Abstract
Epilepsy is a complex disorder, which involves much more than seizures, encompassing a range of associated comorbid health conditions that can have significant health and quality-of-life implications. Of these comorbidities, cognitive impairment is one of the most common and distressing aspects of epilepsy. Clinical studies have demonstrated that refractory seizures, resistant to antiepileptic drugs, occurring early in life have significant adverse effects on cognitive function. Much of what has been learned about the neurobiological underpinnings of cognitive impairment following early-life seizures has come from animal models. While early-life seizures in rodents do not result in cell loss, seizures do result in changes in neurogenesis and synaptogenesis and alteration of excitatory/inhibitory balance, network connectivity and temporal coding. These morphological and physiological changes are accompanied by parallel impairment in cognitive skills. This increased understanding of the pathophysiological basis of seizure-induced cognitive deficits should allow investigators to develop novel targets for therapeutic interventions.
Introduction
Although seizures are the most striking clinical manifestation of childhood epilepsy, children with epilepsy are at risk not only for seizures, but also for a myriad of co-morbid health problems that occur at a higher rate than would be expected by chance1. Among the co-morbidities associated with epilepsy in children, cognitive abnormalities are among the most common and troublesome2,3. The distribution of intelligence quotient (IQ) scores of children with epilepsy is skewed toward lower values4,5 and the number of children with epilepsy experiencing difficulties in school because of learning disabilities is greater than children without epilepsy6–11.
While most children with epilepsy maintain stable IQ scores, there is now strong evidence that some of them slow, or even regress, in their mental development5,12,13. In a community-based cohort study. Berg et al14 assessed and prospectively followed 198 children (aged <8 years) with new-onset epilepsy for 8–9 years. In this cohort refractoriness to antiepileptic drugs (AEDs) was associated with an 11.4 point lower full scale IQ. There were substantial age-resistance to treatment interactions for IQ, indicating a lessening impact of recurrent seizures with increasing age. The authors appropriately concluded that uncontrolled seizures impair cognitive function with effects being most severe in infancy. Cognition is of particular concern in children with an epileptic encephalopathy15–17, a condition characterized by the slowing or regression of development due to seizures, abnormal interictal cortical and subcortical EEG activity, or both, rather than the underlying etiology of the epilepsy15.
Much of the cognitive impairment that occurs in people with epilepsy is related to its underlying etiology. Both acquired disorders such as trauma, hypoxic-ischemic insults, and mesial temporal sclerosis secondary to prolonged febrile seizures and genetic disorders, including tuberous sclerosis, fragile X, Rett, and Dravet syndromes, can lead to significant cognitive impairment in addition to causing epilepsy. While etiology of the epilepsy clearly plays a major role in cognitive development, there are indications that early-life seizures independent of etiology can lead to cognitive impairment18,19. For example, in a study of neuropsychological function in children with focal cortical dysplasia by Korman et al.19 it was found that age of onset of epilepsy and extent of the dysplasia each contributed independently to cognitive dysfunction indicating that early onset of epilepsy disrupted critical periods of development leading to poor cognitive outcomes.
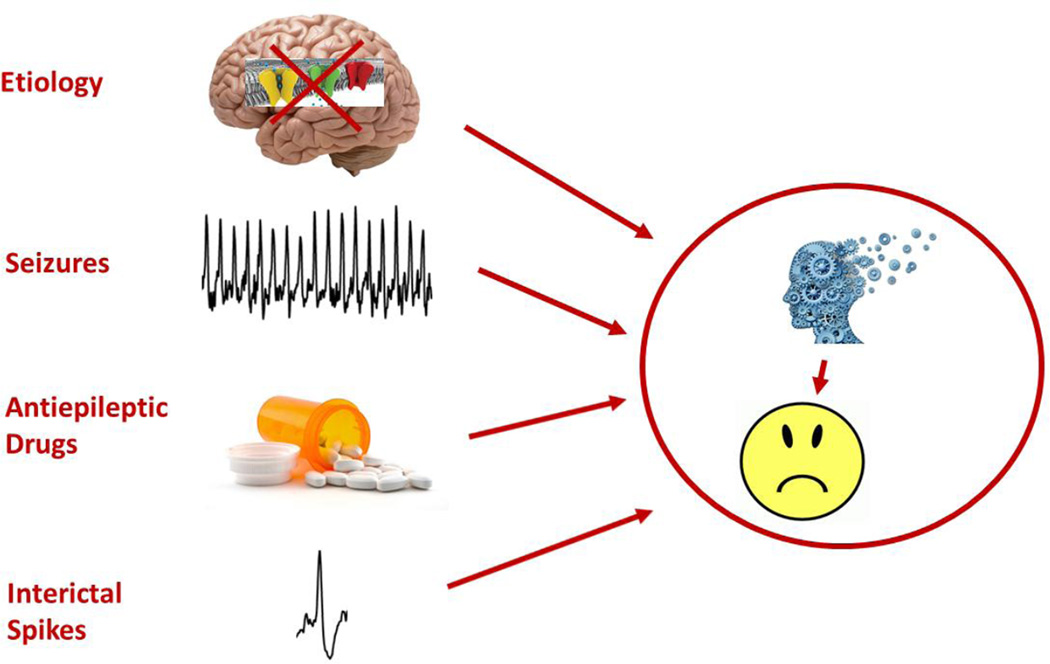
In order to prevent, limit and reverse cognitive comorbidities, it is essential to understand the neurobiological basis of cognitive dysfunction of seizures in children. While studies have indicated that children with seizures occurring early in life, particularly when frequent and resistant to therapy, are at highest risk for cognitive deficits, it is difficult to ascertain the neurobiological disturbances that lead to cognitive impairment. In the clinic it is difficult to differentiate the effects of the number, duration and seizure type, EEG abnormalities and antiepileptic drug therapy from the etiology of the epilepsy (Fig. 1). For this reason, many of the advances in our understanding of the long-term effects of early-life seizures come from rodent studies in which the investigator has control over the etiology and treatment of the seizures.
