Abstract
The purpose of this study was to develop an injectable bone substitute ( IBS) for percutaneous orthopaedic surgery. The multiphasic material used was composed of a 2% aqueous solution of methylhydroxypropylcellulose (MHPC) and biphasic calcium phosphate (BCP: 60% hydroxyapatite and 40% β-tricalcium phosphate) in which HPMC served as the carrier for 80–200 μm BCP granules. The best BCP/polymer ratio was determined by the rheological properties and higher BCP content of the material. Steam sterilization was more effective than gamma irradiation in maintaining the stability of the mixture and conserving its physicochemical and mechanical properties. The in vitro biocompatibility of the composite was checked by direct-contact cytotoxicity and cell-proliferation assays. A preliminary in vivo test was performed in the rabbit using intraosseous implantations in the femoral epiphysis. Histological analysis was done after 1, 2, 4 and 10 weeks. Bone ingrowth into the IBS, in close association with BCP granules, was observed after one week and increased regularly from the surface inward at 2, 4 and 10 weeks. At the same time, smaller BCP granules (less than 80 microns in diameter) were degraded and resorbed. This injectable biomaterial proved suitable for cavity filling. The water-solubility and viscosity of the polymer allow cells to recolonize, with in situ bonding of the mineral phase.
Keywords: Animals, Bone Development, Materials Testing, Rabbits, Rheology, Bone Substitutes, Bone and Bones, Calcium Phosphates, Cell Line, Cell Survival, Cellulose, Female, Giant Cells
1. INTRODUCTION
Calcium phosphates (Ca P) are being increasingly used as bone substitutes in orthopaedic surgery, stomatology and dental applications because of their bioactive properties (1–6). These biomaterials are available as powders, granules or blocks. However, these forms are of limited value when cavities are not easily accessible or when it would be preferable to perform percutaneous surgery. Contrary to practices in visceral surgery, the percutaneous approach has been used much less frequently than open surgery in orthopaedics. At the present time, improvements in specific instrumentation and the use of bioresorbable polymer implants for bone fracture healing are contributing to the development of this noninvasive technique. In this context, it would seem desirable to develop an injectable bone substitute (IBS).
IBS requires suitable rheological properties to ensure bonding of the mineral phase in situ with good cell permeability. Contrary to the use of dense materials which does not have any intrinsic porosity, this approach provides rapid, improved deep bone formation. This new biomaterial could be produced in a sterile ready-to-use cartridge form. Its stable composition and mechanical properties are suitable for the reproducibility of the biological response. Finally, there is less risk of infection since the product is not prepared during surgery.
A high-strength biomaterial which hardens in situ has recently been proposed (7), and calcium phosphates coupled with polymers such as lactic acid are being developed (8). Collagen and elastin have been tested in association with hydroxyapatite (HA) to enhance the bioactive properties of mineral phases (9), and the effect on bone formation of Chitosan, a biological polymer, is also being studied with or without calcium phosphates (10, 11). However, few studies have tested the rheological properties of the biomaterials proposed and their ability to undergo sterilization. Our laboratory previously studied different polymers used for medical purposes, demonstrating the advantages of hydrophilic derivatives of cellulose (e.g. methylhydroxypropylcellulose) as carriers for calcium phosphate granules (unpublished data). Biphasic calcium phosphate (BCP) was selected for the mineral phase because of its efficiency as a bone substitute and in promoting rapid bone ingrowth (4–6).
The purpose of this study was to determine the best ratio of biomaterial components, choose a suitable sterilization process and test the cytocompatibility of the materials by direct-contact cytotoxicity and cell-proliferation assays. A preliminary in vivo study was then performed in the rabbit.
2. MATERIALS AND METHODS
2.1. Composite material
2.1.1. Polymer solution
Methylhydroxypropylcellulose (MHPC; MP 824), with an average molecular weight of 700,000 (as determined by the static light-scattering method) was supplied by Aqualon-Hercules (France). Solutions of 2% MHPC (W/W) were prepared by dissolving the powder in bidistilled water under stirring for 48 h. Solution viscosities were adjusted to 14,000 cps (1 rpm) at 25°C by addition of water or polymer under the control of an LDVI+ Brookfield viscosimeter.
