Abstract
The view of enzymes as punctilious catalysts has been shifting as examples of their promiscuous behavior increase. However, unlike a number of cases where the physiological relevance of breached substrate specificity is questionable, the very synthesis of H2S relies on substrate and reaction promiscuity, which presents the enzymes with a multitude of substrate and reaction choices. The transsulfuration pathway, a major source of H2S, is inherently substrate-ambiguous. A heme-regulated switch embedded in the first enzyme in the pathway can help avert the stochastic production of cysteine versus H2S and control switching between metabolic tracks to meet cellular needs. This review discusses the dominant role of enzyme promiscuity in pathways that double as sulfur catabolic and H2S synthetic tracks.
Graphical Abstract
Enzymes can exhibit considerable laxity in both substrate and reaction specificity, contributing to the growing view that promiscuity and fidelity coexist in biocatalysts [1]. An evolutionary advantage of a specificity cushion is that it affords a latent functional repertoire that is broader than the genome encoding it, and provides an adaptive advantage under pressure for the emergence of new catalytic functions as seen in both natural and laboratory settings [2–4]. Enzymes can exhibit promiscuous behavior toward xenobiotic substrates or towards naturally occurring metabolites. The term “underground metabolism” was coined to refer to the stream of secondary metabolic activity with endogenous substrates that is generally invisible due to low flux but might be phenotypic under certain conditions [5]. At one extreme of the specificity spectrum are enzymes involved in DNA replication, which operate with low albeit nonzero error rates, their ability to slip up and introduce mutations being advantageous from an evolutionary perspective. At the other end of the spectrum, are enzymes involved in some amino acid metabolism pathways such as the ones shared for cysteine and H2S synthesis as discussed in this review.
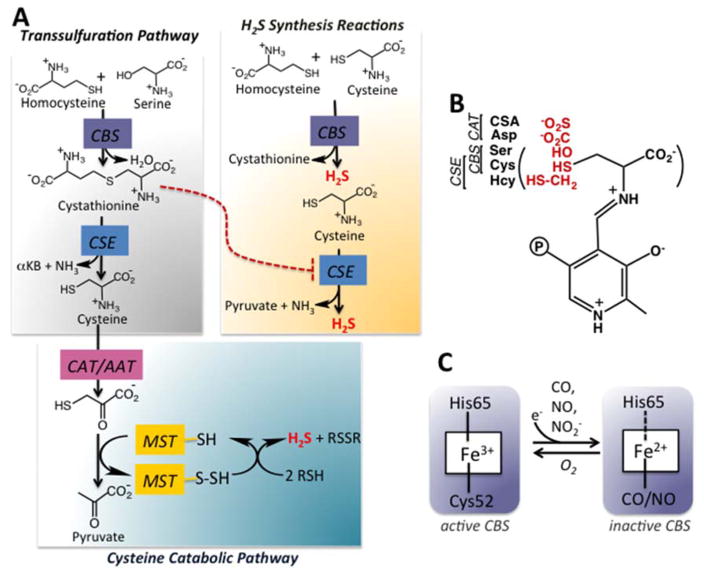
It is posited that ancient enzymes were generalists with broad specificity and that metabolic pathways were inherently leaky [6]. Enzyme promiscuity and underground metabolism play a surprisingly prominent role in multiple facets of H2S synthesis [7–9]. The enzymes involved in H2S biogenesis are distinct from the highly specific nitric oxide synthases and heme oxygenases, dedicated to synthesizing the other two gaseous signaling molecules, NO and CO, respectively. Also in striking contrast to NO and CO synthesis, three unrelated enzymes support H2S synthesis of which two, serve alternative metabolic functions (Fig. 1A). Cystathionine β-synthase (CBS) and γ-cystathionase (CSE) comprise the cytoplasmic transsulfuration pathway that functions to direct homocysteine derived from methionine to cysteine synthesis, particularly under conditions of sulfur excess [10]. The third enzyme, β-mercaptopyruvate sulfurtransferase (MST) resides in the cysteine catabolic branch of the sulfur network and is both cytoplasmic and mitochondrial [11]. The reactions catalyzed by these H2S synthesizing enzymes and their regulation, are discussed in this review.
Figure 1.
Overview of H2S synthesizing reactions. A. H2S can be synthesized by the transsulfuration pathway enzymes, CBS and CSE or by the cysteine catabolism pathway enzymes, CAT/AAT and MST. The canonical transsulfuration reactions catalyzed by CBS and CSE results in the conversion of serine and homocysteine to cysteine. However, these enzymes can also utilize cysteine and homocysteine to generate H2S. Cystathionine, an intermediate in the canonical transsulfuration pathway competes with cysteine for binding to CSE, thus inhibiting H2S synthesis (red dotted line). MST is a sulfurtransferase, which catalyzes the transfer of the sulfur atom from mercaptopyruvate to an active site cysteine thiol to form a cysteine persulfide. The latter, in the presence of reductants can release H2S. αKB denotes α-ketobytyrate. B. The first step in the reactions catalyzed by CBS, CSE and CAT/AAT is the formation of an external aldimine via a Schiff base linkage between PLP and the amino acid. CBS can bind either serine or cysteine, CSE can bind cysteine or homocysteine, while CAT/AAT can bind aspartate or cysteine sulfinic acid (CSA) in addition to cysteine at this position. C. CBS has a regulatory heme cofactor that is ligated by His65 and Cys52 (human protein numbering). One electron reduction to the ferrous state promotes binding of exogenous ligands such as CO or NO leading to inactive enzyme. The heme harbors nitrite reductase activity and forms nitrosyl heme, which is 5-coordinate. The broken line to His65 indicates that this residue serves as a ligand when CO but not when NO is bound. The ferrous nitrosyl and ferrous carbonyl forms of CBS are readily converted to the ferric state in the presence of O2.