Fig. 1.
There are many factors that determine cognitive outcome with early-life seizures. Etiology is the primary determinant of cognitive outcome. For example, channelopathies, such as occur in Dravet syndrome, may have major effects on cognition. Likewise, seizures, antiepileptic drugs and interictal spikes contribute to cognitive impairment.
Genetic Models
Animal models of early-life seizures include genetic and acquired models. The discovery of multiple genetic mutations associated with human epileptic encephalopathies and advances in genetic techniques have resulted in the generation of multiple models in animals carrying the human equivalent of these genes. Mutations in the gene coding for the type-1 alpha subunit of the Nav1.1 sodium channel in neurons, SCN1A, has been linked to the epileptic encephalopathies, especially Dravet syndrome and generalized epilepsy with febrile seizures plus (GEFS+). Models of Dravet syndrome have thermally-induced and spontaneous seizures that develop during the animal’s maturation20–23. Likewise, models of generalized epilepsy + (GEFS+) mimic the clinical features of the condition with increased susceptibility to thermally-induced seizures and generalized tonic-clonic seizures24–27.
There is some evidence that the current Dravet syndrome models have social and spatial cognitive impairment when compared to control animals. Animals with some of them have performed poorly in a social recognition and social novelty task compared to controls28–30. Others, however, have shown similar performance to control animals31. Regarding animal models’ ability to mimic the cognitive decline seen in this disorder, few examples of behavioral testing specific for spatial memory have been reported. However, those that have had such testing have shown significant impairments. This includes Ohmori et al.’s work31 showing impaired performance of mice on a Barnes maze, a cognitive task assessing spatial learning in rodents. Similar results have been repeated in at least one other animal model specific for the SCN1A mutations thought to underlie Dravet syndrome.30 Additional assessments of cognition have shown deficits in the memory requiring components of context-dependent fear conditioning.29,30 Behavioral tests in the GEFS+ model have been similar to those seen in the Dravet models, including hyperactivity, impaired social performance, and deficits in spatial memory32. In addition deficits in cued fear conditioning and risk assessment have also been reported in this model32.
Discerning if the cognitive impairments associated with genetic models of early-life seizures are a result of the seizures seen in the other models, or a result of the mutated protein itself and resultant physiologic changes is important. Bender et al.33 caused a similar decrease in the expression of Nav 1.1 protein seen in the genetic models of Dravet syndrome featuring SCN1A mutations, but through injection of specific small interfering RNA (siRNA) into the medial septum and diagonal band of Broca (MSDB), a brain region important for spatial cognition through its regulation of theta oscillations in the hippocampus, of adult Sprague-Dawley rats. This allowed for an evaluation of the effect of less SCN1A expression on cognition without affecting development of the rats or inducing the spontaneous seizures seen in the Dravet syndrome models. Rats with siRNA-induced firing of interneurons had spatial memory impairment compared to controls indicated by a comparative behavioral lack of response to a change in object location during a reaction-to-novelty task. This specific finding would suggest that the mechanistic changes underlying Dravet syndrome may also play a role in the cognitive impairment seen in the disorder that could be compounded by the effect of the associated developmental seizures.
In general, there has been limited work on the cognitive effects of seizures in the genetic disorders. In addition, the genetic models currently used only affect one specific gene/protein, when multiple genetic changes likely underlie the pathophysiology of many of the epileptic encephalopathies. Finally, while many advances have been made regarding the generation of models of specific syndromes such as West syndrome, Dravet syndrome or GEFS+, there remain few models that exhibit most qualities of the other encephalopathies, like Ohtahara syndrome, beyond the early repetitive seizure models. There are also no animal models that consistently exhibit the characteristics that match some of the syndromes like Lennox-Gastaux syndrome, indicating that further work must be done in order to fully understand the processes behind such disorders.
Acquired Models
Acquired models of early-life seizures have been more extensively studied than the genetic models. With acquired models the investigator has control of age of seizure onset and seizure frequency in normal developing rats and thus can distinguish the effects of seizures from etiology of the seizures. Far more work on the cognitive effects of seizures on the developing brain have been done in the acquired models rather than the genetic models. For that reason, this review deals primarily with the two models of early-life seizures (ELS), experimental febrile status epilepticus34–38 and recurrent flurothyl-induced seizures.
In the febrile status model rat pups are placed in a glass container and their core temperature, which is highly correlated with brain temperature, increased to approximately 40.5°C as a simulation of high fever. Manifested as a set of characteristic behaviors, hyperthermic seizure onset is heralded by sudden freezing, followed by oral automatisms and forelimb clonus. Seizures progress to body flexion and one or more tonic-clonic seizures. To accurately model prolonged febrile seizures in humans, hyperthermia (maximum temperature: 41.5–42.9°C) is maintained for approximately 40 minutes, which results in seizures lasting 36–40 minutes.37 In the repeated flurothyl-induced seizure model, a 10% flurothyl solution (Bis(2,2,2-trifluoroethyl) ether), an inhaled convulsive agent, is delivered to the pups, which are placed in a plastic container located in an airflow hood39–41. Flurothyl (0.1 ml) is injected slowly onto filter paper placed on the inside of the container where it evaporates. Although the behavioral features of the seizures are age-related, the typical sequence of the seizures consists of myoclonic jerks followed by forelimb clonus, wild running, loss of posture and severe tonic posturing.
