Abstract
Background
Direct electrical stimulation applied to the human medial temporal lobe (MTL) typically disrupts performance on memory tasks, however, the mechanism underlying this effect is not known.
Objective
To study the effects of MTL stimulation on memory performance
Methods
We studied the effects of MTL stimulation on memory in five patients undergoing invasive electrocorticographic monitoring during various phases of a memory task (encoding, distractor, recall).
Results
We found that MTL stimulation disrupted memory performance in a timing-dependent manner; we observed greater forgetting when applying stimulation during the delay between encoding and recall, compared to when it was applied during encoding or recall.
Conclusions
The results suggest that recall is most dependent on the MTL between learning and retrieval.
Keywords: free recall memory, medial temporal lobe, electrical stimulation, epilepsy, human
2 Introduction
Following in the tradition established by Wilder Penfield [1], cognitive neuroscientists have begun to use direct electrical stimulation (DES) to uncover the neural basis of human cognition. DES applies a voltage difference on the cortical surface or within the brain parenchyma, and provides a means of modulating local neural elements and their connections [2]. DES creates a short-lived (reversible) lesion, which is used clinically to demonstrate the behavioral function of specific brain regions [3]. Using this paradigm, researchers have shown that DES in the medial temporal lobe (MTL) frequently impairs memory performance [4, 5, 6, 7, 8]. However, the mechanism by which MTL DES impairs performance is not known.
Identifying the specific manner by which MTL DES impairs memory is an increasingly relevant area of research: both for memory theory and for clinical neuroscience. In particular, recent research has suggested that MTL stimulation can, under certain circumstances, enhance memory [9] and has led to the suggestion that electrical stimulation could be used to enhance memory in cases of pathological decreases in mnemonic function [10]. However, before clinical devices can be built to boost memory in the face of pathology, a better understanding of the precise effect of stimulation on memory is needed. A fundamental and unanswered question regarding the mechanistic action of MTL DES is whether it affects a specific mnemonic process or has a global effect on cognitive function.
Human memory function depends on a variety of cognitive processes that can grossly be divided into three categories: those related to stimulus encoding, maintenance, and retrieval. If MTL DES disrupts memory by altering a specific mnemonic processes, one would expect the effects of MTL DES on performance to be stage-dependent (i.e., to have differential effects based on whether it was applied during encoding, maintenance or retrieval). Alternatively, if MTL DES functioned by altering global cognitive function, one would expect MTL DES to have similar effects on memory performance regardless of the stage during which it was applied.
(e.g., one’s car keys) and contextual information (an integrated representation of external and internal features, such as the external environment and emotions, respectively), whereas retrieval involves a cued reinstatement of a previous contextual state. Alternatively, theories of working memory suggest that successful memory involves active maintenance of perceived stimuli or associations until time of test (e.g., by rehearsing a short list of items repeatedly). Both theoretical frameworks posit a distinct set of cognitive functions occur during encoding, delay and recall.
In this study, we leveraged the rare opportunity to study the mechanism by which MTL alters memory performance in patients undergoing invasive electrocortographic monitoring and brain stimulation. Patients performed a verbal memory task as we applied stimulation at eight left-sided medial temporal lobe electrode sites during various phases of the task (encoding, distractor interval, recall). Consistent with previous studies [5, 6, 7], we found that dominant MTL stimulation impairs memory performance. However, this disruptive effect was timing-dependent: we observed greater forgetting when stimulation was applied during the delay between encoding and recall, compared to when it was applied during encoding or recall. Performance on a distractor arithmetic task was not affected by stimulation. Our results suggest that MTL stimulation disrupts memory performance by selectively altering a cognitive process that occurs in between encoding and recall, and not by a global impairment of cognition. Possible mechanisms for this disruptive effect include enhanced contextual drift between encoding and recall (“contextual flushing;”?), disruption of unconscious neural replay of past traces, or impaired conscious maintenance of recently encoded events.
3 Materials and methods
Five patients (age range 19 – 57; two women) with medication-resistant epilepsy underwent surgical procedures at Thomas Jefferson University in which subdural strip or depth electrodes were implanted to localize epileptogenic regions, including left medial temporal lobe sites, for possible surgical resection. All patients were left-language dominant, defined as right-handedness or evidence of left-language dominance on intracarotid sodium amytal injection or fMRI testing. Our research protocol was approved by the institutional review board and informed consent was obtained from the subjects.