H2S Synthesis via the Transsulfuration Pathway
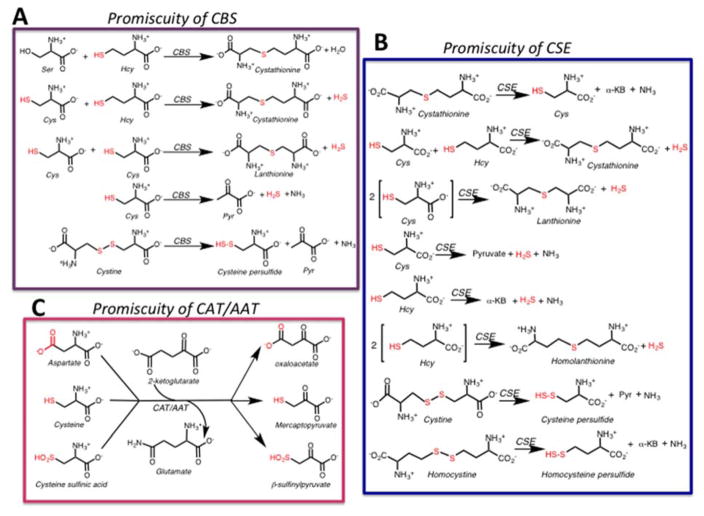
Parallel tracks within the transsulfuration pathway lead to cysteine synthesis from serine and homocysteine and to H2S synthesis from cysteine and homocysteine (Fig. 1A). The first enzyme in the pathway, cystathionine β-synthase (CBS) catalyzes the β-replacement of serine and homocysteine eliminating water and forming cystathionine. The latter is a substrate for γ-cystathionase (CSE), which catalyzes its α-γ eliminination to cysteine, α-ketobutyrate and ammonia. In this configuration of the transsulfuration pathway, sulfur is fated for transfer from homocysteine to cysteine. However, CBS [12] and CSE [13] exhibit both substrate and reaction ambiguity (Fig. 2A, B). Thus, CBS can swap cysteine for serine eliminating H2S while still forming cystathionine in the presence of homocysteine (Fig. 1A). It can also generate H2S from one or two moles of cysteine (Fig. 2A). Of the three routes for CBS-catalyzed H2S-production, the dominant one is β-replacement of cysteine by homocysteine [12].
Figure 2.
Promiscuity of PLP enzymes involved in H2S synthesis. H2S and persulfide-generating reactions catalyzed by the transsulfuration pathway enzymes CBS (A) and CSE (B). Reactions catalyzed by CAT/AAT (C). Pyr and α-KB denote pyruvate and α-ketobutyrate respectively.
CSE, the second enzyme in the transsulfuration pathway, exhibits even greater promiscuity than CBS. In addition to the three H2S generating reactions that it catalyzes in common with CBS, it also produces H2S from one or two moles of homocysteine (Fig. 2B). The major routes for CSE-catalyzed H2S generation are via α-β elimination of cysteine to form pyruvate and ammonia and by α-γ elimination of homocysteine forming α-ketobutyrate and ammonia. The former reaction is favored at physiologically relevant substrate concentrations [12]. In addition to H2S, the transsulfuration enzymes catalyze the synthesis of persulfides from homocystine (CSE only) and cystine (CBS and CSE), the oxidized forms of the respective amino acids (Fig. 2A, B) [14–16]. In the reducing intracellular milieu, cystine and homocystine concentrations are low and the persulfide-generating reactions are predicted to be quantitatively insignificant [15]. However, under oxidizing conditions, these reactions might become significant.
The first step in the CBS and CSE catalyzed reactions involves formation of an external aldimine with the incoming amino acid. While CBS forms a Schiff base with serine or cysteine, CSE can also accommodate homocysteine, with an extra methylene group at this position (Fig. 1B), explaining the wider range of reactions that it catalyzes [12]. H2S synthesis by CSE is responsive to the grade of homocystinuria [13], a metabolic disorder characterized by elevated homocysteine [17]. The physiological relevance of homocysteine-derived H2S is supported by elevated homolanthionine in homocystinuric patients [18,19]. Homolanthionine is a side product of H2S-generation via condensation of two moles of homocysteine (Fig. 2B).
Human CBS does not discriminate between serine and cysteine at the level of the respective specificity constants, which are virtually identical (kcat/Km(Cys) = 2.9×103 M−1s−1 versus kcat/Km(Ser) = 2.7×103 M−1s−1 at pH 7.4 and 37 °C) [12]. However, the Kd for serine is ~7-fold lower than for cysteine [12]. Human CSE exhibits a preference for cystathionine (kcat/Km(Cyst) = 8×103 M−1s−1) over cysteine (kcat/Km(Cys) = 0.3×103 M−1s−1) or homocysteine (kcat/Km(Hcy) = 0.4×103 M−1s−1) [13]. Both CBS and CSE exhibit high Km values for cysteine and homocysteine (2–7 mM) that are 10–100 fold higher than the intracellular concentrations of these substrates in most tissues [12,13]. The corresponding enzymes in other organisms also exhibit high Km values for their substrates [20]. It is not known if small molecule modulators or supramolecular organization of pathway enzymes as seen in purinosomes [21] influence the affinity of the transsulfuration enzymes for their substrates or their kinetic efficiencies in vivo.
