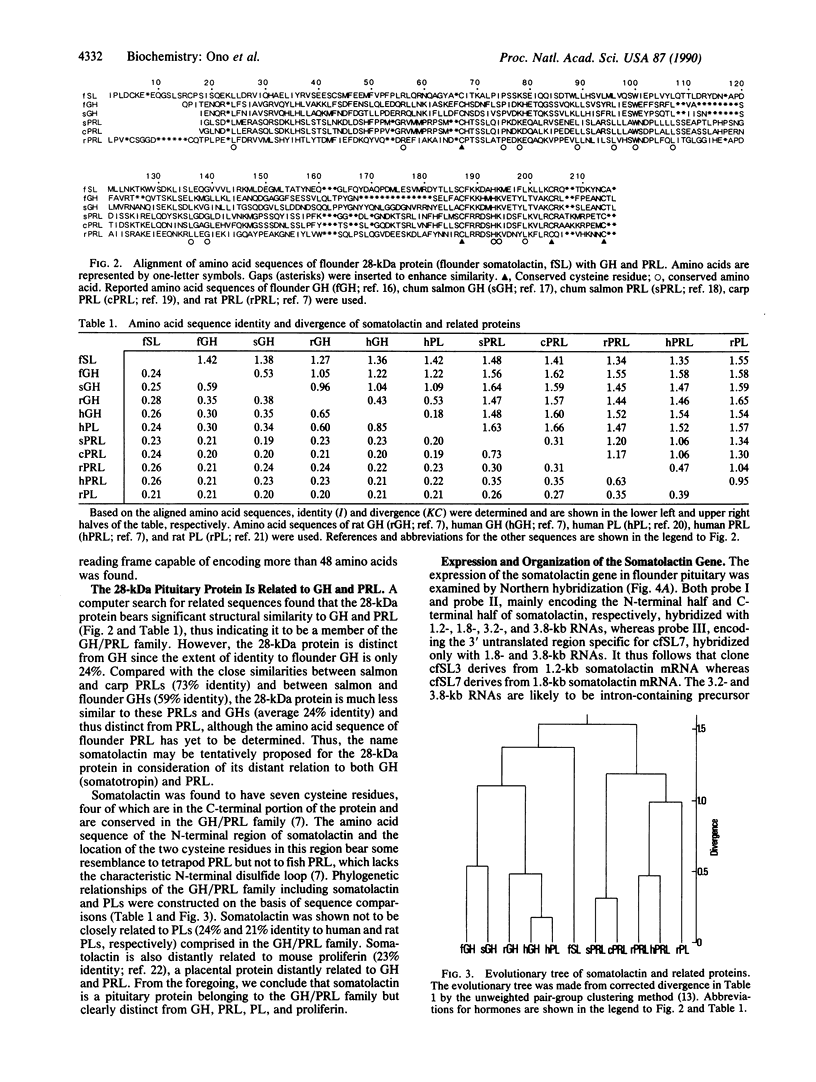
Abstract
From a flounder pituitary cDNA library, cDNA clones encoding a 28-kDa glycoprotein produced by the pars intermedia of the pituitary were isolated and characterized. Nucleotide sequencing demonstrated a precursor of the 28-kDa protein, which consisted of 231 amino acid residues, to be cleaved into a signal peptide (24 amino acids) and a mature protein (207 amino acids) containing one N-glycosylation site. By comparison of amino acid sequences, the 28-kDa protein was found to be distantly and similarly related to growth hormone and prolactin. Consequently, it was named somatolactin. Somatolactin mRNAs were specifically expressed as 1.2 and 1.8 kb poly(A)+ RNAs in flounder pituitary.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball J. N., Batten T. F. Pituitary and melanophore responses to background in Poecilia latipinna (teleostei): role of the pars intermedia PAS cell. Gen Comp Endocrinol. 1981 Jun;44(2):233–248. doi: 10.1016/0016-6480(81)90254-9. [DOI] [PubMed] [Google Scholar]
- Duckworth M. L., Kirk K. L., Friesen H. G. Isolation and identification of a cDNA clone of rat placental lactogen II. J Biol Chem. 1986 Aug 15;261(23):10871–10878. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Kanehisa M. I. Los Alamos sequence analysis package for nucleic acids and proteins. Nucleic Acids Res. 1982 Jan 11;10(1):183–196. doi: 10.1093/nar/10.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathe R. Synthetic oligonucleotide probes deduced from amino acid sequence data. Theoretical and practical considerations. J Mol Biol. 1985 May 5;183(1):1–12. doi: 10.1016/0022-2836(85)90276-1. [DOI] [PubMed] [Google Scholar]
- Linzer D. I., Nathans D. Nucleotide sequence of a growth-related mRNA encoding a member of the prolactin-growth hormone family. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4255–4259. doi: 10.1073/pnas.81.14.4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll C. S., Mayer G. L., Russell S. M. Structural features of prolactins and growth hormones that can be related to their biological properties. Endocr Rev. 1986 May;7(2):169–203. doi: 10.1210/edrv-7-2-169. [DOI] [PubMed] [Google Scholar]
- Olivereau M., Aimar C., Olivereau J. M. PAS-positive cells of the pars intermedia are calcium-sensitive in the goldfish maintained in a hyposmotic milieu. Cell Tissue Res. 1980;212(1):29–38. doi: 10.1007/BF00234030. [DOI] [PubMed] [Google Scholar]
- Olivereau M., Olivereau J. M., Aimar C. Specific effect of calcium ions on the calcium-sensitive cells of the pars intermedia in the goldfish. Cell Tissue Res. 1981;214(1):23–31. doi: 10.1007/BF00235141. [DOI] [PubMed] [Google Scholar]