Effects of Seizures on the Developing Brain
Using the flurothyl model of recurrent ELS in rat pups to human mimic neonatal seizures, we have shown cognitive impairment when the animals are tested during adolescence or adulthood39,42–47. Cognitive deficits seen following ELS include deficits of spatial cognition in the water maze43,48, non-match to sample task40, impaired auditory discrimination49 and reduced behavioral flexibility42. While ELS do not result in cell death46,50, there is evidence for synaptic reorganization as evidenced by sprouting in CA351,52 and reduced neurogenesis53. Functionally, we have demonstrated that ELS lead to persistent decreases in GABA currents in the hippocampus54 and neocortex55,56, enhanced excitation in the neocortex56, impairment in spike frequency adaptation57, marked reductions in after hyperpolarizing potentials following spike trains57 and alterations in short-term58 and long-term plasticity39,59.
One of the major difficulties in deciphering the complex issue of cognitive outcomes following ELS in children is the gulf between the behavioral/mental spheres in which these deficits are substantiated and the underlying physiological/developmental domains that are both the cause of these deficits and the most likely domain for therapy and intervention. While these descriptive studies have added to the body of work describing the effects of ELS on the neurophysiology of the developing brain, there remains a major gap in determining how these myriad of morphological and functional changes result in cognitive impairment.
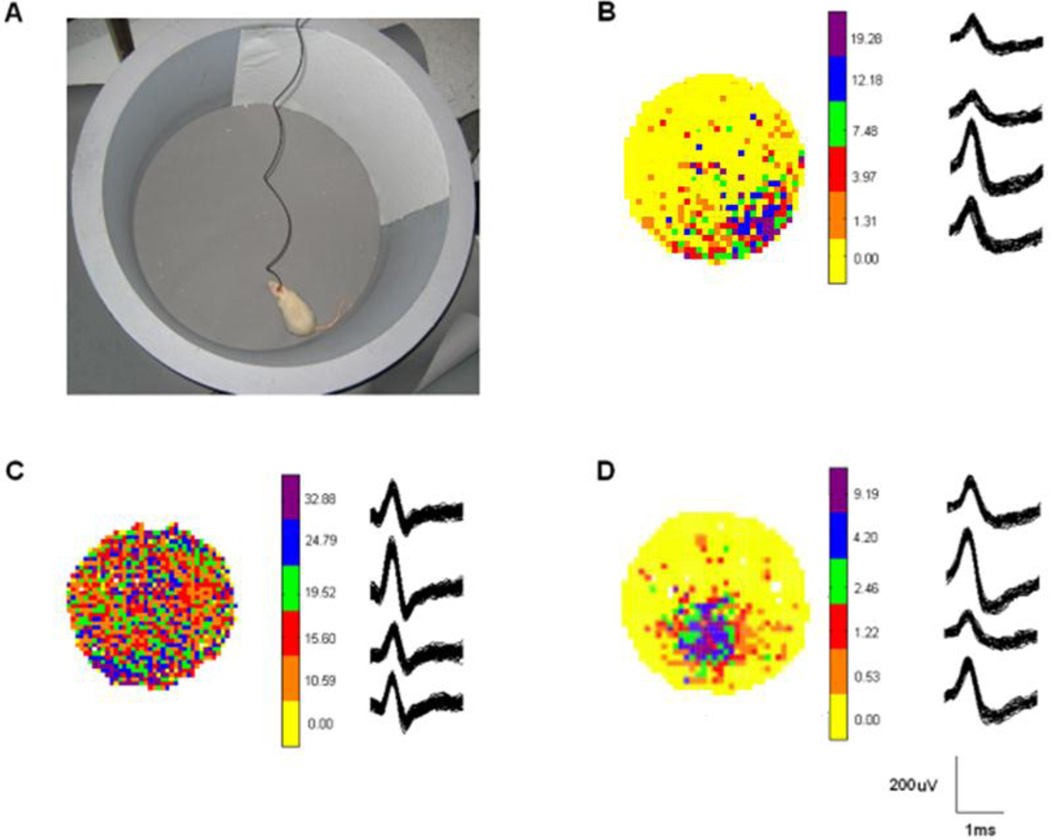
To understand the electrophysiological basis of early-life seizures, investigators have used in vivo electrophysiological techniques to record local field potentials (LFPs) and single cell activity in animals actively engaged in behavioral testing. One of the strongest techniques is the recording of place cells, which fire action potentials (APs), also called units, based on where the animal is within its environment.60,61 (Fig. 2) Specifically, these hippocampal pyramidal neurons selectively discharge (rate coding) when the animal enters certain locations of the environment, called the cells’ firing fields. Field location, size and shape are specific to each cell and each environment and fields tend to cover the surface of the environment homogeneously when a large number of neurons are being recorded simultaneously. Since there is a relationship between place cell activity and the ongoing spatial behavior of rats62,63, such signals provide the animal with a spatial representation in order to navigate efficiently within the environment. Adult rats that experienced status epilepticus (SE) and have impaired learning in the water maze have defective place cells64,65, with imprecise and stable firing fields65. Likewise, flurothyl-induced seizures during the first weeks of life cause impairment in spatial cognition with poor performance in the water maze and radial-arm water maze and have substantial deficits in AP firing with impaired place cell precision and reduced place cell stability43. In the febrile status epilepticus model, rats have spatial cognition deficits and impaired place cell firing field34, indicating that rate coding can be impaired in multiple models of ELS. Furthermore, these findings suggest that abnormalities in single cell firing contribute to impaired spatial cognition.
