Abstract
Background
With climate change, extreme heat (EH) events are increasing, so it is important to understand who is vulnerable to heat-associated morbidity. We determined the association between EH and hospitalizations for all natural causes, cardiovascular, respiratory, and renal diseases, diabetes mellitus, and acute myocardial infarction in Michigan, USA at different intensities and durations. We assessed confounding by ozone and how individual characteristics and health insurance payer (a proxy for income) modified these associations.
Methods
We obtained Michigan Inpatient Database, National Climatic Data Center, and U.S. Environmental Protection Agency ozone data for May–September, 2000–2009 for three Michigan counties. We employed a case-crossover design and modeled EH as an indicator for temperature above the 95th, 97th or 99th percentile thresholds for 1, 2, 3 or 4 days. We examined effect modification by patient age, race, sex, and health insurance payer and pooled the county results.
Results
Among non-whites, the pooled odds ratio for hospitalization on EH (97th-percentile threshold) vs. non-EH days for renal diseases was 1.37 (95% CI = 1.13–1.66), which increased with increasing EH intensity, but was null among whites (OR = 1.00, 95% CI = 0.81, 1.25). We observed a null association between EH and cardiovascular hospitalization. EH (99th-percentile threshold) was associated with myocardial infarction hospitalizations. Confounding by ozone was minimal.
Conclusions
EH was associated with hospitalizations for renal disease among non-whites. This information on vulnerability to heat-associated morbidity helps characterize the public health burden of EH and target interventions including patient education.
Keywords: Hospitalization, Temperature, Morbidity, Heatwave, heat
INTRODUCTION
With climate change, heat waves, or extreme heat (EH) events, have increased in frequency, intensity and duration and are predicted to continue to increase (Martiello and Giacchi 2010). In industrialized countries, heat has been associated with more deaths than all other natural disasters combined (Poumadere et al. 2005). Heat waves are generally defined as extended periods of abnormally high temperature, although the definition of heat wave varies from place to place because the threshold used to identify events depends on the temperature to which the population is accustomed (Luber and McGeehin 2008). The duration and the intensity of heat waves will likely increase in places that are already experiencing extremely hot temperature in the U.S., and cities in the Midwest and the Northeast will likely suffer the most heat-related morbidity and mortality (Luber and McGeehin 2008).
Many studies have examined the association between mortality and heat waves. However, fewer studies have evaluated the association between hospitalizations and EH or heat waves. Reviews and meta-analyses of the association between non-communicable disease morbidity, such as cardiorespiratory morbidity, and extreme temperature in various cities indicated inconsistent associations with the hospitalization outcomes (Turner et al. 2012; Ye et al. 2012). In the United States (U.S.), studies of the 1995 Chicago, Illinois heat wave showed an increase in total hospitalizations during the heat wave period with an increase in admissions for diabetes, hypertension, respiratory diseases, renal diseases, and acute myocardial infarction (Koken et al. 2003; McGeehin and Mirabelli 2001; Semenza et al. 1999). Other studies found an increase in the number of hospitalizations recorded during the 1980 heat wave in Kansas (Mastrangelo et al. 2006; McGeehin and Mirabelli 2001). The 2006 California heat wave was linked with increased risk of admission for acute renal failure, electrolyte imbalance, and nephritis (Knowlton et al. 2009). Time series studies of heat and hospitalizations without focus on a particular heat event have found associations between heat and hospitalization for various respiratory, cardiovascular and renal conditions (Isaksen et al. 2015; Lam et al. 2016; Li et al. 2015; Soneja et al. 2016). A study of heat, heat waves, and hospitalizations among the elderly in 114 cities across the U.S. found associations between renal and respiratory hospitalizations and EH. Additionally, they found an added heat wave effect, whereby the association was stronger following consecutive days of EH. However, associations between cardiovascular hospitalizations and EH were null or slightly inverse (Gronlund et al. 2014). A separate study, also among U.S. elderly, more specifically examining specific hospitalization causes, found increased risk of hospitalization for fluid and electrolyte disorders, renal failure, urinary tract infection, septicemia, and heat stroke on heat_wave vs. non-heat wave days but found a decreased risk of congestive heart failure on heat-wave days (Bobb et al. 2014).
Different factors may compromise a person’s ability to withstand the effects of EH. Most studies suggest that the very old and the very young are most vulnerable (Basu 2002; Gronlund et al. 2016; Isaksen et al. 2015; Kravchenko et al. 2013; Semenza et al. 1999). During the heat waves in 1980 in St. Louis, Missouri and in 1995 in Chicago, mortality rates among people with chronic diseases, the very old or young, those of low socioeconomic status, and urban dwellers tended to be higher when compared to the general population (Jones et al. 1982; Semenza et al. 1999). For reasons likely related to lack of access to air conditioning and cool spaces, low socioeconomic status has been associated with increased vulnerability to heat in many more recent studies of heat morbidity and mortality (Gronlund 2014). Vulnerability may vary according to sex; during the California heat wave of 2006, the mortality rate among women was higher than among men (Knowlton et al. 2009). In addition, several studies in multiple U.S. locations found an increased risk for heat-associated mortality among African Americans as compared to whites (Gronlund et al. 2016; Kravchenko et al. 2013; McGeehin and Mirabelli 2001; Semenza et al. 1999). However, other studies failed to find differences in vulnerability by race (Green et al. 2010; Groulund 2014; Madrigano et al. 2013; Pillai et al. 2014). Heat-associated hospitalizations for renal diseases were significantly higher among African Americans and Hispanics (Fletcher et al. 2012) in a New York study. Marital status may also modify the relationship between temperature and health effects (Bell et al. 2008; Knowlton et al. 2009).
Because the U.S. population life expectancy is high, chronic disease burden in the population is substantial and the population of potentially heat-vulnerable elderly is large (Vogeli et al. 2007). Understanding heat’s association with health outcomes besides mortality, particularly in cooler climates where individuals may not be as well adapted to heat, is important for guiding prevention strategies. The aim of this study was to estimate the association between hospitalizations and high temperatures in the cooler climate of the State of Michigan in recent years (2000–2009). We also evaluated how demographic factors such as: sex, race, age, and income modify the association between high temperature and hospitalizations in this locale. We hope our study results will inform public health preparedness efforts as well as further our understanding of the magnitude of morbidity due to climate change.
MATERIALS AND METHODS
DATA
The study cases included all acute-care hospital admissions of Michigan residents of Ingham, Washtenaw, and Wayne Counties during the warmer months of May–September, 2000–2009. The individual records, stripped of patient and hospital identifiers, were retrieved from a copy of the Michigan Resident Inpatient Files, created using data from the Michigan Inpatient Database (MIDB) obtained with permission from the Michigan Health & Hospital Association Service Corporation and maintained by the Division of Vital Records and Health Statistics, Michigan Department of Health and Human Services (MDHHS). Each MIDB record includes the patient’s sex, age, health insurance payer or payment type, race/ethnicity, and primary and secondary discharge diagnoses classified according to the International Classification of Diseases (ICD) System, 9th Revision, Clinical Modification (ICD-9-CM). Institutional Review Board approvals from the Michigan Department of Health and Human Services and the University of Michigan were obtained for these analyses.
