Abstract
Purpose
To test for the potential presence of novel mutations in the zinc finger protein (ZNF469) gene in patients with sporadic keratoconus (KC) from a Han Chinese population.
Methods
Fifty-three patients with primary KC, 30 patients with high myopia (HM), and 100 unrelated population-matched healthy controls without any ocular or systemic disorders, all of Han Chinese ethnicity, were recruited. Blood samples were donated, and genomic DNA was isolated from peripheral blood leukocytes. Sequence variations in ZNF469 were initially identified in patients with KC with next-generation sequencing and subsequently confirmed using Sanger sequencing. Sequence variants identified in patients with KC were subsequently screened in 30 patients with HM and 100 healthy control subjects. Other genes that were reported to be related to KC were also screened in the patients with KC who carried the mutations in ZNF469. The Sorting Intolerant Form Tolerant (SIFT) program was used to predict the effect of amino acid substitution on the ZNF469 protein.
Results
Sixteen sequence variants in the coding regions of ZNF469 were identified in this Chinese KC cohort. After five known single nucleotide polymorphisms (SNPs), one false-positive result, and three mutations that were also detected in the results of the whole-exome sequencing (WES) data performed in 220 Han Chinese individuals without ocular abnormalities were removed, seven novel mutations in ZNF469 (c.2059G>A, c.2137C>A, c.3466G>A, c.3749C>T, c.4300G>A, c.4684G>A, and c.7262G>A) that were predicted to be potentially damaging were identified. The patient with KC with the c.3466G>A mutation was also shown to carry one dedicator of cytokinesis 9 (DOCK9) mutation (c.1940C>T). None of the mutations were detected in the patients with HM or the healthy controls. All of the seven mutations in the patients with KC were heterozygote.
Conclusions
The results suggested for the first time that ZNF469 has a pathogenic role in Chinese patients with KC and have widened the mutation spectrum of KC in the Han Chinese population.
Introduction
KC is characterized by corneal thinning and ectasia, progressive myopia, and irregular astigmatism [1,2], which affects both genders and all ethnicities [3]. KC usually begins in the late teens or early 20s and progresses until the fourth decade of life [4]. As the most common ectatic disorder, the prevalence of KC has been reported to vary from 8.8 to 54.4 per 100,000 individuals in different studies internationally [2], depending on the diagnostic criteria of KC, population characteristics, and structure [3]. Additionally, because of the limitation of medical treatments, up to 21% of patients with KC worldwide have an indication for corneal transplantation [1,4,5].
Despite extensive studies, the pathophysiology of KC remains unknown and complex. Multiple environmental and genetic factors are thought to be involved in the development of KC. As for its genetic etiology, KC is considered to be compatible with an autosomal inheritance pattern based on familial occurrence, with a higher concordance rate in monozygotic twins compared with dizygotic twins [6] and a 15–67 times higher prevalence in first-degree relatives than in the general population [7]. To date, multiple genes have been reported to be related to KC [8-10], including but not limited to lysyl oxidase (LOX; gene ID:4015, OMIM 153455) [11], interleukin-1 (IL1; gene ID:3552, OMIM 147760) [12], visual system homeo box 1 (VSX1; gene ID:30813, OMIM 605020) [13], microRNA 184 (mir184; gene ID:406960, OMIM 613146) [14], and transforming growth factor beta induced (TGFBI; gene ID:7045, OMIM 601692) [15].
Although the physiologic role of the ZNF469 (gene ID:84627, OMIM 612078) gene is not yet well-established, some evidence suggests that ZNF469 (located on 16q24) may also contribute to the development of KC. Abu et al. reported that ZNF469 shares 30% homology with the helical parts of three types of collagen (COL1A1, COL1A2, and COL4A1), which suggests that ZNF469 may participate in collagen homeostasis in the human cornea [16]. Additionally, the ZNF469 protein may also function as a transcription factor or extranuclear regulator factor in the human cornea [17]. Several genetic analysis studies have been conducted to further ascertain the involvement of ZNF469 in KC. Lu et al. reported that mutations in ZNF469 could confer increased risk of KC [18-20], while recent sequencing analysis of ZNF469 in patients with KC and high myopia showed no significant variants [4]. Thus, the involvement of ZNF469 in KC remains contentious at present, prompting us to conduct the additional genetic studies described here.
ZNF469 has not been thoroughly studied in Chinese patients with KC. In addition, it is unknown whether pathogenic variants identified in largely Caucasian populations are relevant to Asians, in whom the prevalence of KC is much higher and the age of onset of KC is much younger [1]. Therefore, we performed this analysis of the mutations in ZNF469, aiming to identify novel variants that may indicate the potential involvement of ZNF469 in Chinese patients affected by KC.
Methods
This study adhered to the tenets of the Declaration of Helsinki and was approved by the ethics committee of the 2nd Affiliated Hospital, Medical College of Zhejiang University, Hangzhou, China. This study adhered to the Association for Research in Vision and Ophthalmology (ARVO) statement on human subjects. Written informed consent was obtained from every participant.
