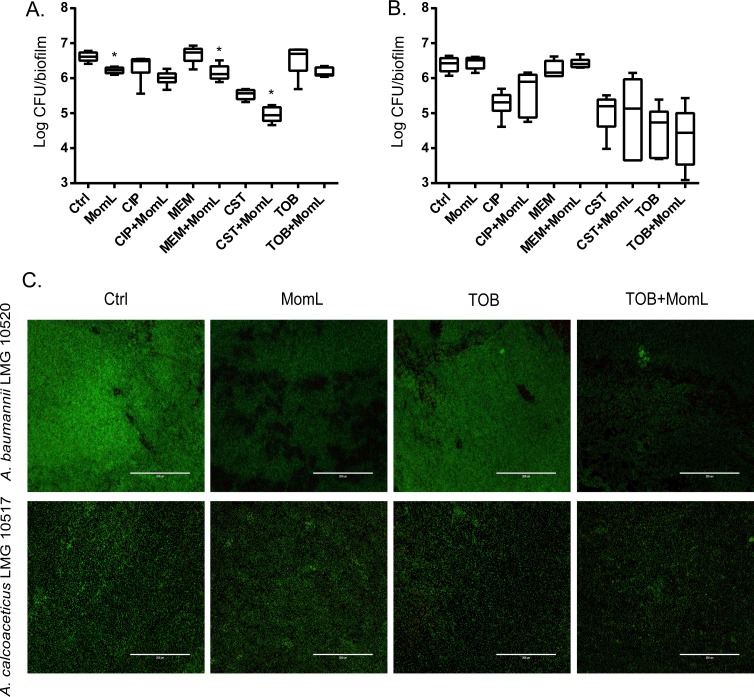
Effect of MomL on biofilm susceptibility to antibiotics
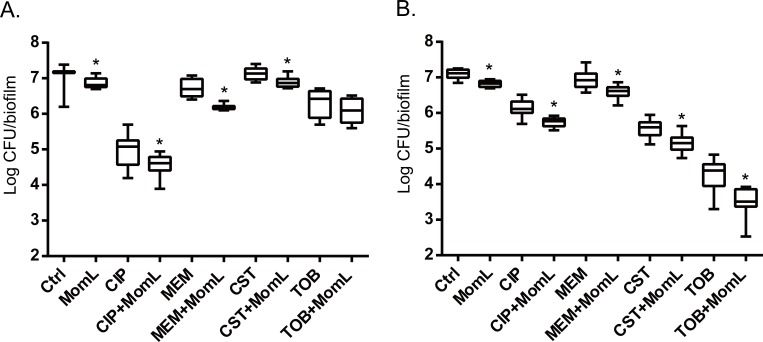
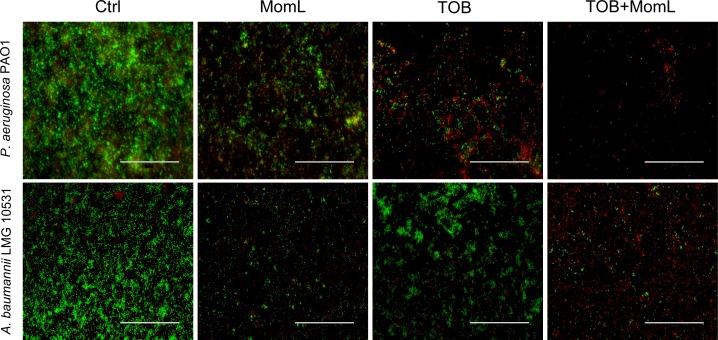
Application of MomL alone (200 μg/mL for P. aeruginosa PAO1 and 10 μg/mL for A. baumannii LMG 10531) reduced the number of cultivable biofilm cells by approximately 50% in both P. aeruginosa PAO1 and A. baumannii LMG 10531. For P. aeruginosa PAO1, combining CIP or MEM with MomL led to >70% more reduction compared to treatment with CIP or MEM alone (Fig. 4A). For A. baumannii LMG 10531, MomL also increased killing of biofilm cells when antibiotics were used together with MomL (Fig. 4B). In case of TOB, cell number was reduced by 80% when used in combination with MomL compared to TOB alone. Consistent with results obtained by plating, fewer living cells were observed in fluorescence microscope images of biofilms treated with MomL, TOB, or a combination of both, compared to control biofilms (Fig. 5).
Figure 4. Effect of MomL on susceptibility of P. aeruginosa PAO1 (A) and A. baumannii LMG 10531 (B) biofilms to different antibiotics.