Daily mean temperatures, defined as the average of the daily maximum and minimum temperatures, were downloaded from the National Climatic Data Center (NCDC) website (NCDC 2011). The temperature monitors were located at the Detroit City Airport (DET/KDET) and Detroit Metropolitan Airport (DTW/KDTW) in Wayne County; Lansing Capital City Airport (LAN/KLAN) in Ingham County; and the University of Michigan in Washtenaw County.
We considered ozone as a potential confounder, as elevated ozone levels have been associated with an increased risk of hospitalizations for conditions such as cardiovascular and respiratory diseases and diabetes mellitus (Hajat et al. 2002; Tsai et al. 2012), and ozone levels are often higher during hot weather. Air pollution data were obtained from the U.S. Environmental Protection Agency (U.S. EPA 2012). These data included hourly ozone levels measured by monitors in Washtenaw, Ingham, and Wayne Counties from 2000–2009. For, both ozone and temperature, daily means were calculated for each monitor. Wayne County had three ozone monitors, so these results were then averaged across all the monitors by day. Ozone was considered to be a confounder of the heat-hospitalization association if the inclusion of ozone terms in the model altered the odds ratio (OR) of hospitalization on EH days vs. non-EH days by more than 10%.
Our seven outcomes of interest were hospitalizations with discharge diagnoses including heat illness (ICD-9-CM codes 992.0–992.9, E900 and E900.9) and all natural causes (ICD-9-CM codes < 800 and heat illness) as well as the specific hospitalizations causes of diabetes mellitus (code 250), cardiovascular diseases (codes 390–448), acute myocardial infarction (code 410), all respiratory diseases (codes 460–519) and renal diseases (codes 580–589). We chose these outcomes based on prior research linking these specific causes of mortality or morbidity with temperature (Knowlton et al. 2009; Schwartz et al. 2004). We defined the outcome two ways: using 1) only the principal diagnosis and 2) the principal or any of the secondary diagnoses. We used only the first 16 of 35 diagnosis fields due to poor data quality in diagnosis field 17. In 96.1% of the admissions records, additional diagnoses were not listed in fields 17 to 35.
STATISTICAL ANALYSIS
EH was modeled as a dummy variable, taking the value of 1 when the daily mean temperature on the day of admission (lag day 0) was above the 97th percentile of the daily mean temperatures from May– September 2000–2009, for each temperature monitor (Basagana et al. 2011). We also examined the effects of heat waves, or daily mean temperature above the 97th percentile for 2, 3 or 4 consecutive days (lag days 2–4) (Basagana et al. 2011). We examined the sensitivity of our results to the percentile threshold by also modeling EH as daily mean temperature above the 95th or 99th percentiles of daily mean temperature. We also examined the sensitivity of our results by including in our models a dummy variable for cold, defined as daily mean temperatures at or below 10°C, which corresponded to the 10th–14th percentiles of temperature in each county. Cold days occur in Michigan in May and September, which would otherwise be included in our reference category of non-EH.
A case-crossover design with a time-stratified control selection approach was used. The case-crossover design is a type of matched case-control design where each person in the study acts as his or her own control, so confounding or bias due to non-time-varying individual characteristics is minimized, and effect modification by unique individual traits can be explored (Basagana et al. 2011; Basu 2002). Three to four control days per case were created and assigned the exposures corresponding to the same days of the week within the same calendar month. For instance, if the case (hospitalization) occurred on the 8th of May, 2000 then the controls were assigned the exposures corresponding to the 1st, 15th, 22nd and 29th of May, 2000. To test the sensitivity of the results to control for season, we divided the year into three-week periods, and used these three-week periods as time strata instead of calendar month.
We employed conditional logistic regression using the PHREG procedure in SAS 9.2 to estimate the association between outcome and exposure, with and without adjustment for daily mean ozone on lag day 0. To examine effect modification, we included interaction terms between the temperature exposure and the following patient characteristics, modeled as dummy variables: age 65 years and older, male sex, non-white race and private health insurance (payer). For example, we modeled:
where nonwhite was a dummy variable taking a value of 1 for patients of non-white race. The model also included dummy variables for each patient, which were conditioned out of the likelihood estimator. The effect modifier was considered statistically significant if the p-value from a two-sample t-test comparing the pooled effects in each group was less than 0.05.
We classified health insurance payer as a proxy for income as follows: those with private insurance (including health maintenance organizations and preferred provider organizations) were included in the high payer category, and the low-payer category included Medicaid and self-pay. We excluded individuals aged 65 or older from the payer analysis because most Americans aged 65 or older are enrolled in Medicare hospital insurance, regardless of income level.
Individual county results were pooled in an inverse-variance weighted fixed effects meta-analysis, i.e., such that the means and variances were averaged with weights equal to the inverse variance of the county-specific result. To test for heterogeneity in the effect modification results between cities, we performed pairwise t-tests, comparing each city’s effect estimate to those of the other two, and applied a Bonferroni correction.
RESULTS
Wayne, Ingham and Washtenaw Counties had a total of 1.035 million, 108,745 and 107,619 hospitalizations, respectively, for all natural causes during the study period (Table S1). Cardiovascular diseases were the most frequent principal discharge diagnosis, comprising approximately 19% of the admissions in each county. Over the entire period in the three counties combined, there were 144 hospitalizations with a primary discharge diagnosis related to heat exposure (Table 1). Therefore, we did not analyze this small number of heat-related hospitalizations separately. For all natural causes, individuals under 65 years of age accounted for 64% of the hospitalizations but 73% of the diabetes mellitus hospitalizations and only 40% of the renal disease, cardiovascular disease and myocardial infarction hospitalizations. Men accounted for a greater proportion of heat-related hospitalizations (69%), while women accounted for a greater percentage of hospitalizations for all natural causes (61%). Counts for other specific causes were similar between the sexes. Counts of hospitalizations by cause were also similar between the two categories of race, with the exception of diabetes mellitus, for which non-whites accounted for about two-thirds of the hospitalizations, and of acute myocardial infarction, for which whites accounted for about two-thirds of the hospitalizations. Though race was categorized into white and non-white, 95% of the non-white population were African American (results not shown). Mean daily temperatures and their 95th, 97th and 99th percentile thresholds were similar between counties (Table 2).
Table 1.