Subject identification
Fifty-three patients with primary KC, 30 patients with HM (defined by a spherical equivalent (SE) of more than −6.00 diopters (D)), and 100 unrelated population-matched healthy controls without any ocular or systemic disorders, all of Han Chinese ethnicity, were recruited from the Eye Center of the 2nd Affiliated Hospital, Medical College of Zhejiang University, Hangzhou, China in 2015. The KC patients were aged from 11 to 43 years, including 31 males and 22 females. The first clinical diagnosis of KC patients was made at ages ranging from 9 to 36 years. The HM controls were aged from 19 to 35 years, including 17 males and 13 females. The healthy controls were aged from 16 to 45 years, including 49 males and 51 females. Only 53 patients with KC were willing to enroll in this study, which to the best of our knowledge was an apparently random sub-set of the total eligible population. The patients with HM were randomly selected with the random data table sampling method. Controls were recruited from healthy people who had undergone routine health examinations at this hospital. In addition, the absence of KC or HM was confirmed in all controls, who received ocular exams, including visual acuity measurement, slit-lamp examination, and corneal topography assessment. All patients with KC who were included had negative family histories for KC. The diagnosis of KC was made based on clinical manifestations: corneal stromal thinning, Vogt’s striae, Fleischer ring, Munson’s sign, conical protrusion of the cornea at the apex, an anterior corneal stromal scar using a slit-lamp microscope, and signs of videokeratography (localized increased surface power and/or inferior superior dioptric asymmetry [Bausch & Lomb Surgical, Orbtek Inc., Salt Lake City, UT]). According to the grading system used in the Collaborative Longitudinal Evaluation of Keratoconus Study, the severity of KC was divided into three categories using average keratometric readings: mild (<45 D), moderate (45–52 D), and severe (>52 D) [21]. The videokeratography pattern was classified into 11 categories [22]. Any patient with KC with coexisting allergy or atopy KC secondary to such causes as trauma, LASIK, or other refractive surgeries or who had Ehlers-Danlos syndrome, Down syndrome, osteogenesis imperfecta, or pellucid marginal degeneration was excluded from this research.
Mutation screening
We collected blood samples (5 ml) of the participates in Vacutainer tubes (Becton-Dickinson, Franklin Lakes, NJ) containing ethylene diamine tetra acetic acid (EDTA),preserved at -80 °C before being extracted genomic DNA with a Simgen DNA Blood Mini Kit (Simgen,Hangzhou,China) according to the manufacturer’s instruction [23,24] All patients with KC and HM and the healthy controls underwent mutation screening. For the patients with KC, the ZNF469 gene was screened with next-generation sequencing technology, which is based on targeted sequence capturing technology with the SureSelect Target Enrichment Kit (Agilent Technologies, Santa Clara, CA) and the Illumina sequencing technology with the HiSeq Sequencer (Illumina , San Diego, CA). To avoid false-positive results and to ascertain the significance of the mutations in ZNF469, mutations (i) with a minor allele frequency (MAF) <0.1% (according to data from the May 2012 release of the 1000 Genomes Project and the Single Nucleotide Polymorphism database) and (ii) absent from the results of the whole-exome sequencing (WES) data acquired from 220 Han Chinese individuals without ocular abnormalities (from a commercial database provided by the Genesky Bio-Tech company) were subsequently confirmed using Sanger sequencing technology. To further test whether the mutations in ZNF469 identified in patients with KC with these two steps were potentially pathogenic mutations and could not be carried by healthy people, we screened these mutations in the patients with HM and the healthy controls with Sanger sequencing.
All coding regions (exons, intron–exon junctions, and promoter regions) of the ZNF469 gene were amplified with PCR using specific primer sequences. Three different PCR conditions were involved in this study. 1) Reaction condition for fragments of 1 and 6:the cycling program was 95 °C 2 min; 35 cycles x (96 °C10 s, 68 °C1 min); 4 °C for ever. 2) Reaction condition for fragments of 3 to 5:the cycling program was 95 °C 2 min; 11 cycles x (94 °C 20 s, 66 °C-0.5 °C /cycle 40 s, 72 °C 1min); 24 cycles x (94 °C 20 s, 60 °C 30 s, 72 °C 1min); 72 °C 2min; 4 °C forever 3 ). Reaction condition for fragments of 2 and 7:the cycling program was 95 °C 2 min; 11 cycles x (94 °C 20 s, 62 °C-0.5 °C /cycle 40 s, 72 °C 1 min); 24 cycles x (94 °C 20 s, 56 °C 30 s, 72 °C 1min); 72 °C 2 min; 4 °C forever. The PCR products were isolated with electrophoresis and sequenced using the BigDye Terminator v3.1 Cycle sequencing kit (Applied Biosystems, Foster City, CA) on an Applied Biosystems ABI 3730 Sequencer Analyzer. Finally, the sequencing results were analyzed using the PolyPhred version and compared with the sequences in the NCBI GenBank database.
To further ensure the significance of the identified mutations in ZNF469, other potentially pathogenic genes related to KC were similarly assessed in the patients with KC with next-generation sequencing and follow-up Sanger sequencing. Details about these genes are shown in the supplemental materials (File S1).
Bioinformatics analysis
The Sorting Intolerant Form Tolerant (SIFT) programs were used to predict the effect of amino acid substitution on the ZNF469 protein. Based on the theory of evolutionary conservation, the amino acid substitution is considered damaging if the SIFT score is ≤0.05; otherwise, the substitution is tolerated.
