Abstract
The recent clinical success of CD19 directed chimeric antigen receptor (CAR) T-cell therapy in chronic and acute leukemia has led to increased interest in broadening this technology to other hematological malignancies and solid tumors. Now, advances are being made using CAR T cell technology to target myeloma antigens such as BCMA, CD138 and kappa-light chain as well as CD19 on putative myeloma stem cells. To date, only a limited multiple myeloma patients have received CAR-T cell therapy but preliminary results have been encouraging. In this review, we summarize the recently reported results of clinical trials conducted utilizing CAR T-cell therapy in MM.
Keywords: Chimeric antigen receptor, multiple myeloma, B-cell maturation antigen, adoptive T cell therapy, clinical trials
Introduction
Multiple Myeloma (MM) is a hematological malignancy defined by the accumulation of clonal plasma cells within the bone marrow with evidence of end organ damage that can be attributed to the underlying plasma cell proliferative disorder. Evidence of disease includes lytic bone lesions and/or pathologic fractures, hypercalcemia, anemia, renal impairment and increased susceptibility to opportunistic infections (1). As of 2016, the incidence of MM has reached 5.6 per 100,000 persons and accounts for approximately 10% of all hematological malignancies in the western world (1, 2). Traditionally, treatment of MM has focused on prolonging patient survival by reduction of malignant plasma cell burden followed by maintenance therapy. Recent insights into the pathophysiology of MM have led to the development of novel therapeutic agents, such as immune-modulatory drugs (e.g. lenalidomide, thalidomide, and pomalidomide) and proteasome inhibitors (e.g. bortezomib and carfilzomib). Over the last decade these new agents have revolutionized disease management and dramatically improved survival in newly diagnosed patients (3–5). When induction therapy utilizing these new agents is followed by autologous stem cell transplant (ASCT) and subsequent maintenance therapy, up to one-third of patients can achieve a complete response (CR) (3). Despite these recent treatment advances, disease recurrence still remains a major obstacle, with nearly all patients eventually relapsing with increasingly refractory disease (6). As MM remains an incurable disease, new treatment options are critical for these patients.
Chimeric Antigen Receptor T cells
The recent success of autologous CAR T-cells transduced to express a chimeric antigen receptor (CAR) targeting CD19 in relapsed/refractory (r/r) chronic lymphocytic leukemia CLL (CLL) (7, 8) and acute lymphoblastic leukemia (ALL) (9–11) has spurred new interest in broadening this technology to other hematological malignancies and solid tumors. CAR T-cells are genetically modified to express an antigen receptor recognizing a tumor associated surface antigen. Ideally, these tumor associated antigens would be uniquely expressed by the malignant cells and absent on normal tissues. In this way, CAR T-cells and their effector functions are redirected towards specific malignant targets in an MHC independent fashion.
Traditionally, CARs consist of an extracellular targeting region linked to various intracellular signaling domains. Targeting is achieved via a single-chain variable fragment (scFv) derived from a monoclonal antibody. The scFv is connected to the intracellular domains by a hinge/transmembrane region, commonly derived from CD8 or IgG4. The intracellular domains have conventionally consisted of either one (2nd generation CAR) or two (3rd generation CAR) co-stimulatory domains e.g. 4-1BB, CD28 or OX-40 linked to the cytoplasmic signaling domain of CD3ζ. The CD3ζ domain alone is not sufficient to drive optimal T-cell proliferation and cytokine production (12, 13), however the incorporation of various co-stimulatory domains can result in increased CAR T cell signaling, persistence and efficacy (14, 15). The effect of various co-stimulatory domains on T cell function differs in accordance with their original function. 4-1BBζ containing CARs have shown increased persistence and reduced exhaustion in vivo compared to CD28ζ CARs (14, 15), whereas CD28ζ CARs display increased cytokine production and may result in different kinetics of expansion following antigen stimulation. Although CD28ζ CARs appear to have faster and more robust functionality initially, they lack the ongoing persistence compared to their 41BBζ counterparts (14). So far, the use of 3rd generation CARs equipped with multiple co-stimulatory domains, have not shown increased efficacy compared to second generation CARs (14, 16).
