Abstract
Telomerase activation via induction of the catalytic component telomerase reverse transcriptase (TERT) plays essential roles in malignant transformation. TERT promoter-activating mutations were recently identified as a novel mechanism to activate telomerase in hepatocellular carcinoma (HCC) and many other malignancies. In addition, single nucleotide polymorphisms (SNPs) in the TERT rs2736098 and rs2736100 are significantly associated with cancer susceptibility. It is currently unclear whether different germline TERT variants modify TERT promoter mutations. Here we analyzed the TERT promoter status and genotyped the TERT SNPs at rs2736098 and rs2736100 in patients with HCC. Thirty percent of HCCs harbored TERT promoter mutations and there was a significant difference in rs2736098 and rs2736100 genotypes between wt and mutant TERT promoter-bearing HCC tumors (P = 0.007 and 0.018, respectively). For rs2736100, the cancer risk genotype CC was significantly associated with a reduced incidence of TERT promoter mutations compared to AA + AC variants [Odds ratio (OR): 0.181, 95% Confidence interval (CI): 0.0543–0.601, P = 0.004]. The rs2736098_CT genotype was significantly associated with the TERT promoter mutation-positive tumors compared to the TT genotype (OR: 5.391, 95% CI: 1.234–23.553, P = 0.025). These differences in genotype distribution did not differ between patients with a wt TERT promoter and controls. The presence of TERT promoter mutations was not associated with clinico-pathological variables. Taken together, the germline TERT genetic background may significantly affect the onset of TERT promoter mutations in HCCs, which provides a better understanding of HCC-related TERT promoter mutations and telomerase regulation in cancer.
Keywords: HCC, promoter mutations, rs2736098, rs2736100, SNP
INTRODUCTION
Hepatocellular carcinoma (HCC) is the dominant primary liver cancer with an estimated number of 466,100 new cases and 422,100 deaths, occurring in China for 2015, which is almost the half of all cases diagnosed globally [1]. During recent last decades, significant advances have been made in the understanding of HCC [2, 3]. Chronic hepatitis B or C infection, alcohol abuse, male gender and cigarette smoking have been identified as important risk factors to contribute to HCC development [1–3]. Nevertheless, the etiology and pathogenesis of HCC remains incompletely elucidated [1]. Clinically, early diagnosis and intervention is the key to patient cures or long-term survival. However, most HCC patients are diagnosed at an advanced stage, and invasive diseases or distant metastasis accounts for the majority of mortalities due to limited treatment choices [1]. Therefore, increased understanding of the HCC pathogenesis, early detection, improved diagnostic and prognostic markers, novel preventive strategies and identification of new therapeutic targets are urgently needed.
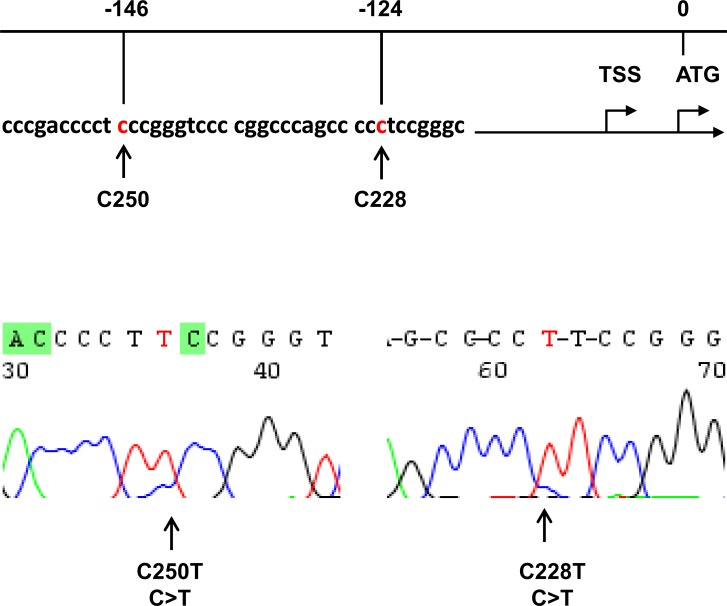
Unlimited proliferation is a key hallmark of cancer cells [4], and activation of telomerase is required to achieve it in the vast majority of cancer types including HCC [5–7]. Telomerase is a ribonucleoprotein enzyme with its catalytic unit telomerase reverse transcriptase (TERT) as rate-limiting factor, and in general silent in normal human differentiated cells due to the transcriptional repression of the TERT gene [5, 6]. In contrast, most HCCs and other malignancies constitutively express TERT and telomerase activity [5, 8]. The mechanism underlying cancer-specific telomerase activation/TERT expression has been extensively studied, and recent findings showed that HCC tumors frequently bear activating mutations in the TERT proximal promoter (−124 and −146 bp from the ATG, so-called C228T and C250T, respectively) [2, 8–20]. These mutations promote the TERT gene transcription, thereby activating telomerase [2, 8–16]. However, TERT promoter mutations occur in up to 50% of HCCs and the mutation frequency varies significantly with tumor types [8]. It remains unclear how and why such variations take place.
The central role of TERT in oncogenesis has also promoted studies of cancer susceptibility and association with single nucleotide polymorphisms (SNPs) in the TERT locus, and the accumulated evidence indeed suggests the association between cancer risk and TERT SNPs [17, 21–36]. Among all the TERT SNPs, rs2736100 at intron 2 and rs2736098 at exon 2 are most studied. The rs2736100 CC genotype was reported to confer an increased risk of different cancer types [21]. Mechanistically, the rs2736100 CC may up-regulate TERT expression through which its oncogenic effect is exerted [34]. The relationship between rs2736098 variants and cancer risk is complicated and risk alleles vary in different types of cancer [25, 31]. Moreover, it remains poorly understood whether and how rs2736098 variants affect TERT activity [37]. Nevertheless, the rs2736098 A allele was shown to significantly increase HCC risk.
