Abstract
Background
Depression is common in Parkinson’s disease (PD) and adversely affects quality of life. Both unilateral and bilateral subthalamic (STN) deep brain stimulation (DBS) effectively treat the motor symptoms of PD, but questions remain regarding the impact of unilateral STN DBS on non-motor symptoms, such as depression.
Methods
We report changes in depression, as measured by the Hamilton Depression Rating Scale (HAMD-17), in 50 consecutive PD patients who underwent unilateral STN DBS. Participants were also evaluated with UPDRS part III, Parkinson’s Disease Questionnaire-39, and Pittsburgh Sleep Quality Index. The primary outcome was change in HAMD-17 at 6 months versus pre-operative baseline, using repeated measures analysis of variance (ANOVA). Secondary outcomes included the change in HAMD-17 at 3, 12, 18, and 24 months post-operatively and correlations amongst outcome variables using Pearson correlation coefficients. As a control, we also evaluated changes in HAMD-17 in 25 advanced PD patients who did not undergo DBS.
Results
Participants with unilateral STN DBS experienced significant improvement in depression 6 months post-operatively (4.94±4.02) compared to preoperative baseline (7.90±4.44) (mean±SD) (p=<0.0001). HAMD-17 scores did not correlate with UPDRS part III at any time-point. Interestingly, the HAMD-17 was significantly correlated with sleep quality and quality of life at baseline, 3 months, and 6 months post-operatively. Participants without DBS experienced no significant change in HAMD-17 over the same interval.
Conclusion
Unilateral STN DBS improves depression 6 months post-operatively in patients with PD. Improvement in depression is maintained over time and correlates with improvement in sleep quality and quality of life.
Keywords: Parkinson’s disease, subthalamic nucleus, deep brain stimulation, depression, sleep, non-motor symptoms
INTRODUCTION
Parkinson’s disease (PD) is a progressive neurodegenerative disease, which is clinically diagnosed by motor symptoms of bradykinesia, rigidity, rest tremor, and postural instability. Many patients also suffer from non-motor symptoms, including autonomic dysfunction, cognitive changes, depression, or sleep disturbances, which in many cases can be more disabling and detrimental to quality of life than the motor symptoms(1, 2). Of these non-motor symptoms, depression is the strongest predictor of negative health-related quality of life(3–5). Importantly, while depression affects 30–40% of PD patients(4, 6), this symptom often cannot be explained as simply a reaction to the diagnosis of a chronic disease(7), nor to therapy with medications used to treat Parkinson’s disease(6, 8). Non-motor symptoms often occur before motor symptoms, and 10–15% patients already have a diagnosis of depression when diagnosed with PD(3, 6, 8). Due to the prevalence and impact of depression in PD, it is important to recognize how current PD treatments affect this disabling symptom.
Like depression, sleep dysfunction also influences quality of life(5, 9). This non-motor symptom affects 74–98% of PD patients and manifests as insomnia, sleep fragmentation, REM behavior disorder, excessive daytime sleepiness, and/or nocturia(2, 10, 11). In the general population, there is a well-recognized association between sleep disorders and depression and sleep dysfunction predicts poor response to treatment of mood disorders(12, 13). Although this association has also been proposed in PD(14, 15), gaps remain in our understanding of the relationship between sleep dysfunction and depression in response to PD therapeutics.
Whether performed bilaterally or unilaterally, subthalamic nucleus (STN) deep brain stimulation (DBS) is safe and effective for motor symptoms, quality of life, and activities of daily living in patients with moderate to advanced PD(16–19). Though bilateral STN DBS improves motor symptoms more than unilateral surgery, PD motor symptoms are typically asymmetric and responsive to dopaminergic therapy. In this context, we and others have argued that unilateral DBS (followed by staged contralateral surgery if needed) is well-tolerated and can provide sufficient motor improvement for up to 5 years(18, 20, 21). The effects of bilateral STN DBS on mood are controversial. Some studies show that DBS ameliorates depressive symptoms and improves quality of life(22, 23), while one case study showed that stimulation can reversibly evoke severe depressive symptoms(24). Further, a large multicenter study reported a higher than expected rate of attempted and completed suicide in PD patients with STN DBS, as compared to World Health Organization statistics, suggesting potential effects on impulsivity and/or depression(25). However, this study did not have control subjects with PD who did not have DBS(25). While unilateral STN DBS improves motor symptoms, its effects on depression have not been extensively investigated(26). To better characterize the impact of DBS therapy on this disabling symptom, we hypothesized that unilateral STN DBS improves depression in patients with moderate to advanced idiopathic PD. Additionally, we examined the relationships between depression and changes in motor symptoms, quality of life, and sleep quality in PD patients following unilateral STN DBS.