Statistical analysis
All statistical analyses were conducted using SPSS software (Version 19.0, SPSS, Chicago, IL). Continuous numeric variables were presented as the mean ± standard deviation (SD). And no other statistical test was used in this study. Only descriptive statistics were used in this study, which were not related to the significance level.
Results
The entire coding region, intron–exon junctions, and promoter regions of ZNF469 were analyzed for mutations in 53 patients with sporadic KC. According to the next-generation sequencing results, 16 sequence variants in ZNF469 were identified in this Chinese KC cohort, which are summarized in Table 1. All sequence variants were nonsynonymous single-nucleotide variants.
Table 1. All ZNF469 sequence variants identified in KC patients.
| Nucleotide change | Amino acid change | SNP ID | Frequency (1KG Project) | Position | Gene region | Mutation effect | SIFT score |
|---|---|---|---|---|---|---|---|
| c.1370C>A |
p.A457D |
|
|
88,495,248 |
Exon |
nonsynonymou |
0.02 |
| c.1471G>A |
p.A491T |
rs117555121 |
0.00998403 |
88,495,349 |
Exon |
nonsynonymou |
0.01 |
| c.2059G>A |
p.E687K |
|
|
88,495,937 |
Exon |
nonsynonymou |
0.02 |
| c.2137C>A |
p.P713T |
|
|
88,496,015 |
Exon |
nonsynonymou |
0.02 |
| c.2653C>G |
p.L885V |
rs139653501 |
0.00459265 |
88,496,531 |
Exon |
nonsynonymou |
0 |
| c.3466G>A |
p.A1156T |
|
|
88,497,428 |
Exon |
nonsynonymou |
0 |
| c.3749C>T |
p.P1250L |
|
|
88,497,711 |
Exon |
nonsynonymou |
0 |
| c.4300G>A |
p.D1434N |
|
0.000199681 |
88,498,262 |
Exon |
nonsynonymou |
0 |
| c.4684G>A |
p.E1562K |
|
|
88,498,646 |
Exon |
nonsynonymou |
0 |
| c.7262G>A |
p.R2421H |
|
0.00119808 |
88,501,224 |
Exon |
nonsynonymou |
0 |
| c.10048G>C |
p.G3350R |
rs192272765 |
0.000599042 |
88,504,010 |
Exon |
nonsynonymou |
0.05 |
| c.10633G>A |
p.G3545R |
rs183149417 |
0.00179712 |
88,504,595 |
Exon |
nonsynonymou |
0.11 |
| c.946G>A |
p.E316K |
|
0.00599042 |
88,494,824 |
Exon |
nonsynonymou |
0 |
| c.2803G>A |
p.E935K |
rs117995699 |
0.00638978 |
88,496,681 |
Exon |
nonsynonymou |
0 |
| c.10244G>A |
p.G3415E |
|
0.000998403 |
88,504,206 |
Exon |
nonsynonymou |
0.01 |
| c.2945G>A | p.R982K | 0.000998403 | 88,496,823 | Exon | nonsynonymou | 0 |
After five known SNPs with MAF>0.1% (c.1471G>A, rs117555121; c.2653C>G, rs139653501; c.10048G>C, rs192272765; c.10633G>A,rs183149417; and c.2803G>A, rs117995699) and three single nucleotide variants (c.946G>A, c.10244G>A, and c.2945G>A) that were also detected in the results of the WES data acquired from the 220 Han Chinese individuals without ocular abnormalities were excluded, eight novel mutations in ZNF469 were selected for further characterization with Sanger sequencing technology to avoid false-positive results. The forward and reverse primers used in the direct PCR sequencing are summarized in Table 2; two mutations (c.2059G>A and c.2137C>A) with similar locations shared the same primer. With the use of Sanger sequencing, one mutation was identified as a false-positive result. The remaining seven mutations in ZNF469 (c.2059G>A, c.2137C>A, c.3466G>A, c.3749C>T, c.4300G>A, c.4684G>A, and c.7262G>A) were screened in 100 healthy controls, and none of the mutations were detected. We also screened the seven mutations in 30 patients with HM. Similarly, none of the mutations were detected.
Table 2. Sequences of primers used in the study.
| Primer name | Fragment size (bp) | Primer sequence (5′ → 3′) |
|---|---|---|
| 1F |
423 |
CCCTGCAGGTCCCCACCAACA |
| 1R |
|
TGGGTTTGGGGCAGGCGGTACT |
| 2F |
399 |
AAGCAGCACAGCCAGTGACTT |
| 2R |
|
CCCCGTGAGTGATTTGGTCT |
| 3F |
383 |
GTTCCTCGGACCCAAAGACCT |
| 3R |
|
GCATCGGGGATACTTCCTCAA |
| 4F |
362 |
ACCCACTCACCCCCAGGAGAC |
| 4R |
|
CCAGGAGCAGGCCACAGAACT |
| 5F |
484 |
CCTAGCTCCCTACCCCAGAGG |
| 5R |
|
GAGCCCCAGGGTCTGTCATT |
| 6F |
467 |
GGGGCCGAGGCGAGAAGAGGA |
| 6R |
|
GGGCGGCGGCTGCTCTGGTAT |
| 7F |
251 |
ATGAGTCACCTGTCCGAGGAT |
| 7R | CGATGTGCAGTGGATTCTCC |
Other genes related to KC were also screened in the patients who carry the mutations in ZNF469. The patient with KC who carries mutation c.3466G>A was detected as carrying one DOCK9 mutation (c.1940C>T). The SIFT score of the DOCK9 mutation was 0, which is considered to be damaging.
