Abstract
Non-small cell lung cancer (NSCLC) is the leading cause of cancer-associated deaths worldwide. Given the efficacy of membrane proteins as therapeutic targets in human malignancies, we examined cell-surface receptors that may act as drivers of lung tumorigenesis. Here we report that the PROTOCADHERIN PCDH7 is overexpressed frequently in NSCLC tumors where this event is associated with poor clinical outcome. PCDH7 overexpression synergized with EGFR and KRAS to induce MAPK signaling and tumorigenesis. Conversely, PCDH7 depletion suppressed ERK activation, sensitized cells to MEK inhibitors and reduced tumor growth. PCDH7 potentiated ERK signaling by facilitating interaction of protein phosphatase PP2A with its potent inhibitor, the SET oncoprotein. By establishing an oncogenic role for PCDH7 in lung tumorigenesis, our results provide a rationale to develop novel PCDH7 targeting therapies which act at the cell surface of NSCLC cells to compromise their growth.
Keywords: PROTOCADHERIN 7, NSCLC, SET oncogene, protein phosphatase 2A, lung tumorigenesis, MAPK signaling, EGFR, KRAS
Introduction
Non-small cell lung cancer (NSCLC) is the most prevalent form of lung cancer and is the leading cause of cancer-associated deaths worldwide (1). Patients have few therapeutic options and disease progression is inevitable, underscoring the critical need to identify new actionable targets. KRAS and EGFR mutations are among the most common alterations in NSCLC and have been identified in 30% and 15% of patients, respectively (1,2). Currently, there are no clinically approved drugs that target mutant KRAS. Significant effort has focused on the development of kinase inhibitors directed against several proteins downstream of KRAS, including the RAF-MAPK and PI3K-AKT signaling cascades (3). These targeted therapies are capable of inducing disease remission, however, the development of drug resistance is inevitable (4). Similarly, patients that harbor EGFR mutations initially respond well to EGFR inhibitor therapy, but drug resistance eventually develops (5,6). Recently, high-throughput sequencing studies have identified hundreds of additional mutations in NSCLC tumors (7,8). In spite of this progress, there remains a significant challenge to distinguish the key alterations that promote lung tumorigenesis, which may lead to the identification of new therapeutic targets.
Protocadherins (PCDHs) are members of the cadherin superfamily that regulate cell adhesion. Their extracellular domains contain cadherin-like repeats, but they differ significantly from classical cadherins with respect to their unique cytoplasmic domains that lack the conserved motifs for binding β-catenin and p120-catenin (9,10). While some PCDHs exhibit cell-cell adhesion properties, a new paradigm has recently emerged suggesting that PCDHs also control signal transduction pathways (9). Moreover, there is a rapidly expanding body of literature demonstrating that PCDH expression is dysregulated and/or mutated in a wide variety of tumor types (11-13). Both tumor suppressive and oncogenic roles of PCDHs have been reported. For example, PCDH8 is inactivated through either mutation or epigenetic silencing in a large fraction of breast carcinomas (14). PCDH10 was identified as a potential tumor suppressor in gastric, colorectal and pancreatic cancers (15). In addition, frequent silencing of PCDH17 has been reported in studies of esophageal and pancreatic carcinoma (16). In contrast, PCDH11Y is overexpressed in advanced prostate cancer primary tumors and metastases, and ectopic expression of PCDH11Y in prostate cancer cell lines enhances tumorigenesis (17). Notably, high expression of PCDH7 has been linked to brain and bone metastasis of breast and lung cancer cells (11,12,18,19). Collectively, these studies suggest that PCDHs may play important roles in tumorigenesis. However, the roles of PCDHs in lung cancer and the mechanisms through which gain- and loss-of function of PCDHs drive tumorigenesis in vivo are poorly understood. Because these molecules are cell surface receptors and therefore are potentially accessible to antibody-based therapeutic modalities, elucidation of the roles of PCDH family members in tumorigenesis, and the resulting consequences on signal transduction pathways, represents a promising area of investigation in cancer biology.
In this study, we describe the unexpected finding that a poorly characterized Protocadherin, PCDH7, functions as an oncogene to promote transformation of human bronchial epithelial cells and lung tumorigenesis. This PCDH came to our attention because it is frequently overexpressed in lung cancer and high expression of PCDH7 in tumors strongly associates with poor survival of NSCLC patients. Moreover, single nucleotide polymorphisms located in PCDH7 have been associated with poor overall survival in early stage NSCLC patients (20). Here we demonstrate that overexpression of PCDH7 potently synergizes with lung cancer drivers including mutant KRAS and EGFR, inducing transformation of human bronchial epithelial cells (HBECs) and promoting tumorigenesis in vivo. These oncogenic effects are associated with the ability of PCDH7 to enhance MAP kinase signaling by binding to Protein Phosphatase 2A (PP2A), which directly de-phosphorylates ERK/2, and the SET oncoprotein, a potent inhibitor of PP2A (21,22), thereby suppressing PP2A activity. Collectively, this work uncovers a new mechanism through which PCDH7 leads to hyperactivation of the MAPK pathway, a critical driver of lung tumorigenesis, and suggests that PCDH7 represents a promising new therapeutic target at the cell surface of lung cancer cells.
Materials and Methods
Mice
Immunocompromised mice NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG mice) were purchased from The Jackson Laboratories. All procedures involving mice were approved by the Institutional Animal Care and Use Committeee (IACUC) of UTSW Medical Center.
Cell lines
CDK4/TERT-immortalized normal HBEC3 (HBEC3-KT) and HBEC14 (HBEC14-KT) lines were provided by John Minna (23). HBECs with stable inhibition of TP53, and ectopic expression of KRASG12V or EGFRL858R were previously described (24). All HBECs were cultured in keratinocyte serum-free medium (KSFM; Life Technologies Inc) containing 50 μg/mL of bovine pituitary extract (BPE; Life Technologies, Inc) and 5 ng/mL of EGF (Life Technologies, Inc). All human NSCLC cells were cultured in RPMI-1640 media supplemented with 10% fetal bovine serum (Life Technlologies, Inc), 100 units/ml of penicillin and streptomycin (Life Technlologies, Inc).
HBECs, PC9, and HCC827 cells with enforced expression of PCDH7 were generated by infecting cells with a PCDH7 lentivirus. Three days after lentiviral transduction, cells were selected in media containing Blasticidin (2-10 μg/mL), or Hygromycin (20 μg/mL) for 7 to 14 days. Ectopic expression of FLAG-tagged SET as well as V5-PCDH7 in HBECs was achieved using lentiviral transduction. PCDH7 depletion in H1944 or H441 was achieved using Lenti-CRISPRv2 followed by selection with puromycin (1 μg/mL). SET knock out cell lines were generated using lenti-CRISPR. Three days after infection, individual GFP+ cells were sorted into 96 well plates single-cell SET knock out cell lines were established.
Cell lines were originally derived in the Minna laboratory with the exception of PC9 cells that were obtained from ATCC. All cell lines have been DNA fingerprinted using the PowerPlex 1.2 kit (Promega) and have been found to be mycoplasma free using the e-Myco Mycoplasma PCR Detection kit V2.0 (Boca Scientific, catalog #25235). Cell lines were obtained by the O'Donnell lab in 2013, last tested for mycoplasma in April 2016, and authenticated in the last year with the PowerPlex 1.2 kit (Promega).
