Abstract
Infections caused by Plasmodium falciparum and P. vivax account for more than 90% of global malaria burden. Exposure to malaria parasite elicits immune responses during natural infection and it is generally believed that the immunity is not only stage specific but also species specific. However, partial genomic similarity for various antigens in different Plasmodium spp. raises the possibility of immunological cross-reactivity at the level of specific antigens. Serum samples collected from children who were permanent residents of a P. falciparum transmission area in Zimbabwe were screened for antibody reactivity against Pfs48/45, a P. falciparum gametocyte antigen and Pvs48/45, a P. vivax homolog of Pfs48/45 using ELISA. Western blotting was used to further confirm identity of the specific antibody reactivity to the Pfs48/45 and Pvs48/45 proteins. Pan Plasmodium PCR and nested PCR were used to confirm infection with the Plasmodium species. Twenty-seven percent (49/181) of the participants were found to be sero-positive for Pfs48/45 and 73% (n=36) of these Pfs48/45 positive sera also showed reactivity with Pvs48/45. Immune cross-reactivity revealed by ELISA was also confirmed by Western blot analysis using a panel of randomly selected 23 Pfs48/45 and Pvs48/45 ELISA positive samples. Nested PCR analysis of 27 blood samples randomly selected from the 36 that showed positive ELISA reactivity to both Pfs48/45 and Pvs48/45 antigens confirmed infection with P. falciparum and generalized absence of P. vivax except for a single sample which revealed PCR positivity for both P. vivax and P. falciparum. Our studies with sera samples from a predominantly P. falciparum transmission area in Zimbabwe suggest immunological cross-reactivity with Pvs48/45, thus raising the possibility of partial species cross-reactive immunity and possible cross-boosting of immunity during co-infection with P. falciparum and P. vivax.
Keywords: Cross reactivity, Plasmodium falciparum, Plasmodium Vivax, antibody responses
Introduction
Current estimates indicate 95 countries and territories as having ongoing malaria transmission (WHO, 2015) in spite of a world-wide reduction in malaria incidence among populations at risk. About 50% of the world’s population is at risk globally among whom, young children, pregnant women and non-immune travellers are especially vulnerable. According to the WHO, out of an estimated 247 million malaria cases, about 88% of malaria cases and 90% of malaria deaths occur in sub-Saharan Africa.
Malaria is transmitted by female Anopheles mosquitoes and is caused by a protozoan of the genus Plasmodium. Four well-established Plasmodium species infect humans, P. vivax, P. malariae, P. falciparum and P. ovale; of these P. falciparum is most prevalent in sub-Saharan Africa while P. vivax dominates outside of sub-Saharan Africa. P. falciparum and P. vivax are endemic and coexist in many countries (Mueller et al., 2009) and account for more than 90% of global malaria burden (WHO, 2015) and in eastern and southern Africa, P. vivax represents around 10% of malaria cases. P. falciparum is responsible for most malaria-related deaths globally, and malaria caused by P. vivax is considered relatively benign, though sometimes causing life-threatening outcomes (Guerra et al., 2010; Price et al., 2007). Additionally, human infection with P. knowlesi have also been detected in some areas and it is believed to be due to zoonoses (Antinori et al., 2013). It is generally accepted that immune responses against the malaria parasite are by and large specific to the infecting species with little known cross reactivity at least between the major human parasites P. falciparum and P. vivax (Clyde, 1990; Nussenzweig and Chen, 1974; Nussenzweig et al., 1972). However, some studies have reported on cross-reactivity of few antigens specific to pre-erythrocytic and erythrocytic asexual stages of multiple Plasmodium species. This includes: (i) recognition of P. falciparum asexual stage antigens by antibodies in sera from people exposed to P. vivax, (Kumar et al., 1992), and suppression of P. falciparum by sera from a case of P. vivax (Nagao et al., 2008), (ii) recognition of circumsporozoite protein (CSP) of P. falciparum and P. berghei by antibodies elicited by VMP001, a P. vivax CSP-based vaccine (VMP00) (Yadava et al., 2012), (iii) induction of cross-reactive and cross-protective antibodies by PfCelTOS, a highly conserved cell-traversal protein expressed on the surface of ookinetes and sporozoites (Bergmann-Leitner et al., 2010), and (iv) reported cross reactivity between apical membrane antigen 1, cytoadherence-linked asexual gene 9 product and merozoite surface protein 5 of P. falciparum and P. vivax (Costa et al., 2013; Igonet et al., 2007; Woodberry et al., 2008).
A search of the genome sequences (www.plasmodb.org) indicates that there are several antigens in the sexual stages which share significant sequence similarity, however the question of possible immunological cross-reactivity has remained a matter of conjecture. The availability of recombinant proteins offers an opportunity to test it directly. More recently, we have shown cross reactive immunity of murine sera raised against Pfs48/45 and Pvs48/45, as well as cross boosting of immune responses against heterologous P48/45 (Cao et al., 2016). Pfs48/45, a protein produced during P. falciparum gametocyte development, is expressed on the surface of male and female gametes and zygotes and plays a key role in male gamete fertility and sexual development. Monoclonal antibodies and polyclonal antibodies against recombinant Pfs48/45 expressed in E. coli have been shown to prevent zygote development and reduced oocyst formation in the mosquito midgut (Chowdhury et al., 2009; Outchkourov et al., 2007; Outchkourov et al., 2008; Rener et al., 1983; van Dijk et al., 2001). Comparison of Pfs48/45 and Pvs48/45 reveals 61% and 55% identity at the level of DNA and protein sequences, respectively; arguing in favour of antigenic and possibly functional immune cross-reactivity between Pfs48/45 and Pvs48/45. The availability of E. coli – expressed recombinant Pfs48/45 and Pvs48/45 allowed us to investigate the immune cross reactivity using human sera from a primarily P. falciparum endemic region in Zimbabwe. In Zimbabwe P. falciparum is the major parasite species responsible for ~100% total malaria cases with no officially documented transmission of P. vivax (WHO, 2015). We reasoned that recognition of Pvs48/45 antigen by sera from individuals resident in P. falciparum endemic Zimbabwe will likely to be due to recognition of cross-reactive epitopes by antibodies elicited by Pfs48/45 during natural exposure to P. falciparum, and not due to a prior P. vivax infection. Demonstration of any immunological cross-reactivity at the level of key vaccine targets is further suggested to likely influence natural dynamics of malaria transmission in the areas co-endemic for P. falciparum and P. vivax.