Both TERT promoter mutations and TERT SNPs play important roles in oncogenesis, but it is unclear whether they interact or associate with each other. To address this issue, we analyzed HCC tumors for TERT promoter mutations and relationship with rs2736100 and rs2736098 variants.
RESULTS
TERT promoter mutations and relation to clinico-pathological variables in HCCs
The TERT promoter was sequenced in DNA from 200 HCC patients and 190 of them were evaluable. Fifty-seven of 190 HCC tumors (30%) harbored TERT promoter mutations, among which 50 were C228T while 7 C250T (Figure 1). Clinic-pathological variables were compared between patients with and without TERT promoter mutations in their tumors, and there were no differences in age, sex, HBV infection, liver cirrhosis, α-fetoprotein levels, tumor sizes, differentiation status and metastasis (Table 1).
Figure 1. Identification of TERT promoter mutations in hepatocellular carcinoma (HCC).
Top: Location of C228T and C250T (in red) in the TERT core promoter. TSS: Transcription start site. Bottom: Sequencing chromatographs of the TERT promoter locus in genomic tumor DNA from two HCC patients obtained by Sanger sequencing.
Table 1. TERT promoter mutations with clinical characteristics in HCC patients.
| TERT promoter mutation | |||
|---|---|---|---|
| Variable informative cases (N = 190 ) | Mutated (N = 57 ) | wild-type (n = 133 ) | P-value |
| Age at diagnosis (n = ) | 56 | 128 | 0.1905 |
| Mean years | 54.71 | 52.63 | |
| Median (range) years | 55.5 (32–75) | 51 (25–76) | |
| Gender (n = ) | 56 | 128 | 0.898 |
| Female | 8 | 19 | |
| Male | 48 | 109 | |
| HBV infection (n = ) | 55 | 129 | 0.105 |
| Yes | 50 | 103 | |
| No | 5 | 26 | |
| Cirrhosis (n = ) | 57 | 130 | 0.394 |
| Yes | 30 | 58 | |
| No | 27 | 72 | |
| α-fetoprotein (ng/ml) | 54 | 120 | 0.927 |
| < 200 | 38 | 82 | |
| ≥ 200 | 16 | 38 | |
| Tumor size (n = ) | 56 | 121 | 0.328 |
| < 5 cm | 32 | 58 | |
| > 5 cm | 24 | 63 | |
| Differentiation | 55 | 123 | 0.609 |
| Well or moderate | 37 | 89 | |
| Poor | 18 | 34 | |
| CTNNB1 (n = ) or TERT (n = ) | |||
| mutated | 6 | 11 | 0.535 |
| wt | 13 | 38 | |
| Metastases (n = ) | 56 | 129 | 0.67 |
| Yes | 1 | 5 | |
| No | 55 | 124 | |
HCC, Hepatocellular carcinoma; HBV, Hepatitis B virus.
TERT rs2736098 and rs2736100 variants and relation to HCC risk
Since SNPs at rs2736098 and rs2736100 have been shown to be associated with increased cancer risk, we determined whether these genetic variants have any effects on HCC susceptibility by comparing their genotype distributions with healthy controls. The genotyping data were available in 240 healthy controls and 231 HCC patients for rs2736098, while in 237 healthy controls and 201 HCC patients for rs2736100, respectively. Table 2 summarizes the genotyping results and both patients and controls exhibited comparable genotype and allele frequencies of rs2736098. However, the rs2736100_CC genotype was significantly lower in HCC patients than in healthy controls (OR: 0.544, 95% CI: 0.320–0.925, P = 0.034) (Table 2).
Table 2. TERT rs2736098 and 2736100 genotyping in healthy adults and HCC patients.
| HA | HCC | Odds ratio (95% CI) | P value | |
|---|---|---|---|---|
| Genotype | ||||
| rs2736098 (N) | 240 (100%) | 231 (100%) | ||
| TT | 31 (12.9) | 19 (8.2) | 1.0 (ref.) | |
| CT | 115 (47.9) | 127 (55.0) | 1.802 (0.965–3.364) | 0.088 |
| CC | 94 (39.2) | 85 (36.8) | 1.475 (0.7763–2.804) | 0.303 |
| CT + CC | 209 (87.1) | 212 (91.8) | 1.655 (0.906–3.023) | 0.133 |
| CC | 94 (39.2) | 85 (36.8) | 1.0 (ref.) | |
| TT + CT | 146 (60.8) | 146 (63.2) | 1.106 (0.762–1.605) | 0.664 |
| rs2736100 (N) | 237 (100%) | 201 (100%) | ||
| AA | 69 (29.1) | 74 (36.8) | 1.0 (ref.) | |
| AC | 108 (45.6) | 92 (45.8) | 0.794 (0.517–1.221) | 0.347 |
| CC | 60 (25.3) | 35 (17.4) | 0.544 (0.320–0.925) | 0.033 |
| AC + CC | 168 (74.7) | 127 (63.2) | 0.705 (0.472–1.053) | 0.107 |
| CC | 60 (25.3) | 35 (17.4) | 1.0 (ref.) | |
| AA + AC | 177 (73.7) | 166 (82.6) | 1.608 (1.007–2.566) | 0.060 |
HCC, Hepatocellular carcinoma; CI, Confidence interval.