Numbers of CFU/biofilm were determined by plating and shown as box-whisker plots. Boxes span the interquartile range; the line within each box denotes the median, and whiskers indicate the minimum and maximum values. MomL was added in a final concentration of 200 µg/mL for P. aeruginosa PAO1 and 10 µg/mL for A. baumannii LMG 10531. Data shown are from three biological replicates with three technical replicates each (n = 9). Mann–Whitney tests were performed to compare different groups (*, P < 0.05)
Figure 5. Representative fluorescence images of biofilms of P. aeruginosa PAO1 and A. baumannii LMG 10531.
Biofilms were treated with MomL alone, TOB alone or a combination of both and stained with Syto9 and propidium iodide. 40 × Objective (numerical aperture: 0.75) was used and the final magnification is 1,200 ×. The scale bar represents 100 µm.
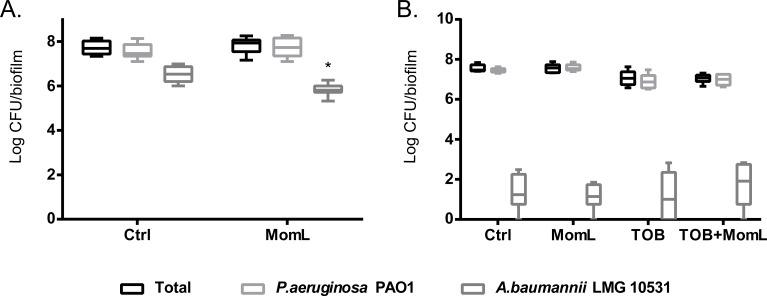
Effect of MomL on dual-species biofilm formed by P. aeruginosa and A. baumannii
We also evaluated the effect of MomL on dual-species biofilm formed by P. aeruginosa PAO1 and A. baumannii LMG 10531. We found that P. aeruginosa PAO1 inhibited growth of A. baumannii LMG 10531 in dual-species biofilm, and most A. baumannii LMG 10531 cells were killed by P. aeruginosa PAO1 after 48 h (Fig. 6). When MomL was added, there was a reduction in A. baumannii LMG 10531 cell numbers; however no difference was observed in either total cell numbers or number of surviving P. aeruginosa PAO1 cells (Fig. 6A). MomL in combination of TOB was also tested, but no change in susceptibility to TOB was observed in the dual-species biofilm (Fig. 6B).
Figure 6. Effect of MomL on dual-species biofilms.
Total number of CFU/biofilm, number of P. aeruginosa PAO1 CFU/biofilm and number of A. baumannii LMG 10531 CFU/biofilm in each dual-species biofilm were determined by plating and shown as box-whisker plots. Boxes span the interquartile range; the line within each box denotes the median, and whiskers indicate the minimum and maximum values. (A). 24 h-biofilm treated with MomL alone; (B). 48 h-biofilm treated with MomL alone, TOB alone or a combination of both. Data shown are from three biological replicates with three (A) or two (B) technical replicates each (n = 9 for A, n = 6 for B). Mann–Whitney tests were performed to compare total, P. aeruginosa PAO1 and A. baumannii LMG 10531 cell numbers respectively between untreated or MomL-treated dual-species biofilm (*, P < 0.05).
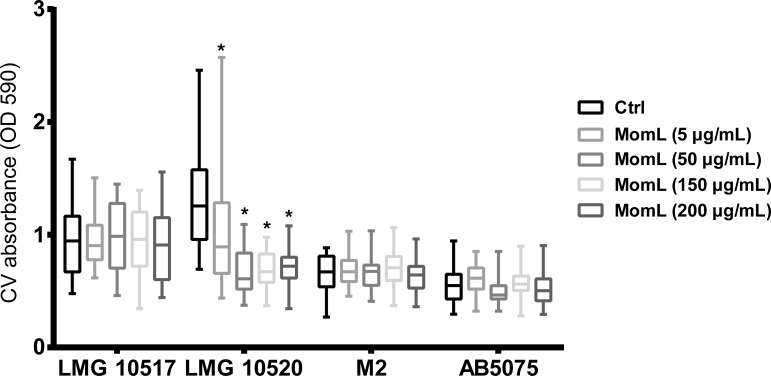
Effect of MomL on other Acinetobacter strains
We also tested MomL on four other Acinetobacter strains. However, only A. baumannii LMG 10520 showed reduction in biofilm biomass when treated with MomL at 50 μg/mL (Fig. 7). No significant difference was observed for A. calcoaceticus LMG 10517, A. nosocomialis M2 and A. baumannii AB5075. The effect of MomL on susceptibility of A. baumannii LMG 10520 and A. calcoaceticus LMG 10517 biofilms was also tested. For A. baumannii LMG 10520, significant differences were detected when MomL was added alone or in combination with several antibiotics (Fig. 8). For A. calcoaceticus LMG 10517, no difference was observed between biofilms receiving MomL treatment and biofilms receiving the control treatment, either by plating or fluorescence microscope.