Materials and Methods
Study area and participants
The study was first reviewed by University of Zimbabwe Institutional Review Board and ethically approved by the Medical Research Council of Zimbabwe (MRCZ/A/1710). The study samples from students attending Bemberi primary school in Mt Darwin district in north eastern Zimbabwe were obtained from an ongoing study on schistosomiasis and malaria co-infection in an endemic area of Zimbabwe (Midzi et al., 2014).
The samples were collected during the malaria transmission season, between February and May. The study design comprised of a cross sectional survey of 203 children aged 6 to 14 and attending school in 2013 with a second follow-up survey and sample collection in 2015. Permission was obtained from the community leaders, provincial medical director, and district medical officer. Parents provided consent for participation of the children and the children signed an assent form after learning the aims, risks and benefits of the study. The primary inclusion criterion for the study included willingness to provide all required samples for parasitology and serology and those children who visibly appeared malnourished or presenting with any unrelated illness were excluded from participation in the study. All the children provided a stool and urine specimen over three consecutive days for detection of schistosomiasis and soil transmitted helminth. Blood specimen were collected on the third day for malaria examination and plasma for immunological investigations. Children positive for any parasitic infection based on urine and stool microscopy received standard treatment of praziquantel at 40 mg/kg body weight and albendazole for the soil transmitted helminths infection. Prevalence of S. haematobium determined in 2013 and 2015 surveys was 33/121 and 31/80, respectively. Prevalence of malaria in the study subjects was 6/121 in 2013 and the target district reported P. falciparum malaria prevalence of 6.6/100 in 2015, as compared to much higher (>16.0/100) prevalence in the surrounding districts and the area ranks 9th out of top 20 malarious districts in Zimbabwe. The packed blood cells and plasma samples were kept frozen at −20°C in the field at a health facility then transported to the laboratory and kept at −80°C until used for the assays.
Rapid diagnostic tests and blood smear microscopy
Immediately upon collection, blood samples were tested for the presence of parasites using rapid malaria diagnostic test (Paracheck™). Thin and thick blood smears stained with 10 % Giemsa were prepared for detection of P. falciparum infection by microscopy. Children found to be positive by either rapid diagnostic test or microscopy were treated immediately at the local health facility with artemisinin-lumefantrine combination. Blood smears were re-examined by an expert microscopist from the National Institute of Health Research in Harare, Zimbabwe for quality assurance.
Recombinant antigens
Details of production, purification and immunogenicity evaluation of recombinant Pfs48/45 and Pvs48/45 are described elsewhere (Cao et al., 2016; Chowdhury et al., 2009). The protein purity was assessed by SDS-PAGE and Western blotting, and the protein concentration was measured by the bicinchoninic acid (BCA) method (Thermo Scientific).
ELISA
Plasma samples were analyzed by ELISA as described previously (Cao et al, 2016) to screen for antibody cross reactivity between Pfs48/45 and Pvs48/45. Briefly, ELISA plates (Immulon 4 HBX) were coated with 100 μl/well of 1 μg/ml antigen in 0.1 M carbonate-bicarbonate buffer (pH 9.6) overnight at 4°C. After coating, the plates were washed three times with PBS 0.05% Tween 20 (wash buffer) and blocked with 5% non-fat milk in PBS for 1 hour at 37°C and washed three times with the wash buffer. Plasma samples were diluted 1:500 in the wash buffer containing 5% non-fat milk, and tested in duplicate. After incubation at 37°C for 1h in a shaking incubator, unbound antibodies were removed by three washes with wash buffer. Bound antibodies were detected with peroxidase-conjugated goat anti-human IgG and IgM (KPL; 1mg/ml stock diluted 1:5,000). The secondary antibody was allowed to react for 1 hour and then wells were washed three times with wash buffer. The amount of antibodies bound was quantified by adding 100μl ABTS peroxidase substrate (KPL) and the color development was measured after 20 minutes (VersaMax Elisa Reader, Molecular Device). A positive control was included on each plate to normalize readings of samples from plate to plate. Briefly, for any given ELISA, positive control readings were averaged for all the plates and used to find the differential for positive control for any given plate to apply as a correction factor for sample readings on the corresponding plate (Clark et al., 2012). The negative cut-off was determined by averaging the negative control readings (pooled sera from 10 North American donors with no prior history of malaria or travel to malaria endemic region) and adding 2 standard deviations.
SDS-PAGE and Western blot analysis
SDS-PAGE and Western blotting was used to confirm antibody cross-reactivity between Pfs48/45 and Pvs48/45 Plasmodium protein antigens. Protein samples reduced with 5% 2-mercaptoethanol were fractionated by 10% SDS-PAGE and blotted on to nitrocellulose membrane. The blots were incubated with PBS containing 0.05% Tween 20 and 2% goat serum to block any non-specific sites and incubated with 1:200 dilution (PBS plus 0.01% tween 20, 2% goat serum) of test human sera for 2 hours. After washing with PBS containing 0.05% Tween 20, the membranes were incubated with horseradish peroxidase (HRP)-conjugated anti-human IgG (1: 5,000) or anti-human IgM (1:20,000) and finally developed using ECL Prime Western blotting detection reagent (GE Healthcare).