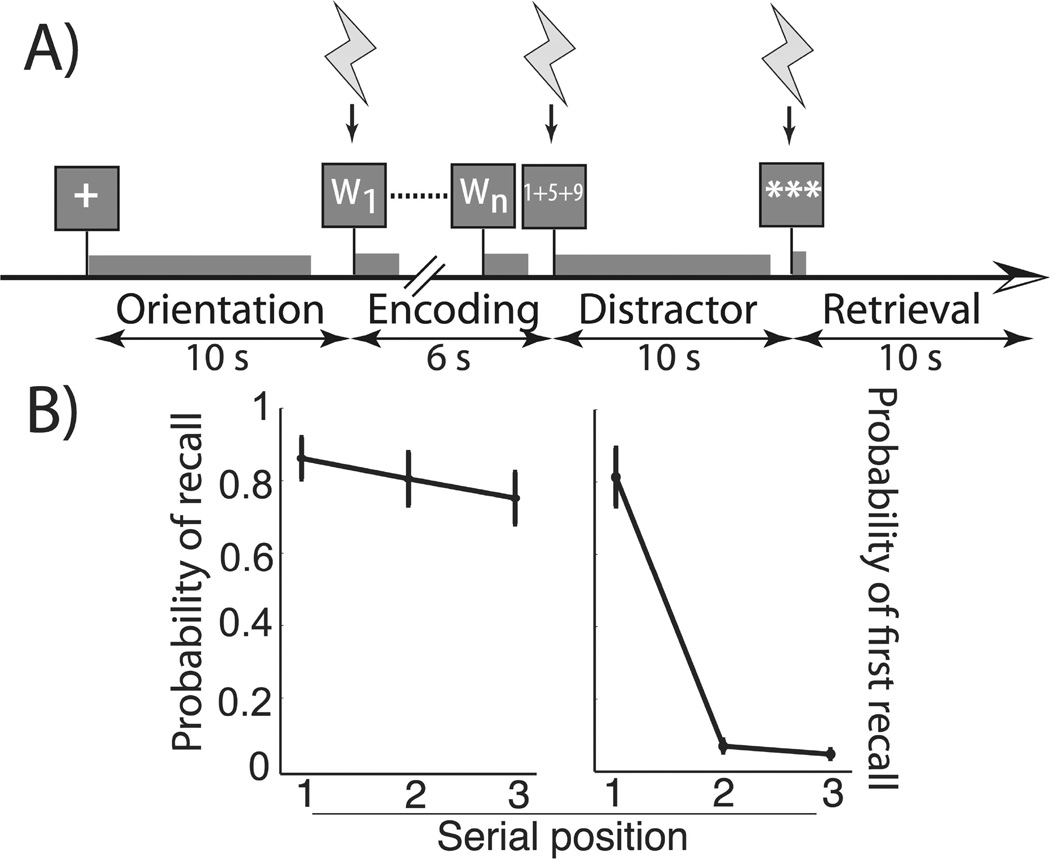
Each patient participated in a free-recall task (see Figure 1A). The task was developed using the python experiment-programming library [PyEPL; see 11] and administered at the subject’s bedside using a laptop computer. A fixation cross presented in the center of the screen for 10 seconds signaled the onset of each study list. Each item in the list was serially presented over a 6 second interval following which, subjects performed a minimum 10 second arithmetic distractor. They then recalled as many words as possible from the most recently presented list in a 10 second recall period. Lists comprised three words chosen randomly and without replacement from a pool of high-frequency nouns (http://memory.psych.upenn.edu/WordPools). In the case of one subject (subject three), we increased the list length to five words at a second electrode site given ceiling behavioral performance. All subjects completed at least 10 trials of each type at each electrode site.
Figure 1.
A. Free-recall stimulation task. The schematic represents one trial of the free-recall task subjects performed. We applied five second stimulation pulses to the left MTL at variable phases of the experiment – encoding, arithmetic distractor, or recall period. B. Sham stimulation probability of recall and probability of first recall by serial position. During sham trials, both the probability of recall and the probability of first response were modulated by serial position (respectively, MSE = 0.024, F2,23 = 5.28, p = 0.020 and MSE = 0.304, F2,23 = 50.8, p < 0.0001). Subjects began recall with the first serial position more commonly than second or third word (respectively, t7 = 8.37, p < 0.0001; t7 = 8.63 and p < 0.0001). Error bars are centered at across-electrode mean and represent ± 1 SEM.
A neuroradiologist experienced in neuroanatomical localizations identified bipolar pairs of electrodes within medial temporal lobe sites [12], which we used to administer DES. Electrodes were either circular 2.4 mm exposed diameter subdural contacts spaced every 10 mm (Integra Lifesciences, N.J., U.S.A) or cylindrical 2.4 mm length, 1.2 mm diameter depth contacts spaced every 8 mm (Adtech, W.I., U.S.A.). A Grass S12 cortical stimulator (Natus, Rhode Island, U.S.A.) generated constant current, 50 Hz, biphasic square wave pulses of 300 microseconds per phase (i.e. each 20ms period began with 600µs of stimulation), 5 second trains, at subafterdischarge threshold, which we administered to the medial temporal lobe synchronized to different phases of the memory task (see below). Prior to participating in the memory task, an epileptologist or neurosurgeon trained in direct cortical stimulation identified the afterdischarge threshold by slowly increasing current levels by 0.50 mA intervals until s/he identified afterdischarge potentials on the clinical recording system. We applied standard electroencephalogram definitions of afterdischarge potentials, which include various rhythmic spike or wave morphologies [13]. Amperage was decreased by 1mA relative to the afterdischarge level for the memory experiment. The clinical recording system was monitored by a neurologist or neurosurgeon during the memory task. When afterdischarge potentials were present during the experiment, the task was paused for at least 2 minutes, the associated trial was discarded, and the amperage was decreased by 5–15%. Patients were tested for clinical symptoms during and after afterdischarge potentials to ensure no seizure had occurred.
There were four trial types: sham (no stimulation provided), or stimulation to the medial temporal lobe during the encoding interval (stimulation onset with the first item presentation), distractor interval (stimulation onset with the first math question), or the retrieval interval (stimulation onset with the “***” that represented the “GO” cue for retrieval). Trial type was ordered pseudorandomly and constrained such that successive series of eight trials included two of each timing condition. For the first four patients, stimulation was manually initiated by the clinician using a pre-determined stimulation schedule for that particular session, whereas for the last patient, stimulation was initiated automatically. We attempted to blind patients from the type of trial in the following ways: first, they were not able to see the monitors that were used by the clinician to monitor for after-discharges, second, when manual initiation of stimulation was used, a button was pressed during all trial types (including sham trials), third, we applied stimulation at currents below the threshold at which subjects became aware of stimulation-evoked sensation.
