Abstract
Purpose
To determine lifetime cost-effectiveness of diagnostic evaluation strategies for individuals with stable chest pain and suspected coronary artery disease (CAD).
Methods
Exercise treadmill testing (ETT), stress echocardiography (SE), myocardial perfusion scintigraphy (MPS), coronary computed tomographic angiography (CCTA), and invasive coronary angiography (ICA) were assessed alone, or in succession to each other.
Results
Initial ETT followed by imaging wherein ETT was equivocal or unable to be performed appeared more cost-effective than any strategy employing initial testing by imaging.
Conclusion
As pre-test likelihood of CAD varies, different modalities including SE, CCTA, and MPS result in improved costs and enhanced effectiveness.
Keywords: Cost Effectiveness, Computed Tomography, Stress Testing, Echocardiography, Myocardial Perfusion SPECT, Invasive Angiography
1. Introduction
Coronary artery disease (CAD) is the leading cause of morbidity and mortality(1). Current clinical practice and appropriateness guidelines recommend either exercise treadmill testing (ETT) or non-invasive cardiac imaging tests—such as stress echocardiography (SE), myocardial perfusion scintigraphy (MPS) and coronary computed tomographic angiography (CCTA)—to diagnose, prognosticate risk and impact therapeutic decision making for patients with an intermediate pre-test likelihood of stable CAD(2–6). Non-invasive cardiac testing with imaging has been favored by some as an initial test for symptomatic patients with at least intermediate pre-test likelihood of obstructive CAD, given its superior ability to diagnose CAD, reclassify CAD likelihood, predict CAD events, and guide subsequent treatment over testing without imaging(3, 7–9). Accordingly, rates of performance of noninvasive cardiac imaging tests have exploded, with growth in imaging outpacing that of other physicians services by more than a factor of two(10). At present, more than 10 million CAD imaging tests are being performed annually in the United States(11). Despite the high utilization and numerous options for noninvasive cardiac testing, uncertainty remains regarding the optimal testing strategies(12, 13). Multiple studies have investigated the value of ETT in comparison with non-invasive imaging modalities, however, a direct comparison of varying diagnostic strategies that employ non-invasive tests in isolation versus in succession to one another has to date not been assessed(9, 13). Further, the opportunity costs of testing strategies that begin with ETT as compared to that that begin with imaging have not been fully evaluated(13).
The aim of the present study was to determine the cost-effectiveness of the most widely available diagnostic evaluation strategies for individuals without known CAD presenting with stable chest pain syndrome.
2. Materials and Methods
We assessed the cost effectiveness of 12 different diagnostic strategies for stable chest pain patients without known CAD: 1) ETT followed by invasive coronary angiography (ICA) for equivocal or positive ETT (ETT-ICA); 2) ETT followed by SE for equivocal ETT and ICA for positive ETT (ETT-SE-ICA); 3) ETT followed by MPS for equivocal ETT and ICA for positive MPS (ETT-MPS-ICA); 4) ETT followed by CCTA for equivocal ETT and ICA for positive ETT (ETT-CCTA-ICA); 5) SE followed by ICA for equivocal or positive SE; 6) SE followed by CCTA for equivocal SE and ICA for positive SE (SE-CCTA-ICA); 7) MPS followed by ICA for equivocal or positive MPS (MPS-ICA); 8) MPS followed by CCTA for equivocal MPS or ICA for positive MPS (MPS-CCTA-ICA); 9) CCTA followed by ICA for equivocal or positive CCTA (CCTA-ICA); 10) CCTA followed by SE for equivocal CCTA or ICA for positive CCTA (CCTA-SE-ICA); 11) CCTA followed by MPS for equivocal CCTA or ICA for positive CCTA (CCTA-MPS-ICA); and 12) direct ICA.
2.1 Economic Model and Assumptions
We developed an economic model over a lifetime horizon in order to evaluate the costs and cost effectiveness of different diagnostic work-up strategies for stable chest pain patients without known CAD. Test sensitivity, specificity, rates of equivocal results, and disease prevalence were used to classify patients undergoing testing as true positive, false positive, true negative, false negative, or equivocal for obstructive CAD. All positive results were assumed to be referred to ICA, and ICA was assumed to have perfect sensitivity and specificity, notwithstanding that this may not be a flawless reflection of clinical practice. Depending on the strategy, patients with equivocal results were assumed to be referred to either additional downstream non-invasive testing or ICA.
For the post-diagnosis period, we employed a Markov model based on 1-year cycles to account for outcomes and costs of treatment for those correctly diagnosed with CAD, diagnosis of false negatives, and clinical events such as coronary revascularization, myocardial infarction and death. Costs were modeled from a payer perspective.
To compare degrees of abnormality of anatomic and functional measurements and their implications for subsequent treatment, we considered 4 categories relating to the extent and severity of abnormality by each method: none, mild, moderate and severe.
CAD was defined angiographically (for ICA and CCTA) as absent, mild, moderate or severe. Mild CAD was defined as non-obstructive coronary artery stenosis ranging from 1–69% in all affected vessels, not including the left main artery. Moderate CAD was defined as ≥70% stenosis in one or two major epicardial coronary artery vessels, not including the left main artery. Severe CAD was defined as ≥50% stenosis in the left main artery or ≥70% stenosis in three major epicardial coronary artery vessels. Following the diagnostic phase, patients experiencing post-test myocardial infarction were also considered to have severe CAD.
For functional cardiac imaging tests—including SE and MPS—the following classification schema was employed: for purposes of considering post-test management and costs, patients with no wall motion abnormalities or perfusion abnormalities were considered to have no CAD. Patients with mild, moderate, and severe SE and MPS test results were considered to have disease of equivalent severity to those defined angiographically.
For ETT, patients with no ST-segment changes were considered to have no CAD. Patients with ST-segment depression or elevation were considered to have obstructive CAD. Patients with positive ETT tests were considered to have moderate or severe CAD, which was confirmed at the time of ICA. For evaluation purposes, individuals were considered ineligible for ETT in the presence of baseline electrocardiogram (ECG) abnormalities, including pre-excitation; electronically paced ventricular rhythm; >1mm of resting ST segment depression or complete left bundle branch block; <1mm of basleine ST depression and taking digoxin; or ECG criteria for left ventricular hypertrophy with <1mm baseline ST depression. For individuals who could not exercise, ETT was considered not able to be performed.
We considered several possible diagnostic outcomes of non-invasive diagnostic test strategies. For ETT, we considered 3 possibilities, which included no exercise-induced ST-segment changes, exercise-induced ST-segment changes or equivocal ST-segment abnormalities, including up-sloping ST segment depression or rapid return to baseline of ST segment depression early during recovery. These findings were interpreted as no CAD, moderate or severe CAD, and equivocal results, respectively. For SE and MPS, we considered 5 possibilities, which included identification of 1) normal myocardial perfusion or wall motion, 2) mild perfusion or wall motion abnormalities, 3) moderate perfusion or wall motion abnormalities, 4) severe perfusion or wall motion abnormalities, and 5) equivocal testing due to inadequate images, low workload, or artifact. All perfusion or wall motion abnormalities that were non-equivocal were assumed to represent flow-limiting coronary artery stenosis.