Limiting CAR T cell related toxicities
CAR related toxicities can be divided into on-target, off-tumor effects and systemic toxicities. One of the major challenges in developing new CAR constructs is the identification of suitable and unique targets. Ideally, the target antigen should be tumor specific and play a fundamental role in maintaining tumorogenicity in order to limit antigen escape, while also lacking expression on normal life-sustaining tissues. Beyond target-mediated toxicities, CAR T-cells can also induce a systemic inflammatory syndrome termed cytokine release syndrome (CRS).
The issue of on-target, off-tumor effects, naturally varies based on the chosen antigen. Given the power of CAR-T cell therapy, targeting an antigen that is also expressed, even in low levels on normal tissues can have fatal consequences (17). The incorporation of a “suicide gene” into CAR T constructs allows for the elimination of CAR modified T cells in the event of toxicity (18, 19). Whether or not this approach will be beneficial depends on the depth and rapidity of CAR T-cell elimination, as CAR T-cell loss may take several days depending on the system used. Additional strategies include engineering CAR T cells to express specific chemokine receptors allowing direct homing to the tumor environment or minimizing trafficking to non-tumor sites (20, 21). Co-inhibitory receptors (iCAR) are also being investigated in an attempt to spare healthy tissue in the event of CAR recognition (22). This strategy is attractive, but requires identification of surface antigens that are only expressed on healthy tissue and not on tumor cells.
CRS is an increasingly recognized toxicity noted in various CAR T –cell trials in hematologic malignancies. In most patients, CRS symptoms are mild and include flu-like symptoms such as fever and mild hypotension. In more severe cases clinical features include: hypotension, hypoxia, capillary leak and coagulopathy all of which can potentially lead to fatal multi-organ dysfunction (23, 24). The hallmark of CRS is immune activation as noted by dramatic elevations in pro-inflammatory cytokines, such as IL-6, IL-10, IFN-γ and granulocyte macrophage-colony stimulating factor (GM-CSF) (17, 23, 24). In general, the presence of CRS correlates with expansion and activation of CAR T cells; in ALL, but not lymphomas, the severity of CRS is associated with increasing disease burden. The incidence and severity of CRS in patients receiving CAR T cells for multiple myeloma has not yet been defined. Although the clinical outcome of CAR therapy cannot be predicted on the basis of CRS severity, the majority of patients who respond to therapy do experience some degree of CRS. One challenge following recognition of CRS has been choosing an appropriate therapy that dampens the uncontrolled inflammatory response without reducing CAR T cell efficacy. Traditionally, management of CRS consisted of systemic administration of glucocorticoids, though prolonged glucocorticoid administration (e.g. > 14 days) may also dampen or ablate the function of infused CAR T cells (9, 23). Given the recognition of IL-6 as a primary mediator of CRS, newer approaches to management have targeted IL-6 itself with anti IL-6 receptor (IL-6R) antibodies such as tocilizumab (11, 23). The effect of IL-6R blockade in relation to CAR T cell proliferation, persistence and anti-tumor efficacy is still under investigation, but early data suggests that the use of anti-cytokine agents does not limit the anti-tumor efficacy of CAR T cells.
CARs in clinical trials for multiple myeloma
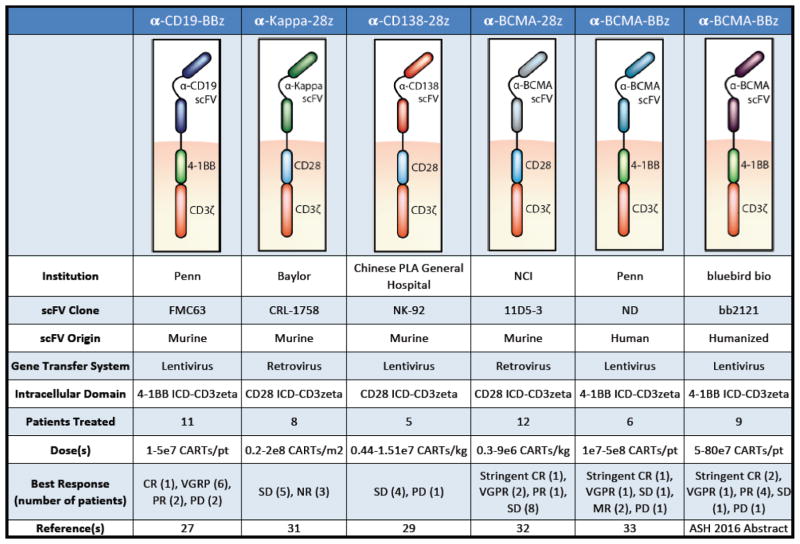
The interest in developing CARs that target MM has dramatically increased over the last few years. To date, the results of four clinical trials have been published and additional constructs are in development. The structure of these various CAR products differs with respect to targeted antigen, incorporation of co-stimulatory domains and reported anti-tumor efficacy in clinical trials (Figure 1).