2.1.2. Mineral phase
BCP granules [60% HA and 40% β-tricalcium phosphate (TCP)] with a granulometry between 80 and 200 μm were used as the mineral phase for the composite. Granules were obtained by crushing blocks of Triosite ™(Zimmer, France) and sifting the powder.
2.1.3. Composite
Granules were added under gentle stirring to MHPC solutions in the range of 20–70% (W/W). The mixture was then conditioned in a 10-ml glass flask.
2.1.4. Sterilization
Two sterilization processes were tested: gamma irradiation at 25 Kgy and steam sterilization at 121°C for 20 min.
2.2. Viscosity measurements
Apparent viscosity was measured to determine the optimal BCP/polymer ratio. Mixture viscosity was measured at 25°C using an RDVI+ Brookfield viscosimeter after each addition of ceramic granules to the polymer solutions.
The rheological properties of the final IBS were checked in the same conditions after sterilization.
2.3. Physicochemical analysis
XRD diffraction according to the AFNOR standard (S 94-066) for calcium phosphates was used to check the HA/βTCP ratio in BCP and the IBS after sterilization and air-drying. Powders were examined using a Siemens D 5000 Kristalloflex diffractometer with 0.02° resolution in the range of 20°< 2θ <40°.
Infrared analysis of the polymer before and after sterilization was performed with a Perkin Elmer FTIR apparatus in the range of 400 to 4,500 cm−1. All materials were observed after dessication and mixing with KBr.
2.4. In vitro cytotoxicity assays
2.4.1. Direct-contact cytotoxicity assay
After air-drying at 37°C, the materials (polymer, BCP, IBS) were compacted in 200 mg discs (11 mm diameter) using a press (Specac Press, France) and sterilized.
An established mouse fibroblast cell line (L929) was used. The medium was Eagle’s minimum essential medium (Gibco, France) supplemented with 7.5% foetal calf serum (FCS), 2 mM glutamine, 100 μg/ml streptomycin and 100 IU/ml penicillin G. Cells were seeded at a density of 5 105 cells in six-well plates (Nunc) and incubated at 37°C under 5% CO2 for 24 h according to French Standard S 90702 and ASTM F 813-83. After checking for confluence, the materials were deposited on the monolayer cells. Assays were done in triplicate. Cultures were continued for 24 h in the same conditions as described above. Thermanox discs (Nunc, Denmark) were used as a positive control. Phenol solutions (6.4 g/l) were used to obtain reproducible cytotoxicity as a negative control.
At the end of the culture time, medium and samples were removed and replaced by 1 ml of a 0.005% neutral red solution (Sigma, France) in MEM for 3 h at 37°C. Cells were then unstuck using a trypsin EDTA solution (200 μl per well, Gibco) and retrieved with MEM/FCS (800 μl). The number of cells and the ratio of living cells to dead cells were evaluated on each sample using a Malassez counting chamber.
2.4.2. Long-term cell culture
The extraction media for polymer, composite and BCP were tested on L 929 cell cultures for 21 days. Extractions were performed according to the ISO -10993-5 standard (1992): BCP granules (20 g/100ml) were incubated in MEM (Gibco) for 5 days at 37°C. The same medium and extraction conditions were used for the composite (33.5 g/100 ml) and polymer (0.270 g/100 ml). Each medium was then stored at 4°C until use.
Cells were inoculated into 24-well plates (Nunc). 2 104 cells (1 ml) were cultured at 37°C and 5% CO2 with MEM (Gibco France) supplemented with 7.5% FCS, 1mM glutamine, 100 μg/ml streptomycin and 100 IU/ml penicillin G. After 72-h incubation (day 3), the medium was renewed and replaced in triplicate by extract media supplemented with FCS, glutamine, and antibiotics, as described above. MEM was used as a control. Medium was renewed every other day. Cell growth was measured in each medium by absolute numeration at day 4, 10, 14 and 21. After media were removed and replaced by neutral red solution, incubation proceeded as described above. The cells were then retrieved by trypsinization and counted in a Malassez counting chamber.
2.5. Experimental animals
Preliminary in vivo tests were performed according to AFNOR standard NF S 90-703. Four New Zealand female rabbits weighing 2.8 kg were used. IBS (0.46 g) was injected into surgical defects in subcutaneous areas (8 implants), paravertebral muscle (16 implants) and in bone defects realized by surgical access. The drilling holes in the femoral and tibial epiphysis (16 implants) were 10 mm length and 5 mm in diameter.