Fig. 2.
Place cell recordings. The recording cylinder with cue card is shown in A. Examples of place cells (B,D) and an interneuron (C) are shown. The place cell in B is at approximately 5 o’clock while the place cell in D is at approximately 7 o’clock but is closer to the center of the cylinder than the cell depicted in B. Firing frequency is color coded. Yellow shows areas of the cylinder in which the cell did not fire while the green, blue and purple colors show high firing rates in the place firing field. Wavefroms from the tetrodes are shown next to the color coded firing rates. The neuron recorded in C is an interneuron which shows a higher firing rate but no clear place preference.
Network oscillations underlie the temporal binding of spatially distributed neuronal populations, enabling information processing for cognition66,67. Theta frequency (5–12 Hz), the largest amplitude oscillation in the hippocampus, is associated with exploratory behavior and has been found to be necessary for spatial memory performance68,69. Gamma oscillations (30–90 Hz) have been hypothesized to “route” information flow in CA170 with slow gamma (20–50 Hz) coupled to CA3 and believed to be essential for memory storage71. There is a slowing of hippocampal CA1 theta in animals with spatial cognition deficits in both the flurothyl50 and hyperthermia34 induced seizures model.
Oscillations are dynamic events that can be modulated by experience. In a study measuring oscillatory activity in the hippocampus and medial prefrontal cortex of adult rats that had experienced flurothyl-induced seizures in the first weeks of life, and were remedially trained on a delayed-nonmatch-to-sample memory task, power was increased among ELS rats42. This enhancement appeared after the first memory-training steps using short delays and plateaued at the most difficult steps, which included both short and long delays. Further, ELS rats showed enhanced CA1-Prefrontal Cortex (PFC) theta coherence in correct trials compared with incorrect trials when long delays were imposed, suggesting hippocampal-PFC enhancements for the task in this group when memory demand was high. ELS rats also showed heightened gamma power and coherence among all three structures during the trials. These results provided the first evidence that training of rats after ELS can dynamically alter oscillatory activity of hippocampal-PFC circuitry.
Effects of Interictal Spikes on the Developing Brain
In addition to seizures, interictal spikes (IIS) can result in cognitive impairment in both rats and humans. Following intrahippocampal pilocarpine infusion, rats develop IIS that result in transient impairment in the delayed-match-to-sample test, a hippocampal-dependent operant behavior task72. Hippocampal IIS that occur during memory retrieval strongly impairs performance. However, IIS that happen during memory encoding or memory maintenance do not affect performance. In a similar study of people with temporal lobe epilepsy (TLE) who have bilateral depth electrodes implanted into their hippocampi for preoperative seizure localization, IIS cause transient memory impairment73,74. Hippocampal IIS occurring during the memory retrieval period decrease the likelihood of a correct response on a short-term memory test, the Sternberg task, when they arise bilaterally in both temporal lobes or in the temporal lobe contralateral to the seizure focus73. Because patients with TLE often have impaired memory in the epileptic hippocampus and require the non-epileptic hippocampus for memory, IIS arising from the damaged epileptic hippocampus have little effect on memory while IIS involving the intact non-epileptic hippocampus interfere with memory retrieval. As with rodents with TLE, patients with TLE and IIS have specific deficits in retrieval of memory while sparing other forms of memory.
IIS in rodents, similar to seizures, result in a sustained reduction of action potentials (APs) in the hippocampus for up to two seconds following IIS75. Furthermore, when occurring in flurries, defined as four or more IIS occurring within 10 sec, IIS can reduce APs firing for up to six seconds. The widespread inhibitory wave immediately following IIS can also reduce the power of gamma (>30 Hz) oscillations and other oscillatory signals in the hippocampus76. As will be discussed further, since gamma oscillations are closely coupled with ongoing learning and memory function, disruption in oscillations may contribute to cognitive deficits.
In adults with IIS, the cognitive effects are typically transient and may have little long-term consequences in cognitive function, unless they occur very frequently. However, in the immature brain, the effects of IIS also have long-term adverse effects on developing neural circuits. In interesting studies from the 1980’s, IIS were elicited by either penicillin77 or bicuculline78 through focal application on the striate cortex of the occipital lobe of immature rabbits. IIS occurred for 6–12 hours following each drug application which was given daily starting from postnatal day 8–9 and continued for 3–4 weeks. None of the rabbits had behavioral or electrographic seizures. In single-unit recordings from the lateral geniculate nucleus, superior colliculus and occipital cortex ipsilateral to the occipital lobe with the IIS, an abnormal distribution of receptive field types was seen, whereas normal recording was present in the contralateral hemisphere. Remarkably, this finding is age-dependent. Adult rabbits with similarly induced IIS have a normal distribution of receptive field types, highlighting the vulnerability of critical developmental periods to cumulative IIS effects over time. Likewise, focally-induced IIS by stereotaxic injections of bicuculline in the prefrontal cortex (PFC) in rat pups for several days result in long-standing increases in short term plasticity in the PFC and marked inattentiveness during a spatial memory task, when the rats are tested as79.