METHODS
Patients
Selection of patients for STN DBS has been previously described(27). Briefly, each potential DBS candidate underwent pre-surgical neuropsychological testing and brain MRI. Our multidisciplinary DBS committee discusses all potential DBS candidates to determine suitability for surgery and recommendations for treatment. Patients were excluded if neuropsychological testing or history revealed active depression (uncontrolled on medications), dementia, or psychosis, or if MRI demonstrated severe cortical or subcortical atrophy or significant ischemic changes. As previously described, surgical targeting for electrode localization was achieved with frame-based stereotaxy, microelectrode recordings, and intra-operative evaluation of clinical effects of stimulation(18). Post-operative 1.5 Tesla volumetric brain MRI was used to confirm appropriate electrode placement and to screen for potential complications. In all cases, the DBS electrode was placed contralateral to the side most affected by motor symptoms. Our routine practice for PD is to place unilateral DBS, followed by a staged implantation on the contralateral side at a later time if necessary(18, 21).
Eighty-seven consecutive patients with PD (UK Brain Bank criteria (2)) who underwent unilateral STN DBS surgery at UAB were evaluated before surgery and at regular intervals post-operatively. Patients who underwent the staged contralateral procedure earlier than 6 months after the initial procedure (N=6), or who had incomplete data at the 3- or 6-month assessment period were excluded, leaving 50 participants in the final analysis. Participant demographics including age, sex, duration of disease, side of DBS placement, and DBS settings 6 months post-operatively are shown in Table 1. This study was approved by the local Institutional Review Board (IRB) at UAB, which approved a waiver of consent for collection of these data as part of routine clinical care and quality control.
Table 1.
Participant Demographics
| Age (years) | |
| Mean ± SD | 60.3 ± 9.6 |
| Range | 37 – 76 |
| Sex (% male) | 62% |
| Duration of Disease (years) | |
| Mean ± S.D. | 11.6 ± 5.5 |
| Range | 2.8 – 30.1 |
| Side of Stimulator Placement (% Left) | 52% |
| DBS settings at 6 months: Mean ± SD | |
| Amplitude (V) | 3.4 ± 0.6 |
| Pulse width (μsec) | 86.4 ± 26.9 |
| Frequency (Hz) | 159.4 ± 14.8 |
Study design
In this prospective, within-subject evaluation, participants completed the Hamilton Depression Rating Scale(28) (HAMD-17) pre-operatively, and at 3, 6, 12, 18, and 24 months following unilateral STN DBS. The HAMD-17 questionnaire has been validated as a screening and diagnostic tool for depression in PD(29). Subgroup analyses were performed on participants with left and right STN DBS and on those who screened positive for depression at baseline (HAMD-17 > 11) and those with HAMD-17 ≤11(29). All participants were also evaluated with the Unified Parkinson’s Disease Rating Scale (30)(UPDRS) Part III in the “practically defined off” medication state (morning assessment following at least 12 hours with no antiparkinsonian medication)(31) at baseline and “off” medicines with stimulation on at the 3 and 6 month post-operative time points. Additional outcomes included the Pittsburg Sleep Quality Index (PSQI)(32) and the Parkinson’s Disease Questionnaire-39 (PDQ-39)(33). The outcomes on the PSQI, UPDRS Part III, and the PDQ-39 in this cohort have previously been reported(18, 21, 34). Levodopa equivalent dose (LED) was calculated for each time point using the following conversion: 100 mg dose of levodopa was defined as equivalent to 133 mg of controlled-release levodopa; 75 mg of levodopa plus entacapone; 1 mg of pramipexole, pergolide, lisuride, or cabergoline; 5 mg of ropinirole; and 10 mg of bromocriptine or apomorphineI (16).
To evaluate longitudinal change in HAMD-17 over a similar time interval in participants receiving best medical therapy who did not undergo DBS, we evaluated 25 age and gender matched PD patients in a cohort of participants being screened for other non-motor symptoms. These values were descriptively compared to those who underwent DBS.
