Abstract
Context
While energy conservation strategies are recommended in clinical practice guidelines, little is known about changes in energy levels in oncology patients undergoing cancer treatment.
Objectives
To identify variations in the trajectories of morning and evening energy levels and to determine which characteristics predicted initial levels as well as the trajectories of morning and evening energy.
Methods
Outpatients receiving chemotherapy (CTX) completed demographic and symptom questionnaires six times over two CTX cycles. Energy was assessed using the Lee Fatigue Scale. Hierarchical linear modeling was used to analyze the data.
Results
A large amount of inter-individual variability was found in the morning and evening energy trajectories. Patients who lived alone, had child care responsibilities, had a lower functional status, did not exercise on a regular basis, had lower hemoglobin levels, had lower attentional function, higher trait anxiety, and higher sleep disturbance reported lower morning energy levels at enrollment. Variations in the trajectories of morning energy were associated with a higher body mass index and higher levels of morning energy and higher sleep disturbance scores. For evening energy, patients who were female, White, had lower functional status, and had lower attentional function and higher sleep disturbance, reported lower evening energy levels at enrollment. Evening energy levels at enrollment were associated with changes in evening energy over time.
Conclusion
Patients undergoing CTX experience decrements in both morning and evening energy. The modifiable characteristics associated with these decrements can be used to design intervention studies to increase energy levels in these patients.
Keywords: morning energy, evening energy, cancer, oncology, chemotherapy, hierarchical linear modeling, symptom trajectories, diurnal variations
Introduction
Energy conservation strategies are recommended in a number of clinical practice guidelines to manage fatigue associated with cancer and its treatments.1,2 In fact, these strategies are listed second to self-monitoring of fatigue levels in the latest guideline published by the National Comprehensive Cancer Network (NCCN).3 However, in most of the symptom management literature, the terms energy and fatigue are used interchangeably.4,5 For example, in the Memorial Symptom Assessment Scale, fatigue is evaluated using the phrase “lack of energy”.6
Of note, a growing body of evidence suggests that energy and fatigue are distinct, but related symptoms.7–9 In fact, in a recent Rasch analysis of the LFS, energy and fatigue were found to be distinct symptoms.10 However, little is known about how energy levels change in oncology patients undergoing cancer treatment. Only one study was identified that evaluated for changes in energy levels in patients who underwent radiation therapy (RT) and their family caregivers.11 In this sample (n=252), the energy subscale scores from the LFS were used to identify groups of participants (i.e., latent classes) with distinct morning and evening energy trajectories. Using growth mixture modeling (GMM), for both morning and evening energy, two latent classes were identified. Participants were more likely to be in the lower morning energy class if they were younger, female, not partnered, Black, had more comorbidities and had a lower functional status. Participants were more likely to be in the lower evening energy class if they were younger, male, had a higher number of comorbidities, had a lower body weight, and had a lower functional status.
No studies were identified that evaluated for changes in energy levels in oncology patients receiving CTX. Therefore, the purpose of this study, in a sample of outpatients with breast, gastrointestinal (GI), gynecological (GYN), and lung cancer who were receiving two cycles of CTX, was to evaluate for variations in the trajectories of morning and evening energy levels. In addition, an evaluation was done to determine which demographic, clinical, and symptom characteristics were associated with initial levels as well as with the trajectories of morning and evening energy.
Methods
Sample and Settings
This study is part of a larger, longitudinal study of the symptom experience of oncology outpatients receiving CTX.12–15 Eligible patients were ≥18 years of age; had a diagnosis of breast, GI, GYN, or lung cancer; had received CTX within the preceding four weeks; were scheduled to receive at least two additional cycles of CTX; were able to read, write, and understand English; and gave written informed consent. Patients were recruited from two Comprehensive Cancer Centers, one Veteran’s Affairs hospital, and four community-based oncology programs.
Instruments
A demographic questionnaire obtained information on age, gender, ethnicity, marital status, living arrangements, education, employment status, and income. In addition, patients indicated if they exercised on a regular basis (yes/no) and if they ever considered themselves a smoker (yes/no). Functional status was rated using the Karnofsky Performance Status (KPS) scale.16 Patients rated their functional status using the KPS scale that ranged from 30 (I feel severely disabled and need to be hospitalized) to 100 (I feel normal; I have no complaints or symptoms).17,18
Self-Administered Comorbidity Questionnaire (SCQ) consists of 13 common medical conditions simplified into language that can be understood without prior medical knowledge.19 Patients indicated if they had the condition (yes/no); if they received treatment for it (proxy for disease severity, yes/no); and if it limited their activities (indication of functional limitations, yes/no). For each condition, the number of “yes” responses was totaled. Across the thirteen conditions, the total SCQ score can range from 0 to 39 with higher scores indicating a worse comorbidity profile. The SCQ has well established validity and reliability.20,21
Lee Fatigue Scale (LFS) consists of 18 items designed to assess physical fatigue and energy.22 Each item was rated on a 0 to 10 numeric rating scale (NRS). Total fatigue and energy scores are calculated as the mean of the 13 fatigue items and the 5 energy items, respectively. Higher scores indicate greater fatigue severity and higher levels of energy. Using separate LFS questionnaires, patients were asked to rate each item based on how they felt within 30 minutes of awakening (i.e., morning fatigue, morning energy) and prior to going to bed (i.e., evening fatigue, evening energy). The LFS has established cut-off scores for clinically meaningful levels of fatigue (i.e., ≥3.2 for morning fatigue, ≥5.6 for evening fatigue)23 and energy (i.e., ≤6.2 for morning energy, ≤3.5 for evening energy).23 It was chosen for this study because it is relatively short, easy to administer, and has well established validity and reliability.4,22,24–27 In the current study, the Cronbach’s alphas were 0.96 for morning and 0.93 for evening fatigue and 0.95 for morning and 0.93 for evening energy.
Spielberger State-Trait Anxiety Inventories (STAI-T and STAI-S) each have 20 items that are rated from 1 to 4. The summed scores for each scale can range from 20 to 80. The STAI-T measures a person’s predisposition to anxiety as part of one’s personality. The STAI-S measures a person’s temporary anxiety response to a specific situation or how anxious or tense a person is “right now” in a specific situation. Cutoff scores of >31.8 and >32.2 indicate high levels of trait and state anxiety, respectively. The STAI-S and STAI-T inventories have well established validity and reliability.28–30 In the current study, the Cronbach’s alphas for the STAI-T and STAI-S were 0.92 and 0.96, respectively.
Center for Epidemiological Studies-Depression scale (CES-D) consists of 20 items selected to represent the major symptoms in the clinical syndrome of depression. A total score can range from 0 to 60, with scores of >16 indicating the need for individuals to seek clinical evaluation for major depression. The CES-D has well established validity and reliability.31–33 In the current study, the Cronbach’s alpha for the CES-D total score was 0.89.
General Sleep Disturbance Scale (GSDS) consists of 21-items designed to assess the quality of sleep in the past week. Each item was rated on a 0 (never) to 7 (everyday) NRS. The GSDS total score is the sum of the seven subscale scores that can range from 0 (no disturbance) to 147 (extreme sleep disturbance). A GSDS total score of >43 indicates a significant level of sleep disturbance.23 The GSDS has well established validity and reliability.25,34,35 In the current study, the Cronbach’s alpha for the GSDS total score was 0.83.
Attentional Function Index (AFI) consists of 16 items designed to measure attentional function.36 A higher total mean score on a 0 to 10 NRS indicates greater capacity to direct attention.36 Total scores are grouped into categories of attentional function (i.e., <5.0 low function, 5.0 to 7.5 moderate function, >7.5 high function).37 The AFI has well established reliability and validity.36 In this study, the Cronbach’s alpha for the AFI total score was 0.93.
Occurrence of pain was evaluated using the Brief Pain Inventory.38 Patients who responded yes to the question about having pain were asked to indicate if their pain was or was not related to their cancer treatment.
Study Procedures
The study was approved by the Institutional Review Board at each of the study sites. Eligible patients were approached in the infusion unit by a member of the research team to discuss participation in the study. Written informed consent was obtained from all patients. Depending on the length of their CTX cycles (i.e., 14-day, 21-day, or 28-day), patients completed study questionnaires in their homes, a total of six times over two cycles of CTX, namely: prior to CTX administration (i.e., recovery from previous CTX cycle; Assessment 1 and 4), approximately 1 week after CTX administration (i.e., acute symptoms; Assessments 2 and 5), and approximately 2 weeks after CTX administration (i.e., potential nadir; Assessments 3 and 6). Research nurses reviewed the patients’ medical records for disease and treatment information (e.g., time since cancer diagnosis, number and types of prior cancer treatments, number of metastatic sites).