No association between TERT promoter mutations and CTNNB1 mutations in HCC tumors
The CTNNB1 gene mutation is frequent in HCCs and recent studies showed its close association with TERT promoter mutations. We thus further sequenced the CTNNB1 exon 3 for the hotspot mutations in 81 HCC tumors [13, 14]. The sequencing data were evaluable in 70 of them and the mutation found in 17 tumors (24.3%). The TERT promoter sequencing was successful in 68 of these tumors. There were 6 (35.3%) and 13 (23.5%) with mutant TERT promoters in 17 CTNNB1 mutation-positive and 51 mutation-negative HCCs, respectively, this difference being non-significant (P = 0.535) (Table 1).
The TERT promoter mutation and association with TERT genetic variants in HCCs
As TERT SNPs at rs2736098 and rs2736100 were observed to regulate TERT expression and telomerase activity [31, 34, 37], we sought to determine the relationship between the TERT promoter mutation and SNPs at these two loci. HCC patients with tumors bearing mutant TERT promoter exhibited remarkably lower frequencies of rs2736098_TT and rs2736100_CC genotypes compared with those of healthy controls (Table 3) (mutant cases vs controls: 3.6% vs 12.9% and 5.8 vs 25.3% for rs2736098_TT and rs2736100_CC, respectively). Compared to rs2736098_TT cases, HCC patients with rs2736098_CT genotype had 5.39-fold increase in TERT promoter mutation-positive tumors (OR: 95% CI: 1.234 – 23.554, P = 0.025). Patients carrying rs2736100_AA and AC variants exhibited 5.5-fold increase in having TERT promoter-mutant tumors (OR: 0.181, 95% CI: 0.054 – 0.601, P = 0.004). Similar differences in the genotype of rs2736098 and rs2736100 were also seen between patients with wt and mutant TERT promoters (mt vs wt: P = 0.007 and 0.018 for rs2736098 and rs2736100, respectively) (Table 4). In contrast, we did not observe significant differences in both rs2736098 and rs2736100 genotype distributions between healthy controls and HCC patients carrying a wt TERT promoter (Table 3).
Table 3. TERT promoter mutations and association with rs2736100 and rs2736098 in HCC patients.
| Genotype | Cases | Healthy controls | Odds ratio (95% CI) | P |
|---|---|---|---|---|
| rs2736098 | ||||
| wt TERT promoter vs controls | 128 (100%) | 240 (100%) | ||
| TT | 13 (10.1) | 31 (12.9 ) | 1.0 (Ref.) | |
| CT | 61 (47.7) | 115 (47.9 ) | 1.265 (0.617–2.594) | 0.643 |
| CC | 54 (42.2) | 94 (39.2 ) | 1.370 (0.661–2.840) | 0.504 |
| CT + CC | 115 (89.9) | 209 (87.1) | 1.310 (0.660–2.607) | 0.543 |
| mt TERT promoter vs controls | 55 (100%) | 240 (100%) | ||
| TT | 2 (3.6) | 31 (12.9 ) | 1.0 (Ref.) | |
| CT | 40 (72.7) | 115 (47.9 ) | 5.391 (1.234–23.553) | 0.025 |
| CC | 13 (23.7) | 94 (39.2) | 2.144 (0.458–10.030) | 0.505 |
| CT + CC | 53 (96.4) | 209 (87.1) | 3.931 (0.912–16.948) | 0.083 |
| rs2736100 | ||||
| wt TERT promoter vs controls | 114 (100%) | 237 (100%) | ||
| AA | 38 (33.3) | 69 (29.1) | 1.0 (Ref.) | |
| CA | 52 (45.6) | 108 (45.6) | 1.141 (0.683–1.916) | 0.705 |
| CC | 24 (21.1) | 60 (25.3) | 0.726 (0.392–1.346) | 0.389 |
| AA + AC | 90 (78.9) | 177 (74.7) | 1.0 (Ref.) | |
| CC | 24 (21.1) | 60 (25.3) | 0.787 ( 0.460–1.346) | 0.457 |
| mt TERT promoter vs controls | 52 (100%) | 237 (100%) | ||
| AA | 15 (28.8) | 69 (29.1) | 1.0 (Ref.) | |
| CA | 34 (65.4) | 108 (45.6) | 1.448 (0.735–2.854) | 0.389 |
| CC | 3 (5.8) | 60 (25.3) | 0.230 (0.0635–0.833) | 0.032 |
| AA + AC | 49 (94.2) | 177 (74.7) | 1.0 (Ref.) | |
| CC | 3 (5.8) | 60 (25.3) | 0.181 (0.0543–0.601) | 0.004 |
HCC, Hepatocellular carcinoma; CI, confidence interval.
Table 4. rs2736098 and rs2736100 genotype frequency in HCC patients bearing wt and mutant TERT promoter in tumors.
| wt | mutant | P value | |
|---|---|---|---|
| rs2736098 | 128 (100%) | 55 (100%) | |
| TT | 13 (10.1) | 2 (3.6) | |
| CT | 61 (47.7) | 40 (72.7) | |
| CC | 54 (42.2) | 13 (23.7) | 0.007 |
| rs2736100 | 114 (100%) | 52 (100%) | |
| AA | 38 (33.3) | 15 (28.8) | |
| CA | 52 (45.6) | 34 (65.4) | |
| CC | 24 (21.1) | 3 (5.8) | 0.018 |
HCC, hepatocellular carcinoma.