For CCTA, we considered 6 possible diagnostic outcomes, which included identification of 1) absence of CAD, 2) mild CAD, 3) moderate CAD, 4) severe CAD; 5) equivocal testing due to artifact or due to presence of a 50–69% stenosis in any epicardial coronary artery vessel for which the functional significance was unclear.
For ICA, we considered 4 possibilities, which included identification of 1) no CAD, 2) mild CAD, 3) moderate CAD and 4) severe CAD. While gradations of CAD severity by ICA were identical to those defined for CCTA, ICA was considered the reference standard and thus, did not produce equivocal or indeterminate test results.
Given the substantial results of the COURAGE and SYNTAX trials, as well as changing practice patterns for treatment of stable CAD, we considered four post-testing treatment strategies: 1) No therapy for patients with absence of CAD; 2) Medical therapy for patients with mild CAD; 3) Percutaneous intervention (PCI) plus optimal medical therapy (OMT) for 50% and OMT alone for 50% of patients with moderate CAD, and 4) Coronary artery bypass surgery (CABG) plus OMT for 50% and PCI plus OMT for 50% patients with severe CAD(14, 15).
2.2 Patient Population
Base case values, sensitivity estimate ranges, costs and sources for our model variables are listed in Table 1. The base case model is a 55-year old man with stable chest pain syndrome and no prior history of CAD with a 20% likelihood of obstructive CAD. Obstructive CAD was defined as a luminal stenosis severity of ≥50% in the left main artery or ≥70% in any other major epicardial artery.
Table 1.
Costs, Effectiveness and Incremental Cost Effectiveness Ratio for Individuals With a 20% Prevalence of Obstructive CAD
| Strategy | Cost | Effect | Δ Cost | Δ Effect | ICER |
|---|---|---|---|---|---|
| ETT-SE-ICA | $10,995 | 16.106 | — | — | — |
| SE-CCTA-ICA | $11,235 | 16.1102 | $240 | 0.0042 | Ext Dominated |
| ETT-MPS-ICA | $11,269 | 16.1045 | $34 | −0.0057 | Dominated |
| SE-ICA | $11,356 | 16.1097 | $122 | −0.0005 | Dominated |
| ETT-CCTA-ICA | $11,564 | 16.1176 | $569 | 0.0116 | $49,021 |
| MPS-CCTA-ICA | $11,677 | 16.1078 | $113 | −0.0098 | Dominated |
| MPS-ICA | $11,798 | 16.1073 | $122 | −0.0005 | Dominated |
| CCTA-SE-ICA | $12,087 | 16.1275 | $524 | 0.0099 | $52,899 |
| CCTA-MPS-ICA | $12,119 | 16.1274 | $32 | −0.0001 | Dominated |
| CCTA-ICA | $12,274 | 16.1283 | $187 | 0.0008 | $233,138 |
| ETT-ICA | $12,635 | 16.1127 | $361 | −0.0156 | Dominated |
| ICA | $14,003 | 16.1205 | $1,729 | −0.0078 | Dominated |
2.3 Test Performance Characteristics
Sensitivity and specificity of non-invasive diagnostic tests within our model were based upon a bivariate analysis of data from published multicenter trials [Table 1](16). This approach of using a bivariate random effects model was chosen to produce unbiased estimates and 95% confidence intervals that preserve the joint distribution or correlation between test sensitivity and specificity.
2.4 Risks of Diagnostic Testing
Invasive coronary angiography was associated with a 0.1% risk of mortality(17, 18). Thus, even though ICA was considered the gold standard diagnostic test, deaths due to ICA were not treated as a correct diagnosis in the diagnostic model.
2.5 Cost Effectiveness
Long-term patient outcomes based upon initial diagnostic imaging strategies were modeled allowing future patient outcome to be determined solely by patient-specific variables (e.g., CAD severity, age, gender) and test-based treatment (e.g., medical therapy or coronary artery revascularization) (Appendix 1).
The relative risks of MI and coronary artery revascularization after the initial diagnostic test-based treatment decision in patients both correctly and incorrectly diagnosed varied according to test-based treatment as well as CAD severity.
We estimated the effects of test-based treatments on the presence or absence of chest pain, in accordance with those that have been reported for the COURAGE quality of life study. Patients were classified as having no CAD, CAD with no pain, CAD with mild pain, and CAD with severe pain. Patients with CAD who were correctly diagnosed and treated obtained a quality of life improvement relative to their undiagnosed counterparts who were not treated.
Costs for imaging tests and downstream clinical events can be observed in Table 1. Costs and QALYs were calculated for all 12 diagnostic strategies. We then ranked all 12 strategies by increasing cost and eliminated strategies by simple dominance (i.e., strategies that were less effective and more costly) and extended dominance (i.e., strategies that were less effective and had a higher incremental cost-effectiveness ratio [ICER]). ICERs were calculated for each remaining strategy relative to the next less costly strategy. The societal willingness to pay (WTP) for additional correct diagnoses or QALYs was used to calculate the probability of cost-effectiveness for different cost perspectives(19). Costs and QALYs were discounted at an annual rate of 3% and all analyses were performed with TreeAge Pro 2008 (Version 1.5.2) (Williamstown, MA).
2.6 Sensitivity Analysis
Probabilistic sensitivity analysis was conducted to assess the impact of uncertainty in model parameters. Monte Carlo simulation was performed to derive mean values for costs and QALYs at CAD prevalence of 20%, 50% and 80%. Ranges and distributional assumptions for model variables were constructed using plausible values and employed actual data or literature estimates where available (Table 1).
3. Results
3.1 Base Case Analysis
The optimal diagnostic strategy for individuals with suspected CAD and stable chest pain syndrome is dependent upon several variables beyond diagnostic test performance, including the prevalence of CAD, the cost of the tests, and the societal willingness to pay (WTP) for additional correct diagnoses or QALYs. Table 1 demonstrates the lifelong costs per QALY saved based upon the 12 diagnostic pathways for 1,000 55-year old males with a 20% CAD prevalence. The least costly strategy was ETT-SE-ICA, at an average cost of $10,995 per patient. The ETT-CCTA-ICA and CCTA-SE-ICA strategies were more effective, with ICERs of $49,021 and $52,899, respectively. The most effective strategy was CCTA-ICA but it was also more costly, with an ICER of $233,138.
Results differed based upon the WTP. The cost-effectiveness acceptability curve (Figure 1) illustrates that there was an 81% chance of ETT-SE-ICA being cost effective at a $20,000 threshold while there was a 41% probability that CCTA-SE-ICA is cost effective at the $50,000 threshold.
Figure 1.