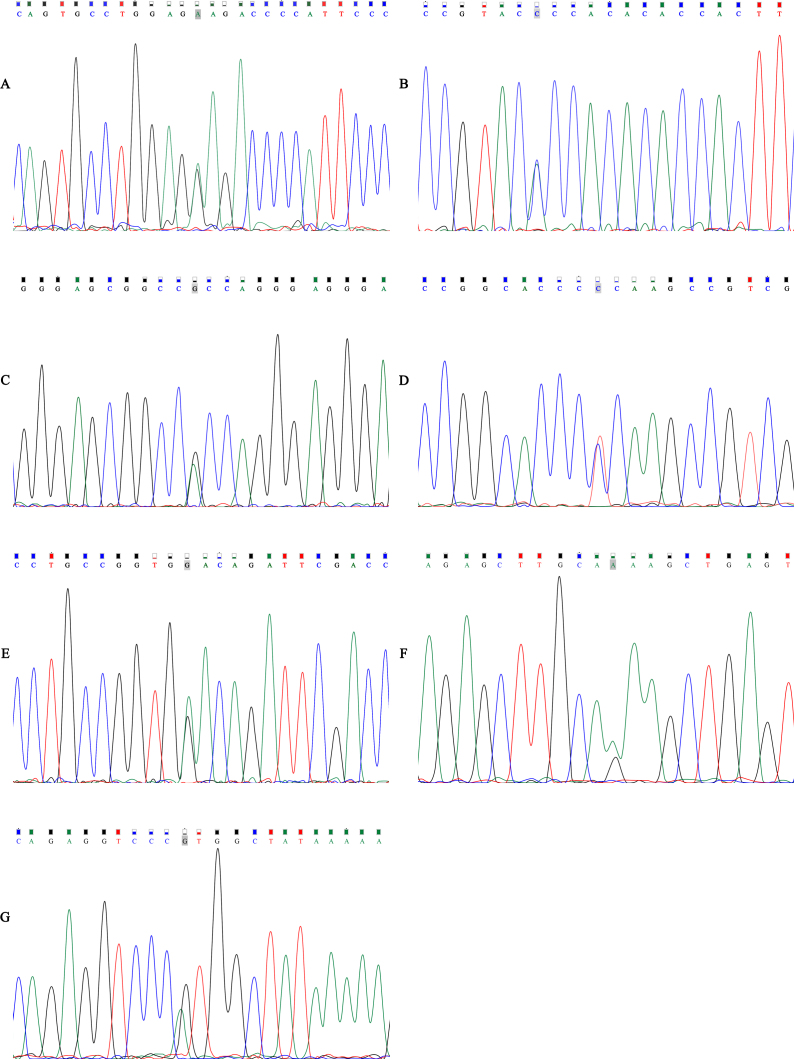
Sequencing chromatograms of the seven novel mutations in ZNF469 identified in this study are shown in Figure 1, and all were located in the exonic region of ZNF469. None of the seven nonsynonymous mutations in ZNF469 were classified as tolerated according to SIFT (Table 1).
Figure 1.
Sequence chromatogram of the seven novel mutations. A: c.2059G>A (p.E687K). B: c.2137C>A (p.P713T). C: c.3466G>A(p.A1156T). D: c.3749C>T (p.P1250L). E: c.4300G>A(p.D1434N). F: c.4684G>A (p.E1562K). G: c.7262G>A(p.R2421H).
Table 3 shows the characteristics of the seven patients with KC carrying the seven novel mutations in ZNF469. All patients with KC were diagnosed with bilateral KC, and 75% of the affected eyes were classified as having severe KC. The mean age of the seven patients was 19.2±4.2 years, and all were male. In terms of the videokeratography patterns, inferior steep (IS) was seen in 83.3% (ten) of the eyes with KC, irregular in 8.3% (one), and pellucid marginal degeneration (PMD) in 8.3% (one). Central corneal thickness (CCT) was examined in the patients with KC carrying the mutations in ZNF469 with videokeratography during the initial visit, and the mean values were 396±85.9 μm.
Table 3. ZNF469 mutations and quantitative and qualitative video-keratographic parameters evaluated in KC patients.