- Ono M., Wada C., Oikawa I., Kawazoe I., Kawauchi H. Structures of two kinds of mRNA encoding the chum salmon melanin-concentrating hormone. Gene. 1988 Nov 30;71(2):433–438. doi: 10.1016/0378-1119(88)90060-1. [DOI] [PubMed] [Google Scholar]
- Rawdon B. B. Immunostaining of eta cells in the rostral pars distalis and PAS-positive cells in the pars intermedia of a teleost (Sarotherodon mossambicus) by antisera to mammalian prolactins. Gen Comp Endocrinol. 1979 Mar;37(3):374–382. doi: 10.1016/0016-6480(79)90011-x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeburg P. H. The human growth hormone gene family: nucleotide sequences show recent divergence and predict a new polypeptide hormone. DNA. 1982;1(3):239–249. doi: 10.1089/dna.1.1982.1.239. [DOI] [PubMed] [Google Scholar]
- Sekine S., Mizukami T., Nishi T., Kuwana Y., Saito A., Sato M., Itoh S., Kawauchi H. Cloning and expression of cDNA for salmon growth hormone in Escherichia coli. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4306–4310. doi: 10.1073/pnas.82.13.4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha Y. N., Jacobsen B. P., Lewis U. J. Antibodies to newly recognized murine 13-18 KDa pituitary peptides crossreact with growth hormone and prolactin from several species, including man. Biochem Biophys Res Commun. 1989 Aug 30;163(1):386–393. doi: 10.1016/0006-291x(89)92147-5. [DOI] [PubMed] [Google Scholar]
- Van Eys G. J., Löwik C. W., Wendelaar Bonga S. E. Isolation of the biosynthetic products of the PAS positive pars intermedia cells in the cichlid teleost Sarotherodon mossambicus. Gen Comp Endocrinol. 1983 Feb;49(2):277–285. doi: 10.1016/0016-6480(83)90145-4. [DOI] [PubMed] [Google Scholar]
- Watahiki M., Yamamoto M., Yamakawa M., Tanaka M., Nakashima K. Conserved and unique amino acid residues in the domains of the growth hormones. Flounder growth hormone deduced from the cDNA sequence has the minimal size in the growth hormone prolactin gene family. J Biol Chem. 1989 Jan 5;264(1):312–316. [PubMed] [Google Scholar]
- Yasuda A., Itoh H., Kawauchi H. Primary structure of chum salmon prolactins: occurrence of highly conserved regions. Arch Biochem Biophys. 1986 Feb 1;244(2):528–541. doi: 10.1016/0003-9861(86)90621-1. [DOI] [PubMed] [Google Scholar]
- Yasuda A., Miyazima K., Kawauchi H., Peter R. E., Lin H. R., Yamaguchi K., Sano H. Primary structure of common carp prolactins. Gen Comp Endocrinol. 1987 May;66(2):280–290. doi: 10.1016/0016-6480(87)90278-4. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]