For every stimulated electrode site we determined the difference in the probability of recall between each stimulation condition and the sham condition. We then determined if the number of words recalled varied as function of stimulation phase by applying a one-way, repeated-measures ANOVA to these distributions of difference values. An analogous analysis was applied to a secondary memory performance measure: response time.
4 Results
We administered a free-recall stimulation task (Figure 1A) to five left-language dominant subjects (two women) at eight unique bipolar electrode sites; clinical characteristics and baseline cognitive data are reported in Table 1. The task assessed memory performance based on randomly varying when sub-afterdischarge stimulation was delivered (during learning, distractor, recall, or sham). Lists initially comprised three words for all electrode stimulations. Subject 3 demonstrated ceiling performance at his first electrode site; we therefore increased list length to five for his second stimulation location. Among bipolar stimulation sites (see Table 1), average current density ranged from 9.0 to 39.8 uC/cm2/phase, all within the range safely tolerated by brain tissue [14, 15].
Table 1.
Subject demographics, clinical characteristics, electrode locations and stimulation parameters
| Subject | Gender | Age | Race | Language Dominance |
IQ/VIQ | Seizure onset |
Pathology | Resection | Anode | Cathode | Elec. Type |
Mean current (mA) |
Mean charge density (µC/cm2/ phase) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 47 | White | Left | N.A./8 7 |
R. frontal, MTL |
-- | None | Hip | PRC | Depth | 5.0 | 9.0 |
| 2 | M | 55 | White | Left | 113/10 5 |
L. frontal lobe, cingulate gyrus |
N.A. | L. frontal lobectomy |
PRC | PRC | Strip | 4.5 | 32.5 |
| 3 | M | 57 | More than 1 |
Left | 88/93 | L. tem- poral and MTL |
-- | None | PRC | TPC | Strip | 5.0 | 36.1 |
| PRC | PRC | Strip | 5.5 | 39.8 | |||||||||
| 4 | F | 43 | White | Left | 95/98 | R. frontal | Subpial gliosis |
R. frontal lobectomy |
Hip | Hip | Depth | 2.5 | 9.0 |
| EC | PRC | Depth | 1.9 | 6.8 | |||||||||
| 5 | F | 19 | African American |
Left | 80/72 | L. tem- poral and MTL |
Dentate gyrus dispersion |
L. anterior temporal lobectomy |
Hip | Hip | Depth | 2.5 | 9.0 |
| PRC | PRC | Depth | 3.5 | 25.3 |
Abbreviations:
(V)IQ : (Verbal) Intelligence Quotient
Hip: Hippocampus
PRC: Perirhinal cortex
EC: Entorhinal cortex
TPC: Temporal polar cortex
Figure 1B illustrates behavioral performance during sham trials. In delayed recall, healthy controls exhibit a strong primacy effect; that is they exhibit better memory for early list items and frequently initiate recall at the start of the list [16]. Our subjects demonstrated these same phenomena: variability in both the probability of recall and probability of first recall during sham trials at each of eight electrodes was modulated by serial position (respectively, MSE = 0.024, F2,23 = 5.28, p = 0.020, and MSE = 0.304, F2,23 = 50.8, p < 0.0001). Beginning recall with the first word of a list was particularly common relative to the second (t7 = 28.63,p < 0.0001) and third (t7 = 8.38, p < 0.0001) words in a list.
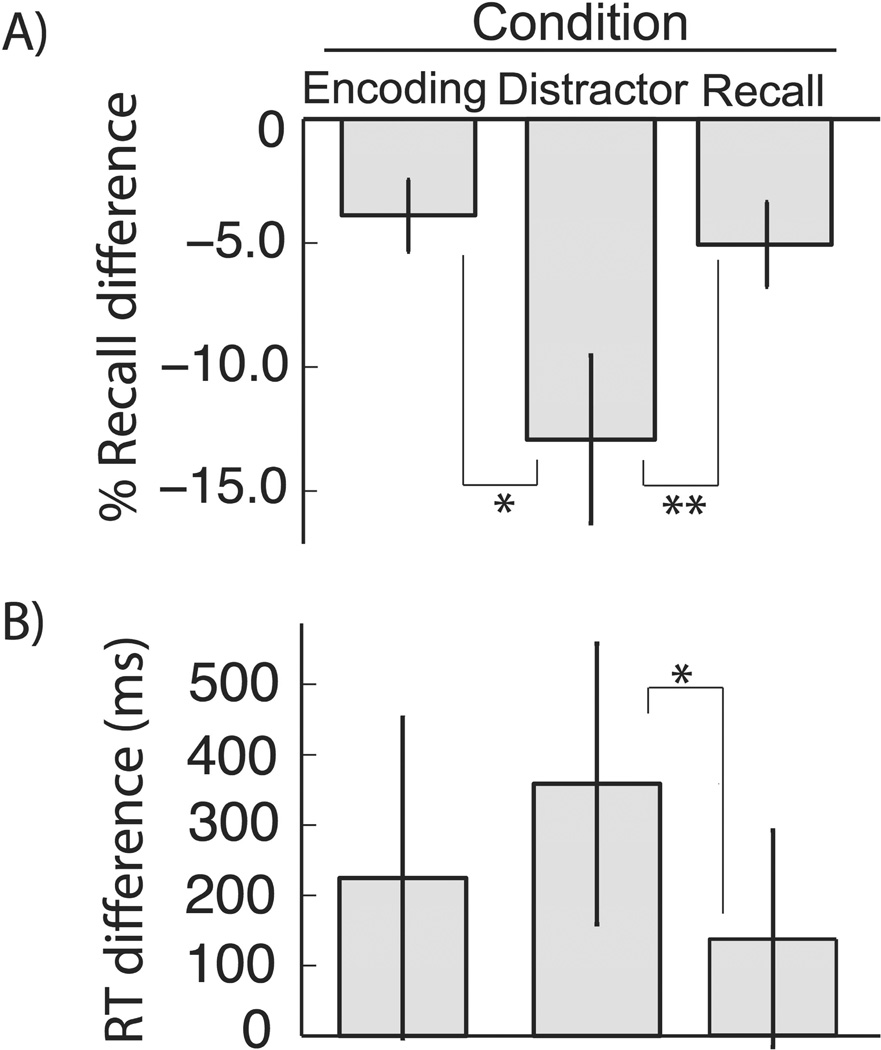
A comparison of all stimulation trials to sham trials revealed a significant decrease in the probability of recall (76% vs. 83%, t7 = 4.37, p = 0.003). As our primary question was to assess if recall was modulated as a function of stimulation task timing, for each stimulation condition (encoding, distractor, and retrieval) we calculated a normalized probability of recall by calculating the difference relative to sham trials for each stimulation site. To assess for differential effects of the timing of stimulation we compared the three distributions of normalized values across stimulation sites. A one-factor, repeated-measures ANOVA revealed that performance at each electrode stimulation site varied as a function of stimulation timing (see Figure 2A; MSE = 0.019, F2,23 = 5.97, p = 0.013). Moreover, paired t-tests demonstrated that stimulation during the distractor period was more disruptive of recall memory (12.9±3.4% worse than sham) than when current was applied during the encoding phase (t7 = 2.41, p = 0.047; 3.9 ± 1.4% worse than sham) or retrieval phase (t7 = 3.18, p = 0.016; 5.1 ± 1.7% worse than sham). In sum, recall was most impaired when medial temporal lobe stimulation was applied during the interval between encoding items and retrieving them, compared to when it was applied during the learning or recall periods.