The implants were retrieved at different time-points (after 7, 14, 30 or 90 days). Samples recovered were fixed with 10% neutral formaldehyde solutions. Half of the samples were embedded in methylmetacrylate. Sections 60 μm thick were obtained using a diamond saw (ISOMET) and then stained with MOVAT and microradiographed at 15 KV. The other half were decalcified by a 5% HNO3 solution and embedded in paraffin. Samples were sectioned as described above, and the sections were stained with Masson’s trichrome.
3. RESULTS
3.1. Rheology
When BCP granules were added to polymer solutions, the viscosity changed according to a biphasic pattern (Fig. 1). With 0 to 50% of BCP, it increased slowly. With over 70 % of granules, the blend became a solid material. In the range of 55 to 65%, the mixture appeared suitable for use as an injectable material. The optimal ratio for the BCP and polymer solution tested was 60/40 (W/W) as determined by the inflexion point of the viscosity curve. The decrease in composite viscosity was very marked with gamma irradiation (to less than 1,000 cps) but relatively slight with steam sterilization (less than 10%).
Figure 1.
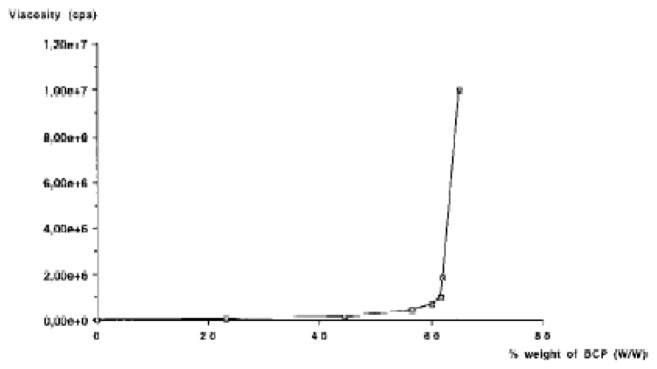
Apparent viscosity of 2% MHPC polymer aqueous solution after each add of BCP powder ( RDVI + brookfield viscosimeter).
3.2. Physicochemical analysis
3.2.1. X-ray diffraction profiles
XRD analysis of BCP confirmed the 60/40 (W/W) HA/βTCP ratio in BCP ceramic granules. This same ratio was found in composite.
3.2.2. Infrared spectroscopy
Polymer FTIR spectra showed absorption bands at 1,100, 2,900 and 3,450 cm−1 (Fig. 2). All peaks were in agreement with those of cellulose derivatives described in the Hummel library and no extra peaks were observed. No differences were observed in polymer IR spectra after sterilization.
Figure 2.
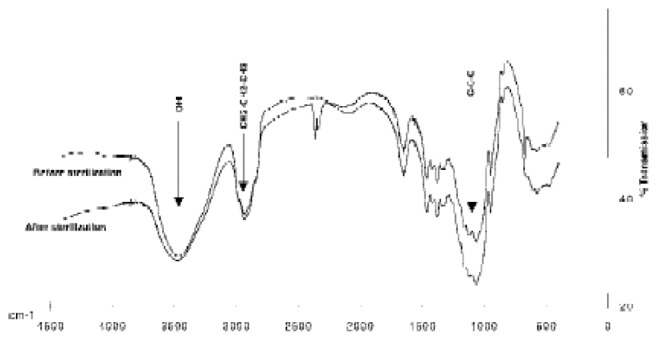
IR spectra of 2% MHPC solutions before and after steam sterilization (121°C, 20 mn).
3.3. In vitro cytotoxicity assays
3.3.1. Direct-contact cytotoxicity assay
(Table 1) shows the percentage of living cells after 24 h of culture with MHPC, BCP, and IBS. All cells were living and there were no significant differences between the values for all materials tested and the control.
Table I.