To further study the effect of recurrent multifocal IIS during development, rat pups were given a low dose of flurothyl for four hours for 10 days during continuous EEG monitoring80. Rats developed IIS without seizures while age-matched controls under similar testing conditions had few IIS. When the rats were tested as adults, there was impairment in reference memory in the Morris water maze, working memory impairment in the four-trial radial-arm water maze and impaired long-term potentiation. Early-life IIS resulted in impaired new cell formation and decreased cell counts in the hippocampus but did not cause an increase in apoptosis. This study, for the first time, demonstrates that IIS in rat pups without seizures can result in long-standing spatial cognitive impairment. These findings suggest that suppressing IIS may be as important as treating seizures during brain development.
Conclusions
In summary, while etiology of the seizures is the primary factor in cognitive outcome, rodent models of childhood epilepsy have shown that both seizures and IIS can be detrimental to brain development. With further refinement of our knowledge of the pathophysiological underpinnings of seizure-related cognitive deficits it is hoped that future therapies will target cognition as much as seizures. While not covered in this review, AEDs can also contribute to cognitive impairment in both children and animals. While suppressing seizures and IIS is an important goal, clinicians should be cognizant of the potentially detriment effects of AEDs on the developing brain when making treatment decisions.
Acknowledgments
Supported by National Institute of Health grants NS074450, NS074450 and NS073083 and the Michael J. Pietroniro Research Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Committee on the Public Health Dimensions of the Epilepsies, Board on Health Sciences Policy, and Institute of Medicine. Epilepsy Across the Spectrum: Promoting Health and Understanding. The National Academies Press; 2012. [PubMed] [Google Scholar]
- 2.Holmes GL. Cognitive impairment in epilepsy: the role of network abnormalities. Epileptic Disord. 2015;17:101–116. doi: 10.1684/epd.2015.0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holmes GL. What is more harmful, seizures or epileptic EEG abnormalities? Is there any clinical data? Epileptic Disord. 2014;16(Suppl 1):12–22. doi: 10.1684/epd.2014.0686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farwell JR, Dodrill CB, Batzel LW. Neuropsychological abilities of children with epilepsy. Epilepsia. 1985;26:395–400. doi: 10.1111/j.1528-1157.1985.tb05670.x. [DOI] [PubMed] [Google Scholar]
- 5.Neyens LG, Aldenkamp AP, Meinardi HM. Prospective follow-up of intellectual development in children with a recent onset of epilepsy. Epilepsy Res. 1999;34:85–90. doi: 10.1016/s0920-1211(98)00118-1. [DOI] [PubMed] [Google Scholar]
- 6.Sillanpaa M, Jalava M, Kaleva O, et al. Long-term prognosis of seizures with onset in childhood. N Engl J Med. 1998;338:1715–1722. doi: 10.1056/NEJM199806113382402. [DOI] [PubMed] [Google Scholar]
- 7.Prasad AN, Burneo JG, Corbett B. Epilepsy, comorbid conditions in Canadian children: analysis of cross-sectional data from cycle 3 of the National Longitudinal Study of Children and Youth. Seizure. 2014;23:869–873. doi: 10.1016/j.seizure.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 8.Buelow JM, Perkins SM, Johnson CS, et al. Adaptive functioning in children with epilepsy and learning problems. J Child Neurol. 2012;27:1241–1249. doi: 10.1177/0883073811432750. [DOI] [PubMed] [Google Scholar]
- 9.Bailet LL, Turk WR. The impact of childhood epilepsy on neurocognitive and behavioral performance: a prospective longitudinal study. Epilepsia. 2000;41:426–431. doi: 10.1111/j.1528-1157.2000.tb00184.x. [DOI] [PubMed] [Google Scholar]
- 10.Wakamoto H, Nagao H, Hayashi M, et al. Long-term medical, educational, and social prognoses of childhood-onset epilepsy: a population-based study in a rural district of Japan. Brain Dev. 2000;22:246–255. doi: 10.1016/s0387-7604(00)00121-2. [DOI] [PubMed] [Google Scholar]
- 11.Williams J, Griebel ML, Dykman RA. Neuropsychological patterns in pediatric epilepsy. Seizure. 1998;7:223–228. doi: 10.1016/s1059-1311(98)80040-x. [DOI] [PubMed] [Google Scholar]
- 12.Bourgeois BFD, Prensky AL, Palkes HS, et al. Intelligence in epilepsy: A prospective study in children. Ann Neurol. 1983;14:438–444. doi: 10.1002/ana.410140407. [DOI] [PubMed] [Google Scholar]
- 13.Hermann BP, Seidenberg M, Bell B. The neurodevelopmental impact of childhood onset temporal lobe epilepsy on brain structure and function and the risk of progressive cognitive effects. Prog Brain Res. 2002;135:429–438. doi: 10.1016/S0079-6123(02)35040-4. [DOI] [PubMed] [Google Scholar]
- 14.Berg AT, Zelko FA, Levy SR, et al. Age at onset of epilepsy, pharmacoresistance, and cognitive outcomes: a prospective cohort study. Neurology. 2012;79:1384–1391. doi: 10.1212/WNL.0b013e31826c1b55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nabbout R, Dulac O. Epileptic encephalopathies: a brief overview. J Clin Neurophysiol. 2003;20:393–397. doi: 10.1097/00004691-200311000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Shields WD. Catastrophic epilepsy in childhood. Epilepsia. 2000;41(Suppl 2):S2–S6. doi: 10.1111/j.1528-1157.2000.tb01518.x. [DOI] [PubMed] [Google Scholar]