Figure 1.
Comparative description of CAR-T cell strategies that have been used as immunotherapies against multiple myeloma
CD19
CAR T-cells directed against CD19 have shown tremendous potential in hematological malignancies such as ALL and CLL (9, 11). CD19 expression is not traditionally associated with MM and is generally not considered a therapeutic target in this disease (25). Despite the lack of CD19 expression on the plasma cell clones, some studies have identified expression on a minor MM stem cell subset (26) making CD19 a potential target for eliminating recurrence following elimination of differentiated plasma cells. These MM stem cells have been associated with increased drug-resistance and are potentially responsible for the incurable nature of MM. This observation was the rationale for conducting a clinical trial using a CAR against CD19(27). Garfall and Maus et al. described one patient treated with an anti-CD19-4-1BB-CD3ζ CAR T-cell product (CTL019) (27). The patient underwent CTL019 infusion following high dose melphalan and autologous stem cell transplant. Following infusion of CAR T cells, no fever or other signs of CRS were noted however CTL019 cells were detected in both blood and bone marrow for up to 47 days following infusion. The patient was started on lenalidomide maintenance treatment 3 months later, as per standard of care. Disease evaluation after 12 months demonstrated maintenance of a complete response (CR) to CTL019. This durable response was achieved despite the absence of CD19 expression on 99.95% of the patient’s malignant cells.
At the annual meeting of the American Society of Hematology (ASH) in December 2016, investigators from U. Penn presented updated data from a safety and feasibility trial of MM patients treated with CTL019. In total, 12 patients were enrolled in the study with 10 patients receiving infusion of CTL019 (5×107 CAR expressing T cells) 12–14 days after high-dose melphalan and ASCT. The treatment resulted in a median progression-free survival after ASCT + CTL019 of 185 days, with patients experiencing very good partial response (VGPR) n=6, partial response (PR) n=2 and progressive disease (PD) n=2. Overall, only minor adverse effects including mild CRS were observed and the product was well tolerated.
Although CD19 is expressed rarely on MM plasma cells, the presence of CD19 on a cancer stem-cell like population makes CD19 a potential target in combination with traditional therapies. Further studies are required to fully understand the mechanisms underlying the results reported in the CTL019 trials.
CD138
CD138, also known as syndecan 1, is a surface protein expressed on most healthy and malignant plasma cells (28). CD138 functions as an adhesion protein, binding collagen and fibronectin molecules located in the extracellular matrix. Because CD138 is highly expressed on MM cells, it is considered an attractive target. In 2016 a group at the Chinese PLA General Hospital reported on the treatment of chemotherapy-refractory MM with a CD138 (CART138) directed, 4-1BBζ CAR construct (29). Five patients received 3–6 CART138 injections following various combinations of pre-conditioning/induction therapy. Four out of five patients experienced mild fever, attributed to the second injection of CAR T cells. Four of five patients achieved stable disease (SD) ranging from three to seven months; CAR T cells were detected in bone marrow for upwards of 3 months. The fifth patient experienced PD and was transitioned to palliative care, despite detectable CART138 cells in bone marrow for 90 days. Data from this study are limited and should be interpreted cautiously. Despite the attractiveness of CD138 as a target for MM it does have potential drawbacks. First, CD138 is known to be expressed on epithelial tissues, and prior clinical trials investigating an anti-CD138-antibody drug-conjugate in MM treatment was notable for skin and/or mucosal toxicity (e.g. mucositis, stomatitis, hand/foot syndrome). Second, CD138 is shed from malignant cells thereby acting as a potential decoy for CD138 targeted T cells. Future studies targeting CD138 with CAR T cells may need to include strategies to avoid on-target toxicity while maintaining potential anti-tumor effects (30).