In vitro direct-contact cytotoxicity assay showing no difference between raw materials and composite (IBS).
| Materials (n=3) | Percentage of Living cells | Standard Deviation | Coefficient of variation |
|---|---|---|---|
| MHPC | 97.9 | ± 0.46 | 0.50% |
| Composite (IBS) | 98.1 | ± 0.64 | 0.65% |
| BCP | 98.6 | ± 0.51 | 0.51% |
| Thermanox | 98.4 | ± 0.35 | 0.30% |
| Phenol | 0 | - | - |
3.3.2. Long-term cell cultures
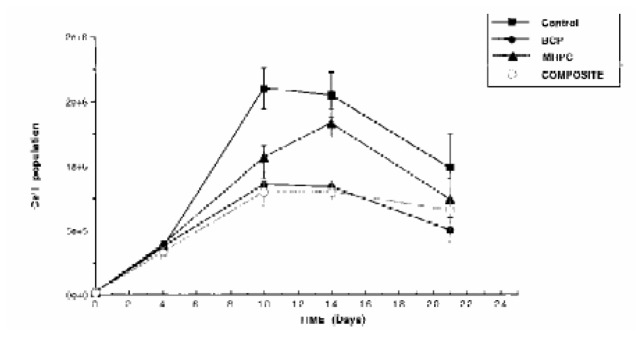
Cell proliferation curves with all tested materials and the control showed different profiles (Fig. 3). Until day 4 (one day of culture with the medium extract), cell number increased in the same manner for all products. From day 4 to 21, the curves for BCP and the composite showed a similar inhibition of cell proliferation. The difference between MHPC and the control was only significant at day 10.
Figure 3.
Long term fibroblasts (L 929) cultures. BCP (n=3) and composite (IBS) (n=3) extracts show the same cell proliferation profiles.
3.3.3. Experimental animals
Non-osseous sites
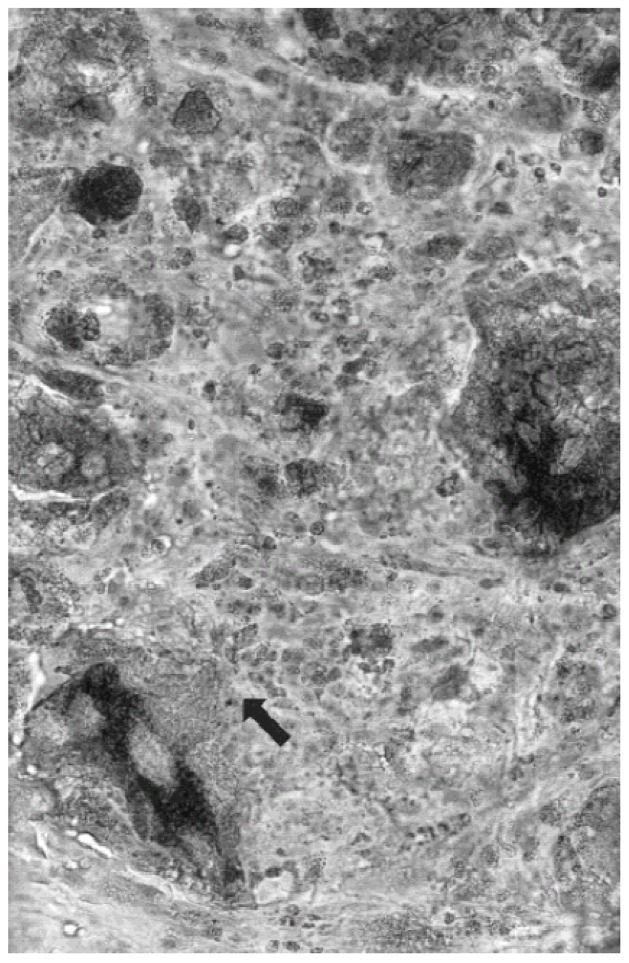
Multinucleate giant cells were observed in spaces occupied by the polymer as of day 7. However, these cells gradually decreased in number. At the same time, the number of calcium phosphate granules decreased, particularly the smaller ones. Fibrous tissue appeared after 15 days (fig 4) both in connective tissue and muscle areas. After 30 and 90 days, the inflammatory processes revealed by macrophages around the smaller particles (less than 80 μm) were no longer observed, and no fibrous encapsulation was seen anywhere around the samples.
Figure 4.
Subcutaneous implantation after 15 days, undecalcified section stained by Movat Pentachrom showing multinucleated giant cells (arrow), and fibrous tissue involving spaces between residual BCP grains.