- 17.Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia. 2010;51:676–685. doi: 10.1111/j.1528-1167.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- 18.Glass HC, Glidden D, Jeremy RJ, et al. Clinical Neonatal Seizures are Independently Associated with Outcome in Infants at Risk for Hypoxic-Ischemic Brain Injury. J Pediatr. 2009;155:318–323. doi: 10.1016/j.jpeds.2009.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korman B, Krsek P, Duchowny M, et al. Early seizure onset and dysplastic lesion extent independently disrupt cognitive networks. Neurology. 2013;81:745–751. doi: 10.1212/WNL.0b013e3182a1aa2a. [DOI] [PubMed] [Google Scholar]
- 20.Kalume F, Yu FH, Westenbroek RE, et al. Reduced sodium current in Purkinje neurons from Nav1.1 mutant mice: implications for ataxia in severe myoclonic epilepsy in infancy. J Neurosci. 2007;27:11065–11074. doi: 10.1523/JNEUROSCI.2162-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oakley JC, Kalume F, Yu FH, et al. Temperature- and age-dependent seizures in a mouse model of severe myoclonic epilepsy in infancy. Proc Natl Acad Sci U S A. 2009;106:3994–3999. doi: 10.1073/pnas.0813330106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogiwara I, Miyamoto H, Morita N, et al. Na(v)1.1 localizes to axons of parvalbumin-positive inhibitory interneurons: a circuit basis for epileptic seizures in mice carrying an Scn1a gene mutation. J Neurosci. 2007;27:5903–5914. doi: 10.1523/JNEUROSCI.5270-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen C, Westenbroek RE, Xu X, et al. Mice lacking sodium channel beta1 subunits display defects in neuronal excitability, sodium channel expression, and nodal architecture. J Neurosci. 2004;24:4030–4042. doi: 10.1523/JNEUROSCI.4139-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang B, Dutt K, Papale L, et al. A BAC transgenic mouse model reveals neuron subtype-specific effects of a Generalized Epilepsy with Febrile Seizures Plus (GEFS+) mutation. Neurobiol Dis. 2009;35:91–102. doi: 10.1016/j.nbd.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin MS, Dutt K, Papale LA, et al. Altered function of the SCN1A voltage-gated sodium channel leads to gamma-aminobutyric acid-ergic (GABAergic) interneuron abnormalities. J Biol Chem. 2010;285:9823–9834. doi: 10.1074/jbc.M109.078568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun L, Gilligan J, Staber C, et al. A knock-in model of human epilepsy in Drosophila reveals a novel cellular mechanism associated with heat-induced seizure. J Neurosci. 2012;32:14145–14155. doi: 10.1523/JNEUROSCI.2932-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schutte RJ, Schutte SS, Algara J, et al. Knock-in model of Dravet syndrome reveals a constitutive and conditional reduction in sodium current. J Neurophysiol. 2014;112:903–912. doi: 10.1152/jn.00135.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wagnon JL, Korn MJ, Parent R, et al. Convulsive seizures and SUDEP in a mouse model of SCN8A epileptic encephalopathy. Hum Mol Genet. 2015;24:506–515. doi: 10.1093/hmg/ddu470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han S, Tai C, Westenbroek RE, et al. Autistic-like behaviour in Scn1a+/− mice and rescue by enhanced GABA-mediated neurotransmission. Nature. 2012;489:385–390. doi: 10.1038/nature11356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ito S, Ogiwara I, Yamada K, et al. Mouse with Nav1.1 haploinsufficiency, a model for Dravet syndrome, exhibits lowered sociability and learning impairment. Neurobiol Dis. 2013;49:29–40. doi: 10.1016/j.nbd.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 31.Ohmori I, Kawakami N, Liu S, et al. Methylphenidate improves learning impairments and hyperthermia-induced seizures caused by an Scn1a mutation. Epilepsia. 2014;55:1558–1567. doi: 10.1111/epi.12750. [DOI] [PubMed] [Google Scholar]
- 32.Sawyer NT, Helvig AW, Makinson CD, et al. Scn1a dysfunction alters behavior but not the effect of stress on seizure response. Genes Brain Behav. 2015 doi: 10.1111/gbb.12281. [DOI] [PubMed] [Google Scholar]
- 33.Bender AC, Natola H, Ndong C, et al. Focal Scn1a knockdown induces cognitive impairment without seizures. Neurobiol Dis. 2013;54:297–307. doi: 10.1016/j.nbd.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barry JM, Choy M, Dube C, et al. T2 relaxation time post febrile status epilepticus predicts cognitive outcome. Exp Neurol. 2015;269:242–252. doi: 10.1016/j.expneurol.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choy M, Dube CM, Patterson K, et al. A novel, noninvasive, predictive epilepsy biomarker with clinical potential. J Neurosci. 2014;34:8672–8684. doi: 10.1523/JNEUROSCI.4806-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dube CM, Zhou JL, Hamamura M, et al. Cognitive dysfunction after experimental febrile seizures. Exp Neurol. 2008;215:167–177. doi: 10.1016/j.expneurol.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dube C, Richichi C, Bender RA, et al. Temporal lobe epilepsy after experimental prolonged febrile seizures: prospective analysis. Brain. 2006;129:911–922. doi: 10.1093/brain/awl018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dubé CM, Baram TZ. Complex febrile seizures - an experimental model in immature rodents. In: Pitkänen A, Schwartzkroin PA, Moshé S, editors. Models of Seizures and Epilepsy. Burlington, Massachusetts: Elsevier; 2006. pp. 333–334. [Google Scholar]
