Abstract
The majority of colon cancer patients will develop advanced disease with the liver being the most common site of metastatic disease. Patients with increased numbers of tumor-infiltrating lymphocytes in primary colon tumors and liver metastases have improved outcomes. However, the molecular factors which could empower anti-tumor immune responses in this setting remained to be elucidated. We reported that the immunostimulatory cytokine LIGHT (TNFSF14) in the microenvironment of colon cancer metastases associates with improved patient survival, and here we demonstrate in an immunocompetent murine model that colon tumors expressing LIGHT stimulate lymphocyte proliferation and tumor-cell specific anti-tumor immune responses. In this model, increasing LIGHT expression in the microenvironment of either primary tumors or liver metastases triggered regression of established tumors and slowed the growth of liver metastases, driven by cytotoxic T-lymphocyte mediated anti-tumor immunity. These responses corresponded with significant increases in tumor-infiltrating lymphocytes and increased expression of lymphocyte-homing signals in the metastatic tumors. Further, we demonstrated evidence of durable tumor-specific anti-tumor immunity. In conclusion, increasing LIGHT expression increased T-cell proliferation, activation, and infiltration, resulting in enhanced tumor-specific immune-mediated tumor regressions in primary tumors and colorectal liver metastases. Mechanisms to increase LIGHT in the colon cancer microenvironment warrant further investigation and hold promise as an immunotherapeutic strategy.
Introduction
Gastrointestinal cancers account for the majority of malignancies worldwide with the highest 5-year mortality. Most patients with colon cancer, the most common gastrointestinal tumor, present with advanced disease, resulting in it being the 2nd leading cause of cancer related deaths in the US.(1) The most common site of metastases in these patients is to the liver, and a combination of advances have increased the survival of patients with surgically resectable colorectal liver metastases (CRLM), however, long-term survival is rare even in these highly selected patients.(2-5) For the vast majority of patients, palliative chemotherapy is the only present option. Improved outcomes through new therapeutic strategies are desperately needed.
It has been demonstrated that tumor biology is accurately governed by the number and location of tumor infiltrating lymphocytes (TIL) invading a primary tumor. Specifically, the presence of activated and proliferating T-cells within primary colon tumors is associated with improved survival.(6,7) We have previously demonstrated an association between increased T-cell infiltrates and improved outcomes in patients with CRLM,(8) and our recent study of resected CRLM from 76 patients confirmed that increased numbers of TIL were associated with improved overall (OS) and recurrence-free survival (RFS) after surgical resection.(9) These observations validated the underlying concept that immunotherapy may play a viable role in managing patients with advanced gastrointestinal malignancies, including colon cancer.(10)
Through gene ontology analysis of resected CRLM, we have shown that LIGHT expression in the tumor microenvironment is significantly associated with both OS and RFS.(9) LIGHT (an acronym for homologous to lymphotoxins, shows inducible expression, and competes with herpes simplex glycoprotein D for HVEM, a receptor expressed by T lymphocytes) is a member of the Tumor Necrosis Factor Superfamily (TNFSF14) and is an immune-stimulatory cytokine that may augment the anti-tumor immune response.(11) CRLM at baseline have low levels of TIL and LIGHT expression(12), therefore, we hypothesized that T-cell infiltration of colorectal tumors and liver metastases could be enhanced by increased LIGHT expression in the tumor microenvironment, and that this strategy could result in tumor-specific immune-mediated tumor regressions.
Materials and methods
Animals, diet and cell lines
All experimental protocols followed NIH guidelines and were approved by the Institutional Animal Care and Use Committees of University of Illinois at Chicago. 6-8 week old female BALB/c mice (Charles River Laboratory, Wilmington, MA) were fed normal or doxycycline diet (TD.08434, Envigo, Madison, WI). 293T cells and a wtCT26 murine colorectal carcinoma cell line were obtained from the American Type Culture Collection (ATCC, Manassas, VA) and were grown in DMEM or RPMI 1640 culture medium (Life technology, Grand Island, NY) supplemented with 10% fetal bovine serum (Invitrogen, Waltham, MA), respectively. All cell lines were authenticated, either by ATCC or IDEXX (4T1), tested for mycoplasma, and used at low passage numbers within 6 months of thawing (2014-2016). To establish a stable CT26LIGHT cell line, transfectants were selected with puromycin or G418 and drug resistant clones were further selected for LIGHT expression by limited dilution cloning. For the inducible expressing LIGHT CT26 cell line (CT26LIGHTi), drug resistant clones were transfected with pLVX-LIGHT. LIGHT expression on the cell surface was confirmed using LTβR-Ig as the primary antibody, confirming its functional ability to bind LTβR. Mouse blood doxycycline concentration was measured at three and 22 days after initiating diet, confirming non-measurable amounts in animals fed regular chow, and significantly increased levels in doxycycline fed animals as determined using an ELISA Kit per manufacturer's protocol. Serum levels three days after returning to normal diet were non-measurable similar to controls. Amount of chow injested was calculated daily and not different between groups.
Antibodies, enzymes, reagents
Reagents were supplied by: anti-mouse CD3 (145-2C11) and anti-mouse CD28 (37.51) mAbs (BD PharMingen, San Diego, CA); Donkey anti-human IgG PE, anti-CD45 FITC, anti-CD8 Pecy7, anti-CD3 APC, anti-CD4 PE, anti-CD25 Pacific blue, anti-CD69 Percp-cy5.5, anti-CD49b Pecy7, anti-CD11c pacific blue, anti-CD80 PE, anti-CD86 APC, IFN-γ ELISA kits, Annexin V Apoptosis Detection Kit , and CFSE (5-(and 6)-Carboxyfluorescein diacetate succinimidyl ester) (eBiosciences , San Diego, CA); Pacific blue conjugated anti-mouse CD4 (GK1.5) (Biolegend, San Diego, CA); anti-mouse LIGHT (R&D, Minneapolis, MN); anti-mouse CD3 (sp7) (Abcam, Cambridge, MA); HRP-conjugated goat anti-rabbit IgG (Santa Cruz Biotechnology, Santa Cruz, CA); CalPhos™ Mammalian Transfection Kit, Lenti-X Tet-One Inducible Expression Systems, G418, and doxycycline (Clontech, (Mountain View, CA); plasmid DNA purification kit (QIAGEN, Valencia, CA); collagen Type I (BD Bioscience San Jose, CA); lipofectamine® 2000, puromycin, and Trizol reagent (Invitrogen Waltham, MA); aqueous non-radioactive cell proliferation assay kit (Promega, Madison, WI); Flt3, recombinant LIGHT, collagenase IV and DNase I (Sigma, St. Louis, MO); MluI, PstI, BamHI, EcoRI, T4 DNA ligase, and DH5 alpha competent E. coli cells (New England Biolabs (Ipswich, MA). For in vivo lymphocyte depletion experiments; anti-CD3 (145-2C11), anti-CD8 (YTS 169.4) and anti-CD4 (GK1.5) were purchased from Bio X Cell (West Lebanon, NH,USA), and anti-asialo GM1 from Wako Chemicals (Richmond, VA, USA). Mice were injected intraperitoneal with 100μg anti-CD3 mAb, 200μg anti-CD8mAb, 200μg anti-CD4 mAb or 20ul anti-asialo GM1 antibody on day -3, 0 and 7 (Day 0 is the day of primary tumor injection). Depletion was confirmed by flow cytometry of peripheral blood four days after the first injection.