Data Analyses
Descriptive statistics and frequency distributions were generated on the sample characteristics and symptom severity scores at enrollment using the Statistical Package for the Social Sciences (SPSS) version 22.39
Hierarchical linear modeling (HLM) based on full maximum likelihood estimation was performed in two stages using software developed by Raudenbush and Bryk.40 The HLM methods are described in detail elsewhere.26,41–44 Separate HLM analyses were done for morning and evening energy. In brief, during stage 1, intra-individual variability in morning and evening energy over time was examined. A piecewise model strategy was employed to evaluate the pattern of change in morning and evening energy over time because the six assessments encompassed two cycles of CTX. The six assessments were coded into two pieces.
Assessments 1, 2, and 3 comprised the first piece (PW1) that was used to model changes over time during the first CTX cycle. Assessments 4, 5, and 6 comprised the second piece (PW2) that was used to model changes over time during the second CTX cycle. A piecewise model can be more sensitive to the timing and sequencing of changes in a dependent variable than conventional HLM models that would have assessed linear, quadratic, or cubic changes over the six assessments and would not have paid attention to the two different CTX cycles.45
The second stage of the HLM analysis examined inter-individual differences in the piecewise trajectories of morning and evening energy by modeling the individual change parameters (i.e., intercept and slope parameters) as a function of proposed predictors at level 2. Supplementary Tables 1 and 2 list the potential predictors for morning and evening energy, respectively, that were evaluated in this study.
To improve estimation efficiency and construct a parsimonious model, exploratory level 2 analyses were completed in which each potential predictor was assessed to determine whether it would result in a better fitting model if it alone were added as a level 2 predictor. Predictors with a t value of <2.0 were excluded from subsequent model testing. All potential significant predictors from the exploratory analyses were entered into the model to predict each individual change parameter. Only predictors that maintained a statistically significant contribution in conjunction with other predictors were retained in the final model. A p-value of <.05 indicated statistical significance.
Results
Morning Energy
Sample Characteristics
The demographic, clinical, and symptom characteristics of the sample (n=1333) are presented Table 1. The sample was predominately female (78%) and white (69%), well educated (16 years), partnered (65%), currently not employed (65%), did not have child care responsibilities (78%), and had a mean age of 57 years. On average, the patients were 2 years from their cancer diagnosis (median = 0.42 year), primarily being treated with 21-day CTX cycles (51%), had one metastatic site, and had received previous cancer treatment (76%). At enrollment, the mean scores on the GSDS, STAI-T, and the STAI-S were above the clinically meaningful cutoff scores for sleep disturbance, trait anxiety, and state anxiety, respectively. In addition, patients reported clinically meaningful decrements in morning energy levels at enrollment, while evening energy levels were at the cut-off score for a clinically meaningful decrement.
Table 1.
Demographic, Clinical, and Symptom Characteristics of the Patients in the Morning Energy Analysis (n=1333)
| Demographic Characteristics | |
|---|---|
| Age (years; mean (SD)) | 57.18 (12.39) |
|
| |
| Gender (% female (n)) | 77.9 (1039) |
|
| |
| Ethnicity (% (n)) | |
| White | 69.5 (926) |
| Black | 9.9 (132) |
| Asian/Pacific Islander | 9.6 (128) |
| Hispanic/Mixed/Other | 11.0 (147) |
|
| |
| Education (years; mean (SD)) | 16.18 (2.98) |
|
| |
| Married or partnered (% yes (n)) | 64.9 (865) |
|
| |
| Lives alone (% yes (n)) | 21.2 (283) |
|
| |
| Currently employed (% yes (n)) | 34.7 (463) |
|
| |
| Child care responsibilities (% yes (n)) | 21.7 (289) |
|
| |
| Income (% yes (n)) | |
| Less than $30,000 | 18.4 (219) |
| $30,000 to <$70,000 | 21.1 (252) |
| $70,000 to < $100,000 | 16.9 (202) |
| More than $100,000 | 43.6 (520) |
|
| |
| Clinical Characteristics | |
|
| |
| Number of comorbidities (mean (SD)) | 2.40 (1.43) |
|
| |
| Self-administered Comorbidity Questionnaire score (mean (SD)) | 5.47 (3.20) |
|
| |
| Body mass index (kg/m2; mean (SD)) | 26.17 (5.63) |
|
| |
| Hemoglobin (gm/dL; mean (SD)) | 11.54 (1.43) |
|
| |
| Karnofsky Performance Status score (mean (SD)) | 80.00 (12.39) |
|
| |
| Have you ever considered yourself a smoker (% yes (n)) | 34.8 (464) |
|
| |
| Exercise on a regular basis (% yes (n)) | 71.5 (953) |
|
| |
| Specific comorbidities reported (% yes (n)) | |
| High blood pressure | 30.2 (402) |
| Back pain | 25.7 (343) |
| Depression | 19.3 (257) |
| Osteoarthritis | 12.0 (160) |
| Anemia or blood disease | 12.2 (163) |
| Lung disease | 11.3 (151) |
| Diabetes | 9.0 (120) |
| Liver disease | 6.5 (86) |
| Heart disease | 5.6 (75) |
| Rheumatoid arthritis | 3.1 (41) |
| Ulcer or stomach disease | 4.9 (65) |
| Kidney disease | 1.4 (19) |
|
| |
| Cancer diagnosis (% yes (n)) | |
| Breast | 40.4 (539) |
| Gastrointestinal | 30.3 (404) |
| Gynecological | 17.5 (233) |
| Lung | 11.8 (157) |
|
| |
| Time since cancer diagnosis (years; mean (SD)) | 1.97 (3.87) |
|
| |
| Time since cancer diagnosis (years; median) | 1.97 (0.42) |
|
| |
| Any prior cancer treatments (% yes (n)) | 75.8 (1010) |
|
| |
| Number prior cancer treatments (mean (SD)) | 1.59 (1.50) |
|
| |
| Chemotherapy cycle length (% (n)) | |
| 14 days | 41.8 (557) |
| 21 days | 50.9 (679) |
| 28 days | 7.3 (97) |
|
| |
| Presence of metastatic disease (% yes (n)) | 67.1 (894) |
|
| |
| Number of metastatic sites including lymph node involvement (mean (SD)) | 1.24 (1.23) |
|
| |
| Number of metastatic sites excluding lymph node involvement (mean (SD)) | 0.78 (1.05) |
|
| |
| Symptom Characteristics at Enrollment | |
|
| |
| Lee Fatigue Scale: evening fatigue score (mean (SD)) | 5.33 (2.15) |
|
| |
| Lee Fatigue Scale: morning fatigue score (mean (SD)) | 3.13 (2.25) |
|
| |
| Lee Fatigue Scale: evening energy score (mean (SD)) | 3.54 (2.04) |
|
| |
| Lee Fatigue Scale: morning energy score (mean (SD)) | 4.40 (2.25) |
|
| |
| Center for Epidemiological Studies-Depression Scale score (mean (SD)) | 12.97 (9.77) |
|
| |
| General Sleep Disturbance Scale score (mean (SD)) | 52.57 (20.17) |
|
| |
| Trait Anxiety score (mean (SD)) | 35.15 (10.39) |
|
| |
| State Anxiety score (mean (SD)) | 33.98 (12.33) |
|
| |
| Attentional Function Index score (mean (SD)) | 6.38 (1.82) |
|
| |
| Pain present (% yes (n)) | 72.8 (971) |
Abbreviations: gm/dL = grams per deciliter; kg/m2 = kilograms per meters squared; SD = standard deviation; RT = radiation therapy.
Changes in Morning Energy Levels Over Time
The first HLM analysis examined how morning energy scores changed within the two cycles of CTX. The linear and quadratic trends for both cycles of CTX were significant (all, p<.0001; see Table 2).
Table 2.
