Abstract
A tug-of-war between the mammalian host and bacterial pathogen for nutrients, including first-row transition metals (e.g. Mn, Fe, Zn), occurs during infection. Here we present recent advances about three metal-chelating metabolites that bacterial pathogens deploy when invading the host: staphylopine, staphyloferrin B, and enterobactin. These highlights provide new insights into the mechanisms of bacterial metal acquisition and regulation, as well as the contributions of host-defense proteins during the human innate immune response. The studies also underscore that the chemical composition of the microenvironment at an infection site can influence bacterial pathogenesis and the innate immune system.
Introduction
The bioinorganic chemistry of infectious disease examines metal homeostasis in the context of the host/pathogen interaction. Transition metal ions are essential nutrients for all organisms, and microbial pathogens must acquire these metal nutrients from the host to replicate and cause infection [1–5]. To counteract pathogenic invaders, the host mounts a metal-withholding response in an attempt to starve microbes and thereby prevent microbial growth. This innate immune response is often termed “nutritional immunity” [6]. The complex machineries employed by the host and microbe when competing for metal nutrients provide opportunities for discovery and mechanistic investigation, as we illustrate below, as well as possibilities for new therapeutic strategies to prevent and treat infectious disease.
In this Current Opinion, we highlight recent fundamental investigations of three different microbial metallophores. These metal-chelating secondary metabolites are biosynthesized and deployed by bacterial pathogens for nutrient metal acquisition in the host (Figure 1). Two vignettes address secondary metabolites of Staphylococcus aureus, a Gram-positive opportunistic human pathogen that causes infections ranging from pneumonia to sepsis [7,8]. We describe the discovery of staphylopine [9], a metallophore that S. aureus employs for acquiring multiple divalent first-row transition metals. Next, we present a link between staphylococcal heme uptake and the biosynthesis of staphyloferrin B, a high-affinity iron chelator or siderophore that is implicated in the virulence of S. aureus [10]. In the third vignette, we discuss enterobactin (Ent), a siderophore of Escherichia coli, and describe work that probes the interplay among Ent, other iron-chelating metabolites, and the human host-defense protein lipocalin-2 (LCN2) [11]. Taken together, these advances illuminate small-molecule metabolite biosynthesis and metal homeostasis, inform our understanding of the competition for transition metals in the host/pathogen interaction, and provide inspiration for further explorations of transition metals in human innate immunity and microbial pathogenesis.
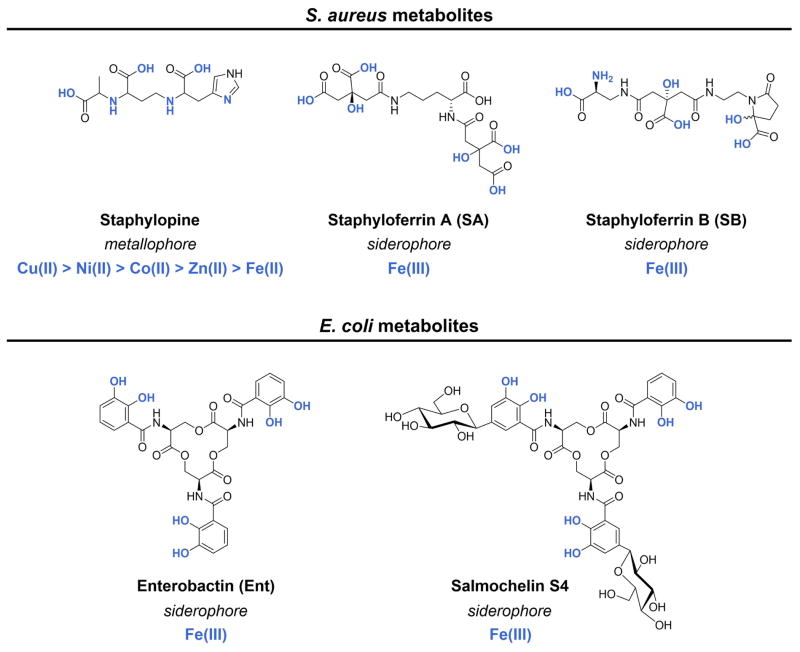
Figure 1.
Chemical structures of select metal-chelating metabolites produced by S. aureus and E. coli. The metal-coordinating groups and the relevant metal ions are shown in blue. Staphylopine coordinates and enables transport of multiple metal ions and the ordering reflects the relative metal-binding affinities.
Discovering staphylopine, a metallophore of Staphylococcus aureus
S. aureus is a Gram-positive opportunistic human pathogen and several of its metal transport systems have been shown to be important for its virulence, especially those involved in iron and manganese acquisition [12–15]. Nonetheless, S. aureus has metal requirements beyond iron and manganese, as exemplified by the recent discovery of staphylopine (Figure 1). This metal chelator allows S. aureus to acquire multiple metals, including nickel and zinc, from the host.
In prior studies, an ATP-binding cassette (ABC) metal transport system named CntABC (Cnt, cobalt-nickel transporter) (Figure 2A) was identified in S. aureus [16–19]. This ABC transporter allows S. aureus to internalize nickel and cobalt under low-zinc conditions, and was shown to contribute to S. aureus virulence in a murine model of urinary tract infection [16,17]. Subsequent biochemical and mass spectrometric investigations revealed that the solute-binding protein CntA recognizes and binds a nickel coordination complex composed of a single Ni(II) ion and a small molecule of unknown identity [20]. This observation suggested that S. aureus biosynthesizes and exports a secondary metabolite that scavenges nickel from the extracellular milieu and is subsequently captured by CntA. A similar scenario occurs in siderophore-mediated iron uptake where high-affinity Fe(III) chelators (siderophores) are biosynthesized and exported to scavenge Fe(III), and the ferric-siderophore complexes are recognized by dedicated transport machineries [21]. In contrast to siderophore-mediated iron transport where hundreds of siderophores are known and many have been shown to be important in the host/microbe interaction [22], only a few examples of secondary metabolites that pathogens use to transport other metal ions have been identified [23,24,25].