| Subject | ZNF469 mutations | Age at detection | Gender | Eye | Keratoplasty | Severity (OS/OD) | Video-keratography pattern |
|---|---|---|---|---|---|---|---|
| 1 |
c.3749C>T:p.P1250L |
23 |
Male |
OU |
No |
Severe/ Severe |
IS/IS |
| 2 |
c.3466G>A:p.A1156T |
20 |
Male |
OU |
No |
Severe/Moderate |
IS/IS |
| 3 |
c.2137C>A:p.P713T |
16 |
Male |
OU |
No |
Severe/ Severe |
IS/Irregular |
| 4 |
c.2059G>A:p.E687K |
13 |
Male |
OU |
No |
Mild/Severe |
IS/PMD |
| 5 |
c.4300G>A:p.D1434N |
24 |
Male |
OU |
Yes |
Severe/ Severe |
IS/IS |
| 6 |
c.4684G>A:p.E1562K |
19 |
Male |
OU |
No |
Severe/Mild |
IS/IS |
| 7 | c.7262G>A:p.R2421H | Na | Male | OU | No | Na/Na | Na/Na |
Discussion
In this study, seven novel mutations (c.2059G>A, c.2137C>A, c.3466G>A, c.3749C>T, c.4300G>A, c.4684G>A, and c.7262G>A) leading to the amino acid substitutions E687K, P713T, A1156T, P1250L, D1434N, E1562K, and R2421H, respectively, were identified in seven of the 53 patients with KC of Han Chinese ethnicity. The mutation in c.3466G>A coexists with a potentially damaging DOCK9 mutation in one patient with KC. All of the mutations were found in sporadic KC cases and were absent in the 30 patients with HM, the 100 healthy controls, and the results of the WES data acquired from the 220 Han Chinese individuals without ocular abnormalities. None of the seven mutations have been previously reported. To date, except the patient who carries mutations in ZNF469 and DOCK9, the identification of the six potentially pathogenic novel mutations in ZNF469 in 11.3% of the patients with KC in this study indicated that ZNF469 might play an important role in corneal diseases in the Han Chinese population.
ZNF469 is a two-exon gene encoding a 413 kDa protein consisting of 3,925 amino acid residues [25]. It is detected in a wide variety of tissues, including human corneas [16]. The five classical C2H2 zinc finger (ZNFs) domains in the C-terminus are the most important parts of the ZNF469 protein [16]. ZNFs can work as sequence-specific DNA-binding motifs to regulate specific transcription processes [16,26]. In addition, the ZNF469 protein shares 30% homology with the helical parts of three types of collagen (COL1A1, COL1A2, and COL4A1), which are the major component of the human cornea [27]. Abu et al. argued that ZNF469 may act as an extranuclear regulator factor for the synthesis and organization of human corneal collagen fibers [16], and functional mutations in ZNF469 may affect the homogeneity and regular properties of corneal collagen fibrils.
Four of the seven novel mutations (E687K, P713T, A1156T, P1250L, and D1434N) lie in the compositionally biased (CB) regions of ZNF469 [28], which are stretches that are primarily made up of a distinct subset of amino acid residues [28,29]. CB regions are highly correlated to the structural roles of the proteins in cells and usually lead to protein disorders [28].
The possible involvement of ZNF469 in corneal disease was first detected in Brittle cornea syndrome (BCS) type 1 [16,25,30]. BCS type 1 is an autosomal recessive disorder characterized by an extremely thin fragile cornea that tends to spontaneously rupture and shares the same corneal characteristics as KC [4]. In 2008, homozygous mutations in ZNF469 were reported to be responsible for BCS type 1 for the first time [16]. A wide variety of mutations in ZNF469 were then reported to confer an increased risk of isolated KC and BCS type 1 in patients of different ethnicities (23% of patients with KC from New Zealand, 50% KC patients of Maori or Polynesian descent, and 12.5% of patients with KC from three European KC cohorts [30-32]). However, there has been no clear consensus about whether ZNF469 is a pathogenic gene for patients with KC of all ethnicities. For example, sequencing analyses of ZNF469 in patients with KC and HM in Poland revealed no significant sequence variants compared with healthy individuals [4]. Similarly, Davidson et al. did not detect any non-synonymous ZNF469 variants segregating in 11 families with KC from the United Kingdom, Dubai, and Saudi Arabia [30]. It seems that the role of ZNF469 in corneal diseases might vary somewhat among different regions and races, and the present results indicated that the gene may have an important pathogenic role in the Han Chinese population.
Although all of the patients with KC carrying the mutations in ZNF469 in this study were heterozygote, which indicates an autosomal dominant inheritance pattern, no parents or siblings of the identified patients with KC were tested. Without this information, it is difficult to determine the genetic pattern and significance of ZNF469 in KC. The majority of recent pedigree studies based on a positive family history of KC suggested an autosomal dominant inheritance pattern [33]. However, some researchers hold the opinion that the inheritance pattern may be autosomal recessive or one that lies between autosomal recessive and dominant inheritance [34,35]. The disagreements about the inheritance pattern may be due to diverse penetrance caused by different mutations in the same gene.
Corneal steepening, as assessed with the use of computer-assisted corneal tomography, is a common feature of KC, even at the early stage, although it is highly variable among patients with KC. The most frequent axial curvature pattern of the KC eyes in this study was IS, quite different from the results of Li et al., who evaluated the characteristics of the corneal topography of patients with KC in a Chinese population [32]. In Li et al.’s study, patients with KC have obvious asymmetry, whereas in the present study, the patients with KC tended to show symmetry. This difference may be due to the different ages at onset, disease duration, disease severity, and inter-observer variability, among other factors [32]. In addition, whether the mutations in ZNF469 are related to the axial curvature pattern or other topography parameters requires further investigation.
In the present study, the mean CCT of patients with mutations in ZNF469 was 396 ± 85.9 μm, significantly thinner than in the HM group. CCT is a quantitative human ocular biometric parameter and a hallmark of KC, which has an estimated heritability of up to 95% [36]. Based on the results of genome-wide association studies (GWASs) from different populations (Croatian, Scottish, Indian, Malay, Caucasian, and Latino), Lu et al. performed a meta-analysis for CCT and KC and identified a significant pathogenic locus upstream of ZNF469 [18]. The combined data across diverse ethnic groups also support a consistent role of ZNF469 for CCT [20]. The fact that the ZNF469 protein shares 30% homology with the helical parts of three main corneal collagens (COL1A2, COL4A1, and COL1A1) of the human cornea suggests a potential mechanism involving structural contributions [16].