Hierarchical Linear Model for Morning Energy
| Morning Energy | Coefficient (SE) | |
|---|---|---|
| Unconditional Model | Final Model | |
| Fixed effects | ||
| Intercept | 4.393 (.062)+ | 4.393 (.058)+ |
| Piecewise 1 – linear rate of change | −0.457 (.113)+ | −0.466 (.110)+ |
| Piecewise 1 – quadratic rate of change | 0.386 (.054)+ | 0.389 (.053)+ |
| Piecewise 2 – linear rate of change | −0.492 (.074)+ | −0.506 (.072)+ |
| Piecewise 2 – quadratic rate of change | 0.166 (.024)+ | 0.170 (.023)+ |
| Time invariant covariates | ||
| Intercept | ||
| Lives alone | −0.250 (.113)* | |
| Child care responsibilities | 0.355 (.112)* | |
| Karnofsky Performance Status | 0.019 (.004)+ | |
| Exercise on a regular basis | 0.461 (.100)+ | |
| Hemoglobin level | 0.104 (.032)* | |
| Trait anxiety | −0.022 (.005)+ | |
| Sleep disturbance | −0.010 (.003)+ | |
| Attentional function | 0.291 (.033)+ | |
| Piecewise 1 – linear rate of change | ||
| Morning energy | −0.134 (.044)* | |
| Piecewise 1 – quadratic rate of change | ||
| Morning energy | 0.026 (.021) | |
| Piecewise 2 – linear rate of change | ||
| Body mass index | −0.031 (.010)* | |
| Sleep disturbance | −0.014 (.003)+ | |
| Piecewise 2 – quadratic rate of change | ||
| Body mass index | 0.010 (.004)* | |
| Sleep disturbance | 0.003 (.001)* | |
| Variance components | ||
| In intercept | 1.618+ | 1.460+ |
| Goodness-of-fit deviance (parameters estimated) | 28656.390 (7)** | 28033.617 (21) |
| Model comparison χ 2 (df) | 622.773 (14)+ | |
p<.05,
p<.001,
p<.0001
Abbreviations: df = degrees of freedom; SE = standard error
The estimates for the initial piecewise model are presented in Table 2. Since the model was unconditional (i.e., no covariates), the intercept represents the average morning energy score at enrollment (i.e., 4.393 on a scale of 0 to 10). The estimated linear piecewise rates of change in morning energy were −0.457 and −0.492 (both p<.0001) for piecewise linear 1 and piecewise linear 2, respectively. The estimated quadratic piecewise rates of change in morning energy were 0.386 and 0.166 (both p<.0001) for piecewise quadratic 1 and piecewise quadratic 2, respectively. The combination of each coefficient determines the curves for the two piecewise components’ changes in morning energy scores over time.
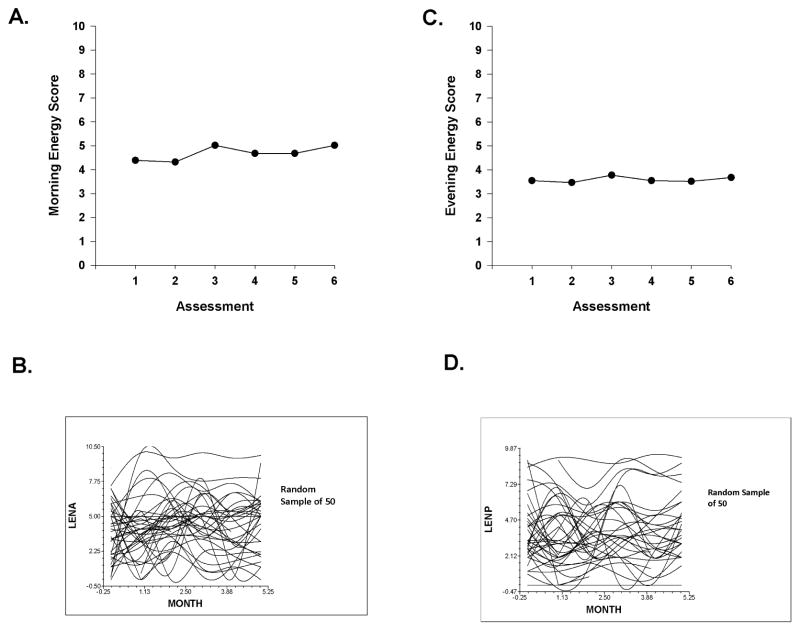
Figure 1A displays the mean morning energy scores over the two cycles of CTX. Morning energy levels declined at assessment 2 and increased with a peak at assessment 3, decreased slightly at assessment 4, remained unchanged at assessment 5, and increased slightly at assessment 6. These results indicate a sample-wide change in morning energy levels over time. However, they do not indicate that all of the patients’ morning energy scores changed at the same rate over time. The variance components (Table 2) suggest that considerable inter-individual variability existed in the trajectories of morning energy (see Figure 1B).
Figure 1.
Figure 1A – Piecewise model of mean morning energy scores for six assessment points over two cycles of chemotherapy (CTX).
Figure 1B – Spaghetti plots of individual morning energy trajectories for a random sample of 50 patients over two cycles of CTX. Abbreviation: LENA = Lee Fatigue Scale - Morning Energy subscale score.
Figure 1C – Piecewise model of mean evening energy scores for six assessment points over two cycles of chemotherapy (CTX).
Figure 1D – Spaghetti plots of individual evening energy trajectories for a random sample of 50 patients over two cycles of CTX. Abbreviation: LENP = Lee Fatigue Scale - Evening Energy subscale score.
Inter-Individual Differences in Initial Levels of Morning Energy
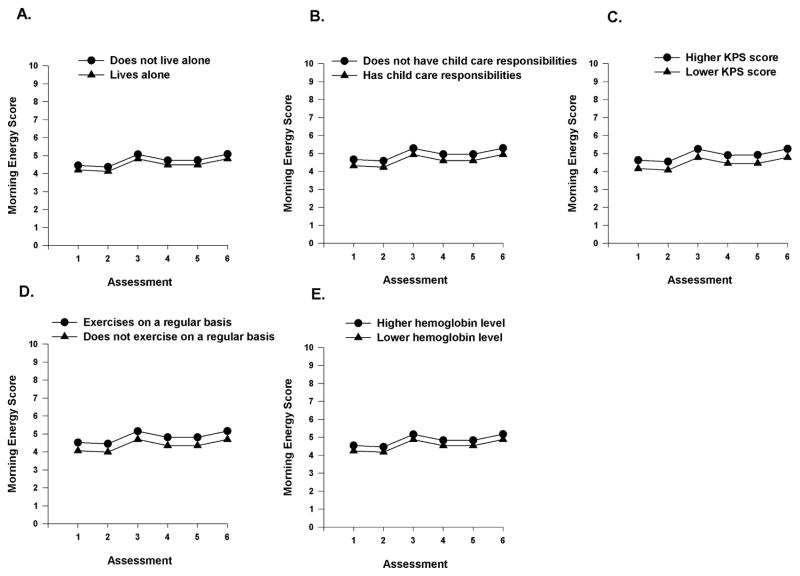
As shown in the final model (Table 2), the demographic characteristics that predicted inter-individual differences in the initial levels (i.e., intercept) of morning energy were living alone and having child care responsibilities. The clinical characteristics that predicted inter-individual differences in the initial levels of morning energy were functional status, exercise on a regular basis, and hemoglobin (Hgb) level. The severity of trait anxiety and attentional function at enrollment were the symptom characteristics that predicted inter-individual differences in initial levels of morning energy.
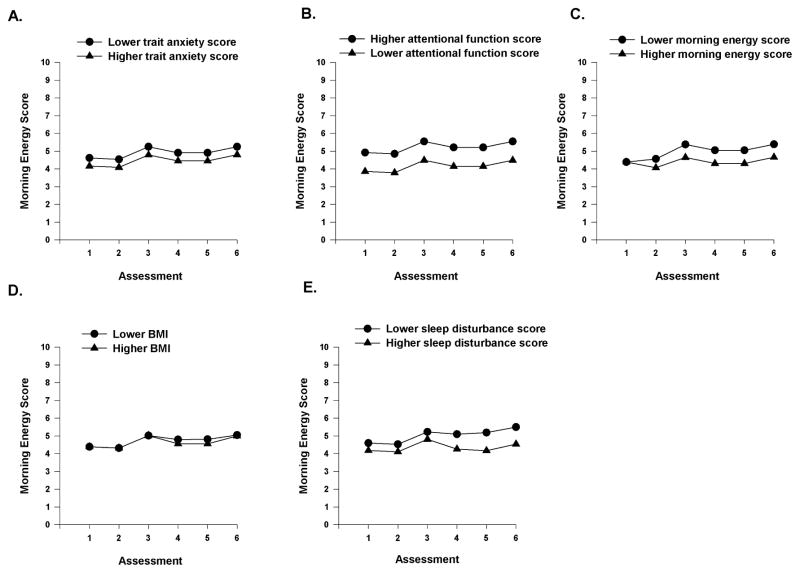
To illustrate the effects of the various demographic, clinical, and symptom characteristics on initial levels of morning energy, Figures 2A–E display the adjusted change curves for morning energy that were estimated based on whether the patient lived alone (i.e., yes or no), had child care responsibilities (i.e., yes or no), had differences in functional status (i.e., lower/higher calculated as one SD above and below the mean KPS score), exercised on a regular basis (i.e., yes or no), and had differences in Hgb levels (i.e., lower/higher calculated as one SD above and below the mean Hgb level). Figures 3A–B display the adjusted change curves for morning energy that were estimated based on differences in trait anxiety (i.e., lower/higher calculated as one SD above and below the mean STAI-T score) and attentional function (i.e., lower/higher calculated as one SD above and below the mean AFI score).