Figure 2.
A. Probability of recall across stimulation conditions. Across-electrode mean and ± 1SEM of normalized probability of recall for each stimulation condition. Stimulation condition modulated probability of recall (MSE = 0.019, F2,23 = 5.97,p = 0.013) and stimulation during distractor led to worse recall than stimulation during encoding (t7 = 2.53, p = 0.039) and retrieval (t7 = 3.78, p = 0.007). We did not find a difference in probability of recall during encoding and retrieval periods (t7 = 0.79, p = 0.789). B. Response time across stimulation conditions. Across electrode mean and ± 1SEM of normalized response times for each stimulation condition. There was a strong trend towards stimulation condition modulating response time (MSE = 99.1 × 103F2,23 = 3.31, p = 0.067). Stimulation during the arithmetic distractor led to slower recall than stimulation during retrieval (t7 = 2.81, p = 0.026), and a comparison between distractor-period stimulation and encoding stimulation trended in the same direction (t7 = 1.85, p = 0.106). We did not identify a difference between response times during encoding or distraction conditions (t7 = 0.83, p = 0.440). In both panels, single and double asterisks mark significance, respectively p < 0.05 and p < 0.01.
Having established a differential effect of medial temporal lobe stimulation timing on probability of recall, we asked whether stimulation modulates response time. We operationalized response time as the interval between retrieval period onset and first correct recall. Analogous to the probability of recall analysis above, we calculated a normalized response time for each stimulation timing condition at each electrode site based on the difference with sham trials at each stimulation site (see Figure 2B). A one-factor, repeated measures ANOVA assessing normalized response time as a function of stimulation timing revealed a trend towards significance (MSE = 99.1 × 103, F2,23 = 3.31,p = 0.067). Although not statistically significant, the qualitative effect of stimulation timing on response time mirrored that of recall probability: the mean response time for distractor stimulation (359 ± 200 ms) was longer than for encoding (224 ± 230 ms) or retrieval (138 ± 155 ms) stimulation. Planned paired t-tests revealed a significant difference between response times during arithmetic distractor stimulation and stimulation during retrieval (t7 = 2.81, p = 0.026) and a trend towards longer response times during arithmetic distractor stimulation as compared to encoding stimulation (t7 = 1.85,p = 0.106). No significant difference was found between arithmetic encoding stimulation and retrieval stimulation (t7 = 0.44, p = 0.828).
Having shown that memory performance is most severely impaired when stimulation is applied during the distractor phase, we wanted to shed light on the cognitive mechanism underlying this effect. While we designed our task with an arithmetic distractor in order to diminish subjects’ ability to rehearse, discouraging this strategy is notoriously difficult. However, if stimulation during the time period between encoding and retrieval led to worse memory performance because it impaired conscious rehearsal, we hypothesized that stimulation not only would impair rehearsal but also the subjects’ global cognitive state. To test this hypothesis we assessed performance of the distractor arithmetic problems during sham and distractor stimulation conditions. While a difference in probability correct may be obscured by ceiling effects (94.1% correct vs. 93.9% correct; t7 = 0.12, p = 0.909), the response time for arithmetic distraction problems was nearly identical between sham and distractor stimulation conditions (4.61 seconds vs. 4.70 seconds, t7 = 0.35, p = 0.74). This analysis highlights the cognitive specificity of our MTL-stimulation effect.