Constructs
RNA was extracted from Hela cells using the RNeasy Mini Kit (Qiagen) with an on-column DNase digestion. cDNA was generated using the Superscript IV First-Strand Synthesis System (Thermo Fisher). PCDH7 isoforms A-D were PCR amplified from cDNA using PrimeSTAR HS DNA polymerase (Clontech) and subsequently cloned into the gateway entry cloning vector pCR8/GW/TOPO (Invitrogen) and confirmed by sequencing. PCDH7 isoforms were then sub-cloned into lentiviral vectors pLX304 (Addgene #25890) or CMV Hygro DEST (Addgene #17454) using the LR Clonase ™ II enzyme (invitrogen). pENTR4-FLAG (Addgene #17423) and then FLAG-tagged SET was introduced into HBECs using the pLX304 vector. Lentiviral packaging was performed in 293T cells to ectopically express PCDH7, SET, or EGFP in HBECs or PC9 cells. Depletion of PCDH7 was performed using Lenti-CRISPR-V2 (Addgene #52961) to introduce Cas9 and sgRNA directed against PCDH7. The sgRNAs of PCDH7 are as follows: sgRNA1, CGACGTCCGCATCGGCAACG; sgRNA3, CCTGGGCATCGTGACCGGAT; sgRNA4, CATCGTGACCGGATCGGGTG; Control sgRNA, CGCTTCCGCGGCCCGTTCAA. A modified lenti-CRISPR-GFP-V2 vector was generated by replacing the puromycin gene with a GFP cDNA. The resulting Lenti-CRISPR-GFP-V2 was used to knock out SET in HBEC-shp53-KRASG12V-PCDH7 cells. To restore SET expression in individual SET KO clones, a SET cDNA (Ultimate™ ORF, Clone ID: IOH56253, Invitrogen) was modified using the Q5 Site-Directed Mutagenesis Kit (New England Biolabs) to construct a mutant SETβ that is resistant to Cas9 cleavage and cloned into the pcDNA6.2 vector. All primers are provided in Supplementary Table S8.
Analysis of lung cancer gene expression and survival data
Lung cancer gene expression and survival data was examined using the Lung Cancer Portal tool, at the following link: https://qbrc.swmed.edu/projects/lungcancer/. A detailed description of quality control criteria, preprocessing, and normalization procedures used to analyze the different microarray datasets is provided in Supplementary Methods. Citations for datasets analyzed in Figure 1 and Supplementary Table S1 are listed in Supplementary References. Affymetrix probe IDs analyzed for each PCDH family member are provided in Supplementary Table S2.
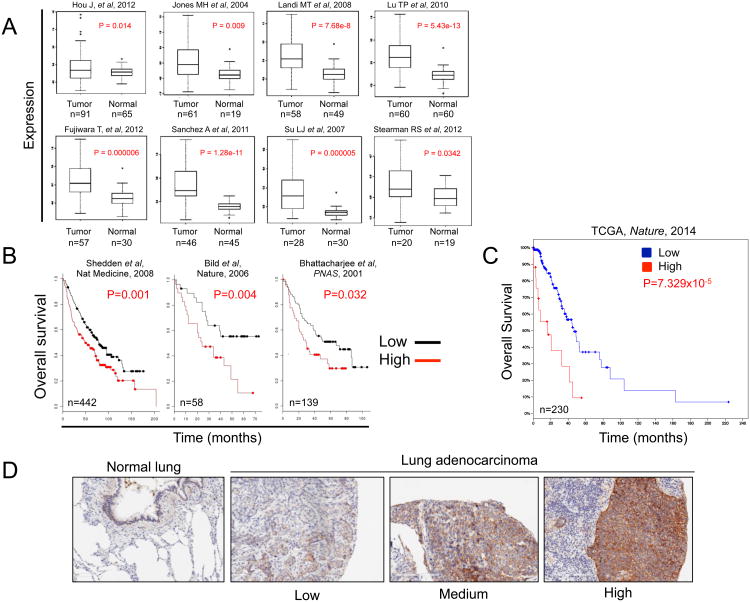
Figure 1. Analysis of PCDH7 in lung cancer datasets and a NSCLC tissue microarray.
A, PCDH7 mRNA expression in lung tumors and adjacent normal tissues of NSCLC patients in data from 8 independent studies. Citations are provided in Supplementary References. B, Overall survival of NSCLC patients clustered by high or low PCDH7 mRNA expression in 3 independent studies. C, Survival analysis of patients with or without PCDH7 mRNA upregulation from The Cancer Genome Atlas (TCGA) 2014 lung adenocarcinoma study (2). D, Immunohistochemistry analysis of PCDH7 in normal human lung and NSCLC tumors.
Western blotting
Approximately 30mg of subcutaneous tumor tissue was homogenized with a Tissue Tearer homogenizer in RIPA buffer (1% NP40, 0.1% SDS, 0.5% sodium deoxycholate in PBS) including phosphatase inhibitor cocktails 2 and 3 (Sigma, P5726 and P0044) diluted 1:100 and protease inhibitor cocktail (Sigma P8430) diluted 1:50. Protein concentrations were determined by BCA assay (Thermo, 23228 and 23224). 25-30μg protein lysate was loaded into each well of a NuPAGE 4-12% gradient Bis-Tris gel (Life Technologies, NP0335), electrophoresed, and transferred to nitrocellulose. Primary antibodies are listed in Supplementary Table S7 and were detected with horseradish peroxidase (HRP)–conjugated anti-rabbit or anti-mouse secondary antibodies (1:5,000-2,000 dilution; BioRad). Cell fractionation was performed using NE-PER™ Nuclear and Cytoplasmic Extraction Reagents (Thermo Fisher).
Co-immunoprecipitation assays
IP Lysis Buffer (Pierce) was used to lyse HBECs or H1944 cells, and immunoprecipitation (IP) was performed using the Dynabeads Protein G Immunoprecipitation Kit (Invitrogen). 2 x 107 HBECs or H1944 cells were harvested and cell lysates prepared using cold IP lysis buffer (Pierce) containing 1 X Halt™ Protease Inhibitor (Pierce). Immunoprecipitations were performed according to manufacturer's instructions of Dynabeads Protein G Immunoprecipitation Kit (Invitrogen) with several modifications. Briefly, 5 ug antibodies were coated on 1.5 mg Dynabeads, and washed with cold IP lysis buffer three times. The antibody-conjugated beads were incubated with 1.0 mg protein lysate at 4°C overnight, and beads were washed extensively with cold IP lysis buffer. IP products were harvested using denaturing elution and subjected to western blot analysis to detect protein-protein interactions. All antibodies utilized in this study are listed in Supplementary Table S7.
Results
PCDH7 is overexpressed in NSCLC and high expression is associated with poor survival of lung adenocarcinoma patients
In order to identify new cell-surface proteins that are oncogenic in NSCLC and might therefore represent potential therapeutic targets, we examined the expression of PCDH family members in existing NSCLC gene expression data. This analysis revealed consistent overexpression of PCDH7 in NSCLC compared to normal lung tissues in 8/8 examined datasets (Fig. 1A; Supplementary Table S1). Moreover, high PCDH7 mRNA correlated with poor survival of lung cancer patients in five independent studies (Fig. 1B-C; Supplementary Table S1). In contrast, other PCDH family members did not exhibit consistent overexpression or correlate with survival in NSCLC.
Next, we performed immunostaining with a PCDH7 antibody on a panel of over 200 clinically and pathologically annotated primary NSCLCs utilizing a tissue microarray (TMA). We observed that PCDH7 protein is expressed at low to medium levels in normal human lung epithelial cells and at medium to high levels in ∼74% of NSCLC cases with predominant expression at the cell membrane (Fig. 1D, Supplementary Fig. S1 and S2A). Using the Log rank test, high expression of PCDH7 protein associated with poor recurrence-free survival of lung adenocarcinoma patients compared to patients with low expression (p= 0.02) (Supplementary Fig. S2B). We also performed univariate and multivariate survival analysis using Cox regression. In univariate analysis, the PCDH7 high group associated with poor recurrence-free survival. In multivariate analysis, both the PCDH7 intermediate group and the PCDH7 high group associated with poor recurrence-free survival when tumor size and final mountain stage were also considered (Supplementary Table S4). Taken together, high expression of PCDH7 in NSCLC tumors is associated with poor clinical outcome, consistent with mRNA studies.