Figure 7. Effect of MomL on biofilms formed by other Acinetobacter strains.
Biofilms of A. calcoaceticus LMG 10517, A. nosocomialis M2, A. baumannii LMG 10520 and A. baumannii AB5075 were treated with different concentration of MomL and quantified by CV staining. Data shown in box-whisker plots are from three biological replicates with variable numbers of technical replicates each (n ≥ 27). Boxes span the interquartile range; the line within each box denotes the median, and whiskers indicate the minimum and maximum values. *, P < 0.05 when compared to untreated control (Kruskal-Wallis test).
Figure 8. Effect of MomL on biofilm susceptibility of A. baumannii LMG 10520 and A. calcoaceticus LMG 10517.
(A). Plating results for biofilms of A. baumannii LMG 10520 exposed to CIP, MEM, CST, TOB alone or in combination with MomL (50 µg/mL); (B), Plating results for biofilms of A. calcoaceticus LMG 10517 exposed to CIP, MEM, CST, TOB alone or in combination with MomL (200 µg/mL). Data shown in box-whisker plots are from two biological replicates with three technical replicates each (n = 6). Boxes span the interquartile range; the line within each box denotes the median, and whiskers indicate the minimum and maximum values. Mann–Whitney tests were performed to compare control and MomL or antibiotic treatment alone and in combination with MomL (*, P < 0.05). (C). Representative fluorescence images of A. baumannii LMG 10520 and A. calcoaceticus LMG 10517. Biofilms were treated with MomL alone or in combination with tobramycin and stained with Syto9 and propidium iodide. 20 × Objective (numerical aperture: 0.65) was used and the final magnification is 599 ×. The scale bar represents 200 µm.
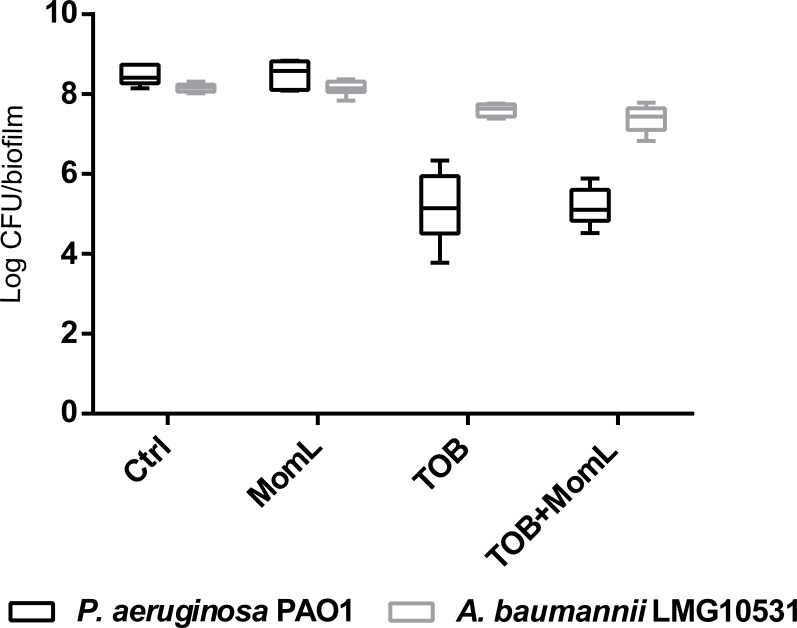
Effect of MomL in a biofilm wound model system and in the C. elegans model
An in vitro wound model was used to mimic the conditions in an infected wound. For both P. aeruginosa PAO1 and A. baumannii LMG 10531, MomL had no effect on biofilm formation in this wound model (Fig. 9).
Figure 9. Effect of MomL on biofilms of P. aeruginosa PAO1 and A. baumannii LMG 10531 formed in wound model.
Data shown in box-whisker plots are from three biological replicates with two technical replicates each (n = 6). Boxes span the interquartile range; the line within each box denotes the median, and whiskers indicate the minimum and maximum values. Mann–Whitney tests were performed to compare control and MomL treatment, or TOB and TOB in combination with MomL.