Western blot
Protein concentrations in cell lysates were determined using a Bio-Rad protein assay kit (Bio-Rad, Hercules, CA). Lysates containing 25μg of protein were resolved on SDS-PAGE and transferred to PVDF membranes (Bio-Rad, Hercules, CA), which were blocked for 1h at room temperature in PBS containing 5% BSA and 0.1% Tween-20, and then incubated 2h with primary antibiodies, washed, and detected using HRP-conjugated IgG revealed by an enhanced chemiluminescence detection system (Amersham).
Cell staining and Flow cytometry analysis
Cells were harvested and suspended in 100μl PBS containing 0.5% fetal bovine serum, incubated at 4°C for 30 minutes after adding fluorochrome conjugated antibodies, washed, and analyzed using a cyan ADP analyzer (Beckman Coulter, Brea, CA). Events were collected and analyzed using Flow Jo software (Tree star Incorporated, Ashland, OR).
In vitro CT26 cell proliferation, apoptosis, and migration analysis
Cell proliferation was measured using a colorimetric non-radioactive cell proliferation assay kit (Promega, Madison, WI) in triplicate. Absorbance at 450nm was determined using a Bio-Rad iMarker™ microplate reader (Hercules, CA, USA). For cell apoptosis studies, cells were seeded in triplicate, harvested, and resuspended with fluorochrome-conjugated Annexin V. Propidium iodide staining solution was added and cells were analyzed using flow cytometry within 4 hours. 3D cell migration assays were performed as previously described.(13) Briefly, 105 cells were embedded in 20μl of type I collagen gel (2.0 mg /mL, BD Biosciences). After gelling, the plug was embedded in cell-free collagen gel (2.0 mg/mL) in a 24-well plate. After culture for another 3 days, invasion distance from the inner collagen plug to the outer collagen gel was determined with a phase contrast microscope (Carl Zeiss, Zeiss Drive Thornwood, NY, USA) and quantified using Image- J software.
T-cell isolation, cytokines production, and proliferation assays in vitro
96-well round bottom plates were coated with anti-CD3 and stored at 4°C overnight. These were then were coated with rLIGHT for 4h at 37°C. T-cells from the spleen or draining lymph nodes, as indicated, were enriched using the Pan T-cell Isolation Kit II (Miltenyi Biotec, Auburn, CA), labeled with 5-and 6-carboxyfluorescein diacetate succinimidyl ester (CFSE) (eBioscience, San Diego, CA), incubated at 37°C for 72 hours, and analyzed by FACS.
For T-cell cocultures with CT26LIGHT, 2×105 purified pan T and 2×104 15,000rad irradiated CT26Control or CT26LIGHT cells were seeded into a round-bottom 96-well plate containing anti-CD3 beads (2.0 μg/ml). The cells were cultured in the presence or absence of low dose (5μg/ml) LTβR-Ig or control-Ig for 3 days. 1μCi 3H-TdR was added for the last 12 hours of culture and incorporation was measured with a topcount microplate scintillation counter. For cytokine production, cell culture supernatants were collected after 48 hours and IFNγ was detected by ELISA following the manufacturer's instructions (eBiosciences).
Bone marrow derived dendritic cells differentiation assay and activation in vitro
Bone marrow derived dendritic cells were harvested from 6-8 week old BALB/c mice and cultured in complete media with Flt3 ligand at 200ng/ml.(14) On day 6, dendritic cells were exposed to 100–1000ng/mL of soluble LIGHT or LPS. On Day 8, cells were stained for FACS analysis with conjugated monoclonal antibodies.
Mouse models of colorectal cancer
For subcutaneous models, 1×106 tumor cells in 100μL PBS were inoculated subcutaneously in the mouse flank. In the inducible models, doxycycline diet was initiated when the tumor size reached ∼200mm3. Tumor growth was monitored and volumes were calculated using the equation 1/2(long diameter × short diameter2). Clinical responses were evaluated by WHO (≥50% decrease in the product of the greatest perpendicular tumor diameters) and RECIST (≥30% decrease in the maximum tumor diameter) criteria. Models of colorectal cancer liver metastases were performed as we have previously described.(12) In brief, 1×106 cells in 50μl of PBS were injected into the spleen parenchyma followed by splenectomy. Mouse MRI was performed as previously described.(15) Briefly, imaging was performed using a 9.4T small-animal magnetic resonance imager (Agilent Technologies, CA), gradient strength 1000mT/m, and 39 mm ID birdcage volume RF coil. Mice were anesthetized and vital signs were continuously monitored. An MRI-compatible small animal gating system (SA Instruments Inc., NY) was used to permit free-breathing acquisition during MRI scanning. Axial images were obtained using a T2-weighted fast-spin echo sequence with external trigger.
Cytotoxic T lymphocyte assay
Splenocytes were cocultured with target cells irradiated at 15000rad in 6 well plates in the presence of 20IU/ml mouse IL-2 for 5 days. WTCT26 cells or 4T1 cells were labeled with 300μCi 51Cr at 37°C for 1 hour and washed. Labeled target cells (1×104 cells/200μl/well) and serial dilutions of effector cells were incubated in RPMI 1640 containing 5% FBS in triplicates in u-bottom 96 well plates at 37°C for 4 hours. 50ul cell culture supernatants were transferred to a 96 well reading plate after being centrifuged and percentage of lysis was determined (experimental –target spontaneous)/(target maximum-target spontaneous)*100. Results were expressed as the percentage of specific or nonspecific lysis.
Isolation of tumor infiltrating cells (TIL) from liver metastases
Tumor infiltrating cells were isolated as we have previously described.(12) In brief, mice were euthanized, livers were immediately perfused via the portal vein with 5mL of PBS, and metastatic liver tumors were isolated and resected. 0.5g sections of each tumor were minced into small pieces and digested in 10mL RPMI containing 2.5% FBS, collagenase IV (1 mg/ml, Sigma), and DNase I (200μg/ml, Sigma). These were then strained to obtain a single cell suspension, and cell viability was determined by trypan blue exclusion, which was >90%.
Peripheral blood mononuclear cell (PMBC) isolation
Peripheral blood mononuclear cells were isolated as previously described.(12) In brief, following euthanasia, mouse peripheral blood was obtained via retro orbital bleed. Specimens were loaded to the top of a Ficoll-Hypaque centrifugation column and the lymphocyte-enriched layer was collected and red blood cells were lysed with ACK lysing buffer (Invitrogen, Grand Island, NY, USA).
RNA extraction from liver tumor metastases
Surgically dissected liver metastases (CRLM) were immediately frozen in liquid nitrogen and stored at -70°C. 1ml of Trizol reagent (Invitrogen) per 50-100mg of tissue was added to tumor tissue after frozen specimens were the pulverized (Biopulverizer, BioSpec Products, Bartlesville, OK). 0.2 ml of chloroform per 1 ml of TRIZOL® Reagent was added to the tissue-Trizol mixture to dissociate nucleoprotein complexes. The RNA pellet was washed and dissolved in RNase-free water.