- 39.Karnam HB, Zhao Q, Shatskikh T, et al. Effect of age on cognitive sequelae following early life seizures in rats. Epilepsy Res. 2009;85:221–230. doi: 10.1016/j.eplepsyres.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kleen JK, Wu EX, Holmes GL, et al. Enhanced oscillatory activity in the hippocampal-prefrontal network is related to short-term memory function after early-life seizures. J Neurosci. 2011;31:15397–15406. doi: 10.1523/JNEUROSCI.2196-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lucas MM, Lenck-Santini PP, Holmes GL, et al. Impaired cognition in rats with cortical dysplasia: additional impact of early-life seizures. Brain. 2011;134:1684–1693. doi: 10.1093/brain/awr087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kleen JK, Sesque A, Wu EX, et al. Early-life seizures produce lasting alterations in the structure and function of the prefrontal cortex. Epilepsy Behav. 2011;22:214–219. doi: 10.1016/j.yebeh.2011.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karnam HB, Zhou JL, Huang LT, et al. Early life seizures cause long-standing impairment of the hippocampal map. Exp Neurol. 2009;217:378–387. doi: 10.1016/j.expneurol.2009.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou JL, Shatskikh TN, Liu X, et al. Impaired single cell firing and long-term potentiation parallels memory impairment following recurrent seizures. Eur J Neurosci. 2007;25:3667–3677. doi: 10.1111/j.1460-9568.2007.05598.x. [DOI] [PubMed] [Google Scholar]
- 45.Hoffmann AF, Zhao Q, Holmes GL. Cognitive impairment following status epilepticus and recurrent seizures during early development: support for the "two-hit hypothesis". Epilepsy Behav. 2004;5:873–877. doi: 10.1016/j.yebeh.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 46.de Rogalski Landrot I, Minokoshi M, Silveira DC, et al. Recurrent neonatal seizures: relationship of pathology to the electroencephalogram and cognition. Brain Res Dev Brain Res. 2001;129:27–38. doi: 10.1016/s0165-3806(01)00177-8. [DOI] [PubMed] [Google Scholar]
- 47.Huang L, Cilio MR, Silveira DC, et al. Long-term effects of neonatal seizures: a behavioral, electrophysiological, and histological study. Brain Res Dev Brain Res. 1999;118:99–107. doi: 10.1016/s0165-3806(99)00135-2. [DOI] [PubMed] [Google Scholar]
- 48.Lugo JN, Swann JW, Anderson AE. Early-life seizures result in deficits in social behavior and learning. Exp Neurol. 2014;256:74–80. doi: 10.1016/j.expneurol.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Neill JC, Liu Z, Sarkisian M, et al. Recurrent seizures in immature rats: effect on auditory and visual discrimination. Brain Res Dev Brain Res. 1996;95:283–292. doi: 10.1016/0165-3806(96)00099-5. [DOI] [PubMed] [Google Scholar]
- 50.Holmes GL, Tian C, Hernan AE, et al. Alterations in sociability and functional brain connectivity caused by earlylife seizures are prevented by bumetanide. Neurobiol Dis. 2015;77:204–219. doi: 10.1016/j.nbd.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Holmes GL, Sarkisian M, Ben-Ari Y, et al. Mossy fiber sprouting after recurrent seizures during early development in rats. J Comp Neurol. 1999;404:537–553. doi: 10.1002/(sici)1096-9861(19990222)404:4<537::aid-cne9>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 52.Holmes GL, Gairsa JL, Chevassus-Au-Louis N, et al. Consequences of neonatal seizures in the rat: morphological and behavioral effects. Ann Neurol. 1998;44:845–857. doi: 10.1002/ana.410440602. [DOI] [PubMed] [Google Scholar]
- 53.McCabe BK, Silveira DC, Cilio MR, et al. Reduced neurogenesis after neonatal seizures. J Neurosci. 2001;21:2094–2103. doi: 10.1523/JNEUROSCI.21-06-02094.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Isaeva E, Isaev D, Khazipov R, et al. Selective impairment of GABAergic synaptic transmission in the flurothyl model of neonatal seizures. Eur J Neurosci. 2006;23:1559–1566. doi: 10.1111/j.1460-9568.2006.04693.x. [DOI] [PubMed] [Google Scholar]
- 55.Isaeva E, Isaev D, Khazipov R, et al. Long-term suppression of GABAergic activity by neonatal seizures in rat somatosensory cortex. Epilepsy Res. 2009;87:286–289. doi: 10.1016/j.eplepsyres.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Isaeva E, Isaev D, Savrasova A, et al. Recurrent neonatal seizures result in long-term increases in neuronal network excitability in the rat neocortex. Eur J Neurosci. 2010;31:1446–1455. doi: 10.1111/j.1460-9568.2010.07179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Villeneuve N, Ben-Ari Y, Holmes GL, et al. Neonatal seizures induced persistent changes in intrinsic properties of CA1 rat hippocampal cells. Ann Neurol. 2000;47:729–738. [PubMed] [Google Scholar]