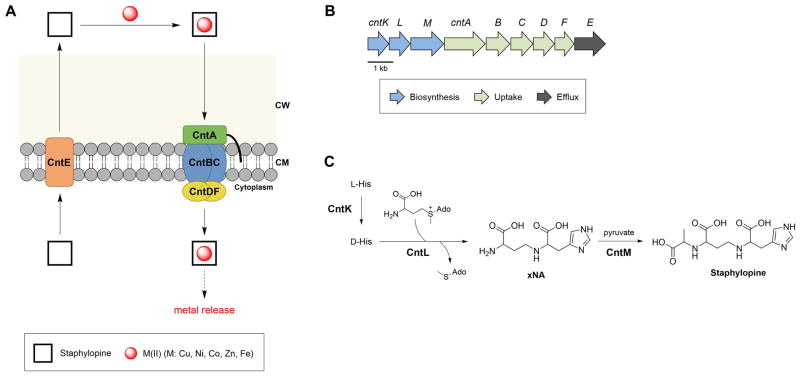
Figure 2.
Overview of staphylopine-based metal acquisition by S. aureus. (A) Cartoon of staphylopine transport by S. aureus. Staphylopine is biosynthesized in the cytosol (not shown) and exported by CntE. Following formation of a coordination complex in the extracellular space, CntA recognizes and binds M(II)-staphylopine, and the complex is transported across the cell membrane and into the cytoplasm by CntBCDF. The mechanism for metal release from staphylopine is unknown. (B) The cnt operon encoding genes for the biosynthesis, efflux, and uptake of staphylopine. (C) Biosynthesis of staphylopine. Abbreviations: CW, cell wall; CM, cell membrane.
Recently, a biosynthetic gene cluster (Figure 2B) for the biosynthesis of a putative staphylococcal metabolite was identified during two bioinformatics searches [9,20]. One investigation was a hunt for plant enzymes involved in the biosynthesis of nicotianamine, a metal chelator and biosynthetic precursor to the phytosiderophores [9]. A gene (hereafter cntL) cotranscribed with cntABC (the three genes encoding CntABC) and with low sequence similarity to the gene encoding Arabidopsis thaliana nicotianamine synthase (NAS) was found in staphylococcal genomes. Moreover, two additional putative biosynthetic genes (hereafter cntKM) were found in the same operon. These observations motivated efforts to isolate the putative metabolite and determine whether CntKLM are involved in its biosynthesis. Treatment of S. aureus culture supernatants with recombinant His6-tagged CntA resulted in the capture of staphylopine coordinated to one of several first-row transition metal ions (Co, Ni, Zn, Cu) [9]. Subsequent in vitro characterization of CntKLM established that these three enzymes are necessary and sufficient for the biosynthesis of staphylopine (Figure 2C). CntK is a L-His racemase, CntL is a NAS-like enzyme that accepts D-His as a substrate and catalyzes the nucleophilic attack of one α-aminobutyric acid moiety of S-adenosylmethionine (SAM) to form an intermediate named xNA, and CntM is a NADPH-dependent dehydrogenase that catalyzes the reductive condensation of xNA with pyruvate to afford staphylopine.
Functional studies of metal binding and metal transport employing staphylopine demonstrated that staphylopine (i) coordinates a number of divalent first-row transition metals with high affinity (Figure 1) and (ii) enables transport of these metals into S. aureus via CntABC (Figure 2A) [9]. These observations indicate that staphylopine is a versatile metal chelator, and that the metal ions bound and transported will depend on metal availability. Moreover, bioinformatics analysis revealed that several other human pathogens, including Gram-negative Pseudomonas aeruginosa, harbor genes for putative biosynthetic enzymes and transport proteins that are homologous to genes of the cnt cluster, which suggests that biosynthesis and utilization of staphylopine (or a structurally related molecule) may not be limited to the staphylococci [9]. Of note, a recent study concluded that an as-yet unidentified siderophore is important for the growth of P. aeruginosa in airway mucus [26]. The genes identified for the production of this molecule share homology with the staphylococcal cnt genes, indicating that either staphylopine or a staphylopine-like molecule contributes to P. aeruginosa pathogenesis in the cystic fibrosis lung. The discovery and structural elucidation of staphylopine provides a foundation for future studies of this molecule in the context of metal homoeostasis, host/microbe and microbe/microbe interactions.
Elucidating a role for heme in the regulation of staphyloferrin B, a siderophore of Staphylococcus aureus
To fulfill its iron requirements, S. aureus employs heme- and siderophore-mediated iron-acquisition systems (Figure 3A) [12,27]. The global transcriptional regulator Fur (Ferric uptake regulator) regulates expression of these iron-uptake pathways [28–30]. S. aureus obtains iron from heme using the iron-regulated surface determinate (Isd) system, which is composed of nine proteins (IsdABCDEFGHI) (Figure 3A) [31]. For siderophore-based iron uptake, S. aureus harbors two gene clusters for the biosynthesis and transport of the citrate-based polycarboxylate siderophores, staphyloferrin A (SA) and staphyloferrin B (SB) (Figure 3B, shown only for SB) [27,32–36]. SB is considered to be a virulence factor of S. aureus [37–39]. The sbn gene locus (sbnABCDEFGHI) encodes machinery for SB biosynthesis (SbnABCEFGH) and efflux (SbnD) [37], and the genes of the nearby sir locus (sirABC) encode an ABC transporter (SirABC) that recognizes and transports Fe(III)-SB into the cytoplasm. (Figure 3A) [40].