Real time quantitative RT-PCR assay
5μg of total RNA was reverse transcribed into complementary DNA by using the high-capacity cDNA reverse transcription kit (AB Applied Biosystems, Waltham, MA) and Real-time quantitative PCR analysis was performed (Bio-Rad CFX connect real time system, Hercules, CA). Each cDNA sample was amplified in triplicate for CCL21, MAdCAM-1 and GAPDH using the Fast SYBR green master mixture kit in accordance with the manufacturer's instructions (AB Applied Biosystems, Waltham, MA). The primer sequence for CCL21 was 5′-AGA CTC AGG AGC CCA AAG CA-3′ (forward primer) and 5′-GTT GAA GCA GGG CAA GGG T-3′ (reverse primer). The primer for MAdCAM-1 was 5′-GAC ACC AGC TTG GGC AGT GT-3′ (forward primer) and 5′-CAG CAT GCC CCG TAC AGA G -3′ (reverse primer). The primer for GAPDH was 5′-AAC TTT GGC ATT GTG GAA GG-3′ (forward primer) and 5′-CAC ATT GGG GGT AGG AAC AC-3′ (reverse primer). PCR conditions were 20sec. at 95C, 3sec. at 95C, 30sec. at 60C for 40 cycles. The concentration of the target gene was determined by the comparative CT method and normalized to the internal GAPDH control.
Tumor tissue Immunofluorescence
Tumors were fixed in 10% neutral buffered formalin and paraffin-embedded. 5μm tissue sections were deparaffinized, microwaved while boiling for 15 minutes in ph6 citrate antigen retrieval buffer, treated with blocking buffer and incubated with anti-CD3 optimized at a dilution of 1:750 (Abcam, clone SP7). Slides were then incubated with ImmPRESS goat anti-rabbit peroxidase polymer (Vector Laboratories, MP-7451). Signal amplification was performed using Opal 520 tyramide signal amplification reagent (PerkinElmer). A second microwave step was performed to remove labeled primary and secondary antibodies, leaving fluorescent label directly bound to the target site. Slides were counterstained with DAPI and coverslipped using Vectashield antifade mounting medium (Vector Laboratories, H-1400). Slides were scanned at 20× on a Vectra multispectral imaging system (PerkinElmer). Using inForm software (PerkinElmer), the CD3-positive cells and total CT26 cells were quantified, and CD3 cells per 100 tumor cells was determined. Individual 20× fields were stitched into whole slide images using a custom routine in Matlab (Mathworks). All imaging analyses were conducted by personnel who were blinded to the treatment status of the samples.
Statistical analysis
Statistical analysis was performed by two tailed Student's t-test. Error bars represent SEM. Statistical significance was considered for *p<0.05 and **p<0.01.
Results
Characterization of constitutive and inducible LIGHT expressing colon cancer cell lines
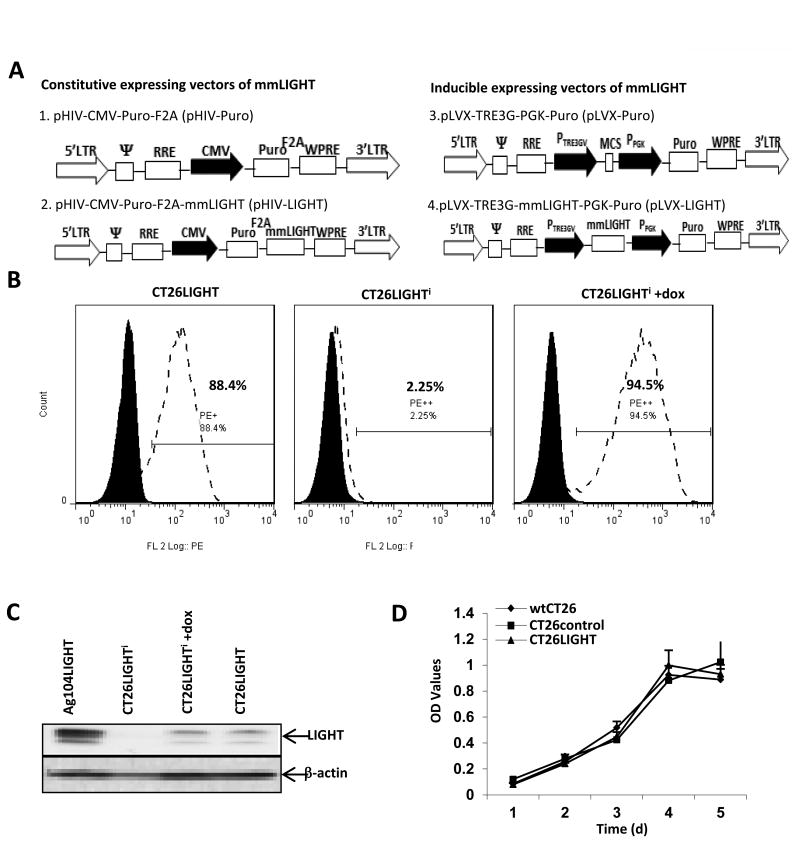
CT26 murine colon carcinoma cells were transfected with pHIV-LIGHT (constitutive expression) or pLVX-LIGHT Plus pTet (inducible expressioni) plasmids (Fig 1a). Stable cell lines demonstrated LIGHT expression in 88% of CT26LIGHT cells and 95% of CT26LIGHTi cells, respectively, on the cell surface. Western blot of whole cell lysates confirmed constitutive and inducible expression (Fig 1b,c). To establish that transfected CT26 cells maintained similar characteristics to wtCT26 cells; proliferation, apoptosis, and migration assays were performed. Cell morphology was assessed with bright-field microscopy and revealed no observable differences between the cells. CT26LIGHT cells demonstrated similar cell growth/proliferation and survival to wtCT26 and CT26control (empty vector) cells. (Figure 1d, Supplemental Figure 1a). 3D migration assays revealed no difference in the relative distance travelled of wtCT26, CT26control, and CT26LIGHT cells (Supplemental Figures 1b, c). CT26LIGHTi cells also displayed similar cell growth and apoptosis to wt and control cells, and did not migrate less than wt or control cells. Thus, the expression of LIGHT on CT26 cells did not alter the growth pattern or tumorigenesis of wtCT26 cells in vitro.
Figure 1. LIGHT expressing colon cancer cell lines maintain wild-type characteristics.
(A) To construct constitutive and doxycycline inducible(i) expressing LIGHT lentiviral vectors, murine LIGHT cDNA was cloned into MluI/PstI sites of the pHIV-Puro (pHIV-LIGHT) and pLVX-TRE3G (pLVX-LIGHT) parental plasmids. (B) CT26 cell clones constitutively expressing LIGHT (CT26LIGHT) or inducible expressing LIGHT (CT26LIGHTi) or its empty control (CT26controli) were established after drug selection and limited dilution, and LIGHT expression was analyzed using FACS after DNA sequencing. (C) LIGHT protein expression was confirmed in CT26LIGHT cells and CT26LIGHTi cells in the presence of doxycycline. The LIGHT expressing murine fibrosarcoma cell line Ag104LIGHT served as a positive control. (D) wtCT26, CT26control (empty vector), CT26controli, CT26LIGHTand CT26LIGHTi cells were evaluated for proliferation, apoptosis, and migration. Representative experiments are shown of the constitutive expressing cell line (see also supplemental figure 1). Cells were seeded in triplicate and MTS/PMS solution was added on days 1 2, 3, 4, and 5 with no difference in cell proliferation or metabolic activity.