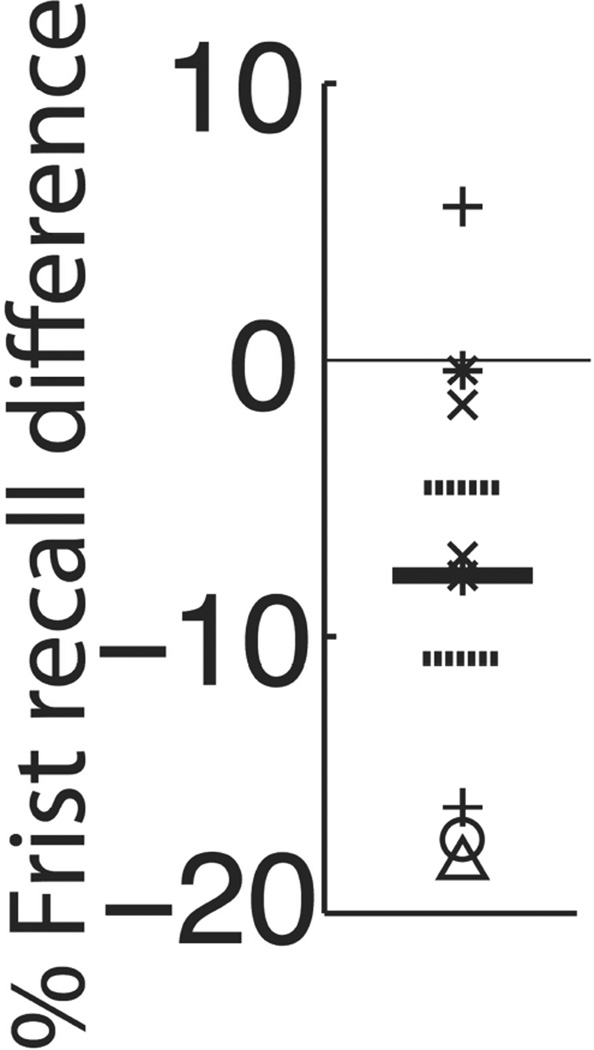
Because distractor period stimulation proved particularly effective in impairing memory, we further assessed its modulation of the strong primacy and first response effects we observed in our sham data. Although performance varied as a function of serial position (MSE = 0.067, F2,47 = 7.01, p = 0.003) and distractor-stimulation vs. sham (MSE = 0.218, F1,47 = 22.8,p < 0.0001), the effect of stimulation did not vary by serial position (interaction term, two-factor repeated-measures ANOVA: MSE = 0.008, F2,47 = 0.78, p = 0.464). In contrast, stimulation during the distractor period significantly diminished the first response effect. Figure 3 shows the difference in probability of beginning recall with the first list item between distrator-stimulation and sham conditions for each electrode site. For lists with at least one word recalled, subjects demonstrated a lower likelihood of beginning with the first word of the list during arithmetic distractor stimulation as compared to sham (73.4% vs. 81.3%, t7 = 2.54;p = 0.039).
Figure 3. Effect of distractor stimulation on probability of first recall.
Symbols represent the difference in beginning recall with the first list item during distractor stimulation relative to the sham condition at each stimulated electrode; each symbol type represents a unique subject. Across electrode mean and ± 1SEM are represented respectively by solid and hatched lines. Overall, distractor stimulation led to a decreased likelihood of beginning recall with the first word of a list (t7 = 2.54, p = 0.039).
5 Discussion
We applied left-sided MTL stimulation in five epilepsy patients as they performed a verbal free recall task to causally test the dependence of memory on the timing of MTL stimulation. Consistent with previous studies [5, 6, 7], we found that MTL stimulation impaired memory performance. Also, we found that the disruptive effect of MTL stimulation on memory performance was timing-dependent: we observed greater forgetting when stimulation was applied between encoding and recall, rather than during encoding or recall. Moreover, performance on a simultaneous arithmetic task was not affected by stimulation. These results suggest that MTL stimulation specifically impairs neural processes that connect our present to our past. That is, MTL stimulation affects the neural processes supporting memory that occur between, rather than those that underlie, learning and retrieval. Nor do our results support MTL stimulation leading to impairment of global cognitive function (e.g., attention).
Theories of memory suggest that successful memory relies on a series of distinct cognitive functions [17, 18] that may be carried out in a distributed manner throughout the brain [19]. Because MTL stimulation induced the greatest amount of forgetting when applied during the interval between encoding and recall, we suggest that the mechanism by which stimulation impairs memory involves altering one of several cognitive functions that occur between encoding and recall. Conversely, the mechanism in question is unlikely to be related to computations that occur during the encoding interval (item encoding and item-context binding), or the retrieval interval (memory search, reactivation of the memory trace, and speech articulation of an item). Our results suggest that additional brain regions may be sufficient to carry out cognitive functions related to encoding and retrieval in a manner that is independent of the MTL.