Osseous sites
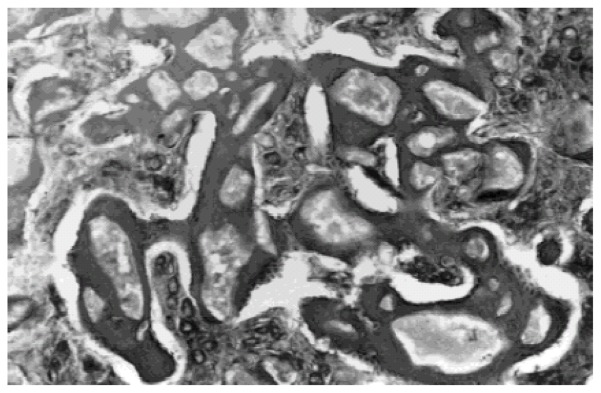
The same process of giant cell invasion of the IBS was observed, with resorption of the smaller particles. After 14 days, fibroblasts and osteoblasts were observed at the same time as extracellular matrix formation. Bone ingrowth around particles in the residual layer gradually increased (Fig. 5). Multinucleate cells smaller than those invading the spaces between the granules were observed in close contact with calcium phosphate. The density of the implants (BCP granules) in terms of the number and size per surface unit, decreased from the host interface to the core of the implant. At the same time, mineralized bone ingrowth was taking place. After 90 days, only larger particles (over 80 μm) were observed coalescing with the newly-formed bone.
Figure 5.
Undecalcified section observed in light microscopy of sample after 14 days of implantation, fibroblasts and osteoblasts were observed at the same time as extracellular matrix formation. Bone ingrowth around particles in the residual layer was observed.
4. DISCUSSION
The purpose of this study was to develop a ready-to-use injectable biomaterial (IBS) with calcium phosphate granules as the bioactive component. The choice of carrier was determined by its biocompatibility, its ability to be sterilized and. suitable rheological properties. Biological materials such as collagen were excluded to avoid any iatrogenic risk of prion or other contamination.
Cellulose and its derivatives have long been used for medical purposes (e.g. haemodialysis membranes, wound-care devices), and their biocompatibility has been studied (12). Hydrophilic derivative products of cellulose appeared to be of particular interest for our applications. Moreover, methylcellulose has already been proposed in a method for testing the cytotoxicity of biomaterials (13).
Methylhydroxypropylcellulose (MHPC) was chosen for its pseudoplastic properties. MHPC is an amorphous linear polymer, and its solutions are colloids. In solutions, gel consistency and supramolecular structure depend on the concentrations used. In a dilute solution, polymer tends toward a spherical form producing globules composed of a single molecule. When the concentration is increased, the polymer aggregates into a linear striated configuration which can cohere and form fibrils (14). As fibril states are unfavourable for the stability and rheologic properties of the composite, a 2% MHPC concentration was chosen for the solutions, in consideration of the gel consistency desired and the risk of aggregate formation. The non-ionic structure of MHPC was also taken into account in the choice of the polymer in order to prevent strong interaction with Ca 2+. Such an effect has been described with carboxymethylcellulose (15).
The mineral phase was BCP, a calcium phosphate ceramic which has been proposed for orthopaedic surgery. This biomaterial, a mixture of hydroxyapatite (HA) and β–tricalcium phosphate (βTCP), benefits from the long-term stability of HA and the enhanced bioactivity of readily soluble TCP (4–6). Rheological studies indicated the quantity of calcium phosphate granules to be blended with the MHPC solutions. The maximum appears to be 65% of the mineral phase since the viscosity of the composite increases rapidly at higher levels, making it unsuitable for injection.
Sterilization was a very important parameter for validation of the materials. It is well known that all sterilization processes commonly used (gamma irradiation, ethylene oxide, steam sterilization) can significantly modify the physical and chemical properties of polymer. Ethylene oxide was excluded since it is not suitable for liquid materials. Gamma irradiation (25 Kgray) was used in a preliminary test in accordance with values recommended in pharmacopeias (16). The composite lost its gel consistency and was transformed into a water suspension. This effect was probably due to a rupture of the polymer chains which often occurs with gamma sterilization (17). Only steam sterilization gave good results at 121°C for 20 min, with a decrease of less than 10% in viscosity. The supramolecular structure of the polymer was probably modified by steam action, but no effect on the chemical structure was seen in IR spectra after treatment. This loss of viscosity did not affect our choice of the BCP/polymer ratio since the rheological properties of the mixture were still suitable for clinical applications. X-ray diffraction studies showed that neither the polymer nor sterilization induced any significant changes in BCP composition.
