Introduction
For decades, radical nephrectomy (RN) has been the gold standard for treatment of renal masses; however, with the understanding of the importance of preserving kidney function and the advent of minimally invasive approaches, the indications for RN, particularly through an open surgical approach, are diminishing [1]. Nephron-sparing surgeries, through open and minimally invasive techniques, are now routinely used to treat localized small renal tumors [2]. The decision to perform a partial nephrectomy, and the choice of which surgical approach to take, involves a complex interaction between various patient factors (e.g. comorbid conditions, age, and body habitus) and renal tumor morphology [3]. Some factors that influence the surgical planning and decision for minimally invasive partial nephrectomy are the anatomic relationship of the tumor with other structures and its location within the kidney [4].
Standardized reporting systems such as the R.E.N.A.L. Nephrometry Score, a renal anatomy scoring system used to assess tumor complexity, have been used to guide surgical approach for renal tumor surgery [5]. The R.E.N.A.L Nephrometry Score measures the following: Radius (maximal diameter in cm) of the tumor, Exophitic/endophytic location of the tumor, Nearness of the tumor to the collecting system or renal sinus (mm), Anterior/posterior location of tumor, and Location of tumor relative to the polar lines. Anatomic complexity as assessed by Nephrometry Score is associated with prolonged warm ischemia time during partial nephrectomy [6], and with volume loss and functional recovery after partial nephrectomy [7]. This descriptive score based approach, although helpful for pre-operative planning and reporting of data, is generally used only as a supplement to visual inspection of the radiographic studies by our urologists, who prefer to examine the images prior to the surgery.
Two-dimensional (2D) and three-dimensional (3D) stacks of images are typically used to assess the tumor anatomy prior to surgery, with 3D reformatted images also available. However, complex relationships between the tumor and various nearby structures may be difficult to obtain from these images alone. As the field of urology moves away from open, RN to minimally invasive and robotic partial nephrectomy, surgeons and trainees often do not obtain a tactile familiarity with the tumor until it is removed [8]. Pre-operative 3D printed models of renal masses may therefore facilitate surgical planning by allowing surgeons to better assess the relationship of the tumor to major anatomic structures such as the renal vasculature and collecting system [9-14], and possibly by providing visuo-haptic input that could further improve understanding of the complex relationship of the tumor with surrounding structures.
Computed tomography (CT) images are generally used to create 3D printed models [13,15]; however, magnetic resonance imaging (MRI) is an attractive alternative, since it offers superior soft-tissue characterization and flexible image contrast mechanisms, and avoids the use of ionizing radiation or iodinated contrast [16]. The objective of the current study was to determine whether patient-specific 3D printed renal tumor models derived from MRI change pre-operative planning decisions made by urological surgeons in preparation for complex renal mass surgical procedures.
Materials and Methods
Patient Selection
A registry of patients who received clinically indicated and research MRI prior to surgery for renal masses between 2011 and 2015 (n=74) was retrospectively reviewed by an attending urologist with expertise in kidney cancer surgery to identify 10 cases with Nephrometry Scores greater than 5. Nephrometry Scores of these 10 cases ranged from 6-10 (average = 8.3). This study was approved by the Institutional Review Board at the New York University School of Medicine.
Image Acquisition and Post-Processing
Pre-operative images were acquired on a 1.5T MR System (Avanto, Siemens, Erlangen, Germany) using a phased array body coil. A 3D post-contrast fat-suppressed gradient-echo T1 weighted sequence with an interpolated spatial resolution of 1.4 mm × 1.4 mm × 2 mm was used to generate the 3D printed models. Standard sequence parameters were the following: TR = 3.58 ms, TE = 1.3 ms, FA = 12°, acquisition time of breath-hold ranged from 13 to 20 seconds. Post-operative images were obtained approximately 6 months after surgery using the same imaging parameters.