There are several cognitive processes in play during the time interval between encoding and recall [20, 17, 21, 22, 18]. Our task lies between common episodic or long-term memory paradigms [16] and working or short-term memory designs [23], which broadens the possible interpretations of our findings. Internal context – the mind’s representations that fluctuate moment to moment – slowly changes over time and provides a unique “time stamp” of events in our lives [16]. This internal contextual stamp is used to cue our memory search for these past events. Thus, MTL stimulation may increase the rate of contextual change, thereby causing one to forget. This finding parallels recent research on directing forgetting [24]. Via a series of psychological [25, 26] and neural recording [?] experiments, researchers have shown that asking a subject to forget a list of items leads to worse memory by means of a rapid change of internally generated context. The instruction to forget speeds up change in mental context, and thus a greater mismatch of context between the recall and learning periods. This contextual mismatch leads to a less effective memory cue (jumping back in time to the learning period is more difficult), and thus worse recall performance. MTL stimulation may have mimicked the forget cue: MTL electrical application may alter the neural circuitry representing the state of internal context thereby impairing performance. Moreover, this is consistent with previous electrophysiological work. Manns et al. described MTL neural activity that may represent internal context [27] and Hanslmayr and colleagues linked decreased electrical synchrony between the MTL and the dorsolateral prefrontal cortex [28], another region know to be involved in the representation of internal context [19], to forgetting.
An alternative explanation is that delay-period MTL stimulation altered the process of unconscious memory replay. Extensive research in animals [29, 30] has found that neuronal activity associated with past experiences occurs in the MTL, and in humans spontaneous replay of BOLD signal associated with an item has been shown to support later memory [31], possibly through memory consolidation to neocortical structures. By applying current to the MTL, we may have disrupted this unconscious neurocognitive process, leading to worse recall performance. A third possible cognitive mechanism that explains our findings is that MTL stimulation interrupted conscious item maintenance or rehearsal. Despite the use of distractor tasks subjects nevertheless have a tendency to rehearse learned items [32]. Although neocortical regions (e.g., the dorsal prefrontal cortex, 33) are more commonly associated with memory maintenance, recent work has linked medial temporal lobe structures to conscious rehearsal [34, 35] that characterizes short lists and the primacy effect [36]. Our finding that arithmetic performance is unchanged during distractor period stimulation relative to sham trials suggests that global cognitive function was not impaired during this time interval.
Healthy subjects begin delayed free-recall of short lists with the first serial position [37]. We found that that delay-period MTL stimulation led to a lower likelihood (7.9% less) of this tendency. Consistent with our interpretations above that stimulation affects contextual drift or conscious rehearsal, several authors have argued that the first item’s strong association with an internal contextual state [38, 39] or prolonged access to the short-term memory buffer [40] confers increased access to the first item during retrieval (see 41 for formalization of the contextual explanation into a model of free-recall). Moreover, that subjects less frequently recalled first list item parallels the analogous behavioral finding in a directed forgetting task [42, see figure 3] intended to maximize contextual change. Thus, the change in first response probability provides further evidence that MTL stimulation between encoding and retrieval increases the rate of internal contextual drift or disrupts MTL-dependent memory maintenance.
Direct brain stimulation likely exerts its physiological effects via membrane potential alterations in both axons and cell bodies, thereby disrupting the functional network (via both anterograde and retrograde propagation) linked to the area directly stimulated [3]. Thus, in addition to disrupting the MTL structures located adjacent to our electrode contacts, we likely affected the connected neocortical inputs and outputs as well. Although speculative, impairment of the neural activity in the MTL [29] or its projections to the prefrontal cortex [19] are both generally consistent with the theoretical account (contextual drift, unconscious replay, active maintenance) of forgetting we propose.
Although the aforementioned cognitive interpretations of our findings are not mutually exclusive, future research is necessary to disambiguate among theoretical accounts of stimulation-induced forgetting during a delay period. Psychological manipulations combined with MTL stimulation may support the claim that disruption of neuronal activity in the MTL with electrical current causes expeditious contextual change. This could be tested with the addition of a category cue to the task [c.f. 43] or with encoding manipulations (e.g., 44). Comparison of neural replay across stimulation conditions and a more challenging distractor test will also be important for testing these cognitive interpretations of our findings. Given the small number of subjects in this study, we compute statistics across electrodes as the random variable. Although no subject contributed more than two electrodes and all bipolar electrode pairs flanked unique tissue volumes, future studies with a greater number of subjects will be able to better control for non-independent properties of same-subject electrodes. Our data cannot specifically implicate subregions within the MTL given the variability of stimulation sites. Stimulation experiments in the future should be aimed at further refining the structure-function relationship we describe here.
Complementary to remembering, forgetting is important to our everyday lives. Forgetting confers us the ability to discard unwanted memories and prioritize recollection of certain events. This study causally links the selective timing of stimulation to forgetting during a free-recall task and thus implicates disruption of specific neurocognitive processes as the mechanism of our findings. Future research will both help further refine our current theoretical framework and determine if our findings apply to broader classes of memories, such as those with strong emotional associations. In this way we may be able to selectively prune unwanted memories, such as those that affect patients with post-traumatic stress disorder
Highlights.
Verbal recall memory is impaired by dominant MTL stimulation.
Stimulation during the interval between learning and recall most strongly disrupts recall.
MTL stimulation does not alter arithmetic performance, demonstrating cognitive specificity of this effect.
The well-known tendency to begin recall of short lists with the first word diminishes with MTL stimulation.
Either enhanced contextual flushing or disruption of unconscious or conscious replay are the likely cognitive mechanisms explaining our results.
Acknowledgments
This work was supported by National Institutes of Health grants MH055687 to Michael J. Kahana and 5T32NS043126 to Douglas Hamilton Smith. We thank Dale H. Wyeth and Edmund Wyeth, for technical assistance at Thomas Jefferson Hospital and Dan Rizzuto for helpful discussion and input. The authors declare no competing financial interests. We are indebted to the patients who have selflessly volunteered their time to participate in our study.
Abbreviations
- DES
Direct Electrical Stimulation
- MTL
Medial Temporal Lobe
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: The authors declare no competing financial interests.
