Abstract
In biparental mammals, the factors facilitating the onset of male parental behavior are not well understood. While hormonal changes in fathers may play a role, prior experience with pups has also been implicated. We evaluated effects of prior exposure to pups on paternal responsiveness in the biparental California mouse (Peromyscus californicus). We analyzed behavioral, neural, and corticosterone responses to pups in adult virgin males that were interacting with a pup for the first time, adult virgin males that had been exposed to pups 3 times for 20 minutes each in the previous week, and new fathers. Control groups of virgins were similarly tested with a novel object (marble). Previous exposure to pups decreased virgins’ latency to approach pups and initiate paternal care, and increased time spent in paternal care. Responses to pups did not differ between virgins with repeated exposure to pups and new fathers. In contrast, repeated exposure to a marble had no effects. Neither basal corticosterone levels nor corticosterone levels following acute pup or marble exposure differed among groups. Finally, Fos expression in the medial preoptic area, and ventral and dorsal bed nucleus of the stria terminalis was higher following exposure to a pup than to a marble. Fos expression was not, however, affected by previous exposure to these stimuli. These results suggest that previous experience with pups can facilitate the onset of parental behavior in male California mice, similar to findings in female rodents, and that this effect is not associated with a general reduction in neophobia.
Keywords: Sensitization, corticosterone, parental care, paternal care, medial preoptic area, bed nucleus of the stria terminalis, biparental, California mouse, Peromyscus californicus
Introduction
In both females and males of some rodent species, parental behavior (i.e., nurturant behavior toward immature individuals) can occur outside of typical reproductive conditions via continuous or repeated exposure to infants, a process called “concaveation” or, more commonly, “sensitization.” Adult, sexually naïve (i.e., virgin) female rats (Rattus norvegicus), for example, typically avoid pups upon first exposure, but display maternal behaviors after repeated or continuous exposure (Bridges et al., 1972; Fleming and Rosenblatt, 1974; Jakubowski and Terkel, 1985a,b; Lonstein et al., 1999; Quadagno et al., 1974; Reisbick et al., 1975; Rosenblatt, 1967; Stern and Mackinnon, 1976; Wiesner and Sheard, 1933). In contrast to rats, adult virgin female house mice (Mus musculus) frequently exhibit maternal behavior upon their first exposure to pups and are often described as “spontaneously maternal” (Gandelman, 1973; Leussis et al., 2008; Martín-Sánchez et al., 2015; Noirot, 1969; Stolzenberg and Rissman, 2011; Stolzenberg et al., 2012); however, repeated or continuous exposure to pups can increase measures of maternal behavior in virgin females even more (Alsina-Llanes et al., 2015; Brown et al., 1999; Ehret and Koch, 1989; Ehret et al., 1987; Pedersen et al., 2006). Virgin female prairie voles (Microtus ochrogaster) may attack, ignore, or care for foster pups at first exposure (Bales et al., 2007; Lonstein and De Vries, 2001), and exposure to pups in adolescence increases some aspects of maternal care in adulthood (Lonstein and De Vries, 2001), similar to rats (Stern and Rogers, 1988). In virgin female Syrian golden hamsters (Mesocricetus auratus), continuous exposure to pups often changes infant-directed behavior from infanticidal to maternal within a few days (Noirot, 1966; Swanson and Campbell, 1979).
Effects of repeated or continuous exposure to pups on paternal care by male rodents have received less attention than those on maternal care. Adult virgin male rats (Bridges et al., 1972; Jakubowski and Terkel, 1985b; Rosenblatt, 1967), mice (Ehret et al., 1987), and golden hamsters (Swanson and Campbell, 1979) can be sensitized to show parental care, with sensitization latencies longer than those of females. These species, however, may not be optimal models for understanding paternal behavior, as male rats, mice, and golden hamsters do not typically provide care for their offspring under naturalistic conditions. In the approximately 5–10% of rodents in which fathers provide care for their offspring in the wild (Dewsbury, 1985; Kleiman and Malcolm, 1981), almost nothing is known about the effects of prior exposure to pups.
The California mouse (Peromyscus californicus) is a monogamous, biparental rodent in which fathers spend as much time as mothers caring for offspring (e.g., huddling, grooming, and retrieving pups) and typically care for unrelated pups during experimental exposure, while adult virgin males may either attack, ignore, or care for experimentally presented pups (Chauke et al., 2012; de Jong et al., 2009, 2010; Gubernick and Addington, 1994; Gubernick and Alberts, 1987; Jasarevic et al., 2013; Rosenfeld et al., 2013). Cohabitation with a younger litter increases the likelihood of males behaving paternally toward an unrelated pup in young juveniles (35–45 days of age), but not in older juveniles (55–65 days) or adults (160 days) (Gubernick and Laskin, 1994). However, whether pup exposure during adulthood alters infant-directed behavior in adult male California mice is not known.
In female rodents, one mechanism underlying the onset of maternal behavior in parturient mothers is suppression of fear-, anxiety-, and stress-related responses to infants. Inhibition of hypothalamic-pituitary-adrenal axis and neuronal responses to aversive stimuli occurs during late pregnancy and lactation, and facilitates expression of maternal care (Brunton et al., 2008; Lightman et al., 2001; Slattery and Neumann, 2008). In virgin males of some biparental rodent species, exposure to pups may dampen some stress-related responses: pup exposure decreases plasma corticosterone levels in response to a handling stressor in virgin male prairie voles (Kenkel et al., 2012). Similar stress response-dampening effects of pup exposure might occur in male California mice. In one study, repeated exposure to pups decreased males’ behavioral and physiologic stress responses to a fox-urine stressor (Bardi et al., 2011); however, other research has found few or no differences in stress response between fathers and virgin male California mice (Chauke et al., 2011, 2012; de Jong et al., 2013; Harris and Saltzman, 2013). The effects of repeated pup exposure on the acute neural and corticosterone responses to pups are unknown.
The aim of this experiment was to determine the effects of repeated pup exposure on behavioral, neural and corticosterone responses to pups in adult, virgin male California mice. To do so, we examined responses to an unfamiliar pup in virgin males that had or had not been exposed to a pup during the preceding week. To control for novelty, we also examined effects of repeated exposure to a novel object on virgin males’ subsequent responses to the same object. Finally, we characterized behavioral, neural, and corticosterone responses to pups in new fathers as a positive control. We predicted that repeated pup exposure would increase paternal behavior in virgin males; alter neural responses to pups, as indicated by Fos expression, in brain regions associated with paternal care, stress and/or anxiety; decrease acute corticosterone responses to pups; and possibly decrease basal plasma corticosterone levels.
Methods
Animals
Fifty-three male California mice, descendants of mice purchased as adults from the Peromyscus Genetic Stock Center (University of South Carolina, Columbia, SC, USA), were used. Animals were housed in 44 × 24 × 20 cm polycarbonate cages containing aspen shavings and cotton wool for nesting material, with food (Purina Rodent Chow 5001) and water available ad libitum. Colony rooms were kept on a 14:10 light:dark cycle (lights on from 0500h to 1900h). At 27–33 days of age, prior to the birth of the next litter of siblings, animals were removed from their parents’ cage and housed in groups of four same-sex, age-matched littermates and/or unrelated juveniles.
All procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals and were approved by the University of California, Riverside (UCR) Institutional Animal Care and Use Committee. UCR is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care.
Experimental design
In adulthood (161–231 days of age), each mouse either remained housed with one of the males from its original group of four (virgin males) or was paired with an unrelated female (new fathers). Thereafter, subjects were weighed twice per week to monitor health and to habituate animals to handling.
Beginning at least 14 days (26.59 ± 1.97, mean ± SE) after pair formation, virgin males underwent data collection over a 10-day period (days 1–10; Table 1). On day 1, we collected a basal blood sample (see below) from each animal at 1200–1500 h. On day 3, each mouse was exposed to either an unrelated pup or a control object (pup-sized glass marble). Each animal subsequently underwent the same pup-exposure, marble-exposure, or handling procedures on days 5 and 7. On day 8, a second basal blood sample was collected from all animals. Finally, on day 10, 21 virgin males underwent a 60-minute exposure to an unfamiliar pup; this included the 11 males that had previously been exposed to a pup on days 3, 5, and 7 (repeated-pup condition) and 10 males that had undergone control handling procedures on the same days (single-pup condition). Similarly, 22 males underwent a 60-minute exposure to a marble on day 10, including the 12 animals that had been exposed to a marble on days 3, 5 and 7 (repeated-object condition) and the 10 remaining mice that had undergone control handling procedures on those days (single-object condition). Finally, 10 breeding males (new fathers) were tested with an unrelated pup 5–7 days after the birth of their first litter, as a positive control. Immediately after the 60-minute pup exposure on day 10, all males were decapitated, and blood and brains were collected. Brains were subsequently analyzed for Fos using immunohistochemistry, and blood was assayed for corticosterone (see below).
Table 1.
Sequence of procedures conducted on adult virgin male California mice that underwent single or repeated exposure to either an unfamiliar object (marble) or an unfamiliar pup, as well as new fathers exposed to an unfamiliar pup. No procedures were conducted on days 2, 4, 6, and 9.
| Day 1 | Day 3 | Day 5 | Day 7 | Day 8 | Day 10 | |
|---|---|---|---|---|---|---|
| Repeated-pup (n=11) | Basal blood sample | 20-min pup exposure | 20-min pup exposure | 20-min pup exposure | Basal blood sample | 60-min pup exposure; blood & brain collection |
| Single-pup (n=10) | Basal blood sample | Handling | Handling | Handling | Basal blood sample | 60-min pup exposure; blood & brain collection |
| Repeated-object (n=12) | Basal blood sample | 20-min marble exposure | 20-min marble exposure | 20-min marble exposure | Basal blood sample | 60-min marble exposure; blood & brain collection |
| Single-object (n=10) | Basal blood sample | Handling | Handling | Handling | Basal blood sample | 60-min marble exposure; blood & brain collection |
| New fathers (n=10) | — | — | — | — | Basal blood sample (3–5 days after birth of first litter) | 60-min pup exposure; blood & brain collection |
Males in the 5 experimental conditions did not differ in age (183.24 ± 2.37 days, mean ± SE; F[1,47]=2.12, p=0.940, η2=0.15; one-way ANOVA) or body mass (46.49 ± 1.0 g, mean ± SE; F[1,47]=0.82, p=0.520, η2=0.06; one-way ANOVA) at the beginning of data collection on day 1.
Pup and marble exposure
On days 3, 5, and 7, virgin males in the repeated-pup and repeated-object conditions were removed from their home cage between 1200h and 1500h and isolated in a clean cage containing bedding, food and water. Animals were tested in new cages to allow testing of both cage mates around the same time under identical conditions. After a 10-minute habituation period, an unfamiliar, unrelated, 1- to 4-day-old pup or a marble was introduced into the corner of the cage farthest from the subject for 20 minutes. If a subject attacked a pup, the exposure was immediately concluded and the pup was euthanized with pentobarbital (ca. 200–300mg/kg i.p.; Fatal-Plus, Vortech Pharmaceuticals, Dearborn, MI, USA). To control for effects of handling, subjects in the single-pup and single-object conditions were placed in a clean cage on days 3, 5, 7 and allowed to habituate for 10 minutes, after which time a gloved hand touched the bedding in the corner farthest from the subject to mimic placement of a pup or marble. Subjects then remained in the cage for an additional 20 minutes before being transferred back to their home cage. All exposures were videotaped.
Pup and marble exposures on day 10 were conducted identically to the earlier exposures, except that animals in all 5 conditions were exposed for 1 hour to either a pup (repeated-pup, single-pup, and new father conditions) or a marble (repeated-object and single-object conditions). One hour after the beginning of the exposure, subjects were decapitated, and trunk blood and brains were collected immediately. Brains were drop fixed in 4% paraformaldehyde for 2 days before being cryoprotected in 30% sucrose and frozen in Fisher Healthcare Optimal Cutting Temperature compound until being sliced and processed for Fos immunohistochemistry as described below.
Behavioral analyses
Behavior during pup exposures and novel-object exposures was scored from videos using JWatcher software (Blumstein and Daniel, 2007) as done previously (Chauke et al., 2012; de Jong et al., 2009). For the entire duration of the 20-minute pup exposures (days 3, 5, 7), and for the first 20 minutes of the 1-hour exposure (day 10), we quantified total durations of sniffing, grooming, and huddling the pup, as well as latencies to perform each of these three behaviors. Grooming and huddling were summed to quantify total time engaged in paternal behavior, and latency to engage in paternal behavior was defined as the latency to groom or huddle, whichever occurred first. For novel-object exposures, latency to approach the marble and total time spent sniffing the marble were quantified.
Immunohistochemical analyses
Immunohistochemistry was performed as described previously (de Jong et al., 2009): 30 μm brains sections were incubated overnight with rabbit-anti-Fos antibody (sc-253, 1:5000, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), followed by donkey anti-rabbit second antibody (1:1500; Jackson ImmunoResearch Laboratories, West Grove, PA, USA), and then stained with 3,3′-diaminobenzidine (DAB) and ammonium nickel sulfate to mark Fos-positive cells as blue-black in color. Individual brain sections were then mounted on chrome alum gelatin-coated glass microscope slides, dehydrated in ethanol, cleared in xylene, then embedded in entellan (EMS, Hatfield, PA, USA).
Because there is no brain atlas for California mice (although micrographs of Nissl-stained sections are available at brainmaps.org), The Mouse Brain in Stereotaxic Coordinates (Paxinos and Franklin, 2013) for Mus musculus was used to locate brain regions. We focused on regions of the hypothalamus and extended amygdala that have known associations with parental behaviors, stress and/or anxiety (medial preoptic area [MPOA], ventral bed nucleus of the stria terminalis [vBNST], medial amygdala [MEA], anterior hypothalamic nucleus [AHN], paraventricular nucleus of the hypothalamus [PVN], dorsal bed nucleus of the stria terminalis [dBNST]), and ventromedial hypothalamus [VMH] (Klampfl et al., 2016; Numan and Insel, 2003; Pêgo et al., 2010; Smith and Lonstein, 2008). An observer blind to the animal’s condition and stimulus took digital photographs of the area with the highest density of Fos immunoreactivity for each brain region in each hemisphere at a magnification of 200x with a digital camera (Canon EOS-40D) mounted on a microscope (Leica Leitz DMRB). ImageJ software (Abramoff et al., 2004) was used to create a 200 × 200 μm square in each photograph that contained either all or the majority of immunoreactive neurons in the brain region. The numbers of Fos-positive cells within the 200 μm square were counted manually and averaged across both hemispheres to produce a measure of neural activity. Due to technical problems, usable Fos-expression data were not available for some of the animals (see Table 3 for final sample sizes).