LIGHT induces T-cell proliferation, activation, and DC maturation in vitro
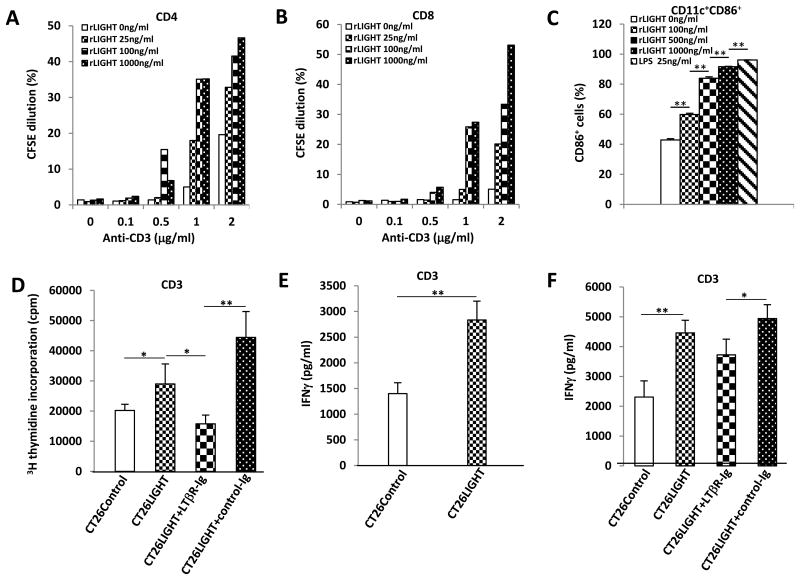
Purified T-cells cultured with immobilized recombinant LIGHT experienced increased CD4 and CD8 cell proliferation with increasing doses of LIGHT (figure 2a, b). Thus, the purified extracellular domain of LIGHT was sufficient to costimulate purified T-cells in vitro. As the adaptive immune response appeared to be activated by LIGHT expression in the tumor microenvironment, its effect on dendritic cells (DCs) was examined. Bone marrow derived DCs were treated in vitro with soluble LIGHT for two days and analyzed for surface expression of the DC cell maturation and activation marker CD86 (Fig. 2c). CD86 increased in a dose-dependent manner to levels comparable with LPS control, indicating that LIGHT can stimulate DC maturation and expression of signals necessary for T-cell activation and survival.
Figure 2. Recombinant LIGHT and LIGHT expressing colon cancer cells enhance T-cell and dendritic cell activation in vitro.
(A,B) CSFE-labeled T-cells were added to round-bottom 96-well plates coated with anti-CD3 and rLIGHT. CD4 and CD8 T-cell proliferation increased with increasing doses of rLIGHT. Shown are representative results of three independent experiments. (C) Bone marrow derived dendritic cells (DC) were exposed to 100–1000ng/mL of soluble LIGHT or LPS as a positive control. Significantly increased DC cell activation was observed with increasing doses of soluble LIGHT. (D) Purified T-cells were cocultured with CT26Control or CT26LIGHT cells revealing increased cell proliferation in the presence of LIGHT that was inhibited by the addition of LTbR-Ig mediated blockade of LIGHT's cognate receptor. (E,F) Cell culture supernatants were collected after 48 hours and IFNγ was detected by ELISA. Increased T-cell IFNγ production was observed in the presence of CT26LIGHT compared to CT26control. (F) Blocking LIGHT with LTbR-Ig decreased T-cell IFNγ production compared to control Ig. (* p<0.05, **p<0.01).
Similar to immobilized recombinant LIGHT, CT26LIGHT cells cocultured with purified T-cells resulted in significantly increased T-cell proliferation. To determine that the proliferation was secondary to LIGHT binding to its receptor on T-cells, cells were co-incubated with LTβR–Ig which substantially blocked cell proliferation compared to CT26LIGHT cells treated with control Ig (Fig. 2d). In addition, T-cells incubated with CT26LIGHT significantly increased IFN-g secretion over control, and treatment with LTβR–Ig significantly decreased IFN-g production compared to treatment with control Ig (Fig. 2e,f). Thus, CT26LIGHT cells stimulated T-cell proliferation and activation, and tumor cell expressed LIGHT could act as a costimulatory signal with engagement of the T-cell receptor.
Colon cancer cell tumors expressing LIGHT stimulated lymphocyte proliferation and tumor-cell specific anti-tumor immune responses in vivo
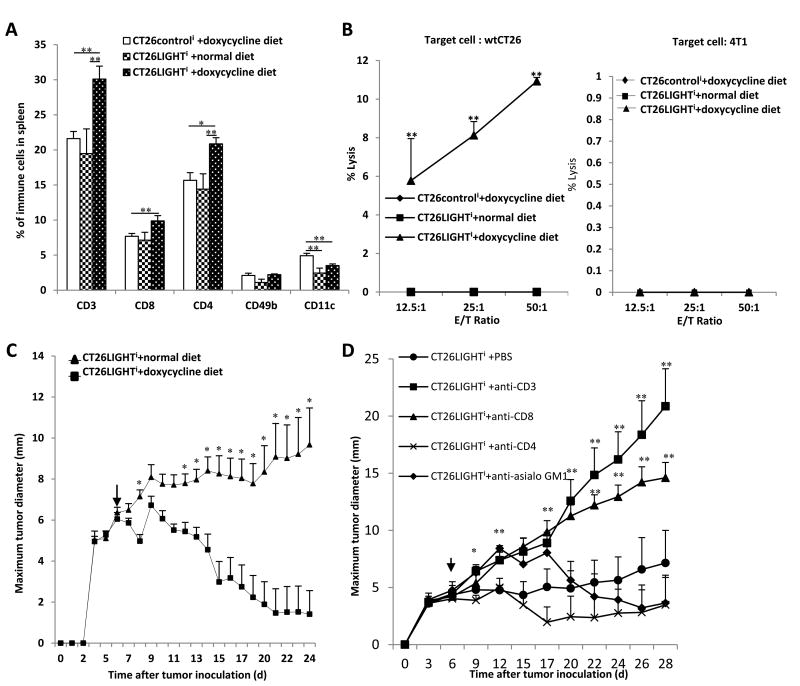
After establishing lymphocyte proliferation and activation in vitro upon exposure to LIGHT expressing colon cancer cells, we wished to determine if LIGHT in the in vivo tumor microenvironment could stimulate a similar systemic adaptive immune response. CT26LIGHTi or CT26controli tumors were established in BALB/c mice. Once palpable and measurable tumors were established, half the CT26LIGHTi mice and all CT26controli mice were fed doxycycline diet to stimulate LIGHT expression. Splenocytes were analyzed after 4 weeks for T-cell populations. The percentage of CD3, CD4 and CD8 positive T-cells significantly increased only in animals with LIGHT expressing tumors compared to both non-induced CT26LIGHTi tumors and CT26control tumors from animals fed doxycycline diet (Figure 3a).
Figure 3. LIGHT expressing tumors generated increased T-cell proliferation, a tumor-specific anti-tumor immune response, and significant T-cell mediated tumor regressions.
(A) Primary flank colorectal cancer cell tumors were established in BALB/c mice. Three weeks after tumor inoculation splenocytes were harvested (n=15). Mice with induced LIGHT expression (+ doxycycline diet) demonstrated increased numbers of CD3, CD4, CD8, and CD11c+ cells compared to CT26LIGHTi tumors fed normal diet and CT26controli tumors fed doxycycline diet. (B) Splenocytes from animals with established colon cancer flank tumors were then used as effector cells, and their cytolytic capacities were assessed against 51Cr-labeled 4T1 cancer cells or wtCT26 tumor cells. Only lymphocytes from animals with LIGHT expressing CT26 tumors demonstrated tumor-specific cytotoxicity against wtCT26 and not 4T1 cells. (C) CT26LIGHTi colon cell tumors were established in animals fed normal diet (n=18) and once palpable and measurable, randomized on day 6 (arrow) to doxycycline diet (LIGHT induction) or normal diet. Significant tumor regression began only in animals with LIGHT induction and continued with partial and complete tumor responses as measured by total tumor volume, WHO criteria, and RECIST clinical tumor response criteria (pictured). (D) Lymphocyte depletion was performed of specific immunocyte subsets. CT26LIGHTi colon cell tumors were established in animals and on day 6 (arrow) received doxycycline diet (LIGHT induction) as before, confirming significant tumor regressions in animals with LIGHT induction. The anti-tumor immune response was abrogated in the absence of CD3 and CD8 T-lymphocytes.