H2S Synthesis via Cysteine Catabolism
The conversion of cysteine to H2S via the cysteine catabolic pathway occurs in two steps catalyzed by a transaminase and by MST (Fig. 1A) [22,23]. Aspartate aminotransferase (AAT) is a notoriously promiscuous pyridoxal phosphate-dependent enzyme that catalyzes the transamination reaction between pairs of amino and keto acids (Fig. 2C). It catalyzes a cysteine aminotransferase (CAT) reaction in which aspartate is substituted with cysteine forming 3-mercaptopyruvate. The promiscuity of CAT/AAT is further demonstrated by its ~10-fold higher activity under Vmax conditions with cysteine sulfinic acid (Fig. 2C) than with aspartate [24]. Cysteine sulfinic acid is the product of cysteine dioxygenase, which is also involved in cysteine catabolism [10].
In the absence of a known mechanism for regulating substrate selectivity, the reaction choice for CAT/AAT is presumably determined by a combination of substrate concentrations and the relevant specificity constants (kcat/Km). Mitochondrial and cytoplasmic isoenzymes of AAT/CAT exist and despite the wealth of structural and mechanistic information on them, direct comparison of the kinetic parameters for the competing reactions at physiologically relevant pH, are not readily available. The Km values for the mitochondrial rat liver CAT/AAT are 22 mM for cysteine and 0.5–1.6 mM for aspartate at pH 9.7 [23] and the specificity constants are estimated to be 1.4×104 M−1 s−1 (aspartate) and 1.4 × 102 M−1 s−1 (cysteine) at pH 9.7 and 37 °C [25]. At pH 7, the CAT activity is ~10-fold lower than at its optimal pH of 9.7 [25]. The CAT activity is potently inhibited by aspartate [23,25], which is more abundant than cysteine in most tissues. Despite the kinetic parameters favoring AAT over CAT activity, the physiological relevance of the CAT reaction is borne out by the accumulation of mercaptolactate disulfide in individuals with a genetic deficiency of MST [26]. Mercaptolactate is the product of lactate dehydrogenase-catalyzed reduction of mercaptopyruvate.
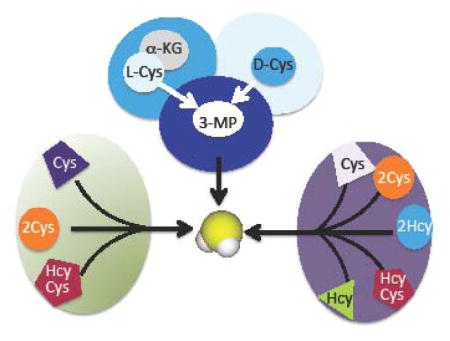
An alternative route to 3-mercaptopyruvate is via the oxidative deamination of D-cysteine catalyzed by the flavoprotein, D-amino acid oxidase, which is yet another promiscuous enzyme [27,28]. While its “physiological” substrate is presumed to be D-serine, it exhibits substantial or even higher activity with other D-amino acids [29]. For the human enzyme, the kcat/Km for D-serine is 0.4 × 103 M−1 s−1 at pH 8.5 and 25 °C [30] while the corresponding value for D-cysteine is not known. D-amino acid oxidase is a peroxisomal enzyme while MST is predominantly mitochondrial. Hence, the contribution of this pair of enzymes to H2S generation in intact cells and the source of D-cysteine are not known.
MST also exhibits promiscuity utilizing either 3-mercaptopyruvate or thiosulfate as substrate (equations 1,2). MST catalyzes a sulfurtransferase reaction forming an enzyme-bound persulfide intermediate, which subsequently donates the sulfane sulfur atom to an acceptor e.g. cyanide (equation 3). The physiological sulfur acceptor is predicted to be thioredoxin [31,32].
| [1] |
| [2] |
| [3] |
The Km values of rat liver MST for mercaptopyruvate (1.2 mM) and thiosulfate (62 mM) are vastly different. Furthermore, their specificity constants (5.6 × 103 M−1 s−1 for mercaptopyruvate (pH 9.55 and 25 °C) and 1.5 × 102 M−1 s−1 for thiosulfate (pH 5.0 and 25 °C)) are difficult to compare given the difference in the assay conditions [33]. The kcat/Km(3-MP) for human MST with mercaptopyruvate as donor and thioredoxin as acceptor is 3.7 × 103 M−1 s−1 at pH 7.4 and 37 °C [31]. In human MST, Arg188, Arg197 and Ser250 make contacts with the carbonyl and carboxyl oxygens of mercaptopyruvate and are important determinants of selectivity against thiosulfate [33].