DNA preparation and molecular diagnosis
Parasite DNA was extracted from 300 μl whole blood using the Qiagen FlexiGene DNA kit. DNA was suspended in 200 μl elution buffer and stored at −20°C. A nested PCR protocol (Mahajan et al., 2012) was used for molecular diagnosis of P. falciparum and P. vivax, First PCR employed pan-Plasmodium primers; PaF (forward) 5′-GAACGAGATCTTAACCTGCTAA-3′, and PaR (reverse) 5′-TCAGCACAATCTGATGAATCAT-3′. Forward and reverse primer pairs used for nested PCR were PfnF 5′-ACATAGGTAACTATACATTTATTCAGT-3′ and PfnR 5′-AGCATCAAAGATACAAATATAAGCA-3′ for P. falciparum; and PvnF 5′-GTGGGACTGAATTCGGTTGA-3′ and PvnR 5′-TACGAAACAGCAAGCTGAATCG-3′ for P. vivax. All the primers were reconstituted to a final concentration of 10 mM and stored at −20°C. The pan Plasmodium PCR mixture (50 μl) consisted of the following: 1 μl each of forward and reverse primers, 5 μl PCR 10X buffer (500 mM Tris.HCl, 20 mM MgCl2, 100 mM KCl, 50 mM (NH4)2SO4, pH 8.3), 1 μl 10 mM dNTPs, 0.5 μl Taq DNA polymerase, and 3 μl genomic DNA. For Plasmodium species–specific nested PCR, 1 μl of amplified pan PCR product was used as the DNA template and species specific pairs of primers. PCR was carried out using the PCR system (Gene Amp 9700, Perkin-Elmer, Waltham, MA) thermal cycler. The following PCR conditions were used: for pan Plasmodium-3 minutes of denaturation at 94°C, 40 cycles of 30 seconds at 94°C, 30 seconds at 57°C, and 30 seconds at 72°C, with a final extension time of 7 minutes at 72°C; and for nested PCR-3 minutes of denaturation at 94°C followed by 30 cycles of 30 seconds at 94°C, 30 seconds at 55°C, and 30 seconds at 72°C, with a final extension time of 7 minutes at 72°C. Purified genomic DNA from P. falciparum (NF54 maintained in culture) and P. vivax (Sal 1 strain dried blood spots on filter papers obtained from MR4) were used as positive and negative controls to establish specificity of nested PCR. P vivax genomic DNA served as a negative control for P. falciparum nested PCR and P. falciparum genomic DNA as negative control for P vivax nested PCR. Amplified products were analyzed using 2% agarose gel and staining with ethidium bromide and photographed using BioRad GelDoc XR+.
Results
Analysis of sera for antibodies against Pfs48/45 and Pvs48/48 by ELISA
Sera/plasma samples collected in 2013 and 2015 from children attending Bemberi primary school in North-Eastern regions of Zimbabwe were analyzed for antibodies against Pfs48/45 protein. Afterwards, sera were tested by ELISA for recognition of Pvs48/45 antigen. ELISA with Bemberi 2013 and 2015 sera (n=181) screened for reactivity against P. falciparum using Pfs48/45 revealed 27 % (n=49) positivity (27.03% positive in 2013 and 27.24% positive in 2015). A pool of N. American normal human sera from ten donors with no past history of travel to malaria endemic areas was used as a negative control and used to assign a cut-off based on mean +2SD (0.26 for Pfs48/45 coated ELISA plates) to identify positive sera. ELISA results against Pfs48/45 for sera collected in 2013 and 2015 from participants are shown in Figs. 1A and 1B, respectively. The Pfs48/45 ELISA positive samples (n=49) were then screened for reactivity against P. vivax antigen Pvs48/45, together with an additional 31 other randomly selected samples to give a total of 80 samples. The negative control cut-off for Pvs48/45 was 0.19 and sixty samples were positive for Pvs48/45 positive by ELISA (Fig. 1A and B). Among Pfs48/45 positive samples a total of 36 (86% samples from 2013 collection and 61% from 2015 collection) showed positive reactivity with Pvs48/45 giving an overall average of 73%. Approximately a similar percentage and low ELISA positive reactivity was also observed for 24/31 randomly selected samples.
Figure 1. ELISA results for Pfs48/45 and Pvs48/45.
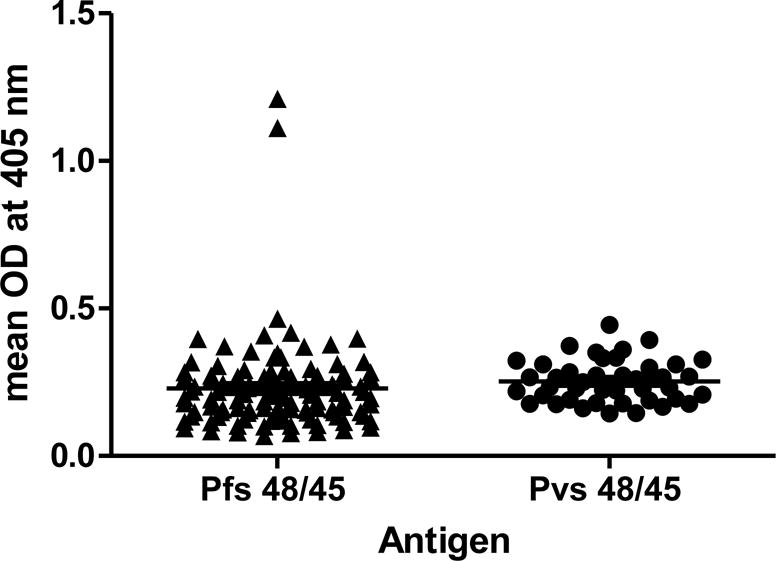
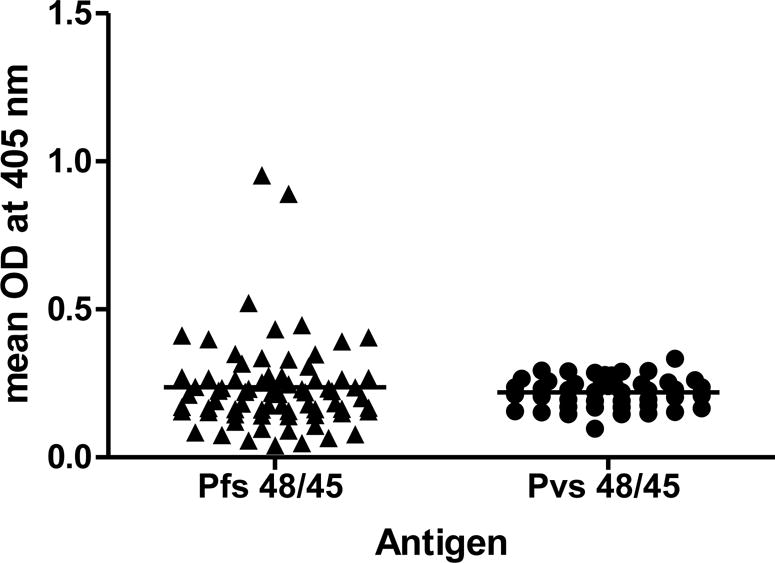
Absorbance (405 nm) with Bemberi 2013 (A) and Bemberi 2015 (B) plasma samples reacting with recombinant Pfs48/45 and Pvs48/45 antigens.