Kappa light chain
Given that mature B lymphocytes express either κ or λ light chains, but not both, Ramos et al. (31) recently developed a kappa-specific CAR construct that could recognize kappa restricted cells, while leaving the lambda-restricted subset intact, thereby maintaining at least partial humoral immunity. Although plasma cells do not express surface immunoglobulins (Igs), they reasoned that targeting the kappa-light chain may also be applicable to MM as several groups have described an MM-initiating population that expresses surface Igs (31). In their phase I clinical trial, seven MM patients were treated with a 2nd generation CD28-CD3ζ CAR T cell construct against the κ light chain (κ.CARTs). Patients received 1 – 2 infusions of κ.CARTs with a 50% reduction in the number of circulating B cells within the first two weeks of treatment. Encouragingly, the elimination of B cells was primarily of κ light chain origin. Four of seven MM patients experienced responses to infused κ.CARTs, with stable disease (>24 months), minimal residual disease, or overall improvement of disease symptoms. More robust responses were seen in the CLL and NHL patients likely due to their higher surface expression of κ light chain. The CAR T cell product was well tolerated and none of the patients experienced severe CRS.
BCMA
Recently, there has been much focus on the B-cell maturation antigen (BCMA) as a CAR target in MM. To date, four clinical trials are registered in the US using various CAR T-cell products against BCMA. BCMA is a cell surface protein exclusively expressed on the B-cell linage and is involved in the differentiation and maturation of B cells into plasma cells. BCMA is highly expressed on malignant MM plasma cells and provides a substantial anti-apoptotic signal making it an encouraging target for BCMA-directed immunotherapy. The first results from a clinical trial, utilizing a 2nd generation anti-BCMA-CAR with CD28, was published by Ali et al., in 2016. The trial was designed as a phase I dose-escalation trial, with 12 patients receiving either 0.3, 1, 3 or 9 × 106 CAR-positive T-cells per kg. Prior to CAR T-cell infusion, patients received lymphodepletion therapy (3 × 300 mg/m2 cyclophosphamide and 3 × 30 mg/m2 fludarabine). The higher dose cohorts experienced more pronounced adverse effects attributed to CAR-T cells, including CRS. CAR-BCMA-positive cells were detected in the blood of all patients in a dose dependent manner. Responses included a stringent CR (n=1), VGPR (n=2), PR (n=1) and SD for up to 16 weeks (n=8). The best responses occurred in the highest dose cohorts. Despite the concern for possible antigenic loss of BCMA following CAR T-cell therapy, only one patient in the CAR-BCMA study was reported to relapse with BCMA-negative MM clones in the bone marrow (32).
Two other BCMA-CAR products are currently being tested in clinical trials. Data from these trials were presented at the recent ASH meeting in 2016. Cohen et al., from the University of Pennsylvania, presented data from an ongoing phase I dose-escalation study, with a 2nd generation 4-1BB-CD3ζ anti-BCMA CAR. To date, six patients received CART-BCMA cells as split dose infusions (10% at day 0, 30% at day 1, and 60% at day 2). Five patients experienced CRS toxicity, with two patients requiring tocilizumab treatment. Interestingly, the two patients receiving tocilizumab had only received 40% of the planned CAR T cell dose due to fever. These patients experienced a robust anti-tumor response as demonstrated by a stringent CR and VGPR despite administration of tocilizumab and incomplete infusion of the targeted CAR T-cell dose. CART-BCMA cells were detected in both blood and bone marrow by PCR, with persistence reported for up to 7 months for the patient experiencing CR. Five months after therapy, the patient achieving a VGPR progressed, associated with a reduction in circulating CART-BCMA cells. Further analysis identified loss of BCMA expression on malignant cells, indicative of antigen escape. The remaining four patients experienced SD (n=1), minimal response (n=2) or PD (n=1), with minimal expansion of CART cells correlating to poorer responses (33). This same group also recently published a case report of posterior reversible encephalopathy syndrome (PRES) after infusion of anti-BCMA CAR T-cells. They reported on a 55-year-old female with high-risk IgA lambda MM who developed worsening neurological toxicity requiring intubation following CAR-T cell infusion despite treatment with tocilizumab and high dose steroids; treatment with cyclophosphamide reversed the syndrome. The rapid reversibility and MRI appearance was felt to be most consistent with PRES. They postulated that high CSF levels of CRS-related cytokines may have caused the syndrome. Additionally, they demonstrated that cyclophosphamide may effectively reverse life-threatening, steroid-refractory CAR T cell neurotoxicity while retaining some CAR T cell efficacy and long-term persistence (34).