References
- 1.Penfield W, Boldrey E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain. 1937;60(4):389–443. [Google Scholar]
- 2.Selimbeyoglu A, Parvizi J. Electrical stimulation of the human brain: perceptual and behavioral phenomena reported in the old and new literature. Frontiers in Human Neuroscience. 2010;4(46) doi: 10.3389/fnhum.2010.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mandonnet E, Winkler PA, Duffau H. Direct electrical stimulation as an input gate into brain functional networks: principles, advantages and limitations. Acta Neurochirurgica. 2010;152(2):185–193. doi: 10.1007/s00701-009-0469-0. [DOI] [PubMed] [Google Scholar]
- 4.Halgren E, Wilson CL, Stapleton JM. Human medial temporal-lobe stimulation disrupts both formation and retrieval of recent memories. Brain and Cognition. 1985 Jul;4(3):287–295. doi: 10.1016/0278-2626(85)90022-3. [DOI] [PubMed] [Google Scholar]
- 5.Loring David W, Lee Gregory P, Flanigin Herman F, Meador Kimford J, Smith Joseph R, Gallagher Brian B, King Don W. Verbal memory performance following unilateral electrical stimulation of the human hippocampus. Journal of Epilepsy. 1988 Jan;1(2):79–85. [Google Scholar]
- 6.Lee Gregory P, Loring David W, Smith Joseph R, Flanigin Herman F. Material specific learning during electrical stimulatin of the human hippocampus. Cortex. 1990;26(3):433–442. doi: 10.1016/s0010-9452(13)80092-5. [DOI] [PubMed] [Google Scholar]
- 7.Coleshill SG, Binnie CD, Morris RG, Alarcon G, Boas Wvan Emde, Velis DN, Simmons A, Polkey CE, Veelen CWMvan, Rijen PCvan. Material-specific recognition memory deficits elicited by unilateral hippocampal electrical stimulation. Journal of Neuroscience. 2004;24(7):1612–1616. doi: 10.1523/JNEUROSCI.4352-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lacruz ME, Valentίn A, Garcίa Seoane JJ, Morris RG, Selway RP, Alarcόn G. Single pulse electrical stimulation of the hippocampus is sufficient to impair human episodic memory. Neuroscience. 2010 Oct;170(2):623–632. doi: 10.1016/j.neuroscience.2010.06.042. [DOI] [PubMed] [Google Scholar]
- 9.Suthana N, Haneef Z, Stern J, Mukamel R, Behnke E, Knowlton B, Fried I. Memory enhancement and deep-brain stimulation of the entorhinal area. The New England Journal of Medicine. 2012;366:502–510. doi: 10.1056/NEJMoa1107212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee DJ, Gurkoff GG, Izadi A, Seidl SE, Echeverri A, Melnik M, Berman RF, Ekstrom AD, Muizelaar JP, Lyeth BG, Shahlaie K. Septohippocampal neuromodulation improves cognition after traumatic brain injury. Journal of Neurotrauma. 2015;32(22):1822–1832. doi: 10.1089/neu.2014.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geller AS, Schleifer IK, Sederberg PB, Jacobs J, Kahana MJ. PyEPL: A cross-platform experiment-programming library. Behavior Research Methods. 2007;39(4):950–958. doi: 10.3758/bf03192990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Insausti R, Juottonen K, Soininen H, Insausti AM, Partanen K, Vainio P, Laakso MP, Pitkanen A. Mr volumetric analysis of the human entorhinal, perirhinal, and temporopolar cortices. American Journal of Neuroradiology. 1998 Apr;19:659–671. [PMC free article] [PubMed] [Google Scholar]
- 13.Blume WT, Jones DC, Pathak P. Properties of after-discharges from cortical electrical stimulation in focal epilepsies. Clinical Neurophysiology. 2004;115:982–989. doi: 10.1016/j.clinph.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 14.Gordon B, Lesser RP, Rance NE, Hart J, Webber R, Uematsu S, Fisher RS. Parameters for direct cortical electrical stimulation in the human: histopathologic confirmation. Electroencephalography and Clinical Neurophysiology. 1990;75(5):371–377. doi: 10.1016/0013-4694(90)90082-u. [DOI] [PubMed] [Google Scholar]
- 15.Merrill DR, Bikson M, Jefferys JGR. Electrical stimulation of excitable tissue: design of efficacious and safe protocols. Journal of Neuroscience Methods. 2005;141(2):171–198. doi: 10.1016/j.jneumeth.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 16.Kahana MJ. Foundations of Human Memory. New York, NY: Oxford University Press; 2012. [Google Scholar]
- 17.Howard MW, Kahana MJ. A distributed representation of temporal context. Journal of Mathematical Psychology. 2002;46:269–299. [Google Scholar]
- 18.Farrell S. Temporal clustering and sequencing in short-term memory and episodic memory. Psychological Review. 2012;119(2):223–271. doi: 10.1037/a0027371. [DOI] [PubMed] [Google Scholar]
- 19.Polyn SM, Kahana MJ. Memory search and the neural representation of context. Trends in Cognitive Sciences. 2008;12:24–30. doi: 10.1016/j.tics.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mensink G-JM, Raaijmakers JGW. A model for interference and forgetting. Psychological Review. 1988;95:434–455. [Google Scholar]
- 21.Sederberg PB, Howard MW, Kahana MJ. A context-based theory of recency and contiguity in free recall. Psychological Review. 2008;115(4):893–812. doi: 10.1037/a0013396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Polyn SM, Norman KA, Kahana MJ. A context maintenance and retrieval model of organizational processes in free recall. Psychological Review. 2009;116:129–156. doi: 10.1037/a0014420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baddeley AD. Working Memory. Oxford, England: Clarendon Press; 1986. [Google Scholar]