Heme-dependent Metabolic Switching
Some cellular strategies for averting the potentially adverse effects of inherently lax substrate specificity are regulation of protein expression levels, limiting active site access via substrate or product inhibition and metabolite repair [34,35]. In the transsulfuration pathway, the single or combinatorial use of even a limited number of amino acids creates a multitude of reaction choices for CBS and CSE (Fig. 2A, B), which must be regulated to service cellular needs for cysteine versus H2S synthesis. CBS is poised at a key metabolic decision point where the choice between recycling or transmuting homocysteine is made. Hence, CBS is a major hub of regulation; it is allosterically stabilized [36] and activated by S-adenosylmethionine (AdoMet), and by glutathionylation [37], but inhibited by CO [38–41], NO [42,43], nitrite [44] and by SUMOylation [45]. Human CBS comprises an N-terminal regulatory domain that houses a heme [46,47] and a C-terminal domain that has a tandem repeat of CBS domains, a secondary structure motif that is often utilized in energy sensing modules [48].
In human CBS, Cys52 and His65 serve as ligands to the heme in the ferric and ferrous states (Fig. 1C). Although located ~20 Å from the catalytic site where the PLP cofactor is housed, the heme exerts long-range allosteric effects [49]. Binding of NO or CO to the ferrous heme results in the formation of 5- and 6-coordinate species, respectively in which Cys52 or both endogenous ligands are displaced (Fig. 1C). The heme also exhibits catalytic activity reducing nitrite to NO and forming the inhibitory ferrous-nitrosyl complex [44]. Changes in the native heme environment are communicated to the PLP pocket [49–51] and results in a shift in the tautomeric equilibrium from the active ketoeneamine to the inactive enolimine form [52]. Inhibition of CBS by CO and NO is readily reversed in the presence of oxygen, which rapidly oxidizes ferrous CBS [53].
The heme in CBS is a key operator that can switch the transsulfuration pathway between the cysteine and H2S production tracks [54]. When the heme is coordinated by endogenous ligands, synthesis of cystathionine via the canonical reaction is favored due to the higher intracellular concentration of serine and its higher affinity for CBS versus cysteine. Cystathionine (Km =0.28 mM) in turn, competes with cysteine (Km = 1.7 mM) and homocysteine (Km = 2.7 mM) for CSE resulting in the transsulfuration pathway operating in the canonical cysteine-producing track (Fig. 3). Under conditions that induce nitric oxide synthase or heme oxygenase, e.g. ER stress [55] or inflammation [56], enhanced production of NO or CO could lead to ferrous nitrosyl or ferrous carbonyl CBS, which are inactive. Consequently, homocysteine levels rise and cystathionine levels fall, promoting H2S synthesis by CSE (Fig. 3). As NO or CO levels drop, or the ferrous heme in CBS is oxidized, the transsulfuration pathway switches back to the cysteine track. Both the transsulfuration pathway and transporters feed the cysteine pool and conditions such as ER stress enhance cysteine import [57]. In liver, where the transsulfuration pathway is best characterized, CSE is estimated to account for ~97% of H2S produced at physiologically relevant substrate concentrations and taking into account differences in the protein levels of CBS and CSE [58].
Figure 3.
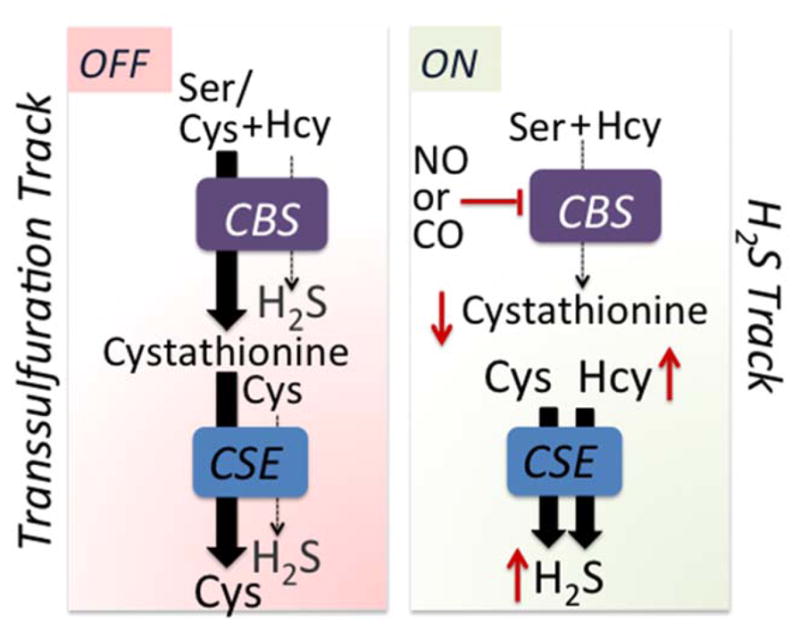
Heme-dependent metabolic track switching. The canonical transsulfuration track operates when the heme in CBS is coordinated by its endogenous ligands and serine, which is more abundant than cysteine and binds with higher affinity, competes effectively for the active site. The product, cystathionine, is then converted by CSE to cysteine. The enzymes switch metabolic tracks when ferrous CBS binds either NO or CO, inhibiting activity, which leads to an increase in homocysteine and a decrease in cystathionine. Under these conditions, H2S synthesis from cysteine, which is catalyzed by CSE, is promoted. The red up and down arrows denote changes in metabolite levels.
Other strategies for controlling H2S production also exist in cells including substrate level activation, posttranslational modification of CBS [37,59] and CSE [60] and regulation of protein levels of the transsulfuration pathway enzymes. While CSE is more abundant than CBS in liver and kidney [58], CBS predominates in brain [61].