Hospitalizations counts (and percentages) by principal discharge diagnosisa and individual characteristics for Wayne, Washtenaw and Ingham Counties, Michigan, May– September, 2000–2009. See Supporting Information Table S1 for county-specific results.
| Heat | Cardiovascular diseases | Respiratory diseases | Diabetes mellitus | Renal diseases | Acute myocardial infarction | All natural causes | |
|---|---|---|---|---|---|---|---|
| Total | 144 | 260,365 | 126,745 | 26,363 | 18,073 | 27,979 | 1,251,481 |
| Age | |||||||
| < 65 years | 74 (52) | 108,514 (42) | 71,797 (57) | 19,156 (73) | 7,285 (40) | 11,755 (42) | 794,920 (64) |
| >= 65 years | 69 (48) | 151,842 (58) | 54,934 (43) | 7,207 (27) | 10,788 (60) | 16,223 (58) | 453,687 (36) |
| Sex | |||||||
| Male | 99 (69) | 127,300 (49) | 59,179 (47) | 13,094 (50) | 9,039 (50) | 14,912 (53) | 489,840 (39) |
| Female | 45 (31) | 133,063 (51) | 67,566 (53) | 13,269 (50) | 9,034 (50) | 13,065 (47) | 761,634 (61) |
| Race | |||||||
| White | 81 (56) | 151,089 (58) | 65,852 (52) | 9,797 (37) | 8,632 (48) | 18,316 (65) | 682,856 (55) |
| Non- white | 63 (44) | 109,209 (42) | 60,837 (48) | 16,555 (63) | 9,435 (52) | 9,658 (35) | 567,819 (45) |
| Payerb | |||||||
| Low | 17 (12) | 22,602 (9) | 21,849 (17) | 5,655 (22) | 1,892 (11) | 2,251 (8) | 214,777 (17) |
| High | 46 (32) | 70,823 (27) | 39,399 (31) | 8,938 (35) | 3,863 (22) | 8,667 (31) | 466,414 (38) |
International Classification of Diseases (ICD), 9th Revision, Clinical Modification (ICD-9-CM) codes for each admissions case diagnosis: heat (992.0–992.3, E900, E900.0, E900.9) cardiovascular diseases (390–448), respiratory diseases (460–519), diabetes mellitus (250), renal diseases (580–589), acute myocardial infarction (410) and all natural causes (< 800).
Payer is among individuals less than 65 years of age. High = private insurance. Low = Medicaid or self-paid.
Table 2.
Mean daily mean temperature and 95th, 97th and 99th percentile thresholds of daily mean temperature in Wayne, Washtenaw and Ingham Counties, Michigan, May–September, 2000–2009.
| County | Mean Daily Mean Temperature, °C | 95th Percentile of Daily Mean Temperature, °C | 97th Percentile of Daily Mean Temperature, °C | 99th Percentile of Daily Mean Temperature, °C |
|---|---|---|---|---|
| Wayne | 18.5 | 26.4 | 26.9 | 28.1 |
| Washtenaw | 18.3 | 26.1 | 26.7 | 27.5 |
| Ingham | 17.0 | 25.3 | 26.1 | 26.9 |
| Overall | 17.9 | 26.1 | 26.7 | 27.8 |
We assessed the associations between EH and hospitalization for residents of the three individual counties and the pooled results in a model without ozone (model 1) and with ozone (model 2), as well as in models where the outcome was based on primary discharge diagnosis alone (model 1) or on both primary and secondary diagnoses (model 3) (Table 3). In model 4, we modeled the time strata as three-week periods instead of calendar months. In county-specific analyses, the ORs did not change by more than 10% when ozone was added to the model. Additionally, model 3 effect estimates were similar to those in model 1. Results were somewhat sensitive to seasonal control, and in contrast to model 1 results, model 4 associations between EH and cardiovascular hospitalizations were null rather than protective in Wayne County and were significantly greater than 1 in Wayne and Ingham counties and in the pooled results for all-natural-cause hospitalizations. Otherwise, we focus our interpretations on model 1. In the pooled analyses, EH and renal disease hospitalizations were positively associated in model 1 (OR = 1.19, 95% CI = 1.03–1.38). EH was not associated with hospitalization for respiratory disease, acute myocardial infarction or diabetes mellitus, in county-specific or pooled results. When we included a term for cold in our models, we found virtually no change in our study results (data not shown).
Table 3.
Odds ratios (95% confidence intervals) for hospitalization for selected causes (using primary only or primary and secondary discharge diagnoses) for the association between hospitalization and extreme heat (EH, daily mean temperature above the 97th percentile on lag day 0) among residents of three Michigan counties in models with and without adjustment for daily mean ozone on lag day 0, May–September, 2000–2009.
| Cardiovascular diseases | Respiratory diseases | Diabetes mellitus | Renal diseases | Acute myocardial infarction | All natural causes | |
|---|---|---|---|---|---|---|
| Wayne | ||||||
| Model 1 | 0.97 (0.94–0.99) | 0.98 (0.95– 1.02) | 0.96 (0.89– 1.04) | 1.22 (1.12– 1.33) | 1.04 (0.96–1.12) | 0.99 (0.98– 1.00) |
| Model 2 | 0.97 (0.95–0.99) | 1.00 (0.96– 1.03) | 0.96 (0.89– 1.05) | 1.17 (1.07– 1.28) | 1.04 (0.96–1.12) | 0.99 (0.98– 1.00) |
| Model 3 | 0.98 (0.97–1.00) | 0.98 (0.96– 1.00) | 0.98 (0.96– 1.01) | 1.05 (1.01– 1.08) | 1.01 (0.95–1.08) | 0.99 (0.98– 1.00) |
| Model 4 | 1.02 (0.99, 1.05) | 1.04 (0.99, 1.08) | 0.98 (0.90, 1.07) | 1.25 (1.13, 1.37) | 1.08 (0.99, 1.17) | 1.02 (1.01, 1.03) |
| Washtenaw | ||||||
| Model 1 | 0.89 (0.82–0.98) | 1.03 (0.90– 1.19) | 1.01 (0.75– 1.36) | 0.94 (0.68– 1.30) | 0.90 (0.68–1.20) | 0.99 (0.95– 1.02) |