Figure 2.
A-E- Influence of enrollment scores for living alone (A), child care responsibilities (B), Karnofsky Performance Status (KPS) score (C), exercise (D), and hemoglobin level (E), on inter-individual differences in the intercept for morning energy.
Figure 3.
A-E Influence of enrollment scores for trait anxiety (A) and attentional function (B), on inter-individual differences in the intercept for morning energy, and influence of morning energy (C) and body mass index (BMI, D) on the slope parameters for morning energy and influence of enrollment scores for sleep disturbance (E) on inter-individual differences in the intercept and slope parameters for morning energy.
Inter-individual Differences in the Trajectories of Morning Energy
As shown in the final model (Table 2), one clinical characteristic (i.e., BMI) and one symptom characteristic (i.e., initial level of morning energy) predicted inter-individual differences in the trajectories of morning energy. Figures 3C–D display the adjusted change curves for morning energy that were evaluated based on differences in morning energy (i.e., lower/higher calculated as one SD above and below the mean LFS morning energy score) and differences in BMI (i.e., lower/higher calculated as one SD above and below the mean BMI).
Inter-Individual Differences in Initial Levels and Trajectories of Morning Energy
As shown in the final model (Table 2), sleep disturbance was the only characteristic that predicted inter-individual differences in both initial levels as well as in the trajectories of morning energy. Figure 3E displays the adjusted change curves for morning energy that were evaluated based on differences in sleep disturbance (i.e., lower/higher calculated as one SD above and below the mean GSDS score).
Evening Energy
Sample Characteristics
The demographic, clinical, and symptom characteristics of the sample (n=1332) are presented Table 3. The sample was predominately female (78%), white (69%), partnered (65%), well educated (16 years), currently not employed (65%), did not have child care responsibilities (78%), with a mean age of 57 years. On average, the patients were 2 years from their cancer diagnosis (median = 0.42 year), primarily being treated with 21-day CTX cycles (51%), had one metastatic site, and had received previous cancer treatment (76%). At enrollment, the mean scores on the GSDS, STAI-T, and the STAI-S were above the clinically meaningful cutoff scores for sleep disturbance, trait anxiety, and state anxiety, respectively. In addition, patients reported clinically meaningful decrements in morning energy levels at enrollment, while evening energy levels were at the cut-off score for a clinically meaningful decrement.
Table 3.
Demographic, Clinical, and Symptom Characteristics of the Patients in the Evening Energy Analysis (n=1332)
| Demographic Characteristics | |
|---|---|
| Age (years; mean (SD)) | 57.16 (12.39) |
|
| |
|
| |
| Gender (% female (n)) | 77.9 (1038) |
|
| |
| Ethnicity (% (n)) | |
| White | 69.4 (925) |
| Black | 9.9 (132) |
| Asian/Pacific Islander | 9.6 (128) |
| Hispanic/Mixed/Other | 11.0 (147) |
|
| |
| Education (years; mean (SD)) | 16.18 (2.99) |
|
| |
| Married or partnered (% yes (n)) | 65.0 (866) |
|
| |
| Lives alone (% yes (n)) | 21.2 (283) |
|
| |
| Currently employed (% yes (n)) | 34.8 (463) |
|
| |
| Child care responsibilities (% yes (n)) | 21.7 (289) |
|
| |
| Income (% yes (n)) | |
| Less than $30,000 | 18.4 (219) |
| $30,000 to <$70,000 | 21.1 (252) |
| $70,000 to < $100,000 | 16.9 (202) |
| More than $100,000 | 43.5 (519) |
|
| |
| Clinical Characteristics | |
|
| |
| Number of comorbidities (mean (SD)) | 2.40 (1.43) |
|
| |
| Self-administered Comorbidity Questionnaire score (mean (SD)) | 5.47 (3.20) |
|
| |
| Body mass index (kg/m2; mean (SD)) | 26.17 (5.63) |
|
| |
| Hemoglobin (gm/dL; mean (SD)) | 11.54 (1.43) |
|
| |
| Karnofsky Performance Status score (mean (SD)) | 80.00 (12.39) |
|
| |
| Have you ever considered yourself a smoker (% yes (n)) | 34.8 (463) |
|
| |
| Exercise on a regular basis (% yes (n)) | 71.5 (952) |
|
| |
| Specific comorbidities reported (% yes (n)) | |
| High blood pressure | 30.1 (401) |
| Back pain | 25.8 (343) |
| Depression | 19.3 (257) |
| Osteoarthritis | 12.0 (160) |
| Anemia or blood disease | 12.3 (164) |
| Lung disease | 11.3 (151) |
| Diabetes | 8.9 (119) |
| Liver disease | 6.5 (86) |
| Heart disease | 5.6 (75) |
| Rheumatoid arthritis | 3.1 (41) |
| Ulcer or stomach disease | 4.9 (65) |
| Kidney disease | 1.4 (19) |
|
| |
| Cancer diagnosis (% yes (n)) | |
| Breast | 40.4 (538) |
| Gastrointestinal | 30.4 (405) |
| Gynecological | 17.4 (232) |
| Lung | 11.8 (157) |
|
| |
| Time since cancer diagnosis (years; mean (SD)) | 1.97 (3.87) |
|
| |
| Time since cancer diagnosis (years; median) | 1.97 (0.42) |
|
| |
| Any prior cancer treatments (% yes (n)) | 75.7 (1008) |
|
| |
| Number prior cancer treatments (mean (SD)) | 1.59 (1.50) |
|
| |
| Chemotherapy cycle length (% (n)) | |
| 14 days | 41.7 (556) |
| 21 days | 51.0 (679) |
| 28 days | 7.3 (97) |
|
| |
| Presence of metastatic disease (% yes (n)) | 67.0 (893) |
|
| |
| Number of metastatic sites including lymph node involvement (mean (SD)) | 1.24 (1.23) |
|
| |
| Number of metastatic sites excluding lymph node involvement (mean (SD)) | 0.78 (1.05) |
|
| |
| Symptom Characteristics at Enrollment | |
|
| |
| Lee Fatigue Scale: evening fatigue score (mean (SD)) | 5.33 (2.15) |
|
| |
| Lee Fatigue Scale: morning fatigue score (mean (SD)) | 3.13 (2.25) |
|
| |
| Lee Fatigue Scale: evening energy score (mean (SD)) | 3.54 (2.04) |
|
| |
| Lee Fatigue Scale: morning energy score (mean (SD)) | 4.40 (2.25) |
|
| |
| Center for Epidemiological Studies-Depression Scale score (mean (SD)) | 12.97 (9.77) |
|
| |
| General Sleep Disturbance Scale score (mean (SD)) | 52.59 (20.19) |
|
| |
| Trait Anxiety score (mean (SD)) | 35.15 (10.39) |
|
| |
| State Anxiety score (mean (SD)) | 33.98 (12.33) |
|
| |
| Attentional Function Index score (mean (SD)) | 6.38 (1.82) |
|
| |
| Pain present (% yes (n)) | 72.8 (970) |
Abbreviations: gm/dL = grams per deciliter; kg/m2 = kilograms per meters squared; SD = standard deviation; RT = radiation therapy.
Changes in Evening Energy Levels Over Time
The first HLM analysis examined how evening energy scores changed within the two cycles of CTX. The linear and quadratic trends for both cycles of CTX were significant (all, p<.05; Table 4). The estimates for the initial piecewise model are presented in Table 4. Since the model was unconditional (i.e., no covariates), the intercept represents the average evening energy score at enrollment (i.e., 3.552 on a scale of 0 to 10).