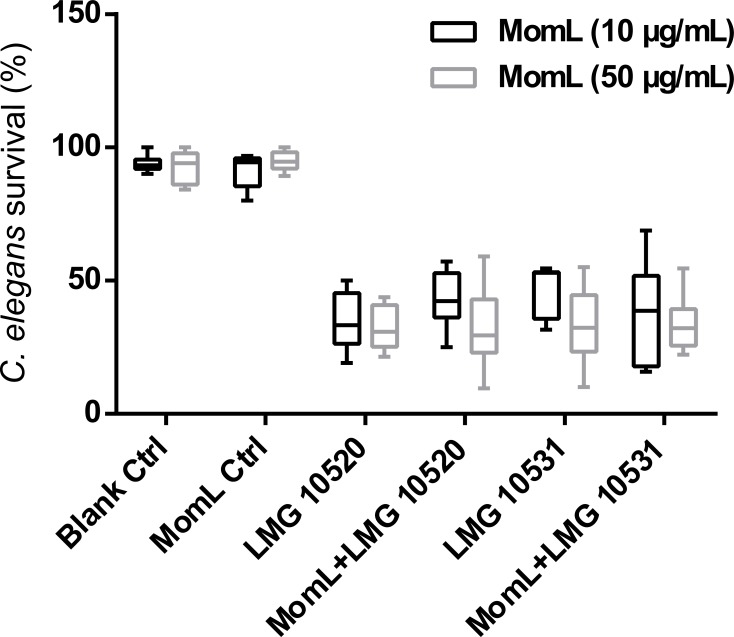
The C. elegans model was used to further evaluate whether MomL can increase survival of nematodes infected with A. baumannii. However, no significant increase of C. elegans survival was found after treating nematodes infected with A. baumannii LMG 10520 or A. baumannii LMG 10531 with MomL (Fig. 10), although AHL-degrading activity was maintained under these test conditions (Fig. S2).
Figure 10. Effect of MomL on the virulence of A. baumanii strains in C. elegans.
Percent survival of C. elegans infected by A. baumannii LMG 10520 and LMG 10531. Data shown in box-whisker plots are from three biological replicates with three technical replicates each (n = 9). Boxes span the interquartile range; the line within each box denotes the median, and whiskers indicate the minimum and maximum values. One-way ANOVA was performed, and no significant differences were found between control and MomL treatment in both uninfected C. elegans and those infected by A. baumannii strains.
Discussion
QS disruption has been considered as a promising anti-infectious strategy to substitute or at least supplement treatment with antibiotics, and could inhibit production of virulence factors and the formation of biofilms (Brackman et al., 2011). Compared to QS inhibitors, QQ enzymes can degrade AHLs from different pathogens and might be more effective in treating multispecies infections. In addition, QQ enzymes do not need to enter the cells as they can act extracellularly, making it less likely resistance will develop (Bzdrenga et al., 2016). The recently-discovered QQ enzyme, MomL, has strong degrading activity towards AHLs with different acyl-chain length and substituents (oxo or hydroxyl) (Tang et al., 2015), and this could be an advantage when targeting bacteria like Acinetobacter strains that produce various AHLs. MomL was reported to reduce the in vitro production of protease and pyocyanin by P. aeruginosa and attenuate the virulence of P. aeruginosa in a C. elegans infection model (Tang et al., 2015). The further application potential of MomL was not determined yet. In the present study we investigated the possible use of MomL for treating biofilm infections, and evaluate its effect on two important Gram-negative nosocomial pathogens, P. aeruginosa and A. baumannii in different models.
First we tested the effect of MomL on single-species biofilms of P. aeruginosa PAO1 and A. baumannii LMG 10531 formed in microtiter plates; a reduction of biofilm biomass was observed for both strains. The maximum decrease in biofilm of A. baumannii LMG 10531 was achieved at a concentration of 5 μg/mL and no further decrease was observed with higher concentrations of MomL, which indicated the presence of other mechanisms besides QS regulating biofilm formation in A. baumannii. Higher concentrations of MomL are needed to show an effect on biofilm of P. aeruginosa PAO1 comparing to A. baumannii LMG 10531 (Fig. 2), possibly due to the higher concentration of AHLs produced by PAO1. When used in combination with antibiotics, fewer biofilm cells survived compared to antibiotic treatment alone, both for P. aeruginosa PAO1 and A. baumannii LMG 10531. In addition, MomL showed no inhibition on planktonic cells of both P. aeruginosa and A. baumannii (Fig. S1), and all these in vitro results seem promising and suggest possible use of MomL to treat biofilm infections of P. aeruginosa and A. baumannii.