Table 3.
Number of Fos-positive cells (back-transformed means and 95% confidence intervals) in 200×200 um sections of brain regions immediately following 1-hour exposure to a pup or a novel object (marble) (day 10). Fos expression was significantly higher (p<0.05) in pup-exposed virgins than marble-exposed virgins, regardless of number of previous exposures, in the medial preoptic area (MPOA), ventral bed nucleus of the stria terminalis (vBNST), and dorsal bed nucleus of the stria terminalis (dBNST), but not in the medial amygdala (MEA), paraventricular nucleus of the hypothalamus (PVN), ventromedial nucleus of the hypothalamus (VMH), or anterior hypothalamic nucleus (AHN). No differences were found between fathers and virgins exposed to pups.
| MPOA
|
vBNST
|
dBNST
|
MEA
|
PVN
|
VMH
|
AHN
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | |
| Single-pup (n=6) | 13.26 | 10.33, 17.02 | 15.83 | 11.71, 21.39 | 27.04 | 19.50, 37.51 | 18.66 | 16.71, 20.84 | 17.78 | 11.93, 26.48 | 13.26 | 8.64, 20.35 | 14.19 | 10.91. 18.45 |
| Repeated-pup (n=6) | 21.79 | 12.36, 38.42 | 16.44 | 10.65, 25.26 | 28.68 | 21.92, 37.52 | 17.41 | 13.27, 22.85 | 24.38 | 14.00, 42.46 | 12.92 | 7.35, 22.72 | 14.84 | 10.45, 21.06 |
| Single-object (n=8) | 7.70 | 4.58, 12.92 | 7.35 | 6.14, 8.81 | 16.61 | 13.97, 19.76 | 14.65 | 10.49, 20.45 | 16.63 | 11.30, 24.47 | 15.92 | 11.20, 22.62 | 13.88 | 10.01, 19.23 |
| Repeated-object (n=8) | 10.26 | 7.33, 14.36 | 10.38 | 7.49, 14.38 | 16.37 | 11.32, 23.68 | 13.44 | 9.69, 18.65 | 14.42 | 10.78, 19.29 | 8.79 | 5.30, 14.58 | 10.73 | 9.13, 12.62 |
| New fathers (n=10) | 15.27 | 13.07, 17.85 | 13.89 | 10.92, 17.68 | 24.11 | 18.80, 30.91 | 19.96 | 12.90, 30.88 | 20.04 | 14.65, 27.42 | 9.84 | 8.03, 12.05 | 13.27 | 10.23, 7.22 |
Blood collection and corticosterone radioimmunoassay
Basal blood samples were collected from the retro-orbital sinus at 1200–1500 h, under undisturbed conditions, on experimental days 1 and 8. Animals were lightly anesthetized with isoflurane, and blood (70 uL) was collected into heparinized glass capillary tubes in < 3 minutes from initial cage disturbance. On day 10, trunk blood was collected into 0.1 mL heparinized weighing boats after decapitation. All blood samples were centrifuged at 13,300 rpm for 12 min at 4 °C, and the plasma was separated and stored at −80°C.
Plasma corticosterone concentration was determined using a commercially available double-antibody radioimmunoassay kit (07120103; MP Biomedicals, Solon, OH) previously validated for use in California mice (Chauke et al., 2011). The assays were run in accordance with the kit instructions, except that the low end of the standard curve was extended down to 12.5 from 25 ng/ml and the samples were diluted 1:400 instead of 1:200 (dilution based on data from previous studies). The new curve ranged from 12.5 ng/ml (91–3% bound) to 1000 ng/ml (19–0% bound). A total of 3 assays was conducted. To minimize variation, samples from all experimental conditions were represented in each assay, and repeated samples from the same animal were always run in the same assay. Intra- and inter-assay coefficients of variability calculated from in-house quality-control pools were 2.23% and 3.36%, respectively.
Statistical analyses
All data (behavioral, immunohistochemical, and horomonal) were analyzed using R software (R Core Team 2014) or SPSS (IBM Corp 2013). Data were tested for normality using the Shapiro-Wilks test. Behavioral data tended to be non-normally distributed and to resist transformations to achieve normality, so between-group comparisons were analyzed using Mann-Whitney U tests or Kruskal-Wallis tests followed by Dunn’s post hoc tests (Dinno, 2016); for these analyses we used a Bonferroni-corrected critical p value of 0.0167 (=0.05/3). We refer to differences with p<0.0167 as significant and those with 0.0167<p<0.05 as nominally significant. Within-group comparisons on behavioral data were analyzed with Friedman tests and Nemenyi post-hoc tests (Pohlert, 2015). Nemenyi’s post-hoc test accounts for family-wise error, and no p–adjustment is required (Nemenyi, 1963; Pohlert, 2015). Effect sizes were determined for each statistical test: Cohen’s d was calculated as the mean difference divided by the pooled standard deviation. Eta squared (η2) was calculated as (SSIndependent Variable/SSTotal) and (SSInteraction/SSTotal) where appropriate. Behavioral data are presented as medians ± 1st and 3rd quartiles.
Corticosterone and Fos expression data were log-transformed to meet assumptions of normality. Basal corticosterone data were analyzed by a three-way ANOVA (stimulus [pup or marble] × condition [single or repeated exposure] × day [1 or 8]). Fos data and corticosterone data from day 10 were analyzed using two-way ANOVAs (stimulus × condition) among virgins, and planned comparisons were conducted between fathers and virgins exposed to pups using Student’s T-tests. Corticosterone and Fos data are presented as the mean and 95% confidence intervals of back-transformed data.
A total of 4 virgin males (3 in the single-pup condition, 1 in the repeated-pup condition) attacked foster pups. The virgin male in the repeated-pup condition attacked a pup during each of his three 20-minute pup exposures as well as his 1-hour exposure. For these animals, latency to approach the pup was included in analyses but all other behaviors were excluded, as the pup was removed from the cage immediately after the attack. Eight virgin males (3 in the repeated-pup condition, 2 in the single-object condition, and 3 in the repeated-object condition) were asleep or inactive during presentation of the stimulus and remained asleep or inactive for the majority, if not the entirety, of one or more exposures, never investigating the stimulus. Data from these animals were not included in any analyses. All new fathers investigated and behaved paternally (i.e., huddled and/or groomed) toward pups.