Two methods were used to study the in vitro biocompatibility of the composite and its components: a direct-contact cytotoxic assay and evaluation of fibroblast cell proliferation using extract media obtained from the raw materials and the composite. The first test is simple and well-coded by AFNOR and ASTM standards. It is the initial step in screening primary acute toxicity, providing rapid detection (24 h) of toxic compounds (18-21). However, other cytocompatibility tests are also required (18-21). Cell cultures with extracts of the materials (18-22) can be applied for long-term studies (14 to 21 days) which reveal delayed toxicity due to the release of products from the materials and indicate the precise relationships between cells and materials (22). The direct-contact assay showed no differences between the composite, its components and the control. In this assay, MHPC was tested in the same conditions as the mineral phase (same weight and contact areas). This provided a large safety margin compared with the ratio used in the composite. The usual tests for determining cell growth and vitality, such as the MTT test and neutral red incorporation read by spectrophotometry (22,23), were not applied since the polymer produced a gel with the medium after 24 h of incubation, making it impossible to remove the materials entirely. However, cell counting, which is recognized by AFNOR standard S 90702, appear to be a successful method for direct-contact cytotoxicity (24,25).
In long-term cell cultures, extract media were obtained from BCP by placing granules in MEM with a ratio of about 1 g/5 ml as in other studies (19). The significance of the test depended on the concentration of the product in the medium. Accordingly, the amount of composite was calculated so as to provide the same weight in calcium phosphate as in the BCP assay. For MHPC, the same weight was used as in composite. However, in this case polymer was not extracted but dissolved in the medium because of its water solubility. Cell responses to the three products were different. MHPC showed a moderate inhibition of growth, essentially at day 10, whereas composite and BCP displayed the same pattern of cell-growth inhibition. Such in vitro inhibition of cell proliferation with calcium phosphates is often noted in the literature and probably results from changes in the ionic homeostasis of media (22, 26). In particular, Hyakuna et al. (27) have described a decrease in calcium and phosphate concentrations in media after only 3 h of incubation with HA, the major component of BCP (19). Long-term cultures with BCP extract may enhance this effect since it appears that TCP is transformed into HA after 1 week of incubation in medium (28). However, there are no correlations between these observations and the efficiency of bone substitutes (HA, TCP, BCP) in vivo (1–6).
Macrophage cells frequently appear during the biological colonization of calcium phosphate implants (29). However, these cells gradually decrease in number and are replaced by multinucleate TRAP cells identified as osteoclasts (30). Bone ingrowth is observed at the same time that resorption of calcium phosphate ceramics occurs. Calcium phosphate ceramics thus act as a support for osteoblast adhesion (30). These processes were observed in this preliminary in vivo study, as evidenced by the decrease in BCP content, particularly the largest granules. It acts as a scaffold for the spread of osteoblasts and for osteocoalescence with newly-formed bone by means of the BCP materials.
In conclusion, this study shows that MHPC is a non-toxic material which can be associated with calcium phosphates to produce an injectable bone substitute. In vitro studies indicate that it does not alter the physicochemical and biological properties of the mineral phase. MHPC is not intended to produce a high-strength material in vivo, in the manner of PMMA or a calcium phosphate cement (SRS Norian), but to create a matrix for deep cell colonization providing intense osteogenesis. Preliminary results of animal experimentation in vivo have been encouraging, and further implantation studies are now in progress.
Acknowledgments
This work was supported primarily by CNRS EP 59 and INSERM C.J. F 93-05. We thanks particularly Prof. N. Passuti, director of the Experimental surgery Center of the Medicine faculty for helping in animal experiment, and J.M. Bouler for its contribution to the XRD analysis.