We subsequently investigated the effect of MomL in a dual-species biofilm formed by P. aeruginosa and A. baumannii and in a wound biofilm model. MomL had no effect on the overall cell number in the mixed species biofilm and the same disappointing results were obtained in biofilms formed in wound model system. In this wound model system, medium containing plasma, serum, horse blood and heparin were used to reflect nutritional condition in wounds. An artificial dermis was used to mimic a wound like surface and an inoculum of 104 cells was used to reflect the microbial load of a wound prior to infection. Additionally, in contrast to what we observed for the mono-species biofilms formed in 96-well microtiter plates, MomL did not potentiate the activity of TOB in this model system. Possible explanations for this are that component(s) present in this wound biofilm model protect AHL from degradation and/or interfere with the activity of MomL (potentially through interactions with proteins in the plasma), or that QS is not essential for biofilm formation and/or resistance in these conditions. Further experiments will be required to clarify this. Although MomL showed strong activity against 3-OH-C12-HSL in the medium used in the C. elegans model (Fig. S2), no effect of MomL on the virulence of A. baumanii was observed. As previously reported, an A. baumannii QS mutant did not differ from the wild type with regards to killing in a Galleria mellonella infection model (Peleg et al., 2009). These results indicated that although QS is known to play an important role in A. baumanii biofilm formation, it might only have limited role in the virulence in the C. elegans and G. mellonella models.
Thus far, a series of promising results about in vivo application of QQ enzymes have been reported. Phosphotriesterase-like lactonase SsoPox-I has been reported to reduce biofilm formation of P. aeruginosa at a concentration higher than 170 μg/mL, and the early use of SsoPox-I reduced the mortality of rats with acute pneumonia from 75% to 20% (Hraiech et al., 2014). In another study, acylase-containing coatings on silicone urinary catheters reduced formation of P. aeruginosa biofilms and mixed-species P. aeruginosa-E. coli biofilms (Ivanova et al., 2015). Our data obtained in a dual-species biofilm formed by P. aeruginosa and A. baumannii as well as in a wound model strongly suggest that the effect of MomL (and potentially also other QQ enzymes) on in vivo grown bacterial biofilms may be much less pronounced than the effect observed with biofilms formed under simple in vitro conditions. Factors affecting the anti-biofilm activity in more complex systems could include stability of the enzyme, penetration of the enzyme through the biofilm matrix, and the composition of the environment.
Different outcomes were also observed when we evaluated the effect of MomL on different Acinetobacter strains, and no effects of MomL on biofilm formation was detected for three out of five Acinetobacter strains tested. In addition, for A. baumanii LMG 10520, a considerably higher concentration of MomL was required to obtain a pronounced inhibitory effect than for A. baumannii LMG 10531. These results confirm that the anti-biofilm activity of QQ enzymes is strain-dependent, which is likely to reduce their clinical efficacy.
Conclusion
The results of the present study highlight that there are considerable hurdles to be cleared before QQ enzymes could potentially be used to combat infections. Our data indicate that demonstrating AHL degrading activity in vitro and/or anti-biofilm activity in simple in vitro biofilm model systems is not sufficient to predict an anti-biofilm effect in more complex systems.
Supplemental Information
The growth of P. aeruginosa PAO1 and A. baumannii LMG 10531 was spectrophotometrically determined at OD 590 after being incubated with different concentrations of MomL at 37 °C for 24 h. No MomL was added in control. Data shown in box-whisker plots are from three biological replicates with two technical replicates each (n = 6). Boxes span the interquartile range; the line within each box denotes the median, and whiskers indicate the minimum and maximum values.
3-OH-C12-HSL (10 µM) was mixed with MomL under the same medium condition in C. elegans model and incubated at 37°C for 1h. No MomL was added in control. The amount of residual 3-OH-C12-HSL was quantified using A. tumefaciens A136 liquid X-gal assay and expressed as the normalized β-galactosidase activity.
Acknowledgments
We thank prof. Xiao-Hua Zhang for providing Escherichia coli BL21(DE3) harboring the MomL expression plasmid pET24a(+)-momL-(−SP), prof. Wim Quax for providing A. nosocomialis M2 and prof. Colin Manoil for providing A. baumannii AB5075.
Funding Statement
This work was supported by the China Scholarschip Counil (CSC) and the Special Research Fund of Ghent University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
Tom Coenye is an Academic Editor for PeerJ.
Author Contributions
Yunhui Zhang conceived and designed the experiments, performed the experiments, analyzed the data, wrote the paper, prepared figures and/or tables.
Gilles Brackman conceived and designed the experiments, analyzed the data, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Tom Coenye conceived and designed the experiments, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Data Availability
The following information was supplied regarding data availability:
The raw data has been supplied as Supplementary File.