Conclusions
The rampant promiscuity of enzymes involved in mammalian H2S synthesis is not surprising from an evolutionary perspective. CSE and CBS orthologs in lower organisms condense cysteine and H2S (or thiosulfate) with O-phosphohomoserine and O-acetylserine forming cystathionine and cysteine (or sulfocysteine), respectively [20,62,63]. While metabolic regulation during evolution has resulted in reversal of the transsulfuration pathway from sulfur assimilation in lower organisms to dissimilation in higher organisms, lax substrate specificity in the pathway enzymes has endured. Other enzymes such as AAT/CAT and D-amino acid oxidase, which feed MST-dependent H2S synthesis, are inherently broad specificity enzymes. The heme-regulated metabolic track switching discussed here is one strategy for regulating the multipurpose enzymes involved in H2S biogenesis; other strategies must exist and remain to be identified. Whether the use of small molecule regulators for switching enzyme specificity and redirecting flux might is a strategy deployed by other metabolic pathways remains to be elucidated.
Highlights.
This review highlights the prevalence of promiscuity not only in the enzymes that synthesize hydrogen sulfide, a signaling molecule, but also in the metabolic pathways in which they reside.
The role of heme-dependent metabolic track switching as a mechanism of regulating flux between competing pathways is discussed.
Acknowledgments
This work was supported by grants from the National Institutes of Health (HL58984 and GM112455).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jensen RA. Enzyme recruitment in evolution of new function. Annu Rev Microbiol. 1976;30:409–425. doi: 10.1146/annurev.mi.30.100176.002205. [DOI] [PubMed] [Google Scholar]
- 2.Yang K, Metcalf WW. A new activity for an old enzyme: Escherichia coli bacterial alkaline phosphatase is a phosphite-dependent hydrogenase. Proc Natl Acad Sci U S A. 2004;101(21):7919–7924. doi: 10.1073/pnas.0400664101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bridgham JT, Carroll SM, Thornton JW. Evolution of hormone-receptor complexity by molecular exploitation. Science. 2006;312(5770):97–101. doi: 10.1126/science.1123348. [DOI] [PubMed] [Google Scholar]
- 4.Aharoni A, Gaidukov L, Khersonsky O, Mc QGS, Roodveldt C, Tawfik DS. The ‘evolvability’ of promiscuous protein functions. Nat Genet. 2005;37(1):73–76. doi: 10.1038/ng1482. [DOI] [PubMed] [Google Scholar]
- 5.D’Ari R, Casadesus J. Underground metabolism. Bioessays. 1998;20(2):181–186. doi: 10.1002/(SICI)1521-1878(199802)20:2<181::AID-BIES10>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 6.Tawfik DS. Messy biology and the origins of evolutionary innovations. Nat Chem Biol. 2010;6(10):692–696. doi: 10.1038/nchembio.441. [DOI] [PubMed] [Google Scholar]
- 7.Kabil O, Banerjee R. Redox biochemistry of hydrogen sulfide. J Biol Chem. 2010;285:21903–21907. doi: 10.1074/jbc.R110.128363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh S, Banerjee R. PLP-dependent H2S biogenesis. Biochim Biophys Acta. 2011;1814:1518–1527. doi: 10.1016/j.bbapap.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kabil O, Banerjee R. Enzymology of H2S biogenesis, decay and signaling. Antioxid Redox Signal. 2014;20(5):770–782. doi: 10.1089/ars.2013.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stipanuk MH. Sulfur amino acid metabolism: Pathways for production and removal of homocysteine and cysteine. Annu Rev Nutr. 2004;24:539–577. doi: 10.1146/annurev.nutr.24.012003.132418. [DOI] [PubMed] [Google Scholar]
- 11.Nagahara N, Ito T, Kitamura H, Nishino T. Tissue and subcellular distribution of mercaptopyruvate sulfurtransferase in the rat: Confocal laser fluorescence and immunoelectron microscopic studies combined with biochemical analysis. Histochem Cell Biol. 1998;110(3):243–250. doi: 10.1007/s004180050286. [DOI] [PubMed] [Google Scholar]
- 12•.Singh S, Padovani D, Leslie RA, Chiku T, Banerjee R. Relative contributions of cystathionine beta-synthase and gamma-cystathionase to H2S biogenesis via alternative trans-sulfuration reactions. J Biol Chem. 2009;284(33):22457–22466. doi: 10.1074/jbc.M109.010868. Evaluates the relative contributions of the transsulfuration pathway enzymes to H2S versus persulfide synthesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13•.Chiku T, Padovani D, Zhu W, Singh S, Vitvitsky V, Banerjee R. H2S biogenesis by cystathionine gamma-lyase leads to the novel sulfur metabolites, lanthionine and homolanthionine, and is responsive to the grade of hyperhomocysteinemia. J Biol Chem. 2009;284:11601–11612. doi: 10.1074/jbc.M808026200. Describes the catalytic promiscuity of CSE and links homolanthionine formation to H2S synthesis from homocysteine. Shows that H2S synthesis is increased in homocystinuria. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ida T, Sawa T, Ihara H, Tsuchiya Y, Watanabe Y, Kumagai Y, Suematsu M, Motohashi H, Fujii S, Matsunaga T, Yamamoto M, et al. Reactive cysteine persulfides and S-polythiolation regulate oxidative stress and redox signaling. Proc Natl Acad Sci U S A. 2014;111(21):7606–7611. doi: 10.1073/pnas.1321232111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yadav PK, Martinov M, Vitvitsky V, Seravalli J, Wedmann R, Filipovic MR, Banerjee R. Biosynthesis and reactivity of cysteine persulfides in signaling. J Am Chem Soc. 2016;138(1):289–299. doi: 10.1021/jacs.5b10494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cavallini D, Mondovi B, De Marco C, Sciosciasantoro A. Inhibitory effect of mercaptoethanol and hypotaurine on the desulfhydration of cysteine by cystathionase. Arch Biochem Biophys. 1962;96:456–457. doi: 10.1016/0003-9861(62)90436-8. [DOI] [PubMed] [Google Scholar]
- 17.Mudd SH, Finkelstein JD, Irreverre F, Laster L. Homocystinuria: An enzymatic defect. Science. 1964;143:1443–1445. doi: 10.1126/science.143.3613.1443. [DOI] [PubMed] [Google Scholar]
- 18.Perry TL, Hansen S, MacDougall L. Homolanthionine excretion in homocystinuria. Science. 1966;152(730):1750–1752. doi: 10.1126/science.152.3730.1750. [DOI] [PubMed] [Google Scholar]
- 19.Kozich V, Krijt J, Sokolova J, Melenovska P, Jesina P, Vozdek R, Majtan T, Kraus JP. Thioethers as markers of hydrogen sulfide production in homocystinurias. Biochimie. 2016;126:14–20. doi: 10.1016/j.biochi.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Ravanel S, Gakiere B, Job D, Douce R. The specific features of methionine biosynthesis and metabolism in plants. Proc Natl Acad Sci U S A. 1998;95(13):7805–7812. doi: 10.1073/pnas.95.13.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.An S, Kumar R, Sheets ED, Benkovic SJ. Reversible compartmentalization of de novo purine biosynthetic complexes in living cells. Science. 2008;320(5872):103–106. doi: 10.1126/science.1152241. [DOI] [PubMed] [Google Scholar]
- 22.Meister A, Fraser PE, Tice SV. Enzymatic desulfuration of beta-mercaptopyruvate to pyruvate. J Biol Chem. 1954;206(2):561–575. [PubMed] [Google Scholar]
- 23.Ubuka T, Umemura S, Yuasa S, Kinuta M, Watanabe K. Purification and characterization of mitochondrial cysteine aminotransferase from rat liver. Physiol Chem Phys. 1978;10(6):483–500. [PubMed] [Google Scholar]
- 24.Recasens M, Benezra R, Basset P, Mandel P. Cysteine sulfinate aminotransferase and aspartate aminotransferase isoenzymes of rat brain. Purification, characterization, and further evidence for identity. Biochemistry. 1980;19(20):4583–4589. doi: 10.1021/bi00561a007. [DOI] [PubMed] [Google Scholar]
- 25.Akagi R. Purification and characterization of cysteine aminotransferase from rat liver cytosol. Acta Med Okayama. 1982;36(3):187–197. doi: 10.18926/AMO/30697. [DOI] [PubMed] [Google Scholar]
- 26.Crawhall JC, Parker R, Sneddon W, Young EP, Ampola MG, Efron ML, Bixby EM. Beta mercaptolactate-cysteine disulfide: Analog of cystine in the urine of a mentally retarded patient. Science. 1968;160(3826):419–420. doi: 10.1126/science.160.3826.419. [DOI] [PubMed] [Google Scholar]
- 27.Huang J, Niknahad H, Khan S, O’Brien PJ. Hepatocyte-catalysed detoxification of cyanide by L- and D-cysteine. Biochem Pharmacol. 1998;55(12):1983–1990. doi: 10.1016/s0006-2952(98)00072-0. [DOI] [PubMed] [Google Scholar]
- 28•.Shibuya N, Koike S, Tanaka M, Ishigami-Yuasa M, Kimura Y, Ogasawara Y, Fukui K, Nagahara N, Kimura H. A novel pathway for the production of hydrogen sulfide from D-cysteine in mammalian cells. Nat Commun. 2013;4:1366. doi: 10.1038/ncomms2371. Describes D-cysteine as a source of mercaptpyruvate, which feeds into MST-dependent H2S synthesis. [DOI] [PubMed] [Google Scholar]
- 29.Dixon M, Kleppe K. D-amino acid oxidase II. Specificity, competitive inhibition and reaction sequence. Biochim Biophys Acta. 1965;96(3):368–382. [Google Scholar]
- 30.Molla G, Sacchi S, Bernasconi M, Pilone MS, Fukui K, Polegioni L. Characterization of human D-amino acid oxidase. FEBS Lett. 2006;580(9):2358–2364. doi: 10.1016/j.febslet.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 31.Yadav PK, Yamada K, Chiku T, Koutmos M, Banerjee R. Structure and kinetic analysis of H2S production by human mercaptopyruvate sulfurtransferase. J Biol Chem. 2013;288:20002–20013. doi: 10.1074/jbc.M113.466177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagahara N, Yoshii T, Abe Y, Matsumura T. Thioredoxin-dependent enzymatic activation of mercaptopyruvate sulfurtransferase. An intersubunit disulfide bond serves as a redox switch for activation. J Biol Chem. 2007;282(3):1561–1569. doi: 10.1074/jbc.M605931200. [DOI] [PubMed] [Google Scholar]