SDS-PAGE and Western blot analysis
Specific human lgG and lgM responses to P. falciparum antigen Pfs48/45 and P. vivax antigen Pvs48/45 was confirmed in 23 randomly selected samples (ELISA positive for Pfs48/45 and Pvs48/45) using SDS-PAGE and western blot analysis using recombinant Pfs48/45 and Pvs48/45. Serum samples that produced double bands or a single band of apparent molecular weight of 50 kDa were considered to have anti-Pfs48/45 and anti-Pvs48/45 antibodies. Figs. 2A and 2B show Western blot reactivity of 14 representative samples to reduced forms Pfs48/45 and Pvs48/45 proteins, respectively. These studies also revealed some discrepancies: one ELISA positive sample (#33) was negative by Western blot analysis for both Pfs48/45 and Pvs48/45 and a second sample (#34) that was ELISA reactive with both antigens did not recognize Pvs48/45 by Western blotting.
Figure 2. Western blot Analysis.
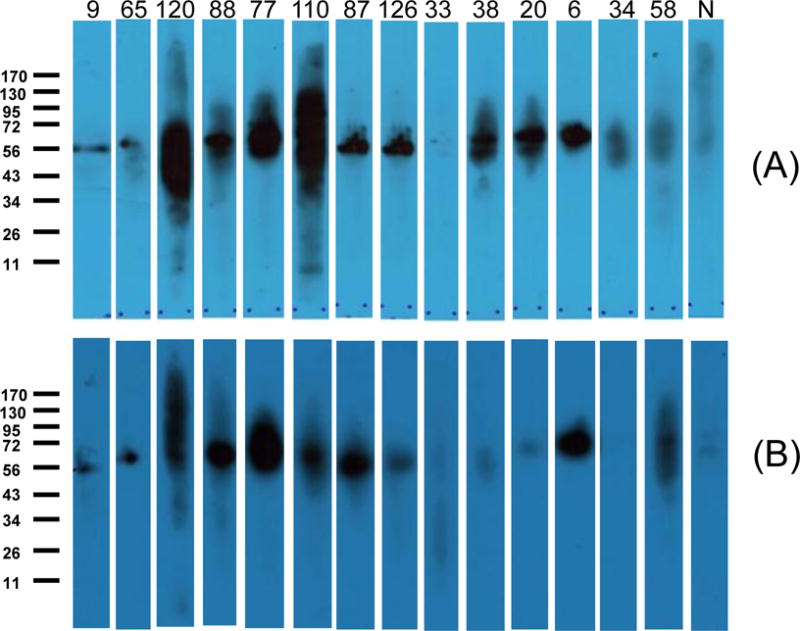
Images of representative plasma samples (1:200 dilutions) reacting with Pfs48/45 (A) and Pvs48/45 (B).
PCR detection of human Plasmodium species
Pan Plasmodium PCR and nested PCR were used to identify Plasmodium species using DNA extracted from whole blood samples (n=27) available for individuals who had revealed ELISA positivity for both Pfs48/45 and Pvs48/45. Pan-Plasmodium PCR sense and antisense primers used were designed based on 18s ribosomal RNA gene that has 100% sequence homology for P. falciparum and P. vivax. In order to detect species of Plasmodium, primers specific for P. falciparum and P. vivax were used in a second nested PCR amplification. PCR amplification using P. falciparum and P. vivax species specific primers gave specific amplicons of expected size (Figs. 3A and 3B for P. falciparum and P. vivax, respectively). PCR analysis revealed 24 out of 27 participants positive for P. falciparum, and only one participant revealed a PCR band for P. vivax. This individual also showed P. falciparum specific band, suggesting co-infection with both species. One sample was found to be negative by nested PCR for both P. falciparum and P. vivax. The specificity of nested PCR was established using standard P. falciparum (lab grown NF54 isolate DNA) and P. vivax (Sal 1 strain from MR4).
Figure 3. Results of nested PCR for P. falciparum (A) and P. vivax (B).
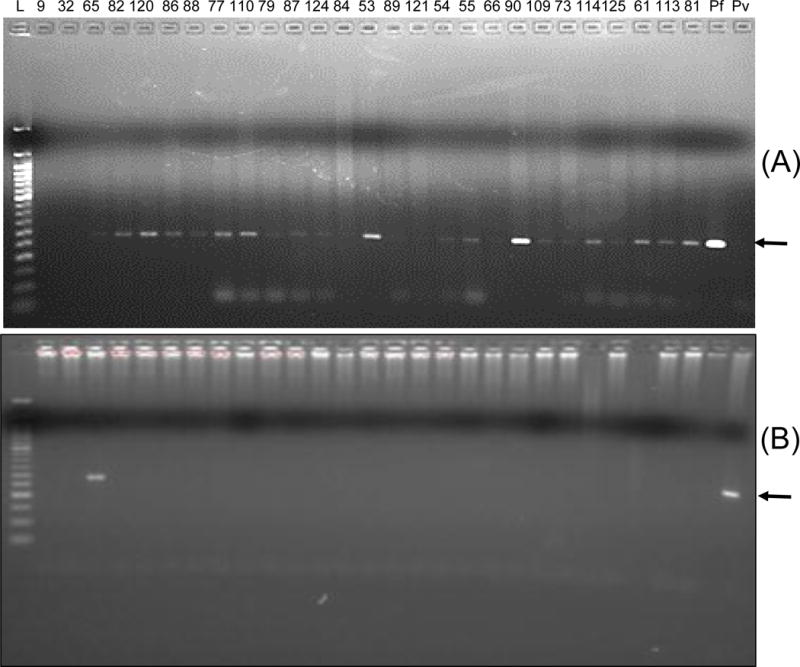
Lane marked L shows a 50 base pair molecular mass marker ladder and the number on top show the samples IDs of Bemberi 2013 participants. Lane marked Pf contained P. falciparum (NF54) genomic DNA and lane marked Pv contained P. vivax (Sal1 strain) genomic DNA.