Table 4.
Hierarchical Linear Model for Evening Energy
| Evening Energy | Coefficient (SE) | |
|---|---|---|
| Unconditional Model | Final Model | |
| Fixed effects | ||
| Intercept | 3.552 (.058)+ | 3.551 (.058)+ |
| Piecewise 1 – linear rate of change | −0.275 (.109)* | −0.277 (.107)* |
| Piecewise 1 – quadratic rate of change | 0.195 (.052)+ | 0.196 (.051)+ |
| Piecewise 2 – linear rate of change | −0.323 (.071)+ | −0.327 (.070)+ |
| Piecewise 2 – quadratic rate of change | 0.096 (.023)+ | 0.097 (.022)+ |
| Time invariant covariates | ||
| Intercept | ||
| Female | −0.337 (.108)* | |
| Nonwhite | 0.372 (.097)+ | |
| Karnofsky Performance Status | 0.019 (.004)+ | |
| Sleep disturbance | −0.009 (.003)* | |
| Attentional function | 0.178 (.030)+ | |
| Piecewise 1 – linear rate of change | ||
| Evening energy | −0.128 (.047)* | |
| Piecewise 1 – quadratic rate of change | ||
| Evening energy | 0.037 (.023) | |
| Variance components | ||
| In intercept | 1.462+ | 1.467+ |
| Goodness-of-fit deviance (parameters estimated) | 27573.766 (7)** | 27365.202 (14) |
| Model comparison χ2 (df) | 208.564 (7)+ | |
p<.05,
p<.001,
p<.0001
Abbreviations: df = degrees of freedom; SE = standard error
The estimated linear piecewise rates of change in evening energy were −0.275 (p<.05) and −0.323 (p<.0001) for piecewise linear 1 and piecewise linear 2, respectively. The estimated quadratic piecewise rates of change in evening energy were 0.195 and 0.096 (both p<.0001) for piecewise quadratic 1 and piecewise quadratic 2, respectively. The combination of each coefficient determines the curves for the two piecewise components’ changes in evening energy scores over time.
Figure 1C displays the mean evening energy scores over the two cycles of CTX. Evening energy levels declined at assessment 2 and increased slightly at assessment 3, decreased through assessment 5, and then increased slightly at assessment 6.
The results indicate a sample-wide change in evening energy levels over time. However, they do not indicate that all of the patients’ evening energy level scores changed at the same rate over time. The variance components (Table 4) suggest that a considerable amount of inter-individual variability existed in the trajectories of evening energy scores (See Figure 1D).
Inter-Individual Differences in Initial Levels of Evening Energy
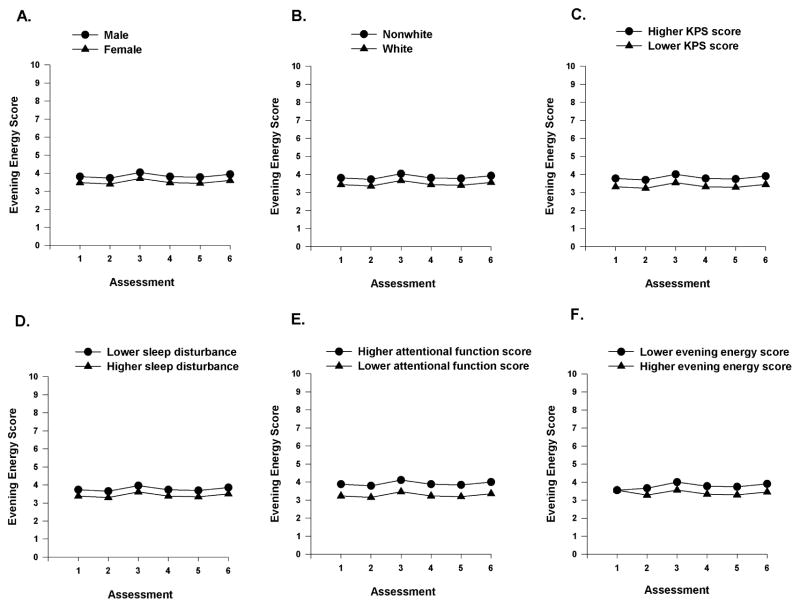
As shown in the final model (Table 4), the demographic characteristics that predicted inter-individual differences in the initial levels (i.e., intercept) of evening energy were gender and ethnicity. Functional status was the only clinical characteristic that predicted inter-individual differences in the initial levels of evening energy. Sleep disturbance and attentional function at enrollment were the symptom characteristics that predicted inter-individual differences in initial levels of evening energy.
To illustrate the effects of the various demographic, clinical, and symptom characteristics, Figures 4A–C display the adjusted change curves for evening energy that were estimated based on gender (i.e., male or female), ethnicity (i.e., white or nonwhite), and performance status (i.e., lower/higher calculated as one SD above and below the mean KPS score). Figures 4D–E display the adjusted change curves for evening energy that were estimated based on differences in sleep disturbance (i.e., lower/higher calculated as one SD above and below the mean GSDS score) and attentional function (i.e., lower/higher calculated as one SD above and below the mean AFI score).
Figure 4.
A-F Influence of gender (A), ethnicity (B), and enrollment scores Karnofsky Performance Status (KPS, C), sleep disturbance (D) and attentional function (E) on inter-individual differences in the intercept for evening energy, and influence of evening energy score at enrollment (F) on the slope parameters for evening energy.
Inter-Individual Differences in Trajectories of Evening Energy
As shown in the final model (Table 4), one symptom characteristic (i.e., initial level of evening energy) predicted inter-individual differences in the trajectories of evening energy. Figure 4F displays the adjusted change curves for evening energy that were evaluated based on differences in evening energy (i.e., lower/higher calculated as one SD above and below the mean LFS evening energy score).
Discussion
This study is the first to evaluate for inter-individual differences in morning and evening energy levels in oncology patients undergoing two cycles of CTX. In addition, common and distinct demographic, clinical, and symptom characteristics associated with more severe decrements in morning and evening energy were determined. As shown in Table 5, only three characteristics (i.e., functional status, sleep disturbance, and attentional function) were associated with decrements in both morning and evening energy. Findings regarding morning energy will be presented first. Contrasts will be made between the characteristics associated with decrements in initial levels as well as in the trajectories of morning and evening energy (see Table 5).
Table 5.
Comparison of Intercept and Slope Predictors of Morning and Evening Energy
| Characteristics | Morning Energy | Evening Energy |
|---|---|---|
| Intercept predictors | ||
| Nonwhite | ◆ | |
| Lives alone | ◆ | |
| Female | ◆ | |
| Child care responsibilities | ◆ | |
| Functional status | ◆ | ◆ |
| Exercise on a regular basis | ◆ | |
| Hemoglobin level | ◆ | |
| Trait anxiety | ◆ | |
| Sleep disturbance | ◆ | ◆ |
| Attentional function | ◆ | ◆ |
| Slope predictors | ||
| Morning energy | ◆ | |
| Evening energy | ◆ | |
| Body mass index | ◆ | |
| Sleep disturbance | ◆ | |
Morning Energy
It should be noted that across the entire sample, mean scores for morning energy at the first assessment (i.e., 4.40) were below the clinically meaningful cutoff score of ≤6.2. While Figure 1A illustrates that the changes in morning energy levels were relatively stable over the two cycles of CTX, a large amount of inter-individual variability in morning energy levels was found in the sample. Taken together, these findings suggest that prior to their next dose of CTX patients were experiencing clinically meaningful decrements in morning energy that persisted over the next 6 to 8 weeks.
Direct comparisons of our findings regarding the characteristics associated with initial levels, as well as the trajectories of morning energy over the two cycles of CTX, are not possible, because no studies were identified that used the same energy measure; the same assessment time points; and HLM as the analysis method. However, some information is available on the characteristics associated with decrements in morning energy levels. In a study that evaluated patients who underwent radiation therapy (RT) and their family caregivers,11 GMM identified two classes of participants (i.e., low and moderate morning energy). Similar to the current study, participants in the low energy class reported morning energy scores of 4.7 (±1.6) prior to the initiation of RT. Consistent with the findings in the RT study,11 in the current study, poorer functional status, higher trait anxiety, and poorer attentional function were associated with lower morning energy scores at enrollment. In addition, higher levels of sleep disturbance, as well as more severe decrements in morning energy were associated with worse trajectories of morning energy. While the timing of the assessments, the types of cancer treatments, and the statistical approaches used to identify the specific characteristics associated with more severe decrements in morning energy differed between the previous11 and the current study, a relatively large number of characteristics were similar in both studies. While these findings warrant confirmation, oncology clinicians can use these characteristics to identify patients who are at increased risk for decrements in morning energy.