Blubird Bio reported data in December 2016, from a cohort of 9 patients with relapsed/refractory MM who received infusion of an anti-BCMA CAR product (bb2121). Bb2121 is a 2nd generation CAR T cell product with 4-1BB co-stimulation. Patients received a single infusion of bb2121 at various doses (5, 15 or 45 × 107 CAR T cells) after cyclophosphamide/fludarabine pre-conditioning. In all patients, CAR T cell expansion and persistence were observed, ranging from 4 to 24 weeks after infusion. The best response was achieved in the cohort treated with 15 × 107 CAR T cells, with two patients showing stringent CR and one patient VGPR. In the low-dose cohort, three patients initially achieved PR, SD and PD respectively. All patients in this cohort experienced disease progression between eight and 11 weeks post CAR infusion. Overall, BCMA as antigen target for CART based immunotherapy seems promising with various clinical outcomes reflecting the various CAR construct design, dose, and baseline disease burden. Further studies are warranted, to determine the best BCMA CAR design for treatment of MM (35).
Conclusion
In this review, we have outlined the recent clinical data with CAR T cells as they transition as a platform from targeting B cell malignancies to targeting multiple myeloma with a variety of antigen targets. Multiple questions remain, including a thorough understanding of the potential toxicities specific to multiple myeloma, including cytokine release syndrome, the depth and duration of anti-tumor effects, and the role that CAR T cells may play in the management of MM, where there are multiple therapies available. So far, CARs utilizing BCMA seem to offer the most promise. As the field moves forward, new strategies to overcome the described limitations are being developed. With the introduction of dual-targeting CARs (e.g. iCAR or CARs recognizing two antigens), future treatment of MM with CAR T cell therapy may result in longer-lasting efficacy and avoid antigen escape. As in other hematologic diseases, antigen escape is emerging as a new hurdle, and is likely due to the selective pressure exerted by CARs. Therefore, more research and development into the optimal CAR construct, with limited toxicities, no antigen escape and high anti-tumor efficacy is still needed. However, with combined efforts between academic institutions and their industry partners, CARs have the potential for offering definitive therapy for MM.
Abbreviations
- MM
Multiple Myeloma
- ASCT
autologous stem cell transplant
- CAR
Chimeric Antigen Receptor
- r/r
relapsed/refractory
- CLL
Chronic Lymphocytic Leukemia
- ALL
Acute Lymphoblastic Leukemia
- scFv
single-chain variable fragment
- CRS
cytokine release syndrome
- IL-6R
IL-6 receptor
- iCAR
inhibitory CAR
- PRES
posterior reversible encephalopathy syndrome
- PCR
polymerase chain reaction
- BCMA
B-Cell maturation antigen
- Ig
immunoglobulin
- ASH
American Society of Hematology
- VGPR
very good partial response
- PR
partial response
- PD
progressive disease
- SD
stable disease
- CR
complete response
- NHL
Non Hodgkin’s lymphoma
Footnotes
Conflict of Interest
Maria Ormhøj declare no potential conflicts of interest.
Felipe Bedoya reports a patent pending.
Matthew J. Frigault reports a patent issued and royalties received for compositions and methods for generating a persisting population of t cells useful for the treatment of cancer.