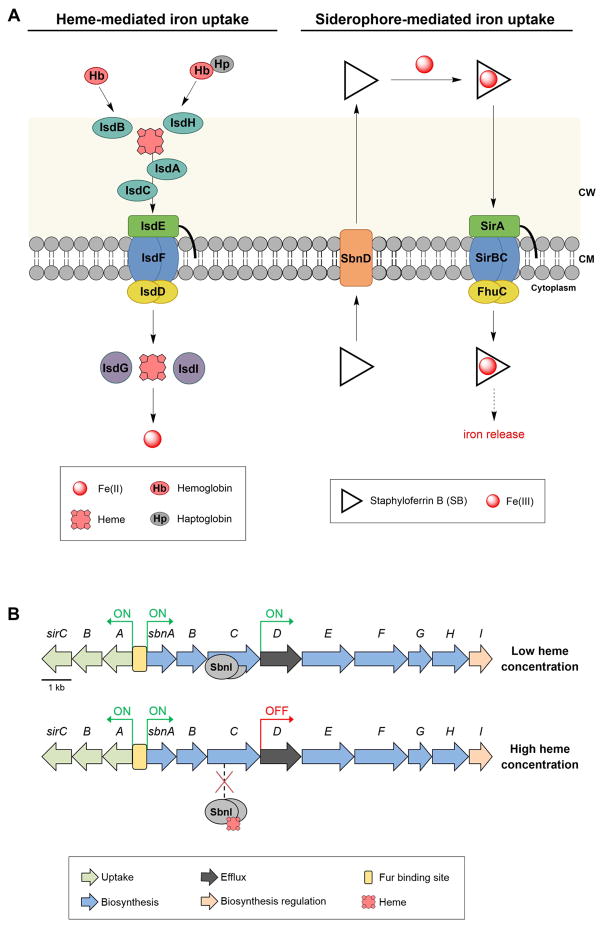
Figure 3.
Overview of heme- and siderophore-based iron acquisition by S. aureus. (A) Cartoon of heme- and SB-mediated iron uptake. Left: heme-mediated iron uptake. Four proteins (IsdABCH) are anchored to the peptidoglycan layer and are involved in the capture of the heme-binding proteins hemoglobin(Hb) and haptoglobin (Hp), removal of heme from these proteins, and transfer of heme across the cell wall, which results in delivery of heme to an ABC transporter (IsdDEF). Following cytoplasmic entry, heme oxygenases (IsdGI) degrade the heme and release iron. Right: SB-mediated iron uptake. SB is biosynthesized in the cytoplasm (not shown) and exported by SbnD. Following capture of Fe(III) in the extracellular space, Fe(III)-SB is recognized and transported into the cytoplasm by SirABC. The mechanism for iron release from SB is unknown, but likely involves a reductase. (B) The sir and sbn loci encoding gens for the biosynthesis, efflux, and uptake of SB. Under low-iron conditions, both operons can be expressed because the global repressor Fur (not shown) is in the apo form and does not bind to the DNA.[30] Further regulation of the SB biosynthesis occurs as a result of the presence or absence of heme. Under low-heme conditions, the SbnI dimer binds to an internal promoter in the coding region of sbnC and promotes expression of sbnD-H, whereas under high-heme conditions, heme binding to SbnI prevents binding to the DNA and thereby reduces the production of SB. Abbreviations: CW, cell wall; CM, cell membrane.
Until recently, the function of sbnI, a gene conserved among S. aureus strains, was unknown. A sbnI-deficient mutant strain of S. aureus exhibited a severe growth defect under iron-limited growth conditions [10]; however, enzymology studies revealed that SbnI is not required for the in vitro biosynthesis of SB [40], and no evidence for a role of sbnI in SB transport was found. Indeed, recent investigations demonstrated that SbnI is a regulatory protein. It binds DNA upstream of sbnD and is required for the expression of SbnD and the biosynthetic enzymes SbnEFGH (Figure 3B) [10]. Further interrogation revealed that SbnI binds heme, and that formation of the SbnI-heme complex prevents the SbnI-DNA interaction. Thus, this study (i) indicates that S. aureus regulates SB production by switching SB biosynthesis on or off in the absence or presence of heme, respectively (Figure 3B), and (ii) suggests that SbnI is a heme sensor and regulatory protein, providing new insight into the regulation of SB biosynthesis [10]. This model is reminiscent of prior work that demonstrated S. aureus will preferentially use heme as an iron source when heme is available [40].
The heme-regulated biosynthesis of SB illustrates a new example where the regulation of siderophore biosynthesis extends beyond global regulators like Fur. It is noteworthy that expression of the sir gene locus is independent of SbnI and heme availability (Figure 3B). This organization indicates that S. aureus may import Fe(III)-SB when biosynthesis of the metabolite is turned off, which may provide a competitive advantage. S. aureus also biosynthesizes heme [42], and investigation of whether there is crosstalk between the heme and SB biosynthetic pathways will be informative.