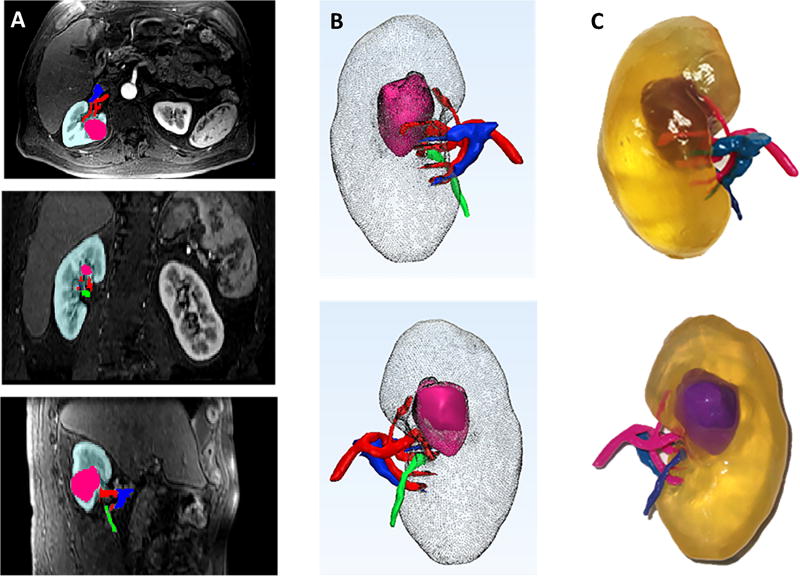
All MR images were imported to a dedicated software platform (Mimics, Materialise, Leuven, BE), which was used for 3D visualization, image segmentation, and generation of Stereolithography (STL) files. Image post-processing was performed by a research scientist with five years of post-processing experience. The kidney tumor, kidney cortex and medulla, main renal artery, main renal vein, and ureter were segmented as five separate anatomical regions of interest (ROIs) (Fig. 1a). For all ROIs, both thresholding and manual editing was performed to ensure that only the anatomy of interest was selected. Each ROI was converted to a separate 3D object and combined into a 3D virtual model (Fig. 1b). The segmentation data, which were in DICOM format, were converted to STL format so that they could be recognized by the 3D printer. Smoothing was performed to minimize the pixelated appearance (3-matic, Materialise, Leuven, BE). Each kidney was printed in a clear, transparent flexible material (HeartPrint Flex, Materialise, Leuven, BE). Different combinations of rigid cyan and rigid magenta (Vero Cyan and Vero Magenta, Stratasys, Eden Prairie, MN) were used and 3D printing was performed (Connex 500, Stratasys, Eden Prairie, MN) (Fig. 1c). Image post-processing and printing times were recorded.
Fig. 1.
(a) Axial, coronal, and sagittal views with segmentation masks for one representative case. Kidney = teal, tumor = pink, artery = red, vein = blue, collecting system = green (b) Anterior and Posterior 3D projections. Kidney = gray, tumor = pink, artery = red, vein = blue, ureter = green. (c) Photographs of 3D printed model. Kidney = transparent, tumor = purple, artery = pink, vein = light blue, ureter = dark blue.
The FDA currently requires medical 3D printing software to be approved. Certifying the accuracy of 3D printers is however out of the realm of the FDA [17]. It is important to ensure the accuracy of 3D printed medical models. Therefore, in order to assess the accuracy of the 3D printed kidney models, a measurement phantom was designed using computer-aided design (CAD) software (Fig. 2a) and 3D printed in the same materials as the kidneys (Fig. 2b). The phantom was 50 × 50 mm2 in in total dimension. It consisted of six 5mm × 10mm rectangles, six 2mm × 10mm rectangles, six 1mm × 10mm rectangles, one 3mm diameter circle, one 6mm diameter circle, and one 10mm diameter circle. Each circle diameter was measured four times (twice in both the x and y directions) and the length and width of each rectangle were measured twice, on the 3D printed phantom. Measurements made on the 3D printed phantom were compared to CAD file measurements. In addition, tumor diameter measurements were made on the 2D images and were compared to caliper measurements made on the exophytic tumors of the 3D printed models. Six exophytic tumors were measured in two dimensions, right to left and craniocaudal (CC), and one partially exophytic tumor was measured in one dimension (CC); therefore allowing 13 total measurements. Measurements performed on the 3D printed models were performed in a similar manner to those shown in a recent study by Ripley et al [18].