References
- Aybey & Demirkan (2016).Aybey A, Demirkan E. Inhibition of quorum sensing-controlled virulence factors in Pseudomonas aeruginosa by human serum paraoxonase. Journal of Medical Microbiology. 2016;65:105–113. doi: 10.1099/jmm.0.000206. [DOI] [PubMed] [Google Scholar]
- Bassler & Losick (2006).Bassler BL, Losick R. Bacterially speaking. Cell. 2006;125:237–246. doi: 10.1016/j.cell.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Bhargava, Sharma & Capalash (2010).Bhargava N, Sharma P, Capalash N. Quorum sensing in Acinetobacter: an emerging pathogen. Critical Reviews in Microbiology. 2010;36:349–360. doi: 10.3109/1040841X.2010.512269. [DOI] [PubMed] [Google Scholar]
- Bhargava et al. (2015).Bhargava N, Singh SP, Sharma A, Sharma P, Capalash N. Attenuation of quorum sensing-mediated virulence of Acinetobacter baumannii by Glycyrrhiza glabra flavonoids. Future Microbiology. 2015;10:1953–1968. doi: 10.2217/fmb.15.107. [DOI] [PubMed] [Google Scholar]
- Brackman & Coenye (2015).Brackman G, Coenye T. Quorum sensing inhibitors as anti-biofilm agents. Current Pharmaceutical Design. 2015;21:5–11. doi: 10.2174/1381612820666140905114627. [DOI] [PubMed] [Google Scholar]
- Brackman et al. (2011).Brackman G, Cos P, Maes L, Nelis HJ, Coenye T. Quorum sensing inhibitors increase the susceptibility of bacterial biofilms to antibiotics in vitro and in vivo. Antimicrobial Agents and Chemotherapy. 2011;55:2655–2661. doi: 10.1128/AAC.00045-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackman et al. (2016).Brackman G, Garcia-Fernandez MJ, Lenoir J, De Meyer L, Remon JP, De Beer T, Concheiro A, Alvarez-Lorenzo C, Coenye T. Dressings loaded with cyclodextrin–hamamelitannin complexes increase Staphylococcus aureus susceptibility toward antibiotics both in single as well as in mixed biofilm communities. Macromolecular Bioscience. 2016;16(6):859–869. doi: 10.1002/mabi.201500437. [DOI] [PubMed] [Google Scholar]
- Bzdrenga et al. (2016).Bzdrenga J, Daudé D, Rémy B, Jacquet P, Plener L, Elias M, Chabrière E. Biotechnological applications of quorum quenching enzymes. Chemico-Biological Interactions. 2016;267:104–115. doi: 10.1016/j.cbi.2016.05.028. [DOI] [PubMed] [Google Scholar]
- Chow et al. (2014).Chow JY, Yang Y, Tay SB, Chua KL, Yew WS. Disruption of biofilm formation by the human pathogen Acinetobacter baumannii using engineered quorum-quenching lactonases. Antimicrobial Agents and Chemotherapy. 2014;58:1802–1805. doi: 10.1128/AAC.02410-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donlan & Costerton (2002).Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clinical Microbiology Reviews. 2002;15:167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetzner (2015).Fetzner S. Quorum quenching enzymes. Journal of Biotechnology. 2015;201:2–14. doi: 10.1016/j.jbiotec.2014.09.001. [DOI] [PubMed] [Google Scholar]
- Furiga et al. (2016).Furiga A, Lajoie B, El Hage S, Baziard G, Roques C. Impairment of Pseudomonas aeruginosa biofilm resistance to antibiotics by bombining the drugs with a new quorum-sensing inhibitor. Antimicrobial Agents and Chemotherapy. 2016;60:1676–1686. doi: 10.1128/AAC.02533-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González et al. (2009).González R, Dijkshoorn L, Van den Barselaar M, Nudel C. Quorum sensing signal profile of Acinetobacter strains from nosocomial and environmental sources. Revista Argentina De Microbiologia. 2009;41:73–78. [PubMed] [Google Scholar]
- Gonzalez-Villoria & Valverde-Garduno (2016).Gonzalez-Villoria AM, Valverde-Garduno V. Antibiotic-resistant Acinetobacter baumannii increasing success remains a challenge as a nosocomial pathogen. Journal of Pathogens. 2016;2016 doi: 10.1155/2016/7318075. Epub ahead of print Feb 04 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall-Stoodley, Costerton & Stoodley (2004).Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nature Reviews Microbiology. 2004;2:95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- Hentzer & Givskov (2003).Hentzer M, Givskov M. Pharmacological inhibition of quorum sensing for the treatment of chronic bacterial infections. The Journal of Clinical Investigation. 2003;112:1300–1307. doi: 10.1172/JCI20074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentzer et al. (2002).Hentzer M, Riedel K, Rasmussen TB, Heydorn A, Andersen JB, Parsek MR, Rice SA, Eberl L, Molin S, Høiby N. Inhibition of quorum sensing in Pseudomonas aeruginosa biofilm bacteria by a halogenated furanone compound. Microbiology. 2002;148:87–102. doi: 10.1099/00221287-148-1-87. [DOI] [PubMed] [Google Scholar]