For both morning and evening energy, higher levels of sleep disturbance were associated with lower energy levels. At enrollment, the mean sleep disturbance score of this sample (i.e., 52.6 + 20.2) was well above the clinically meaningful GSDS cutoff score of >43.0. At one standard deviation above the mean score (i.e., 72.8), these patients would have sleep disturbance scores above those reported by shift workers46 and parents of newborn infants.4 Additional research is warranted on the specific types and causes of sleep disturbance in oncology patients undergoing CTX. Clinicians need to assess for sleep disturbance in these patients and initiate appropriate interventions.
It should be noted that in in the current study, an additional five characteristics were associated with decrements in morning energy. As shown in Table 5, living alone, having child care responsibilities, not exercising on regular basis, having a lower Hgb level, and having a higher BMI were associated with lower levels of morning energy. While no studies were found that examined the relationship between living alone and morning energy levels, our findings make clinical sense in that individuals who live alone may lack immediate support to care for themselves or their living situation. Clinicians may need to involve social workers to assist patients to obtain the needed services and support during CTX.
In terms of child care responsibilities, in our previous RT study,11 no associations were found between this characteristic and decrements in either morning or evening energy. However, in the current study, it is interesting to note, that having child care responsibilities was associated with decrements in both morning and evening energy. Given its impact on both morning and evening energy levels, clinicians may need to counsel patients or involve social workers to evaluate the need for assistance with child care during CTX.
The association between increased exercise and decreased levels of fatigue in oncology patients is well established.47,48 In fact, the current fatigue guidelines published by the National Comprehensive Cancer Network recommended exercise as the only evidenced-based intervention to decrease fatigue in oncology patients.3 To our knowledge, this study is the first to demonstrate an association between lack of regular exercise and more severe decrements in morning energy levels. While this finding warrants replication, patients should receive ongoing education and encouragement to participate in a regular exercise program during and following CTX.
Anemia is implicated as a potential mechanism for the development of fatigue in oncology patients.49 In the current study, lower Hgb levels were associated with more severe decrements in morning energy levels. The mean Hgb level of our sample was 11.54 gm/dL. At one standard deviation below this mean level (i.e., 10.11 gm/dL), these patients would be classified as anemic. While this association makes sense clinically, it warrants confirmation in future studies.
The mean BMI of our sample (i.e., 26.17 kg/m2) is considered overweight.50 While no studies were found on the association between BMI and energy, studies of patients with breast cancer,51,52 and patients receiving CTX,13,53 reported that a higher BMI was associated with higher levels of fatigue. The exact reasons why a higher BMI is associated with higher levels of fatigue, as well as decrements in morning energy are not readily apparent. One potential explanation is that patients with higher BMI are more likely to be diagnosed with obstructive sleep apnea (OSA).54 Increased levels of sleep disturbance associated with OSA may contribute to the decrements in morning energy levels found in the current study. Another potential explanation for the decrements in morning energy associated with a higher BMI is that obesity is associated with increases in oxidative stress and metabolic disorders.55 This hypothesis is supported by recent studies that found an association between higher levels of oxidative stress and higher levels of fatigue in patients with chronic fatigue syndrome56 and children undergoing treatment for leukemia.57
Evening Energy
Mean scores for evening energy at the first assessment (i.e., 3.54) were at the clinically meaningful cutoff score of ≤3.5. Like morning energy, a large amount of inter-individual variability was found in this symptom (see Figure 1D). Again, at the initiation of their next dose of CTX and throughout the remaining 6 to 8 weeks, these patients experienced clinically meaningful decrements in evening energy.
While no studies were found that evaluated for changes in evening energy over two cycles of CTX, findings from the previously cited RT study11 are worth noting. In this RT study, two classes of participants (i.e., moderate and high evening energy) were identified using GMM. Prior to the initiation of RT, participants in the moderate energy class had evening energy scores of 4.1 (±1.6) which were slightly above the clinically meaningful cutoff score. Consistent with the findings in the RT study,11 in the current study, poorer functional status and poorer attentional function were associated with lower evening energy scores at enrollment. In addition, higher levels of sleep disturbance, as well as more severe decrements in evening energy were associated with worse trajectories of evening energy. Although differences exist between the previous11 and the current study (i.e., assessment time points, statistical analysis, and cancer treatment), a considerable number of the characteristics associated with decrements in evening energy were similar in both studies. From a clinical perspective, all three of these characteristics can be modified through targeted interventions. For example, functional status can be improved with regular exercise;58,59 improvements in attentional function can occur through the use of cognitive training tasks;60 and sleep hygiene interventions can be used to reduce sleep disturbance.61
Two additional characteristics (i.e., gender, ethnicity) were associated with decrements in evening energy. Our finding that female gender was associated with more severe decrements in evening energy contrasts with the findings from the previously cited RT study,11 in which male patients were more likely to be classified in the lower evening energy class. Given that the findings on gender differences in a variety of symptoms are not consistent,62,63 this inconsistency warrants additional investigation.
In terms of ethnic differences in symptom severity, findings for a variety of symptoms (e.g., pain,64,65 sleep disturbance66,67) are also inconsistent. In our previous RT study,11 while no association was found between ethnicity and evening fatigue, compared to White patients, Black patients were more likely to be classified in the lower morning energy class. However, in the current study, White patients were more likely to report decrements in evening energy at enrollment. Reasons for these inconsistent findings, in terms of both gender and ethnicity, may be related to differences in sample characteristics; differences in statistical methods used to evaluate the associations between gender or ethnicity and energy levels; and differences in the characterization of energy levels (i.e., mean energy levels versus diurnal variations in energy).
Limitations and Strengths
Several limitations and strengths need to be acknowledged. Because patients were recruited at various points in their CTX treatment, changes in energy levels from the initiation of CTX cannot be evaluated. In addition, the relationships between decrements in morning and evening energy levels and specific CTX regimens were not evaluated in this study. Patients rated their experience of morning and evening energy over a one week period of time. Daily assessments may provide more accurate information and insights into the variability of morning and evening energy during two cycles of CTX.68 Finally, while a number of characteristics associated with morning and evening energy (e.g., higher BMI, lack of exercise) that are interrelated remained significant in the final HLM models, interaction effects were not evaluated. However, this large, representative sample of oncology outpatients undergoing CTX; the assessment and evaluation of changes in morning and evening energy over two cycles of CTX; and the use of HLM to identify characteristics associated with decrements in morning and evening energy are major strengths of this study. In addition, this study is the first to evaluate for variations in the trajectories of morning and evening energy levels and to determine which demographic, clinical, and symptom characteristics were associated with initial levels as well as with the trajectories of these symptoms.
Clinical Implications
Our findings have a number of clinical implications. Assessment of diurnal variations in energy levels, as well as associated risk factors, need to be incorporated into clinical practice. These assessments may allow oncology clinicians to focus interventions on one or both of these symptoms. Several modifiable risk factors for decrements in morning and evening energy levels were identified. For morning energy, the modifiable risk factors were living alone, having child care responsibilities, lower functional status, lack of regular exercise, lower Hgb level, higher BMI, higher levels of sleep disturbance and lower levels of attentional function. For evening energy, the modifiable risk factors included lower functional status, higher levels of sleep disturbance, and lower levels of attentional function. These findings suggest that clinicians need to do a multidimensional symptom assessment in patients undergoing CTX. Interventions that improve functional status and decrease sleep disturbance may have the greatest potential to increase both morning and evening energy levels.
Future Research
Longitudinal studies are needed to evaluate for decrements in morning and evening energy levels in oncology patients prior to the initiation of CTX, as well as during treatment and into survivorship. Studies are needed that evaluate for changes in morning and evening energy among patients undergoing different types of cancer treatment (e.g., surgery, RT). Additional research is needed that evaluates the impact of different types of CTX on morning and evening energy. Studies of how genetic variations contribute to decrements in morning and evening energy levels in oncology patients may increase our understanding of the mechanisms that underlie diurnal variations in energy levels.