Illuminating the tug-of-war for iron between the host and enterobactin, a siderophore of Escherichia coli
E. coli are both human commensal gut microbes and pathogens, and pathogenic strains cause diseases ranging from diarrhea to urinary tract infections and sepsis [43,44]. All E. coli biosynthesize the triscatecholate siderophore enterobactin (Ent, Figure 1) [45], and many pathogenic strains harbor additional siderophore biosynthesis and transport systems (e.g. salmochelins, yersiniabactin, aerobactin) [43]. The human innate immune system blocks Ent-mediated iron acquisition by deploying a host-defense protein named lipocalin-2 (LCN2; also known as neutrophil gelatinase-associated lipocalin (NGAL), siderocalin (Scn), uterocalin, 24p3) [46,47]. In the canonical model for LCN2-mediated host defense, neutrophils or epithelial cells release this protein into the extracellular space where it captures Fe(III)-Ent (Figure 4) [46,47]. Structural and biophysical studies of LCN2 revealed that positively charged residues within its calyx provide electrostatic and cation-π interactions with this ferric siderophore [46,48], enabling the protein to coordinate Fe(III)-Ent with high affinity. When abundant, LCN2 starves E. coli strains that rely on Ent for iron acquisition in the host [47,49,50]. Pathogens that biosynthesize so-called “stealth siderophores” (e.g. salmochelin S4, Figure 1) are able to evade the LCN2-mediated immune response because the sugar moieties prevent these molecules from being captured by LCN2 [4,51,52] LCN2 levels are elevated in women with urinary tract infections caused by E. coli [11,53], and how this host-defense protein contributes to innate immunity in the urinary tract is a topic of current interest.
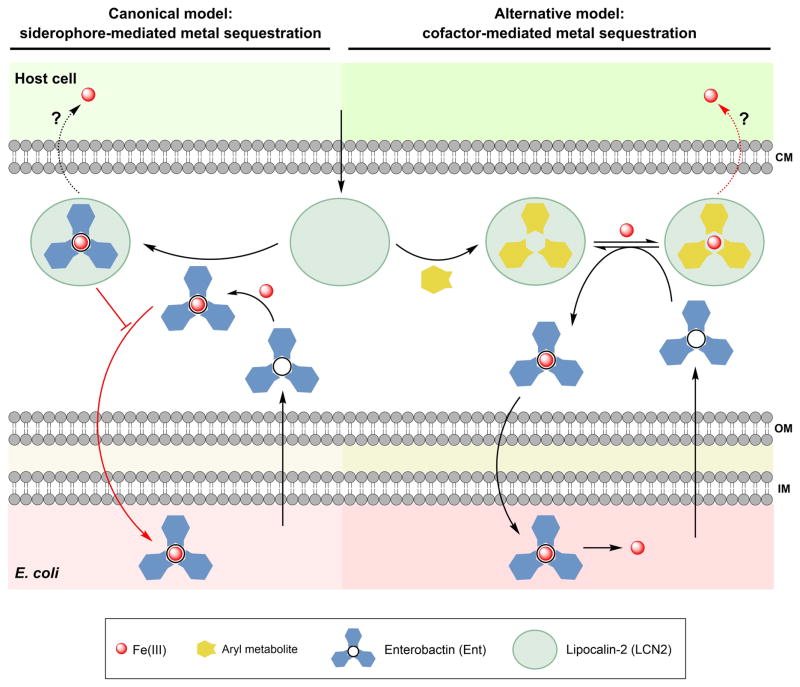
Figure 4.
Cartoon depicting two models for how the host and E. coli compete for iron that involve enterobactin (Ent) and lipocalin-2 (LCN2). Canonical model: LCN2 sequesters Fe(III)-Ent in the extracellular space and thereby prevents Fe uptake by E. coli. Alternative model based on studies in human urine: LCN2 binds aryl metabolites present in urine and sequesters Fe(III). Metallation of Ent contributes to the demetallation of LCN2. Ent may prevent Fe(III) from binding to LCN2 and/or sequester Fe(III) from the Fe(III)-catechol-LCN2 complex, and the resulting Fe(III)-Ent provides nutrient iron to E. coli. Iron-bound LCN2 might be transported into host cells by receptor-mediated uptake. Abbreviations: CM, cell membrane; OM, outer membrane; IM, inner membrane.
Here, we highlight a recent investigation that evaluated how the chemical composition of urine influences LCN2-mediated growth inhibition of uropathogenic E. coli UTI89. This report provides an alternative model for the interplay between Ent and this host-defense protein (Figure 4) [11]. E. coli UTI89 biosynthesizes multiple siderophores (Ent, salmochelins, and yersiniabactin) [54]. In this work, growth studies performed in a chemically defined medium were in agreement with the canonical model and demonstrated that LCN2 provided maximal growth inhibition of a UTI89 mutant strain that can only biosynthesize Ent. In contrast, growth assays conducted in pooled human urine showed that LCN2 afforded maximal growth inhibition of a UTI89 mutant strain that cannot biosynthesize Ent. Under the same conditions, negligible growth inhibition was observed for mutant strains that produce Ent but are deficient in the biosynthesis of yersiniabactin and the salmochelins. It should also be noted that the antibacterial activity of LCN2 against wild-type UTI89 was attenuated in pooled urine relative to the activity observed in chemically defined medium by ≈ 10-fold.