Results
Behavioral responses to pups
Initial exposure to pups
During their first experimental exposure, virgins in the repeated-pup condition, virgins in the single-pup condition, and new fathers differed significantly in their behavioral responses to pups, as revealed by Kruskal-Wallis tests and Dunn’s post-hoc tests, using the Bonferroni-corrected critical p value (0.0167) (Fig. 1). Virgin males in the repeated-pup (day 3) and single-pup (day 10) conditions and new fathers (day 10; see Table 1) differed in latency to initiate paternal care (χ2=12.14, df=2, p=0.002, η2=0.20): new fathers initiated paternal care more quickly than virgin males in both the repeated-pup and single-pup conditions (new fathers vs. single-pup: Z=−2.90, p=0.002, d=1.14; new fathers vs. repeated-pup: Z=−3.08, p=0.001, d=1.07; Fig. 1B), whereas the two groups of virgin males did not differ from each other, as expected (Z=0.09, p=0.463, d=0.22). No significant differences were found among virgins in the repeated-pup condition, virgins in the single-pup condition, and new fathers in latency to approach pups (χ2=5.65, df=2, p=0.059, η2=0.17; Fig 1A), time spent in paternal behavior (i.e., huddling or grooming) (χ2=4.34, df=2, p=0.114, η2=0.18; Fig 1D), or time spent sniffing the pup (χ2=4.95, df=2, p=0.08, η2=0.22; Fig 1C) during their first experimental exposure.
Fig. 1.
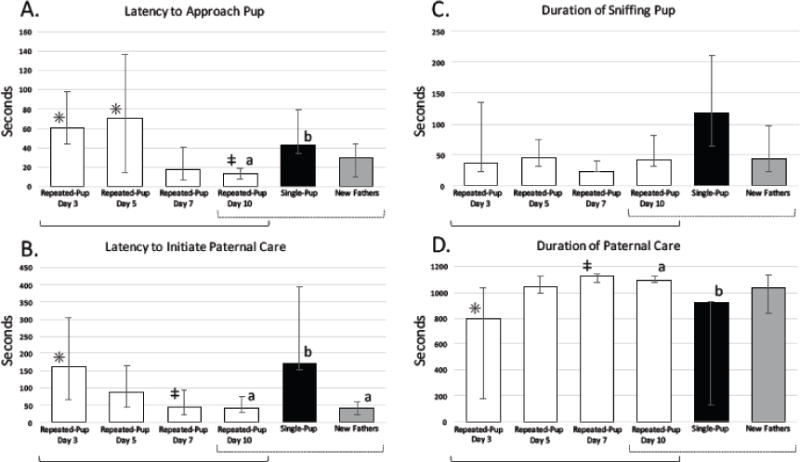
Pup-directed behaviors (median ± 1st and 3rd quartiles) for new fathers, virgin males in the single-pup condition (day 10) and virgin males in the repeated-pup condition in their first (day 3), second (day 5), third (day 7), and fourth (day 10) exposure to pups. Within-group comparisons of virgin males in the repeated-pup condition across the four exposures are indicated by solid brackets; time points with different symbols (*,‡) differed significantly (p≤0.05) from one another. Between-group comparisons of repeated-pup males in their fourth exposure, single-pup males, and new fathers (all on day 10) are indicated by dotted brackets; groups with different letters (a, b) differed significantly (p≤0.017) from one another. A: Latency to approach a pup decreased across repeated exposures to a pup in virgin males, and was lower in previously exposed virgins and new fathers than in virgins exposed to a pup for the first time. B: Latency to initiate paternal care decreased across repeated exposures in virgin males, and was lower in previously exposed virgins and new fathers than in virgins exposed to a pup for the first time. C: Duration of time spent sniffing a pup did not change across repeated exposures in virgin males and did not differ among experimental groups. D: Duration of time spent engaging in paternal care increased across exposures to a pup in virgin males, and was higher in previously exposed virgins and new fathers than in virgin males exposed to a pup for the first time.
Final exposure to pups
In their 1-hour exposure to a pup on day 10, virgins in the repeated-pup condition, virgins in the single-pup condition, and new fathers differed significantly in latency to approach pups (χ2=11.23, df=2, p=0.004, η2=0.39; Fig. 1A), latency to initiate paternal care (χ2=9.79, df=2, p=0.007, η2=0.14; Fig. 1B), and duration of paternal care (χ2=6.88, df=2, p=0.032, η2=0.22; Fig. 1D; Kruskal-Wallis tests). Males in the repeated-pup condition, compared to males in the single-pup condition, approached pups sooner (z=3.34, p<0.001), engaged in paternal care sooner (z=2.63, p=0.004, d=1.68) and spent more time engaged in paternal care (z=2.57, p=0.005, d=0.95). Similarly, new fathers, compared to virgin males in the single-pup condition, engaged in paternal care sooner (z=−2.88, p=0.002, d=1.14), approached pups nominally sooner (z=−1.91, p=0.028, d=1.04) and spent nominally more time engaged in paternal care (z=1.87, p=0.031, d=1.14). Virgin males in the repeated-pup condition did not differ significantly from new fathers in latency to approach a pup (z=1.34, p=0.091, d=0.75), latency to behave paternally (z=−0.27, p=0.393, η2=0.22), or duration of paternal care (z=−0.75, p=0.226, d=0.03). Time spent sniffing the pup did not differ significantly across the three groups (χ2=5.29, df=2, p=0.071, η2=0.32; Fig. 1C).
Within-animal changes across exposures
To identify effects of repeated pup exposure within individual animals, we compared behavioral responses to pups across all four exposures in virgin mice in the repeated-pup condition using Friedman tests, with Nemenyi post-hoc tests when merited (Fig. 1). Latency to approach a pup decreased across the four exposures (χ2=14.52, df=3, p=0.002, η2=0.37), with shorter latency to approach on day 10 (fourth exposure) compared to day 3 (first exposure; p=0.005, d=0.51) and on day 10 compared to day 5 (p=0.029, d=1.12). Latency to behave paternally also decreased across exposures (χ2=1125, df=3, p=0.010, η2=0.17), with a shorter latency on day 7 (third exposure) compared to day 3 (p=0.018), d=0.48. Duration of paternal behavior increased across exposures (χ2=11.25, df=3, p=0.010, η2=0.26), with an increased duration of paternal care on day 7 compared to day 3 (p=0.018, d=0.76). Duration of sniffing the pup did not change across exposures (χ2=5.93, df=3, p=0.115, η2=0.14).
Behavioral responses to marbles
Initial exposure to marbles
During their first exposure to a marble, virgin males in the repeated-object (day 3) and single-object (day 10) conditions did not differ in latency to approach the marble (U=23.00, p=0.242, d=0.99; Mann-Whitney U test) or duration of time spent sniffing the marble (U=21.00, p=0.172, d=0.67; Mann-Whitney U test).
Final exposure to marbles
During their final exposure, on day 10, virgin males in the single-object condition took nominally longer to approach the marble than virgin males in the repeated-object condition (U=13.00, p=0.032, d=1.34; Mann-Whitney U test). Duration of time spent sniffing the marble during the final exposure did not differ between groups (U=24.00, p=0.283, d=0.51; Mann-Whitney U test).
Within-animal changes across exposures
To identify possible effects of repeated exposure to marbles within individual animals, we compared behavioral responses to marbles across all four exposures in virgins in the repeated-object condition. Friedman tests found no change in latency to approach (χ2=3.51, df=3, p=0.319, η2=0.32) or time spent sniffing the marble (χ2=7.63, df=3, p=0.054, η2=0.31).