Fig. 2.
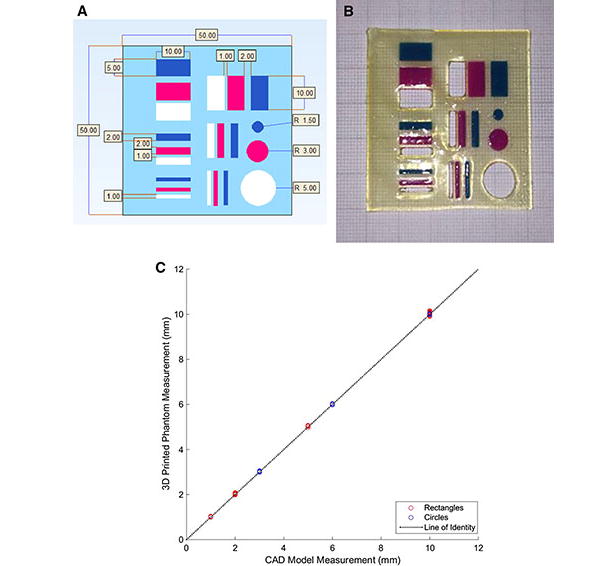
(a) CAD file of measurement phantom showing desired measurements in mm. Light blue = Heart print flex material, Dark blue = Vero Cyan material, Pink = Vero Magenta material, White = No material and (b) 3D printed phantom shown overlaid on graph paper in order to demonstrate accuracy of 3D printing. Each dark line on the graph paper represents 1cm and each light line represents 1mm. (c) Correlation plot showing agreement between CAD model measurements and caliper measurements made of 3D printed phantom model.
Pre- and post- operative renal volumes were calculated using the segmentation data. The degree of volume loss in the operated kidney was correlated with ischemia time, Nephrometry Score, and changes in decisions between hypothetically selected pre-surgical planning decisions with and without the 3D model.
Pre-Operative Planning
Renal mass cases were reviewed individually by three experienced urologists, one with 17 years and two with 12 years of experience. First, cases were reviewed with imaging alone on the picture archiving and communication system (PACS). Next, in a separate session with at least one week separation, cases were then reviewed with imaging in addition to the 3D printed model. All cases were blinded and the case order was randomized in both sessions. All urologists had extensive experience performing pre-operative planning using the PACS system which has 3D multi-planar reformatting capability.
A questionnaire was completed during each session; and the planned surgical approach, including decisions regarding (1) partial or radical nephrectomy, (2) open or robotic approach, (3) transperitoneal or retroperitoneal approach, and (4) clamping were evaluated with and without the 3D model (Table 1). Operative notes were reviewed to determine how the actual procedures were performed. The hypothetically preferred pre-operative approaches with and without the models were compared to each other as well as to the actual surgical intervention as determined in the operative notes.
Table 1.
Pre-surgical physician questionnaire regarding surgical approach and procedure.
| Question | Response Choices |
|---|---|
| 1. What kind of tumor removal would you perform? | Partial or Radical? |
| 2. How would you perform the procedure? | Open or Robotic? |
| 3. What approach would you take in order to perform this procedure? | Transperitoneal or Retroperitoneal? |
| 4. What type of clamping would you use? | None, Selective, or Complete? |
At the end of review of all cases, the surgeons were asked if 3D models helped significantly with comprehension of anatomy and surgical planning and whether the models helped to increase their confidence that the surgery was planned correctly.