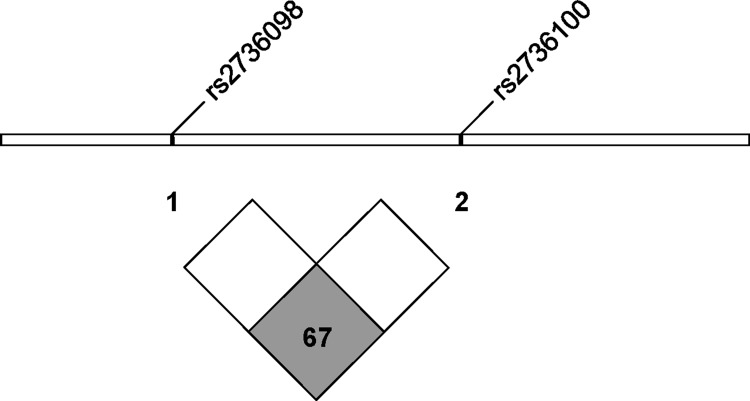
In addition, we further performed the linkage disequilibrium analysis of rs2736098 and rs2736100 in the Han Chinese population (Figure 2). LD or D’ and r2 values are 0.67 and 0.41, respectively, which indicates a non-significant association between these two SNPs (< 0.8).
Figure 2. The linkage disequilibrium analysis of rs2736098 and rs2736100 in the Han Chinese population.
D′ = 0.67 (< 0.8 as non-significantly associated) and r2 = 0.41. (From http://www.ensembl.org).
DISCUSSION
Multiple oncogenic signalings contribute to the aberrant TERT induction and telomerase activation, an essential step towards malignant transformation, and the recent identification of cancer-specific hotspot mutations in the TERT proximal promoter reveals a novel mechanism behind telomerase activation [8]. It has been documented that the frequency of TERT promoter mutations varies significantly from cancer to cancer, but the underlying mechanism is unclear. In the present study, we determined TERT promoter mutations and TERT gene SNPs in HCC patients, and our results show a negative association between patients having TERT promoter mutation-positive tumors and rs2736098_TT and rs2736100_CC genotypes.
TERT promoter mutations, thus identified in multiple-types of human malignancies including HCCs, function as oncogenic drivers due to their stimulatory effects on TERT transcription and telomerase activation [8, 38, 39]. Both C228T and C250T mutations create extra de novo ETS binding motifs that are selectively bound by the ETS family transcription factor GABPA [39]. The GABPA binding leads to opened chromatin at the TERT proximal promoter, thereby aberrantly activating TERT transcription and telomerase [39]. Consistently, higher levels of TERT mRNA and telomerase activity were frequently observed in tumors carrying TERT promoter mutations compared with those having a wt promoter [8, 18, 40, 41]. On the other hand, the rs2736100_CC genotype has also been shown to promote TERT transcription and to maintain telomere length much more strongly than its AA and AC variants [17, 34]; whereas tumors with shorter telomere are prone to undergo TERT promoter mutations [42]. From this standpoint of view, HCC tumors derived from patients with rs2736100_AA or AC genotype are more genetically stressed for telomerase activation than those bearing the CC variant. It is thus conceivable that TERT promoter mutations occur predominantly in HCC patients with rs2736100_AA and AC genotypes, as observed in the present study.
We also found a significantly lower frequency of the rs2736098_TT genotype in HCCs with a TERT promoter mutation, which was similar to that of the rs2736100_CC variant. However, in contrast to the rs2736100_CC genotype that up-regulates TERT transcription, rs2736098_TT has been shown to be associated with lower TERT expression and shorter telomere length [25, 31, 43, 44]. Therefore, the reduced rs2736098_TT variant in mutant TERT promoter-bearing HCCs cannot simply be explained by its effect on TERT expression. Of note, it should also be pointed out that both rs2736100_CC and rs2736098_TT genotypes increase cancer risk, despite their opposite impacts on TERT transcription and telomere length regulation. Nevertheless, patients with TERT promoter mutation-positive HCCs have lower frequencies of cancer susceptibility genotypes rs2736098_TT and rs2736100_CC.
Up to 50% of HCCs were previously reported to have TERT promoter mutations, and those studies showed an association of the mutation with certain clinico-pathological variables including age, sex, HBV or HCV infection, α-fetoprotein, tumor sizes/numbers, differentiation status and metastasis [2, 9–16]. However, we did not find that the presence of TERT promoter mutations was associated with any of the above variables in this cohort of HCC patients.
Co-occurrence of TERT promoter mutations together with activating mutation of oncogenic drivers that facilitate cellular replication is frequent. The fibroblast growth factor receptor 3 (FGFR3) gene mutation is highly prevalent in urothelial carcinomas, and the mutant FGFR3 promotes malignant development by over-stimulating the RAS-mitogen-activated protein kinase and phosphatidylinositide-3 kinase–AKT pathway [45, 46]. TERT promoter mutations are shown to be tightly associated with the presence of FGFR3 mutations in urothelial carcinomas (UC) [45]. In HCCs, the CTNNB1 gene, encoding β-catenin, is frequently mutated and its mutant version plays similarly important parts in enhancing HCC cell proliferation [3]. A few of studies demonstrated that the presence of the TERT promoter and CTNNB1 mutations was correlated with each other in HCCs [12–14]. However, our results show that these two genetic events were independent of each other. Likely, different genetic susceptibility and/or environment exposure may contribute to different mutation profiles. For instance, we found that the FGFR3 mutation was rare in Chinese patients with UC, despite its high incidence in those UC patients from Western countries [45, 47, 48].