- Hentzer et al. (2003).Hentzer M, Wu H, Andersen JB, Riedel K, Rasmussen TB, Bagge N, Kumar N, Schembri MA, Song Z, Kristoffersen P. Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. The EMBO Journal. 2003;22:3803–3815. doi: 10.1093/emboj/cdg366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hraiech et al. (2014).Hraiech S, Hiblot J, Lafleur J, Lepidi H, Papazian L, Rolain J-M, Raoult D, Elias M, Silby MW, Bzdrenga J. Inhaled lactonase reduces Pseudomonas aeruginosa quorum sensing and mortality in rat pneumonia. PLOS ONE. 2014;9:e107125. doi: 10.1371/journal.pone.0107125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova et al. (2015).Ivanova K, Fernandes MM, Francesko A, Mendoza E, Guezguez J, Burnet M, Tzanov T. Quorum-quenching and matrix-degrading enzymes in multilayer coatings synergistically prevent bacterial biofilm formation on urinary catheters. ACS Applied Materials & Interfaces. 2015;7:27066–27077. doi: 10.1021/acsami.5b09489. [DOI] [PubMed] [Google Scholar]
- LaSarre & Federle (2013).LaSarre B, Federle MJ. Exploiting quorum sensing to confuse bacterial pathogens. Microbiology and Molecular Biology Reviews. 2013;77:73–111. doi: 10.1128/MMBR.00046-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo, Vuotto & Donelli (2014).Longo F, Vuotto C, Donelli G. Biofilm formation in Acinetobacter baumannii. New Microbiologica. 2014;37:119–127. [PubMed] [Google Scholar]
- Migiyama et al. (2013).Migiyama Y, Kaneko Y, Yanagihara K, Morohoshi T, Morinaga Y, Nakamura S, Miyazaki T, Hasegawa H, Izumikawa K, Kakeya H. Efficacy of AiiM, an N-acylhomoserine lactonase, against Pseudomonas aeruginosa in a mouse model of acute pneumonia. Antimicrobial Agents and Chemotherapy. 2013;57:3653–3658. doi: 10.1128/AAC.00456-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu et al. (2008).Niu C, Clemmer KM, Bonomo RA, Rather PN. Isolation and characterization of an autoinducer synthase from Acinetobacter baumannii. Journal of Bacteriology. 2008;190:3386–3392. doi: 10.1128/JB.01929-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Loughlin et al. (2013).O’Loughlin CT, Miller LC, Siryaporn A, Drescher K, Semmelhack MF, Bassler BL. A quorum-sensing inhibitor blocks Pseudomonas aeruginosa virulence and biofilm formation. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:17981–17986. doi: 10.1073/pnas.1316981110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters, Nelis & Coenye (2008).Peeters E, Nelis HJ, Coenye T. Comparison of multiple methods for quantification of microbial biofilms grown in microtiter plates. Journal of Microbiological Methods. 2008;72:157–165. doi: 10.1016/j.mimet.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Peleg et al. (2009).Peleg AY, Jara S, Monga D, Eliopoulos GM, Moellering RC, Mylonakis E. Galleria mellonella as a model system to study Acinetobacter baumannii pathogenesis and therapeutics. Antimicrobial Agents and Chemotherapy. 2009;53:2605–2609. doi: 10.1128/AAC.01533-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peleg, Seifert & Paterson (2008).Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen. Clinical Microbiology Reviews. 2008;21:538–582. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesci et al. (1997).Pesci EC, Pearson JP, Seed PC, Iglewski BH. Regulation of las and rhl quorum sensing in Pseudomonas aeruginosa. Journal of Bacteriology. 1997;179:3127–3132. doi: 10.1128/jb.179.10.3127-3132.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potron, Poirel & Nordmann (2015).Potron A, Poirel L, Nordmann P. Emerging broad-spectrum resistance in Pseudomonas aeruginosa and Acinetobacter baumannii: mechanisms and epidemiology. International Journal of Antimicrobial Agents. 2015;45:568–585. doi: 10.1016/j.ijantimicag.2015.03.001. [DOI] [PubMed] [Google Scholar]