- 24.Sahakyan L, Delaney PF, Foster NL, Abushanab B. List-method directed forgetting in cognitive and clinical research: a theoretical and methodological review. Psychology of Learning and Motivation. 2013;59:131–189. [Google Scholar]
- 25.Sahakyan L, Kelley CM. A contextual change account of the directed forgetting effect. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2002;28(6):1064–1072. doi: 10.1037//0278-7393.28.6.1064. [DOI] [PubMed] [Google Scholar]
- 26.Sahakyan Lili, Delaney Peter F, Waldum Emily R. Intentional forgetting is easier after two “shots” than one. Journal of Experimental Psychology Learning Memory and Cognition. 2008;34(2):408–414. doi: 10.1037/0278-7393.34.2.408. [DOI] [PubMed] [Google Scholar]
- 27.Manns Joseph R, Howard Marc W, Eichenbaum Howard. Gradual changes in hippocampal activity support remembering the order of events. Neuron. 2007;56(3):530–540. doi: 10.1016/j.neuron.2007.08.017. ISSN 0896-6273 (Print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanslmayr S, Staudigl T, Fellner MC. Oscillatory power decreases and long-term memory: the information via desynchronization hypothesis. Frontiers in Human Neuroscience. 2012;6(74) doi: 10.3389/fnhum.2012.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karlsson MP, Frank LM. Awake replay of remote experiences in the hippocampus. Nature Neuroscience. 2009 Jul;12(7):913–918. doi: 10.1038/nn.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davidson TJ, Kloosterman F, Wilson MA. Hippocampal replay of extended experience. Neuron. 2009;63(4):497–407. doi: 10.1016/j.neuron.2009.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deuker L, Olligs J, Fell fJ, Kranz TA, Mormann F, Montag C, Reuter M, Elger CE¿, Axmacher N. Memory Consolidation by Replay of Stimulus-Specific Neural Activity. The Journal of neuroscience. 2013 Dec;33(49):19373–19383. doi: 10.1523/JNEUROSCI.0414-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tulving E. Elements of Episodic Memory. New York: Oxford; 1983. [Google Scholar]
- 33.Chao LL, Knight RT. Prefrontal and posterior cortical activation during auditory working memory. Brain Research : Cognitive Brain Research. 1996;4:27–37. doi: 10.1016/0926-6410(95)00043-7. [DOI] [PubMed] [Google Scholar]
- 34.Ranganath C, Cohen MX, Brozinsky CJ. Working memory maintenance contributes to long-term memory formation: neural and behavioral evidence. Journal of Cognitive Neuroscience. 2005;17:994–1010. doi: 10.1162/0898929054475118. [DOI] [PubMed] [Google Scholar]
- 35.Axmacher N, Mormann F, Ferna`ndez G, Cohen MX, Elger CE, Fell J. Sustained neural activity patterns during working memory in the human medial temporal lobe. Journal of Neuroscience. 2007;27(29):7807–7816. doi: 10.1523/JNEUROSCI.0962-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Axmacher N, Elger CE, Fell J. Working memory-related hippocampal deactivations interferes with long-term memory formation. Journal of Neuroscience. 2009;29(4):1052–1060. doi: 10.1523/JNEUROSCI.5277-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spurgeon J, Ward G, Matthews WJ. Why do participants initiate free recall of short lists of words with the first list item? Toward a general episodic memory explanation. Journal of Experimental Psychology: Learning Memory and Cognition. 2014 Nov;40(6):1551–1567. doi: 10.1037/xlm0000028. [DOI] [PubMed] [Google Scholar]
- 38.Metcalfe J, Murdock BB. An encoding and retrieval model of single-trial free recall. Journal of Verbal Learning and Verbal Behavior. 1981;20:161–189. [Google Scholar]
- 39.Laming D. Testing the idea of distinct storage mechanisms in memory. International Journal of Psychology. 1999;34:419–426. [Google Scholar]
- 40.Atkinson RC, Shiffrin RM. Human memory: A proposed system and its control processes. In: Spence KW, Spence JT, editors. The psychology of learning and motivation. Vol. 2. New York: Academic Press; 1968. pp. 89–105. [Google Scholar]
- 41.Kragel JE, Morton NW, Polyn SM. Neural activity in the medial temporal lobe reveals the fidelity of mental time travel. Journal of Neuroscience. 2015 Feb;35(7):2914–2926. doi: 10.1523/JNEUROSCI.3378-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lehman M, Malmberg KJ. A global theory of remembering and forgetting from multilple lists. Journal Experimental Psychology: Learning, Memory and Cognition. 2009;35(4):970–988. doi: 10.1037/a0015728. [DOI] [PubMed] [Google Scholar]
- 43.Lehman Melissa, Malmberg Kenneth J. Overcoming the effects of intentional forgetting. Memory and Cognition. 2011;39(2):335–347. doi: 10.3758/s13421-010-0025-4. [DOI] [PubMed] [Google Scholar]
- 44.Malmberg KJ, Shiffrin RM. The “one-shot” hypothesis for context storage. Journal Experimental Psychology: Learning, Memory and Cognition. 2005;31(2):322–336. doi: 10.1037/0278-7393.31.2.322. [DOI] [PubMed] [Google Scholar]