The rs2736098_TT genotype was previously found to be associated with HCC risk in a Chinese population by Zhang et al. [25], however, we failed to demonstrate such an association. Of note, those authors showed the association of the rs2736098_TT variant only with HBV infection-negative HCC [25]. Because our cohort of HCC patients was predominantly HBV-infected, we were unable to do this same analysis. Further investigations by recruiting larger cohorts of HBV-negative HCC are apparently required to solve this discrepancy. Intriguingly, the vast majority of studies have shown rs2736100_CC genotype as a cancer risk variant [17, 21], while our present findings suggest its association with a lower risk of HCC. However, this result was obtained from relatively small cohorts of subjects (< 300), which calls for further evaluations on more healthy individuals and HCC patients for validation.
MATERIALS AND METHODS
Study population
Two hundred and forty-five patients with newly diagnosed histologically confirmed HCC were recruited from Shandong University Second Hospital and Shandong Provincial Hospital (Table 1). Adult sex-matched healthy individuals (N = 257) served as controls. Age (mean ± SD) for cases and controls was 45 ± 16 yrs and 54 ± 10 yrs, respectively. The ethnic background of both cases and controls was Han Chinese from the Shandong area. Tumor specimens and/or blood samples were obtained from all the participants after informed content. The study was approved by the Shandong University Second Hospital Ethics Committee. All experiments were performed in accordance with relevant guidelines and regulations.
DNA extraction and Sanger sequencing of the TERT promoter and CTNNB1 gene
Genomic DNA was extracted using QIAGEN DNA extraction kits. DNA derived from HCC tumors was analyzed for the TERT promoter and CTNNB1 gene mutations using Sanger sequencing. The two mutations defined as C228T and C250T in the TERT core promoter correspond to positions 124 and 146 bp upstream of the ATG site [38, 49] (Figure 1). The primer pair for the TERT promoter sequencing was previously described [40, 42]: 5′-CAC CCG TCC TGC CCC TTC ACC TT-3′ (forward) and 5′-GGC TTC CCA CGT GCG CAG CAG GA-3′ (reverse). The CTNNB1 gene hotspot mutations occur in exon 3 and we chose the following primers for sequencing analysis of this region: 5′-GGG TAT TTG AAG TAT ACC ATA C-3′ (forward) and 5′-TGG TCC TCG TCA TTT AGC AG-3′ (reverse). All the mutations were verified by sequencing from both directions.
The TERT rs2736100 (AC) and rs2736098 (TC) genotyping
The TERT rs2736100 (AC) and rs2736098 (TC) genotyping was carried out using pre-designed TaqMan SNP genotyping assay kits on an ABI PRISM 7900 HT Sequence Detection System (Applied Biosystems), as described [17, 24, 50]. Both positive and negative controls were included in all assays and the running condition was as followed: 95°C for 10 min, followed by 40 cycles of 92°C for 15 sec and 60°C for 1 min.
Statistical analyses
Sex was compared between patients and controls using Chi-square (χ2) test. Student's T-test or χ2 test was used to analyze differences in clinico-pathological variables between patients with the TERT promoter mutation-positive and negative tumors, respectively. The evaluation of distribution differences of selected variables and alleles of the TERT rs2736100 and rs2736098 between patients and healthy controls were done using χ2 test. Hardy–Weinberg equilibrium of the genotype distribution among the controls was tested by a goodness-of-fit χ2 test. Unconditional univariate and multivariate logistic regression analyses were used to estimate Odds ratios (OR) for risk of HCC or tumors with and without TERT promoter mutation and their 95% confidence interval (CI). All the tests were computed using SigmaStat3.1® software (Systat Software, Inc., Richmond, CA). P values of < 0.05 were considered as statistically significant.
CONCLUSIONS
Our results show that 30% of Chinese HCCs carry TERT promoter mutations and there was a significant difference in genotype distributions of rs2736098 and rs2736100 between patients with wt versus mutant TERT promoter tumors, respectively. The cancer risk genotypes rs2736098_TT and rs2736100_CC are intimately associated with HCC tumors bearing a wt TERT promoter. Based on the present findings, together with experimental and epidemiological data reported by others, we thus suggest that hepatocytes with rs2736098_TT or rs2736100_CC variants are more prone to telomerase activation in oncogenesis, thereby less likely undergoing TERT promoter mutation. Collectively, the germline TERT genetic background may significantly affect the onset of TERT promoter mutations in HCCs, which contributes to better understandings of the mechanism underlying cancer-related telomerase activation.
ACKNOWLEDGMENTS AND FUNDING
This study was supported by grants from the National Basic Research Program of China (Grant No. 2012CB911202), the National Natural Science Foundation of China (#81502409), Shandong Provincial Natural Science Foundation (2016ZDJS07A09) Swedish Cancer Society, the Swedish Research Council, Cancer Society in Stockholm.