PCDH7 promotes transformation of HBECs and synergizes with mutant KRAS to promote MAPK pathway activation and tumorigenesis in vivo
Given the data demonstrating significant correlations between high PCDH7 expression and poor clinical outcomes in NSCLC, we next examined whether PCDH7 has oncogenic activity in this tumor type. Transformation of CDK4/TERT-immortalized HBECs is assessed by the ability to efficiently form large colonies in soft agar or tumors after transplantation into immunocompromised mice (24). These cells progress to full malignancy upon stable inhibition of TP53 and simultaneous expression of KRASG12V or c-MYC (23,25). To determine whether PCDH7 can substitute for these oncogenes in HBEC transformation, HBEC cells with short hairpin RNA-mediated repression of p53 (HBEC-shp53) were infected with lentiviruses expressing each of the four full-length PCDH7 isoforms, which are produced by alternative splicing (Fig. 2A). Each of these isoforms shares the same extracellular domain, trans-membrane domain and juxtamembrane cytoplasmic region. Expression of each isoform resulted in robust anchorage-independent growth of HBEC-shp53 cells (Fig. 2B-C). In contrast, PCDH10, another δPCDH family member, did not promote transformation in these assays (Supplementary Fig. S3A).
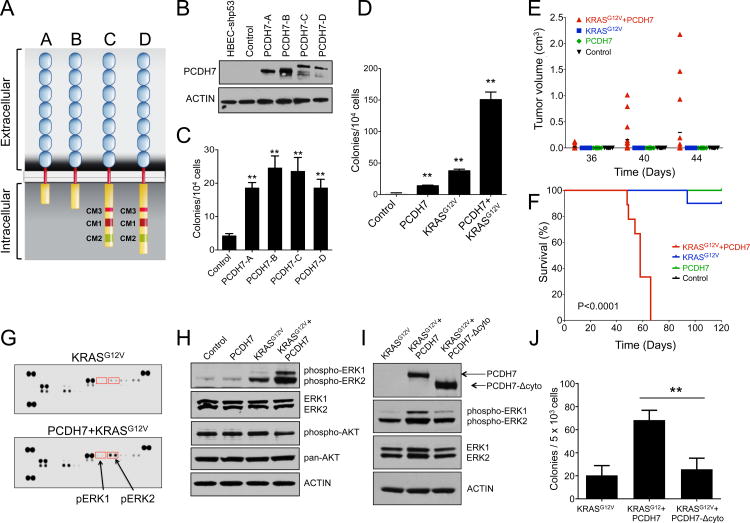
Figure 2. PCDH7 promotes transformation of HBECs and synergizes with mutant KRAS to promote MAPK pathway activation and tumorigenesis.
A, Four isoforms of PCDH7 generated by alternative splicing. B, Western blot demonstrating overexpression of four isoforms of PCDH7 in HBEC-shp53 cells. For this and all subsequent western blots, Beta-ACTIN was used as a loading control. C, Enforced expression of PCDH7 isoforms increased colony formation of HBEC-shp53 cells in soft agar. Error bars indicate standard deviation for this and all subsequent data. D, Quantification of soft agar assays demonstrating that PCDH7 (isoform A) significantly increased colony formation of HBEC-shp53 and HBEC-shp53-KRASG12V cells in soft agar. E, Quantification of tumor volume in NSG mice injected with control or HBEC-shp53 cells expressing PCDH7, KRASG12V, or both. F, Survival analysis of mice transplanted subcutaneously with HBEC-shp53 cells expressing PCDH7, KRASG12V, or both. (n=9 mice/group). P-value<0.0001, Mantel-Cox test. G, Phospho-MAPK antibody array demonstrating that PCDH7 enhanced ERK activation in HBEC-shp53-KRASG12V cells. H, Western blot demonstrating increased levels of phospho-ERK1/2 in HBEC-shp53-KRASG12V-PCDH7 cells. I, Western blot analysis showing that overexpression of V5-tagged PCDH7 lacking the intracellular domain (Δcyto) results in reduced phospho-ERK as compared to full length PCDH7. J, Quantification of soft agar assays demonstrating that the cytoplasmic domain of PCDH7 is required to promote transformation of HBEC-shp53-KRASG12V cells (**p<0.01, one way ANOVA test).
Analysis of RNA sequencing (RNAseq) data from 138 human lung cancer cell lines from the Cancer Cell Line Encyclopedia (CCLE) revealed that PCDH7 isoform A is expressed at higher levels compared to other isoforms (Supplementary Fig. S3B) (26). Indeed, western blotting confirmed that a ∼135kD species consistent with the size of isoform A is the dominantly expressed PCDH7 isoform in NSCLC cell lines (Supplementary Fig. S3C). Therefore, we utilized PCDH7 isoform A for the majority of subsequent experiments.
We next tested whether PCDH7 could potentiate transformation by mutant KRAS, the most commonly mutated gene in NSCLC (2). These experiments were further motivated by our observation that PCDH7 overexpression frequently occurs in the setting of KRAS mutations in NSCLC cell lines (Supplementary Table S5). PCDH7 was overexpressed in HBEC-shp53-KRASG12V cells and anchorage independent growth in vitro and tumorigenesis in vivo was evaluated. HBEC-shp53-KRASG12V-PCDH7 cells formed significantly more colonies than HBEC-shp53-KRASG12V cells in soft agar, suggesting functional cooperation between PCDH7 and KRASG12V (Fig. 2D). Furthermore, HBEC-shp53-KRASG12V-PCDH7 cells rapidly formed tumors within 3-6 weeks in immunocompromised NOD/SCID IL2Rγnull (NSG) mice, resulting in fully penetrant lethality (Fig. 2E-F). In contrast, HBEC-shp53-KRASG12V cells formed tumors in only 30% of mice and only minimally affected survival at the four-month experimental endpoint (Fig. 2F). Neither HBEC-shp53 nor HBEC-shp53-PCDH7 cells formed tumors. These data demonstrate that PCDH7 exhibits oncogenic activity in lung epithelial cells and suggest that this protein may stimulate KRAS-activated signaling pathways.
To begin to investigate the mechanisms through which PCDH7 promotes transformation and tumorigenesis, we used RNA-seq to assess global gene expression changes in response to enforced PCDH7 expression in HBEC-shp53 cells (Supplementary Table S6). Gene Set Enrichment Analysis (GSEA) identified several upregulated gene sets associated with cell proliferation, including the G1-S cell cycle phase, DNA replication, E2F3 Oncogenic Signature, and pRb Pathways (Supplementary Fig. S3D-E). We validated the upregulation of a set of these transcripts using quantitative real-time PCR (Supplementary Fig. S3F). Consistent with these gene expression changes, HBEC-shp53-PCDH7 cells grew significantly faster than control cells (Supplementary Fig. S3G).
To determine how PCDH7 triggers enhanced proliferation and tumorigenesis, we interrogated a set of core signaling pathways using phospho-antibody arrays. This revealed significantly enhanced activation of the MAPK-ERK1/2 pathway in HBEC-shp53-KRASG12V-PCDH7 cells as compared to HBEC-shp53-KRASG12V cells (Fig. 2G-H, Supplementary Figure S4). Phosphorylation of AKT was not effected by either KRASG12V or PCDH7 in this cell line (Fig. 2H). These data demonstrate that enforced expression of PCDH7 potentiates the activation of MAPK signaling downstream of oncogenic KRAS.