Statistical Analysis
Correlation between measurements made on 2D data sets and 3D printed models were calculated using the Pearson correlation coefficient. In order to determine the difference between the measurements, a Bland Altman analysis was performed. The planned operative approach as determined on the 2D image review and 3D printed model were compared to the actual surgical approach and agreement was calculated for each surgeon. Correlation between volume loss and nephrometry score was also calculated using the Pearson correlation coefficient. Statistical analysis was performed using MiniTab 17 (MiniTab Inc, State College, Pennsylvania).
Results
Image Post–Processing/Printing
Image post-processing time was approximately 7 hours per kidney model. Each model took approximately 10 hours to print and cost about $US 1000. (See discussion for further consideration of time and cost). There was a high degree of correlation between CAD model dimensions and measurements made on the 3D printed phantom model (84 measurements total, r=1.000, r2 =1.000, p < 0.001) (Fig. 2c). Measurements in 3D printed phantom models exceeded the size compared to the reference CAD measurements by an average of 0.04mm (1.2 ± 1.6 %). Tumor diameter measurements made on the 2D image sets were in good agreement to measurements made on the corresponding 3D printed models (n=13, r = 0.988, r2 =0.974, p <0.001). Measurements on the 3D printed model also exceeded 2D measurements by an average of 0.76mm (0.6 ± 1.9 %) (Fig. 3).
Fig. 3.
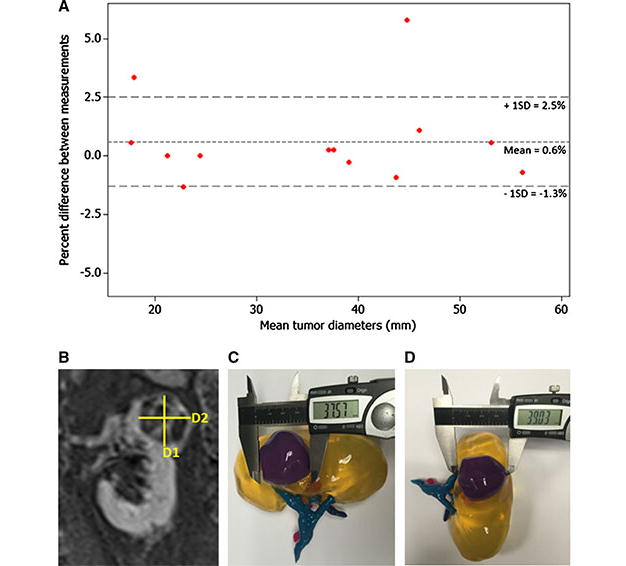
3D models accurately depict tumor size. (a) Bland-Altman plot of diameter measurements made on 2D images versus 3D models. The fact that the points lie around the mean demonstrates that there is no inherent bias between the two methods. (b) Coronal MRI showing two diameter measurements (D1 = 39.1mm and D2 =37.5mm). (c) Diameter measurement D1 of 3D printed model by calipers. (d) Diameter measurement D2 measured on the same 3D printed model.
Pre-Operative Planning
Results for the planned surgical decisions made with and without the 3D model are discussed below (Table 2).
Partial or Radical Nephrectomy: Compared to conventional image interpretation on PACS, with use of the 3D model, one of the surgeons changed the potential surgical approach in 1 (10%) case, and another surgeon changed the approach in 2 (20%) cases.
Open or Robotic Approach: Compared to conventional image interpretation on PACS, with use of the 3D model, two of the three surgeons changed their approach in 1 case (10%) each.
Retroperitoneal or Transperitoneal approach: Compared to conventional imaging interpretation, with use of the 3D model one surgeon changed the approach in 4 cases (40%), and two surgeons changed the approach in 3 cases (30%) each.
Clamping: With use of 3D model, one surgeon changed the clamping approach in 5 cases (50%), whereas two surgeons changed approach in 4 cases (40%) each.
Table 2.