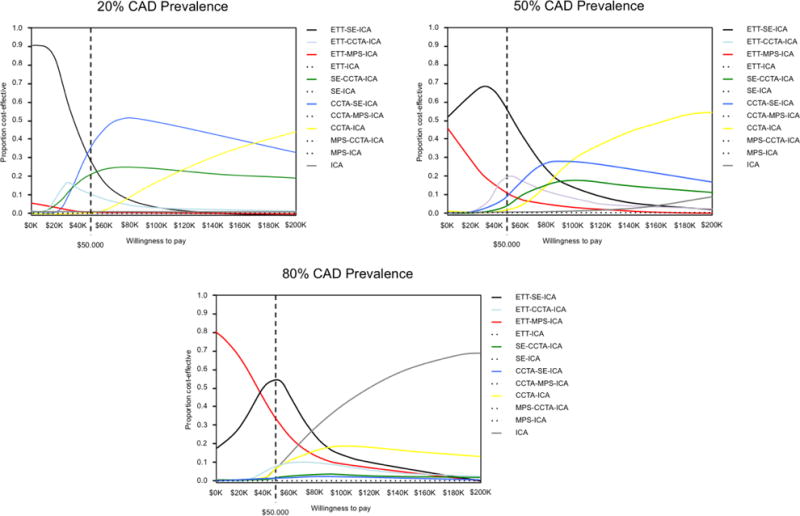
Cost-Effectiveness Acceptability Curves Based Upon Differing Willingess to Pay Thresholds
When the CAD prevalence increased to 50%, similar relationships held as ETT-SE-ICA remained the least expensive strategy (Table 2). However, the ICERs for the ETT-CCTA-ICA ($63,294) and CCTA-SE-ICA ($73,734) strategies increased, and there was a 49% probability that ETT-SE-ICA is cost effective at the $50,000 threshold (Figure 1). A total of five strategies were dominated at 20% and 50% pre-test likelihood of CAD, including ETT-MPS-ICA, MPS-CCTA-ICA, SE-ICA, MPS-ICA and CCTA-MPS-ICA.
Table 2.
Costs, Effectiveness and Incremental Cost Effectiveness Ratio for Individuals With a 50% Prevalence of Obstructive CAD
| Strategy | Cost | Effect | Δ Cost | Δ Effect | ICER |
|---|---|---|---|---|---|
| ETT-SE-ICA | $21,386 | 15.0391 | — | — | — |
| ETT-MPS-ICA | $21,397 | 15.035 | $10 | −0.0041 | Dominated |
| ETT-CCTA-ICA | $22,070 | 15.0499 | $684 | 0.0108 | $63,294 |
| SE-CCTA-ICA | $22,100 | 15.048 | $30 | −0.0019 | Dominated |
| MPS-CCTA-ICA | $22,116 | 15.0414 | $46 | −0.0085 | Dominated |
| SE-ICA | $22,195 | 15.0481 | $125 | −0.0018 | Dominated |
| MPS-ICA | $22,211 | 15.0414 | $141 | −0.0085 | Dominated |
| ETT-ICA | $22,929 | 15.0502 | $858 | 0.0003 | Ext Dominated |
| CCTA-SE-ICA | $23,124 | 15.0642 | $1,054 | 0.0143 | $73,734 |
| CCTA-MPS-ICA | $23,126 | 15.0637 | $2 | −0.0005 | Dominated |
| CCTA-ICA | $23,295 | 15.0654 | $170 | 0.0012 | $141,900 |
| ICA | $24,675 | 15.0659 | $1,380 | 0.0005 | $2,760,880 |
At an 80% prevalence of CAD, ETT-MPS-ICA became the least expensive strategy at an average cost of $31,498 (Table 3). An ETT-SE-ICA strategy was slightly more effective, with an ICER of $38,234 per QALY, and the ICER for CCTA-SE-ICA with an incremental improvement of 0.02 QALYs was $85,523. Using a $20,000 WTP threshold, there was a 66% probability that ETT-MPS-ICA is cost effective, and a 54% probability that ETT-SE-ICA is cost effective at a $50,000 threshold.
Table 3.
Costs, Effectiveness and Incremental Cost Effectiveness Ratio for Individuals With a 80% Prevalence of Obstructive CAD
| Strategy | Cost | Effect | Δ Cost | Δ Effect | ICER |
|---|---|---|---|---|---|
| ETT-MPS-ICA | $31,498 | 13.9581 | — | — | — |
| ETT-SE-ICA | $31,747 | 13.9646 | $249 | 0.0065 | $38,234 |
| MPS-CCTA-ICA | $32,554 | 13.9678 | $807 | 0.0032 | Ext Dominated |
| ETT-CCTA-ICA | $32,554 | 13.9749 | $808 | 0.0103 | $78,404 |
| MPS-ICA | $32,624 | 13.9684 | $69 | −0.0065 | Dominated |
| SE-CCTA-ICA | $32,956 | 13.9784 | $401 | 0.0035 | Ext Dominated |
| SE-ICA | $33,026 | 13.979 | $70 | 0.0006 | Ext Dominated |
| ETT-ICA | $33,196 | 13.9804 | $170 | 0.0014 | Ext Dominated |
| CCTA-MPS-ICA | $34,144 | 13.993 | $948 | 0.0126 | Ext Dominated |
| CCTA-SE-ICA | $34,171 | 13.9938 | $1,616 | 0.0189 | $85,523 |
| CCTA-ICA | $34,330 | 13.9955 | $160 | 0.0017 | $93,841 |
| ICA | $35,366 | 14.0044 | $1,035 | 0.0089 | $116,337 |
3.2 Influence of Post-Test Treatment Strategy on Cost-Effectiveness of Diagnostic Evaluation Strategies
In order to evaluate the potential effects of post-test treatment on the cost-effectiveness of different diagnostic evaluation pathways, we compared three distinct treatment approaches (Table 4). A “conservative” treatment approach was defined by the use of optimal medical therapy alone for all patients with 1–2 vessel CAD and PCI for all patients with 3-vessel or left main CAD. An “aggressive” treatment approach was defined by PCI for all patients with 1–2 vessel CAD and coronary artery bypass surgery for all patients with 3-vessel or LM CAD. An “intermediate” treatment approach was defined similarly to that used for our base case analysis, in which 50% of patients with 1–2 vessel CAD would be treated with optimal medical therapy alone and 50% would be treated with PCI; and in which 50% of patients with 3-vessel or LM CAD would be treated with PCI and 50% would be treated with CABG. For this analysis, an example of a 55-year old man without known CAD presenting with stable chest pain and a 30% likelihood of obstructive CAD was employed. Using a conservative treatment approach, a CCTA-SE-ICA strategy demonstrated an ICER of $11,570 relative to the least expensive ETT-CCTA-ICA. Employment of an aggressive treatment approach, an ETT-SE-ICA strategy was the least expensive strategy. Both ETT-CCTA-ICA and CCTA-SE-ICA approaches were more effective but resulted in ICERs of $190,465 for both. For the intermediate treatment approach, ETT-SE-ICA was the least expensive strategy with a favorable $20,701 ICER for both ETT-CCTA-ICA and CCTA-SE-ICA.
Table 4.