| Model 2 | 0.89 (0.81–0.98) | 1.02 (0.89– 1.18) | 1.07 (0.79– 1.44) | 0.89 (0.64– 1.24) | 0.88 (0.66–1.18) | 1.00 (0.96– 1.04) |
| Model 3 | 0.99 (0.94–1.04) | 0.93 (0.86– 1.01) | 0.98 (0.90– 1.08) | 1.01 (0.89– 1.15) | 0.89 (0.71–1.13) | 0.99 (0.95– 1.02) |
| Model 4 | 0.93 (0.84, 1.02) | 1.00 (0.86, 1.17) | 1.01 (0.74, 1.39) | 0.82 (0.59, 1.15) | 1.13 (0.83, 1.53) | 1.01 (0.97, 1.05) |
| Ingham | ||||||
| Model 1 | 0.98 (0.87–1.11) | 1.00 (0.83– 1.21) | 1.00 (0.70– 1.44) | 0.86 (0.44– 1.67) | 1.14 (0.77–1.70) | 1.03 (0.98– 1.09) |
| Model 2 | 0.98 (0.86–1.11) | 1.00 (0.83– 1.21) | 1.03 (0.71– 1.49) | 0.82 (0.42– 1.61) | 1.16 (0.78–1.74) | 1.03 (0.98– 1.08) |
| Model 3 | 1.06 (0.99–1.13) | 1.08 (0.97– 1.19) | 1.01 (0.90– 1.14) | 1.17 (0.97– 1.41) | 1.19 (0.85–1.65) | 1.03 (0.98– 1.09) |
| Model 4 | 1.06 (0.93, 1.21) | 1.11 (0.91, 1.36) | 1.13 (0.76, 1.67) | 0.79 (0.40, 1.58) | 1.18 (0.77, 1.80) | 1.12 (1.06, 1.19) |
| Pooled | ||||||
| Model 1 | 0.96 (0.92, 1.01) | 0.99 (0.93, 1.05) | 0.97 (0.85, 1.10) | 1.19 (1.03, 1.38) | 1.03 (0.91, 1.17) | 0.99 (0.97– 1.01) |
| Model 2 | 0.97 (0.93, 1.01) | 1.00 (0.94, 1.07) | 0.97 (0.85, 1.11) | 1.14 (0.98, 1.33) | 1.03 (0.90, 1.17) | 0.99 (0.97– 1.01) |
| Model 3 | 0.99 (0.96, 1.01) | 0.98 (0.95, 1.02) | 0.99 (0.95, 1.03) | 1.05 (1.00, 1.11) | 1.01 (0.91, 1.12) | 0.99 (0.97– 1.01) |
| Model 4 | 1.01 (0.97, 1.06) | 1.04 (0.97, 1.11) | 0.99 (0.86, 1.14) | 1.20 (1.03, 1.40) | 1.09 (0.94, 1.25) | 1.02 (1.00, 1.05) |
Model 1 : Logit(admission) = β1EH (Outcome = Principal diagnosis only)
Model 2 : Logit(admission) = β1EH+β2ozone0 (Outcome =Principal diagnosis only)
Model 3 : Logit(admission) = β1EH (Outcome = Principal and Secondary diagnoses)
Model 4 : Model 1 except time strata were three-week periods instead of calendar months
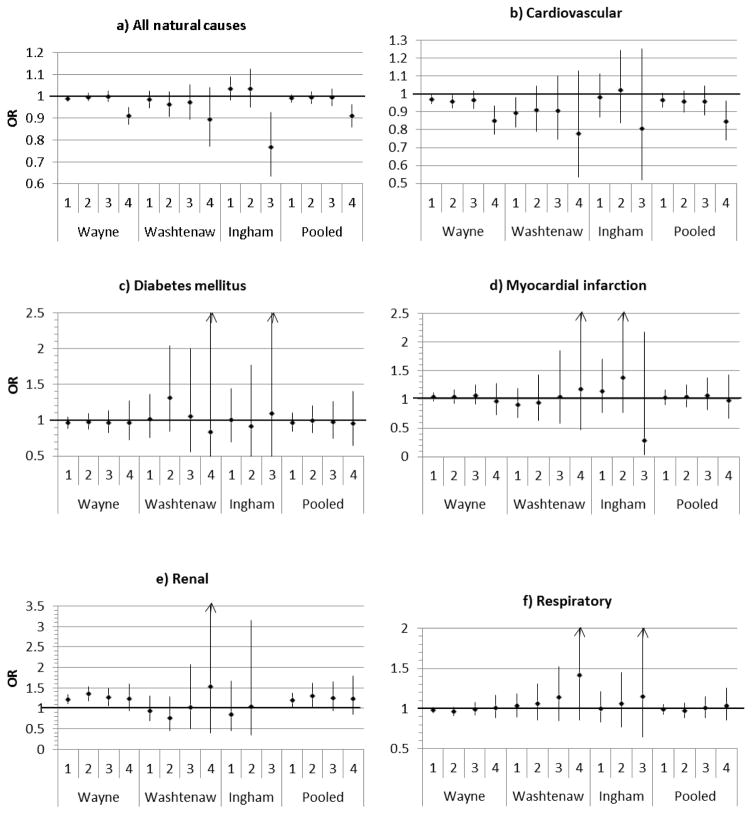
The odds of hospitalization after a four-day heat wave were significantly decreased for admissions for all natural causes (OR = 0.91, 95% CI = 0.86–0.96) and cardiovascular diseases (OR = 0.84, 95% CI = 0.74–0.96) when pooled across the two counties which experienced four-day heat waves (Figure 1). Similar to one day of EH, we found an increased odds of hospitalization for renal diseases associated with a two-day heat wave (pooled OR=1.30, 95% CI=1.05–1.61). However, we did not see a further increase in hospitalizations as the length of the heat wave increased. Heat waves of any length, with EH defined at the 97th percentile of temperature, were not associated with hospitalization for diabetes mellitus, myocardial infarction or respiratory diseases.
Figure 1.
Figure 1a–1f. Odds ratios and 95% confidence intervals for the association between extreme heat defined as daily mean temperature above the 97th percentile for 1, 2, 3 or 4 consecutive days and cause-specific hospitalizations, based on the principal diagnoses, for Wayne, Washtenaw, Ingham Counties and pooled results among the three counties, May–September 2000–2009. (Arrows indicate that confidence intervals extend beyond the graph boundaries.)
In general, after pooling the results among counties, effect modification of the association between EH and hospitalization by age, sex, race or payer was not observed (Table 4). The one exception was for renal diseases, for which we had also found a significant main effect. Among non-whites, we found a 1.37 (95% CI = 1.13–1.66) increased odds of hospitalization for renal diseases during EH vs. non-EH, while among whites, we found a null association between hospitalization for renal diseases and EH (OR = 1.00, 95% CI = 0.81–1.25, two-sample t-test p = 0.037). In the county-specific analyses (Table S2), we did also find an increased odds of cardiovascular and all-natural-cause hospitalizations among individuals in the low payer category in Ingham County (OR = 5.67, 95% CI = 0.94–34.0 for cardiovascular and OR = 3.17, 95% CI = 1.16–8.77 for all natural cause) and an association suggestive of a protective effect in the high payer category (OR = 0.46, 95% CI = 0.21–1.02, two-sample t-test p = 0.012). However, in pairwise t-tests, we did not find significant differences between any two counties in the associations between EH and any hospitalization cause within any category of age, sex, race or payer. In the pooled results, ORs were consistently higher among the low payer category than the high payer category for each hospitalization cause, although the differences between the two payer categories were not statistically significant.
Table 4.
Odds ratios and 95% confidence intervals for the association between hospitalization for the principal diagnoses of cardiovascular disease, acute myocardial infarction, respiratory disease, diabetes mellitus, and all natural causes and EH (defined as mean daily temperature above the 97th percentile on lag day 0) by categories of age, sex, race and health insurance payer pooled across Wayne, Ingham, and Washtenaw Counties in Michigan. See Supporting Information Table S2 for county-specific results.