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
REFERENCES
- 1.Chen W, Zheng R, Baade P, Zhang S, Zeng H, Bray F, Jemal A, Yu X, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Tornesello ML, Buonaguro L, Izzo F, Buonaguro FM. Molecular alterations in hepatocellular carcinoma associated with hepatitis B and hepatitis C infections. Oncotarget. 2016;7:25087–25102. doi: 10.18632/oncotarget.7837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zucman-Rossi J, Villanueva A, Nault JC, Llovet JM. Genetic Landscape and Biomarkers of Hepatocellular Carcinoma. Gastroenterology. 2015;149:1226–1239. doi: 10.1053/j.gastro.2015.05.061. e1224. [DOI] [PubMed] [Google Scholar]
- 4.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 5.Daniel M, Peek GW, Tollefsbol TO. Regulation of the human catalytic subunit of telomerase (hTERT) Gene. 2012;498:135–146. doi: 10.1016/j.gene.2012.01.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kong F, Zheng C, Xu D. Telomerase as a “stemness” enzyme. Sci China Life Sci. 2014;57:564–570. doi: 10.1007/s11427-014-4666-6. [DOI] [PubMed] [Google Scholar]
- 7.Cong YS, Wright WE, Shay JW. Human telomerase and its regulation. Microbiol Mol Biol Rev. 2002;66:407–425. doi: 10.1128/MMBR.66.3.407-425.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu T, Yuan X, Xu D. Cancer-Specific Telomerase Reverse Transcriptase (TERT) Promoter Mutations: Biological and Clinical Implications. Genes (Basel) 2016:7. doi: 10.3390/genes7070038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen YL, Jeng YM, Chang CN, Lee HJ, Hsu HC, Lai PL, Yuan RH. TERT promoter mutation in resectable hepatocellular carcinomas: a strong association with hepatitis C infection and absence of hepatitis B infection. Int J Surg. 2014;12:659–665. doi: 10.1016/j.ijsu.2014.05.066. [DOI] [PubMed] [Google Scholar]
- 10.Kawai-Kitahata F, Asahina Y, Tanaka S, Kakinuma S, Murakawa M, Nitta S, Watanabe T, Otani S, Taniguchi M, Goto F, Nagata H, Kaneko S, Tasaka-Fujita M, et al. Comprehensive analyses of mutations and hepatitis B virus integration in hepatocellular carcinoma with clinicopathological features. J Gastroenterol. 2016;51:473–486. doi: 10.1007/s00535-015-1126-4. [DOI] [PubMed] [Google Scholar]
- 11.Nault JC, Zucman-Rossi J. TERT promoter mutations in primary liver tumors. Clin Res Hepatol Gastroenterol. 2016;40:9–14. doi: 10.1016/j.clinre.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 12.Nault JC, Calderaro J, Di Tommaso L, Balabaud C, Zafrani ES, Bioulac-Sage P, Roncalli M, Zucman-Rossi J. Telomerase reverse transcriptase promoter mutation is an early somatic genetic alteration in the transformation of premalignant nodules in hepatocellular carcinoma on cirrhosis. Hepatology. 2014;60:1983–1992. doi: 10.1002/hep.27372. [DOI] [PubMed] [Google Scholar]
- 13.Nault JC, Mallet M, Pilati C, Calderaro J, Bioulac-Sage P, Laurent C, Laurent A, Cherqui D, Balabaud C, Zucman Rossi J. High frequency of telomerase reverse-transcriptase promoter somatic mutations in hepatocellular carcinoma and preneoplastic lesions. Nat Commun. 2013;4:2218. doi: 10.1038/ncomms3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pezzuto F, Izzo F, Buonaguro L, Annunziata C, Tatangelo F, Botti G, Buonaguro FM, Tornesello ML. Tumor specific mutations in TERT promoter and CTNNB1 gene in hepatitis B and hepatitis C related hepatocellular carcinoma. Oncotarget. 2016;7:54253–54262. doi: 10.18632/oncotarget.9801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schulze K, Imbeaud S, Letouze E, Alexandrov LB, Calderaro J, Rebouissou S, Couchy G, Meiller C, Shinde J, Soysouvanh F, Calatayud AL, Pinyol R, Pelletier L, et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet. 2015;47:505–511. doi: 10.1038/ng.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang X, Guo X, Chen Y, Chen G, Ma Y, Huang K, Zhang Y, Zhao Q, Winkler CA, An P, Lyu J. Telomerase reverse transcriptase promoter mutations in hepatitis B virus-associated hepatocellular carcinoma. Oncotarget. 2016;7:27838–27847. doi: 10.18632/oncotarget.8539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dahlström J, Liu T, Saft L, Ghaderi M, Wei Y, Lavebratt C, Li P, Zheng C, Björkholm M, Xu D. TERT rs2736100 Genotypes are associated with differential risk of myeloproliferative neoplasms in Swedish and Chinese male patient populations. Ann Hematol. 2016;95:1825–1832. doi: 10.1007/s00277-016-2787-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang K, Liu T, Liu L, Liu J, Liu C, Wang C, Ge N, Ren H, Yan K, Hu S, Bjorkholm M, Fan Y, Xu D. TERT promoter mutations in renal cell carcinomas and upper tract urothelial carcinomas. Oncotarget. 2014;5:1829–1836. doi: 10.18632/oncotarget.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang K, Liu T, Ge N, Liu L, Yuan X, Liu J, Kong F, Wang C, Ren H, Yan K, Hu S, Xu Z, Bjorkholm M, et al. TERT promoter mutations are associated with distant metastases in upper tract urothelial carcinomas and serve as urinary biomarkers detected by a sensitive castPCR. Oncotarget. 