- Rasmussen & Givskov (2006).Rasmussen TB, Givskov M. Quorum sensing inhibitors: a bargain of effects. Microbiology. 2006;152:895–904. doi: 10.1099/mic.0.28601-0. [DOI] [PubMed] [Google Scholar]
- Rutherford & Bassler (2012).Rutherford ST, Bassler BL. Bacterial quorum sensing: its role in virulence and possibilities for its control. Cold Spring Harbor Perspectives in Medicine. 2012;2:a012427. doi: 10.1101/cshperspect.a012427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saroj & Rather (2013).Saroj SD, Rather PN. Streptomycin inhibits quorum sensing in Acinetobacter baumannii. Antimicrobial Agents and Chemotherapy. 2013;57:1926–1929. doi: 10.1128/AAC.02161-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiernagle (1999).Stiernagle T. Maintenance of C. elegans. C Elegans. 1999;2:51–67. doi: 10.1895/wormbook.1.101.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang et al. (2015).Tang K, Su Y, Brackman G, Cui F, Zhang Y, Shi X, Coenye T, Zhang X-H. MomL, a novel marine-derived N-acyl homoserine lactonase from Muricauda olearia. Applied and Environmental Microbiology. 2015;81:774–782. doi: 10.1128/AEM.02805-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang & Zhang (2014).Tang K, Zhang X-H. Quorum quenching agents: resources for antivirulence therapy. Marine Drugs. 2014;12:3245–3282. doi: 10.3390/md12063245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang et al. (2013).Tang K, Zhang Y, Yu M, Shi X, Coenye T, Bossier P, Zhang X-H. Evaluation of a new high-throughput method for identifying quorum quenching bacteria. Scientific Reports. 2013;3:2935. doi: 10.1038/srep02935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempé et al. (1977).Tempé J, Petit A, Holsters M, Van Montagu M, Schell J. Thermosensitive step associated with transfer of the Ti plasmid during conjugation: possible relation to transformation in crown gall. Proceedings of the National Academy of Sciences of the United States of America. 1977;74:2848–2849. doi: 10.1073/pnas.74.7.2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams et al. (2007).Williams P, Winzer K, Chan WC, Camara M. Look who’s talking: communication and quorum sensing in the bacterial world. Philosophical Transactions of the Royal Society B: Biological Sciences. 2007;362:1119–1134. doi: 10.1098/rstb.2007.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu et al. (2004).Wu H, Song Z, Hentzer M, Andersen JB, Molin S, Givskov M, Høiby N. Synthetic furanones inhibit quorum-sensing and enhance bacterial clearance in Pseudomonas aeruginosa lung infection in mice. Journal of Antimicrobial Chemotherapy. 2004;53:1054–1061. doi: 10.1093/jac/dkh223. [DOI] [PubMed] [Google Scholar]
- Yin et al. (2015).Yin H, Deng Y, Wang H, Liu W, Zhuang X, Chu W. Tea polyphenols as an antivirulence compound disrupt quorum-sensing regulated pathogenicity of Pseudomonas aeruginosa. Scientific Reports. 2015;5:16158. doi: 10.1038/srep16158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu et al. (1998).Zhu J, Beaber JW, Moré MI, Fuqua C, Eberhard A, Winans SC. Analogs of the autoinducer 3-oxooctanoyl-homoserine lactone strongly inhibit activity of the TraR protein of Agrobacterium tumefaciens. Journal of Bacteriology. 1998;180:5398–5405. doi: 10.1128/jb.180.20.5398-5405.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The growth of P. aeruginosa PAO1 and A. baumannii LMG 10531 was spectrophotometrically determined at OD 590 after being incubated with different concentrations of MomL at 37 °C for 24 h. No MomL was added in control. Data shown in box-whisker plots are from three biological replicates with two technical replicates each (n = 6). Boxes span the interquartile range; the line within each box denotes the median, and whiskers indicate the minimum and maximum values.
3-OH-C12-HSL (10 µM) was mixed with MomL under the same medium condition in C. elegans model and incubated at 37°C for 1h. No MomL was added in control. The amount of residual 3-OH-C12-HSL was quantified using A. tumefaciens A136 liquid X-gal assay and expressed as the normalized β-galactosidase activity.
Data Availability Statement
The following information was supplied regarding data availability:
The raw data has been supplied as Supplementary File.