Effect of Post-Test Treatment Approach on the Cost Effectiveness of Diagnostic Testing Strategies
| Strategy | Cost ($) | QALY | ICER | Cost ($) | QALY | ICER | Cost ($) | QALY | ICER |
|---|---|---|---|---|---|---|---|---|---|
| “CONSERVATIVE” | “AGGRESSIVE” | “INTERMEDIATE” | |||||||
| ETT-CCTA-ICA | $14,873.41 | 15.7952 | $16,325.65 | 15.7693 | $190,465 | $15,599.53 | 15.7822 | $20,701 | |
| ETT-SE-ICA | $14,889.03 | 15.7938 | (Dominated) | $16,273.70 | 15.769 | $15,581.37 | 15.7814 | ||
| CCTA-SE-ICA | $14,967.24 | 15.8034 | $11,570 | $16,757.73 | 15.7713 | $190,465 | $15,862.49 | 15.7873 | $20,701 |
| SE-CCTA-ICA | $15,008.85 | 15.7994 | (Dominated) | $16,619.34 | 15.7706 | $221,026 | $15,814.10 | 15.785 | $78,166 |
| CCTA-MPS-ICA | $15,010.82 | 15.8033 | (Dominated) | $16,802.19 | 15.7712 | (Dominated) | $15,906.50 | 15.7873 | (Dominated) |
| CCTA-ICA | $15,051.37 | 15.8031 | (Dominated) | $16,858.69 | 15.7707 | (Dominated) | $15,955.03 | 15.7869 | (Dominated) |
| SE-ICA | $15,096.10 | 15.7988 | (Dominated) | $16,709.93 | 15.77 | (Dominated) | $15,903.02 | 15.7844 | (Dominated) |
| ETT-MPS-ICA | $15,107.38 | 15.7935 | (Dominated) | $16,496.46 | 15.7687 | (Dominated) | $15,801.92 | 15.7811 | (Dominated) |
| ETT-ICA | $15,310.56 | 15.7924 | (Dominated) | $16,779.54 | 15.7661 | (Dominated) | $16,045.05 | 15.7793 | (Dominated) |
| MPS-CCTA-ICA | $15,634.11 | 15.7988 | (Dominated) | $17,257.24 | 15.7697 | (Dominated) | $16,445.68 | 15.7843 | (Dominated) |
| MPS-ICA | $15,721.36 | 15.7982 | (Dominated) | $17,347.83 | 15.7691 | (Dominated) | $16,534.60 | 15.7784 | (Dominated) |
4. Discussion
We examined different diagnostic strategies for symptomatic individuals without known CAD undergoing a wide range of diagnostic testing strategies in order to identify preferred strategies that demonstrate favorable long-term costs per QALY gained. The main results indicate that, depending on the WTP per QALY, ETT followed by SE or CCTA before referral to ICA were being the preferred strategies in a population with a 20–50% prevalence of obstructive CAD, whilst for patients with a higher likelihood of obstructive CAD (80%) ETT followed by SE or MPS should be favored before referral to ICA. To our knowledge, this study represents the first analysis of cost-effectiveness of CAD evaluation for symptomatic individuals with stable chest pain syndrome that examined strategies that do or do not incorporate successive non-invasive diagnostic testing. Further, within the diagnostic strategies evaluated, we evaluated the cost-effectiveness of those strategies that employed ETT without imaging as an initial test as well as strategies that employed imaging as initial tests. Finally, we examined the cost-effectiveness of diagnostic evaluation strategies based upon different post-test treatment approaches.
At a $50,000 WTP threshold, the most cost-effective strategy for stable chest pain patients with a 20% pre-test likelihood of obstructive CAD was initial testing by ETT, followed by CCTA for ETT that was equivocal or not able to be performed, and ICA for positive ETT. ETT-CCTA-ICA remained more effective than the less expensive ETT-SE-ICA when the pre-test likelihood of obstructive CAD rose to 50%, albeit with a less favorable ICER of $63,294. As pre-test likelihood rose to 80%, ETT-MPS-ICA emerged as the least expensive strategy, although ETT-SE-ICA continued to demonstrate a favorable ICER of $38,234 per QALY.
From these data, testing strategies that employed initial ETT without imaging were more cost effective than those that employed initial testing with imaging, albeit with modest savings per QALY. These data are nevertheless in direct accordance with ACC/AHA guidelines on management of patients with chronic stable angina, which favor initial testing by ETT without imaging as a Class IIa recommendation over stress testing with imaging as a Class IIb recommendation(18). Indeed, across a range of pre-test likelihood of obstructive CAD that extended from intermediate to high, no strategy that employed initial testing by imaging was cost-effective.
Further, the present data underscore the potential value of different tests for different individuals based upon prevalence of CAD, and suggest that exclusive use of a single modality for all individuals presenting with stable chest pain syndrome may not be uniformly cost-effective. These findings should be considered within the context of their implications to the US healthcare system. At present, while many payers direct diagnostic evaluation by policy-based algorithms that favor certain tests over others as the initial evaluation, this “one size fits all” policy may potentially both reduce clinical effectiveness as well as increase healthcare costs.
While ICERs were used as the primary outcome in the present study, no single diagnostic evaluation strategy proved to be uniformly cost effective. Indeed, no single testing strategy was wholly dominant across different CAD prevalence levels or even within CAD prevalence strata, and several testing strategies yielded ICERs that were very close to those found to be most effective. As was observed by cost-effectiveness acceptability curves, there is significant overlap for the probability of cost effectiveness among different testing strategies, representing uncertainty about the most cost-effective strategy. This finding underscores the potential difficulty of policies that mandate a single evaluation strategy. Indeed, numerous factors beyond the variables examined in this analysis—that includes local site expertise, test availability, and specific patient characteristics—may also affect diagnostic test performance and ensuant cost effectiveness of testing. Given the wide uncertainty that exists with the multiple available testing strategies, future trials and registries will be needed to determine the cost effectiveness of testing strategies, but will do well to empower analyses that are genuinely representative of specific patient cohorts, physician expertise, and test location.
The present study analyzed twelve distinct diagnostic evaluation pathways, 5 of which permitted 2 successive non-invasive diagnostic imaging tests in cases of equivocal initial testing. As multiple non-invasive imaging tests are available to many practitioners and as non-negligible rates of equivocal testing are known to occur, successive downstream imaging testing reflects a growing clinical reality. Prior studies that have examined the cost-effectiveness of non-invasive cardiac testing have generally limited the diagnostic evaluation to a single test, followed by ICA for equivocal or abnormal tests(20–22). The results of the present study suggest that succession of imaging tests does not enhance overall cost-effectiveness across a wide range of CAD prevalence and thus, should not be advocated. In contrast, when initial testing is performed by ETT without imaging, successive testing by imaging for individuals in whom ETT is either equivocal or unable to be performed appears to enhance cost-effectiveness. Reinforcing this concept is our finding that ETT followed by direct ICA without the option for intermediary imaging for equivocal ETT does not appear to be cost-effective.
Interestingly, the present data suggest that in individuals up to a high likelihood of significant CAD, SE or CCTA following ETT represent more cost effective strategies as compared to testing algorithms that routinely employ MPS. At high prevalence levels of significant CAD, MPS following ETT emerged as a less costly strategy. However, an 80% pre-test likelihood of CAD in a population without prior cardiac history is relatively high for referral for non-invasive imaging. Therefore, using MPS will rarely be the most cos-efficient diagnostic strategy. We have previously observed in large retrospective analyses that in a direct comparison of CCTA to MPS, CCTA is more cost-efficient for patients without known CAD while MPS appears to be more cost-efficient for patients with known CAD, and the present results are in keeping with those findings(23).
Further, this study—to our knowledge—represents the first analysis to examine the effects of post-test treatment approaches on the cost effectivess of diagnostic cardiac imaging for suspected CAD. Numerous recent clinical trials have challenged the historical treatment of CAD, with varying degrees of medical versus invasive therapy now being utilized in daily clinical practice(14, 15). The results of the present study indicate that the cost effectiveness of diagnostic evaluation strategies is not only dependent upon test and patient characteristics, but also contingent upon how treatment approaches are tailored to patient care based upon test findings. These data highlight the complexity of assessing cost-effectiveness of diagnostic testing, and should serve to inform future investigators examining this topic of the importance of accounting for diverse clinical practice patterns when attempting to identify the most efficient diagnostic pathways.