References
- 1.JARCHO M. Calcium phosphate ceramics as hard tissue prosthetics. Clin Orthop. 1981;157:259–278. [PubMed] [Google Scholar]
- 2.WILLIAMS DF. Prosthesis stabilization by tissue ingrowth into porous ceramics. In: WILLIAMS DF, editor. Biocompatibility of Orthopaedic Implant. CRC Press; Boca Raton: 1982. [Google Scholar]
- 3.LEGEROS RZ. Calcium phosphate materials in restorative dentistery: a review. Adv Dent Res. 1988;2:164–168. doi: 10.1177/08959374880020011101. [DOI] [PubMed] [Google Scholar]
- 4.DACULSI G, PASSUTI N. Macroporous calcium phosphate ceramic performance in human spine fusion. Clin Orthop. 1989;248:169–176. [PubMed] [Google Scholar]
- 5.DACULSI G, PASSUTI N, MARTIN S, DEUDON C, LeGEROS RZ, RAHER S. Macroporous calcium phosphate ceramic for long bone surgery in humans and dogs. J Biomed Mater Res. 1990;24:379–396. doi: 10.1002/jbm.820240309. [DOI] [PubMed] [Google Scholar]
- 6.DACULSI G, LEGEROS RZ, NERY E, LYNCH K, KEREBEL B. Transformattion of biphasic calcium phosphate ceramics in vivo: Ultrastructural and physicochemical characterization. J Biomed Mater Res. 1989;23:883–894. doi: 10.1002/jbm.820230806. [DOI] [PubMed] [Google Scholar]
- 7.CONSTANTZ BR, ISON IC, FULMER MT, POSER RD, SMITH ST, VAN-WAGONER M, ROSS J, GOLDSTEIN SA, JUPITER JB, ROSENTHAL DI. Skeletal repair by in situ formation of the mineral phase of bone. Science. 1995;267:1796–1799. doi: 10.1126/science.7892603. [DOI] [PubMed] [Google Scholar]
- 8.VERHEYEN CCPM, KLEIN CPAT, DE BLIECK-HOGERVORST JMA, WOLKE JGC, VAN BLITTERSWIJN CT, DE GROOT K. Evaluation of hydroxylapatite/Poly(L lactide) composites: physicochemical properties. J Mater Sci: Materials in Medicine. 1993;4:58–65. [Google Scholar]
- 9.ROVIRA A, BAREILLE R, LOPEZ I, ROUAIS F, BORDENAVE L, REY C, RABAUD M. Preliminary report on a new composite material made of calcium phosphate, elastin peptides and collagens. J Mater Sci: Materials in Medicine. 1993;4:372–380. [Google Scholar]
- 10.MUZARELLI RAA, ZUCCHINI C. Osteoconductive properties of methyl pyrolidone chitosan in an animal model. Biomaterials. 1993;14:925–929. doi: 10.1016/0142-9612(93)90134-n. [DOI] [PubMed] [Google Scholar]
- 11.MUZARELLI RAA, MATTIOLI-BELMONTE M, TIETZ C, BIAGINI R, FERIOLI RG. Stimulatory effect on bone formation exerted by a modified chitosan. Biomaterials. 1994;15:1075–1081. doi: 10.1016/0142-9612(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 12.MIYAMOTO T, TAKAHASHI S, ITO H, INAGAKI H, NOISHIKI Y. Tissue biocompatibility of cellulose and its derivatives. J Biomed Mater Res. 1989;23:125–133. doi: 10.1002/jbm.820230110. [DOI] [PubMed] [Google Scholar]
- 13.VAN LUYN MJA, VAN WACHEM PB, NIEUWENHUIS H, OLDE DAMINK L, TEN HOOPEN H, FEIJEN J. Methylcellulose cell culture as a new cytotoxicity test system for biomaterials. Mater Sci: Materials in Medicine. 1991;2:142–148. [Google Scholar]
- 14.DUMITRIU S, DUMITRIU-MEDVICHI C. Hydrogel and general properties of Biomaterials. In: DUMITRIU S, editor. Polymeric Biomaterials. Marcel Dekker; New York: 1994. pp. 3–97. [Google Scholar]
- 15.JUST EK, MAJEWICZ TG. Encyclopedia of Polymer science and Engeneering. John Wiley and sons; NYC: 1985. p. 226. [Google Scholar]