Statistical analysis
The primary outcome measure was the change in HAMD-17 at 6 months compared to the pre-operative baseline. Secondary outcomes included the change in HAMD-17 at 3, 12, 18 and 24 months post-operatively. The UPDRS, Part III in the “off” medication state and the LED were evaluated as covariates. Additionally, correlations between the HAMD-17 and sleep quality, measured by PSQI; quality of life, measured by PDQ-39; and motor symptoms, measured by UPDRS Part III were calculated. Descriptive statistics were employed to calculate mean, standard deviation, and standard error of the mean. Multivariable regression analysis with repeated measures within patients was used to analyze outcome measures using changes from baseline to assess incremental changes per patient. Correlations amongst variables were calculated with Pearson Correlation Coefficients. Statistical significance was considered achieved when the p-value was <0.05.
RESULTS
Depression improved at 6 months post-operatively and was sustained over time
Unilateral STN DBS significantly improved depression scores 6 months post-operatively compared to pre-operative baseline, with the average HAMD-17 score decreasing from 7.9±4.4 (mean±SD) to 4.9±4.0 (p<0.001). These outcomes did not change after adjustment for age, sex, disease duration, or side of surgery. Specifically, there were similar improvements in HAMD-17 scores in those with left (baseline: 7.3±4.4; 6-month: 4.7±44; p=0.0013) and right (baseline: 8.5±4.5; 6-month:5.2±3.7; p=0.0044) STN DBS.
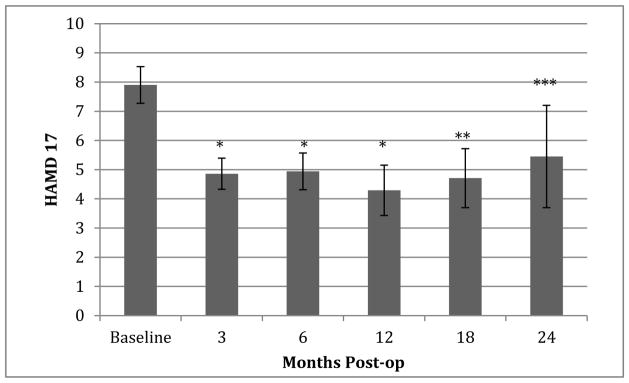
To determine the influence of baseline depression status on HAMD-17 outcomes, we performed subgroup analyses on participants who screened positive for depression (HAMD-17 > 11) (N=11) prior to surgery and those who did not (HAMD-17 ≤ 11) (N=39). Those with a positive screen for depression showed improvement from pre-operative baseline (13.1 ± 2.4) to 6 months post-operatively (6.7 ± 4.4) (p<0.001). Interestingly, improvements in HAMD-17 scores were significant even among participants who did not screen positive for depression at baseline, improving from 6.2 ± 3.2 to 4.4 ± 3.8 (p=0.0035). Only 3 participants had HAMD-17 scores >11 at the 6-month time point. Improvement in depression was apparent as early as 3 months post-operatively (p<0.001) and was sustained over time through the last available assessments at 24 months (Figure 1).
Figure 1.
Improvement in depression is sustained over time. Fifty participants were evaluated at baseline, 3 months, and 6 months. Fewer participants were available for follow-up evaluation at the 12-month (N=31), 18-month (N=24), and 24-months (N=11) post-operative time points.
*p<0.0001, **p=0.0002, ***p=0.0291 compared to baseline
Use of antidepressant and anxiolytic medications was similar between the pre-operative baseline and 6-month follow up. Specifically, at pre-operative baseline, 48% of participants were taking antidepressant medications and 24% were on anxiolytics. At 6-months post-operatively, 46% and 24% of participants were taking antidepressants and anxiolytics, respectively. This was stable at the time of last follow up, with 46% and 17% on antidepressants and anxiolytics, respectively.
In a cohort of 25 age- and sex-matched participants who did not undergo DBS, there was no change in HAMD-17 over time (6.96 ± 5.19 at baseline and 5.48 ± 4.59 at follow up; p=0.138). Those in this group did have shorter disease duration (8.1 ± 4.7 years) compared to those in the DBS group (11.6 ± 5.5 years) (p=0.009), but with no significant difference in age or sex between groups.