This study is not without limitations. While our study examined 12 possible diagnostic pathways that include the most widely utilized non-invasive diagnostic tests, we did not include stress testing by magnetic resonance imaging (MRI) or positron emission tomography (PET), given their significantly lower availability, expertise and rates of utilization. In addition, a ≥ 70%stenosis on CCTA was deemed significant to maintain uniformity with ICA, however the results may differ for other CCTA stenosis thresholds. Further, the present study examined only patients without known CAD presenting with stable chest pain syndrome. Therefore, whether the present results are applicable to patients with known CAD or more acute forms of chest pain remains uncertain.
Additionally, the economic model required assuming that all positive diagnostic test results were referred to ICA, and that ICA holds perfect sensitivity and specificity. However, this may be different in actual clinical practice. Finally, practice patterns associated with treatment of CAD are in transition. Data from such studies as COURAGE and SYNTAX have challenged the paradigm of early automatic coronary revascularization for stable patients with CAD and coronary artery bypass surgery for severe forms of CAD such as left main stenosis, respectively(14, 15). We have attempted to account for these transitional patterns in our present analysis by employing realistic estimates of patterns of care that represent both present clinical reality as well as near-term anticipated adoption of clinical practice patterns.
5. Conclusions
Evaluation of individuals without known CAD presenting with stable chest pain syndrome is most cost effective when ETT is performed first, followed by imaging tests for ETT that is equivocal or not able to be performed. As pre-test likelihood of CAD varies, different modalities—including SE, CCTA, and MPS—result in lower overall costs and enhanced effectiveness. In individuals up to high likelihood of significant CAD, SE or CCTA following ETT represent the most cost effective strategies, however, at high prevalence of significant CAD, MPS following ETT emerged as a less costly strategy.
Highlights.
At 20% risk of obstructive CAD, ETT-CCTA-ICA displayed a favorable ICER of $49,021.
At 50% risk of obstructive CAD, ETT-CCTA-ICA strategy cost $63,294 per QALY gained.
At 80% risk of obstructive CAD, ETT-ECHO-ICA strategy cost $38,234 per QALY gained.
Various diagnostic imaging modalities augment lifetime cost-effectiveness.
Acknowledgments
Funding: Research reported in this publication was supported by the National Heart, Lung, and Blood Institute under Award number R01HL111141 and R01HL118019 and also in part by a generous gift from the Dalio Institute of Cardiovascular Imaging (New York NY) and the Michael Wolk Foundation. This study was also funded by an unrestricted educational grant from GE Healthcare.
Abbreviations
- CAD
Coronary artery disease
- CCTA
Coronary computed tomographic angiography
- ECHO
Echocardiogram
- ETT
Exercise treadmill testing
- ICA
Invasive coronary angiography
- ICER
Incremental cost-effectiveness ratio
- MPS
Myocardial perfusion scintigraphy
- SE
Stress echocardiography
- QALY
Quality adjusted life year
Appendix A
Table A.1.
Model Parameter Values and Distributional Assumptions
| Model Parameter | Value | Calculation | Distributional Assumptions |
|---|---|---|---|
| Cost of aspirin(24) | $15 | Based on 3 prescriptions annually | None |
| Cost of atenolol(24) | $103 | Based on 12 prescriptions annually | None |
| Cost of coronary artery bypass graft surgery(25) | $29,518 | Based on mean cost of CABG $20,574 in 2000$ = $29,518 in 2009$, SD $5230 in 2000$ = $7503 in 2009$ (SE = $531 in 2009$ based on 200 observations). Inflated based on the Medical Care Component of the Consumer Price Index (US Bureau of Labor Statistics). | Gamma, alpha = (29518^2)/(531^2), lambda = 29518/(531^2); Expected value: 29518 |
| Cost of CCTA(26) | $445.54 | Calculated value based on cost of test probability of incidental findings and cost of follow-up scan for incidental findings | None |
| Cost of CT scan for incidental findings(26) | $344 | Technical component $285 + professional component $59 = $344 | +/−20%: Gamma, alpha = (344^2)/(35^2), lambda = 344/(35^2); Expected value: 344 |
| Cost of CCTA test(27) | $394 | Technical component $293 + professional component $101 = $394 | +/−20%, Gamma, alpha = (394^2)/(40^2), lambda = 394/(40^2); Expected value: 394 |
| Cost of diagnosis | n/a | Variable used to calculate the total diagnosis costs on each branch using a formula defined at the appropriate node | None |
| Cost of SE | $340 | Technical component $268 + professional component $72 = $340. | +/−20%, Gamma, alpha = (340^2)/(35^2), lambda = 340/(35^2); Expected value: 340 |
| Cost of ETT | $100 | $100 (includes interpretation and report). | +/−20% Gamma, alpha = (100^2)/(10^2), lambda = 100/(10^2); Expected value: 100 |
| Cost of ICA test | $3,081 | Cath placement CPT 93508 $1,061 + professional component $236 + left heart cath CPT 93510 $1334 + professional component $249 + injection for heart x-rays CPT 93543 $16 + injection for coronary x-rays CPT 93545 $22 + imaging CPT 93555 $118 + $45 = $3,081 | +/− 20% Gamma, alpha = (3081^2)/(314^2), lambda = 3081/(314^2); Expected value: 3081 |
| Cost of isosorbide mononitrate(24) | $110 | Based on 12 prescriptions annually | None |
| Cost of acute myocardial infarction(27) | $26,034 | $26,034 (standard error = $4017) | Gamma, alpha = (26034^2)/(4017^2), lambda = 26034/(4017^2); Expected value: 26034 $26,034 (95% CI: $19,469, $35,213) in 2009 US$ based on $16,845 (95% CI: $12,597; $22,784) in 1998 US$, inflated using the Medical Care Component of the CPI from the BLS. |
| Cost of angioplasty and stent(28) | $11,609 | $11,609 (standard error = $150) | Gamma, alpha = (11609^2)/(150^2), lambda = 11609/(150^2); Expected value: 11609 $11,609 in 2009 US$ based on $8464 in 2001 US$ and reported SD of $2497 (and a sample of 522) inflated using the Medical Care Component of the Consumer Price Index (US Bureau of Labor Statistics) |
| Cost of simvastatin(24) | $216 | Based on 12 annual prescriptions | None |
| Cost of MPS test | $819 | SPECT, multiple studies CPT 78465 $485 + professional component $79 + MPS with wall motion CPT 78478 $60 + professional component $27 + MPS study with ejection fraction CPT 78480 $50 + $18 + cardiovascular stress test CPT 93015 $100 = $819 | +/−20% Gamma, alpha = (819^2)/(84^2), lambda = 819/(84^2); Expected value: 819 |
| Patient age | 55 | 55 years | None |