| Cardiovascular diseases | Respiratory diseases | Diabetes mellitus | Renal diseases | Acute myocardial infarction | All natural cause | |
|---|---|---|---|---|---|---|
| Age | ||||||
| < 65 years | 0.97 (0.91, 1.04) | 0.97 (0.89, 1.05) | 0.92 (0.79, 1.08) | 1.18 (0.94, 1.48) | 0.99 (0.82, 1.21) | 0.99 (0.97, 1.01) |
| >= 65 years | 0.96 (0.91, 1.01) | 1.01 (0.92, 1.11) | 1.09 (0.85, 1.39) | 1.20 (0.99, 1.45) | 1.06 (0.90, 1.26) | 0.99 (0.96, 1.03) |
| Sex | ||||||
| Male | 0.96 (0.90, 1.01) | 0.99 (0.91, 1.08) | 0.98 (0.82, 1.18) | 1.24 (1.01, 1.52) | 0.98 (0.81, 1.18) | 0.99 (0.96, 1.01) |
| Female | 0.97 (0.92, 1.03) | 0.98 (0.90, 1.08) | 0.95 (0.79, 1.14) | 1.14 (0.93, 1.40) | 1.09 (0.91, 1.29) | 0.99 (0.96, 1.02) |
| Race | ||||||
| White | 0.96 (0.91, 1.01) | 1.02 (0.94, 1.11) | 0.98 (0.79, 1.22) | 1.00 (0.81, 1.25) | 1.02 (0.87, 1.19) | 0.99 (0.96, 1.02) |
| Non- white | 0.97 (0.91, 1.03) | 0.95 (0.87, 1.04) | 0.96 (0.81, 1.13) | 1.37 (1.13, 1.66)* | 1.06 (0.86, 1.32) | 0.99 (0.96, 1.02) |
| Payera | ||||||
| High | 0.95 (0.78, 1.16) | 1.00 (0.70, 1.42) | 1.12 (0.47, 2.71) | 0.87 (0.39, 1.93) | 0.84 (0.45, 1.57) | 0.96 (0.85, 1.08) |
| Low | 1.28 (0.58, 2.84) | 1.25 (0.43, 3.65) | b | 1.03 (0.21, 4.99) | 1.59 (0.08, 31.6) | 1.09 (0.69, 1.72) |
Significant difference (p < 0.05) in effects between the two categories.
Payer is among individuals less than 65 years of age. High = private insurance. Low = Medicaid or self-paid.
Too few cases on heat-wave days in this group to estimate an effect.
Finally, when comparing effects for different definitions of EH, we found a weak inverse association between EH, defined at the 95th percentile of temperature, and hospitalization for cardiovascular disease in the pooled analysis (OR = 0.96, 95% CI = 0.93–0.99). Although not reaching statistical significance, the odds ratios for respiratory diseases as well as diabetes mellitus were also all consistently less than one at each of the percentile thresholds of EH in the pooled analyses. For both renal disease and myocardial infarction, we observed an increased odds of hospitalization with increasingly hot definitions of EH. Furthermore, in Wayne and Ingham Counties, EH, defined at the 99th percentile of temperature, and hospitalization for myocardial infarction were significantly associated, whereas these associations were not evident when EH was defined at lower thresholds.
DISCUSSION
We evaluated the effect of EH on hospitalizations and how demographic factors such as age, sex, race, and income, as proxied by health insurance payer, could modify the association between EH and hospitalization in three temperate counties in Michigan. Hospitalization for renal disease was positively associated with EH, which supports most studies that have looked at the association between renal pathology and morbidity due to EH exposure (Gronlund 2014; Li et al. 2015). This association might be because cases of primary heat- related diseases often present as water and electrolyte imbalance due to fluid loss through increased sweating and/or that individuals with pre-existing renal disease are at increased risk for this same presentation/hospitalization. Additionally, the medications commonly used to treat renal disease, such as diuretics, may also impair thermoregulatory responses (Levine 2012).
We found a significant decrease in heat-associated hospitalizations for cardiovascular disease with the coolest EH definition, at or above the 95th percentile threshold of temperature, and a null association at hotter definitions of EH. Additionally, our results were also suggestive of protective associations between EH and respiratory hospitalizations as well as hospitalizations for diabetes mellitus. However, our results for cardiovascular hospitalizations were sensitive to how season was controlled for, with protective effects becoming null when the time stratum from which controls were drawn was shortened from a month to three weeks. This null or inverse association of EH with cardiovascular hospitalizations is consistent with several other recent studies (Gronlund et al. 2014, Turner et al. 2012, Petitti et al. 2016; Ye et al. 2012), including a meta-analysis (Phung et al. 2016). As in our study, Wichmann et al. (2013) also found a significant decrease in the odds of hospitalization for acute myocardial infarction on hot days when compared to non-hot days. However, not all studies have found this inverse association, and a recent study in Korea found a 4.5% increase in cardiovascular hospitalizations with EH (Son et al. 2014). It is not clear why an inverse or null association between hospitalizations and EH exists in many locations, given the positive association between EH and cardiovascular mortality. One hypothesis is that of competing risks between mortality and hospitalization: the cardiovascular symptoms might be so severe during the first day of EH exposure that they result in death instead of hospitalization (Gronlund et al. 2014). A second explanation is that persons with cardiovascular disease are managed by emergency department or outpatient treatment that is not followed by a hospitalization. A third, more optimistic alternative is that public health measures to protect Michiganders from EH are effective, and professional urgent medical care is not sought by individuals who might otherwise have experienced hospitalizations for cardiovascular or respiratory diseases or diabetes mellitus. Future analyses of mortality, emergency department and outpatient records are necessary to shed light on this issue.
The decreased odds of hospitalization for cardiovascular disease following four consecutive days of EH might be due to competing risks between mortality and morbidity or a displacement, or harvesting, effect. With the harvesting hypothesis, a decrease in hospitalizations occurs in the days following an EH event because these relatively frail individuals who were hospitalized on the day of the event were removed from the pool of un-hospitalized at-risk individuals (Schwartz 2000).
We also observed an increase in hospitalization for acute myocardial infarction at temperatures above the 99th percentile in all three counties, consistent with other studies (Koken et al. 2003; Semenza et al. 1999). Grouping cardiovascular diseases together may underestimate the effect of heat exposure on different specific cardiovascular diseases, such as acute myocardial infarction. We lacked the sample size and statistical power to disaggregate our cardiovascular diseases into diagnoses other than acute myocardial infarction. However, this phenomenon was noted in a California study that showed no association between emergency room visits for all cardiovascular diseases and temperature but significant associations for some specific cardiovascular diseases, including ischemic stroke, ischemic heart disease, and cardiac dysrhythmia with temperature (Basu et al. 2012).
Hospitalization for diabetes mellitus was not associated with EH. This result is similar to that observed by Semenza et al. during the 1995 Chicago heat wave, where no significant vulnerability by diabetes mellitus status was seen when type 1 and type 2 diabetes mellitus were combined. However, they saw a significant increases in hospitalizations for type 1 and type 2 diabetes when they were analyzed as two separate outcomes (Semenza et al. 1999).
We looked at ozone as a confounder as previous studies differed on the confounding effect of ozone for the association between temperature and heat-related hospitalization for chronic diseases such as respiratory and cardiovascular diseases. Our finding that the OR changed by less than 10% in models with vs. without ozone was consistent with other U.S. studies, which found ozone to be a weak confounder or not a confounder of the association between heat and respiratory morbidity (Basu et al. 2012; Green et al. 2010; Gronlund et al. 2014). Ozone may also be considered a mediator of the association between extreme heat and health, given that increased temperatures aid the chemical reactions which form ozone (Buckley et al.2014). In this case, it would actually be inappropriate to include a term for ozone in the model if one was interested in the total effects—direct and indirect—of EH. Given that our results did not change by more than 10%, ozone is unlikely to be a substantial mediator of the association.