Motor improvements
As previously reported(34), patients who underwent unilateral STN DBS had a significant improvement in motor symptoms, measured by the UPDRS Part III in the “practically defined off” medication state, at 3 months post-operatively compared to pre-operative baseline. This improvement continued to 6 months, with little change between the 3- and 6-month post-operative evaluations (Table 2). This motor improvement allowed for decreased anti-Parkinsonian medications by 24.8% as measured by LED (Table 2).
Table 2.
Motor symptoms scores and Levodopa equivalent dose at baseline and follow up
| Pre-operative baseline | 3 months post-op | 6 months post-op | |
|---|---|---|---|
| UPDRS part III, “off” medication | 35.16 ± 1.39 | 20.95 ± 1.42* | 20.42 ± 1.41* |
| Levodopa equivalent dose | 1180.55 ± 76.29 | 909.02 ± 68.73* | 887.08 ± 61.21* |
Values are mean ± SEM
p-value <0.0001 compared to baseline
Depression correlates with sleep quality and quality of life
Scores on the HAMD-17 were significantly positively correlated with sleep quality, as measured by the Pittsburgh Sleep Quality Index at baseline (p<0.001), 3-months (p<0.001), and 6-month post-operatively (p<0.001), indicating that depression correlates with worse sleep quality. Additionally, the degree of depression was significantly correlated with the quality of life measurements at the pre-operative (p<0.001), 3-month (p<0.001), and 6-month (p=0.005) time points (Table 3). Interestingly, HAMD-17 scores did not correlate with motor symptoms, as measured by the UPDRS, Part III, at any time point (p=0.963 pre-operatively and p=0.076 6 months post-operatively). Further, there was no correlation between the change in HAMD-17 and UPDRS from pre-operative baseline to 6-months post-operative follow-up (p=0.663).
Table 3.
Correlation of depression with sleep quality and quality of life
| HAMD-17 baseline r (p-value) |
HAMD-17 3 months r (p-value) |
HAMD-17 6 months r (p-value) |
|
|---|---|---|---|
| PSQI at baseline | 0.62 (p < 0.0001) | ||
| PSQI at 3 months | 0.67 (p < 0.0001) | ||
| PSQI at 6 months | 0.61 (p < 0.0001) | ||
| PDQ-39 at baseline | 0.47 (p = 0.0008) | ||
| PDQ-39 at 3 months | 0.58 (p < 0.0001) | ||
| PDQ-39 at 6 months | 0.39 (p = 0.0050) |
Adverse behaviors
Of the 50 participants in the study, 3 reported adverse behavioral events. One participant was incarcerated for sexual assault after staged placement of the second stimulator, which occurred 22 months after the initial stimulator was placed. These behaviors ceased once dopamine agonists were discontinued. An additional participant developed impulsive behavior with hypersexuality and alcohol abuse 2 years after left STN DBS placement. These symptoms also resolved with reduction of dopamine agonists. Another participant had two suicide attempts, the first occurred 6 months after placement of right STN DBS. This participant’s suicidal ideation seemed to improve for several months by turning the DBS off at night, but he attempted suicide again 14 months post-operation. He did not have any reported history of suicidal ideation or attempt prior to surgery. Baseline HAMD-17 for these participants was 9, 14, and 3, respectively.
DISCUSSION
This within-subject, prospective, longitudinal study provides evidence that unilateral STN DBS improves depression for up to 2 years after surgery in patients with moderate to advanced idiopathic PD. In contrast, an age- and sex-matched sample of 25 PD patients without DBS demonstrated no significant improvement in depression over 6 months. Additionally, we demonstrate robust correlations between depression and subjective sleep quality and quality of life at baseline and at 3 and 6 months after unilateral STN DBS surgery. This is the only study of which we are aware to demonstrate this relationship between mood and sleep following treatment with unilateral STN DBS. Further, we did not find a correlatation between depression and motor symptoms, supporting the idea that depression is not simply a reaction to motor deficits, but also a separate symptom of the underlying neurodegenerative process of advanced PD(35).