Marcela V. Maus reports a patent pending with some licensed to Novartis for a portfolio relevant to the use of CART cells in general, and to multiple myeloma specifically, including CD19.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Rajkumar SV. Multiple myeloma: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol. 2016 Jul;91(7):719–34. doi: 10.1002/ajh.24402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011 Mar 17;364(11):1046–60. doi: 10.1056/NEJMra1011442. [DOI] [PubMed] [Google Scholar]
- 3.McCarthy PL, Owzar K, Hofmeister CC, Hurd DD, Hassoun H, Richardson PG, et al. Lenalidomide after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012 May 10;366(19):1770–81. doi: 10.1056/NEJMoa1114083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar SK, Dispenzieri A, Lacy MQ, Gertz MA, Buadi FK, Pandey S, et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia. 2014 May;28(5):1122–8. doi: 10.1038/leu.2013.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richardson PG, Xie W, Mitsiades C, Chanan-Khan AA, Lonial S, Hassoun H, et al. Single-agent bortezomib in previously untreated multiple myeloma: efficacy, characterization of peripheral neuropathy, and molecular correlations with response and neuropathy. J Clin Oncol. 2009 Jul 20;27(21):3518–25. doi: 10.1200/JCO.2008.18.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Majithia N, Rajkumar SV, Lacy MQ, Buadi FK, Dispenzieri A, Gertz MA, et al. Early relapse following initial therapy for multiple myeloma predicts poor outcomes in the era of novel agents. Leukemia. 2016 Nov;30(11):2208–13. doi: 10.1038/leu.2016.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011 Aug 10;3(95):95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Porter DL, Hwang WT, Frey NV, Lacey SF, Shaw PA, Loren AW, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015 Sep 2;7(303):303ra139. doi: 10.1126/scitranslmed.aac5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013 Mar 20;5(177):177ra38. doi: 10.1126/scitranslmed.3005930. Epub 2013/03/22. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turtle CJ, Hanafi LA, Berger C, Gooley TA, Cherian S, Hudecek M, et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest. 2016 Jun 01;126(6):2123–38. doi: 10.1172/JCI85309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013 Apr 18;368(16):1509–18. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gross G, Gorochov G, Waks T, Eshhar Z. Generation of effector T cells expressing chimeric T cell receptor with antibody type-specificity. Transplant Proc. 1989 Feb;21(1 Pt 1):127–30. [PubMed] [Google Scholar]
- 13.Brocker T. Chimeric Fv-zeta or Fv-epsilon receptors are not sufficient to induce activation or cytokine production in peripheral T cells. Blood. 2000;96(5):1999–2001. [PubMed] [Google Scholar]
- 14.Milone MC, Fish JD, Carpenito C, Carroll RG, Binder GK, Teachey D, et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther. 2009 Aug;17(8):1453–64. doi: 10.1038/mt.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Long AH, Haso WM, Shern JF, Wanhainen KM, Murgai M, Ingaramo M, et al. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat Med. 2015 Jun;21(6):581–90. doi: 10.1038/nm.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carpenito C, Milone MC, Hassan R, Simonet JC, Lakhal M, Suhoski MM, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci U S A. 2009 Mar 3;106(9):3360–5. doi: 10.1073/pnas.0813101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010 Apr;18(4):843–51. doi: 10.1038/mt.2010.24. Epub 2010/02/25. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoyos V, Savoldo B, Quintarelli C, Mahendravada A, Zhang M, Vera J, et al. Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their anti-lymphoma/leukemia effects and safety. Leukemia. 2010 Jun;24(6):1160–70. doi: 10.1038/leu.2010.75. Epub 2010/04/30. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Minagawa K, Jamil MO, Al-Obaidi M, Pereboeva L, Salzman D, Erba HP, et al. In Vitro Pre-Clinical Validation of Suicide Gene Modified Anti-CD33 Redirected Chimeric Antigen Receptor T-Cells for Acute Myeloid Leukemia. PLoS One. 2016;11(12):e0166891. doi: 10.1371/journal.pone.0166891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moon EK, Carpenito C, Sun J, Wang LC, Kapoor V, Predina J, et al. Expression of a functional CCR2 receptor enhances tumor localization and tumor eradication by retargeted human T cells expressing a mesothelin-specific chimeric antibody receptor. Clin Cancer Res. 2011 Jul 15;17(14):4719–30. doi: 10.1158/1078-0432.CCR-11-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Craddock JA, Lu A, Bear A, Pule M, Brenner MK, Rooney CM, et al. Enhanced tumor trafficking of GD2 chimeric antigen receptor T cells by expression of the chemokine receptor CCR2b. J Immunother. 