Further examination of urine samples from individual patients revealed that pH and aryl metabolites (e.g. aryl alcohols, catecholates) influence the antibacterial activity of LCN2 [11]. In particular, the presence of aryl metabolites in urine enhanced the growth inhibitory activity of LCN2, which suggested that LCN2 can withhold iron from E. coli in a siderophore-independent manner by using aryl metabolites as cofactors (Figure 4). The ability of LCN2 to harbor Fe(III) complexes in the calyx upon binding catecholate metabolites was previously reported in studies of LCN2-mediated iron trafficking in the circulatory system [55], and later for LCN2 isolated from human urine [56]. Moreover, the recent work indicates that Ent appears to have the capacity to liberate Fe(III) from the LCN2-bound aryl metabolites in urine, thereby promoting E. coli growth [11]. Taken together, these in vitro assays indicate that the interaction of Ent and LCN2 depends on the growth conditions, and provide the basis for an alternative model where Ent enables UTI89 to overcome LCN2-mediated growth restriction in urine.
The interplay among Ent, aryl metabolites and LCN2 appears to be markedly dependent on the individualistic chemical features of human urine [11], which underscores the complexity of the host environment and emphasizes that host-to-host variations exist and can alter the course of an infection. Whether aryl metabolites affect the growth inhibitory activity of LCN2 and growth of E. coli at other sites where infections occur (e.g. gut, blood) warrants consideration. Lastly, the sequestration of Fe(III)-Ent (or another siderophore) by LCN2 presents a rather narrow-spectrum antibacterial mechanism where only strains that require the entrapped ferric siderophore for growth are affected. In contrast, employing available metabolites implicates a more general mechanism of iron-withholding that may allow for growth inhibition of multiple bacteria strains, and that can only be overcome by siderophores or other as-yet undetermined microbial metal acquisition machinery able to sequester the bound iron from LCN2.
Summary and perspectives
The recent advances about bacterial metallophores employed by human pathogens summarized here have provided remarkable insights into the chemistry and biology of the host/pathogen interaction. The discovery of staphylopine has provided a new broad-spectrum metallophore that appears to be an important virulence factor for S. aureus and will enrich future studies at the microbe/microbe and host/microbe interface. The recent demonstration of heme-dependent siderophore biosynthesis exemplifies how a pathogen can exquisitely regulate specific iron-acquisition strategies in response to available iron sources. Lastly, the new model for the iron-withholding function of LCN2 and proposed role of Ent in liberating Fe(III) from this host-defense protein in the urinary tract emphasizes the need to consider how subtle changes in the chemical composition of an infection site or microenvironment affect virulence factors and pathogenicity. It also serves as a reminder that standard laboratory conditions and the host environment are markedly different. In closing, the outcomes of these three initiatives provide foundations for both fundamental and applied investigations that interface bioinorganic chemistry with microbial pathogenesis and infectious disease, including explorations of novel approaches to prevent and treat bacterial infections.
Highlights.
Staphylopine is a metallophore that S. aureus deploys to acquire first-row transition metals.
The protein SbnI provides a link between heme uptake and siderophore biosynthesis in S. aureus.
Aryl metabolites may participate in the competition for iron between the host and microbe.
Acknowledgments
We acknowledge NIH Grants 1R01AI114625 and 1R21AI126465 for supporting our current research on siderophores. WN is a recipient of a Leopoldina Fellowship of the German National Academy of Sciences Leopoldina (LPDS 2015-08).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hood MI, Skaar EP. Nutritional immunity: Transition metals at the pathogen-host interface. Nat Rev Microbiol. 2012;10:525–537. doi: 10.1038/nrmicro2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cassat JE, Skaar EP. Iron in infection and immunity. Cell Host Microbe. 2013;13:509–519. doi: 10.1016/j.chom.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ma Z, Jacobsen FE, Giedroc DP. Coordination chemistry of bacterial metal transport and sensing. Chem Rev. 2009;109:4644–4681. doi: 10.1021/cr900077w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fischbach MA, Lin H, Liu DR, Walsh CT. How pathogenic bacteria evade mammalian sabotage in the battle for iron. Nat Chem Biol. 2006;2:132–138. doi: 10.1038/nchembio771. [DOI] [PubMed] [Google Scholar]
- 5.Brophy MB, Nolan EM. Manganese and microbial pathogenesis: Sequestration by the mammalian immune system and utilization by microorganisms. ACS Chem Biol. 2015;10:641–651. doi: 10.1021/cb500792b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weinberg ED. Nutritional immunity. Host’s attempt to withold iron from microbial invaders. JAMA. 1975;231:39–41. doi: 10.1001/jama.231.1.39. [DOI] [PubMed] [Google Scholar]