| CAD prevalence | 0.2 | 20% prevalence | None |
| Discount rate | 0.03 | 3% discount rate | None |
| Patient sex (1 = male, 2 = female) | 1 | Male | None |
| Mortality risk of CABG | 0.020749 | Based on 252 deaths out of 12146 procedures | None |
| Probability of subsequent CAD diagnosis | 0.043976 | 0.044 annually (reflects 20% over 5 years, with an assumed 95% CI between 0.1 and 0.3 over 5 years) | Beta, Real-numbered parameters, alpha = 12.3, beta = 267.4; Expected value: 0.043975688 |
| Proportion of positives with 3 vessel or LM disease(29) | 0.226 | n/a | None |
| Proportion of negatives (<70% stenosis) with low likelihood of CAD (1–69% stenosis)(29) | 0.367 | n/a | None |
| Probability of incidental findings | 0.14982 | 0.15, with an assumed 95% CI between 0.1 and 0.2 | None |
| CCTA Indeterminate Test | 0.069697 | 0.07, with an assumed 95% CI between 0.02 and 0.12 | None |
| CTA Negative Predictive Value | 0.981744 | Calculated | None |
| CTA Positive Predictive Value | 0.604893 | Calculated | None |
| CCTA Sensitivity(29–31) | 0.937 | 93.7% with 95% CI(86.9%, 97.1%), based on bivariate analysis of multicenter studies | beta dist (alpha = 80.76, beta = 5.43) |
| CCTA Specificity(29–31) | 0.846991 | 84.7% with 95% CI (80.8%, 87.9%), based on bivariate analysis of multicenter studies | beta dist (alpha = 333.74, beta = 60.29) |
| SE indeterminate(32) | 0.069697 | 0.07, with an assumed 95% CI between 0.02 and 0.12 | None |
| SE negative predictive value(32) | 0.960424 | Calculated | None |
| SE positive predictive value(32) | 0.528775 | Calculated | None |
| SE sensitivity(32) | 0.86701 | 86.7% with 95% CI (83.8%, 89.2%), based on bivariate analysis of multicenter studies | beta dist (alpha = 526.98, beta = 80.68) |
| SE specificity(32) | 0.806838 | 80.7% with 95% CI (60.1%, 92.0%), based on bivariate analysis of multicenter studies | beta dist (alpha = 18.17, beta = 4.35) |
| ETT indeterminate(33) | 0.619881 | 62%, with an assumed 95% CI between 55.8% and 68.2% Based on 41.6% who cannot achieve Stage III or IV of Bruce protocol and 35% of the remainder being uninterpretable (35% × 58.4% = 20.4%). Thus, 41.6% + 20.4% = 62% of ETT were not performable or uninterpretable | None |
| ETT negative predictive value(33) | 0.905886 | Calculated | None |
| ETT positive predictive value(33) | 0.42501 | Calculated | None |
| ETT sensitivity(33) | 0.680011 | 68%, using an assumed 30% prevalence this yields 4906 correct out of 7214 (mean = 0.68, SE = 0.00549) | Beta dist (alpha = 4909 beta = 2310) |
| ETT specificity(33) | 0.770006 | 77%, using an assumed 30% prevalence this yields 12,961 correct out of 16,833 (mean 0.77, SE = 0.00324) | Beta dist (alpha = 12990, beta = 3880) |
| Probability that a treated patient with severe CAD will be treated with PCI | 0.5 | 1.0(conservative Tx strategy), 0.5 (normal Tx strategy), 0.0 (aggressive Tx strategy) | None |
| ICA Mortality Rate(17) | 0.001 | n/a | None |
| Probability that a treated patient with moderate CAD will be treated with medicine | 0.5 | 1.0 (conservative Tx strategy), 0.5 (normal Tx strategy), 0.0 (aggressive Tx strategy) | None |
| Probability of MI for high risk patients(34) | 0.032014 | 3.2% annual probability of MI, based on a PROCAM risk score of 54–61 (“high risk”) which is associated with a 28.1% incidence of acute coronary events in 10 years (adjusted to 1 year probability) | None |
| Probability of MI for low risk patients(34) | 0.006992 | 0.7% annual probability of MI, based on a PROCAM risk score of 38–44 which is associated with a 6.6% incidence of acute coronary events in 10 years (adjusted to 1 year probability) | None |
| Probability of MI for medium risk patients(34) | 0.016 | 1.6% annual probability of MI, based on a PROCAM risk score of 45–53 which is associated with a 14.8% incidence of acute coronary events in 10 years (adjusted to 1 year probability) | None |
| Probability of a non-fatal MI | 0.590226 | n/a | None |
| Mortality risk of PTCA(35) | 0.006302 | 0.0063 (0.63%), distribution calculated based on 0.63% or 894 deaths out a total of 141,865 non-emergency cases | Beta, Real-numbered parameters, alpha = 894.0, beta = 140970.0; Expected value: 0.00630181 |
| CABG revascularization rate for severe CAD patient treated with CABG(15) | 0.012972 | 1.3% 1 year rate | None |
| PCI revascularization rate for severe CAD patient treated with CABG(15) | 0.04717 | 4.7% 1 year rate | None |
| CABG revascularization rate for severe CAD patient treated with PCI(15) | 0.02809 | 2.8% 1 year rate | None |
| PCI revascularization rate for severe CAD patient treated with PCI(15) | 0.114494 | 11.4% 1 year rate | None |
| Probability of revascularization for low risk patients | 0.01 | Assumption - based on lower value than medium risk group | None |
| CABG revascularization rate for moderate CAD patient treated with medicine(14) | 0.015919 | 1.59% per 1 year, based on 7.11 % per 4.6 years; 81 out of 1138 patients (over 4.6 years) mean = 0.0711, SE = 0.007622, calculating the 1 year TP from the 4.6 year rate, mean = 0.0159235, SE = 0.00166192 | None |
| PCI revascularization rate for moderate CAD patient treated with medicine(14) | 0.061696 | 6.17% per year, based on 25.4% per 4.6 years; 289 out 1138 patients (over 4.6 years) mean = 0.254, SE = 0.012903, calculating the 1 year TP from the 4.6 year rate, mean = 0.06170, SE = 0.002819 | None |
| CABG revascularization rate for moderate CAD patient treated with PCI(14, 15) | 0.014962 | 1.50% per year, based on 6.71% per 4.6 years; 77 out of 1149 patients (over 4.6 years) mean = 0.0671, SE = 0.007377, calculating the 1 year TP from the 4.6 year rate, mean = 0.01497, SE = 0.00161 | None |
| PCI revascularization rate for moderate CAD patient treated with PCI(14) | 0.033133 | 3.31% for 1 year, based on 14.39% for 4.6 years; 165 out of 1149 patients (over 4.6 years) mean = 0.1439%, SE = 0.01035, calculating the 1 year TP from the 4.6 year rate, mean = 0.033139, SE = 0.002259 | None |
| MPS Indeterminate Test | 0.069697 | 0.07 | 95% CI between 0.02 and 0.12 |
| SPECT Negative Predictive Value(4) | 0.939038 | Calculated | None |
| SPECT Positive Predictive Value(4) | 0.443372 | Calculated | None |
| MPS Sensitivity(4) | 0.806014 | 80.6% with 95% CI (74.9%, 85.3%), based on bivariate analysis of multicenter studies | beta dist (alpha = 178.25, beta = 42.90) |
| MPS Specificity(4) | 0.747024 | 74.7% with 95% CI (67.2%, 80.9%), based on bivariate analysis of multicenter studies | beta dist (alpha = 114.84, beta = 38.89) |
| Relative risk of MI for high risk patients treated with CABG(20) | 0.583938 | n/a | 95% CI: 0.45 – 0.71 |