- 58.Hernan AE, Holmes GL, Isaev D, et al. Altered short-term plasticity in the prefrontal cortex after early life seizures. Neurobiol Dis. 2013;50:120–126. doi: 10.1016/j.nbd.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Isaeva E, Isaev D, Holmes GL. Alteration of synaptic plasticity by neonatal seizures in rat somatosensory cortex. Epilepsy Res. 2013 doi: 10.1016/j.eplepsyres.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.O'Keefe J, Dostrovsky J. The hippocampus as a spatial map: preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34:171175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- 61.O'Keefe J. Place units in the hippocampus of freely moving rats. Exp Neurol. 1973;51:78–109. doi: 10.1016/0014-4886(76)90055-8. [DOI] [PubMed] [Google Scholar]
- 62.Lenck-Santini PP, Muller RU, Save E, et al. Relationships between place cell firing fields and navigational decisions by rats. J Neurosci. 2002;22:9035–9047. doi: 10.1523/JNEUROSCI.22-20-09035.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lenck-Santini PP, Save E, Poucet B. Evidence for a relationship between place-cell spatial firing and spatial memory performance. Hippocampus. 2001;11:377–390. doi: 10.1002/hipo.1052. [DOI] [PubMed] [Google Scholar]
- 64.Lenck-Santini PP, Holmes GL. Altered phase precession and compression of temporal sequences by place cells in epileptic rats. J Neurosci. 2008;28:5053–5062. doi: 10.1523/JNEUROSCI.5024-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu X, Muller RU, Huang LT, et al. Seizure-induced changes in place cell physiology: relationship to spatial memory. J Neurosci. 2003;23:11505–11515. doi: 10.1523/JNEUROSCI.23-37-11505.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.O'Keefe J, Recce M. Phase relationships between hippocampal place units and the EEG theta rhythm. Hippocampus. 1993;3:317–330. doi: 10.1002/hipo.450030307. [DOI] [PubMed] [Google Scholar]
- 67.Skaggs WE, McNaughton BL, Gothard KM, et al. An information-theoretic approach to deciphering the hippocampal code. In: Hanson SJ, Cowan JD, Giles CL, editors. Advances in Neural Information Processing Systems. Vol. 5. San Francisco: Morgan Kaufmann; 1993. pp. 1030–1037. [Google Scholar]
- 68.Winson J. Loss of hippocampal theta rhythm results in spatial memory deficit in the rat. Science. 1978;201:160–163. doi: 10.1126/science.663646. [DOI] [PubMed] [Google Scholar]
- 69.Gupta AS, van der Meer MA, Touretzky DS, et al. Segmentation of spatial experience by hippocampal theta sequences. Nat Neurosci. 2012;15:1032–1039. doi: 10.1038/nn.3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Colgin LL, Denninger T, Fyhn M, et al. Frequency of gamma oscillations routes flow of information in the hippocampus. Nature. 2009;462:353–357. doi: 10.1038/nature08573. [DOI] [PubMed] [Google Scholar]
- 71.Steffenach HA, Sloviter RS, Moser EI, et al. Impaired retention of spatial memory after transection of longitudinally oriented axons of hippocampal CA3 pyramidal cells. Proc Natl Acad Sci U S A. 2002;99:3194–3198. doi: 10.1073/pnas.042700999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kleen JK, Scott RC, Holmes GL, et al. Hippocampal interictal spikes disrupt cognition in rats. Ann Neurol. 2010;67:250–257. doi: 10.1002/ana.21896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kleen JK, Scott RC, Holmes GL, et al. Hippocampal interictal epileptiform activity disrupts cognition in humans. Neurology. 2013;81:18–24. doi: 10.1212/WNL.0b013e318297ee50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Krauss GL, Summerfield M, Brandt J, et al. Mesial temporal spikes interfere with working memory. Neurology. 1997;49:975–980. doi: 10.1212/wnl.49.4.975. [DOI] [PubMed] [Google Scholar]
- 75.Zhou JL, Lenck-Santini PP, Zhao Q, et al. Effect of interictal spikes on single-cell firing patterns in the hippocampus. Epilepsia. 2007;48:720–731. doi: 10.1111/j.1528-1167.2006.00972.x. [DOI] [PubMed] [Google Scholar]
- 76.Urrestarazu E, Jirsch JD, Levan P, et al. High-frequency intracerebral EEG activity (100–500 Hz) following interictal spikes. Epilepsia. 2006;47:1465–1476. doi: 10.1111/j.1528-1167.2006.00618.x. [DOI] [PubMed] [Google Scholar]
- 77.Baumbach HD, Chow KL. Visuocortical epileptiform discharges in rabbits: differential effects on neuronal development in the lateral geniculate nucleus and superior colliculus. Brain Res. 1981;209:61–76. doi: 10.1016/0006-8993(81)91172-0. [DOI] [PubMed] [Google Scholar]
- 78.Ostrach LH, Crabtree JW, Campbell BG, et al. Effects of bicuculline-induced epileptiform activity on development of receptive field properties in striate cortex and lateral geniculate nucleus of the rabbit. Brain Res. 1984;317:113–123. doi: 10.1016/0165-3806(84)90146-9. [DOI] [PubMed] [Google Scholar]
- 79.Hernan AE, Alexander A, Jenks KR, et al. Focal epileptiform activity in the prefrontal cortex is associated with long-term attention and sociability deficits. Neurobiol Dis. 2014;63:25–34. doi: 10.1016/j.nbd.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Khan OI, Zhao Q, Miller F, et al. Interictal spikes in developing rats cause long-standing cognitive deficits. Neurobiol Dis. 2010;39:362–371. doi: 10.1016/j.nbd.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]