- 7.Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, Craig AS, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–1771. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 8.Kraus D, Peschel A. Staphylococcus aureus evasion of innate antimicrobial defense. Future Microbiol. 2008;3:437–451. doi: 10.2217/17460913.3.4.437. [DOI] [PubMed] [Google Scholar]
- 9••.Ghssein G, Brutesco C, Ouerdane L, Fojcik C, Izaute A, Wang S, Hajjar C, Lobinski R, Lemaire D, Richaud P, Voulhoux R, et al. Biosynthesis of a broad-spectrum nicotianamine-like metallophore in Staphylococcus aureus. Science. 2016;352:1105–1109. doi: 10.1126/science.aaf1018. Studies of the structure and biosynthesis of staphylopine, and its function in metal transport, are reported for the first time. The discovery of staphylopine informs prior studies of metal homeostasis in the human pathogens Staphylococcus aureus and Pseudomonas aeruginosa and reveals that one metal chelator and ABC transport system can provide import of various first-row transition metals depending on the growth conditions. [DOI] [PubMed] [Google Scholar]
- 10••.Laakso HA, Marolda CL, Pinter TB, Stillman MJ, Heinrichs DE. A heme-responsive regulator controls synthesis of staphyloferrin B in Staphylococcus aureus. J Biol Chem. 2016;291:29–40. doi: 10.1074/jbc.M115.696625. This work elucidates the function of SbnI, a protein encoded in the gene cluster for staphyloferrin B biosynthesis and export, in the regulation of siderophore biosynthesis. A model is presented that involves the interplay between the heme- and SB-mediated iron acquisition pathways utilized by S. aureus. SbnI is a heme sensor and regulatory protein that binds both heme and DNA. Under low-heme conditions, SbnI binds DNA only and turns on the biosynthesis of SB. Under high-heme conditions, SbnI binds heme and dissociates from the DNA, which turns off SB biosynthesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11••.Shields-Cutler RR, Crowley JR, Hung CS, Stapleton AE, Aldrich CC, Marschall J, Henderson JP. Human urinary composition controls antibacterial activity of siderocalin. J Biol Chem. 2015;290:15949–15960. doi: 10.1074/jbc.M115.645812. This work examines how the chemical composition of urine influences LCN2 (siderocalin) activity and presents a new model where enterobactin can help E. coli overcome the LCN2-mediated immune response. In this model, LCN2 is suggested to sequester Fe(III) by using aryl metabolites present in urine as cofactors rather than sequestering Fe(III)-bound bacterial siderophores. Growth studies performed in human urine indicated that the antibacterial activity of LCN2 against E. coli was promoted by the presence of aryl metabolites. Moreover, Ent seems to be able to outcompete the LCN2 complex for Fe(III) and thereby provide E. coli with this nutrient metal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hammer ND, Skaar EP. Molecular mechanisms of Staphylococcus aureus iron acquisition. Annu Rev Microbiol. 2011;65:129–147. doi: 10.1146/annurev-micro-090110-102851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheldon JR, Heinrichs DE. Recent developments in understanding the iron acquisition strategies of Gram positive pathogens. FEMS Microbiol Rev. 2015;39:592–630. doi: 10.1093/femsre/fuv009. [DOI] [PubMed] [Google Scholar]
- 14.Lisher JP, Giedroc DP. Manganese acquisition and homeostasis at the host-pathogen interface. Front Cell Infect Microbiol. 2013;3:91. doi: 10.3389/fcimb.2013.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corbin BD, Seeley EH, Raab A, Feldmann J, Miller MR, Torres VJ, Anderson KL, Dattilo BM, Dunman PM, Gerads R, Caprioli RM, et al. Metal chelation and inhibition of bacterial growth in tissue abscesses. Science. 2008;319:962–965. doi: 10.1126/science.1152449. [DOI] [PubMed] [Google Scholar]
- 16.Remy L, Carrière M, Derré-Bobillot A, Martini C, Sanguinetti M, Borezée-Durant E. The Staphylococcus aureus Opp1 ABC transporter imports nickel and cobalt in zinc-depleted conditions and contributes to virulence. Mol Microbiol. 2013;87:730–743. doi: 10.1111/mmi.12126. [DOI] [PubMed] [Google Scholar]
- 17.Hiron A, Posteraro B, Carrière M, Remy L, Delporte C, La Sorda M, Sanguinetti M, Juillard V, Borezée-Durant E. A nickel ABC-transporter of Staphylococcus aureus is involved in urinary tract infection. Mol Microbiol. 2010;77:1246–1260. doi: 10.1111/j.1365-2958.2010.07287.x. [DOI] [PubMed] [Google Scholar]
- 18.Coulter SN, Schwan WR, Ng EYW, Langhorne MH, Ritchie HD, Westbrock-Wadman S, Hufnagle WO, Folger KR, Bayer AS, Stover CK. Staphylococcus aureus genetic loci impacting growth and survival in multiple infection environments. Mol Microbiol. 1998;30:393–404. doi: 10.1046/j.1365-2958.1998.01075.x. [DOI] [PubMed] [Google Scholar]
- 19.Hiron A, Borezée-Durant E, Piard J-C, Juillard V. Only one of four oligopeptide transport systems mediates nitrogen nutrition in Staphylococcus aureus. J Bacteriol. 2007;189:5119–5129. doi: 10.1128/JB.00274-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20•.Lebrette H, Borezée-Durant E, Martin L, Richaud P, Boeri Erba E, Cavazza C. Novel insights into nickel import in Staphylococcus aureus: The positive role of free histidine and structural characterization of a new thiazolidine-type nickel chelator. Metallomics. 2015;7:613–621. doi: 10.1039/c4mt00295d. Native mass spectrometry reveals that the solute-binding protein CntA of S. aureus binds a Ni(II) complex where the ligand is a small molecule with a mass of 327 Da. Subsequent studies demonstrated that this ligand is staphyopine (ref. 9). Moreover, this work presents a bioinformatics analysis that unveils three putative biosynthetic genes upstream of cntABC, which are cntKLM required for staphyopine biosynthesis (ref. 9) [DOI] [PubMed] [Google Scholar]