| Relative risk of MI for low risk patients treated with medicines(36) | 0.712437 | n/a | 95% CI: 0.60 – 0.83 |
| Relative risk of MI for medium risk patient on medicines(20) | 0.831113 | Relative risk of MI for patients treated with medicine (assumed to be same as for PTCA) | +/−10% CI |
| Relative risk of MI for medium risk patients treated with PCI(20) | 0.831113 | n/a | +/−10% CI |
| Mortality risk of 3 vessel or LM disease(37) | 3.554842 | Calculated as the weighted average of mortality relative risk for 3 vessel and LMD: Relative risk for 3 vessel 2.2 × 62.5% plus relative risk for LMD 5.8 × 37.5% = 3.55 | +/−10% CI |
| Mortality risk for patients with 1/2 vessel disease(37) | 1.401875 | n/a | +/−10% CI |
| Mortality risk for patients with no CAD(38) | 0.734 | Based on heart disease causing 26.6% | None |
| Relative risk of mortality for treated high risk patients(36) | 0.770983 | n/a | +/−10% CI |
| Relative risk of mortality for treated low risk patients(36) | 0.930783 | n/a | 95% CI: 0.86 – 1.01 |
| Relative risk of mortality for 1/2 vessel patient treated with PCI(20, 39) | 0.851131 | Relative risk for medium risk patients treated with PCI is the same as for patients treated with medicines | +/−10% CI |
| Relative risk of mortality for 1/2 vessel patient treated with medicines(20, 39) | 0.851131 | Relative risk for patients treated with medicines | +/−10% CI |
| Utility of CAD with mild pain(39, 40) | 0.969669 | Based on standard gamble value for Class II angina. | +/−10% CI |
| Utility of CAD with no pain(39, 40) | 0.969669 | Based on standard gamble value for Class I angina. | +/−10% CI |
| Utility of CAD with severe pain(39, 40) | 0.879938 | Based on standard gamble value for Class III/IV angina. | +/−10% CI |
| Utility decrement for myocardial infarction(39, 40) | −0.10009 | n/a | +/−10% CI |
| Utility of no CAD | 1 | n/a | None |
| Utility improvement of revascularization | 0.100087 | This is attenuated in the calculations, so that patients who receive a utility improvement from revascularization can obtain a maximum utility of 1.0 | +/−10% CI |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures and Conflicts of Interest:
James Min serves on the speaker’s bureau and receives research support from GE Healthcare.
References
- 1.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, et al. Heart disease and stroke statistics–2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119(3):480–6. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 2.Brindis RG, Douglas PS, Hendel RC, Peterson ED, Wolk MJ, Allen JM, et al. ACCF/ASNC appropriateness criteria for single-photon emission computed tomography myocardial perfusion imaging (SPECT MPI): a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group and the American Society of Nuclear Cardiology endorsed by the American Heart Association. J Am Coll Cardiol. 2005;46(8):1587–605. doi: 10.1016/j.jacc.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 3.Hendel RC, Berman DS, Di Carli MF, Heidenreich PA, Henkin RE, Pellikka PA, et al. ACCF/ASNC/ACR/AHA/ASE/SCCT/SCMR/SNM 2009 Appropriate Use Criteria for Cardiac Radionuclide Imaging: A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the American Society of Nuclear Cardiology, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the Society of Cardiovascular Computed Tomography, the Society for Cardiovascular Magnetic Resonance, and the Society of Nuclear Medicine. J Am Coll Cardiol. 2009;53(23):2201–29. doi: 10.1016/j.jacc.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Klocke FJ, Baird MG, Lorell BH, Bateman TM, Messer JV, Berman DS, et al. ACC/AHA/ASNC guidelines for the clinical use of cardiac radionuclide imaging–executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASNC Committee to Revise the 1995 Guidelines for the Clinical Use of Cardiac Radionuclide Imaging) J Am Coll Cardiol. 2003;42(7):1318–33. doi: 10.1016/j.jacc.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 5.Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2012;126(25):e354–471. doi: 10.1161/CIR.0b013e318277d6a0. [DOI] [PubMed] [Google Scholar]
- 6.Fihn SD, Blankenship JC, Alexander KP, Bittl JA, Byrne JG, Fletcher BJ, et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2014;130(19):1749–67. doi: 10.1161/CIR.0000000000000095. [DOI] [PubMed] [Google Scholar]
- 7.Berman DS, Hachamovitch R, Shaw LJ, Friedman JD, Hayes SW, Thomson LE, et al. Roles of nuclear cardiology, cardiac computed tomography, and cardiac magnetic resonance: Noninvasive risk stratification and a conceptual framework for the selection of noninvasive imaging tests in patients with known or suspected coronary artery disease. J Nucl Med. 2006;47(7):1107–18. [PubMed] [Google Scholar]
- 8.Rodgers GP, Ayanian JZ, Balady G, Beasley JW, Brown KA, Gervino EV, et al. American College of Cardiology/American Heart Association Clinical Competence statement on stress testing: a report of the American College of Cardiology/American Heart Association/American College of Physicians–American Society of Internal Medicine Task Force on Clinical Competence. J Am Coll Cardiol. 2000;36(4):1441–53. doi: 10.1016/s0735-1097(00)01029-9. [DOI] [PubMed] [Google Scholar]
- 9.Zacharias K, Ahmed A, Shah BN, Gurunathan S, Young G, Acosta D, et al. Relative clinical and economic impact of exercise echocardiography vs. exercise electrocardiography, as first line investigation in patients without known coronary artery disease and new stable angina: a randomized prospective study. Eur Heart J Cardiovasc Imaging. 2016 doi: 10.1093/ehjci/jew049. [DOI] [PubMed] [Google Scholar]
- 10.Committee on Quality of Health Care in America IoM. Crossing the Quality Chasm - A New Health System for the 21st Century. Washington (DC): National Academies Press (US); 2001. [PubMed] [Google Scholar]
- 11.Present Practices & Future Directions in Cardiac Imaging: The Cardiologist’s Perspective. Des Plaines, IL: IMV Medical Information Division; 2006. [Google Scholar]
- 12.Shaw LJ, Min JK, Hachamovitch R, Peterson ED, Hendel RC, Woodard PK, et al. Cardiovascular imaging research at the crossroads. JACC Cardiovasc Imaging. 2010;3(3):316–24. doi: 10.1016/j.jcmg.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 13.Bourque JM, Beller GA. Value of Exercise ECG for Risk Stratification in Suspected or Known CAD in the Era of Advanced Imaging Technologies. JACC Cardiovasc Imaging. 2015;8(11):1309–21. doi: 10.1016/j.jcmg.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boden WE, O’Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356(15):1503–16. doi: 10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]