Our results suggest that unilateral STN DBS has beneficial effects on depression for up to 2 years. Further, our findings do not support the notion that STN DBS is behaviorally adverse at the group level, as even the most severely depressed patients in our sample showed improvement in depression following unilateral surgery. Similar to our findings, Chopra and colleagues observed a reduction in depression by HAMD in 49 participants 6 months after STN DBS(22) and Funkiewiez and colleagues showed improvement in depression for up to 3 years following bilateral STN DBS(23). In contrast, Weaver and colleagues found no improvement in depression following bilateral surgery(19). Few other studies have investigated the effects of unilateral STN DBS on mood outcomes. One such study randomized PD patients to receive unilateral STN or globus pallidus interna (GPi) DBS and found no differences in mood between the two groups, but an evaluation of all the participants regardless of stimulation target did show an improvement in “happiness” on a visual analog mood scale(36).
Given its clinical heterogeneity, improvement in depression following unilateral STN DBS is likely multifactorial and related to improvements in motor function, sleep quality, quality of life, and/or to medication reduction in individual patients. However, levodopa therapy can reduce depression(37), which would further support efficacy of unilateral STN DBS improving depression, even in the setting of reduced LED. Further, it is possible that STN DBS stimulation could influence brain structures responsible for mood. Specifically, STN has limbic territories, and spread of stimulation to these sites could influence depression(38). Stimulation could also be affecting other monoaminergic pathways important for mediating mood. Though dopaminergic dysfunction can certainly play a role in depression, other monoaminergic structures are also implicated in depression (serotonergic raphe nucleus and the noradrenergic locus coeruleus) and are involved in the neurodegenerative pathogenic process of PD(39). These systems are also involved in regulation of behavioral state and may therefore explain the correlation noted in this study between depression and sleep dysfunction.
This study has several strengths and some limitations. Strengths include the repeated assessment of depression over time, the relatively large sample size, the novel exploration of relationships between depression and sleep quality in the context of DBS, and the inclusion of age- and sex-matched PD patients who did not have DBS. Additionally, this study provides reassuring safety data regarding the effects of unilateral STN DBS for PD on mood and behavior. The most important limitation of this study is the lack of a sham stimulation group. Thus, a potential contribution of the placebo effect is unmeasured. Despite this, we would not have expected a placebo effect to persist over the entire 24-month study duration. Second, although HAMD has been verified as an appropriate questionnaire to use for dichotimizing PD patients into depressed and not depressesd groups and used as a secondary outcome in numerous DBS studies, it is not PD-specific and some of the items could be worsened by physical symptoms of PD and not depression per se. Third, a relatively small minority of patients (N=6) underwent the contralateral DBS surgery before the 6 month time point and were excluded, potentially exluding patients with more severe motor and depression symptoms. However, we calculated the baseline “off” UPDRS part III and HAMD-17 scores for the 6 participants who underwent staged surgery before 6 months and found no difference between that group and the 50 participants included in this analysis (UPDRS p=0.511; HAMD-17 p=0.324).
Although some severe adverse behavioral events occurred, these are difficult to interpret because of the lack of a parallel control group and the infrequency of these events. Importantly, without a sham stimulation or randomized medical control group, the conclusion that DBS caused behavioral side effects can seldom be made with great confidence, especially given the severity of PD symptoms in these patients and their ongoing need for dopamine agonists and other medications with potentially potent effects on behavior. In fact, in two studies that randomized patients to DBS or best medical therapy, one showed a similar number of adverse psychiatric events in both groups, while another showed depression as an adverse event more frequently in the DBS group(19, 40). In an evaluation of potential contributors to suicide in PD patients following STN DBS, Voon and colleagues found that pre-operative history of depression, younger age, being single, previous suicide attempts, and impulse control disorders were associated with attempted suicides(25). For our cases, impulse control disorders associated with dopamine agonist use appeared to contribute in at least two of the three cases. Regardless of underlying cause, the current findings emphasize the importance of discussion of both potential positive and negative mood/behavioral outcomes with candidates for DBS prior to surgery. Further research is needed to understand the etiology of these outcomes in the setting of overall improvement in subjective mood symptoms.
In summary, this study provides evidence that unilateral STN DBS improves depression in patients with moderate to advanced PD and that this improvement correlates with improved sleep quality and quality of life. Further, improvement in depression did not correlate with changes in motor symptoms. Our findings support that well selected patients who undergo unilateral STN DBS surgery will benefit not only from motor improvement but also from improvement of these two disabling non-motor symptoms. These findings also support the bidirectional relationship between mood and sleep. Further research is needed to understand the mechanisms of improvement in mood dysfunction induced by both unilateral and bilateral STN DBS.