2014;5:12428–12439. doi: 10.18632/oncotarget.2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mosrati MA, Malmstrom A, Lysiak M, Krysztofiak A, Hallbeck M, Milos P, Hallbeck AL, Bratthall C, Strandeus M, Stenmark-Askmalm M, Soderkvist P. TERT promoter mutations and polymorphisms as prognostic factors in primary glioblastoma. Oncotarget. 2015;6:16663–16673. doi: 10.18632/oncotarget.4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mocellin S, Verdi D, Pooley KA, Landi MT, Egan KM, Baird DM, Prescott J, De Vivo I, Nitti D. Telomerase reverse transcriptase locus polymorphisms and cancer risk: a field synopsis and meta-analysis. J Nat Cancer Inst. 2012;104:840–854. doi: 10.1093/jnci/djs222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qi HY, Zou P, Zhao L, Zhu J, Gu AH. TERT rs2736098 polymorphism and cancer risk: results of a meta-analysis. Asian Pac J Cancer Prev. 2012;13:3483–3488. doi: 10.7314/apjcp.2012.13.7.3483. [DOI] [PubMed] [Google Scholar]
- 23.Shete S, Hosking FJ, Robertson LB, Dobbins SE, Sanson M, Malmer B, Simon M, Marie Y, Boisselier B, Delattre JY, Hoang-Xuan K, El Hallani S, Idbaih A, et al. Genome-wide association study identifies five susceptibility loci for glioma. Nat Genet. 2009;41:899–904. doi: 10.1038/ng.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuan X, Meng Y, Li P, Ge N, Kong F, Yang L, Bjorkholm M, Zhao S, Xu D. The association between the TERT rs2736100 AC genotype and reduced risk of upper tract urothelial carcinomas in a Han Chinese population. Oncotarget. 2016;7:31972–31979. doi: 10.18632/oncotarget.7777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang C, Tian YP, Wang Y, Guo FH, Qin JF, Ni H. hTERT rs2736098 genetic variants and susceptibility of hepatocellular carcinoma in the Chinese population: a case-control study. Hepatobiliary Pancreat Dis Int. 2013;12:74–79. doi: 10.1016/s1499-3872(13)60009-0. [DOI] [PubMed] [Google Scholar]
- 26.Oddsson A, Kristinsson SY, Helgason H, Gudbjartsson DF, Masson G, Sigurdsson A, Jonasdottir A, Jonasdottir A, Steingrimsdottir H, Vidarsson B, Reykdal S, Eyjolfsson GI, Olafsson I, et al. The germline sequence variant rs2736100_C in TERT associates with myeloproliferative neoplasms. Leukemia. 2014;28:1371–1374. doi: 10.1038/leu.2014.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melin BS, Nordfjall K, Andersson U, Roos G. hTERT cancer risk genotypes are associated with telomere length. Genet Epidemiol. 2012;36:368–372. doi: 10.1002/gepi.21630. [DOI] [PubMed] [Google Scholar]
- 28.McKay JD, Hung RJ, Gaborieau V, Boffetta P, Chabrier A, Byrnes G, Zaridze D, Mukeria A, Szeszenia-Dabrowska N, Lissowska J, Rudnai P, Fabianova E, Mates D, et al. Lung cancer susceptibility locus at 5p15.33. Nat Genet. 2008;40:1404–1406. doi: 10.1038/ng.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kinnersley B, Migliorini G, Broderick P, Whiffin N, Dobbins SE, Casey G, Hopper J, Sieber O, Lipton L, Kerr DJ, Dunlop MG, Tomlinson IP, Houlston RS. The TERT variant rs2736100 is associated with colorectal cancer risk. Br J Cancer. 2012;107:1001–1008. doi: 10.1038/bjc.2012.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gago-Dominguez M, Jiang X, Conti DV, Castelao JE, Stern MC, Cortessis VK, Pike MC, Xiang YB, Gao YT, Yuan JM, Van Den Berg DJ. Genetic variations on chromosomes 5p15 and 15q25 and bladder cancer risk: findings from the Los Angeles-Shanghai bladder case-control study. Carcinogenesis. 2011;32:197–202. doi: 10.1093/carcin/bgq233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Martino M, Taus C, Lucca I, Hofbauer SL, Haitel A, Shariat SF, Klatte T. Association of human telomerase reverse transcriptase gene polymorphisms, serum levels, and telomere length with renal cell carcinoma risk and pathology. Mol Carcinog. 2016;55:1458–66. doi: 10.1002/mc.22388. [DOI] [PubMed] [Google Scholar]
- 32.Chen XF, Cai S, Chen QG, Ni ZH, Tang JH, Xu DW, Wang XB. Multiple variants of TERT and CLPTM1L constitute risk factors for lung adenocarcinoma. Genet Mol Res. 2012;11:370–378. doi: 10.4238/2012.February.16.2. [DOI] [PubMed] [Google Scholar]
- 33.Bojesen SE, Pooley KA, Johnatty SE, Beesley J, Michailidou K, Tyrer JP, Edwards SL, Pickett HA, Shen HC, Smart CE, Hillman KM, Mai PL, Lawrenson K. Multiple independent variants at the TERT locus are associated with telomere length and risks of breast and ovarian cancer. Nat Genet. 2013;45:371–384. doi: 10.1038/ng.2566. 384e371–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei R, Cao L, Pu H, Wang H, Zheng Y, Niu X, Weng X, Zhang H, Favus MJ, Zhang L, Jia W, Zeng Y, Amos CI. TERT Polymorphism rs2736100-C Is Associated with EGFR Mutation-Positive Non-Small Cell Lung Cancer. Clin Cancer Res. 2015;21:5173–5180. doi: 10.1158/1078-0432.CCR-15-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krahling T, Balassa K, Kiss KP, Bors A, Batai A, Halm G, Egyed M, Fekete S, Remenyi P, Masszi T, Tordai A, Andrikovics H. Co-occurrence of Myeloproliferative Neoplasms and Solid Tumors Is Attributed to a Synergism Between Cytoreductive Therapy and the Common TERT Polymorphism rs2736100. Cancer Epidemiol Biomarkers Prev. 2015;25:98–104. doi: 10.1158/1055-9965.EPI-15-0805. [DOI] [PubMed] [Google Scholar]