Number of times each surgeon changed his/her survey answer to the survey questions when the 3D model was provided (when compared to conventional imaging) during the surgical planning work-up.
| Radical or Partial? | Open or Robotic? | Trans or Retro? | Clamping? | |
|---|---|---|---|---|
| Surgeon 1 | 1 (10%) | 1 (10%) | 4 (40%) | 5 (50%) |
| Surgeon 2 | 0 | 0 | 3 (30%) | 4 (40%) |
| Surgeon 3 | 2 (20%) | 1 (10%) | 3 (30%) | 4 (40%) |
Nine out of the ten subjects were included in the analysis comparing pre-operative planning decisions to what procedure was actually performed in the operating room. One subject was excluded from the analysis since the surgery was not performed at our institution. Concordance between preoperative plan as determined on the conventional imaging and 3D model with actual surgical intervention is as follows (Table 3):
Partial or Radical Nephrectomy: Planned decisions matched what was performed in the actual surgical procedure 100% of the time with the 3D model as compared to 92.6% with the conventional image evaluation.
Open or Robotic Approach: The proposed approach matched the actual surgical approach 81.5% of the time with the 3D printed model versus 77.8% with the conventional image interpretation.
Retroperitoneal or Transperitoneal Approach: The proposed approach matched the actual surgery performed 55.6% of the time with use of 3D model versus 59.3% with conventional imaging.
Clamping: Use of 3D model resulted in a 85.2% match with actual surgical approach compared to a 81.5% match with the conventional image evaluation.
Table 3.
Concordance between pre-operative decisions using imaging alone versus imaging plus 3D printed model as compared to actual surgical procedure. Percentage indicates the percentage of time that the pre-operative decision matched the actual surgical approach (for 9 cases*, 27 total survey answers for each question). *One surgical procedure was not performed at our institution.
| Pre-operative Decision | Imaging | Imaging + 3D Model |
|---|---|---|
| Q1: Radical or Partial Nephrectomy? | 92.6% | 100% |
| Q2: Robotic Laparoscopic or Open? | 77.8% | 81.5% |
| Q3: Retroperitoneal or Transperitoneal? | 59.3% | 55.6% |
| Q4: Clamping? | 81.5% | 85.2% |
After reviewing all ten cases with and without the model, all three surgeons reported that (1) the 3D printed model helped with comprehension of anatomy, (2) with regards to decisions on surgical approach, and (3) increased their confidence that they correctly planned the procedure.
In terms of actual surgical outcomes, mean parenchymal volume loss for the operated kidney was 21.4cc (minimum = 3.2cc, maximum = 90.5cc) six months post-operatively. Volume losses corresponding to >20% (mean = 29.8cc) were associated with increased ischemia times (Fig. 4a). In addition, surgeons tended to alter their planned surgical approach more often with use of the 3D printed models in these cases with >20% volume loss (Fig. 4b), with decisions regarding radical or partial nephrectomy (20%), retroperitoneal or transperitoneal approach (26.7%), and open or robotic approach (7%) changing the most. For this set of complex patients, post-operative volume loss did not correlate with Nephrometry Score (r=0.345, p=0.363).
Fig. 4.
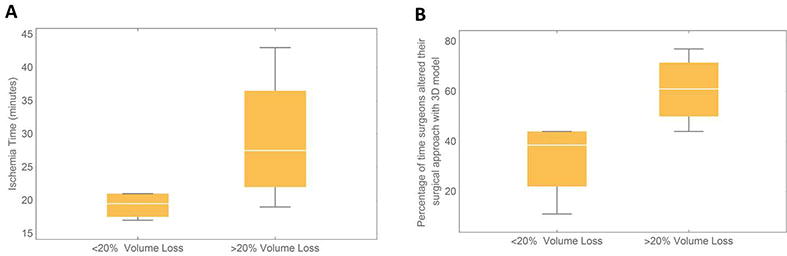
Box plots demonstrating that operated kidney volume loss (a) correlates with ischemia time (b) correlates with changes in decision making regarding surgical approach with and without 3D model. *Whiskers extend to maximum and minimum values.