Supplementary Material
Acknowledgments
This study was funded by the National Cancer Institute (NCI, CA134900). Dr. Miaskowski is supported by a grant from the American Cancer Society and NCI (CA168960).
Footnotes
Disclosures: None to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barsevick AM, Whitmer K, Sweeney C, et al. A pilot study examining energy conservation for cancer treatment-related fatigue. Cancer Nurs. 2002;25:333–341. doi: 10.1097/00002820-200210000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Barsevick AM, Dudley W, Beck S, et al. A randomized clinical trial of energy conservation for patients with cancer-related fatigue. Cancer. 2004;100:1302–1310. doi: 10.1002/cncr.20111. [DOI] [PubMed] [Google Scholar]
- 3.Berger AM, Mooney K, Alvarez-Perez A, et al. Cancer-Related Fatigue, Version 2. 2015. J Natl Compr Canc Netw. 2015;13:1012–1039. doi: 10.6004/jnccn.2015.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gay CL, Lee KA, Lee SY. Sleep patterns and fatigue in new mothers and fathers. Biol Res Nurs. 2004;5:311–318. doi: 10.1177/1099800403262142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee KA, Gay C, Portillo CJ, et al. Symptom experience in HIV-infected adults: a function of demographic and clinical characteristics. J Pain Symptom Manage. 2009;38:882–893. doi: 10.1016/j.jpainsymman.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Portenoy RK, Thaler HT, Kornblith AB, et al. The Memorial Symptom Assessment Scale: an instrument for the evaluation of symptom prevalence, characteristics and distress. Eur J Cancer. 1994;30A:1326–1336. doi: 10.1016/0959-8049(94)90182-1. [DOI] [PubMed] [Google Scholar]
- 7.Lerdal A. A concept analysis of energy. Its meaning in the lives of three individuals with chronic illness. Scand J Caring Sci. 1998;12:3–10. doi: 10.1080/02839319850163075. [DOI] [PubMed] [Google Scholar]
- 8.Lerdal A. A theoretical extension of the concept of energy through an empirical study. Scand J Caring Sci. 2002;16:197–206. doi: 10.1046/j.1471-6712.2002.00079.x. [DOI] [PubMed] [Google Scholar]
- 9.O'Connor PJ. Mental energy: Assessing the mood dimension. Nutr Rev. 2006;64:S7–9. doi: 10.1111/j.1753-4887.2006.tb00256.x. [DOI] [PubMed] [Google Scholar]
- 10.Lerdal A, Kottorp A, Gay CL, et al. Development of a short version of the Lee Visual Analogue Fatigue Scale in a sample of women with HIV/AIDS: a Rasch analysis application. Qual Life Res. 2013;22:1467–1472. doi: 10.1007/s11136-012-0279-3. [DOI] [PubMed] [Google Scholar]
- 11.Aouizerat BE, Dhruva A, Paul SM, et al. Phenotypic and molecular evidence suggests that decrements in morning and evening energy are distinct but related symptoms. J Pain Symptom Manage. 2015;50:599–614. doi: 10.1016/j.jpainsymman.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wright F, D'Eramo Melkus G, Hammer M, et al. Trajectories of evening fatigue in oncology outpatients receiving chemotherapy. J Pain Symptom Manage. 2015;50:163–175. doi: 10.1016/j.jpainsymman.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wright F, D'Eramo Melkus G, Hammer M, et al. Predictors and trajectories of morning fatigue are distinct from evening fatigue. J Pain Symptom Manage. 2015;50:176–189. doi: 10.1016/j.jpainsymman.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hammer MJ, Aouizerat BE, Schmidt BL, et al. Glycosylated hemoglobin A1c and lack of association with symptom severity in patients undergoing chemotherapy for solid tumors. Oncol Nurs Forum. 2015;42:581–590. doi: 10.1188/15.ONF.581-590. [DOI] [PubMed] [Google Scholar]
- 15.Miaskowski C, Cooper BA, Melisko M, et al. Disease and treatment characteristics do not predict symptom occurrence profiles in oncology outpatients receiving chemotherapy. Cancer. 2014;120:2371–2378. doi: 10.1002/cncr.28699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karnofsky D, Abelmann WH, Craver LV, et al. The use of nitrogen mustards in the palliative treatment of carcinoma. Cancer. 1948;1:634–656. [Google Scholar]
- 17.Schnadig ID, Fromme EK, Loprinzi CL, et al. Patient-physician disagreement regarding performance status is associated with worse survivorship in patients with advanced cancer. Cancer. 2008;113:2205–2214. doi: 10.1002/cncr.23856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ando M, Ando Y, Hasegawa Y, et al. Prognostic value of performance status assessed by patients themselves, nurses, and oncologists in advanced non-small cell lung cancer. Br J Cancer. 2001;85:1634–1639. doi: 10.1054/bjoc.2001.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sangha O, Stucki G, Liang MH, et al. The Self-Administered Comorbidity Questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum. 2003;49:156–163. doi: 10.1002/art.10993. [DOI] [PubMed] [Google Scholar]
- 20.Brunner F, Bachmann LM, Weber U, et al. Complex regional pain syndrome 1--the Swiss cohort study. BMC Musculoskelet Disord. 2008;9:92. doi: 10.1186/1471-2474-9-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cieza A, Geyh S, Chatterji S, et al. Identification of candidate categories of the International Classification of Functioning Disability and Health (ICF) for a Generic ICF Core Set based on regression modelling. BMC Med Res Methodol. 2006;6:36. doi: 10.1186/1471-2288-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee KA, Hicks G, Nino-Murcia G. Validity and reliability of a scale to assess fatigue. Psychiatry Res. 1991;36:291–298. doi: 10.1016/0165-1781(91)90027-m. [DOI] [PubMed] [Google Scholar]
- 23.Fletcher BS, Paul SM, Dodd MJ, et al. Prevalence, severity, and impact of symptoms on female family caregivers of patients at the initiation of radiation therapy for prostate cancer. J Clin Oncol. 2008;26:599–605. doi: 10.1200/JCO.2007.12.2838. [DOI] [PubMed] [Google Scholar]
- 24.Lee KA, Portillo CJ, Miramontes H. The fatigue experience for women with human immunodeficiency virus. J Obstet Gynecol Neonatal Nurs. 1999;28:193–200. doi: 10.1111/j.1552-6909.1999.tb01984.x. [DOI] [PubMed] [Google Scholar]
- 25.Miaskowski C, Lee KA. Pain, fatigue, and sleep disturbances in oncology outpatients receiving radiation therapy for bone metastasis: a pilot study. J Pain Symptom Manage. 1999;17:320–332. doi: 10.1016/s0885-3924(99)00008-1. [DOI] [PubMed] [Google Scholar]
- 26.Miaskowski C, Paul SM, Cooper BA, et al. Trajectories of fatigue in men with prostate cancer before, during, and after radiation therapy. J Pain Symptom Manage. 2008;35:632–643. doi: 10.1016/j.jpainsymman.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miaskowski C, Cooper BA, Paul SM, et al. Subgroups of patients with cancer with different symptom experiences and quality-of-life outcomes: a cluster analysis. Oncol Nurs Forum. 2006;33:E79–89. doi: 10.1188/06.ONF.E79-E89. [DOI] [PubMed] [Google Scholar]
- 28.Kennedy BL, Schwab JJ, Morris RL, et al. Assessment of state and trait anxiety in subjects with anxiety and depressive disorders. Psychiatr Q. 2001;72:263–276. doi: 10.1023/a:1010305200087. [DOI] [PubMed] [Google Scholar]
- 29.Bieling PJ, Antony MM, Swinson RP. The State-Trait Anxiety Inventory, Trait version: structure and content re-examined. Behav Res Ther. 1998;36:777–788. doi: 10.1016/s0005-7967(98)00023-0. [DOI] [PubMed] [Google Scholar]
- 30.Spielberger CG, Gorsuch RL, Suchene R, et al. Manual for the State-Anxiety (Form Y): Self Evaluation Questionnaire. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 31.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 32.Sheehan TJ, Fifield J, Reisine S, et al. The measurement structure of the Center for Epidemiologic Studies Depression Scale. J Pers Assess. 1995;64:507–521. doi: 10.1207/s15327752jpa6403_9. [DOI] [PubMed] [Google Scholar]
- 33.Carpenter JS, Andrykowski MA, Wilson J, et al. Psychometrics for two short forms of the Center for Epidemiologic Studies-Depression Scale. Issues Ment Health Nurs. 1998;19:481–494. doi: 10.1080/016128498248917. [DOI] [PubMed] [Google Scholar]