To establish whether the intracellular domain of PCDH7 is required for PCDH7-mediated induction of MAPK signaling and transformation, we generated a mutant V5-tagged PCDH7 in which the cytoplasmic domain was deleted (PCDH7-Δcyto). Enforced expression of PCDH7-Δcyto did not stimulate ERK activation nor anchorage-independent growth in HBEC-shp53-KRASG12V cells (Fig. 2I-J). Thus, the cytoplasmic domain of PCDH7 is necessary to induce ERK hyperactivation and transformation of KRAS-mutant cells.
PCDH7 synergizes with mutant EGFR to enhance MAPK pathway activation and tumor growth of NSCLCs
EGFR mutations occur in ∼15% of NSCLC patients (6). Because we found that NSCLC cell lines expressing high levels of PCDH7 also frequently harbor EGFR alterations (Supplementary Table S5), we sought to determine whether enforced expression of PCDH7 cooperates with mutant EGFR to activate MAPK signaling and promote lung tumorigenesis in vivo. We infected HBEC-shp53 cells with a lentivirus expressing mutant EGFRL858R. As expected, expression of EGFRL858R alone enhanced activation of EGFR and ERK (Fig. 3A). Expression of PCDH7 in HBEC-shp53-EGFRL858R cells dramatically enhanced ERK activation and further stimulated colony formation (Fig. 3A-B). We injected HBEC-shp53-EGFRL858R and HBEC-shp53-EGFRL858R-PCDH7 cells into NSG mice and analyzed animals for tumor growth over time. Neither group of animals formed tumors within 4 months after injection (data not shown), suggesting that additional hits must be required for EGFRL858R and PCDH7 to promote tumor growth in the HBEC system.
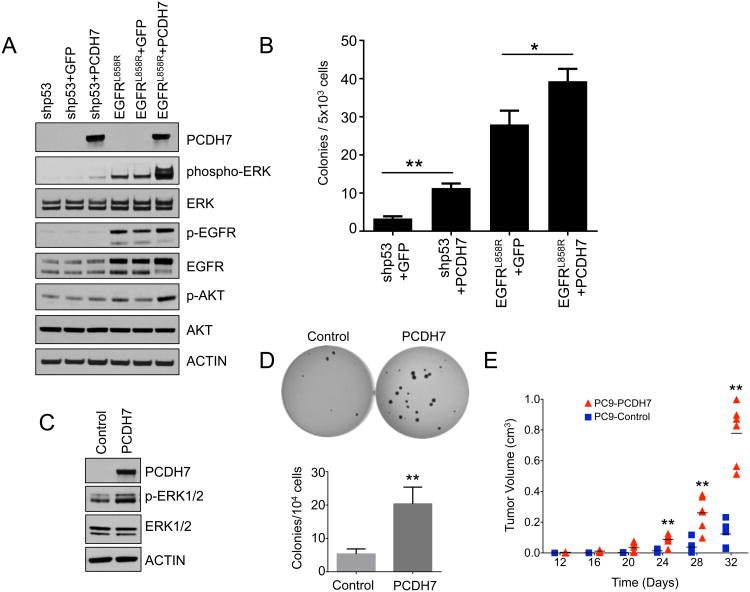
Figure 3. PCDH7 and mutant EGFR cooperate to promote MAPK activation and tumor growth of NSCLC cells.
A, Western blot analysis demonstrating strong ERK activation in HBEC-shp53-EGFRL858R cells overexpressing PCDH7. B, Quantification of soft agar assays demonstrating enhanced transformation of HBEC-shp53 cells and HBEC-shp53-EGFRRRL858R cells with PCDH7 expression. C, Western blot demonstrating elevated ERK activation in PC9 cells overexpressing PCDH7. D, Soft agar assays and quantification in EGFR mutant PC9 cells showing that enforced expression of PCDH7 increased colony formation. E, Quantification of tumor volume in NSG mice injected with PC9 cells with and without enforced PCDH7 expression (**p<0.01 vs. control, Student's t-test).
We next examined the effects of PCDH7 overexpression in NSCLC cells that harbor an EGFR mutation in the endogenous gene. Expression of PCDH7 augmented ERK signaling (Fig. 3C), enhanced colony formation in soft agar (Fig. 3D), and accelerated tumor growth after transplantation into NSG mice (Fig. 3E, Supplementary Fig. S5). These data demonstrate that PCDH7 cooperates with oncogenic EGFR to activate ERK signaling and promote tumorgenesis of lung cancer cells in vivo.
PCDH7 loss of function suppresses tumor growth and sensitizes NSCLC cells to MAPK inhibitors
To establish whether inhibition of PCDH7 impairs tumorigenesis, we next utilized the CRISPR/Cas9 nuclease system to inactivate PCDH7 in KRAS mutant NSCLC cells. H1944 cells were infected with a lentivirus expressing the Cas9 nuclease and a control single-guide RNA (sgRNA) or one of five sgRNAs directed against PCDH7. Western blotting demonstrated a near complete loss of PCDH7 with multiple sgRNAs (Fig. 4A). Depletion of PCDH7 in H1944 cells reduced colony formation in vitro and tumorigenesis in vivo (Fig. 4B-4F). Tumor lysates were utilized to assess the effects on downstream signaling using a phospho-MAPK antibody array. This confirmed a significant reduction in ERK phosphorylation in PCDH7 knockout tumors (Fig. 4G).
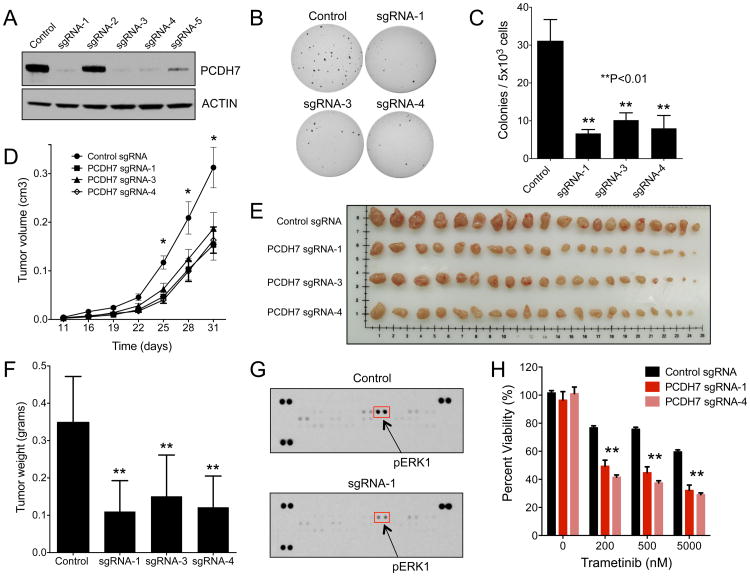
Figure 4. Genetic inactivation of PCDH7 suppresses tumor growth and sensitizes NSCLC cells to MAPK inhibitors.
A, Western blot analysis of PCDH7 in H1944 KRAS mutant NSCLC cells infected with a lentivirus expressing Cas9 and a control sgRNA or sgRNAs targeting PCDH7. B-C, Soft agar colony formation of PCDH7 knockout H1944 cells (B) and associated quantification (C) (**p<0.01 vs. control, one-way ANOVA test). D, Quantification of tumor volume over time (*p<0.05 vs. control, Student's t-test). E, Tumors from NSG mice injected with H1944 cells with control or PCDH7 sgRNA lentiviruses. F, Quantification of tumor weights shown in (E), (**p<0.01 vs. control, Student's t-test). G, Phospho-MAPK antibody array demonstrating that PCDH7 knockout tumors exhibit reduced ERK activation. H, Cell viability assay demonstrating that PCDH7 knockout in KRAS mutant cells enhanced sensitivity to the MEK inhibitor Trametinib (*p<0.05, **p<0.01 vs. control, Student's t-test).