Discussion
In this study, we created ten anatomically accurate, patient-specific 3D printed renal tumor models from MRI data and evaluated their impact in pre-surgical planning. A strong correlation was noted between the actual measurement on the CAD model and the measurements on the 3D printed phantom. Although there was excellent correlation, the Bland-Altman analysis suggested that there was a slight systematic overestimation in size on both the 3D printed measurement phantom and the 3D printed kidney tumor models.
The translucency of the 3D printed models allows easy visualization of the location and size of the tumor as well as the relationship of the tumor to key anatomical structures such as the renal artery and renal vein. Furthermore, 3D printed models allow surgeons to touch the renal tumor and renal parenchyma, thereby enhancing their understanding of the anatomy and facilitating surgical planning.
Our results indicate that even experienced urologists may potentially benefit from the 3D printed models for planning of complex surgeries. Specifically, pre-operative 3D printed renal mass models could potentially promote nephron-sparing surgery and preservation of healthy parenchyma, as surgeons gain a better understanding of the size and location of a tumor in relation to normal tissue and vital structures such as the arteries and veins.
In this study, pre-operative decisions, including decisions regarding (1) partial or radical nephrectomy, (2) open or laparoscopic procedure, (3) transperitoneal or retroperitoneal approach, and (4) clamping were altered with the 3D model. The most frequent changes in pre-surgical planning were seen in decisions regarding transperitoneal or retroperitoneal approach and clamping (30-50%).
Additionally, the concordance between the actual surgical approach and pre-operative surgical decisions regarding nephrectomy type, laparoscopic or open approach, and clamping improved with the use of the 3D model. Since the concordance with what was actually performed improved with the 3D model, it is possible that 3D printed models may facilitate better anticipation of patient-specific anatomy and better planning for a complex surgery, potentially allowing for less changes to be made in the operating room, therefore reducing duration of induced ischemia or complications related to complex tumor anatomy.
For the complex surgeries that resulted in an actual parenchymal volume loss of more than 20%, surgeons altered their planned approach more often when a 3D model was available, which suggests that in patients with high degree of anatomic complexity, the pre-operative 3D printed model might be useful in surgical planning of complex renal masses. Furthermore, we found that for this set of cases, with Nephrometry, post-operative volume loss correlated with warm ischemia time, but did not depend on Nephrometry Score. It is possible that the lack of correlation with Nephrometry Score may be due to the Nephrometry Score in part being driven by the size. For example, a Nephrometry Score of 7 due to large size does not reflect the same degree of surgical difficulty as a small mass that is completely endophytic or touching the collecting system. This will be investigated in future studies.
Similar to the findings by Zhang et al [12], who created 3D printed kidney tumor models from CT data and evaluated the usefulness of the models, the physicians in our study reported that the 3D models are useful. One key difference is that our 3D printed models were derived from MRI and not CT data. Although CT is the most widely used technique for the characterization of renal masses [19, 20] MRI is considered comparable to CT by the American College of Radiology. MRI is advantageous as compared to CT since it allows a highly flexible choice of protocols and provides exquisite soft tissue contrast without ionizing exposure. In addition, Zhang et al did not address specific questions regarding surgical planning or describe how the 3D printed models impacted specific surgical planning decisions. The present study addressed these questions and demonstrated how pre-surgical planning decisions regarding nephrectomy type, surgical approach, clamping, and collecting system repair may be impacted with the use of the 3D model.