- 33.Nagahara N, Nishino T. Role of amino acid residues in the active site of rat liver mercaptopyruvate sulfurtransferase. J Biol Chem. 1996;271(44):27395–27401. doi: 10.1074/jbc.271.44.27395. [DOI] [PubMed] [Google Scholar]
- 34.Linster CL, Van Schaftingen E, Hanson AD. Metabolite damage and its repair or preemption. Nat Chem Biol. 2013;9(2):72–80. doi: 10.1038/nchembio.1141. [DOI] [PubMed] [Google Scholar]
- 35.Khersonsky O, Tawfik DS. Enzyme promiscuity: A mechanistic and evolutionary perspective. Annu Rev Biochem. 2010;79:471–505. doi: 10.1146/annurev-biochem-030409-143718. [DOI] [PubMed] [Google Scholar]
- 36•.Prudova A, Bauman Z, Braun A, Vitvitsky V, Lu SC, Banerjee R. S-adenosylmethionine stabilizes cystathionine beta-synthase and modulates redox capacity. Proc Natl Acad Sci U S A. 2006;103(17):6489–6494. doi: 10.1073/pnas.0509531103. Reports an additional layer of regulation on heme-dependent CBS inhibition by the allosteric effector AdoMet with implications for controlling H2S synthesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Niu WN, Yadav PK, Adamec J, Banerjee R. S-glutathionylation enhances human cystathionine beta-synthase activity under oxidative stress conditions. Antioxid Redox Signal. 2015;22(5):350–361. doi: 10.1089/ars.2014.5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taoka S, West M, Banerjee R. Characterization of the heme and pyridoxal phosphate cofactors of human cystathionine β-synthase reveals nonequivalent active sites. Biochemistry. 1999;38(9):2738–2744. doi: 10.1021/bi9826052. [DOI] [PubMed] [Google Scholar]
- 39.Puranik M, Weeks CL, Lahaye D, Kabil O, Taoka S, Nielsen SB, Groves JT, Banerjee R, Spiro TG. Dynamics of carbon monoxide binding to cystathionine beta-synthase. J Biol Chem. 2006;281(19):13433–13438. doi: 10.1074/jbc.M600246200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vicente JB, Colaco HG, Sarti P, Leandro P, Giuffre A. S-adenosyl-l-methionine modulates CO and NO* binding to the human H2S-generating enzyme cystathionine beta-synthase. J Biol Chem. 2016;291(2):572–581. doi: 10.1074/jbc.M115.681221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41••.Kabil O, Weeks CL, Carballal S, Gherasim C, Alvarez B, Spiro TG, Banerjee R. Reversible heme-dependent regulation of human cystathionine beta-synthase by a flavoprotein oxidoreductase. Biochemistry. 2011;50(39):8261–8263. doi: 10.1021/bi201270q. Demonstrates that despite the low redox potential of the CBS heme, it can be reduced by flavoproteins in the presence of CO or NO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taoka S, Banerjee R. Characterization of NO binding to human cystathionine [beta]-synthase:Possible implications of the effects of CO and NO binding to the human enzyme. J Inorg Biochem. 2001;87(4):245–251. doi: 10.1016/s0162-0134(01)00335-x. [DOI] [PubMed] [Google Scholar]
- 43.Vicente JB, Colaco HG, Mendes MI, Sarti P, Leandro P, Giuffre A. NO* binds human cystathionine beta-synthase quickly and tightly. J Biol Chem. 2014;289(12):8579–8587. doi: 10.1074/jbc.M113.507533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gherasim C, Yadav PK, Kabil O, Niu WN, Banerjee R. Nitrite reductase activity and inhibition of H2S biogenesis by human cystathionine beta-synthase. PLoS One. 2014;9(1):e85544. doi: 10.1371/journal.pone.0085544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Agrawal N, Banerjee R. Human Polycomb 2 protein is a Sumo E3 ligase and alleviates substrate-induced inhibition of cystathionine beta-synthase sumoylation. PLoS One. 2008;3(12):e4032. doi: 10.1371/journal.pone.0004032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meier M, Janosik M, Kery V, Kraus JP, Burkhard P. Structure of human cystathionine beta-synthase: A unique pyridoxal 5′-phosphate-dependent heme protein. EMBO J. 2001;20(15):3910–3916. doi: 10.1093/emboj/20.15.3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taoka S, Lepore BW, Kabil O, Ojha S, Ringe D, Banerjee R. Human cystathionine beta-synthase is a heme sensor protein. Evidence that the redox sensor is heme and not the vicinal cysteines in the cxxc motif seen in the crystal structure of the truncated enzyme. Biochemistry. 2002;41(33):10454–10461. doi: 10.1021/bi026052d. [DOI] [PubMed] [Google Scholar]
- 48.Bateman A. The structure of a domain common to archaebacteria and the homocystinuria disease protein. Trends Biochem Sci. 1997;22:12–13. doi: 10.1016/s0968-0004(96)30046-7. [DOI] [PubMed] [Google Scholar]