Discussion
The most widely distributed malaria parasite in the world is P. vivax, but it was believed to be “absent” from Africa, because of the fact that majority of the Africans are Duffy negative. The high prevalence of Duffy negativity in Africa provided a rationale for excluding the possibility of P. vivax transmission in Africa, since the Duffy antigen is the only known receptor for P. vivax infection. However, a recent paper (Howes et al., 2015) reviewed evidence sources such as local clinical case reports, entomological and serological studies that contradicts this general perspective. Other reports also described the presence of P. vivax parasites in Duffy negative individuals in Africa and Latin America (Cavasini et al., 2007; Ryan et al., 2006), and it has been proposed that the parasite is in the process of developing mechanisms that permit the infection of people that do not express the Duffy antigen on their red blood cells. Such findings highlight the requirement for a vibrant study of the prevalence and population dynamics of P. vivax in Africa, using accurate molecular species diagnostic tools (Culleton et al., 2008).
P48/45 proteins are highly conserved among Plasmodium species. Pvs48/45 shares 70.4% identity at the level of DNA sequence with other Plasmodium species. Greater homology is observed between P. vivax and P. knowlesi (84.0%), whereas P. falciparum shares 60.08 % homology with P. vivax at the level of DNA sequence, and 55% at the level of protein sequence (Arévalo-Herrera et al., 2015). Availability of recombinant Pfs48/45 and Pv48/45 proteins offered the opportunity to investigate the question of immune cross-reactivity between P. falciparum and P. vivax using human sera from a known, low to moderate P. falciparum transmission area. The cross-species immune reactivity was observed by the fact that 73 % anti-Pfs48/45 ELISA positive sera also reacted with recombinant Pvs48/45. A previous study using plasma from acute malaria infected patients living in central China where P. vivax is endemic has described cross reactivity of antibodies against two P. vivax antigens with P. falciparum homologous proteins, (Xia et al., 2015). They found an elevated level of antibodies to total P. vivax proteins and low levels of antibodies to total P. falciparum proteins in acute P. vivax infected samples, suggesting antibody cross reactivity between P. vivax and P. falciparum antigens.
In order to experimentally rule out local prevalence of P. vivax in our study area nested PCR detection method was used to analyze the parasite species in the blood samples evaluated by ELISA and Western blotting, As shown, 25 out of 27 samples analyzed by nested PCR showed the presence of P. falciparum infection and only 1 out of 27 samples showed presence of both P. falciparum and P. vivax specific PCR bands. Based on these results, we believe it will be incorrect to state that P. vivax is completely absent in Zimbabwe. However, propose that there is always a possibility that low level undocumented concurrent P, vivax infection may account for the apparent observed cross-reactivity demonstrated by our findings. However, it seems more reasonable to conclude that the higher sero-prevalence of Pvs48/45 positivity is more likely a reflection of cross-reactivity of antibodies elicited by predominant P. falciparum infection in Zimbabwe. Tables 1 and 2 summarize data on reported cross-reactivity by ELISA and Western blot analysis and apparent lack of P. vivax infection by PCR analysis.
Table 1.
Summary of the cross reactivity study using Bemberi 2013 serum samples
| Method | ELISA | SDS-PAGE and Western blot | P. falciparum Nested PCR | P. vivax Nested PCR | ||||
|---|---|---|---|---|---|---|---|---|
| Antigen | Pfs 48/45 | Pvs 48/45 | Pfs 48/45 | Pvs 48/45 | ||||
| Secondary antibodies | Anti-human IgG/IgM | Anti-human IgG/IgM | Anti-human IgG | Anti-human IgM | Anti-human IgG | Anti-human IgM | ||
| Participant | ||||||||
| 9 | + | + | + | + | + | + | − | − |
| 58 | + | − | + | + | − | +\− | + | − |
| 32 | + | − | − | − | + | + | − | − |
| 65 | + | + | + | + | + | + | + | + |
| 82 | + | + | + | + | + | − | ||
| 120 | + | + | − | + | +\− | + | + | − |
| 86 | + | + | + | +\− | + | + | + | − |
| 88 | + | + | + | + | + | + | + | − |
| 77 | + | + | + | + | + | + | + | − |
| 110 | + | + | + | + | + | + | + | − |
| 79 | + | + | +\− | +\− | − | + | + | − |
| 87 | + | + | + | + | + | + | + | − |
| 126 | + | + | + | + | + | + | ||
| 53 | + | + | + | + | + | + | + | − |
| 89 | + | + | + | +\− | + | + | Faint bands | − |
| 121 | + | + | − | − | − | + | Faint bands | − |
| 84 | + | + | ND | ND | ND | ND | + | − |
| 116 | + | − | ND | ND | ND | ND | No sample | No sample |
| 55 | + | + | ND | ND | ND | ND | + | − |
| 66 | + | + | ND | ND | ND | ND | Faint bands | − |
| 90 | + | + | ND | ND | ND | ND | + | − |
| 61 | + | + | ND | ND | ND | ND | + | − |
| 125 | + | + | ND | ND | ND | ND | + | − |
| 114 | + | + | ND | ND | ND | ND | + | − |
| 78 | + | − | ND | ND | ND | ND | + | − |
| 91 | + | + | ND | ND | ND | ND | ND | ND |
| 109 | + | + | ND | ND | ND | ND | + | − |
| 81 | + | + | ND | ND | ND | ND | + | − |
| 113 | + | + | ND | ND | ND | ND | + | − |
| 124 | + | No | ND | ND | ND | ND | + | − |
| 70 | − | + | ND | ND | ND | ND | ND | ND |
| 64 | − | − | ND | ND | ND | ND | ND | ND |
| 31 | − | + | ND | ND | ND | ND | ND | ND |
| 42 | − | + | ND | ND | ND | ND | ND | ND |
| 38 | − | − | ND | ND | ND | ND | ND | ND |
| 7 | − | + | ND | ND | ND | ND | ND | ND |
| 48 | − | − | ND | ND | ND | ND | ND | ND |
| 62 | − | + | ND | ND | ND | ND | ND | ND |
| 54 | − | − | ND | ND | ND | ND | ND | ND |
| 72 | − | + | ND | ND | ND | ND | ND | ND |
| 69 | − | + | ND | ND | ND | ND | ND | ND |
| 60 | − | + | ND | ND | ND | ND | ND | ND |
| 71 | − | − | ND | ND | ND | ND | ND | ND |
| 75 | − | − | ND | ND | ND | ND | ND | ND |
ND: Not determined
Table 2.