According to Karolak et al. [4], many potentially causative variants related to KC are also found in patients with HM. However, the pathogenesis mechanism of axial HM is completely different from that of KC. Thus, identification of the same variants in patients with axial HM and KC suggests that these variants are not causative for KC. In the present study, we also included an axial HM group. None of the seven mutations identified in the patients with KC were detected in the patients with HM.
The mutation c.3466G>A coexists with a potentially damaging DOCK9 mutation in one patient with KC. The patent is male and was diagnosed with bilateral KC at the age of 20 (Table 3). Both mutations are predicted to be damaging according to the SIFT score. Thus, the pathogenesis of this patient with KC is more complicated than for the others, and it is difficult to evaluate the effect of the ZNF469 mutation on this patient with KC.
Only mutations in ZNF469 identified in the patients with KC were screened in the controls. The negative results indicated only that there is a great chance that these seven mutations in ZNF469 are harmful nonsynonymous variants and that healthy people would not carry any of them. However, healthy controls may carry other mutations in ZNF469 that would not cause any ocular abnormality.
To date, the exact etiology of KC is still unclear. The present results suggest a possible role for ZNF469 in patients with KC in the Chinese population. Wright et al. reported that ZNF469 may be involved in the transforming growth factor beta (TGFβ) pathway, whose disturbance would lead to the disarray of collagens in human cornea [33,34]. However, it is still too early to definitively resolve whether variants in ZNF469 are causative for KC. Further analysis of the corneal characteristics of genetic manipulation of ZNF469 in animal models and corneal materials obtained from keratoplasty surgeries of carriers of mutations in ZNF469 may help uncover the potential role of ZNF469 in KC etiology. However, only a few parents or siblings of the identified patients with KC in this study were willing to take genetic tests for mutations in ZNF469. Thus, it is difficult to define the genetic pattern of ZNF469 and trace the origins of the mutations; a pedigree study would help to solve this difficulty. In conclusion, despite some caveats, the present results revealed the enrichment of the mutations in ZNF469 in patients with sporadic KC from a Han Chinese population for the first time, which indicate alleles in ZNF469 are potentially important genetic factors contributing to pathogenesis in Chinese patients with KC.
Acknowledgments
This work was supported by the Natural Science Foundation of China (Grant NO. 81371000 to Xingchao Shentu and Grant NO. 81670834 to Xingchao Shentu), the Zhejiang Key Laboratory Fund of China (Grant No. 2011E10006), the Natural Science Foundation of Zhejiang Province (Grant No. LY17H120002 to Binbin Chen and Grant No. LY14H120002 to Xiajiang Tang).
Appendix 1. Details about other KC related genes screened in this study
To access the data, click or select the words “Appendix 1.”
References
- 1.Kok YO, Tan GF, Loon SC. Review: keratoconus in Asia. Cornea. 2012;31:581–93. doi: 10.1097/ICO.0b013e31820cd61d. [DOI] [PubMed] [Google Scholar]
- 2.M. Edwards M S. Dean. The genetics of keratoconus. Clin Experiment Ophthalmol. 2001;29:345–51. doi: 10.1046/j.1442-9071.2001.d01-16.x. [DOI] [PubMed] [Google Scholar]
- 3.Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998;42:297–319. doi: 10.1016/s0039-6257(97)00119-7. [DOI] [PubMed] [Google Scholar]
- 4.Karolak JA, Gambin T, Rydzanicz M, Szaflik JP, Polakowski P, Frajdenberg A, Mrugacz M, Podfigurna-Musielak M, Stankiewicz P, Gajecka M. Evidence against ZNF469 being causative for keratoconus in Polish patients. Acta Ophthalmol. 2016;94:289–94. doi: 10.1111/aos.12968. [DOI] [PubMed] [Google Scholar]
- 5.Williams KALM, Keane MC, Jones VJ, Loh RSK, Coster DJ. The Australian Corneal Graft Registry 2012 Report. 2012. Available at: http://hdl.handle.net/2328/25860. 2012.