2010 Oct;33(8):780–8. doi: 10.1097/CJI.0b013e3181ee6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fedorov VD, Themeli M, Sadelain M. PD-1- and CTLA-4-based inhibitory chimeric antigen receptors (iCARs) divert off-target immunotherapy responses. Sci Transl Med. 2013 Dec 11;5(215):215ra172. doi: 10.1126/scitranslmed.3006597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014 Jul 10;124(2):188–95. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brentjens R, Yeh R, Bernal Y, Riviere I, Sadelain M. Treatment of chronic lymphocytic leukemia with genetically targeted autologous T cells: case report of an unforeseen adverse event in a phase I clinical trial. Mol Ther. 2010 Apr;18(4):666–8. doi: 10.1038/mt.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tembhare PR, Yuan CM, Venzon D, Braylan R, Korde N, Manasanch E, et al. Flow cytometric differentiation of abnormal and normal plasma cells in the bone marrow in patients with multiple myeloma and its precursor diseases. Leuk Res. 2014 Mar;38(3):371–6. doi: 10.1016/j.leukres.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hajek R, Okubote SA, Svachova H. Myeloma stem cell concepts, heterogeneity and plasticity of multiple myeloma. Br J Haematol. 2013 Dec;163(5):551–64. doi: 10.1111/bjh.12563. [DOI] [PubMed] [Google Scholar]
- 27**.Garfall AL, Maus MV, Hwang WT, Lacey SF, Mahnke YD, Melenhorst JJ, et al. Chimeric Antigen Receptor T Cells against CD19 for Multiple Myeloma. N Engl J Med. 2015 Sep 10;373(11):1040–7. doi: 10.1056/NEJMoa1504542. Important paper presenting results from a clinical trial with a patient treated for multiple myeloma with CD19 CAR T cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wijdenes J, Vooijs WC, Clement C, Post J, Morard F, Vita N, et al. A plasmocyte selective monoclonal antibody (B-B4) recognizes syndecan-1. Br J Haematol. 1996 Aug;94(2):318–23. doi: 10.1046/j.1365-2141.1996.d01-1811.x. [DOI] [PubMed] [Google Scholar]
- 29.Guo B, Chen M, Han Q, Hui F, Dai H, Zhang W, et al. CD138-directed adoptive immunotherapy of chimeric antigen receptor (CAR)-modified T cells for multiple myeloma. Journal of Cellular Immunotherapy. 2016;2:28–35. [Google Scholar]
- 30.Heffner LT, Jagannath S, Zimmerman TM, Lee KP, Rosenblatt J, Lonial S, et al. BT062, an Antibody-Drug Conjugate Directed Against CD138, Given Weekly for 3 Weeks in Each 4 Week Cycle: Safety and Further Evidence of Clinical Activity. Blood. 2012;120:4042. [Google Scholar]
- 31**.Ramos CA, Savoldo B, Torrano V, Ballard B, Zhang H, Dakhova O, et al. Clinical responses with T lymphocytes targeting malignancy-associated kappa light chains. J Clin Invest. 2016 Jul 1;126(7):2588–96. doi: 10.1172/JCI86000. Results from a clinical trial using CAR T cells directed against κ light chain as treatment of multiple myeloma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32*.Ali SA, Shi V, Maric I, Wang M, Stroncek DF, Rose JJ, et al. T cells expressing an anti-B-cell-maturation-antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood. 2016 Jul 13; doi: 10.1182/blood-2016-04-711903. First published data in clinical trials for multiple myeloma using a BCMA-CAR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen AD, Garfall AL, Stadtmauer EA, Lacey SF, Lancaster E, Vogl DT, et al., editors. ASH. San Diego, CA: 2016. B-Cell Maturation Antigen (BCMA)-Specific Chimeric Antigen Receptor T Cells (CART-BCMA) for Multiple Myeloma (MM): Initial Safety and Efficacy from a Phase I Study. [Google Scholar]
- 34.Garfall AL, Lancaster E, Stadtmauer EA, Lacey SF, Dengel K, Ambrose DE, et al. Posterior Reversible Encephalopathy Syndrome (PRES) after Infusion of Anti-Bcma CAR T Cells (CART-BCMA) for Multiple Myeloma: Successful Treatment with Cyclophosphamide. Blood. 2016;128(22):5702. [Google Scholar]
- 35.Berdeja JGYL, Raje N, Siegel D, Munshi N, Turka A, Lam LP, Quigley MT, Kochenderfer JN. Clinical remissions and limited toxicity in a first-in-human multicenter study of bb2121, a novel anti-BCMA CAR T cell therapy for relapsed/refractory multiple myeloma; Annual Meeting of the EORTC/NCI/AACR; December 1, 2016; Munich, Germany. 2016. [Google Scholar]