Our study had several limitations. First, our study was limited by a small number of subjects. Also, since this was a retrospective evaluation, we could not determine from the operative reports whether the surgical plan was altered by the surgeon during the procedure. In addition, the impact of the 3D models on patient understanding of their disease and treatment plan or on renal function preservation could not be evaluated. Furthermore, although 3D printing from MRI data is feasible, present implementation is time consuming (mean image post-processing time of 7 hours and mean printing time 10 hours) and costly ($US 1000 per kidney tumor case). Indirect costs including Information Technology support and opportunity costs were not measured in the current study. In the future, automated image segmentation methods specific for MRI data and new 3D printing technologies may facilitate this 3D printing workflow and may also decrease cost. Two urologic surgeons reported that more of the internal anatomy (i.e. distal arterial branches and renal calyces) should be shown in the 3D printed models in order to determine if anything was directly feeding the tumor and whether or not to anticipate collecting system repair. This would facilitate planning for selective arterial clamping and could further decrease the region of ischemia. Further work is needed to improve the resolution of the 3D printed models to determine the proximity of the tumor to the vasculature and to study differences in planned vessel clamping and functional outcomes. Finally, printing materials do not accurately mimic tissue properties, and therefore currently do not allow realistic simulations of surgery for training.
Conclusion
Our study demonstrates that anatomically accurate 3D models can be generated from MRI data and these 3D printed models may influence pre-operative planning for anatomically complex renal masses by experienced urologists. There was high correlation between percentage of volume loss and changes in surgical planning decisions with the 3D printed model, which suggests that the 3D printed models may provide the surgeons with a better ability to plan the for nephron sparing surgeries. A larger, prospective study is currently being implemented at our institution to determine the overall impact of the 3D printed models on pre-operative planning for renal cancer.
Acknowledgments
Grant Support: This work was supported by the Center for Advanced Imaging Innovation and Research (www.cai2r.net), an NIBIB Biomedical Technology Resource Center (NIH P41 EB017183).
Abbreviations
- MRI
Magnetic resonance imaging
- CT
Computed tomography
- 3D
Three-dimensional
- 2D
Two-dimensional
- RN
Radical nephrectomy
- STL
Stereolithography
- PACS
Picture archiving and communication system
- CAD
Computer aided design
- ROI
Region of interest
Footnotes
IRB Approval: The Institutional Review Board of the New York University School of Medicine approved this study.
Conflict of Interest: All authors of this manuscript disclose no conflict of interest.
Compliance with Ethical Standards: Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all patients at the time of recruitment to the research study.
References
- 1.Campbell SC, Novick AC, Belldegrun A, et al. Guideline for management of the clinical T1 renal mass. J Urol. 2009;182(4):1271–9. doi: 10.1016/j.juro.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Zargar H, Allaf ME, Bhayani S, et al. Trifecta and optimal perioperative outcomes of robotic and laparoscopic partial nephrectomy in surgical treatment of small renal masses: a multi-institutional study. BJU Int. 2015;116(3):407–14. doi: 10.1111/bju.12933. [DOI] [PubMed] [Google Scholar]
- 3.Broughton GJ, Clark PE, Barocas DA, et al. Tumour size, tumour complexity, and surgical approach are associated with nephrectomy type in small renal cortical tumours treated electively. BJU Int. 2012;109(11):1607–13. doi: 10.1111/j.1464-410X.2011.10607.x. [DOI] [PubMed] [Google Scholar]
- 4.Funahashi Y, Murotani K, Yoshino Y, Sassa N, Ishida S, Gotoh M. The renal tumor morphological characteristics that affect surgical planning for laparoscopic or open partial nephrectomy. Nagoya J Med Sci. 2015;77(1-2):229–35. [PMC free article] [PubMed] [Google Scholar]