The EH effect on hospitalizations for renal diseases was modified by race. Non-whites, a population which consisted mainly of African Americans in our study area, had significantly higher odds of hospitalization for renal diseases during EH vs. non-EH compared to the white population. Studies of the effect of heat-wave events on mortality have also shown that African Americans have a higher risk of mortality from heat-related illness when compared to whites. This difference is often attributed to social and economic factors (McGeehin and Mirabelli 2001; Gronlund 2014). However, we did not see effect modification by health insurance payer. This discrepancy may exist solely because we had much less statistical power in our payer analysis given our need to restrict the analysis to non-Medicare recipients under the age of 65. However, among African Americans as compared to whites, there is a marked difference in the progression of end-stage renal disease, a genetic predisposition to renal disease due to variants in the APOL1 gene and evidence that renal function decline may be faster (Genovese et al. 2010; Peralta et al. 2013; Pollak et al. 2012). Therefore, African Americans may be more susceptible to hospitalization for renal disease during EH given a tendency towards more advanced renal disease than whites. On the other hand, Wayne County accounted for a large proportion of our total sample and also had a larger proportion of African Americans than the other two counties. Therefore, the significant association between EH and renal hospitalizations and the apparent effect modification by race may be driven by unaccounted risk factors, such as urban heat island effects, born more heavily by Wayne County (which contains the predominately African American city of Detroit) than the other two counties.
The strengths of our study include the use of MIDB, which is a robust database and contains virtually all inpatient hospitalizations that occurred in Michigan for all ages. We were also able to look at the effect of income, as proxied by health insurance payer, on chronic disease hospitalizations that were potentially related to heat exposure in those under age 65. Furthermore, we examined the effect of temperature exposure at different levels of intensity and duration. However, our cases are persons admitted to acute care hospitals. Other studies have shown that emergency room visits may be a more sensitive indicator of the association between heat waves and heat-associated morbidity (Knowlton et al. 2009). This may also be true in Michigan, where syndromic surveillance of emergency room visits has shown increased numbers of heat-related chief complaints during heat events (Mamou et al. 2013).
Additional limitations include the exclusion of individuals aged 65 and older from the payer analysis; the use of an ecologic exposure—daily temperature at the nearest monitor, which was assigned to residents as far as 55 km from the monitor—rather than personal EH exposure based on microclimates such as urban heat islands and air conditioning access; and not accounting for differences in risk over the course of the summer related to acclimatization to EH earlier in the season. We also did not test the sensitivity of our results to using a temperature metric that accounts for humidity, such as apparent temperature or heat index. It is unlikely that our results would be substantially different given that, in a recent national study of hospitalizations and heat among the elderly, Gronlund et al. (2014) found similar results between extreme-heat effects estimated using mean temperature and extreme-heat effects estimated using apparent temperature. Furthermore, the Ann Arbor temperature monitor, which we selected based on its location within the city limits of Ann Arbor, did not measure humidity or dew point.
In conclusion, our study confirms an association between EH and hospitalizations for renal diseases in this temperate-climate population. We also observed differences in the odds of hospitalization for renal diseases by race. These results suggest that patients with renal conditions may particularly benefit from education on the increased risk of renal health effects during EH. Further research is needed to compare mortality and morbidity data for heat-associated events during EH in the same time period and population, to determine differences in risk factors and vulnerable populations. We also recommend more research on specific diagnoses of cardiovascular disease (i.e., ischemic stroke, ischemic heart disease and cardiac dysrhythmia) and diabetes mellitus (both Type 1 and Type 2), as well as on the competing effects of mortality and morbidity, in order to further understand the public health burden of EH.
Supplementary Material
Table S1. Hospitalizations counts (and percentages) by principal discharge diagnosisa and individual characteristics for Wayne, Washtenaw and Ingham Counties, Michigan, May– September, 2000–2009.
Table S2. Odds ratios and 95% confidence intervals for the association between hospitalization for the principal diagnoses of cardiovascular disease, acute myocardial infarction, respiratory disease, diabetes mellitus and all natural causes and extreme heat (defined as mean daily temperature above the 97th percentile on lag day 0) within categories of age, sex, race and income in Wayne, Ingham and Washtenaw Counties in Michigan, 2000–2009.
Table 5.
Odds ratios and 95% confidence intervals for the associations between cause-specific hospitalizations (principal diagnosis only) and EH defined as daily mean temperature above the 95th, 97th and the 99th percentile for daily mean temperature in three Michigan counties, May–September, 2000–2009.
| Cardiovascular | Respiratory | Diabetes mellitus | Renal | Acute myocardial infarction | All natural cause | |
|---|---|---|---|---|---|---|
| Wayne | ||||||
| 95th percentile | 0.97 (0.95, 0.99) | 0.99 (0.96, 1.02) | 0.98 (0.92, 1.04) | 1.14 (1.07, 1.22) | 1.01 (0.95, 1.07) | 0.99 (0.98, 1.00) |
| 97th percentile | 0.97 (0.94, 0.99) | 0.98 (0.95, 1.02) | 0.96 (0.89, 1.04) | 1.22 (1.12, 1.33) | 1.04 (0.96, 1.12) | 0.99 (0.98, 1.00) |
| 99th percentile | 0.98 (0.95, 1.02) | 0.99 (0.94, 1.05) | 0.97 (0.87, 1.08) | 1.37 (1.21, 1.55) | 1.12 (1.01, 1.25) | 1.00 (0.98, 1.02) |
| Washtenaw | ||||||
| 95th percentile | 0.92 (0.86, 0.98) | 1.06 (0.96, 1.17) | 1.03 (0.83, 1.29) | 1.16 (0.91, 1.46) | 0.91 (0.74, 1.12) | 1.00 (0.97, 1.03) |
| 97th percentile | 0.89 (0.81, 0.98) | 1.03 (0.90, 1.19) | 1.01 (0.75, 1.36) | 0.94 (0.68, 1.30) | 0.90 (0.68, 1.20) | 0.99 (0.95, 1.02) |
| 99th percentile | 0.87 (0.74, 1.04) | 0.97 (0.75, 1.26) | 1.16 (0.69, 1.94) | 0.67 (0.34, 1.30) | 1.06 (0.66, 1.70) | 0.96 (0.90, 1.03) |
| Ingham | ||||||
| 95th percentile | 0.91 (0.83, 0.99) | 0.97 (0.85, 1.10) | 0.87 (0.67, 1.15) | 0.86 (0.57, 1.31) | 0.90 (0.69, 1.18) | 0.96 (0.92, 0.99) |
| 97th percentile | 0.98 (0.87, 1.11) | 1.00 (0.83, 1.21) | 1.00 (0.70, 1.44) | 0.86 (0.44, 1.67) | 1.14 (0.77, 1.70) | 1.03 (0.98, 1.09) |
| 99th percentile | 0.95 (0.73, 1.23) | 0.99 (0.64, 1.53) | 0.65 (0.25, 1.69) | 1.62 (0.42, 6.26) | 2.80 (1.27, 6.18) | 1.07 (0.96, 1.20) |
| Pooled | ||||||
| 95th percentile | 0.96 (0.93, 0.99) | 0.99 (0.95, 1.04) | 0.98 (0.89, 1.07) | 1.14 (1.02, 1.27) | 1.00 (0.91, 1.10) | 0.99 (0.98, 1.01) |
| 97th percentile | 0.96 (0.92, 1.01) | 0.99 (0.93, 1.05) | 0.97 (0.85, 1.10) | 1.19 (1.03, 1.38) | 1.03 (0.91, 1.17) | 0.99 (0.97, 1.01) |
| 99th percentile | 0.98 (0.92, 1.03) | 0.99 (0.91, 1.09) | 0.97 (0.81, 1.17) | 1.34 (1.09, 1.66) | 1.14 (0.95, 1.36) | 1.00 (0.97, 1.03) |
Acknowledgments
This research was funded by the U.S. Centers for Disease Control and Prevention (grant EH000348); the National Institute of Environmental Health Sciences (grant R21-ES020156); the National Institute on Aging Interdisciplinary Research Training in Health and Aging (grant T32AG027708) and a University of Michigan Graham Sustainability Institute Dow Sustainability Fellowship. We would also like to thank members of the “Climate change and health: Residential energy-efficiency for comfort and equity” project for their input and guidance and the Division of Vital Records and Health Statistics of the Michigan Department of Health and Human Services for MIDB data access and support. RW and LC were associated with CDC Cooperative Agreement IUE 1EH000744 during the time this study was conducted. The contents of this study are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention.