Summary of the cross reactivity study using Bemberi 2015 samples
| Method | ELISA | SDS-PAGE and Western blot | |||||
|---|---|---|---|---|---|---|---|
| Antigen | Pfs 48/45 | Pvs 48/45 | Pfs 48/45 | Pvs 48/45 | |||
| Secondary antibody | Anti-human IgG/IgM | Anti-human IgG/IgM | Anti-human IgG | Anti-human IgM | Anti-human IgG | Anti-human IgM | |
| Participants | |||||||
| 110 | + | + | + | High background | − | +\− | |
| 76 | + | + | +\− | + | +/− | + | |
| 36 | + | + | + | + | + | + | |
| 58 | + | − | − | + | − | + | |
| 20 | + | + | + | + | + | + | |
| 86 | + | No sample | No sample | No sample | No sample | No sample | |
| 6 | + | + | + | +|− | + | + | |
| 34 | + | + | +|− | − | + | + | |
| 61 | + | + | + | + | + | + | |
| 1 | + | − | + | + | + | + | |
| 99 | + | + | + | High background | + | + | |
| 32 | + | − | High background | +\− | High background | + | |
| 98 | + | − | + | +\− | − | − | |
| 7 | + | − | + | + | +|− | +|− | |
| 93 | + | + | ND | ND | ND | ND | |
| 77 | + | − | ND | ND | ND | ND | |
| 96 | + | + | ND | ND | ND | ND | |
| 94 | + | + | ND | ND | ND | ND | |
| 27 | + | ND | ND | ND | ND | ND | |
| 16 | − | ND | − | − | ND | ND | |
| 17 | − | − | − | − | ND | ND | |
| 21 | − | + | ND | ND | ND | ND | |
| 120 | − | − | ND | ND | ND | ND | |
| 29 | − | + | ND | ND | ND | ND | |
| 44 | − | + | ND | ND | ND | ND | |
| 75 | − | + | ND | ND | ND | ND | |
| 57 | − | + | ND | ND | ND | ND | |
| 123 | − | + | ND | ND | ND | ND | |
| 17 | − | − | ND | ND | ND | ND | |
| 78 | − | + | ND | ND | ND | ND | |
| 108 | − | − | ND | ND | ND | ND | |
| 106 | − | − | ND | ND | ND | ND | |
| 5 | − | + | ND | ND | ND | ND | |
| 92 | − | + | ND | ND | ND | ND | |
| 65 | − | + | ND | ND | ND | ND | |
| 10 | − | + | ND | ND | ND | ND | |
| 14 | − | + | ND | ND | ND | ND | |
| 80 | − | + | ND | ND | ND | ND | |
| 71 | − | + | ND | ND | ND | ND | |
| 9 | − | + | ND | ND | ND | ND | |
| 90 | − | + | ND | ND | ND | ND | |
| 40 | − | − | ND | ND | ND | ND | |
| 63 | − | + | ND | ND | ND | ND | |
| 74 | − | − | ND | ND | ND | ND | |
ND: Not determined
Officially, malaria in Zimbabwe is almost exclusively attributed to P. falciparum. Current diagnostic, treatment and surveillance systems in much of sub-Saharan Africa including Zimbabwe are not designed to identify or report non P. falciparum human malaria infections accurately. Recent reports on high occurrence of P. vivax in some parts of Africa, south of Sahara, it is possible that both Duffy negative and 1–5% of the human population who are Duffy positive might maintain the low level transmission of P. vivax (Culleton and Carter, 2012). In order to ascertain exposure of serum-donors to P. vivax malaria the reactivity of antibodies to Pvs48/45 protein was determined in the test serum by ELISA and Western blotting. Human IgG and IgM reacted with both Pfs48/45 and Pvs48/45 as shown by the ELISA data and Western blots suggesting that there might be undetected or underappreciated low level transmission of P. vivax malaria in some areas of Zimbabwe. With the reduction in malaria transmission globally and many countries heading towards malaria elimination, management of P. vivax malaria is gaining importance because of its unique inherent biological features (Barbosa et al., 2014). As the Zimbabwe national malaria control program is embarking towards elimination of malaria, the current data will be important in planning malaria control interventions as the strategy for P. falciparum malaria control may be different from P. vivax malaria control. For example, the current P. falciparum malaria control depends mainly on artemisinin based treatment while the elimination of P. vivax malaria requires robust surveillance for case recognition and radical management to prevent relapse malaria (Sharma et al., 2015).