- 36.Ko E, Seo HW, Jung ES, Kim BH, Jung G. The TERT promoter SNP rs2853669 decreases E2F1 transcription factor binding and increases mortality and recurrence risks in liver cancer. Oncotarget. 2016;7:684–699. doi: 10.18632/oncotarget.6331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rafnar T, Sulem P, Stacey SN, Geller F, Gudmundsson J, Sigurdsson A, Jakobsdottir M, Helgadottir H, Thorlacius S, Aben KK, Blondal T, Thorgeirsson TE, Thorleifsson G, et al. Sequence variants at the TERT-CLPTM1L locus associate with many cancer types. Nat Genet. 2009;41:221–227. doi: 10.1038/ng.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang FW, Hodis E, Xu MJ, Kryukov GV, Chin L, Garraway LA. Highly recurrent TERT promoter mutations in human melanoma. Science. 2013;339:957–959. doi: 10.1126/science.1229259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bell RJ, Rube HT, Kreig A, Mancini A, Fouse SD, Nagarajan RP, Choi S, Hong C, He D, Pekmezci M, Wiencke JK, Wrensch MR, Chang SM, et al. The transcription factor GABP selectively binds and activates the mutant TERT promoter in cancer. Science. 2015;348:1036–1039. doi: 10.1126/science.aab0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang N, Liu T, Sofiadis A, Juhlin CC, Zedenius J, Hoog A, Larsson C, Xu D. TERT promoter mutation as an early genetic event activating telomerase in follicular thyroid adenoma (FTA) and atypical FTA. Cancer. 2014;120:2965–2979. doi: 10.1002/cncr.28800. [DOI] [PubMed] [Google Scholar]
- 41.Arita H, Narita Y, Takami H, Fukushima S, Matsushita Y, Yoshida A, Miyakita Y, Ohno M, Shibui S, Ichimura K. TERT promoter mutations rather than methylation are the main mechanism for TERT upregulation in adult gliomas. Acta Neuropathol. 2013;126:939–941. doi: 10.1007/s00401-013-1203-9. [DOI] [PubMed] [Google Scholar]
- 42.Liu T, Wang N, Cao J, Dinets A, Sofiadis A, Zedenius J, Larsson C, Xu D. The age- and shorter telomere-dependent TERT promoter mutation in follicular thyroid cell-derived carcinomas. Oncogene. 2014;33:4978–4984. doi: 10.1038/onc.2013.446. [DOI] [PubMed] [Google Scholar]
- 43.Soerensen M, Thinggaard M, Nygaard M, Dato S, Tan Q, Hjelmborg J, Andersen-Ranberg K, Stevnsner T, Bohr VA, Kimura M, Aviv A, Christensen K, Christiansen L. Genetic variation in TERT and TERC and human leukocyte telomere length and longevity: a cross-sectional and longitudinal analysis. Aging Cell. 2012;11:223–227. doi: 10.1111/j.1474-9726.2011.00775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Atzmon G, Cho M, Cawthon RM, Budagov T, Katz M, Yang X, Siegel G, Bergman A, Huffman DM, Schechter CB, Wright WE, Shay JW, Barzilai N, et al. Evolution in health and medicine Sackler colloquium: Genetic variation in human telomerase is associated with telomere length in Ashkenazi centenarians. Proc Nat Acad Sci USA. 2011;107:1710–1717. doi: 10.1073/pnas.0906191106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Allory Y, Beukers W, Sagrera A, Flandez M, Marques M, Marquez M, van der Keur KA, Dyrskjot L, Lurkin I, Vermeij M, Carrato A, Lloreta J, Lorente JA, et al. Telomerase Reverse Transcriptase Promoter Mutations in Bladder Cancer: High Frequency Across Stages, Detection in Urine, and Lack of Association with Outcome. Eur Urol. 2014;65:360–366. doi: 10.1016/j.eururo.2013.08.052. [DOI] [PubMed] [Google Scholar]
- 46.Hurst CD, Platt FM, Knowles MA. Comprehensive mutation analysis of the TERT promoter in bladder cancer and detection of mutations in voided urine. Eur Urol. 2014;65:367–368. doi: 10.1016/j.eururo.2013.08.057. [DOI] [PubMed] [Google Scholar]
- 47.Yuan X, Liu C, Wang K, Liu L, Liu T, Ge N, Kong F, Yang L, Bjorkholm M, Fan Y, Zhao S, Xu D. The genetic difference between Western and Chinese urothelial cell carcinomas: infrequent FGFR3 mutation in Han Chinese patients. Oncotarget. 2016;7:25826–25835. doi: 10.18632/oncotarget.8404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang K, Liu T, Liu C, Meng Y, Yuan X, Liu L, Ge N, Liu J, Wang C, Ren H, Yan K, Hu S, Xu Z. TERT promoter mutations and TERT mRNA but not FGFR3 mutations are urinary biomarkers in Han Chinese patients with urothelial bladder cancer. Oncologist. 2015;20:263–269. doi: 10.1634/theoncologist.2014-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Horn S, Figl A, Rachakonda PS, Fischer C, Sucker A, Gast A, Kadel S, Moll I, Nagore E, Hemminki K, Schadendorf D, Kumar R. TERT promoter mutations in familial and sporadic melanoma. Science. 2013;339:959–961. doi: 10.1126/science.1230062. [DOI] [PubMed] [Google Scholar]
- 50.Wei YB, Martinsson L, Liu JJ, Forsell Y, Schalling M, Backlund L, Lavebratt C. hTERT genetic variation in depression. J Affect Disord. 2015;189:62–69. doi: 10.1016/j.jad.2015.09.025. [DOI] [PubMed] [Google Scholar]