Portions of this data are taken from a proprietary database owned and maintained by the Michigan Health & Hospital Association Service Corporation (MHASC). All rights reserved. This data may not be used for commercial purposes without first obtaining written permission from the MHASC. Contact MHASC at datakoala@mha.org for more information.
Footnotes
COMPLIANCE WITH ETHICAL STANDARDS
The authors have no conflicts of interest to declare. The research was determined exempt by the University of Michigan Health Sciences and Behavioral Sciences Institutional Review Board.
Contributor Information
Adesuwa S. Ogbomo, University of Michigan, Department of Environmental Health Sciences, School of Public Health. Phone 734-478-1870. Address: University of Michigan, School of Public Health, 1415 Washington Heights, Ann Arbor, Michigan 48109. USA. Affiliation- University of Michigan.
Carina J. Gronlund, University of Michigan, Department of Epidemiology, School of Public Health. Phone 734-615-9215. Address: University of Michigan, School of Public Health, 1415 Washington Heights, Ann Arbor, Michigan 48109. USA. Affiliation- University of Michigan.
Marie S. O’Neill, University of Michigan, Departments of Epidemiology and Environmental Health Sciences, School of Public Health. Phone 734-615-5135. Address: University of Michigan, School of Public Health, 1415 Washington Heights, Ann Arbor, Michigan. 48109, USA. Affiliation- University of Michigan.
Tess Konen, Address: University of Michigan, Department of Environmental Health Sciences, School of Public Health, 1415 Washington Heights, Ann Arbor, Michigan. USA. Affiliation-University of Michigan 48109, USA. Affiliation- University of Michigan.
Lorraine Cameron, Division of Environmental Health, Michigan Department of Health and Human Services. Phone- 517-284-4795. Address- 333 South Grand Ave., Lansing Michigan. 48913. Affiliation- Michigan Department of Health and Human Services.
Robert Wahl, Lifecourse Epidemiology and Genomics Division, Michigan Department of Health and Human Services. Phone- 517-335- 9151. Address- 333 South Grand Ave., Lansing Michigan. 48913. Affiliation- Michigan Department of Health and Human Services.
References
- U.S. Environmental Protection Agency. Technology Transfer Network (TTN) Air Quality System (AQS) 2012 http://www.epa.gov/ttn/airs/airsaqs/detaildata/downloadaqsdata.htm.
- Basagana X, et al. Heat waves and cause-specific mortality at all ages. Epidemiology. 2011;22:765–772. doi: 10.1097/EDE.0b013e31823031c5. [DOI] [PubMed] [Google Scholar]
- Basu R. Relation between Elevated Ambient Temperature and Mortality: A Review of the Epidemiologic Evidence. Epidemiologic Reviews. 2002;24:190–202. doi: 10.1093/epirev/mxf007. [DOI] [PubMed] [Google Scholar]
- Basu R, Pearson D, Malig B, Broadwin R, Green R. The effect of high ambient temperature on emergency room visits. Epidemiology. 2012;23:813–820. doi: 10.1097/EDE.0b013e31826b7f97. [DOI] [PubMed] [Google Scholar]
- Bell ML, O’Neill MS, Ranjit N, Borja-Aburto VH, Cifuentes LA, Gouveia NC. Vulnerability to heat-related mortality in Latin America: a case-crossover study in Sao Paulo, Brazil, Santiago, Chile and Mexico City, Mexico. Int J of Epidemiol. 2008;37:796–804. doi: 10.1093/ije/dyn094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobb JF, Obermeyer Z, Wang Y, Dominici F. Cause-specific risk of hospital admission related to extreme heat in older adults. JAMA. 2014;312:2659–2667. doi: 10.1001/jama.2014.15715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher BA, Lin S, Fitzgerald EF, Hwang S-A. Association of Summer Temperatures With Hospital Admissions for Renal Diseases in New York State: A Case-Crossover Study. Am J Epidemiol. 2012 doi: 10.1093/aje/kwr417. [DOI] [PubMed] [Google Scholar]
- Genovese G, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans Science. 2010;329:841–845. doi: 10.1126/science.1193032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green RS, Basu R, Malig B, Broadwin R, Kim JJ, Ostro B. The effect of temperature on hospital admissions in nine California counties. Int J Public Health. 2010;55:113–121. doi: 10.1007/s00038-009-0076-0. [DOI] [PubMed] [Google Scholar]
- Gronlund CJ, Zanobetti A, Schwartz JD, Wellenius GA, O’Neill MS. Heat, Heat Waves, and Hospital Admissions among the Elderly in the United States, 1992–2006. Environ Health Perspect. 2014 doi: 10.1289/ehp.1206132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronlund CJ, Zanobetti A, Wellenius GA, Schwartz JD, O’Neill MS. Vulnerability to renal, heat and respiratory hospitalizations during extreme heat among US elderly. Climatic Change. 2016;136:1–15. doi: 10.1007/s10584-016-1638-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronlund CJ. Racial and socioeconomic disparities in heat-related health effects and their mechanisms: a review. Curr Epidemiol Rep. 2014 doi: 10.1007/s40471-014-0014-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajat S, Kovats RS, Atkinson WR, Haine A. Impact of hot temperatures on death in London: a time series approach. J Epidemiol Commun Health. 2002;56:367–372. doi: 10.1136/jech. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T, Liang AP, Kilbourne EM, et al. Morbidity and mortality associated with the July 1980 heat wave in St. Louis and Kansas City, MO. JAMA. 1982;247:3327–3331. doi: 10.1001/jama.1982.03320490025030. [DOI] [PubMed] [Google Scholar]
- Knowlton K, et al. The 2006 California heat wave: impacts on hospitalizations and emergency department visits. Environ Health Perspect. 2009;117:61–67. doi: 10.1289/ehp.11594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koken PJM, Piver WT, Ye F, Elixhauser A, Olsen LM, Portier CJ. Temperature, Air Pollution, and Hospitalization for Cardiovascular Diseases among Elderly People in Denver. Environ Health Perspect. 2003;111:1312–1317. doi: 10.1289/ehp.5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravchenko J, Abernethy AP, Fawzy M, Lyerly HK. Minimization of heatwave morbidity and mortality. Am J Prev Med. 2013;44:274–282. doi: 10.1016/j.amepre.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Lam HC, Li AM, Chan EY, Goggins WB., 3rd The short-term association between asthma hospitalisations, ambient temperature, other meteorological factors and air pollutants in Hong Kong: a time-series study. Thorax. 2016 doi: 10.1136/thoraxjnl-2015-208054. [DOI] [PubMed]