Plasma corticosterone concentrations
To determine effects of treatment and stimulus on basal plasma corticosterone levels, a three-way mixed ANOVA (stimulus [pup or marble] × condition [single or repeated exposure] × day [1 or 8]) was conducted on log-transformed data. We found no main effects of day (F[1,27]=0.02, p=0.896, η2<0.01), stimulus (F[1,27]=0.26, p=0.612, η2=0.01), or condition (F[1,27]=3.10, p=0.090, η2=0.12). Moreover, we found no significant two- or three-way interactions (day × stimulus: F[1,27]=0.08, p=0.928, η2<0.01; day × condition: F[1,27]=0.006, p=0.939, η2<0.01; stimulus × condition: F[1,27]=3.57, p=0.070, η2=0.12; day × stimulus × condition: F[1,2]=2.53, p=0.124, η2=0.10).
To examine effects of repeated exposure on the acute corticosterone response to a pup or marble, we collected trunk blood immediately after the 60-minute exposure on day 10. A two-way ANOVA on virgin males revealed no significant main effect of stimulus (F[1,29]=0.76, p=0.392, η2=0.02) or condition (F[1,29]=0.14, p=0.707, η2<0.01), nor a significant stimulus × condition interaction (F[1,29]=3.36, p=0.077, η2=0.10).
Planned comparisons were conducted on plasma corticosterone levels of new fathers, single-pup virgin males, and repeated-pup virgin males immediately after the 60-minute pup exposure on day 10. Acute corticosterone responses to pups did not differ between new fathers and virgins in the repeated-pup condition (t=0.68, df=16, p=0.507, d=0.24), between new fathers and virgins in the single-pup condition (t=1.336, df=14, p=0.20, d=0.20), or between virgins in the single-pup and repeated-pup conditions (t=1.60, df=15, p=0.131, d=0.01) (Table 2).
Table 2.
Plasma corticosterone concentrations (ng/ml; back-transformed means and 95% confidence intervals) in blood samples collected under resting conditions (days 1, 8) or immediately after 1-hour exposure to a pup or a novel object (marble) (day 10). No significant differences were found within or among groups.
| Day 1: Basal | Day 8: Basal | Day 10: After exposure | ||||
|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | |
| Single-pup (n=10) | 255.86 | 186.21, 354.81 | 192.31 | 131.83, 281.84 | 274.79 | 147.91, 512.86 |
| Repeated-pup (n=10) | 266.83 | 158.49, 446.68 | 306.19 | 208.93, 446.68 | 323.59 | 173.78, 602.56 |
| Single-object (n=5−10) | 169.82 | 97.72, 295.12 | 277.33 | 162.18, 467.74 | 435.51 | 295.12, 645.65 |
| Repeated-object (n=7) | 119.83 | 67.61, 213.80 | 91.50 | 51.29, 162.18 | 215.97 | 107.15, 436.52 |
| New fathers (n=10) | — | — | 202.30 | 141.25, 288.40 | 400.87 | 229.09, 691.83 |
Fos expression
Two-way ANOVAs (stimulus [pup or marble] × condition [single or repeated exposure]) were conducted on log-transformed Fos expression data from virgin males in seven brain regions: MPOA, vBNST, dBNST, MEA, PVN, VMH, and AHN. Significant main effects of stimulus were found in the MPOA (F[1,22]=9.18, p=0.006, η2=0.26), vBNST (F[1,22]=15.19, p=0.001, η2=0.37), and dBNST (F[1,22]=10.31, p=0.004, η2=0.34), with all of these regions having more Fos expression in virgins exposed to pups than in those exposed to marbles. None of these regions showed a significant main effect of condition or a stimulus × condition interaction. Fos expression in the MeA, VMH, PVN, and AHN did not show a main effect of stimulus, condition, or a stimulus × condition interaction (Table 3).
Planned comparisons were conducted on Fos expression among new fathers and virgin males in the single-pup and repeated-pup conditions. T-tests found no significant differences between fathers and virgins in either condition in any brain region (Table 3).
Discussion
The mechanisms underlying the onset of paternal behavior in rodents are not well understood in any species. This experiment is, to our knowledge, the first to investigate the effects of brief, repeated pup exposure on paternal care in adult virgin males of a naturally biparental mammal.
Three 20-minute exposures to a pup over 8 days increased indices of paternal responsiveness in virgin male California mice. Specifically, repeated exposure decreased latency to approach the pup and latency to initiate paternal care, and increased total duration of time engaged in paternal care (i.e., grooming and/or huddling). No differences were seen between the first and second exposures for individual mice, suggesting that at least two previous exposures were necessary for sensitization. In the third exposure to a pup, virgin males showed a significant decrease in their latency to initiate paternal behavior and a significant increase in time spent behaving paternally, and during the fourth exposure, paternal behavior in virgins did not differ from that in new fathers.
Many sensitization paradigms involve constant exposure to pups over several days (e.g., Rosenblatt, 1967). Our data suggest that brief contact with pups is sufficient to increase paternal behavior in virgin male California mice. To investigate whether this effect might be mediated by a reduction in neophobia, we investigated the behavioral effects of repeated exposure to a pup-sized marble. No evidence of decreased neophobia was seen in the males exposed to a marble multiple times. Thus, unknown cues from pups in particular, rather than decreased neophobia in general, are likely to facilitate attraction to pups and engagement in paternal behavior in virgin male California mice.
We found no effect of prior pup exposure on either basal plasma corticosterone concentrations or corticosterone responses to pups in virgin males. Furthermore, these measures did not differ between virgin males and new fathers, nor between virgins exposed to pups and those exposed to marbles. These results are consistent with previous findings from our lab, in which neither basal plasma corticosterone levels nor plasma corticosterone levels following a predator-odor stressor differed among virgin males, vasectomized males cohabiting with a female, and new fathers (Chauke et al., 2011). Similarly, in separate studies, measures of hypothalamic-pituitary-adrenal activity and reactivity did not differ among virgin males, males housed with a tubally ligated female, and new fathers (de Jong et al., 2013; Harris and Saltzman, 2013; Harris et al., 2013). In virgin male prairie voles, which exhibit high levels of alloparental behavior toward unrelated young, exposure to pups for 10 minutes prevents acute corticosterone elevations induced by handling; however, this effect is not seen after 20 minutes of exposure to pups (Kenkel et al., 2012). It is possible that in our study, in which blood was collected after 60 minutes of continuous exposure to a pup, initial corticosterone responses to stimuli differed among the groups and/or between animals exposed to pups and those exposed to marbles, but that these differences dissipated by the end of the 60-minute exposure. It is also possible that corticosterone responses to a pup or novel object were dwarfed by the response to handling and placement in a new cage, although all animals were allowed to habituate to the new cage for 10 minutes before being tested.
Fos expression in several brain regions previously implicated in paternal behavior – the MPOA, vBNST, and dBNST (Bales and Saltzman, 2016) – was higher in virgin males exposed to pups than in those exposed to marbles. We found no evidence, however, that Fos responses to a pup were altered by previous exposure to pups: Fos did not differ among new fathers and virgin males in either the repeated-pup or single-pup conditions. In contrast, virgin male Mus musculus that behaved paternally in two pup tests two days apart exhibited more Fos expression in the central MPOA, as well as the rhomboid nucleus of the BNST, after pup exposure than did fathers (Tsuneoka et al., 2015).