- 49.Kabil Ö, Taoka S, LoBrutto R, Shoemaker R, Banerjee R. The pyridoxal phosphate binding sites are similar in human heme-dependent and yeast heme-independent cystathionine beta synthases. Evidence from 31p nmr and pulsed epr spectroscopy that the heme and the plp cofactors are not proximal in the human enzyme. J Biol Chem. 2001;276:19350–19355. doi: 10.1074/jbc.M100029200. [DOI] [PubMed] [Google Scholar]
- 50.Taoka S, Green EL, Loehr TM, Banerjee R. Mercuric chloride-induced spin or ligation state changes in ferric or ferrous human cystathionine beta-synthase inhibit enzyme activity. J Inorg Bioc. 2001;87:253–259. doi: 10.1016/s0162-0134(01)00336-1. [DOI] [PubMed] [Google Scholar]
- 51.Singh S, Madzelan P, Banerjee R. Properties of an unusual heme cofactor in plp-dependent cystathionine beta-synthase. Nat Prod Rep. 2007;24:631–639. doi: 10.1039/b604182p. [DOI] [PubMed] [Google Scholar]
- 52••.Weeks CL, Singh S, Madzelan P, Banerjee R, Spiro TG. Heme regulation of human cystathionine beta-synthase activity: Insights from fluorescence and raman spectroscopy. J Am Chem Soc. 2009;131(35):12809–12816. doi: 10.1021/ja904468w. Reveals the molecular basis of long range allosteric regulation from the heme to the catalytic site in CBS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carballal S, Madzelan P, Zinola CF, Grana M, Radi R, Banerjee R, Alvarez B. Dioxygen reactivity and heme redox potential of truncated human cystathionine beta-synthase. Biochemistry. 2008;47(10):3194–3201. doi: 10.1021/bi700912k. [DOI] [PubMed] [Google Scholar]
- 54••.Kabil O, Yadav V, Banerjee R. Heme-dependent metabolite switching regulates H2S synthesis in response to endoplasmic reticulum (ER) stress. J Biol Chem. 2016;291(32):16418–16423. doi: 10.1074/jbc.C116.742213. Reveals how the heme in CBS can regulate switching between metabolic tracks for cysteine versus H2S synthesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu XM, Peyton KJ, Ensenat D, Wang H, Schafer AI, Alam J, Durante W. Endoplasmic reticulum stress stimulates heme oxygenase-1 gene expression in vascular smooth muscle. Role in cell survival. J Biol Chem. 2005;280(2):872–877. doi: 10.1074/jbc.M410413200. [DOI] [PubMed] [Google Scholar]
- 56.Vane JR, Mitchell JA, Appleton I, Tomlinson A, Bishop-Bailey D, Croxtall J, Willoughby DA. Inducible isoforms of cyclooxygenase and nitric-oxide synthase in inflammation. Proc Natl Acad Sci U S A. 1994;91(6):2046–2050. doi: 10.1073/pnas.91.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57••.Gao XH, Krokowski D, Guan BJ, Bederman I, Majumder M, Parisien M, Diatchenko L, Kabil O, Willard B, Banerjee R, Wang B, et al. Quantitative H2S-mediated protein sulfhydration reveals metabolic reprogramming during the integrated stress response. eLife. 2015:4. doi: 10.7554/eLife.10067. Reports a robust method for persulfide tagging in the proteome and the role of this postranslational modification in reprogramming energy metabolism during ER stress. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kabil O, Vitvitsky V, Xie P, Banerjee R. The quantitative significance of the transsulfuration enzymes for H2S production in murine tissues. Antioxid Redox Signal. 2011;15(2):363–372. doi: 10.1089/ars.2010.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.d’Emmanuele di Villa Bianca R, Mitidieri E, Esposito D, Donnarumma E, Russo A, Fusco F, Ianaro A, Mirone V, Cirino G, Russo G, Sorrentino R. Human cystathionine-beta-synthase phosphorylation on serine227 modulates hydrogen sulfide production in human urothelium. PLoS One. 2015;10(9):e0136859. doi: 10.1371/journal.pone.0136859. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 60.Yuan G, Vasavda C, Peng YJ, Makarenko VV, Raghuraman G, Nanduri J, Gadalla MM, Semenza GL, Kumar GK, Snyder SH, Prabhakar NR. Protein kinase G-regulated production of H2S governs oxygen sensing. Sci Signal. 2015;8(373):ra37. doi: 10.1126/scisignal.2005846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Enokido Y, Suzuki E, Iwasawa K, Namekata K, Okazawa H, Kimura H. Cystathionine beta-synthase, a key enzyme for homocysteine metabolism, is preferentially expressed in the radial glia/astrocyte lineage of developing mouse CNS. FASEB J. 2005;19(13):1854–1856. doi: 10.1096/fj.05-3724fje. [DOI] [PubMed] [Google Scholar]
- 62.Leustek T, Martin MN, Bick JA, Davies JP. Pathways and regulation of sulfur metabolism revealed through molecular and genetic studies. Annu Rev Plant Physiol Plant Mol Biol. 2000;51:141–165. doi: 10.1146/annurev.arplant.51.1.141. [DOI] [PubMed] [Google Scholar]
- 63.Sekowska A, Kung HF, Danchin A. Sulfur metabolism in Escherichia coli and related bacteria: Facts and fiction. J Mol Microbiol Biotechnol. 2000;2(2):145–177. [PubMed] [Google Scholar]