- 34.Lee KA. Self-reported sleep disturbances in employed women. Sleep. 1992;15:493–498. doi: 10.1093/sleep/15.6.493. [DOI] [PubMed] [Google Scholar]
- 35.Lee KA, DeJoseph JF. Sleep disturbances, vitality, and fatigue among a select group of employed childbearing women. Birth. 1992;19:208–213. doi: 10.1111/j.1523-536x.1992.tb00404.x. [DOI] [PubMed] [Google Scholar]
- 36.Cimprich B, Visovatti M, Ronis DL. The Attentional Function Index--a self-report cognitive measure. Psychooncology. 2011;20:194–202. doi: 10.1002/pon.1729. [DOI] [PubMed] [Google Scholar]
- 37.Cimprich B, So H, Ronis DL, et al. Pre-treatment factors related to cognitive functioning in women newly diagnosed with breast cancer. Psychooncology. 2005;14:70–78. doi: 10.1002/pon.821. [DOI] [PubMed] [Google Scholar]
- 38.Daut RL, Cleeland CS, Flanery RC. Development of the Wisconsin Brief Pain Questionnaire to assess pain in cancer and other diseases. Pain. 1983;17:197–210. doi: 10.1016/0304-3959(83)90143-4. [DOI] [PubMed] [Google Scholar]
- 39.IBM. IBM SPSS Statistics for Windows, Version 22. Armonk, NY: IBM Corp; Released 2013. [Google Scholar]
- 40.Raudenbush SW, Bryk A. Hierarchical linear models: Applications and data analysis methods. 2. Thousand Oaks, CA: Sage Publications; 2002. [Google Scholar]
- 41.Aouizerat BE, Dodd M, Lee K, et al. Preliminary evidence of a genetic association between tumor necrosis factor alpha and the severity of sleep disturbance and morning fatigue. Biol Res Nurs. 2009;11:27–41. doi: 10.1177/1099800409333871. [DOI] [PubMed] [Google Scholar]
- 42.Dhruva A, Dodd M, Paul SM, et al. Trajectories of fatigue in patients with breast cancer before, during, and after radiation therapy. Cancer Nurs. 2010;33:201–212. doi: 10.1097/NCC.0b013e3181c75f2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Langford DJ, Tripathy D, Paul SM, et al. Trajectories of pain and analgesics in oncology outpatients with metastatic bone pain. J Pain. 2011;12:495–507. doi: 10.1016/j.jpain.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miaskowski C, Paul SM, Cooper BA, et al. Predictors of the trajectories of self-reported sleep disturbance in men with prostate cancer during and following radiation therapy. Sleep. 2011;34:171–179. doi: 10.1093/sleep/34.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Osborne C, Berger LM, Magnuson K. Family structure transitions and changes in maternal resources and well-being. Demography. 2012;49:23–47. doi: 10.1007/s13524-011-0080-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee KA, Lipscomb J. Sleep among shiftworkers--a priority for clinical practice and research in occupational health nursing. AAOHN J. 2003;51:418–420. [PubMed] [Google Scholar]
- 47.Meneses-Echavez JF, Gonzalez-Jimenez E, Ramirez-Velez R. Effects of supervised multimodal exercise interventions on cancer-related fatigue: Systematic review and meta-analysis of randomized controlled trials. Biomed Res Int. 2015:328636. doi: 10.1155/2015/328636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tomlinson D, Diorio C, Beyene J, et al. Effect of exercise on cancer-related fatigue: a meta-analysis. Am J Phys Med Rehabil. 2014;93:675–686. doi: 10.1097/PHM.0000000000000083. [DOI] [PubMed] [Google Scholar]
- 49.Saligan LN, Olson K, Filler K, et al. The biology of cancer–related fatigue: a review of the literature. Support Care Cancer. 2015;23:2461–2478. doi: 10.1007/s00520-015-2763-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.World Health Organization. Obesity: Preventing and managing the global epidemic. Report of a WHO consultation World Health Organization Techinical Report Series. 2000;894:1–253. [PubMed] [Google Scholar]
- 51.Donovan KA, Small BJ, Andrykowski MA, et al. Utility of a cognitive-behavioral model to predict fatigue following breast cancer treatment. Health Psychol. 2007;26:464–472. doi: 10.1037/0278-6133.26.4.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Andrykowski MA, Donovan KA, Laronga C, et al. Prevalence, predictors, and characteristics of off-treatment fatigue in breast cancer survivors. Cancer. 2010;116:5740–5748. doi: 10.1002/cncr.25294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kober KM, Cooper BA, Paul SM, et al. Subgroups of chemotherapy patients with distinct morning and evening fatigue trajectories. Support Care Cancer. 2016;24:1473–1485. doi: 10.1007/s00520-015-2895-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haensel A, Norman D, Natarajan L, et al. Effect of a 2 week CPAP treatment on mood states in patients with obstructive sleep apnea: a double-blind trial. Sleep Breath. 2007;11:239–244. doi: 10.1007/s11325-007-0115-0. [DOI] [PubMed] [Google Scholar]
- 55.Le Lay S, Simard G, Martinez MC, et al. Oxidative stress and metabolic pathologies: from an adipocentric point of view. Oxid Med Cell Longev. 2014:908539. doi: 10.1155/2014/908539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fukuda S, Nojima J, Motoki Y, et al. A potential biomarker for fatigue: Oxidative stress and anti-oxidative activity. Biol Psychol. 2016;118:88–93. doi: 10.1016/j.biopsycho.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 57.Rodgers C, Sanborn C, Taylor O, et al. Fatigue and oxidative stress in children undergoing leukemia treatment. Biol Res Nurs. 2016 doi: 10.1177/1099800416647794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mishra SI, Scherer RW, Geigle PM, et al. Exercise interventions on health-related quality of life for cancer survivors. Cochrane Database Syst Rev. 2012;8:CD007566. doi: 10.1002/14651858.CD007566.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mishra SI, Scherer RW, Snyder C, et al. Exercise interventions on health-related quality of life for people with cancer during active treatment. Cochrane Database Syst Rev. 2012;8:CD008465. doi: 10.1002/14651858.CD008465.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zeng Y, Cheng AS, Chan CC. Meta-Analysis of the effects of neuropsychological interventions on cognitive function in non-central nervous system cancer survivors. Integr Cancer Ther. 2016 doi: 10.1177/1534735416638737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Langford DJ, Lee K, Miaskowski C. Sleep disturbance interventions in oncology patients and family caregivers: a comprehensive review and meta-analysis. Sleep Med Rev. 2012;16:397–414. doi: 10.1016/j.smrv.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 62.Kvachadze I, Tsagareli MG, Dumbadze Z. An overview of ethnic and gender differences in pain sensation. Georgian Med News. 2015:102–108. [PubMed] [Google Scholar]
- 63.Linden W, Vodermaier A, Mackenzie R, et al. Anxiety and depression after cancer diagnosis: prevalence rates by cancer type, gender, and age. J Affect Disord. 2012;141:343–351. doi: 10.1016/j.jad.2012.03.025. [DOI] [PubMed] [Google Scholar]
- 64.Campbell CM, Edwards RR, Fillingim RB. Ethnic differences in responses to multiple experimental pain stimuli. Pain. 2005;113:20–26. doi: 10.1016/j.pain.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 65.Edwards RR, Doleys DM, Fillingim RB, et al. Ethnic differences in pain tolerance: clinical implications in a chronic pain population. Psychosom Med. 2001;63:316–323. doi: 10.1097/00006842-200103000-00018. [DOI] [PubMed] [Google Scholar]
- 66.Baldwin CM, Ervin AM, Mays MZ, et al. Sleep disturbances, quality of life, and ethnicity: the Sleep Heart Health Study. J Clin Sleep Med. 2010;6:176–183. [PMC free article] [PubMed] [Google Scholar]
- 67.Jean-Louis G, Magai C, Casimir GJ, et al. Insomnia symptoms in a multiethnic sample of American women. J Womens Health. 2008;17:15–25. doi: 10.1089/jwh.2006.0310. [DOI] [PubMed] [Google Scholar]
- 68.Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annu Rev Clin Psychol. 2008;4:1–32. doi: 10.1146/annurev.clinpsy.3.022806.091415. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.