Given that LIGHT expression on CT26 colon cancer tumor cells induced systemic lymphocyte proliferation, we wished to determine if these lymphocytes were tumor-specific and cytotoxic. The lymphocytes were used as effector cells and their cytolytic potential was assessed against 51Cr-labeled target tumor cells in a standard 51Cr release assay (Fig. 3b). Splenocytes from mice with LIGHT expressing tumors lysed wtCT26 cells with increasing potency and to a significantly greater degree than splenocytes from either CT26control mice fed doxycycline or CT26LIGHTi mice fed regular diet, where no cytolytic activity was demonstrated. These same lymphocytes from all three groups had no cytotoxic activity against syngeneic breast cancer 4T1 cells, suggesting an anti-tumor immune specific response (Fig 3b).
LIGHT expression in the tumor microenvironment induces significant T-cell infiltration and tumor regression of established tumors
Since LIGHT expressing tumors appeared to stimulate T-cell proliferation and tumor cell-specific cytotoxicity in vivo, we wished to evaluate for a clinically relevant effect of LIGHT expression in the tumor microenvironment by using established colon cancer cell tumors. CT26LIGHTi palpable and measurable tumors were established. Animals were then randomized and half received doxycycline diet to stimulate LIGHT expression. Mice with induced LIGHT expression experienced rapid and significant anti-tumor responses that were durable and persisted, as compared to control animals that approached humane endpoints and had to be sacrificed (Fig. 3c). Tumors experienced both partial and complete responses and met clinical WHO and RECIST tumor response criteria for significant tumor regressions. Responders experienced durable tumor regressions that persisted for over five weeks. When LIGHT induction (doxycycline diet) was discontinued, responders continued to experience durable tumor regression for weeks until sacrifice and many had complete tumor regression.
T-cell depletion experiments were performed to further confirm the induction of anti-tumor immunity in the model. The anti-tumor immune response induced by LIGHT was significantly abrogated when animals were depleted of CD3 T-cells and, more specifically, depleted of CD8 T-cells in particular (Figure 3d).
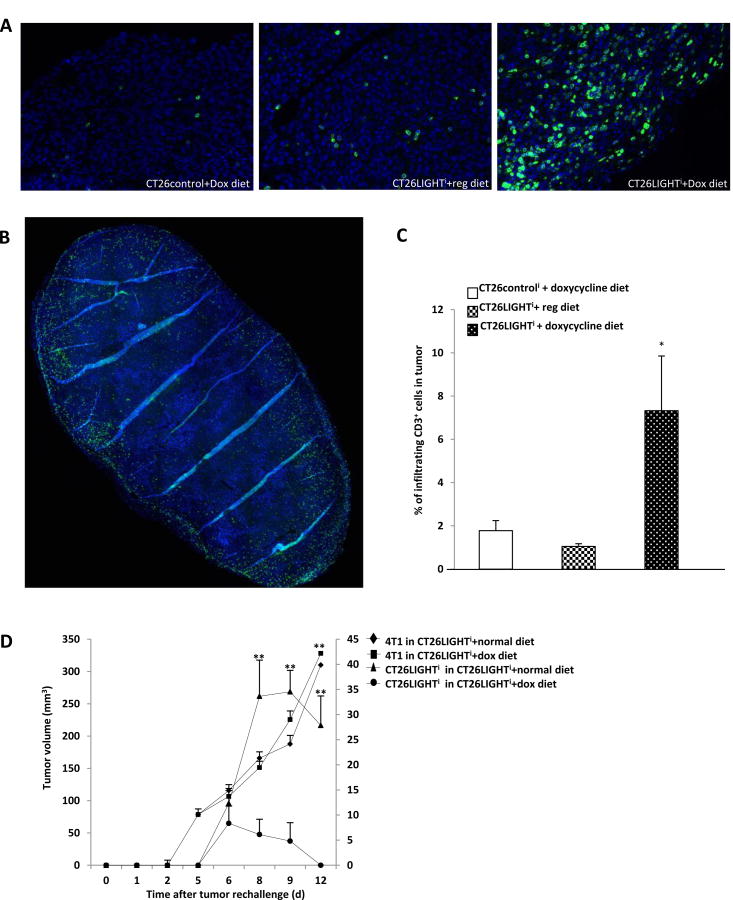
In additional experiments, tumors were harvested prior to complete pathologic response in order to evaluate and quantify tumor infiltrating lymphocytes (Fig. 4a,b). LIGHT expressing tumors contained greater than 7 times the percentage of CD3+ cells in the lesions compared to non-LIGHT expressing tumors (Fig. 4c). The amount of tumor infiltrating lymphocytes was similarly significantly increased compared to CT26controli cells that were also fed doxycycline diet.
Figure 4. LIGHT expressing tumors induced increased tumor infiltrating lymphocyte infiltration and generated persistent anti-tumor immunity.
(A) Tumors were harvested 21 days after tumor inoculation (n=15). Individual 20× fields on Vectra multispectral imaging system were stitched into whole tumor images (B), the total number of CD3-positive cells and colon cancer cells in the tumor were quantified, and CD3 cells per 100 tumor cells was determined to be significantly increased in LIGHT expressing tumors (C). (D) Mice with established CT26LIGHTi tumors (n=8 per group) were injected subcutaneously with 4T1 syngeneic breast cancer cells in the contralateral superior flank and with CT26LIGHTi in the contralateral inferior flank. 4T1 tumors grew rapidly and to similar size in mice from both groups after the tumor challenge. On the other hand, CT26LIGHTi challenge tumors from mice previously exposed to LIGHT expressing tumors grew minimally and then disappeared, while the challenge tumors in mice fed regular diet grew significantly larger. Tumor volumes of 4T1 are on the left y-axis, and volumes of CT26 are on the right y-axis. *p<0.05 and **p<0.01.
LIGHT-induced tumor regression instills tumor specific anti-tumor immunity
To determine whether the anti-tumor immunostimulatory effect of LIGHT expression in the tumor microenvironment could induce long-term immune responses to distal tumors, mice (n=16) were inoculated with CT26LIGHTi cells in the right flank. After establishment of palpable tumors, half the mice were fed with doxycycline diet to stimulate LIGHT expression. Fourteen days later, the eight mice fed doxycycline diet were switched back to normal diet. Three days later, after all doxycycline was confirmed to have left the system, mice were injected subcutaneously with 4T1 syngeneic breast cancer cells in the contralateral superior flank and with CT26LIGHTi in the contralateral inferior flank. 4T1 tumors grew rapidly and to a similar size in mice from both groups after the tumor challenge. On the other hand, CT26LIGHTi challenge tumors from mice previously exposed to LIGHT expressing tumors grew minimally and then disappeared, while the challenge tumors in mice fed regular diet grew significantly larger (Fig. 4d). The same anti-tumor immune response and inability for tumors to grow was established whether wtCT26 or CT26LIGHTi were utilized as challenge tumors in this experiment. Mice were sacrificed when humane endpoints were reached with 4T1 tumors and CT26 tumors in the control animals.