The observation that PCDH7 activates the MAPK pathway in cooperation with mutant KRAS suggested that PCDH7 may modulate the responsiveness of KRAS-mutant NSCLC cells to clinically approved MAPK inhibitors. In support of this concept, PCDH7 was recently identified in a genome-wide CRISPR/Cas9 activation screen for genes that mediate resistance to BRAF inhibitors (27). To translate these findings to lung cancer, we assessed whether loss of PCDH7 sensitized KRAS mutant NSCLC cells to MAPK inhibitors. Depletion of PCDH7 from H1944 and H441 cells, which normally express high PCDH7 levels (Supplementary Fig. S3C), increased sensitivity to Trametinib, a FDA approved MEK inhibitor, and GDC-0944, an ERK inhibitor (Fig. 4H, Supplementary Fig. S6). Taken together, these data demonstrate that inhibition of PCDH7 impairs tumorigenesis and sensitizes KRAS mutant NSCLC cells to FDA approved MAPK inhibitors, raising the intriguing possibility that targeting PCDH7 in combination with MAPK pathway inhibitors may provide a novel therapeutic strategy in NSCLC.
PCDH7 interacts with SET and PP2A and suppresses PP2A activity in NSCLC cells
Previous studies demonstrated an interaction between the cytoplasmic domain of NFPC, the Xenopus PCDH7 ortholog, and SET/TAF1 (28). Initially identified as gene fusion with CAN in acute undifferentiated leukemia (29), SET is a known oncogene, which encodes an epigenetic regulator of transcription (21,30). Recent studies have demonstrated that SET overexpression is common in lung cancer tissues (31), and in other tumor types (32,33). Importantly, SET is also a potent physiologic inhibitor of PP2A (21,22), a major serine/threonine phosphatase that is known to directly de-phosphorylate and inhibit ERK1/2 signaling (34). Two isoforms of the structural subunit, PP2A-Aα and PP2A-Aβ, are mutated in a variety of human malignancies, including lung cancer (35). Since SET is known to inhibit PP2A and both have been implicated in lung cancer, we reasoned that PCDH7 might facilitate repression of PP2A by mediating inhibitory interactions with SET, thereby potentiating MAPK-ERK signaling.
Co-immunoprecipitation assays were used to determine whether PCDH7 interacts with SET in human cells. Indeed, V5-tagged PCDH7 robustly co-purifies with FLAG-tagged SETβ in HBEC-shp53 cells (Fig. 5A). The shortest PCDH7 isoform (A) and the longer isoforms (C and D) all interact with SET (Supplementary Figure S7). We also confirmed that SET localizes to both the nucleus and cytoplasm in HBECs and in NSCLC cells using cell fractionation and western blotting (Supplementary Fig. S8A-B).
Figure 5. PCDH7 represses PP2A by facilitating inhibitory interactions with SET.
A, Western blot analysis of FLAG-tagged SET immunoprecipitates in HBEC-shp53 cells. B, Reciprocal co-immunoprecipitation of endogenous PCDH7 and SET in KRAS mutant H1944 cells. C, Reciprocal co-immunoprecipitation of endogenous PCDH7 and PP2A-A or PP2A-C in KRAS mutant H1944 cells. D, Western blot analysis of FLAG-tagged SET immunoprecipitates in HBEC-shp53-KRASG12V and HBEC-shp53-KRASG12V-PCDH7 cells. E, Quantification of PP2A activity in HBEC-shp53-KRASG12V cells with overexpression of PCDH7 and knockout of SET. F, Western blot showing reduced phospho-ERK1/2 in clonal SET knockout HBEC-shp53-KRASG12V-PCDH7 cells and rescued phospho-ERK1/2 levels upon expression of a mutant SET-β cDNA that is resistant to Cas9 cleavage (Lenti-SET-CR). G, Quantification of soft agar assays demonstrating that SET is required for PCDH7-induced transformation of HBEC-shp53-KRASG12V cells. H, Western blot demonstrating that FTY720 treatment reduced ERK activation in HBEC-shp53-KRASG12V-PCDH7 cells. I, Western blot analysis demonstrating a dose-dependent effect of FTY720 on phospho-ERK in HBEC-shp53-KRASG12V-PCDH7 cells. J, Western blot showing increased ERK activation in HBEC-shp53-KRASG12V-PCDH7 cells treated with Okadaic Acid for 75 min. A longer exposure is shown to highlight the baseline level of phospho-ERK1/2 in untreated cells.
PP2A consists of a holoenzyme that contains a core dimer composed of the catalytic subunit (PP2A-C) and a scaffold protein termed A subunit (PP2A-A). PP2A-A mediates interaction of the core dimer with a wide variety of regulatory subunits (PP2A-B) that dictate specificity of PP2A (36). SET was previously reported to bind the catalytic subunit PP2A-C and inhibit its activity (37). We detected an interaction between SET and PP2A-A in HBEC-shp53 cells (Fig. 5A). Although the abundance of PP2A subunits may vary in different tissues, our findings are consistent with another study demonstrating that SET binds PP2A-A in murine neurons (38).
We next extended our findings to lung cancer cells by examining PCDH7-SET-PP2A interactions in KRAS mutant H1944 cells, which express high levels of the endogenous proteins. Reciprocal co-IP experiments demonstrated that PCDH7 indeed binds to SET (Fig. 5B). We also found that PCDH7 interacts with PP2A-A and PP2A-C (Fig. 5C), suggesting that PCDH7 may facilitate SET:PP2A interactions and thereby inhibit PP2A activity. Given that SET can potently inhibit PP2A, it is plausible that modest changes in the SET pool may affect SET-PP2A binding and PP2A activity.
To further investigate the role of PCDH7 in promoting SET:PP2A complex formation, we introduced FLAG-tagged SETβ into HBEC-shp53-KRASG12V or HBEC-shp53-KRASG12V-PCDH7 cells, followed by co-immunoprecipitation using an anti-FLAG antibody. As expected, SET bound to both PCDH7 and PP2A-A in both cell lines, but the PP2A-A interaction was enhanced by the presence of PCDH7 (Fig. 5D). Furthermore, we observed a significant reduction of PP2A activity in HBEC-shp53-KRASG12V-PCDH7 cells as compared to HBEC-shp53-KRASG12V cells (Fig. 5E). Taken together, these studies suggest that when PCDH7 is overexpressed in lung cancer cells, the cytoplasmic domain serves as a scaffold that binds to SET and PP2A, thereby forming an inhibitory complex to facilitate activation of the MAPK signaling pathway.
SET is required for PCDH7-induced suppression of PP2A and ERK activation
To assess the role of SET in PCDH7-induced ERK activation, we used CRISPR/Cas9 to generate SET knockout HBEC-shp53-KRASG12V-PCDH7 cells. There are two splicing variants of SET, SET-α and SET-β, and a common region in both isoforms was targeted. Nine independent SET knockout (SET-KO) cell lines were established by single cell cloning and ERK activation was markedly reduced in all lines (Fig. 5F, Supplementary Fig. S8C). These effects were specifically due to SET loss of function since restoring SET expression with a SETβ cDNA with silent mutations in the sgRNA targeting site rescued ERK phosphorylation (Fig. 5F). Importantly, the previously documented inhibition of PP2A by PCDH7 in HBEC-shp53-KRASG12V cells was reversed by SET knockout (Fig. 5E), demonstrating an essential role for SET downstream of PCDH7 in regulating PP2A activity. Furthermore, anchorage-independent growth of HBEC-shp53-KRASG12V-PCDH7 cells was greatly impaired by SET knockout (Fig. 5G).