- 21.Krewulak KD, Vogel HJ. Structural biology of bacterial iron uptake. Biochim Biophys Acta. 2008;1778:1781–1804. doi: 10.1016/j.bbamem.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 22.Hider RC, Kong X. Chemistry and biology of siderophores. Nat Prod Rep. 2010;27:637–657. doi: 10.1039/b906679a. [DOI] [PubMed] [Google Scholar]
- 23.Johnstone TC, Nolan EM. Beyond iron: Non-classical biological functions of bacterial siderophores. Dalton Trans. 2015;44:6320–6339. doi: 10.1039/c4dt03559c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24•.Bobrov AG, Kirillina O, Fetherston JD, Miller MC, Burlison JA, Perry RD. The Yersinia pestis siderophore, yersiniabactin, and the ZnuABC system both contribute to zinc acquisition and the development of lethal septicaemic plague in mice. Mol Microbiol. 2014;93:759–775. doi: 10.1111/mmi.12693. This work describes a new function for the heterocyclic siderophore yersiniabactin in zinc acquisition, indicating that this metabolite is versatile and can provide Yersinia with transition metals beyond Fe(III) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koh EI, Hung CS, Parker KS, Crowley JR, Giblin DE, Henderson JP. Metal selectivity by the virulence-associated yersiniabactin metallophor esystem. Metallomics. 2015;7:1011–1022. doi: 10.1039/c4mt00341a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26•.Gi M, Lee K-M, Kim SC, Yoon J-H, Yoon SS, Choi JY. A novel siderophore system is essential for the growth of Pseudomonas aeruginosa in airway mucus. Sci Rep. 2015;5:14644. doi: 10.1038/srep14644. This work indicated that a gene, pa4834, is important for P. aeruginosa growth in airyway mucus and involved in metal homeostasis. It was hypothesized that this gene encodes a protein involved in the biosynthesis of nicotanamine, which is a plant metabolite and biosynthetic precursor to phytosiderophores. Based on susbequent studies in S. aureus and bioinformatics analysis (ref. 9), this molecule is likely staphylopine or a staphylopine-like molecule. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beasley FC, Heinrichs DE. Siderophore-mediated iron acquisition in the staphylococci. J Inorg Biochem. 2010;104:282–288. doi: 10.1016/j.jinorgbio.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 28.Torres VJ, Pishchany G, Humayun M, Schneewind O, Skaar EP. Staphylococcus aureus IsdB is a hemoglobin receptor required for heme iron utilization. J Bacteriol. 2006;188:8421–8429. doi: 10.1128/JB.01335-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friedman DB, Stauff DL, Pishchany G, Whitwell CW, Torres VJ, Skaar EP. Staphylococcus aureus redirects central metabolism to increase iron availability. PLoS Pathog. 2006;2:e87. doi: 10.1371/journal.ppat.0020087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Troxell B, Hassan HM. Transcriptional regulation by ferric uptake regulator (Fur) in pathogenic bacteria. Front Cell Infect Microbiol. 2013;3:59. doi: 10.3389/fcimb.2013.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mazmanian SK, Skaar EP, Gaspar AH, Humayun M, Gornicki P, Jelenska J, Joachmiak A, Missiakas DM, Schneewind O. Passage of heme-iron across the envelope of Staphylococcus aureus. Science. 2003;299:906–909. doi: 10.1126/science.1081147. [DOI] [PubMed] [Google Scholar]
- 32.Drechsel H, Freund S, Nicholson G, Haag H, Jung O, Zähner H, Jung G. Purification and chemical characterization of staphyloferrin B, a hydrophilic siderophore from staphylococci. Biometals. 1993;6:185–192. doi: 10.1007/BF00205858. [DOI] [PubMed] [Google Scholar]
- 33.Haag H, Fiedler H-P, Meiwes J, Drechsel H, Jung G, Zähner H. Isolation and biological characterization of staphyloferrin B, a compound with siderophore activity from staphylococci. FEMS Microbiol Lett. 1994;115:125–130. doi: 10.1111/j.1574-6968.1994.tb06626.x. [DOI] [PubMed] [Google Scholar]
- 34.Münzinger M, Taraz K, Budzikiewicz H. Staphyloferrin B, a citrate siderophore of Ralstonia eutropha. Z Naturforsch C. 1999;54:867–875. [Google Scholar]
- 35.Madsen JL, Johnstone TC, Nolan EM. Chemical synthesis of staphyloferrin B affords insight into the molecular structure, iron chelation, and biological activity of a polycarboxylate siderophore deployed by the human pathogen Staphylococcus aureus. J Am Chem Soc. 2015;137:9117–9127. doi: 10.1021/jacs.5b04557. [DOI] [PubMed] [Google Scholar]
- 36.Konetschny-Rapp S, Jung G, Meiwes J, Zähner H. Staphyloferrin A. A structurally new siderophore from staphylococci. Eur J Biochem. 1990;191:65–74. doi: 10.1111/j.1432-1033.1990.tb19094.x. [DOI] [PubMed] [Google Scholar]
- 37.Dale SE, Doherty-Kirby A, Lajoie G, Heinrichs DE. Role of siderophore biosynthesis in virulence of Staphylococcus aureus: Identification and characterization of genes involved in production of a siderophore. Infect Immun. 2004;72:29–37. doi: 10.1128/IAI.72.1.29-37.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beasley FC, Vinés ED, Grigg JC, Zheng Q, Liu S, Lajoie GA, Murphy MEP, Heinrichs DE. Characterization of staphyloferrin A biosynthetic and transport mutants in Staphylococcus aureus. Mol Microbiol. 2009;72:947–963. doi: 10.1111/j.1365-2958.2009.06698.x. [DOI] [PubMed] [Google Scholar]