- 15.Serruys PW, Morice MC, Kappetein AP, Colombo A, Holmes DR, Mack MJ, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360(10):961–72. doi: 10.1056/NEJMoa0804626. [DOI] [PubMed] [Google Scholar]
- 16.Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol. 2005;58(10):982–90. doi: 10.1016/j.jclinepi.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 17.Bernstein SJLM, Hilborne LH, Leapse LL, Kahan JP, Park RE, Kamberg JC, Brook RH. oronary angiography – A literature review and ratings of appropriateness and necessity. 1992 [Google Scholar]
- 18.Gibbons RJ, Chatterjee K, Daley J, Douglas JS, Fihn SD, Gardin JM, et al. ACC/AHA/ACP-ASIM guidelines for the management of patients with chronic stable angina: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Patients With Chronic Stable Angina) J Am Coll Cardiol. 1999;33(7):2092–197. doi: 10.1016/s0735-1097(99)00150-3. [DOI] [PubMed] [Google Scholar]
- 19.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness–the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371(9):796–7. doi: 10.1056/NEJMp1405158. [DOI] [PubMed] [Google Scholar]
- 20.Kuntz KM, Fleischmann KE, Hunink MG, Douglas PS. Cost-effectiveness of diagnostic strategies for patients with chest pain. Ann Intern Med. 1999;130(9):709–18. doi: 10.7326/0003-4819-130-9-199905040-00002. [DOI] [PubMed] [Google Scholar]
- 21.Garber AM, Solomon NA. Cost-effectiveness of alternative test strategies for the diagnosis of coronary artery disease. Ann Intern Med. 1999;130(9):719–28. doi: 10.7326/0003-4819-130-9-199905040-00003. [DOI] [PubMed] [Google Scholar]
- 22.Ladapo JA, Jaffer FA, Hoffmann U, Thomson CC, Bamberg F, Dec W, et al. Clinical outcomes and cost-effectiveness of coronary computed tomography angiography in the evaluation of patients with chest pain. J Am Coll Cardiol. 2009;54(25):2409–22. doi: 10.1016/j.jacc.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 23.Min JK, Shaw LJ, Berman DS, Gilmore A, Kang N. Costs and clinical outcomes in individuals without known coronary artery disease undergoing coronary computed tomographic angiography from an analysis of Medicare category III transaction codes. Am J Cardiol. 2008;102(6):672–8. doi: 10.1016/j.amjcard.2008.04.045. [DOI] [PubMed] [Google Scholar]
- 24.Murray L. Pharmacy’s Fundamental Reference. 1133. Montvale, NJ: Physicians’ Desk; 2009. [Google Scholar]
- 25.Reynolds MR, Neil N, Ho KK, Berezin R, Cosgrove RS, Lager RA, et al. Clinical and economic outcomes of multivessel coronary stenting compared with bypass surgery: a single-center US experience. Am Heart J. 2003;145(2):334–42. doi: 10.1067/mhj.2003.38. [DOI] [PubMed] [Google Scholar]
- 26.Physicians’ Fee & Coding Guide. Mag Mutual. 2009 20 Annual Edition. [Google Scholar]
- 27.Sloss EM, Wickstrom SL, McCaffrey DF, Garber S, Rector TS, Levin RA, et al. Direct medical costs attributable to acute myocardial infarction and ischemic stroke in cohorts with atherosclerotic conditions. Cerebrovasc Dis. 2004;18(1):8–15. doi: 10.1159/000078602. [DOI] [PubMed] [Google Scholar]
- 28.Cohen DJ, Bakhai A, Shi C, Githiora L, Lavelle T, Berezin RH, et al. Cost-effectiveness of sirolimus-eluting stents for treatment of complex coronary stenoses: results from the Sirolimus-Eluting Balloon Expandable Stent in the Treatment of Patients With De Novo Native Coronary Artery Lesions (SIRIUS) trial. Circulation. 2004;110(5):508–14. doi: 10.1161/01.CIR.0000136821.99814.43. [DOI] [PubMed] [Google Scholar]
- 29.Budoff MJ, Dowe D, Jollis JG, Gitter M, Sutherland J, Halamert E, et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol. 2008;52(21):1724–32. doi: 10.1016/j.jacc.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 30.Meijboom WB, Meijs MF, Schuijf JD, Cramer MJ, Mollet NR, van Mieghem CA, et al. Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J Am Coll Cardiol. 2008;52(25):2135–44. doi: 10.1016/j.jacc.2008.08.058. [DOI] [PubMed] [Google Scholar]
- 31.Miller JM, Rochitte CE, Dewey M, Arbab-Zadeh A, Niinuma H, Gottlieb I, et al. Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med. 2008;359(22):2324–36. doi: 10.1056/NEJMoa0806576. [DOI] [PubMed] [Google Scholar]
- 32.Cheitlin MD, Armstrong WF, Aurigemma GP, Beller GA, Bierman FZ, Davis JL, et al. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography–summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography) J Am Coll Cardiol. 2003;42(5):954–70. doi: 10.1016/s0735-1097(03)01065-9. [DOI] [PubMed] [Google Scholar]
- 33.McNeer JF, Margolis JR, Lee KL, Kisslo JA, Peter RH, Kong Y, et al. The role of the exercise test in the evaluation of patients for ischemic heart disease. Circulation. 1978;57(1):64–70. doi: 10.1161/01.cir.57.1.64. [DOI] [PubMed] [Google Scholar]
- 34.Assmann G, Cullen P, Schulte H. Simple scoring scheme for calculating the risk of acute coronary events based on the 10-year follow-up of the prospective cardiovascular Munster (PROCAM) study. Circulation. 2002;105(3):310–5. doi: 10.1161/hc0302.102575. [DOI] [PubMed] [Google Scholar]
- 35.Lampe FC, Whincup PH, Wannamethee SG, Shaper AG, Walker M, Ebrahim S. The natural history of prevalent ischaemic heart disease in middle-aged men. Eur Heart J. 2000;21(13):1052–62. doi: 10.1053/euhj.1999.1866. [DOI] [PubMed] [Google Scholar]
- 36.Thavendiranathan P, Bagai A, Brookhart MA, Choudhry NK. Primary prevention of cardiovascular diseases with statin therapy: a meta-analysis of randomized controlled trials. Arch Intern Med. 2006;166(21):2307–13. doi: 10.1001/archinte.166.21.2307. [DOI] [PubMed] [Google Scholar]
- 37.Institute for clinical and economic review. Final appreaisal document - Coronary computed tomographic angiography for detection of coronary artery disease. 2009 http://icer-review.org/wp-content/uploads/2013/04/CCTA_Final.pdf.
- 38.National Heart, Lung, and Blood Institute. NHLBI Morbidity and Mortality Chart Book. 2012 https://www.nhlbi.nih.gov/research/reports/2012-mortality-chart-book.
- 39.Weintraub WS, Spertus JA, Kolm P, Maron DJ, Zhang Z, Jurkovitz C, et al. Effect of PCI on quality of life in patients with stable coronary disease. N Engl J Med. 2008;359(7):677–87. doi: 10.1056/NEJMoa072771. [DOI] [PubMed] [Google Scholar]
- 40.Nease RF, Jr, Kneeland T, O’Connor GT, Sumner W, Lumpkins C, Shaw L, et al. Variation in patient utilities for outcomes of the management of chronic stable angina. Implications for clinical practice guidelines. Ischemic Heart Disease Patient Outcomes Research Team. JAMA. 1995;273(15):1185–90. [PubMed] [Google Scholar]