A previous study in our lab showed Fos expression to differ between California mouse fathers and virgin males after exposure to a pup. Specifically, fathers had higher Fos expression than virgins in the MPOA, medial posteromedial division of the BNST, ventral medial division of the BNST, and caudal dorsal raphe nucleus (de Jong et al. 2009). The reason for the disparity between the findings of that study and the present one is not clear. In the earlier study, however, Fos expression was quantified 1 hour after a 5-minute exposure to a pup confined in a wire mesh ball, which precluded direct contact between the adult male and the pup. Thus, male mice in both studies could engage in appetitive behavior towards pups (e.g., approach, sniff), but only the mice in the present study could engage in consummatory aspects of paternal care (i.e., direct physical interaction). The precise stimulus eliciting Fos expression may therefore have differed between the two studies, and consummatory and appetitive aspects of parental behavior may be controlled by somewhat different neural circuitry (Stolzenberg and Numan, 2011). It is also possible that in the present study, previous exposure altered Fos responses to pups in brain regions other than the ones we investigated and/or that neuronal activation in response to pups was associated with expression of an immediate-early gene(s) other than c-fos, such as egr-1 or c-jun (Kawashima et al., 2014).
In summary, this study demonstrates that in the biparental California mouse, repeated, brief exposure to a pup can increase paternal responses to pups in virgin males, similar to pup-induced paternal care in uniparental rats, mice, and golden hamsters (Bridges et al., 1972; Ehret et al., 1987; Jakubowski and Terkel, 1985b; Rosenblatt, 1967, Swanson and Campbell, 1979). On the other hand, we found no evidence that repeated exposure to pups altered basal plasma corticosterone levels, or either corticosterone or neural responses to pups. Thus, although male California mice exhibit paternal care immediately at the birth of their offspring (Gubernick and Alberts, 1987; Lee and Brown, 2002), and chemosensory cues from their mates facilitate the maintenance of this paternal care (Gubernick, 1990; Gubernick and Alberts 1989), we found that cues from pups alone can facilitate the onset of paternal care. This suggests that cues from a female pairmate are not necessary for the onset of paternal behavior in male California mice.
The mechanisms by which pup exposure facilitates paternal care are not understood. Our findings suggest that the induction of paternal care is not mediated by changes in generalized neophobia or corticosterone response to pups in California mice. Future research investigating the effects of pup exposure on other aspects of endocrine and neural signaling may reveal the mechanisms of sensitized paternal behavior.
Fig. 2.
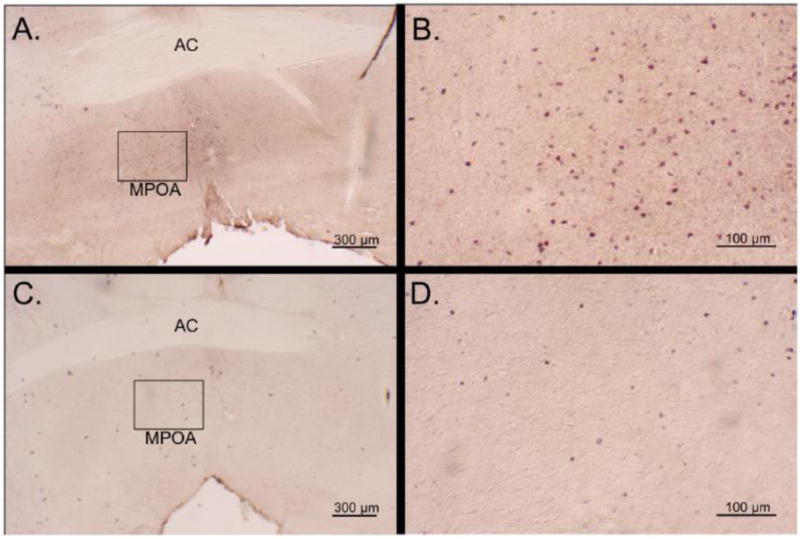
Photomicrographs of 30-um Fos-stained coronal sections of virgin male California mice following a 60-minute exposure to a pup (A and B) or marble (C and D). Images A and C are at 25× magnification and show a box containing the medial preoptic area (MPOA), which is enhanced to 100× magnification in images B and D. AC - anterior commissure.
Table 4.
Marble-directed behaviors (medians and quartiles) for virgin males in the repeated-marble condition in their first (day 3), second (day 5), third (day 7), and fourth (day 10) exposure to a marble and virgin males in the single-marble condition in their only exposure to a marble (day 10). No differences in marble-directed behavior were seen across exposures within the repeated-object exposure condition. Virgin males in the single-object condition took nominally longer to approach the marble than virgin males in the repeated object exposure condition on day 10 (p=0.032).
| Repeated-object condition | Single-object condition | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Day 3
|
Day 5
|
Day 7
|
Day 10
|
Day 10
|
||||||
| Median | 1st & 3rd Quartiles | Median | 1st & 3rd Quartiles | Median | 1st & 3rd Quartiles | Median | 1st & 3rd Quartiles | Median | 1st & 3rd Quartiles | |
| Latency to approach | 133.94 | 74.61, 145.60 | 34.12 | 28.89, 73.65 | 100.02 | 41.84, 112.92 | 30.19 | 27.87, 112.25 | 292.10 | 102.43, 486.51 |
| Duration of sniffing | 161.16 | 100.39, 258.01 | 80.59 | 37.25, 80.59 | 65.68 | 16.08, 187.47 | 68.78 | 36.30, 136.78 | 111.86 | 63.39, 168.99 |
Highlights.
Prior exposure to pups increased paternal behavior in virgin male California mice.
Responses to pups did not differ between fathers and previously pup-exposed virgins.
Responses to a novel object were not affected by prior exposure to the same object.
Previous exposure to pups did not alter basal or pup-induced corticosterone levels.
Prior exposure to pups did not alter Fos responses to pups in several limbic regions.