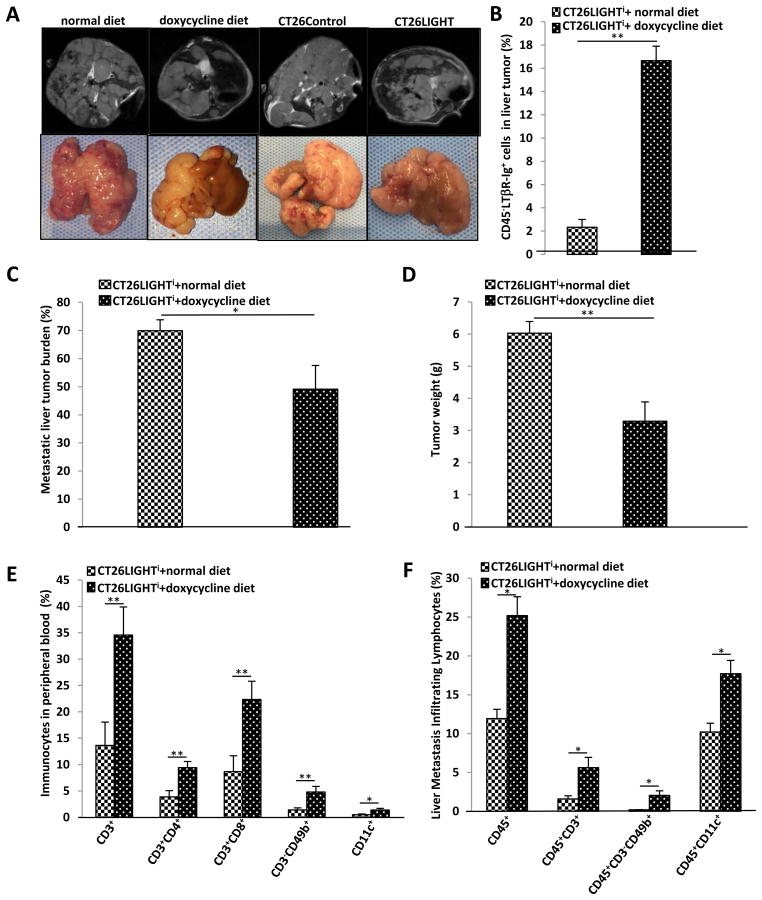
Enhanced LIGHT expression increased tumor infiltrating lymphocytes and lymphocyte homing signals in metastatic liver tumors with associated inhibited growth of colorectal liver metastases
Colorectal liver metastases were established in animals (Fig. 5a) and half of the animals were randomized to express LIGHT. On day 25 livers were harvested for tumor assays. Significantly increased LIGHT expression was confirmed in the induced tumors as compared to control tumors (Fig. 5b). The experiment was also repeated with constitutively expressing CT26LIGHT tumors. In both models, tumor burden was significantly decreased in the LIGHT expressing tumors. Quantitative measurements of metastatic burden were determined by liver tumor area and total liver weights. In both measurements, LIGHT expression was associated with significant decreases in metastatic liver tumor burden (Fig. 5c,d)
Figure 5. LIGHT inhibits colorectal liver metastases (CRLM) growth and increases metastatic tumor infiltrating lymphocytes.
(A) CRLM were established with CT26LIGHT and CT26LIGHTi colon cancer cells and tumor burden was followed with MRI imaging. (B) CT26LIGHTi CRLM (n=7 per group) were resected on day25. Increased LIGHT expression was confirmed in CRLM tumors from animals fed doxycycline. (C) The percent of affected liver was quantified with imaging software and was significantly decreased in tumors expressing LIGHT. (D) Total tumor burden as measured with liver weight was similarly significantly decreased. (E) Peripheral blood mononuclear cells from animals harboring LIGHT expressing CRLM demonstrated increased populations of CD3, CD4, and CD8 T-cells, NK cells, and DC cells. (F) LIGHT expressing CRLM similarly recruited increased numbers of total lymphocytes, CD3 cells, NK cells, and dendritic cells into the tumor.
Peripheral blood from these animals revealed significantly increased percentages of CD3+, CD4+, CD8+, CD49b+, and CD11c+ cells from LIGHT expressing animals compared to controls (Fig. 5e). Evaluation of liver tumor single cell suspensions revealed significantly increased CD45+ tumor infiltrating leucocytes in LIGHT expressing compared to non-LIGHT expressing metastatic liver tumors. Specifically, CD3+ lymphocytes, CD3-CD49b+ natural killer cells, and CD11c+ DCs were found in significantly increased percentages in LIGHT expressing metastases compared to controls (Fig. 4f). Interestingly, CD86 was significantly increased in CD11c DC cell populations only in LIGHT expressing metastases, similar to results observed in the in vitro experiments (Supplemental Fig 2a). In addition, transcript expression of the lymphocyte homing signals CCL21 and MadCAM-1 were found to be increased 3-fold and 2-fold, respectively, in LIGHT expressing metastases (Supplemental Fig 2b).
Discussion
For patients with CRLM, the best current chemotherapy regimens are not curative and provide a median overall survival of approximately 20 months.(16-18) To improve outcomes for these patients new strategies are needed. Our previous studies, along with those from other groups, have demonstrated an association between increased T-cell infiltration in resected tumors and long-term survival. (6,19-26) From gene analysis data and immunohistochemistry of resected CRLM it was inferred that overall survival may be a function of multiple host and tumor variables, including T-cell infiltration, but that tumor recurrence is predominantly determined by the ability of the host to mount, and the tumor to support, lymphocyte proliferation, activation, and cytotoxicity.(9) Thus, it follows that immunotherapy may be promising approach for advanced colon cancer.
The use of immunotherapy for gastrointestinal cancers, however, has met significant challenges. Vaccines may increase circulating tumor specific lymphocytes but appear to lack the ability to impart significant tumor regressions even when cancer-associated antigens are utilized.(27,28) Checkpoint blockade with antibodies including Ipilimumab, which have demonstrated anti-tumor activity in other malignancies,(29,30) have not been successful in microsatellite stable advanced colon cancer(31), and furthermore, function via imprecise immune activation that impart significant autoimmunity in a proportion of patients. Adoptive cell transfer, a strategy that has been used successfully in a subset of patients with melanoma, renal cell cancer, and synovial cell sarcoma, is challenging to utilize in colon cancer patients due to self-antigens, and a recent trial using T-cells engineered against the carcinoembryonic antigen demonstrated dose limiting toxicity.(32) The main limitation to this approach has been the lack of known colon cancer specific tumor antigens and in identifying a treatment strategy that can be utilized across a spectrum of patients. Thus, there is a gap in our knowledge of how to enhance TIL activation and proliferation in the tumor microenvironment of CRLM, and we propose a strategy that focuses on immunostimulation of naturally occurring TIL at the level of the tumor.
LIGHT plays a key role in T-cell homeostasis and proliferation, is produced by activated T-cells, and mediates T-cell activation, survival, and death.(33-35) Our current research supports the ability of LIGHT to stimulate effector T-cell proliferation and differentiation both in vitro and in vivo, and highlights its potential in impacting tumor growth and recurrence. We therefore utilized a reliable immunocompetent model of CRLM in which to study LIGHT expression that mimics the human course of metastases.(12) We previously determined that in CRLM, the number of TIL and LIGHT+ T-cells infiltrating the tumor were very low.(12) The CD4+ and CD8+ TIL were activated compared to non-tumor liver T-cells, and the tumor microenvironment reflected a Th1 cell-mediated immune response, however, this was not sufficient to halt tumor growth. The majority of T-cells in the tumor microenvironment did not infiltrate the tumor margin and expressed low levels of LIGHT.(12) Since LIGHT can stimulate proliferation and activation of T-cells in tumors, tumor regression, and was associated with improved OS in CRLM, it is possible that increasing LIGHT within CRLM could amplify an existing anti-tumor immune response.