- 6.Parker J, Pavlopoulos G, Wolfe PJ, Rabinowitz YS. Videokeratopography of keratoconus in monozygotic twins. J Refract Surg. 1996;12:180–3. doi: 10.3928/1081-597X-19960101-31. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Rotter JI, Yang H. Genetic epidemiological study of keratoconus: evidence for major gene determination. Am J Med Genet. 2000;93:403–9. [PubMed] [Google Scholar]
- 8.Hamilton JB. Significance of heredity in ophthalmology: preliminary survey of hereditary eye diseases in Tasmania. Br J Ophthalmol. 1938;22:83–108. doi: 10.1136/bjo.22.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falls HFAA. Dominantly inherited keratoconus: report of a family. J Genet Hum. 1969;17:317–24. [PubMed] [Google Scholar]
- 10.Ihalainen A. Clinical and epidemiological features of keratoconus: genetic and external factors in the pathogenesis of the disease. Acta Ophthalmol. 1986;178:1–66. [PubMed] [Google Scholar]
- 11.Hao XD, Chen P, Chen ZL, Li SX, Wang Y. Evaluating the Association between Keratoconus and Reported Genetic Loci in a Han Chinese Population. Ophthalmic Genet. 2015;36:132–6. doi: 10.3109/13816810.2015.1005317. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Wei W, Zhang C, Zhang X, Liu M, Zhu X, Xu K. Association of Interleukin-1 Gene Single Nucleotide Polymorphisms with Keratoconus in Chinese Han Population. Curr Eye Res. 2016;41:630–5. doi: 10.3109/02713683.2015.1045083. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Jin T, Zhang X, Wei W, Cui Y, Geng T, Liu Q, Gao J, Liu M, Chen C, Zhang C, Zhu X. Common single nucleotide polymorphisms and keratoconus in the Han Chinese population. Ophthalmic Genet. 2013;34:160–6. doi: 10.3109/13816810.2012.743569. [DOI] [PubMed] [Google Scholar]
- 14.Lechner J, Bae HA, Guduric-Fuchs J, Rice A, Govindarajan G, Siddiqui S, Abi Farraj L, Yip SP, Yap M, Das M, Souzeau E, Coster D, Mills RA, Lindsay R, Phillips T, Mitchell P, Ali M, Inglehearn CF, Sundaresan P, Craig JE, Simpson DA, Burdon KP, Willoughby CE. Mutational analysis of MIR184 in sporadic keratoconus and myopia. Invest Ophthalmol Vis Sci. 2013;54:5266–72. doi: 10.1167/iovs.13-12035. [DOI] [PubMed] [Google Scholar]
- 15.Guan T, Liu C, Ma Z, Ding S. The point mutation and polymorphism in keratoconus candidate gene TGFBI in Chinese population. Gene. 2012;503:137–9. doi: 10.1016/j.gene.2012.04.061. [DOI] [PubMed] [Google Scholar]
- 16.Abu A, Frydman M, Marek D, Pras E, Nir U, Reznik-Wolf H, Pras E. Deleterious mutations in the Zinc-Finger 469 gene cause brittle cornea syndrome. Am J Hum Genet. 2008;82:1217–22. doi: 10.1016/j.ajhg.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Emma MM. Burkitt Wright, Helen L. Spencer, Sarah B. Daly, Forbes D.C. Manson, Leo A.H. Zeef, Jill Urquhart, Nicoletta Zoppi, Richard Bonshek, Ioannis Tosounidis, Meyyammai Mohan, Colm Madden, Annabel Dodds, Kate E. Chandler, Siddharth Banka, Leon Au, Jill Clayton-Smith, Naz Khan, Leslie G. Biesecker, Meredith Wilson, Marianne Rohrbach, Marina Colombi, Cecilia Giunta, Graeme C.M. Black. Mutations in PRDM5 in brittle cornea syndrome identify a pathway regulating extracellular matrix development and maintenance. Am J Hum Genet. 2011;88:767–77. doi: 10.1016/j.ajhg.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu Y, Vitart V, Burdon KP, Khor CC, Bykhovskaya Y, Mirshahi A, Hewitt AW, Koehn D, Hysi PG, Ramdas WD, Zeller T, Vithana EN, Cornes BK, Tay WT, Tai ES, Cheng CY, Liu J, Foo JN, Saw SM, Thorleifsson G, Stefansson K, Dimasi DP, Mills RA, Mountain J, Ang W, Hoehn R, Verhoeven VJ, Grus F, Wolfs R, Castagne R, Lackner KJ, Springelkamp H, Yang J, Jonasson F, Leung DY, Chen LJ, Tham CC, Rudan I, Vatavuk Z, Hayward C, Gibson J, Cree AJ, MacLeod A, Ennis S, Polasek O, Campbell H, Wilson JF, Viswanathan AC, Fleck B, Li X, Siscovick D, Taylor KD, Rotter JI, Yazar S, Ulmer M, Li J, Yaspan BL, Ozel AB, Richards JE, Moroi SE, Haines JL, Kang JH, Pasquale LR, Allingham RR, Ashley-Koch A, NEIGHBOR Consortium Mitchell P, Wang JJ, Wright AF, Pennell C, Spector TD, Young TL, Klaver CC, Martin NG, Montgomery GW, Anderson MG, Aung T, Willoughby CE, Wiggs JL, Pang CP, Thorsteinsdottir U, Lotery AJ, Hammond CJ, van Duijn CM, Hauser MA, Rabinowitz YS, Pfeiffer N, Mackey DA, Craig JE, Macgregor S, Wong TY. Genome-wide association analyses identify multiple loci associated with central corneal thickness and keratoconus. Nat Genet. 2013;45:155–63. doi: 10.1038/ng.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu Y, Dimasi DP, Hysi PG, Hewitt AW, Burdon KP, Toh T, Ruddle JB, Li YJ, Mitchell P, Healey PR, Montgomery GW, Hansell N, Spector TD, Martin NG, Young TL, Hammond CJ, Macgregor S, Craig JE, Mackey DA. Common genetic variants near the Brittle Cornea Syndrome locus ZNF469 influence the blinding disease risk factor central corneal thickness. PLoS Genet. 2010;6:e1000947. doi: 10.1371/journal.pgen.1000947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vithana EN, Aung T, Khor CC, Cornes BK, Tay WT, Sim X, Lavanya R, Wu R, Zheng Y, Hibberd ML, Chia KS, Seielstad M, Goh LK, Saw SM, Tai ES, Wong TY. Collagen-related genes influence the glaucoma risk factor, central corneal thickness. Hum Mol Genet. 2011;20:649–58. doi: 10.1093/hmg/ddq511. [DOI] [PubMed] [Google Scholar]