HIGHLIGHTS.
Unilateral STN DBS improves depression in patients with Parkinson’s disease.
Improvement in depression correlates with improvements in subjective sleep quality and quality of life, but not with motor symptoms.
Improvement in depression following unilateral STN DBS is sustained over up to 2 years.
Acknowledgments
This work was supported by grant funding from the National Institute of Neurological Disorders and Stroke (grant nos: K23 NS080912 [to A.W.A.] and K23 NS067053 [to H.C.W.], the American Sleep Medicine Foundation, and the CCTS (UL1 TR00141). Dr. Walker receives grant funding from Medtronic (grant no.: 25677). The authors declare that there are no conflicts of interest related to this work.
Abbreviations
- DBS
deep brain stimulation
- ESS
Epworth Sleepiness Scale
- GPi
globus pallidus interna
- HAMD-17
Hamilton Depression Rating Scale
- LED
Levodopa Equivalent Dose
- PD
Parkinson’s disease
- PDQ-39
Parkinson’s disease Questionnaire-39
- PSQI
Pittsburgh Sleep Quality Index
- STN
subthalamic nucleus
- UPDRS
Unified Parkinson’s disease Rating Scale
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Qin Z, Zhang L, Sun F, Fang X, Meng C, Tanner C, et al. Health related quality of life in early Parkinson’s disease: impact of motor and non-motor symptoms, results from Chinese levodopa exposed cohort. Parkinsonism Relat Disord. 2009;15(10):767–71. doi: 10.1016/j.parkreldis.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 2.Rye DB, Bliwise DL. Movement Disorders Specific to Sleep and the Nocturnal Manifestations of Waking Movement Disorders. In: Watts RL, Koller WC, editors. Movement Disorders: Neurologic Principles and Practice. 2. McGraw-Hill; 2004. pp. 855–90. [Google Scholar]
- 3.Duncan GW, Khoo TK, Yarnall AJ, O’Brien JT, Coleman SY, Brooks DJ, et al. Health-related quality of life in early Parkinson’s disease: the impact of nonmotor symptoms. Mov Disord. 2014;29(2):195–202. doi: 10.1002/mds.25664. [DOI] [PubMed] [Google Scholar]
- 4.Schrag A. Quality of life and depression in Parkinson’s disease. J Neurol Sci. 2006;248(1–2):151–7. doi: 10.1016/j.jns.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 5.Naismith SL, Hickie IB, Lewis SJ. The role of mild depression in sleep disturbance and quality of life in Parkinson’s disease. J Neuropsychiatry Clin Neurosci. 2010;22(4):384–9. doi: 10.1176/jnp.2010.22.4.384. [DOI] [PubMed] [Google Scholar]
- 6.Aarsland D, Pahlhagen S, Ballard CG, Ehrt U, Svenningsson P. Depression in Parkinson disease--epidemiology, mechanisms and management. Nat Rev Neurol. 2012;8(1):35–47. doi: 10.1038/nrneurol.2011.189. [DOI] [PubMed] [Google Scholar]
- 7.Nilsson FM, Kessing LV, Sorensen TM, Andersen PK, Bolwig TG. Major depressive disorder in Parkinson’s disease: a register-based study. Acta Psychiatr Scand. 2002;106(3):202–11. doi: 10.1034/j.1600-0447.2002.02229.x. [DOI] [PubMed] [Google Scholar]
- 8.de la Riva P, Smith K, Xie SX, Weintraub D. Course of psychiatric symptoms and global cognition in early Parkinson disease. Neurology. 2014 doi: 10.1212/WNL.0000000000000801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomez-Esteban JC, Tijero B, Somme J, Ciordia R, Berganzo K, Rouco I, et al. Impact of psychiatric symptoms and sleep disorders on the quality of life of patients with Parkinson’s disease. J Neurol. 2011;258(3):494–9. doi: 10.1007/s00415-010-5786-y. [DOI] [PubMed] [Google Scholar]
- 10.Lees AJ, Blackburn NA, Campbell VL. The nighttime problems of Parkinson’s disease. Clin Neuropharmacol. 1988;11(6):512–9. doi: 10.1097/00002826-198812000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Simuni T, Sethi K. Nonmotor manifestations of Parkinson’s disease. Ann Neurol. 2008;64(Suppl 2):S65–80. doi: 10.1002/ana.21472. [DOI] [PubMed] [Google Scholar]