We initially tested the effect of soluble LIGHT on lymphocytes and found that proliferation and activation could be profoundly enhanced with only CD3 co-engagement. Thus, LIGHT was confirmed to be an immunostimulatory cytokine that could activate T-cells in a CD28-independent fashion. This property supported further investigation in vivo, as tumor immunoediting often inhibits or masks co-stimulatory signals needed to engage a T-cell response. Therefore, we created a syngeneic LIGHT expressing colon cancer cell line in order to use an immunocompetent host system. For clinical applicability, we distinctly did not engineer increased expression of tumor-associated antigens, overexpress recognizable epitopes, or transfer antigen-recognizing T-cells into the animal model in order to more accurately reflect the human course. This would more than likely increase the difficulty of the assays, consistency of the results, and the magnitude of anti-tumor immune responses, however, critically, it would more accurately reflect the human condition and serve as a translational pre-clinical model.
Two LIGHT expressing colon cancer cells were created and tested, one that constitutively expressed LIGHT and another that inducibly expressed LIGHT. With the inducible expression of LIGHT, we could test it's effect in established tumors which would more accurately reflect the way this intervention would be used clinically, and also allow for the same cells to be used as internal controls. Similar to soluble LIGHT, in vitro culture of CT26LIGHT with lymphocytes stimulated proliferation and activation, confirming the ability of the cell line to bind to cognate receptors on T-cells and to affect a clinical response. When splenocytes were harvested from animals with LIGHT expressing colon cancer tumors, they were found to be tumor-cell specific and capable of cytotoxic T-cell responses. Using whole tumor 3-D images we calculated TIL concentrations in the entire tumor, not just in a representative slice, and found that TIL were increased in the LIGHT expressing tumors corresponding with significant anti-tumor responses by both WHO and RECIST criteria. Lymphocyte subset depletion experiments demonstrated that CD8 cytotoxic T-cells were critically responsible for in vivo anti-tumor immune responses to LIGHT. To further establish that the anti-tumor response was secondary to LIGHT-enhanced tumor-specific immunity, animals exposed to LIGHT expressing tumors were later challenged with the wild-type colon tumor or an irrelevant syngeneic tumor. Wild-type colon tumors grew minimally and regressed in these animals while syngeneic non-colon cancers grew unaffected. In these experiments, LIGHT-induced tumor regressions instilled tumor specific anti-tumor immunity in tumors distant to the primary, suggesting that treating tumors with immunostimulatory agents like LIGHT could act as a tumor vaccine preventing establishment of future metastases. Thus, it followed that the effect of LIGHT on the establishment of CRLM should be tested.
Subcutaneous tumors have tremendous advantages for monitoring tumor progression and for assessing the effects of therapeutic intervention. However, a major disadvantage is that the subcutaneous microenvironment differs from that of the liver, the most common site of metastases. Interactions between the host environment and the tumor determine tumor cell behavior, levels of growth factors and nutrients, and tumor angiogenesis.(36) Thus, a metastatic CRLM model was utilized where cells metastasize from a distant organ via the portal vein. The model is well-established for these experiments, reliable, and mimics the human course of disease.(12,37-39) We found that enhanced LIGHT expression in the tumor microenvironment inhibited growth of colorectal liver metastases and increased tumor infiltrating lymphocytes and lymphocyte homing signals in the metastatic tumors. The impact on metastases was impressive, and importantly it was measured grossly, as is performed in our patients with CRLM, and not by colonogenic assay, which measures single cells in metastatic organs. Therefore, this represents the first study that has evaluated the effect of LIGHT expression in the tumor microenvironment on the growth of metastatic liver tumors from a gastrointestinal/colon primary.
There are limitations to the study. In the inducible model, the animals with LIGHT expressing tumors received a doxycycline diet. Therefore, we added CT26 control tumor groups with an empty vector on the same promoter that were fed doxycycline and demonstrated significant differences in tumor regressions compared to the LIGHT expressing group and similar to the animals fed regular chow. Furthermore, the doxycycline diet resulted in green stool, and this was confirmed daily in the mice fed doxycycline chow to insure consistent injestion. In addition, the amount of chow injested daily was measured and not different between doxy chow and regular chow fed animals. This corresponded with measured serum levels of doxycycline throughout the experimental time period in the experimental groups. In addition, additional colon cancer cell lines would have been useful to test in this model. However, the only syngeneic Balb/C colon cancer cell line available is CT26 and the use of other colon cancer cell lines would require an immune-incompetent model. Therefore, 4T1 murine mammary cancer cells were used in challenge experiments and as controls in cytotoxicity assays, as has been well-established in this model. Furthermore, LIGHT was presented in the tumor microenvironment on the colon cancer tumor cells. The chosen model insured reliably increased and stable levels of expression only in the tumor, and served as proof-of-principal for the experimental strategy, though other mechanisms to deliver LIGHT into the tumors are warranted for study and applicability in human clinical trials.
In conclusion, increasing LIGHT expression in colon cancer cells increased T-cell proliferation and activation resulting in enhanced tumor-specific immune mediated tumor regressions in primary tumors and colorectal liver metastases.