The reactivity of the human sera with P. vivax antigen, Pvs48/45, measured by ELISA and Western blot using P. vivax antigen was likely due to the cross reactivity of antibodies elicited in response to P. falciparum exposure, rather than to exposure with P. vivax. The cross-reactivity of the antigens may have important consequences for species specific and species transcending immunity.
Acknowledgments
We would like to thank the participants and community of Bemberi in Mt Darwin for their support. We also thank the NIHR team and the Biochemistry staff, University of Zimbabwe for assisting in fieldwork. This work received financial support from NIH/FIC2D43TW001587 Malaria Training and Research Capacity Building in Southern Africa (MTCBSA) Project (PI: Dr. Nirbhay Kumar).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contribution
GB and NK conceived the idea, designed the experiments, analyzed the data and developed the final written manuscript; AV performed the assays and analyzed the data; GB, NK and YC provided technical guidance to AV; TM coordinated collection of samples. All authors contributed to the writing of the manuscript.
Conflict of interest: The authors declare no conflict of interest.
References
- WHO. World Malaria Report 2015. World Health Organization; Geneva: 2015. http://www.who.int/malaria/publications/world-malaria-report-2015/report/en. [Google Scholar]
- Antinori S, Galimberti L, Milazzo L, Corbellino M. Plasmodium knowlesi: The emerging zoonotic malaria parasite. Acta Tropica. 2013;125:191–201. doi: 10.1016/j.actatropica.2012.10.008. [DOI] [PubMed] [Google Scholar]
- Arévalo-Herrera M, Vallejo AF, Rubiano K, Solarte Y, Marin C, Castellanos A. Recombinant Pvs 48/45 antigen expressed in E. coli generates antibodies that block malaria transmission in Anopheles albimanus mosquitoes. PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0119335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa S, Gozze AB, Lima NF, Batista CL, da Silva Bastos M, Nicolete VC, Fontoura PS, Gonçalves RM, Viana SAS, Menezes MJ. Epidemiology of disappearing Plasmodium vivax malaria: a case study in rural Amazonia. PLoS Negl Trop Dis. 2014;8:e3109. doi: 10.1371/journal.pntd.0003109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann-Leitner ES, Mease RM, De La Vega P, Savranskaya T, Polhemus M, Ockenhouse C, Angov E. Immunization with pre-erythrocytic antigen CelTOS from Plasmodium falciparum elicits cross-species protection against heterologous challenge with Plasmodium berghei. PloS one. 2010;5:e12294. doi: 10.1371/journal.pone.0012294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Bansal GP, Merino K, Kumar N. Immunological Cross-Reactivity between Malaria Vaccine Target Antigen P48/45 in Plasmodium vivax and P. falciparum and Cross–Boosting of Immune Responses. PLoS ONE. 2016;11:e0158212. doi: 10.1371/journal.pone.0158212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavasini CE, de Mattos LC, Couto ÁADA, Bonini-Domingos CR, Valencia SH, de Souza Neiras WC, Alves RT, Rossit ARB, Castilho L, Machado RLD. Plasmodium vivax infection among Duffy antigen-negative individuals from the Brazilian Amazon region: an exception? Transactions of the Royal Society of Tropical Medicine and Hygiene. 2007;101:1042–1044. doi: 10.1016/j.trstmh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Chowdhury DR, Angov E, Kariuki T, Kumar N. A Potent Malaria Transmission Blocking Vaccine Based on Codon Harmonized Full Length Pfs48/45 Expressed in Escherichia coli. PLoS ONE. 2009;4:e6352. doi: 10.1371/journal.pone.0006352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark EH, Silva CJ, Weiss GE, Li S, Padilla C, Crompton PD, Hernandez JN, Branch OH. Plasmodium falciparum malaria in the Peruvian Amazon, a region of low transmission, is associated with immunologic memory. Infection and immunity. 2012;80:1583–1592. doi: 10.1128/IAI.05961-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clyde D. Immunity to falciparum and vivax malaria induced by irradiated sporozoites: a review of the University of Maryland studies, 1971–75. Bulletin of the World Health Organization. 1990;68:9. [PMC free article] [PubMed] [Google Scholar]
- Costa JD, Zanchi FB, Rodrigues FLdS, Honda ER, Katsuragawa TH, Pereira DB, Taborda RLM, Tada MS, Ferreira RdGM, Pereira-da-Silva LH. Cross-reactive anti-PfCLAG9 antibodies in the sera of asymptomatic parasite carriers of Plasmodium vivax. Memórias do Instituto Oswaldo Cruz. 2013;108:98–105. doi: 10.1590/S0074-02762013000100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culleton R, Carter R. African Plasmodium vivax: Distribution and origins. International Journal for Parasitology. 2012;42:1091–1097. doi: 10.1016/j.ijpara.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Culleton RL, Mita T, Ndounga M, Unger H, Cravo PV, Paganotti GM, Takahashi N, Kaneko A, Eto H, Tinto H. Failure to detect Plasmodium vivax in West and Central Africa by PCR species typing. Malaria Journal. 2008;7:1. doi: 10.1186/1475-2875-7-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra CA, Howes RE, Patil AP, Gething PW, Van Boeckel TP, Temperley WH, Kabaria CW, Tatem AJ, Manh BH, Elyazar IR. The international limits and population at risk of Plasmodium vivax transmission in 2009. PLoS Negl Trop Dis. 2010;4:e774. doi: 10.1371/journal.pntd.0000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes RE, Reiner RC, Jr, Battle KE, Longbottom J, Mappin B, Ordanovich D, Tatem AJ, Drakeley C, Gething PW, Zimmerman PA. Plasmodium vivax transmission in Africa. PLoS Negl Trop Dis. 2015;9:e0004222. doi: 10.1371/journal.pntd.0004222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igonet S, Vulliez-Le Normand B, Faure G, Riottot MM, Kocken CH, Thomas AW, Bentley GA. Cross-reactivity studies of an anti-Plasmodium vivax apical membrane antigen 1 monoclonal antibody: binding and structural characterisation. Journal of molecular biology. 2007;366:1523–1537. doi: 10.1016/j.jmb.2006.12.028. [DOI] [PubMed] [Google Scholar]