- 12.Maglione JE, Ancoli-Israel S, Peters KW, Paudel ML, Yaffe K, Ensrud KE, et al. Subjective and objective sleep disturbance and longitudinal risk of depression in a cohort of older women. Sleep. 2014;37(7):1179–87. doi: 10.5665/sleep.3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee E, Cho HJ, Olmstead R, Levin MJ, Oxman MN, Irwin MR. Persistent sleep disturbance: a risk factor for recurrent depression in community-dwelling older adults. Sleep. 2013;36(11):1685–91. doi: 10.5665/sleep.3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chung S, Bohnen NI, Albin RL, Frey KA, Muller ML, Chervin RD. Insomnia and sleepiness in Parkinson disease: associations with symptoms and comorbidities. J Clin Sleep Med. 2013;9(11):1131–7. doi: 10.5664/jcsm.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neikrug AB, Maglione JE, Liu L, Natarajan L, Avanzino JA, Corey-Bloom J, et al. Effects of sleep disorders on the non-motor symptoms of Parkinson disease. J Clin Sleep Med. 2013;9(11):1119–29. doi: 10.5664/jcsm.3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deuschl G, Schade-Brittinger C, Krack P, Volkmann J, Schafer H, Botzel K, et al. A randomized trial of deep-brain stimulation for Parkinson’s disease. N Engl J Med. 2006;355(9):896–908. doi: 10.1056/NEJMoa060281. [DOI] [PubMed] [Google Scholar]
- 17.Krack P, Batir A, Van Blercom N, Chabardes S, Fraix V, Ardouin C, et al. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N Engl J Med. 2003;349(20):1925–34. doi: 10.1056/NEJMoa035275. [DOI] [PubMed] [Google Scholar]
- 18.Walker HC, Watts RL, Guthrie S, Wang D, Guthrie BL. Bilateral effects of unilateral subthalamic deep brain stimulation on Parkinson’s disease at 1 year. Neurosurgery. 2009;65(2):302–9. doi: 10.1227/01.NEU.0000349764.34211.74. discussion 9–10. [DOI] [PubMed] [Google Scholar]
- 19.Weaver FM, Follett K, Stern M, Hur K, Harris C, Marks WJ, Jr, et al. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA. 2009;301(1):63–73. doi: 10.1001/jama.2008.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taba HA, Wu SS, Foote KD, Hass CJ, Fernandez HH, Malaty IA, et al. A closer look at unilateral versus bilateral deep brain stimulation: results of the National Institutes of Health COMPARE cohort. J Neurosurg. 2010;113(6):1224–9. doi: 10.3171/2010.8.JNS10312. [DOI] [PubMed] [Google Scholar]
- 21.Sung VW, Watts RL, Schrandt CJ, Guthrie S, Wang D, Amara AW, et al. The relationship between clinical phenotype and early staged bilateral deep brain stimulation in Parkinson disease. J Neurosurg. 2013;119(6):1530–6. doi: 10.3171/2013.8.JNS122025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chopra A, Abulseoud OA, Sampson S, Lee KH, Klassen BT, Fields JA, et al. Mood stability in Parkinson disease following deep brain stimulation: a 6-month prospective follow-up study. Psychosomatics. 2014;55(5):478–84. doi: 10.1016/j.psym.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Funkiewiez A, Ardouin C, Caputo E, Krack P, Fraix V, Klinger H, et al. Long term effects of bilateral subthalamic nucleus stimulation on cognitive function, mood, and behaviour in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2004;75(6):834–9. doi: 10.1136/jnnp.2002.009803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bejjani BP, Damier P, Arnulf I, Thivard L, Bonnet AM, Dormont D, et al. Transient acute depression induced by high-frequency deep-brain stimulation. N Engl J Med. 1999;340(19):1476–80. doi: 10.1056/NEJM199905133401905. [DOI] [PubMed] [Google Scholar]