- Li M, Gu S, Bi P, Yang J, Liu Q. Heat Waves and Morbidity: Current Knowledge and Further Direction-A Comprehensive Literature Review. International Journal of Environmental Research and Public Health. 2015;12:5256–5283. doi: 10.3390/ijerph120505256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luber G, McGeehin M. Climate Change and Extreme Heat Events. Am J Prev Med. 2008;35:429–435. doi: 10.1016/j.amepre.2008.08.021. [DOI] [PubMed] [Google Scholar]
- Madrigano J, Mittleman MA, Baccarelli A, Goldberg R, Melly S, von Klot S, Schwartz J. Temperature, myocardial infarction, and mortality: effect modification by individual- and area-level characteristics. Epidemiology. 2013;24:439–446. doi: 10.1097/EDE.0b013e3182878397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamou F, Racine R, Henderson T, Fiedler J. Michigan Heat-Related Illness, Emergency Department Visits: 2013 Summary. Michigan Department of Health and Human Services; Lansing, Michigan: 2013. http://www.michigan.gov/documents/mdch/Michigan_Heat_Summary_Summer_2013__437200_7.pdf. [Google Scholar]
- Martiello MA, Giacchi MV. High temperatures and health outcomes: a review of the literature. Scandinavian J Public Health. 2010;38:826–837. doi: 10.1177/1403494810377685. [DOI] [PubMed] [Google Scholar]
- Mastrangelo G, Hajat S, Fadda E, Buja A, Fedeli U, Spolaore P. Contrasting patterns of hospital admissions and mortality during heat waves: Are deaths from circulatory disease a real excess or an artifact? Medical Hypotheses. 2006;66:1025–1028. doi: 10.1016/j.mehy.2005.09.053. [DOI] [PubMed] [Google Scholar]
- McGeehin MA, Mirabelli M. The Potential Impacts of Climate Variability and Change on Temperature-Related Morbidity and Mortality in the United States. Environ Health Perspect. 2001;109:185–189. doi: 10.1289/ehp.109-1240665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Oceanic and Atmospheric Administration (NOAA) National Climatic Data Center (NCDC) Temperature data. 2011 http://lwf.ncdc.noaa.gov/oa/ncdc.html.
- Petitti DB, Hondula DM, Yang S, Harlan SL, Chowell G. Multiple Trigger Points for Quantifying Heat-Health Impacts. New Evidence from a Hot Climate Environmental Health Perspectives. 2016;124:176–183. doi: 10.1289/ehp.1409119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phung D, Thai PK, Guo Y, Morawska L, Rutherford S, Chu C. Ambient temperature and risk of cardiovascular hospitalization: An updated systematic review and meta-analysis. Sci Total Environ. 2016;550:1084–1102. doi: 10.1016/j.scitotenv.2016.01.154. http://dx.doi.org/10.1016/j.scitotenv.2016.01.154. [DOI] [PubMed] [Google Scholar]
- Pillai SK, et al. Heat illness: predictors of hospital admissions among emergency department visits-Georgia, 2002–2008. J Commun Health. 2014;39:90–98. doi: 10.1007/s10900-013-9743-4. [DOI] [PubMed] [Google Scholar]
- Pollak MR, Genovese G, Friedman DJ. APOL1 and kidney disease. Current opinion in nephrology and hypertension. 2012;21:179–182. doi: 10.1097/MNH.0b013e32835012ab. [DOI] [PubMed] [Google Scholar]
- Poumadère M, Mays C, Le Mer S, Blong R. The 2003 Heat Wave in France: Dangerous Climate Change Here and Now. Risk Analysis. 2005;25:1483–1494. doi: 10.1111/j.1539-6924.2005.00694.x. [DOI] [PubMed] [Google Scholar]
- Schwartz J. Harvesting and long term exposure effects in the relation between air pollution and mortality. Am J Epidemiol. 2000;151:440–448. doi: 10.1093/oxfordjournals.aje.a010228. [DOI] [PubMed] [Google Scholar]
- Schwartz J, Samet JM, Patz JA. Hospital Admissions for Heart Disease: The Effects of Temperature and Humidity. Epidemiology. 2004;15:755–761. doi: 10.1097/01.ede.0000134875.15919.0f. [DOI] [PubMed] [Google Scholar]
- Semenza JC, McCullough JE, Flanders WD, McGeehin MA, Lumpkin JR. Excess hospital admissions during the July 1995 heat wave in Chicago. Am J Prev Med. 1999;16:269–277. doi: 10.1016/s0749-3797(99)00025-2. [DOI] [PubMed] [Google Scholar]
- Son JY, Bell ML, Lee JT. The impact of heat, cold, and heat waves on hospital admissions in eight cities in Korea. Int J Biometeorol. 2014;58:1893–1903. doi: 10.1007/s00484-014-0791-y. [DOI] [PubMed] [Google Scholar]
- Tsai SS, Chen PS, Yang YH, Liou SH, Wu TN, Sung FC, Yang CY. Air pollution and hospital admissions for myocardial infarction: are there potentially sensitive groups? J Toxicol Environ Health Part A. 2012;75:242–251. doi: 10.1080/15287394.2012.641202. [DOI] [PubMed] [Google Scholar]
- Turner LR, Barnett AG, Connell D, Tong S. Ambient Temperature and Cardiorespiratory Morbidity: A Systematic Review and Meta-analysis. Epidemiology. 2012;23:594–606. doi: 10.1097/EDE.0b013e3182572795. 510.1097/EDE.1090b1013e3182572795. [DOI] [PubMed] [Google Scholar]
- Vogeli C, Shields AE, Lee TA, Gibson TB, Marder WD, Weiss KB, Blumenthal D. Multiple chronic conditions: prevalence, health consequences, and implications for quality, care management, and costs. J Gen Intern Med. 2007;22(Suppl 3):391–395. doi: 10.1007/s11606-007-0322-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann J, Rosengren A, Sjoberg K, Barregard L, Sallsten G. Association between ambient temperature and acute myocardial infarction hospitalisations in Gothenburg, Sweden: 1985–2010. PLoS One. 2013;8:e62059. doi: 10.1371/journal.pone.0062059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Wolff R, Yu W, Vaneckova P, Pan X, Tong S. Ambient Temperature and Morbidity: A Review of Epidemiological Evidence. Environ Health Perspect. 2012;120:19–28. doi: 10.1289/ehp.1003198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Hospitalizations counts (and percentages) by principal discharge diagnosisa and individual characteristics for Wayne, Washtenaw and Ingham Counties, Michigan, May– September, 2000–2009.
Table S2. Odds ratios and 95% confidence intervals for the association between hospitalization for the principal diagnoses of cardiovascular disease, acute myocardial infarction, respiratory disease, diabetes mellitus and all natural causes and extreme heat (defined as mean daily temperature above the 97th percentile on lag day 0) within categories of age, sex, race and income in Wayne, Ingham and Washtenaw Counties in Michigan, 2000–2009.