- 16.JACOBS . Radiation in the sterilization of pharmaceuticals. In: GROVES MJ, POLSON W, HANISFELD M, editors. Sterile pharmaceuticals manufacturing applications for the 1990’. Vol. 1. Interpharm Press Inc; Buffalo grove Il: 1991. pp. 57–58. [Google Scholar]
- 17.DEMPSEY DJ, THIRUCOTE RR. Sterilization of medical devices: A review. Journal of Biomaterials Applications. 1989;3:454–523. doi: 10.1177/088532828800300303. [DOI] [PubMed] [Google Scholar]
- 18.CINGI MR, DE ANGELIS I, FORTUNATI E, REGGIANI D, BIANCHI V, TIOZZO R, ZUCCO F. Choice and standardization of test protocols in cytotoxicology: a multicenter approach. Toxic in Vitro. 1991;5:119–125. doi: 10.1016/0887-2333(91)90031-8. [DOI] [PubMed] [Google Scholar]
- 19.PIZZOFERRATO A, CIAPETTI G, STEA S, CENNI E, ARCIOLA CR, GRANCHI D, AVARINO L. Cell culture methods for testing biocompatibilite. Clinical Materials. 1994;15:173–190. doi: 10.1016/0267-6605(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 20.HARMAND MF. Evaluation of high-tech biomaterials biocompatibility using human cell cultures. Biomaterials. 1992:163–170. [Google Scholar]
- 21.JOHNSON HJ, NORTHUP SJ, SEAGRAVES PA, GARVIN PJ, WALLIN RF. Biocompatibility test procedures for materials evaluation in vitro. I. Comparative test system sensitivity. J Biomed Mater Res. 1983;17:571–586. doi: 10.1002/jbm.820170403. [DOI] [PubMed] [Google Scholar]
- 22.LI J, LIU Y, HERMANSSON L, SOREMARK R. Evaluation of biocompatibility of various ceramic powders with human fibroblasts in vitro. Clinical Materials. 1993;12:197–201. doi: 10.1016/0267-6605(93)90073-g. [DOI] [PubMed] [Google Scholar]
- 23.GUILLEMIN G, LAUNAY M, MEUNIER A. Natural Coral as a substitute for fibroblastic growth in vitro. J Mat Sci: Materials in Medicine. 1993;4:575–581. [Google Scholar]
- 24.KIRKPATRICK CJ, MITTERMAYER C. Theoretical and practical aspects of testing potential biomaterials in vitro. J Mater Sci: Materials in Medicine. 1990;1:9–13. [Google Scholar]
- 25.CERVINKA M, PUZA V. In vitro testing of implantation materials used in Medicine: Effects on cell morphology, cell proliferation and DNA synthesis. Toxic in vitro. 1990;4:711–716. doi: 10.1016/0887-2333(90)90149-n. [DOI] [PubMed] [Google Scholar]
- 26.LENGHEDEN A. Influence of pH and calcium on growth and attachment of human fibroblasts in vitro. Scand J Dent Res. 1994;102:130–136. doi: 10.1111/j.1600-0722.1994.tb01168.x. [DOI] [PubMed] [Google Scholar]
- 27.HYAKUNA K, YAMAMURO T, KOTOURA Y, KAKUTANI Y, KITSUGI T, TAKAGI H, OKA M, KOKUBO T. The influence of calcium phosphate ceramics and glass ceramics on cultured cells and their surrounding media. 2J Biomed Mater Res. 1989;23:1049–1066. doi: 10.1002/jbm.820230906. [DOI] [PubMed] [Google Scholar]
- 28.KOHRI M, MIKI K, WAITE DE, OKABE T. In vitro stability of biphasic calcium phosphate ceramics. Biomaterials. 1993;14:299–304. doi: 10.1016/0142-9612(93)90122-i. [DOI] [PubMed] [Google Scholar]
- 29.BASLE M, DACULSI G, GRIZON F, FILMON R, PASSUTI N, REBEL A. Cellular response to sintered calcium phosphate ceramics implanted in rabbit bone. J Mat Sci Mat Medicine. 1993;4:273–280. [Google Scholar]
- 30.BASLE MF, CHAPPARD D, GRIZON F, FILMON R, DELECRIN J, DACULSI G, REBEL A. Osteoclastic Resorption of CaP biomaterials implanted in rabbit bone. Calcif Tiss Int. 1993;53:348–356. doi: 10.1007/BF01351842. [DOI] [PubMed] [Google Scholar]