- 25.Voon V, Krack P, Lang AE, Lozano AM, Dujardin K, Schupbach M, et al. A multicentre study on suicide outcomes following subthalamic stimulation for Parkinson’s disease. Brain. 2008;131(Pt 10):2720–8. doi: 10.1093/brain/awn214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zahodne LB, Okun MS, Foote KD, Fernandez HH, Rodriguez RL, Wu SS, et al. Greater improvement in quality of life following unilateral deep brain stimulation surgery in the globus pallidus as compared to the subthalamic nucleus. J Neurol. 2009;256(8):1321–9. doi: 10.1007/s00415-009-5121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deuschl G, Wenzelburger R, Kopper F, Volkmann J. Deep brain stimulation of the subthalamic nucleus for Parkinson’s disease: a therapy approaching evidence-based standards. J Neurol. 2003;250(Suppl 1):I43–6. doi: 10.1007/s00415-003-1109-8. [DOI] [PubMed] [Google Scholar]
- 28.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leentjens AF, Verhey FR, Lousberg R, Spitsbergen H, Wilmink FW. The validity of the Hamilton and Montgomery-Asberg depression rating scales as screening and diagnostic tools for depression in Parkinson’s disease. Int J Geriatr Psychiatry. 2000;15(7):644–9. doi: 10.1002/1099-1166(200007)15:7<644::aid-gps167>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 30.Fahn S, Elton RL . Members of the UPDRS Development Committee. The Unified Parkinson’s Disease Rating Scale. In: Fahn S, Marsden CD, Calne DB, Goldstein M, editors. Recent Developments in Parkinson’s Disease. Florham Park: Macmillan Healthcare Information; 1987. pp. 153–63. [Google Scholar]
- 31.Langston JW, Widner H, Goetz CG, Brooks D, Fahn S, Freeman T, et al. Core assessment program for intracerebral transplantations (CAPIT) Mov Disord. 1992;7(1):2–13. doi: 10.1002/mds.870070103. [DOI] [PubMed] [Google Scholar]
- 32.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 33.Peto V, Jenkinson C, Fitzpatrick R, Greenhall R. The development and validation of a short measure of functioning and well being for individuals with Parkinson’s disease. Qual Life Res. 1995;4(3):241–8. doi: 10.1007/BF02260863. [DOI] [PubMed] [Google Scholar]
- 34.Amara AW, Standaert DG, Guthrie S, Cutter G, Watts RL, Walker H. Unilateral subthalamic nucleus deep brain stimulation improves sleep quality in Parkinson’s disease. Parkinsonism Relat Disord. 2012;18(1):63–8. doi: 10.1016/j.parkreldis.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lemke MR. Depressive symptoms in Parkinson’s disease. Eur J Neurol. 2008;15(Suppl 1):21–5. doi: 10.1111/j.1468-1331.2008.02058.x. [DOI] [PubMed] [Google Scholar]
- 36.Okun MS, Fernandez HH, Wu SS, Kirsch-Darrow L, Bowers D, Bova F, et al. Cognition and mood in Parkinson’s disease in subthalamic nucleus versus globus pallidus interna deep brain stimulation: the COMPARE trial. Ann Neurol. 2009;65(5):586–95. doi: 10.1002/ana.21596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fetoni V, Soliveri P, Monza D, Testa D, Girotti F. Affective symptoms in multiple system atrophy and Parkinson’s disease: response to levodopa therapy. J Neurol Neurosurg Psychiatry. 1999;66(4):541–4. doi: 10.1136/jnnp.66.4.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim HJ, Jeon BS, Paek SH. Nonmotor Symptoms and Subthalamic Deep Brain Stimulation in Parkinson’s Disease. J Mov Disord. 2015;8(2):83–91. doi: 10.14802/jmd.15010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gallagher DA, Schrag A. Psychosis, apathy, depression and anxiety in Parkinson’s disease. Neurobiol Dis. 2012;46(3):581–9. doi: 10.1016/j.nbd.2011.12.041. [DOI] [PubMed] [Google Scholar]
- 40.Witt K, Daniels C, Reiff J, Krack P, Volkmann J, Pinsker MO, et al. Neuropsychological and psychiatric changes after deep brain stimulation for Parkinson’s disease: a randomised, multicentre study. Lancet Neurol. 2008;7(7):605–14. doi: 10.1016/S1474-4422(08)70114-5. [DOI] [PubMed] [Google Scholar]