- 5.Canter D, Kutikov A, Manley B, Egleston B, et al. Utility of the R.E.N.A.L. nephrometry scoring system in objectifying treatment decision-making of the enhancing renal mass. Urology. 2011;78(5):1089–94. doi: 10.1016/j.urology.2011.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tomaszewski JJ, Smaldone MC, Mehrazin R, et al. Anatomic complexity quantitated by nephrometry score is associated with prolonged warm ischemia time during robotic partial nephrectomy. Urology. 2014;84(2):340–4. doi: 10.1016/j.urology.2014.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simmons MN, Hillyer SP, Lee BH, Fergany AF, Kaouk J, Campbell SC. Nephrometry score is associated with volume loss and functional recovery after partial nephrectomy. J Urol. 2012;188(1):39–44. doi: 10.1016/j.juro.2012.02.2574. [DOI] [PubMed] [Google Scholar]
- 8.Poon SA, Silberstein JL, Chen LY, Ehdaie B, Kim PH, Russo P. Trends in partial and radical nephrectomy: an analysis of case logs from certifying urologists. J Urol. 2013;190(2):464–9. doi: 10.1016/j.juro.2013.02.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitsouras D, Liacouras P, Imanzadeh A, et al. Medical 3D Printing for the Radiologist. Radiographics. 2015;35(7):1965–88. doi: 10.1148/rg.2015140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsumoto JS, Morris JM, Foley TA, et al. Three-dimensional Physical Modeling: Applications and Experience at Mayo Clinic. Radiographics. 2015;35(7):1989–2006. doi: 10.1148/rg.2015140260. [DOI] [PubMed] [Google Scholar]
- 11.Silberstein JL, Maddox MM, Dorsey P, Feibus A, Thomas R, Lee BR. Physical models of renal malignancies using standard cross-sectional imaging and 3-dimensional printers: a pilot study. Urology. 2014;84(2):268–72. doi: 10.1016/j.urology.2014.03.042. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Ge HW, Li NC, et al. Evaluation of three-dimensional printing for laparoscopic partial nephrectomy of renal tumors: a preliminary report. World J Urol. 2015;34(4):533–7. doi: 10.1007/s00345-015-1530-7. [DOI] [PubMed] [Google Scholar]
- 13.Rengier F, Mehndiratta A, von Tengg-Kobligk H, et al. 3D printing based on imaging data: review of medical applications. Int J Comput Assist Radiol Surg. 2010;5(4):335–41. doi: 10.1007/s11548-010-0476-x. [DOI] [PubMed] [Google Scholar]
- 14.Wake N, Rude T, Huang C, Stifelman MD, Borin JF, Sodickson DK, Chandarana H. 3D Printed Renal Cancer Models Derived from MRI data: Application in Pre-surgical Planning. Proceedings of the ISMRM Singapore. 2015 doi: 10.1007/s00261-016-1022-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Esses SJ, Berman P, Bloom AI, Sosna J. Clinical applications of physical 3D models derived from MDCT data and created by rapid prototyping. AJR. 2011;196(6):W683–8. doi: 10.2214/AJR.10.5681. [DOI] [PubMed] [Google Scholar]
- 16.Wake N, Chandarana H, Huang WC, Taneja SS, Rosenkrantz AB. Application of anatomically accurate, patient-specific 3D printed models from MRI data in urological oncology. Clin Radiol. 2016;71:610–614. doi: 10.1016/j.crad.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 17.DiParma M, Coburn J, Hwang D, Kelly J, Khairuzzaman A, Ricles L. Additively manufactured medical products – the FDA perspective. 3D Printing in Medicine. 2016;2(1):1–6. doi: 10.1186/s41205-016-0005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ripley B, Kelil T, Cheezum MK, Goncalves A, Di Carli MF, Rybicki FJ, Steigner M, Mitsouras D, Blankstein R. 3D printing based on cardiac CT assists anatomic visualization prior to transcatheter aortic valve replacement. JCCT. 2016;10:28–36. doi: 10.1016/j.jcct.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang SK, Huang WC, Pandharipande PV, Chandarana H. Solid renal masses: what the numbers tell us. AJR. 2014;202:1196–1206. doi: 10.2214/AJR.14.12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heilbrun ME, Casilano DD, Beland MD, Bishoff JT, Blaufox MD, Coursey CA, Goldfarb S, Harvin HJ, Nikolaidis P, Preminger GM, Raman SS, Sahni VA, Vikram R, Weinfeld RM, Remer EM. ACR Appropriateness Criteria: indeterminate renal mass. ACR; Reston, VA: 2014. p. 11. [DOI] [PubMed] [Google Scholar]