- 39.Lindsay JA, Riley TV, Mee BJ. Production of siderophore by coagulase-negative staphylococci and its relation to virulence. Eur J Clin Microbiol Infect Dis. 1994;13:1063–1066. doi: 10.1007/BF02111829. [DOI] [PubMed] [Google Scholar]
- 40.Cheung J, Beasley FC, Liu S, Lajoie GA, Heinrichs DE. Molecular characterization of staphyloferrin B biosynthesis in Staphylococcus aureus. Mol Microbiol. 2009;74:594–608. doi: 10.1111/j.1365-2958.2009.06880.x. [DOI] [PubMed] [Google Scholar]
- 41.Skaar EP, Humayun M, Bae T, DeBord KL, Schneewind O. Iron-source preference of Staphylococcus aureus infections. Science. 2004;305:1626–1628. doi: 10.1126/science.1099930. [DOI] [PubMed] [Google Scholar]
- 42.Lobo SA, Scott A, Videira MA, Winpenny D, Gardner M, Palmer MJ, Schroeder S, Lawrence AD, Parkinson T, Warren MJ, Saraiva LM. Staphylococcus aureus haem biosynthesis: Characterisation of the enzymes involved in final steps of the pathway. Mol Microbiol. 2015;97:472–487. doi: 10.1111/mmi.13041. [DOI] [PubMed] [Google Scholar]
- 43.Kaper JB, Nataro JP, Mobley HL. Pathogenic Escherichia coli. Nat Rev Microbiol. 2004;2:123–140. doi: 10.1038/nrmicro818. [DOI] [PubMed] [Google Scholar]
- 44.Alteri CJ, Mobley HL. Escherichia coli physiology and metabolism dictates adaptation to diverse host microenvironments. Curr Opin Microbiol. 2012;15:3–9. doi: 10.1016/j.mib.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raymond KN, Dertz EA, Kim SS. Enterobactin. An archetype for microbial iron transport. Proc Natl Acad Sci U S A. 2003;100:3584–3588. doi: 10.1073/pnas.0630018100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goetz DH, Holmes MA, Borregaard N, Bluhm ME, Raymond KN, Strong RK. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol Cell. 2002;10:1033–1043. doi: 10.1016/s1097-2765(02)00708-6. [DOI] [PubMed] [Google Scholar]
- 47.Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, Akira S, Aderem A. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004;432:917–921. doi: 10.1038/nature03104. [DOI] [PubMed] [Google Scholar]
- 48.Bao G-H, Ho C-T, Barasch J. The ligands of neutrophil gelatinase-associated lipocalin. RSC Adv. 2016;5:104363–104374. doi: 10.1039/C5RA18736B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49•.Raymond KN, Allred BE, Sia AK. Coordination chemistry of microbial iron transport. Acc Chem Res. 2015;48:2496–2505. doi: 10.1021/acs.accounts.5b00301. This Accounts provides a ≈ 40-year perspective on studies of siderophores and microbial iron transport, and includes a discussion of siderophore-sequestering innate immune proteins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sia AK, Allred BE, Raymond KN. Siderocalins. Siderophore binding proteins evolved for primary pathogen host defense. Curr Opin Chem Biol. 2013;17:150–157. doi: 10.1016/j.cbpa.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fischbach MA, Lin H, Zhou L, Yu Y, Abergel RJ, Liu DR, Raymond KN, Wanner BL, Strong RK, Walsh CT, Aderem A, et al. The pathogen-associated iroA gene cluster mediates bacterial evasion of lipocalin 2. Proc Natl Acad Sci U S A. 2006;103:16502–16507. doi: 10.1073/pnas.0604636103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raffatellu M, George MD, Akiyama Y, Hornsby MJ, Nuccio S-P, Paixao TA, Butler BP, Chu H, Santos RL, Berger T, Mak TW, et al. Lipocalin-2 resistance confers an advantage to Salmonella enterica serotype Typhimurium for growth and survival in the inflamed intestine. Cell Host Microbe. 2009;5:476–486. doi: 10.1016/j.chom.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53•.Steigedal M, Marstad A, Haug M, Damås JK, Strong RK, Roberts PL, Himpsl SD, Stapleton A, Hooton TM, Mobley HLT, Hawn TR, et al. Lipocalin 2 imparts selective pressure on bacterial growth in the bladder and is elevated in women with urinary tract infection. J Immunol. 2014;193:6081–6089. doi: 10.4049/jimmunol.1401528. This study reports that LCN2 is secreted not only by the kidneys, but also by the urinary tract muscosa in response to uropathogenic E. coli. It also demonstrated a growth advantage for uropathogens that express receptors for stealth siderophores (salmochelin, aerobactin, yersiniabactin) in the presence of LCN2. The results from this study are consistent with the canonical model for LCN2-mediated growth inhibition of E. coli. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Henderson JP, Crowley JR, Pinkner JS, Walker JN, Tsukayama P, Stamm WE, Hooton TM, Hultgren SJ. Quantitative metabolomics reveals an epigenetic blueprint for iron acquisition in uropathogenic Escherichia coli. PLoS Pathog. 2009;5:e1000305. doi: 10.1371/journal.ppat.1000305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bao G, Clifton M, Hoette TM, Mori K, Deng S-X, Qiu A, Viltard M, Williams D, Paragas N, Leete T, Kulkarni R, et al. Iron traffics in circulation bound to a siderocalin (NGAL)-catechol complex. Nat Chem Biol. 2010;6:602–609. doi: 10.1038/nchembio.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bao G-H, Barasch J, Xu J, Wang W, Hu F-L, Deng S-X. Purification and structural characterization of “simple catechol”, the NGAL-siderocalin siderophore in human urine. RSC Adv. 2015;5:28527–28535. doi: 10.1039/C5RA02509E. [DOI] [PMC free article] [PubMed] [Google Scholar]