Restoration of PP2A activity through silencing of SET, or pharmacologic activation of PP2A by the SET antagonist FTY720 inhibits proliferation of NSCLC cells in vitro and tumorigeneisis in vivo (31,39). FTY720 has also been shown to similarly suppress growth of hematopoietic cancer cells (37). We confirmed that FTY720 treatment inhibited the growth of NSCLC cells (Supplementary Fig. S9). We reasoned that FTY720 treatment might also inhibit PCDH7-induced ERK activation. Accordingly, treatment of HBEC-shp53-KRASG12V-PCDH7 cells with FTY720 reduced ERK phosphorylation in a dose-dependent manner (Fig. 5H-I). Conversely, treatment of HBEC-shp53-KRASG12V-PCDH7 cells with the PP2A inhibitor Okadaic acid further enhanced ERK phosphorylation (Fig. 5J). Importantly, these experiments were performed using concentrations of Okadaic acid that inhibit PP2A but not other phosphatases such as PP1. Taken together, these results provide evidence that SET is required for PCDH7-mediated PP2A inhibition, ERK activation, and transformation of NSCLC cells.
Discussion
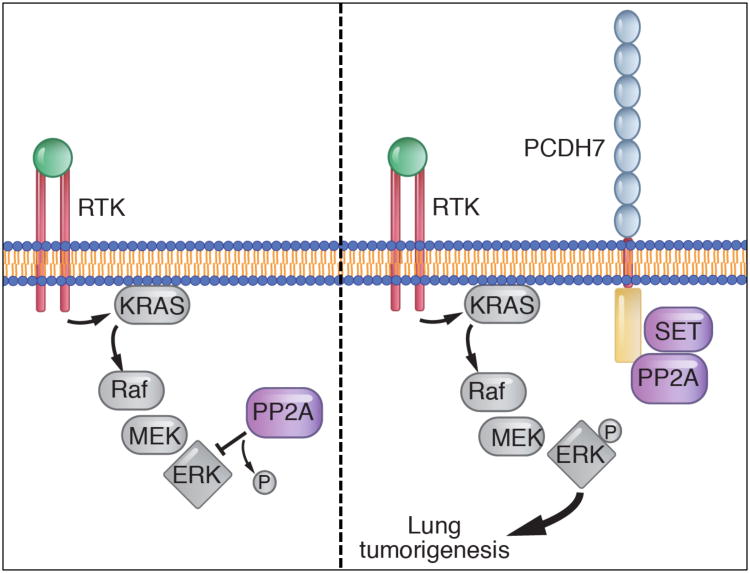
Targeting cell-surface proteins with therapeutic antibodies has proven to be an effective anti-cancer therapeutic strategy (40,41). To extend this approach to lung cancer, we set out to identify new membrane proteins that promote oncogenic signaling in NSCLC cells. These studies led to the identification of PCDH7 as a cell-surface receptor that is highly expressed in NSCLC patients and is strongly associated with poor clinical outcome. PCDH7 induces cellular transformation and promotes tumor growth in vitro and in vivo, while depletion of PCDH7 reduces the tumor growth of NSCLC cells in mice, supporting an oncogenic function for PCDH7 in lung tumorigenesis. Our mechanistic studies support a model whereby PCDH7 interacts with SET and PP2A, facilitating PP2A inhibition and thereby potentiating MAPK-ERK signaling in cooperation with common lung cancer driver mutations (Fig. 6). These results raise the possibility that PCDH7 represents a new therapeutic target in NSCLC. Moreover, these findings introduce the concept that the intracellular domain of PCDH proteins can function as scaffolds that facilitate protein complex assembly to activate key signal transduction pathways.
Figure 6. Model of PCDH7-mediated activation of MAPK signaling in lung tumorigenesis.
When overexpressed in lung cancer, PCDH7 interacts with SET and PP2A and facilitates inhibitory PP2A-SET interaction, thereby potentiating MAPK-ERK signaling in cooperation with oncogenic drivers such as KRAS or EGFR (depicted as a receptor tyrosine kinase, RTK).
A previous study demonstrated that SET interacts with the cytoplasmic domain of NFPC, the Xenopus ortholog of PCDH7 (28). The intracellular domains of NFPC and human PCDH7 exhibit 98% identity and we confirmed that PCDH7 interacts with SET in HBECs and in lung cancer cells. Although PCDH7-A is the predominant isoform that is frequently overexpressed in human lung cancers, all four isoforms of PCDH7 are sufficient to transform HBEC-shp53 cells and activate MAPK signaling, and may therefore be important for lung cancer pathogenesis. Notably, SET binding was mapped to the juxtamembrane region of Xenopus PCDH7, within the first 80 amino acids of the cytoplasmic domain (28), which is shared by all four isoforms. Taken together, our data suggest that the membrane proximal region of PCDH7 binds to SET and plays an important role in PCDH7-induced transformation and tumorigenesis. Interestingly, PCDH7-SET interactions are believed to be important for central nervous system development (28,38,42,43). Thus, this physiologic interaction, which normally functions in a developmental context, is co-opted by cancer cells to enhance oncogenic signaling. Given that PCDH7 overexpression has been detected in several tumor types (44,45), our findings may extend beyond lung cancer.
SET, also known as TAF1, is a known oncogene and was first identified as a fusion gene with CAN in acute undifferentiated leukemia (29). SET encodes an epigenetic regulator of transcription and a potent inhibitor of PP2A (21,22,30). Overexpression of SET has been documented in multiple tumor types, including NSCLC, and the pro-oncogenic role of SET is associated with PP2A inhibition (31,37,46). A clinically-approved SET inhibitor, FTY720, was recently demonstrated to directly bind SET and disrupt the interaction between SET and PP2A, thereby resulting in re-activation of PP2A, de-phosphorylation of ERK, and inhibition of NSCLC tumor growth in xenograft assays (39). Our work uncovered interactions between PCDH7 and PP2A subunits in human NSCLC cells (Fig. 5C) and demonstrated that PCDH7 strengthened the interaction between SET and PP2A, likely contributing to PP2A suppression (Fig. 5D). These results suggest that overexpression of PCDH7 may represent a useful biomarker for selection of patients who would benefit from treatment with FTY720. Additionally, since depletion of PCDH7 increased the sensitivity of KRAS mutant lung adenocarcinoma cells to MEK and ERK inhibitors, our data suggest that therapies that target PCDH7 or SET may have a synergistic effect with EGFR or MAPK inhibitors to inhibit the growth of NSCLC cells.
PP2A is a well-characterized tumor suppressor, and its loss of function results in aberrant phosphorylation of substrates in a variety of pathways that are linked to neoplastic transformation, such as the MAPK, pRB, AKT, and JAK/STAT pathways (47,48). Indeed, mutations in PPP2R1A and PPP2R1B, which encode PP2A regulatory subunits, are common in lung cancer (35). Two targets of PP2A, ERK1 and ERK2, are key intermediates of the MAPK pathway that are activated upon phosphorylation and subsequently translocate to the nucleus where they induce gene expression to promote proliferation and inhibit apoptosis. While the critical role of MAPK signaling in lung cancer points to a central role for this pathway in PCDH7-mediated tumorigenesis, additional studies are warranted to examine the contribution of other PP2A targets.