Acknowledgments
We thank the staff of the UCR Spieth Hall Vivarium and Dr. Akiko Sato for care of the animals, and members of the Saltzman lab for assistance with the animal colony and processing of brains. This project was supported by the National Institutes of Health (R21HD075021) and the National Science Foundation (IOS-1256572).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abramoff MD, Magelhaes PJ, Ram SJ. Image Processing with ImageJ. Biophotonics Intern. 2004;11:36–42. [Google Scholar]
- Alsina-Llanes M, De Brun V, Olazabal DE. Development and expression of maternal behavior in naive female C57BL/6 mice. Dev Psychobiol. 2015;57:189–200. doi: 10.1002/dev.21276. [DOI] [PubMed] [Google Scholar]
- Bales KL, Saltzman W. Fathering in rodents: Neurobiological substrates and consequences for offspring. Horm Behav. 2016;77:249–259. doi: 10.1016/j.yhbeh.2015.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales KL, van Westerhuyzen JA, Lewis-Reese AD, Grotte ND, Lanter JA, Carter CS. Oxytocin has dose-dependent developmental effects on pair-bonding and alloparental care in female prairie voles. Horm Behav. 2007;52:274–279. doi: 10.1016/j.yhbeh.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardi M, Franssen CL, Hampton JE, Shea EA, Fanean AP, Lambert KG. Paternal experience and stress responses in California mice (Peromyscus californicus) Comp Med. 2011;61:20–30. [PMC free article] [PubMed] [Google Scholar]
- Blumstein DT, Daniel JC. Quantifying Behavior the JWatcher Way. Sinauer; Sunderland, MA: 2007. [Google Scholar]
- Bridges R, Zarrow MX, Gandelman R, Denenberg VH. Differences in maternal responsiveness between lactating and sensitized rats. Dev Psychobiol. 1972;5:123–127. doi: 10.1002/dev.420050205. [DOI] [PubMed] [Google Scholar]
- Brown RE, Mathieson WB, Stapleton J, Neumann PE. Maternal behavior in female C57BL/6J and DBA/2J inbred mice. Physiol Behav. 1999;67:599–605. doi: 10.1016/s0031-9384(99)00109-2. [DOI] [PubMed] [Google Scholar]
- Brunton PJ, Russell JA, Douglas AJ. Adaptive responses of the maternal hypothalamic-pituitary-adrenal axis during pregnancy and lactation. J Neuroendocrinol. 2008;20:764–776. doi: 10.1111/j.1365-2826.2008.01735.x. [DOI] [PubMed] [Google Scholar]
- Chauke M, de Jong TR, Garland T, Jr, Saltzman W. Paternal responsiveness is associated with, but not mediated by reduced neophobia in male California mice (Peromyscus californicus) Physiol Behav. 2012;107:65–75. doi: 10.1016/j.physbeh.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauke M, Malisch JL, Robinson C, de Jong TR, Saltzman W. Effects of reproductive status on behavioral and endocrine responses to acute stress in a biparental rodent, the California mouse (Peromyscus californicus) Horm Behav. 2011;60:128–138. doi: 10.1016/j.yhbeh.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong TR, Chauke M, Harris BN, Saltzman W. From here to paternity: neural correlates of the onset of paternal behavior in California mice (Peromyscus californicus) Horm Behav. 2009;56:220–231. doi: 10.1016/j.yhbeh.2009.05.001. [DOI] [PubMed] [Google Scholar]
- de Jong TR, Harris BN, Perea-Rodriguez JP, Saltzman W. Physiological and neuroendocrine responses to chronic variable stress in male California mice (Peromyscus californicus): Influence of social environment and paternal state. Psychoneuroendocrinology. 2013;38:2023–2033. doi: 10.1016/j.psyneuen.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong TR, Measor KR, Chauke M, Harris BN, Saltzman W. Brief pup exposure induces Fos expression in the lateral habenula and serotonergic caudal dorsal raphe nucleus of paternally experienced male California mice (Peromyscus californicus) Neuroscience. 2010;169:1094–1104. doi: 10.1016/j.neuroscience.2010.06.012. [DOI] [PubMed] [Google Scholar]
- Dewsbury DA. Paternal behavior in rodents. Integr Comp Biol. 1985;25:841–852. [Google Scholar]
- Dinno A. Dunn’s test of multiple comparisons using rank sums. R package 2016 [Google Scholar]
- Ehret G, Koch M. Ultrasound-induced parental behaviour in house mice is controlled by female sex hormones and parental experience. Ethology. 1989;80:81–93. [Google Scholar]
- Ehret G, Koch M, Haack B, Markl H. Sex and parental experience determine the onset of an instinctive behavior in mice. Naturwissenschaften. 1987;74:47. doi: 10.1007/BF00367047. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Rosenblatt JS. Maternal behavior in the virgin and lactating rat. J Comp Physiol Psychol. 1974;86:957–972. doi: 10.1037/h0036414. [DOI] [PubMed] [Google Scholar]
- Gandelman R. Induction of maternal nest building in virgin female mice by the presentation of young. Horm Behav. 1973;4:191–197. doi: 10.1016/0018-506x(73)90003-2. [DOI] [PubMed] [Google Scholar]
- Gubernick DJ. A maternal chemosignal maintains paternal behaviour in the biparental California mouse, Peromyscus californicus. Anim Behav. 1990;39:936–942. [Google Scholar]
- Gubernick DJ, Addington RL. The stability of female social and mating preferences in the monogamous California mouse, Peromyscus californicus. Anim Behav. 1994;47:559–567. [Google Scholar]
- Gubernick DJ, Alberts JR. The biparental care system of the California mouse, Peromyscus californicus. J Comp Psychol. 1987;101:169–177. [PubMed] [Google Scholar]
- Gubernick DJ, Alberts JR. Postpartum maintenance of paternal behaviour in the biparental California mouse, Peromyscus californicus. Anim Behav. 1989;37:656–664. [Google Scholar]
- Gubernick DJ, Laskin B. Mechanisms influencing sibling care in the monogamous biparental California mouse, Peromyscus californicus. Anim Behav. 1994;48:1235–1237. [Google Scholar]
- Harris BN, de Jong TR, Yang V, Saltzman W. Chronic variable stress in fathers alters paternal and social behavior but not pup development in the biparental California mouse (Peromyscus californicus) Horm Behav. 2013;64:799–811. doi: 10.1016/j.yhbeh.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris BN, Saltzman W. Effect of reproductive status on hypothalamic–pituitary– adrenal (HPA) activity and reactivity in male California mice (Peromyscus californicus) Physiol Behav. 2013;112:70–76. doi: 10.1016/j.physbeh.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM Corp. IBM SPSS Statistics for Windows, Version 22.0. IBM Corp; Armonk, New York: 2013. [Google Scholar]
- Jakubowski M, Terkel J. Incidence of pup killing and parental behavior in virgin female and male rats (Rattus norvegicus): differences between Wistar and Sprague-Dawley stocks. J Comp Psychol. 1985a;99:93–97. [PubMed] [Google Scholar]
- Jakubowski M, Terkel J. Transition from pup killing to parental behavior in male and virgin female albino rats. Physiol Behav. 1985b;34:683–686. doi: 10.1016/0031-9384(85)90364-6. [DOI] [PubMed] [Google Scholar]
- Jasarevic E, Bailey DH, Crossland JP, Dawson WD, Szalai G, Ellersieck MR, Rosenfeld CS, Geary DC. Evolution of monogamy, paternal investment, and female life history in Peromyscus. J Comp Psychol. 2013;127:91–102. doi: 10.1037/a0027936. [DOI] [PubMed] [Google Scholar]