Supplementary Material
Acknowledgments
Financial Support: NIH/NCI K08CA190855 (A.V. Maker)
Footnotes
The authors have declared that no conflict to interest exists
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: a cancer journal for clinicians. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Bismuth H, Adam R, Lévi F, Farabos C, Waechter F, Castaing D, et al. Resection of nonresectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Annals of Surgery. 1996;224(4):509–22. doi: 10.1097/00000658-199610000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leonard GD, Brenner B, Kemeny NE. Neoadjuvant chemotherapy before liver resection for patients with unresectable liver metastases from colorectal carcinoma. Journal of Clinical Oncology. 2005;23(9):2038–48. doi: 10.1200/JCO.2005.00.349. [DOI] [PubMed] [Google Scholar]
- 4.Steele G, Jr, Ravikumar TS. Resection of hepatic metastases from colorectal cancer: Biologic perspectives. Annals of Surgery. 1989;210(2):127–38. doi: 10.1097/00000658-198908000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomlinson JS, Jarnagin WR, DeMatteo RP, Fong Y, Kornprat P, Gonen M, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. Journal of Clinical Oncology. 2007;25(29):4575–80. doi: 10.1200/JCO.2007.11.0833. [DOI] [PubMed] [Google Scholar]
- 6.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–4. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 7.Pagès F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. New England Journal of Medicine. 2005;353(25):2654–66. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 8.Katz SC, Bamboat ZM, Maker AV, Shia J, Pillarisetty VG, Yopp AC, et al. Regulatory T cell infiltration predicts outcome following resection of colorectal cancer liver metastases. Ann Surg Oncol. 2013;20(3):946–55. doi: 10.1245/s10434-012-2668-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maker AV, Ito H, Mo Q, Weisenberg E, Qin LX, Turcotte S, et al. Genetic evidence that intratumoral T-cell proliferation and activation are associated with recurrence and survival in patients with resected colorectal liver metastases. Cancer immunology research. 2015;3(4):380–8. doi: 10.1158/2326-6066.CIR-14-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maker AV. Precise identification of immunotherapeutic targets for solid malignancies using clues within the tumor microenvironment-Evidence to turn on the LIGHT. Oncoimmunology. 2016;5(1):e1069937. doi: 10.1080/2162402X.2015.1069937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu P, Lee Y, Liu W, Chin RK, Wang J, Wang Y, et al. Priming of naive T cells inside tumors leads to eradication of established tumors. Nature immunology. 2004;5(2):141–9. doi: 10.1038/ni1029. [DOI] [PubMed] [Google Scholar]
- 12.Qin JZ, Upadhyay V, Prabhakar B, Maker AV. Shedding LIGHT (TNFSF14) on the tumor microenvironment of colorectal cancer liver metastases. J Transl Med. 2013;11:70. doi: 10.1186/1479-5876-11-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang K, Corsa CA, Ponik SM, Prior JL, Piwnica-Worms D, Eliceiri KW, et al. The collagen receptor discoidin domain receptor 2 stabilizes SNAIL1 to facilitate breast cancer metastasis. Nature cell biology. 2013;15(6):677–87. doi: 10.1038/ncb2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brasel K, De Smedt T, Smith JL, Maliszewski CR. Generation of murine dendritic cells from flt3-ligand-supplemented bone marrow cultures. Blood. 2000;96(9):3029–39. [PubMed] [Google Scholar]
- 15.Zhang Z, Li W, Blatner NR, Dennis KL, Procissi D, Khazaie K, et al. Quantitative magnetic resonance imaging in the transgenic APC(Delta468) mouse model of hereditary colon cancer. Molecular imaging. 2013;12(1):59–66. [PubMed] [Google Scholar]
- 16.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–42. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 17.Saltz LB, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26(12):2013–9. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 18.Van Cutsem E, Kohne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360(14):1408–17. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 19.Chiba T, Ohtani H, Mizoi T, Naito Y, Sato E, Nagura H, et al. Intraepithelial CD8+ T-cell-count becomes a prognostic factor after a longer follow-up period in human colorectal carcinoma: possible association with suppression of micrometastasis. Br J Cancer. 2004;91(9):1711–7. doi: 10.1038/sj.bjc.6602201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clemente CG, Mihm MC, Jr, Bufalino R, Zurrida S, Collini P, Cascinelli N. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer. 1996;77(7):1303–10. doi: 10.1002/(SICI)1097-0142(19960401)77:7<1303::AID-CNCR12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 21.Diederichsen ACP, Hjelmborg JVB, Christensen PB, Zeuthen J, Fenge C. Prognostic value of the CD4+/CD8+ ratio of tumour infiltrating lymphocytes in colorectal cancer and HLA-DR expression on tumour cells. Cancer Immunology, Immunotherapy. 2003;52(7):423–28. doi: 10.1007/s00262-003-0388-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naito Y, Saito K, Shiiba K, Ohuchi A, Saigenji K, Nagura H, et al. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Research. 1998;58(16):3491–94. [PubMed] [Google Scholar]
- 23.Prall F, Duhrkop T, Weirich V, Ostwald C, Lenz P, Nizze H, et al. Prognostic role of CD8+ tumor-infiltrating lymphocytes in stage III colorectal cancer with and without microsatellite instability. Hum Pathol. 2004;35(7):808–16. doi: 10.1016/j.humpath.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 24.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(51):18538–43. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang L. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348(3):203–13. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 26.Katz SC, Pillarisetty V, Bamboat ZM, Shia J, Hedvat C, Gonen M, et al. T cell infiltrate predicts long-term survival following resection of colorectal cancer liver metastases. Ann Surg Oncol. 2009;16(9):2524–30. doi: 10.1245/s10434-009-0585-3. [DOI] [PubMed] [Google Scholar]
- 27.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10(9):909–15. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Le DT, Wang-Gillam A, Picozzi V, Greten TF, Crocenzi T, Springett G, et al. Safety and Survival With GVAX Pancreas Prime and Listeria Monocytogenes-Expressing Mesothelin (CRS-207) Boost Vaccines for Metastatic Pancreatic Cancer. J Clin Oncol. 2015;33(12):1325–33. doi: 10.1200/JCO.2014.57.4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maker AV, Attia P, Rosenberg SA. Analysis of the cellular mechanism of antitumor responses and autoimmunity in patients treated with CTLA-4 blockade. J Immunol. 2005;175(11):7746–54. doi: 10.4049/jimmunol.175.11.7746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maker AV, Phan GQ, Attia P, Yang JC, Sherry RM, Topalian SL, et al. Tumor regression and autoimmunity in patients treated with cytotoxic T lymphocyte-associated antigen 4 blockade and interleukin 2: a phase I/II study. Ann Surg Oncol. 2005;12(12):1005–16. doi: 10.1245/ASO.2005.03.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chung KY, Gore I, Fong L, Venook A, Beck SB, Dorazio P, et al. Phase II study of the anti-cytotoxic T-lymphocyte-associated antigen 4 monoclonal antibody, tremelimumab, in patients with refractory metastatic colorectal cancer. J Clin Oncol. 2010;28(21):3485–90. doi: 10.1200/JCO.2010.28.3994. [DOI] [PubMed] [Google Scholar]
- 32.Parkhurst MR, Yang JC, Langan RC, Dudley ME, Nathan DA, Feldman SA, et al. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther. 2010;19(3):620–6. doi: 10.1038/mt.2010.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Granger SW, Ware CF. Turning on LIGHT. J Clin Invest. 2001;108(12):1741–2. doi: 10.1172/JCI14651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harrop JA, McDonnell PC, Brigham-Burke M, Lyn SD, Minton J, Tan KB, et al. Herpesvirus entry mediator ligand (HVEM-L), a novel ligand for HVEM/TR2, stimulates proliferation of T cells and inhibits HT29 cell growth. J Biol Chem. 1998;273(42):27548–56. doi: 10.1074/jbc.273.42.27548. [DOI] [PubMed] [Google Scholar]
- 35.Tamada K, Shimozaki K, Chapoval AI, Zhai Y, Su J, Chen SF, et al. LIGHT, a TNF-like molecule, costimulates T cell proliferation and is required for dendritic cell-mediated allogeneic T cell response. J Immunol. 2000;164(8):4105–10. doi: 10.4049/jimmunol.164.8.4105. [DOI] [PubMed] [Google Scholar]
- 36.Heijstek MW, Kranenburg O, Borel Rinkes IH. Mouse models of colorectal cancer and liver metastases. Digestive surgery. 2005;22(1-2):16–25. doi: 10.1159/000085342. [DOI] [PubMed] [Google Scholar]
- 37.Grimm M, Gasser M, Bueter M, Strehl J, Wang J, Nichiporuk E, et al. Evaluation of immunological escape mechanisms in a mouse model of colorectal liver metastases. BMC Cancer. 10:82. doi: 10.1186/1471-2407-10-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Endo T, Toda M, Watanabe M, Iizuka Y, Kubota T, Kitajima M, et al. In situ cancer vaccination with a replication-conditional HSV for the treatment of liver metastasis of colon cancer. Cancer gene therapy. 2002;9(2):142–8. doi: 10.1038/sj.cgt.7700407. [DOI] [PubMed] [Google Scholar]
- 39.Jain A, Slansky JE, Matey LC, Allen HE, Pardoll DM, Schulick RD. Synergistic effect of a granulocyte-macrophage colony-stimulating factor-transduced tumor vaccine and systemic interleukin-2 in the treatment of murine colorectal cancer hepatic metastases. Ann Surg Oncol. 2003;10(7):810–20. doi: 10.1245/aso.2003.10.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.