- 21.Zadnik K, Barr JT, Edrington TB, Everett DF, Jameson M, McMahon TT, Shin JA, Sterling JL, Wagner H, Gordon MO. Baseline findings in the Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study. Invest Ophthalmol Vis Sci. 1998;39:2537–46. [PubMed] [Google Scholar]
- 22.Zadnik K, Gordon MO, Edrington TB. Biomicroscopic signs and disease severity in keratoconus: Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study Group. Cornea. 1996;15:139–46. doi: 10.1097/00003226-199603000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Shentu X, Miao Q, Tang X, Yin H, Zhao Y. Identification and Functional Analysis of a Novel MIP Gene Mutation Associated with Congenital Cataract in a Chinese Family. PLoS One. 2015;10:e0126679. doi: 10.1371/journal.pone.0126679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shentu XC, Zhao SJ, Zhang L, Miao Q. A novel p.R890C mutation in EPHA2 gene associated with progressive childhood posterior cataract in a Chinese family. Int J Ophthalmol. 2013;6:34–8. doi: 10.3980/j.issn.2222-3959.2013.01.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rohrbach M, Spencer HL, Porter LF, Burkitt-Wright EM, Burer C, Janecke A, Bakshi M, Sillence D, Al-Hussain H, Baumgartner M, Steinmann B, Black GC, Manson FD, Giunta C. ZNF469 frequently mutated in the brittle cornea syndrome (BCS) is a single exon gene possibly regulating the expression of several extracellular matrix components. Mol Genet Metab. 2013;109:289–95. doi: 10.1016/j.ymgme.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gamsjaeger R, Liew CK, Loughlin FE, Crossley M, Mackay JP. Sticky fingers: zinc-fingers as protein-recognition motifs. Trends Biochem Sci. 2007;32:63–70. doi: 10.1016/j.tibs.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 27.Ihanamaki T, Pelliniemi LJ, Vuorio E. Collagens and collagen-related matrix components in the human and mouse eye. Prog Retin Eye Res. 2004;23:403–34. doi: 10.1016/j.preteyeres.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Dyson HJWP. Intrinsically unstructured proteins and their functions. Nat Rev Mol Cell Biol. 2005;6:197–208. doi: 10.1038/nrm1589. [DOI] [PubMed] [Google Scholar]
- 29.Hariison PM. Exhaustive assignment of compositional bias reveals universally prevalent biased regions: analysis of functional associations in human and Drosophila. BMC Bioinformatics. 2006;7 doi: 10.1186/1471-2105-7-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davidson AE, Borasio E, Liskova P, Khan AO, Hassan H, Cheetham ME, Plagnol V, Alkuraya FS, Tuft SJ, Hardcastle AJ. Brittle cornea syndrome ZNF469 mutation carrier phenotype and segregation analysis of rare ZNF469 variants in familial keratoconus. Invest Ophthalmol Vis Sci. 2015;56:578–86. doi: 10.1167/iovs.14-15792. [DOI] [PubMed] [Google Scholar]
- 31.Bykhovskaya Y, Margines B, Rabinowitz YS. Genetics in Keratoconus: where are we? Eye and vision (London, England). 2016;3:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vincent AL, Jordan CA, Cadzow MJ, Merriman TR, McGhee CN. Mutations in the zinc finger protein gene, ZNF469, contribute to the pathogenesis of keratoconus. Invest Ophthalmol Vis Sci. 2014;55:5629–35. doi: 10.1167/iovs.14-14532. [DOI] [PubMed] [Google Scholar]
- 33.Vincent AL. Vincent CAJ, Murray J. Cadzow, Tony R. Merriman, Charles N. McGhee. Mutations in the Zinc Finger Protein Gene, ZNF469, Contribute to the Pathogenesis of Keratoconus. Invest Ophthalmol Vis Sci. 2014;55:5629–35. doi: 10.1167/iovs.14-14532. [DOI] [PubMed] [Google Scholar]
- 34.Fullerton J, Foote S, Mackey DA, Williamson R, Forrest S. Identity-by-descent approach to gene localisation in eight individuals affected by keratoconus from north-west Tasmania, Australia. Hum Genet. 2002;110:462–70. doi: 10.1007/s00439-002-0705-7. [DOI] [PubMed] [Google Scholar]
- 35.Tyynismaa H, Sistonen P, Tuupanen S, Tervo T, Dammert A, Latvala T, Alitalo T. a locus for autosomal dominant keratoconus: linkage to 16q22.3-q23.1 in Finnish families. Invest Ophthalmol Vis Sci. 2002;43:3160–4. [PubMed] [Google Scholar]
- 36.Dimasi DP, Burdon KP, Craig JE. The genetics of central corneal thickness. Br J Ophthalmol. 2010;94:971–6. doi: 10.1136/bjo.2009.162735. [DOI] [PubMed] [Google Scholar]