- Kawashima T, Okuno H, Bito H. A new era for functional labeling of neurons: activity-dependent promoters have come of age. Fron Neural Ciruits. 2014;8:109–117. doi: 10.3389/fncir.2014.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenkel WM, Paredes J, Lewis GF, Yee JR, Pournajafi-Nazarloo H, Grippo AJ, Porges SW, Carter CS. Autonomic substrates of the response to pups in male prairie voles. PLoS One. 2013;8:e69965. doi: 10.1371/journal.pone.0069965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenkel WM, Paredes J, Yee JR, Pournajafi-Nazarloo H, Bales KL, Carter CS. Neuroendocrine and behavioural responses to exposure to an infant in male prairie voles. J Neuroendocrinol. 2012;24:874–886. doi: 10.1111/j.1365-2826.2012.02301.x. [DOI] [PubMed] [Google Scholar]
- Kenkel WM, Suboc G, Carter CS. Autonomic, behavioral and neuroendocrine correlates of paternal behavior in male prairie voles. Physiol Behav. 2014;128:252–259. doi: 10.1016/j.physbeh.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klampfl SM, Schramm MM, Stinnett GS, Bayerl DS, Seasholtz AF, Bosch OJ. Brain CRF-binding protein modulates aspects of maternal behavior under stressful conditions and supports a hypo-anxious state in lactating rats. Horm Behav. 2016;84:136–144. doi: 10.1016/j.yhbeh.2016.06.009. [DOI] [PubMed] [Google Scholar]
- Kleiman DG, Malcolm JR. The evolution of male parental investment in mammals. In: Gubernick DJ, Klopfer PH, editors. Parental Care in Mammals. Plenum Press; New York: 1981. pp. 347–387. [Google Scholar]
- Lee AW, Brown RE. The presence of the male facilitates parturition in California mice (Peromyscus californicus) Can J Zool. 2002;80:926–933. [Google Scholar]
- Leussis MP, Bond TL, Hawken CM, Brown RE. Attenuation of maternal behavior in virgin CD-1 mice by methylphenidate hydrochloride. Physiol Behav. 2008;95:395–399. doi: 10.1016/j.physbeh.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Lightman SL, Windle RJ, Wood SA, Kershaw YM, Shanks N, Ingram CD. Peripartum plasticity within the hypothalamo-pituitary-adrenal axis. Prog Brain Res. 2001;133:111–129. doi: 10.1016/s0079-6123(01)33009-1. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, De Vries GJ. Social influences on parental and nonparental responses toward pups in virgin female prairie voles (Microtus ochrogaster) J Comp Psychol. 2001;115:53–61. doi: 10.1037/0735-7036.115.1.53. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Wagner CK, De Vries GJ. Comparison of the “nursing” and other parental behaviors of nulliparous and lactating female rats. Horm Behav. 1999;36:242–251. doi: 10.1006/hbeh.1999.1544. [DOI] [PubMed] [Google Scholar]
- Martín-Sánchez A, Valera-Marín G, Hernández-Martínez A, Lanuza E, Martínez-García F, Agustín-Pavón C. Wired for motherhood: induction of maternal care but not maternal aggression in virgin female CD1 mice. Front Behav Neurosci. 2015;9:197. doi: 10.3389/fnbeh.2015.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemenyi Peter. PhD thesis. Princeton University; Princeton, NJ: 1963. Distribution-free mulitple comparisons. [Google Scholar]
- Noirot E. Changes in responsiveness to young in the adult mouse. V Priming Anim Behav. 1969;17:542–546. doi: 10.1016/0003-3472(69)90161-4. [DOI] [PubMed] [Google Scholar]
- Noirot E, Richards MPM. Maternal behaviour in virgin female golden hamsters: Changes consequent upon initial contact with pups. Anim Behav. 1966;14:7–10. doi: 10.1016/s0003-3472(66)80003-9. [DOI] [PubMed] [Google Scholar]
- Numan M, Insel TR. The Neurobiology of Parental Behavior. Springer; New York: 2003. [Google Scholar]
- Paxinos G, Franklin KB. The Mouse Brain in Stereotaxic Coordinates. 3rd. Academic Press; New York: 2013. [Google Scholar]
- Pedersen CA, Vadlamudi SV, Boccia ML, Amico JA. Maternal behavior deficits in nulliparous oxytocin knockout mice. Genes Brain Behav. 2006;5:274–281. doi: 10.1111/j.1601-183X.2005.00162.x. [DOI] [PubMed] [Google Scholar]
- Pêgo JM, Sousa JC, Almeida OF, Sousa N. Stress and the neuroendocrinology of aniety disorders. Curr Top Behav Neurosci. 2010;2:97–117. doi: 10.1007/7854_2009_13. [DOI] [PubMed] [Google Scholar]
- Pohlert T. The pairwise multiple comparison of mean ranks package (PMCMR) R package. 2014 http://CRAN.R-project.org/package=PMCMR.
- Quadagno DM, Debold JF, Gorzalka BB, Whalen RE. Maternal behavior in the rat: Aspects of concaveation and neonatal androgen treatment. Physiol Behav. 1974;12:1071–1074. doi: 10.1016/0031-9384(74)90157-7. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: the R Foundation for Statistical Computing; 2011. Available online at http://www.R-project.org/ [Google Scholar]
- Reisbick S, Rosenblatt JS, Mayer AD. Decline of maternal behavior in the virgin and lactating rat. J Comp Physiol Psychol. 1975;89:722–732. doi: 10.1037/h0077059. [DOI] [PubMed] [Google Scholar]
- Rosenblatt JS. Nonhormonal basis of maternal behavior in the rat. Science. 1967;156:1512–1513. doi: 10.1126/science.156.3781.1512. [DOI] [PubMed] [Google Scholar]
- Rosenfeld CS, Johnson SA, Ellersieck MR, Roberts RM. Interactions between parents and pups in the monogamous California mouse (Peromyscus californicus) PLoS One. 2013;8:e75725. doi: 10.1371/journal.pone.0075725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery DA, Neumann ID. No stress please! Mechanisms of stress hyporesponsiveness of the maternal brain. J Physiol. 2008;586:377–385. doi: 10.1113/jphysiol.2007.145896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CD, Lonstein JS. Contact with infants modulates anxiety-generated c-fos activity in the brains of postpartum rats. Behav Brain Res. 2008;190:193–200. doi: 10.1016/j.bbr.2008.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern JM, Mackinnon DA. Postpartum, hormonal, and nonhormonal induction of maternal behavior in rats: Effects on T-maze retrieval of pups. Horm Behav. 1976;7:305–316. doi: 10.1016/0018-506x(76)90036-2. [DOI] [PubMed] [Google Scholar]
- Stern JM, Rogers L. Experience with younger siblings facilitates maternal responsiveness in pubertal Norway rats. Dev Psychobiol. 1988;21:575–589. doi: 10.1002/dev.420210608. [DOI] [PubMed] [Google Scholar]
- Stolzenberg DS, Numan M. Hypothalamic interaction with the mesolimbic DA system in the control of the maternal and sexual behaviors in rats. Neuosci Biobehav Rev. 2011;25:826–847. doi: 10.1016/j.neubiorev.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Stolzenberg DS, Rissman EF. Oestrogen-independent, experience-induced maternal behaviour in female mice. J Neuroendocrinol. 2011;23:345–354. doi: 10.1111/j.1365-2826.2011.02112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolzenberg DS, Stevens JS, Rissman EF. Experience-facilitated improvements in pup retrieval; evidence for an epigenetic effect. Horm Behavior. 2012;62:128–135. doi: 10.1016/j.yhbeh.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LJ, Campbell CS. Induction of maternal behavior in nulliparous golden hamsters (Mesocricetus auratus) Behav Neural Biol. 1979;26:364–371. doi: 10.1016/s0163-1047(79)91344-x. [DOI] [PubMed] [Google Scholar]
- Tsuneoka Y, Tokita K, Yoshihara C, Amano T, Esposito G, Huang AJ, Yu ML, Odaka Y, Shinozuka K, McHugh TJ, Kuroda KO. Distinct preoptic-BST nuclei dissociate paternal and infanticidal behavior in mice. EMBO J. 2015;34:2652–2670. doi: 10.15252/embj.201591942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesner BP, Sheard NM. Maternal Behavior in the Rat. Oliver & Boyd; Oxford: 1933. [Google Scholar]