The mechanism by which PCDH7 is upregulated in lung cancer is presently unknown. Previously, a genome-wide ChIP-PET study identified a p53 binding site within the PCDH7 gene, and demonstrated that PCDH7 expression is suppressed by p53 activation in colon cancer cells (49). Additionally, PCDH7 was one of 185 upregulated genes in EGFR-mutant NSCLC cells (50). Future studies are required to better understand the mechanisms by which PCDH7 is upregulated in lung cancer cells. This work will enable new therapeutic strategies based on targeting PCDH7, its upstream regulators, or its downstream effectors in NSCLC.
Supplementary Material
Acknowledgments
We thank Suzie Hight for sharing cell lines, Feng Zhang, David Root, and Eric Campeau for plasmids, and Joshua Mendell, and members of the O'Donnell laboratory for critical reading of the manuscript. We also thank the McDermott Center Next Generation Sequencing Core, and Jose Cabrera for assistance with figures. K.A.O. is a CPRIT Scholar in Cancer Research and a Kimmel Scholar. RNAseq data will be deposited to the National Center for Biotechnology Information (NCBI) GEO repository, http://www.ncbi.nlm.nih.gov/geo/ (Accession #).
Financial Support: K.A.O'Donnell was supported by The Cancer Prevention Research Institute of Texas (CPRIT, R1101), The Welch Foundation (I-1881), The Sidney Kimmel Foundation (SKF-15-067), the LUNGevity Foundation, and a SPORE in Lung Cancer CDA (P50CA70907-17). B.L.Updegraff was supported by CPRIT (RP140110) and NIH Award (T32GM008203). J.D.Minna was supported by CPRIT (RP110708), a SPORE in Lung Cancer (P50CA70907), and NIH Award (U01 CA176284). X.Zhou was supported by the Lung Cancer Research Foundation (LCRF 2015) and a National Natural Science Foundation of China grant (NSFC: 81571527).
Footnotes
Conflicts of Interest: None
References
- 1.Swanton C, Govindan R. Clinical Implications of Genomic Discoveries in Lung Cancer. N Engl J Med. 2016;374:1864–73. doi: 10.1056/NEJMra1504688. [DOI] [PubMed] [Google Scholar]
- 2.Cancer Genome Atlas Research N. Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511:543–50. doi: 10.1038/nature13385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu Z, Golay HG, Barbie DA. Targeting pathways downstream of KRAS in lung adenocarcinoma. Pharmacogenomics. 2014;15:1507–18. doi: 10.2217/pgs.14.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niederst MJ, Engelman JA. Bypass mechanisms of resistance to receptor tyrosine kinase inhibition in lung cancer. Science Signaling. 2013;6:re6. doi: 10.1126/scisignal.2004652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 6.Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3:75ra26. doi: 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Govindan R, Ding L, Griffith M, Subramanian J, Dees ND, Kanchi KL, et al. Genomic landscape of non-small cell lung cancer in smokers and never-smokers. Cell. 2012;150:1121–34. doi: 10.1016/j.cell.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imielinski M, Berger AH, Hammerman PS, Hernandez B, Pugh TJ, Hodis E, et al. Mapping the hallmarks of lung adenocarcinoma with massively parallel sequencing. Cell. 2012;150:1107–20. doi: 10.1016/j.cell.2012.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kahr I, Vandepoele K, van Roy F. Delta-protocadherins in health and disease. Progress in molecular biology and translational science. 2013;116:169–92. doi: 10.1016/B978-0-12-394311-8.00008-X. [DOI] [PubMed] [Google Scholar]
- 10.Pan D. The hippo signaling pathway in development and cancer. Developmental Cell. 2010;19:491–505. doi: 10.1016/j.devcel.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bos PD, Zhang XH, Nadal C, Shu W, Gomis RR, Nguyen DX, et al. Genes that mediate breast cancer metastasis to the brain. Nature. 2009;459:1005–9. doi: 10.1038/nature08021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li AM, Tian AX, Zhang RX, Ge J, Sun X, Cao XC. Protocadherin-7 induces bone metastasis of breast cancer. Biochem Biophys Res Commun. 2013;436:486–90. doi: 10.1016/j.bbrc.2013.05.131. [DOI] [PubMed] [Google Scholar]
- 13.van Roy F. Beyond E-cadherin: roles of other cadherin superfamily members in cancer. Nat Rev Cancer. 2014;14:121–34. doi: 10.1038/nrc3647. [DOI] [PubMed] [Google Scholar]
- 14.Yu JS, Koujak S, Nagase S, Li CM, Su T, Wang X, et al. PCDH8, the human homolog of PAPC, is a candidate tumor suppressor of breast cancer. Oncogene. 2008;27:4657–65. doi: 10.1038/onc.2008.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu B, Yang H, Zhang C, Wu Q, Shao Y, Zhang J, et al. High-resolution melting analysis of PCDH10 methylation levels in gastric, colorectal and pancreatic cancers. Neoplasma. 2010;57:247–52. doi: 10.4149/neo_2010_03_247. [DOI] [PubMed] [Google Scholar]
- 16.Vincent A, Omura N, Hong SM, Jaffe A, Eshleman J, Goggins M. Genome-wide analysis of promoter methylation associated with gene expression profile in pancreatic adenocarcinoma. Clin Cancer Res. 2011;17:4341–54. doi: 10.1158/1078-0432.CCR-10-3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang X, Chen MW, Terry S, Vacherot F, Chopin DK, Bemis DL, et al. A human-and male-specific protocadherin that acts through the wnt signaling pathway to induce neuroendocrine transdifferentiation of prostate cancer cells. Cancer Res. 2005;65:5263–71. doi: 10.1158/0008-5472.CAN-05-0162. [DOI] [PubMed] [Google Scholar]
- 18.Valiente M, Obenauf AC, Jin X, Chen Q, Zhang XH, Lee DJ, et al. Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell. 2014;156:1002–16. doi: 10.1016/j.cell.2014.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Q, Boire A, Jin X, Valiente M, Er EE, Lopez-Soto A, et al. Carcinoma-astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nature. 2016;533:493–8. doi: 10.1038/nature18268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang YT, Heist RS, Chirieac LR, Lin X, Skaug V, Zienolddiny S, et al. Genome-wide analysis of survival in early-stage non-small-cell lung cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27:2660–7. doi: 10.1200/JCO.2008.18.7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li M, Makkinje A, Damuni Z. The myeloid leukemia-associated protein SET is a potent inhibitor of protein phosphatase 2A. J Biol Chem. 1996;271:11059–62. doi: 10.1074/jbc.271.19.11059. [DOI] [PubMed] [Google Scholar]
- 22.Neviani P, Santhanam R, Trotta R, Notari M, Blaser BW, Liu S, et al. The tumor suppressor PP2A is functionally inactivated in blast crisis CML through the inhibitory activity of the BCR/ABL-regulated SET protein. Cancer Cell. 2005;8:355–68. doi: 10.1016/j.ccr.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 23.Ramirez RD, Sheridan S, Girard L, Sato M, Kim Y, Pollack J, et al. Immortalization of human bronchial epithelial cells in the absence of viral oncoproteins. Cancer Res. 2004;64:9027–34. doi: 10.1158/0008-5472.CAN-04-3703. [DOI] [PubMed] [Google Scholar]
- 24.Sato M, Vaughan MB, Girard L, Peyton M, Lee W, Shames DS, et al. Multiple oncogenic changes (K-RAS(V12), p53 knockdown, mutant EGFRs, p16 bypass, telomerase) are not sufficient to confer a full malignant phenotype on human bronchial epithelial cells. Cancer Res. 2006;66:2116–28. doi: 10.1158/0008-5472.CAN-05-2521. [DOI] [PubMed] [Google Scholar]