- Kumar N, Folgar JP, Lubega P. Recognition of Plasmodium falciparum asexual stage antigens by antibodies in sera from people exposed to Plasmodium vivax. The American journal of tropical medicine and hygiene. 1992;47:422–428. doi: 10.4269/ajtmh.1992.47.422. [DOI] [PubMed] [Google Scholar]
- Mahajan B, Zheng H, Pham PT, Sedegah MY, Majam VF, Akolkar N, Rios M, Ankrah I, Madjitey P, Amoah G. Polymerase chain reaction–based tests for pan‐species and species‐specific detection of human Plasmodium parasites. Transfusion. 2012;52:1949–1956. doi: 10.1111/j.1537-2995.2011.03541.x. [DOI] [PubMed] [Google Scholar]
- Midzi N, Mduluza T, Chimbari MJ, Tshuma C, Charimari L, Mhlanga G, Manangazira P, Munyati SM, Phiri I, Mutambu SL, Midzi SS, Ncube A, Muranzi LP, Rusakaniko S, Mutapi F. Distribution of Schistosomiasis and Soil Transmitted Helminthiasis in Zimbabwe: Towards a National Plan of Action for Control and Elimination. PLoS Neglected Tropical Diseases. 2014;8:e3014. doi: 10.1371/journal.pntd.0003014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller I, Galinski MR, Baird JK, Carlton JM, Kochar DK, Alonso PL, del Portillo HA. Key gaps in the knowledge of Plasmodium vivax, a neglected human malaria parasite. The Lancet infectious diseases. 2009;9:555–566. doi: 10.1016/S1473-3099(09)70177-X. [DOI] [PubMed] [Google Scholar]
- Nagao Y, Kimura-Sato M, Chavalitshewinkoon-Petmitr P, Thongrungkiat S, Wilairatana P, Ishida T, Tan-ariya P, de Souza JB, Krudsood S, Looareesuwan S. Suppression of Plasmodium falciparum by serum collected from a case of Plasmodium vivax infection. Malaria Journal. 2008;7:1. doi: 10.1186/1475-2875-7-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussenzweig R, Chen D. The antibody response to sporozoites of simian and human malaria parasites: Its stage and species specificity and strain cross-reactivity. Bulletin of the World Health Organization. 1974;50:293. [PMC free article] [PubMed] [Google Scholar]
- Nussenzweig R, Vanderberg J, Spitalny G, Rivera C, Orton C, Most H. Sporozoite-Induced Immunity in Mammalian Malaria A Review. The American journal of tropical medicine and hygiene. 1972;21:722–728. doi: 10.4269/ajtmh.1972.21.722. [DOI] [PubMed] [Google Scholar]
- Outchkourov N, Vermunt A, Jansen J, Kaan A, Roeffen W, Teelen K. Epitope analysis of the malaria surface antigen pfs48/45 identifies a subdomain that elicits transmission blocking antibodies. J Biol Chem. 2007;282 doi: 10.1074/jbc.M700948200. [DOI] [PubMed] [Google Scholar]
- Outchkourov NS, Roeffen W, Kaan A, Jansen J, Luty A, Schuiffel D. Correctly folded Pfs48/45 protein of Plasmodium falciparum elicits malaria transmission-blocking immunity in mice. Proc Natl Acad Sci USA. 2008;105 doi: 10.1073/pnas.0800459105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RN, Tjitra E, Guerra CA, Yeung S, White NJ, Anstey NM. Vivax malaria: neglected and not benign. The American journal of tropical medicine and hygiene. 2007;77:79–87. [PMC free article] [PubMed] [Google Scholar]
- Rener J, Graves P, Carter R, Williams J, Burkot T. Target antigens of transmission-blocking immunity on gametes of Plasmodium falciparum. J Exp Med. 1983;158:976–981. doi: 10.1084/jem.158.3.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan JR, Stoute JA, Amon J, Dunton RF, Mtalib R, Koros J, Owour B, Luckhart S, Wirtz RA, Barnwell JW. Evidence for transmission of Plasmodium vivax among a duffy antigen negative population in Western Kenya. The American journal of tropical medicine and hygiene. 2006;75:575–581. [PubMed] [Google Scholar]
- Sharma VP, Dev V, Phookan S. Neglected Plasmodium vivax malaria in northeastern States of India. The Indian journal of medical research. 2015;141:546. doi: 10.4103/0971-5916.159511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk MR, Janse CJ, Thompson J, Waters AP, Braks JAM, Dodemont HJ, Stunnenberg HG, van Gemert GJ, Sauerwein RW, Eling W. A Central Role for P48/45 in Malaria Parasite Male Gamete Fertility. Cell. 2001;104:153–164. doi: 10.1016/s0092-8674(01)00199-4. [DOI] [PubMed] [Google Scholar]
- WHO. World Malaria Report 2015 [Google Scholar]
- Woodberry T, Minigo G, Piera KA, Hanley JC, de Silva HD, Salwati E, Kenangalem E, Tjitra E, Coppel RL, Price RN. Antibodies to Plasmodium falciparum and Plasmodium vivax merozoite surface protein 5 in Indonesia: species-specific and cross-reactive responses. Journal of Infectious Diseases. 2008;198:134–142. doi: 10.1086/588711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia H, Fang Q, Jangpatarapongsa K, Zhiyong T, Cui L, Li B, Udomsangpetch R. A comparative study of natural immune responses against Plasmodium vivax C-terminal merozoite surface protein-1 (PvMSP-1) and apical membrane antigen-1 (PvAMA-1) in two endemic settings. EXCLI journal. 2015;14:926. doi: 10.17179/excli2015-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadava A, Nurmukhambetova S, Pichugin AV, Lumsden JM. Cross-Species Immunity Following Immunization With a Circumsporozoite Protein–Based Vaccine for Malaria. Journal of Infectious Diseases. 2012;205:1456–1463. doi: 10.1093/infdis/jis220. [DOI] [PubMed] [Google Scholar]