- 25.Sato M, Larsen JE, Lee W, Sun H, Shames DS, Dalvi MP, et al. Human lung epithelial cells progressed to malignancy through specific oncogenic manipulations. Mol Cancer Res. 2013;11:638–50. doi: 10.1158/1541-7786.MCR-12-0634-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barretina J, Caponigro G, Stransky N, Venkatesan K, Margolin AA, Kim S, et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483:603–7. doi: 10.1038/nature11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Konermann S, Brigham MD, Trevino AE, Joung J, Abudayyeh OO, Barcena C, et al. Genome-scale transcriptional activation by an engineered CRISPR-Cas9 complex. Nature. 2015;517:583–8. doi: 10.1038/nature14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heggem MA, Bradley RS. The cytoplasmic domain of Xenopus NF-protocadherin interacts with TAF1/set. Developmental Cell. 2003;4:419–29. doi: 10.1016/s1534-5807(03)00036-4. [DOI] [PubMed] [Google Scholar]
- 29.Adachi Y, Pavlakis GN, Copeland TD. Identification and characterization of SET, a nuclear phosphoprotein encoded by the translocation break point in acute undifferentiated leukemia. J Biol Chem. 1994;269:2258–62. [PubMed] [Google Scholar]
- 30.Seo SB, McNamara P, Heo S, Turner A, Lane WS, Chakravarti D. Regulation of histone acetylation and transcription by INHAT, a human cellular complex containing the set oncoprotein. Cell. 2001;104:119–30. doi: 10.1016/s0092-8674(01)00196-9. [DOI] [PubMed] [Google Scholar]
- 31.Liu H, Gu Y, Wang H, Yin J, Zheng G, Zhang Z, et al. Overexpression of PP2A inhibitor SET oncoprotein is associated with tumor progression and poor prognosis in human non-small cell lung cancer. Oncotarget. 2015;6:14913–25. doi: 10.18632/oncotarget.3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Christensen DJ, Chen Y, Oddo J, Matta KM, Neil J, Davis ED, et al. SET oncoprotein overexpression in B-cell chronic lymphocytic leukemia and non-Hodgkin lymphoma: a predictor of aggressive disease and a new treatment target. Blood. 2011;118:4150–8. doi: 10.1182/blood-2011-04-351072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carlson SG, Eng E, Kim EG, Perlman EJ, Copeland TD, Ballermann BJ. Expression of SET, an inhibitor of protein phosphatase 2A, in renal development and Wilms' tumor. Journal of the American Society of Nephrology: JASN. 1998;9:1873–80. doi: 10.1681/ASN.V9101873. [DOI] [PubMed] [Google Scholar]
- 34.Adams DG, Coffee RL, Jr, Zhang H, Pelech S, Strack S, Wadzinski BE. Positive regulation of Raf1-MEK1/2-ERK1/2 signaling by protein serine/threonine phosphatase 2A holoenzymes. J Biol Chem. 2005;280:42644–54. doi: 10.1074/jbc.M502464200. [DOI] [PubMed] [Google Scholar]
- 35.Wang SS, Esplin ED, Li JL, Huang L, Gazdar A, Minna J, et al. Alterations of the PPP2R1B gene in human lung and colon cancer. Science. 1998;282:284–7. doi: 10.1126/science.282.5387.284. [DOI] [PubMed] [Google Scholar]
- 36.Perrotti D, Neviani P. Protein phosphatase 2A: a target for anticancer therapy. Lancet Oncol. 2013;14:e229–38. doi: 10.1016/S1470-2045(12)70558-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pippa R, Dominguez A, Christensen DJ, Moreno-Miralles I, Blanco-Prieto MJ, Vitek MP, et al. Effect of FTY720 on the SET-PP2A complex in acute myeloid leukemia; SET binding drugs have antagonistic activity. Leukemia. 2014;28:1915–8. doi: 10.1038/leu.2014.141. [DOI] [PubMed] [Google Scholar]
- 38.Trakhtenberg EF, Wang Y, Morkin MI, Fernandez SG, Mlacker GM, Shechter JM, et al. Regulating Set-beta's Subcellular Localization Toggles Its Function between Inhibiting and Promoting Axon Growth and Regeneration. J Neurosci. 2014;34:7361–74. doi: 10.1523/JNEUROSCI.3658-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saddoughi SA, Gencer S, Peterson YK, Ward KE, Mukhopadhyay A, Oaks J, et al. Sphingosine analogue drug FTY720 targets I2PP2A/SET and mediates lung tumour suppression via activation of PP2A-RIPK1-dependent necroptosis. EMBO Mol Med. 2013;5:105–21. doi: 10.1002/emmm.201201283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anagnostou VK, Brahmer JR. Cancer immunotherapy: a future paradigm shift in the treatment of non-small cell lung cancer. Clin Cancer Res. 2015;21:976–84. doi: 10.1158/1078-0432.CCR-14-1187. [DOI] [PubMed] [Google Scholar]
- 41.Pirker R. EGFR-directed monoclonal antibodies in non-small cell lung cancer: how to predict efficacy? Transl Lung Cancer Res. 2012;1:269–75. doi: 10.3978/j.issn.2218-6751.2012.10.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bradley RS, Espeseth A, Kintner C. NF-protocadherin, a novel member of the cadherin superfamily, is required for Xenopus ectodermal differentiation. Curr Biol. 1998;8:325–34. doi: 10.1016/s0960-9822(98)70132-0. [DOI] [PubMed] [Google Scholar]
- 43.Piper M, Dwivedy A, Leung L, Bradley RS, Holt CE. NF-protocadherin and TAF1 regulate retinal axon initiation and elongation in vivo. J Neurosci. 2008;28:100–5. doi: 10.1523/JNEUROSCI.4490-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jaeger J, Koczan D, Thiesen HJ, Ibrahim SM, Gross G, Spang R, et al. Gene expression signatures for tumor progression, tumor subtype, and tumor thickness in laser-microdissected melanoma tissues. Clin Cancer Res. 2007;13:806–15. doi: 10.1158/1078-0432.CCR-06-1820. [DOI] [PubMed] [Google Scholar]
- 45.Singh AP, Bafna S, Chaudhary K, Venkatraman G, Smith L, Eudy JD, et al. Genome-wide expression profiling reveals transcriptomic variation and perturbed gene networks in androgen-dependent and androgen-independent prostate cancer cells. Cancer letters. 2008;259:28–38. doi: 10.1016/j.canlet.2007.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Janghorban M, Farrell AS, Allen-Petersen BL, Pelz C, Daniel CJ, Oddo J, et al. Targeting c-MYC by antagonizing PP2A inhibitors in breast cancer. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:9157–62. doi: 10.1073/pnas.1317630111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kurimchak A, Grana X. PP2A: more than a reset switch to activate pRB proteins during the cell cycle and in response to signaling cues. Cell Cycle. 2015;14:18–30. doi: 10.4161/15384101.2014.985069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ross JA, Cheng H, Nagy ZS, Frost JA, Kirken RA. Protein phosphatase 2A regulates interleukin-2 receptor complex formation and JAK3/STAT5 activation. J Biol Chem. 2010;285:3582–91. doi: 10.1074/jbc.M109.053843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wei CL, Wu Q, Vega VB, Chiu KP, Ng P, Zhang T, et al. A global map of p53 transcription-factor binding sites in the human genome. Cell. 2006;124:207–19. doi: 10.1016/j.cell.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 50.Choi K, Creighton CJ, Stivers D, Fujimoto N, Kurie JM. Transcriptional profiling of non-small cell lung cancer cells with activating EGFR somatic mutations. PLoS One. 2007;2:e1226. doi: 10.1371/journal.pone.0001226. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.